Abstract
OBJECTIVE
To assess 7-year associations between magnesium intake and incident prediabetes and/or insulin resistance (IR), and progression from these states to type 2 diabetes.
RESEARCH DESIGN AND METHODS
In 2,582 community-dwelling participants 26–81 years old at baseline, magnesium intake and risk of incident “metabolic impairment,” defined as impaired fasting glucose (FG) (≥5.6 to <7.0 mmol/L), impaired glucose tolerance (2-h postload glucose ≥7.8 to <11.1 mmol/L), IR, or hyperinsulinemia (≥90th percentile of homeostasis model assessment of IR or fasting insulin, respectively), was estimated among those with normal baseline status, and risk of incident diabetes was estimated among those with baseline metabolic impairment. In participants without incident diabetes, we examined magnesium intake in relation to 7-year changes in fasting and postload glucose and insulin, IR, and insulin sensitivity.
RESULTS
After adjusting for age, sex, and energy intake, compared with those with the lowest magnesium intake, those with the highest intake had 37% lower risk of incident metabolic impairment (P trend = 0.02), and in those with baseline metabolic impairment, higher intake was associated with 32% lower risk of incident diabetes (P trend = 0.05). In the combined population, the risk in those with the highest intake was 53% (P trend = 0.0004) of those with the lowest intake. Adjusting for risk factors and dietary fiber attenuated associations in the baseline normal population but did not substantially affect associations in the metabolically impaired. Higher magnesium intake tended to associate with lower follow-up FG and IR, but not fasting insulin, postload values, or insulin sensitivity.
CONCLUSIONS
Magnesium intake may be particularly beneficial in offsetting risk of developing diabetes among those at high risk. Magnesium’s long-term associations with non–steady-state (dynamic) measures deserve further research.
Introduction
Prediabetes and diabetes affected an estimated 45% of U.S. adults in 2010 (1). Diabetes significantly raises the risk of heart disease and stroke morbidity and mortality and is the leading cause of adult blindness and kidney failure. An estimated $245 billion in indirect and direct medical costs are attributable annually to diabetes (2). Diet modification is recommended as an important prevention strategy at any stage of progression from health to overt type 2 diabetes (3). Prospective studies (4–6) have shown that individuals with higher magnesium intake are 10–47% less likely to develop type 2 diabetes. However, only 50% of Americans 1 year of age or older achieve the recommended dietary allowance for magnesium, which is 400–420 mg/day for adult men and 300–310 mg/day for adult women (7,8).
A body of clinical evidence (9–14) supports a role for magnesium supplementation in glucose and insulin metabolism. A meta-analysis of nine magnesium supplement trials in those with type 2 diabetes found that a median magnesium dose of 360 mg/day was associated with significantly lower postintervention fasting glucose (FG) in treatment groups, suggesting improved glucose control (12). A recent small, randomized, placebo-controlled trial in obese, nondiabetic, insulin-resistant individuals demonstrated that 365 mg/day of magnesium for 6 months significantly lowered FG, fasting insulin (FI), and insulin resistance (IR) and improved insulin sensitivity (13). Three-month supplementation with magnesium in individuals with other risk factors, such as mild hypertension or hypomagnesaemia, has been found to improve insulin sensitivity and pancreatic β-cell function (9–11), whereas low-magnesium diets given to otherwise healthy individuals have been shown to impair insulin sensitivity after just 3 weeks (14).
Few prospective studies have evaluated magnesium intake in relation to various stages of progression of disordered glucose and insulin metabolism, i.e., from normal to impaired states, including prediabetes and IR, over the long-term (i.e., >5 years), even though these states are significant risk factors for diabetes as well as cardiovascular disease (15–17). In addition, few studies have examined magnesium’s associations with long-term progression from baseline impaired states to type 2 diabetes (4,18). One study of magnesium intake in U.S. adults estimated that the optimal magnesium intake level in relation to insulin sensitivity measured 5 years later was at least 325 mg/day (18), and another study in U.S. adults reported lower long-term IR with higher magnesium intake (4).
In the present analysis, we evaluated the prospective association between magnesium intake and incidence of metabolic impairment, defined as impaired FG (IFG), impaired glucose tolerance (IGT), IR, or hyperinsulinemia, in otherwise healthy individuals. Further, in those with baseline metabolic impairment (as defined above), we examined whether magnesium intake was associated with incident diabetes. We split the population by metabolic impairment status to assess whether magnesium intake may have differing associations with progression of disease at varying stages of underlying metabolic impairment.
Research Design and Methods
Study Sample
The National Heart, Lung, and Blood Institute’s Framingham Heart Study (FHS) Offspring cohort is a community-based, longitudinal study of cardiovascular disease that began in 1971 whose participants are among the offspring of the original FHS cohort (19). In the fifth examination cycle (1991–1995) of the Offspring cohort, 3,799 participants underwent a standard medical examination, including laboratory and anthropometric measurements, as well as dietary assessment. Participants were followed from baseline at the fifth through seventh (1998–2001) examinations. Individuals were excluded if they had a history of diabetes or were identified as having diabetes at the baseline examination (n = 400), if they had invalid dietary data at baseline (n = 326), if they were missing necessary covariates (n = 109), if they were not present at the final follow-up examination (n = 329, 135 of whom were lost to follow-up owing to death), or if they had invalid or missing dietary data over follow-up (n = 53). The final sample size for the primary analysis was 2,582 participants.
A 2-h 75-g oral glucose tolerance test (OGTT) was administered to all participants at exam 5 and in a subset of participants at exam 7 who had undergone OGTT at exam 5, based on glucose tolerance at exam 5 (sex block-randomly selected from five quintile strata of FG). A total of 863 participants had follow-up OGTT measures available for the present analysis.
The original data collection protocols were approved by the institutional review board at Boston University Medical Center, and written informed consent was obtained from all participants. The current study protocol was reviewed by the Tufts Medical Center and Tufts University Health Sciences institutional review board.
Dietary Assessment
The Harvard semiquantitative, 126-item food frequency questionnaire (FFQ) was used to assess dietary intake at each exam (20). The FFQ included a list of foods together with a standard serving size and nine consumption frequency categories ranging from “never, or less than once per month” to “6+ per day.” Participants were asked to report consumption of each food item over the previous year. Invalid FFQs were defined as those that estimated daily caloric intake as <600 kcal/day or ≥4,000 kcal/day for women or ≥4,200 kcal/day for men or those that had ≥12 blank items. The exposure of interest, total magnesium intake, included both dietary and nondietary (i.e., supplemental) sources. Magnesium intake from nondietary sources contributed ∼5% of total intake.
The relative validity of the FFQ for energy-adjusted magnesium intake has been previously reported (20–22) and shows reasonable correlation with estimates from dietary records (r = 0.67–0.71) (20). All nutrients were adjusted for total energy using the residual method (23).
To account for long-term dietary exposure and to reduce within-person variability, intake of nutrients is presented as mean intake obtained from the dietary data of the fifth (baseline), sixth, and/or seventh examinations. For those with incident type 2 diabetes, intake of nutrients and energy was averaged across dietary data from the fifth examination up to but not including the examination at which diabetes incidence was ascertained. For those without incident diabetes, intake was averaged across all exams (5, 6, and/or 7) for which dietary data were available.
Outcome Measures and Definitions
FG and 2-h OGTT glucose were measured in fresh specimens with a hexokinase reagent kit (A-Gent glucose test; Abbot, South Pasadena, CA). At baseline (exam 5), fasting plasma insulin (FI) and 2-h OGTT insulin were measured using Coat-A-Count total insulin radioimmunoassay (RIA) (Diagnostic Products Corp., Los Angeles, CA), and at exam 7, FI and 2-h OGTT insulin were measured using a different assay, the human-specific insulin RIA (Linco Research Inc., St. Charles, MO). Owing to the different assays used to measure insulin at exams 5 and 7, a calibration study was conducted using FI in frozen plasma from 87 participants. These samples from exam 5 were reanalyzed ∼9 years later using the human-specific insulin RIA assay, and a regression equation was derived to calibrate total insulin RIA measures at exam 5 to human-specific RIA-equivalent values. The calibrated measures were used in the present analysis.
We defined metabolic impairment or type 2 diabetes based in part on impaired glucose criteria from the American Diabetes Association (ADA) (24), in addition to impaired insulin criteria (Supplementary Table 1); participants were classified as having type 2 diabetes if they had a diagnosis of type 2 diabetes, reported use of an oral hypoglycemic drug or insulin, or had FG ≥7.0 mmol/L (≥126 mg/dL) or 2-h OGTT glucose ≥11.1 mmol/L (≥200 mg/dL). Metabolic impairment was defined as having one or more of the following: IFG, IGT, IR, or hyperinsulinemia, per criteria that follow. Participants were classified as having normal FG (NFG) if they had FG <5.6 mmol/L (<100 mg/dL). IFG was classified as FG ≥5.6 to <7.0 mmol/L (≥100 to <126 mg/dL). Normal glucose tolerance (NGT) was classified as 2-h OGTT glucose <7.8 mmol/L (<140 mg/dL). IGT was classified as 2-h OGTT glucose ≥7.8 to <11.1 mmol/L (≥140 to <200 mg/dayL). Homeostasis model assessment of IR (HOMA-IR), a measure of hepatic IR, was calculated as FI (µU/mL) × FG (mmol/L)/22.5 (25). IR was defined as HOMA-IR ≥90th percentile. Hyperinsulinemia was defined as FI ≥90th percentile. Gutt’s insulin sensitivity index0,120 (ISI), a measure of peripheral tissue insulin sensitivity, was calculated as ISI = (m/mean plasma glucose)/log(mean serum insulin), where the glucose uptake rate in peripheral tissues (m) = {75,000 mg + [FG (mg/dL) – 2-h OGTT glucose (mg/dL)] × 0.19 × weight (kg)}/120 min; mean plasma glucose = mean of FG (mmol/L) and 2-h OGTT glucose (mmol/L); and mean serum insulin = mean of serum FI (µU/L) and 2-h OGTT serum insulin (µU/L) (26).
Covariates
Potential confounders of the relationship between diet and progression to metabolic impairment or diabetes were considered as covariates. Covariates were assessed at baseline as follows. Age was measured in years. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). Waist circumference (cm) was measured at the umbilicus with the participant standing. Parental history of diabetes was based on self-reported history in one or both natural parents. Blood pressure (BP) was measured twice by a physician and averaged to calculate the systolic and diastolic BP (mmHg). Hypertension (yes/no) was defined as BP ≥130/85 mmHg or undergoing treatment for hypertension. Information on regular smoking during the year prior to the examination (yes/no) was assessed via questionnaire. Physical activity was quantified as a continuous score based on activity levels as well as intensities of these activities, as previously described (27).
Statistical Analyses
We generated energy-adjusted quintile categories of averaged magnesium intake. Participant characteristics adjusted for age, sex, and energy (in the case of foods and nutrients) are presented across quintile categories. Tests for linear trend across increasing categories of intake were performed by assigning the median value of intake within each category and treating these as a continuous variable.
Because we sought to characterize magnesium’s associations with progression from normal to metabolic impairment, we assessed the association of magnesium intake with 1) incident metabolic impairment (defined as having IFG, IGT, IR, or hyperinsulinemia), among participants with normal status (NFG, NGT, no IR, and normoinsulinemia) at baseline, and 2) incident type 2 diabetes among participants who had baseline metabolic impairment, as defined above. Because there were few cases of incident diabetes among those with normal baseline status (n = 25), these cases were incorporated into our definition of incident metabolic impairment. In secondary sensitivity analyses, we redefined metabolic impairment by use of the ADA prediabetes criteria of IFG and IGT only, as these are frequently used in other clinical and research contexts. This redefinition also allowed us to examine whether excluding IR and hyperinsulinemia from the definition of metabolic impairment impacts magnesium’s associations with incident disorder. Relative risks (RRs) and 95% CIs across quintile categories of magnesium intake were estimated from multivariable logistic regression analyses for incident metabolic impairment or diabetes. P for trend was estimated using the median value in each category of intake.
In secondary analyses in participants without incident diabetes, we assessed the association between magnesium intake and change in continuous measures of FG, FI, HOMA-IR, 2-h OGTT glucose and insulin, and ISI over an average 7-year period. Change was modeled in each case as the final measure adjusted for the baseline measure. For these continuous outcomes, we estimated least squares adjusted means of values in each quintile category of energy-adjusted magnesium intake. P for trend was estimated using the median value in each category of intake. Natural-logged values were used for FI, HOMA-IR, and 2-h OGTT insulin, which were back transformed to geometric means for presentation.
For all outcomes, the initial analysis was adjusted for age, sex, and energy intake (model 1). Model 2 was adjusted as for model 1, plus parental history of diabetes, BMI, physical activity, smoking status, alcohol intake, and hypertension. In model 3, we further adjusted for dietary fiber. Additional adjustment for caffeine did not change the results, and therefore we do not include those results. Dietary fiber and caffeine were initially chosen because they represent surrogates of nonmagnesium constituents of commonly consumed magnesium-containing foods (i.e., whole grains and coffee), which themselves have been associated with lower risk of type 2 diabetes (6,28–30). Adjusting for these dietary variables allows us to at least partially distinguish the associations of magnesium from the associations of the foods themselves, their constituents (e.g., phytochemicals), or health behaviors associated with these nutrients (e.g., higher fiber may also be a surrogate for a healthy lifestyle).
In post hoc analyses, we modeled energy-adjusted dietary magnesium intake, adjusted for magnesium from supplements, to assess whether dietary intake specifically accounted for the observed associations. The results of dietary magnesium paralleled those of total magnesium, and without an a priori hypothesis regarding the mechanism of magnesium from dietary versus supplemental sources, we present the results from the original analyses of total magnesium intake described above.
Finally, we separately tested for statistical interaction between magnesium and age, sex, and BMI in the final models using cross-product terms. No interaction was statistically significant (all P > 0.1). Substituting waist circumference for BMI, or including waist circumference or change in weight between baseline and follow-up, did not substantively alter results.
All analyses were conducted in SAS (version 9.3; SAS Institute, Cary, NC). Statistical significance was set at the 0.05 level. All tests were two tailed.
Results
Baseline clinical and dietary characteristics of 2,582 participants are presented across quartile categories of energy-adjusted magnesium intake in Table 1. The average age of the population was 54 years; 55% were women, 42% were overweight, and 21% were obese. Average magnesium intake was 308 mg/day, which parallels intake reported in other U.S. adult populations (31). Approximately 50% of women and 75% of men reported magnesium intake below the recommended dietary allowance. In analyses of trend from lowest to highest quartile category of magnesium intake, those in the highest category were more likely to be female, older, and have lower BMI. They were less likely to have hypertension or to have smoked regularly in the prior year. Intake of energy and most other nutrients increased along with increasing magnesium intake, except for alcohol. Baseline characteristics of almost all glucose and insulin parameters tended to be lower in participants with higher magnesium intake. Magnesium intake was moderately correlated with dietary fiber (r = 0.67, P < 0.001) but not with caffeine (r = 0.03, P = 0.08).
Table 1.
Characteristics of study population free of type 2 diabetes at baseline
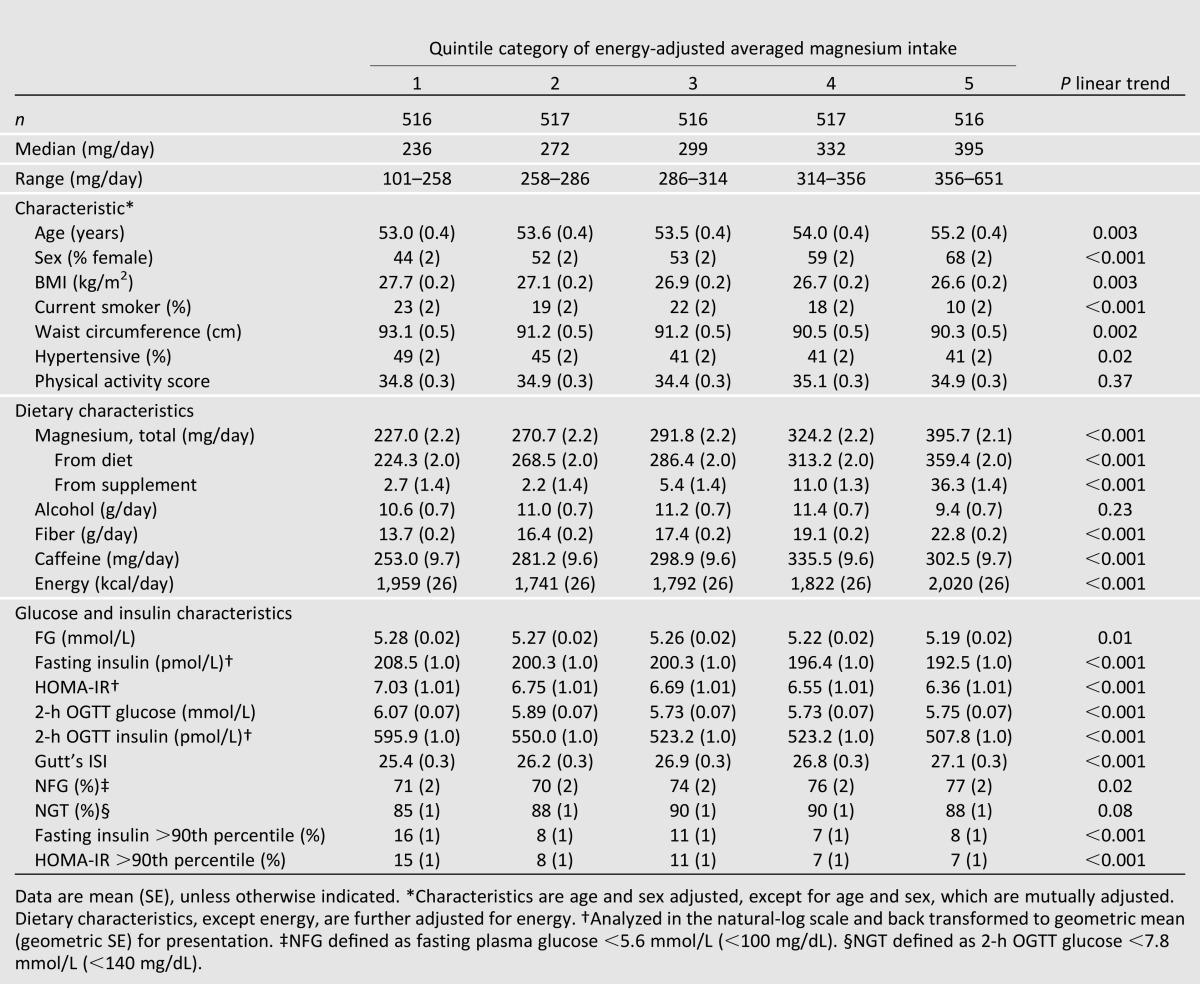
Incident Metabolic Impairment Among Those With Normal Status at Baseline
Among the 1,654 (64.1%) participants without metabolic impairment at baseline, there were 307 (18.6%) cases of incident metabolic impairment, of which 25 were cases of incident diabetes over an average 6.9-year follow-up. Risks of incident metabolic impairment and diabetes in those with normal status at baseline, according to magnesium intake, are presented in Table 2. In the basic model, adjusted for age, sex, and energy intake, higher magnesium intake was associated with 37% lower risk of incident metabolic impairment (Q1 [reference] vs. Q5 RR [95% CI]: 0.63 [0.45–0.87], P trend = 0.02), which was attenuated after adjusting for risk factors (P trend = 0.08) and further attenuated after adjusting for dietary fiber (P trend = 0.26).
Table 2.
RR of progression from normal to metabolically impaired (IFG, IGT, insulin resistant, or hyperinsulinemic) and metabolically impaired to type 2 diabetes, by quintile categories of energy-adjusted magnesium intake*
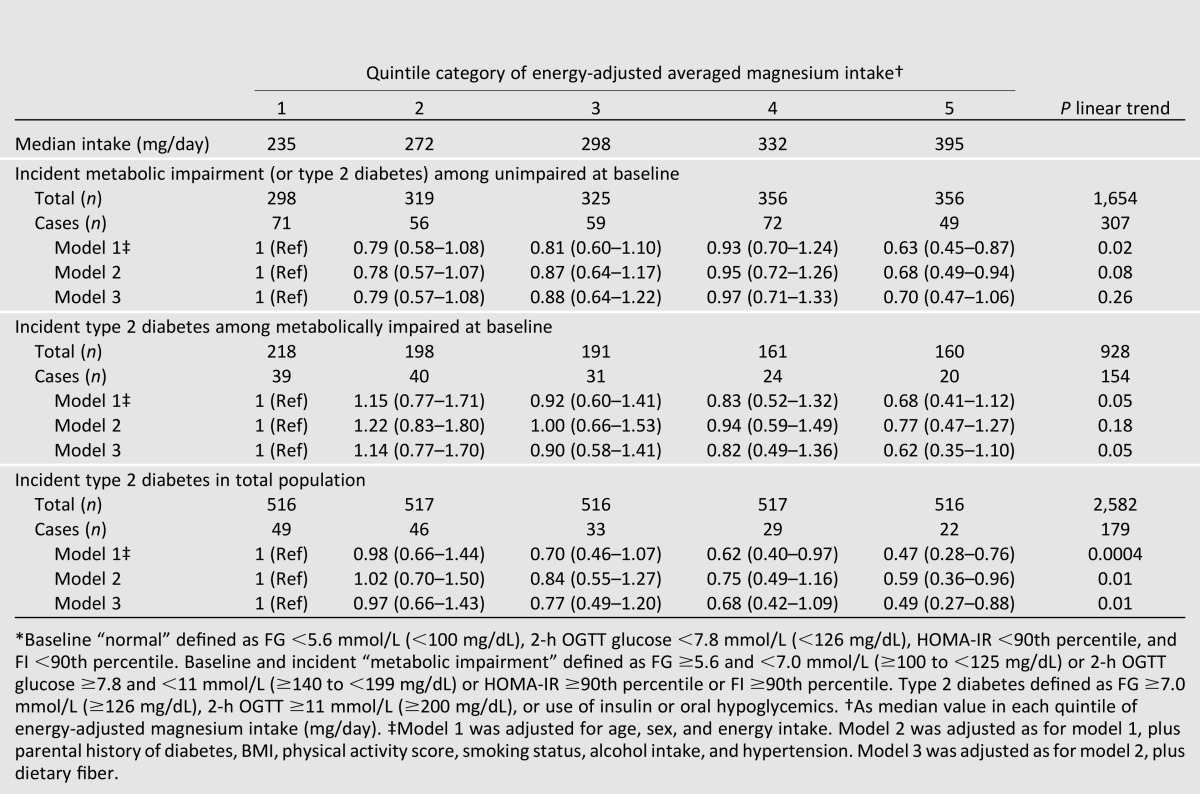
Incident Type 2 Diabetes Among Metabolically Impaired at Baseline
Among the 928 (35.9%) participants impaired at baseline, there were 154 (16.6%) cases of incident diabetes over an average 6.9-year follow-up. After adjusting for age, sex, and energy intake, higher magnesium intake was associated with 32% lower risk of incident diabetes (0.68 [0.41–1.12], P trend = 0.05) (Table 2). The trend was attenuated after adjusting for risk factors (P trend = 0.18), but further adjusting for fiber intake de-attenuated the estimate such that the final estimate was 38% lower risk in the highest compared with the lowest category of magnesium intake (0.62 [0.35–1.10)], P trend = 0.05).
In the total study population, there were 179 (6.9%) incident cases of diabetes over an average 6.9-year follow-up. In fully adjusted models, higher magnesium intake was associated with 51% lower risk of incident diabetes (0.49 [0.27–0.88], P trend = 0.01) (Table 2).
Secondary Analyses
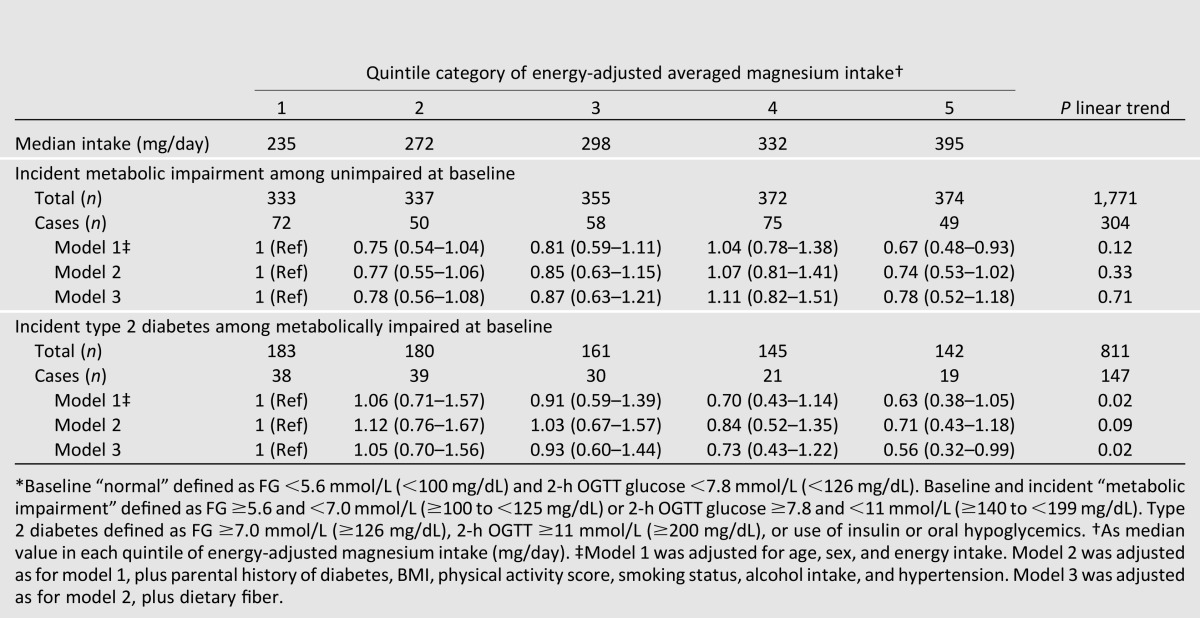
In secondary analyses, IR and hyperinsulinemia were excluded from the working definition of baseline or incident metabolic impairment and, as such, more closely aligned with ADA prediabetes criteria (IGT and/or IFG). Prevalence of baseline metabolic impairment, as a percentage of the total sample, decreased from 35.9 to 31.4%, and incident metabolic impairment, as a percentage of those who were normal at baseline, also decreased from 18.6 to 17.2%.
Results of these analyses were similar to those using the primary definition. Among those with normal status at baseline when impairment was defined by IGT and/or IFG, higher magnesium intake was not associated with risk of incident metabolic impairment after adjusting for age, sex, and energy intake (P trend = 0.12) (Table 3). However, among those initially impaired at baseline, trends for lower risk of incident diabetes across increasing quintile categories of magnesium showed associations similar to those observed when the definition of metabolic impairment included insulin-based criteria; in the fully adjusted model, those with the highest magnesium intake had 44% lower risk of developing diabetes compared with the lowest magnesium intake (RR 0.56 [0.32–0.99], P trend = 0.02).
Table 3.
RR of progression from normal to metabolically impaired (IFG or IGT) or metabolically impaired to type 2 diabetes by ADA criteria, by quintile categories of energy-adjusted magnesium intake*
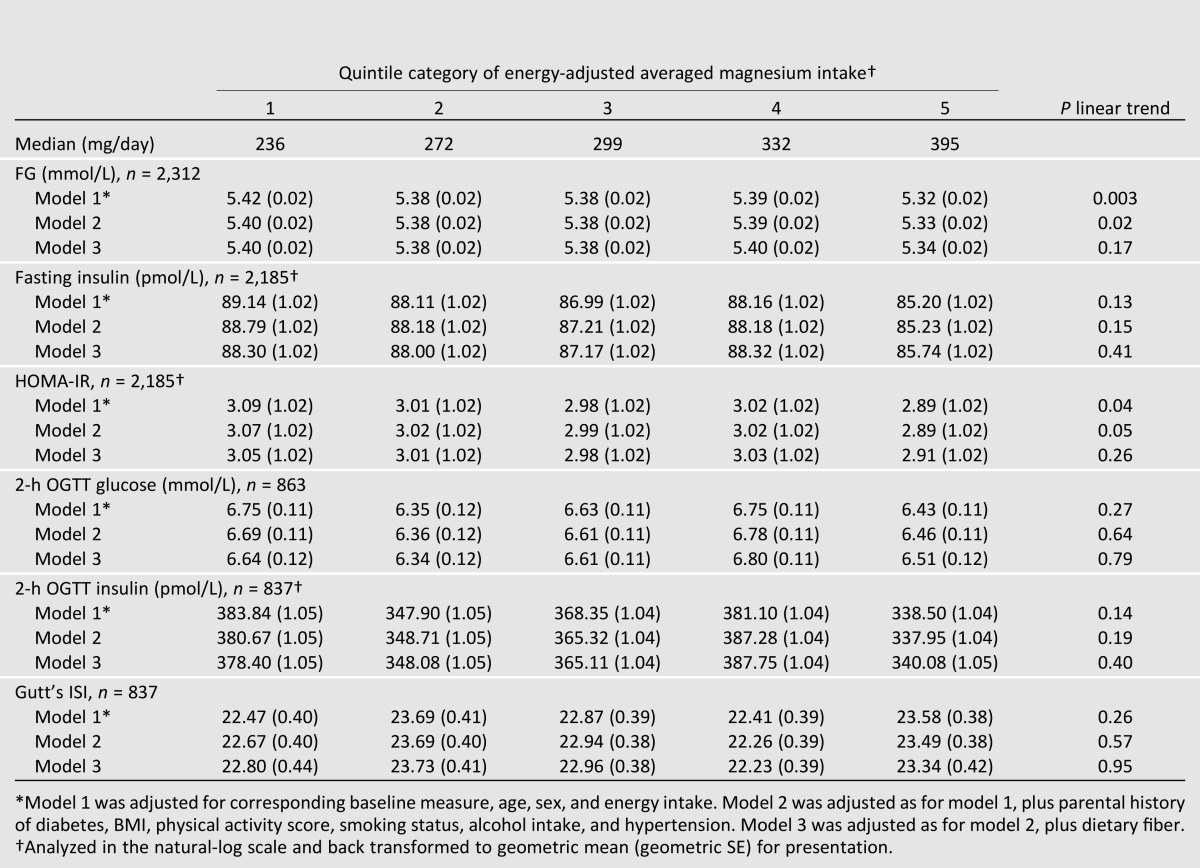
Linear Outcomes in the Total Sample
Adjusted means of various measures of glucose and insulin homeostasis and metabolism after ∼7 years of follow-up in those without incident diabetes are presented in Table 4. In basic models adjusted for age, sex, energy intake, and the corresponding baseline measure, there were significant inverse trends with higher magnesium intake and subsequent FG (Q1 vs. Q5: 5.42 vs. 5.32 mmol/L, P trend = 0.003) and HOMA-IR (3.08 vs. 2.89, P trend = 0.05). However, all trends were attenuated after additionally adjusting the risk factor model (model 2) for dietary fiber (model 3).
Table 4.
Adjusted means of measures of glucose and insulin by quintile categories of energy-adjusted magnesium intake over 7 years of follow-up in participants without incident type 2 diabetes
Conclusions
Our results support previously reported longitudinal associations between higher magnesium intake and lower risk of type 2 diabetes (5,6). Across 7 years of follow-up, higher magnesium intake appeared to partially offset risk of developing metabolic impairment in those with normal baseline glucose and insulin homeostasis. In addition, in those with baseline metabolic impairment, magnesium intake was also associated with lower risk of type 2 diabetes. Interestingly, magnesium’s associations with incident impairment were stronger when the definition of metabolic impairment included hyperinsulinemia and IR than when they included hyperglycemia or impaired glycemic response alone. This is intriguing, since elevated insulin and IR are etiological predecessors of chronically elevated FG concentrations (15), perhaps indicating that magnesium intake is more important for maintaining long-term healthy insulin metabolism. This is supported by our observation that those with the highest magnesium intake had, on average, 6% lower HOMA-IR after 7 years than those with the lowest magnesium intake, after adjusting for risk factors. However, as our results indicate, once metabolic impairment had taken hold, magnesium intake seemed to be associated with lower risk of type 2 diabetes, regardless of whether baseline metabolic impairment was defined by both glucose and insulin criteria or glucose criteria alone.
Our observation of lower risk of type 2 diabetes with higher magnesium intake is one that is fairly well established in the magnesium literature (4–6,32,33). In addition, several clinical studies of magnesium supplementation in those with and without diabetes indicate that magnesium supplementation can improve glycemic control, insulin sensitivity, and β-cell function (9–11,13,34). However, the durations of these clinical studies have been relatively short (≤6 months), and most of the observational studies of magnesium intake in relation to insulin homeostasis or metabolism have been cross-sectional (33,35–37). As such, there is a relative dearth of knowledge on the long-term impact of magnesium intake on insulin metabolism.
Our results related to HOMA-IR are consistent with another study in younger American adults (18–30 years at baseline) evaluating magnesium intake against repeated measures of HOMA-IR over 20 years, in which a significant inverse association was observed between IR and magnesium intake, after adjusting for risk factors similar to those adjusted for in the present analysis (4). Although our follow-up was shorter, our population was older, and we excluded those with incident diabetes in our analyses, we nevertheless also observed an inverse trend between higher magnesium intake and long-term HOMA-IR. However, this association did not persist after adjustment for dietary fiber. One other prospective study examined magnesium intake and insulin sensitivity in 1,036 U.S. adults (56.4% women) participating in the Insulin Resistance Atherosclerosis Study (18). In that study, a threshold effect of magnesium intake (at 325 mg/day) was observed in relation to insulin sensitivity, derived from intravenous glucose tolerance test (18). Progressively poorer 5-year insulin sensitivity was observed below that threshold, with no evidence for improvement of sensitivity above that threshold.
Magnesium’s associations with insulin sensitivity are supported by experimental evidence in animals fed magnesium-deficient diets, in which insulin sensitivity of peripheral tissue decreases via reduced autophosphorylation of tyrosine kinase, a component of the β-subunit of the insulin receptor for which magnesium is a cofactor (38). In addition, hypomagnesemia is thought to deleteriously impact the proliferation and mass of β-cells, thus affecting insulin production (39,40). Insulin itself may also be regulating magnesium metabolism, as prolonged high concentrations of circulating insulin, such as those known to occur in IR, induce increases in renal magnesium excretion, thus perpetuating a deleterious cycle (40).
Whereas we observed that higher magnesium intake was inversely associated with long-term changes in FG and IR in those without incident diabetes (attenuated after adjustment for fiber intake), we did not observe significant trends of magnesium intake with fasting insulin, glucose clearance or insulin metabolism (as post-OGTT measures), or insulin sensitivity (as ISI), although we had >80% power to observe, for example, the observed difference in 2-h glucose between extreme quintiles. However, our findings are consistent with a recent 6-month trial in nondiabetic, insulin-resistant individuals that demonstrated that treatment with 365 mg/day of magnesium results in significantly lowered FG and HOMA-IR and improved insulin sensitivity (Matsuda index, but not Gutt’s ISI), with no effect on 2-h glucose or insulin and only marginal effects on FI (13). It may be that Gutt’s ISI, measured both in the trial and in the present analysis with null associations, is measuring peripheral IR, whereas other insulin-related measures, such as HOMA-IR or the Matsuda index, reflect hepatic IR (13).
We included fiber as a potential confounder owing to the body of literature on fiber’s protective effects against diabetes (6), and to shared dietary sources of magnesium and fiber, such as whole grains and vegetables. Interestingly, including fiber in our models (model 3) had differential effects on magnesium’s diabetes risk–lowering associations, depending on whether the population was initially normal or impaired. In those with normal baseline status, fiber attenuated the observed associations of magnesium on risk of metabolic impairment, suggesting that magnesium intake is not acting independently of the effects of fiber in those who are initially healthy. However, in those with impaired baseline status, fiber de-attenuated the association of magnesium, suggesting that higher magnesium intake may be more important to those with existing metabolic impairment, irrespective of fiber intake. This may, in part, be related to the deficient magnesium status generally observed in those with metabolic impairment (39). Of note is that there was no interaction between magnesium intake and fiber, or between magnesium intake and impairment status. Fiber intake was only ∼0.5 g/day higher, and magnesium intake ∼8 mg/day higher, on average, in those with normal versus impaired status at baseline.
Our study has several strengths. We benefitted from a large sample in a well-characterized community-based cohort with repeated dietary measures (up to three) for estimation of magnesium intake over 7 years. Incident metabolic impairment and diabetes were classified based on fasting and postload measures, rather than relying on self-report alone. This study also has several limitations. First, different insulin assays were used at baseline and final exams. Although we calibrated fasting values at exam 5 to those at exam 7, no calibration was possible for postload insulin. Therefore, the null findings observed between magnesium intake and fasting and postload insulin and ISI may be a partial result of this. Second, higher magnesium intake may also be reflective of better health consciousness, a confounder that we may have inadequately controlled for despite adjusting for fiber intake, cigarette smoking status, and physical activity, which may serve as surrogate markers of a healthy lifestyle. Although residual confounding of lifestyle factors may remain an issue, the attenuation by fiber intake of our estimates may also represent an overadjustment of the model, owing to magnesium and fiber’s shared food sources (namely, whole grains). Finally, the generalizability of our findings may be limited, as ours was a relatively homogenous, middle-aged Caucasian population.
In conclusion, higher magnesium intake may lower the risk of progressing to diabetes among those with the highest risk of doing so, namely, those with IR or prediabetes. These findings support a role for higher magnesium intake in those at high risk of developing diabetes and the need for large, randomized trials to confirm these observations.
Supplementary Material
Article Information
Acknowledgments. The authors thank the participants of the FHS for their contributions.
Funding. At the time of writing, A.H. was an American Heart Association Predoctoral Fellow. This work was also supported by the National Heart, Lung, and Blood Institute’s FHS (Contract N01-HC-25195) and the U.S. Department of Agriculture (Agreement 58-1950-0-014). J.B.M. is supported by K24-DK-080140.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.H. designed the analysis, analyzed the data, drafted the manuscript, and had primary responsibility for the final content. J.B.M., C.J.O., and P.F.J. oversaw data collection and management. N.M.M. had primary responsibility for the concept of the study and final content. All authors contributed to writing the manuscript and approved the final version. A.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-1397/-/DC1.
References
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011
- 2.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DJ, Xun P, Liu K, et al. Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes Care 2010;33:2604–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong J-Y, Xun P, He K, Qin L-Q. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care 2011;34:2116–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med 2007;167:956–965 [DOI] [PubMed] [Google Scholar]
- 7.Moshfegh A, Goldman J, Ahuja J, Rhodes D, LaComb R. What we eat in America, NHANES 2005–2006: usual nutrient intakes from food and water compared to 1997 dietary reference intakes for vitamin D, calcium, phosphorus, and magnesium. U.S. Department of Agriculture, Agricultural Research Service, 2009
- 8.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC, National Academy Press, 1997 [Google Scholar]
- 9.Guerrero-Romero F, Tamez-Perez HE, González-González G, et al. Oral magnesium supplementation improves insulin sensitivity in non-diabetic subjects with insulin resistance. A double-blind placebo-controlled randomized trial. Diabetes Metab 2004;30:253–258 [DOI] [PubMed] [Google Scholar]
- 10.Guerrero-Romero F, Rodríguez-Morán M. Magnesium improves the beta-cell function to compensate variation of insulin sensitivity: double-blind, randomized clinical trial. Eur J Clin Invest 2011;41:405–410 [DOI] [PubMed] [Google Scholar]
- 11.Hadjistavri LS, Sarafidis PA, Georgianos PI, et al. Beneficial effects of oral magnesium supplementation on insulin sensitivity and serum lipid profile. Med Sci Monit 2010;16:CR307–CR312 [PubMed] [Google Scholar]
- 12.Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabet Med 2006;23:1050–1056 [DOI] [PubMed] [Google Scholar]
- 13.Mooren FC, Krüger K, Völker K, Golf SW, Wadepuhl M, Kraus A. Oral magnesium supplementation reduces insulin resistance in non-diabetic subjects - a double-blind, placebo-controlled, randomized trial. Diabetes Obes Metab 2011;13:281–284 [DOI] [PubMed] [Google Scholar]
- 14.Nadler JL, Buchanan T, Natarajan R, Antonipillai I, Bergman R, Rude R. Magnesium deficiency produces insulin resistance and increased thromboxane synthesis. Hypertension 1993;21:1024–1029 [DOI] [PubMed] [Google Scholar]
- 15.Pratley RE, Weyer C. The role of impaired early insulin secretion in the pathogenesis of type II diabetes mellitus. Diabetologia 2001;44:929–945 [DOI] [PubMed] [Google Scholar]
- 16.Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB, Sr, Wilson PW. Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the Framingham Offspring Study. Diabetes 2005;54:3252–3257 [DOI] [PubMed] [Google Scholar]
- 17.Meigs JB, Nathan DM, D’Agostino RB, Sr, Wilson PWF, Framingham Offspring Study Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care 2002;25:1845–1850 [DOI] [PubMed] [Google Scholar]
- 18.Ma B, Lawson AB, Liese AD, Bell RA, Mayer-Davis EJ. Dairy, magnesium, and calcium intake in relation to insulin sensitivity: approaches to modeling a dose-dependent association. Am J Epidemiol 2006;164:449–458 [DOI] [PubMed] [Google Scholar]
- 19.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med 1975;4:518–525 [DOI] [PubMed] [Google Scholar]
- 20.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–1126; discussion 1127–1136 [DOI] [PubMed] [Google Scholar]
- 21.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–867 [DOI] [PubMed] [Google Scholar]
- 22.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 23.Willett W. Nutritional Epidemiology. New York, Oxford University Press, 1998 [Google Scholar]
- 24.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 26.Gutt M, Davis CL, Spitzer SB, et al. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract 2000;47:177–184 [DOI] [PubMed] [Google Scholar]
- 27.Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med 1979;139:857–861 [PubMed] [Google Scholar]
- 28.Bhupathiraju SN, Pan A, Malik VS, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr 2013;97:155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Dam RM, Willett WC, Manson JE, Hu FB. Coffee, caffeine, and risk of type 2 diabetes: a prospective cohort study in younger and middle-aged U.S. women. Diabetes Care 2006;29:398–403 [DOI] [PubMed] [Google Scholar]
- 30.Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr 2012;142:1304–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burnett-Hartman AN, Fitzpatrick AL, Gao K, Jackson SA, Schreiner PJ. Supplement use contributes to meeting recommended dietary intakes for calcium, magnesium, and vitamin C in four ethnicities of middle-aged and older Americans: the Multi-Ethnic Study of Atherosclerosis. J Am Diet Assoc 2009;109:422–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Ridaura R, Willett WC, Rimm EB, et al. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care 2004;27:134–140 [DOI] [PubMed] [Google Scholar]
- 33.Song Y, Manson JE, Buring JE, Liu S. Dietary magnesium intake in relation to plasma insulin levels and risk of type 2 diabetes in women. Diabetes Care 2004;27:59–65 [DOI] [PubMed] [Google Scholar]
- 34.Yokota K, Kato M, Lister F, et al. Clinical efficacy of magnesium supplementation in patients with type 2 diabetes. J Am Coll Nutr 2004;23:506S–509S [DOI] [PubMed] [Google Scholar]
- 35.Rumawas ME, McKeown NM, Rogers G, Meigs JB, Wilson PWF, Jacques PF. Magnesium intake is related to improved insulin homeostasis in the Framingham Offspring cohort. J Am Coll Nutr 2006;25:486–492 [DOI] [PubMed] [Google Scholar]
- 36.Fung TT, Manson JE, Solomon CG, Liu S, Willett WC, Hu FB. The association between magnesium intake and fasting insulin concentration in healthy middle-aged women. J Am Coll Nutr 2003;22:533–538 [DOI] [PubMed] [Google Scholar]
- 37.Humphries S, Kushner H, Falkner B. Low dietary magnesium is associated with insulin resistance in a sample of young, nondiabetic black Americans. Am J Hypertens 1999;12:747–756 [DOI] [PubMed] [Google Scholar]
- 38.Suárez A, Pulido N, Casla A, Casanova B, Arrieta FJ, Rovira A. Impaired tyrosine-kinase activity of muscle insulin receptors from hypomagnesaemic rats. Diabetologia 1995;38:1262–1270 [DOI] [PubMed] [Google Scholar]
- 39.Barbagallo M, Dominguez LJ. Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Arch Biochem Biophys 2007;458:40–47 [DOI] [PubMed] [Google Scholar]
- 40.Günther T. The biochemical function of Mg2+ in insulin secretion, insulin signal transduction and insulin resistance. Magnes Res 2010;23:5–18 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


