Abstract
OBJECTIVE
In the population-based Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) cohort, we sought to examine whether a decline in the prevalence and incidence of end-stage renal disease (ESRD) was evident with increasing calendar year of type 1 diabetes diagnosis among people followed for 25 years. Factors associated with the hazard of incident ESRD that may mediate a decline were also investigated.
RESEARCH DESIGN AND METHODS
Participants were examined at baseline in 1980 (n = 996) and at 4–25 years of follow-up. ESRD was defined by self-reported renal transplant or dialysis. Cumulative incidence with competing risk of death was determined. Incident ESRD was modeled by period of diagnosis, adjusting for other known risk factors using discrete time hazard models.
RESULTS
When diabetes was diagnosed during 1970–1980, the unadjusted cumulative incidence of ESRD at 25 years was 9.3%. The unadjusted hazard of ESRD was reduced by 70% (P < 0.001), compared with those diagnosed with diabetes in 1922–1969; however, the association was attenuated by glycosylated hemoglobin level (HbA1c), systolic blood pressure, and antihypertensive use (hazard ratio [HR] 0.89 [95% CI 0.55–1.45]). HbA1c, age, and male sex remained associated with ESRD hazard after adjustment for kidney function and proliferative retinopathy.
CONCLUSIONS
A lower incidence of ESRD among those more recently diagnosed with type 1 diabetes was explained by improvements in glycemic and blood pressure control over the last several decades. Intensive diabetes management, especially for glycemic control, remains important even in long-standing diabetes as it may delay the development of ESRD.
Introduction
It is well known that diabetic nephropathy is the leading cause of end-stage renal disease (ESRD) in many regions, including the U.S. (1). Type 1 diabetes accounts for >45,000 cases of ESRD per year (2), and the incidence may be higher than in people with type 2 diabetes (3). Despite this, there are few population-based data available regarding the prevalence and incidence of ESRD in people with type 1 diabetes in the U.S. (4). A declining incidence of ESRD has been suggested by findings of lower incidence with increasing calendar year of diagnosis and in comparison with older reports in some studies in Europe and the U.S. (5–8). This is consistent with better diabetes management tools becoming available and increased renoprotective efforts, including the greater use of ACE inhibitors and angiotensin type II receptor blockers, over the past two to three decades (9). Conversely, no reduction in the incidence of ESRD across enrollment cohorts was found in a recent clinic-based study (9). Further, an increase in ESRD has been suggested for older but not younger people (9). Recent improvements in diabetes care have been suggested to delay rather than prevent the development of renal disease in people with type 1 diabetes (4).
A decrease in the prevalence of proliferative retinopathy by increasing calendar year of type 1 diabetes diagnosis was previously reported in the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) cohort (10); therefore, we sought to determine if a similar pattern of decline in ESRD would be evident over 25 years of follow-up. Further, we investigated factors that may mediate a possible decline in ESRD as well as other factors associated with incident ESRD over time.
RESEARCH DESIGN AND METHODS
Study Population
The WESDR cohort and methods have been described in detail elsewhere (10–15). In brief, 10,135 patients with diabetes who were receiving primary care in an 11-county area in southern Wisconsin during 1979–1980 were classified as “older” and “younger” onset. All 1,210 individuals with “younger-onset” diabetes, taking insulin and diagnosed before 30 years of age, and later confirmed by C-peptide as having type 1 diabetes (16) were invited for a baseline examination. During 1980–1982, 996 individuals were examined with subsequent examinations made on the cohort at 4 (1984–1986), 10 (1990–1992), 14 (1995–1996), 20 (2000–2001), and 25 (2005–2007) years after the baseline examination. Participation was >80% at each exam phase among the living. Reasons for nonparticipation and comparison of characteristics by participation status for each examination have also been described previously (10–15). This study was approved by the institutional review board of the University of Wisconsin and conformed to the tenets of the Declaration of Helsinki, with written informed consent obtained for all study participants.
Data Collection
ESRD was defined by self-reported history of renal transplant or dialysis. A structured interview captured information on medications including antihypertensives and daily aspirin use, health behaviors such as current cigarette smoking, diabetes management, and sociodemographic factors. Weight was measured on a physician beam scale and height by standard stadiometer height rod; BMI was calculated. Two seated blood pressures were measured according to the Hypertension Detection and Follow-up Program protocol (11,17) and the mean used for analysis. The presence of proliferative retinopathy was ascertained by grading of seven-field stereoscopic fundus photographs at each examination (10,11), with an exception for the 20-year visit when stereoscopic photographs were not taken.
Glycosylated hemoglobin A1 (HbA1) was determined through the 14-year exams using a resin microcolumn technique (Quik-Step Fast Hemoglobin; Isolab, Akron, OH). HbA1c was calculated using an equation previously determined by comparing test values performed in tandem with the core laboratory of the Diabetes Control and Complications Trial (DCCT) at the University of Minnesota (18). HbA1c was then determined at the 20- and 25-year exams by high-performance liquid chromatography (Tosoh Medics, Inc., San Francisco, CA) at the University of Minnesota laboratory, calibrated using standard values derived by the National Glycohemoglobin Standardization Program. At the 4–14-year visits, serum creatinine was measured using a modification of the Jaffe reaction (13) and at subsequent visits by an enzymatic rate reflectance spectrophotometric method found similar to the Jaffe method (regression equation R2 = 0.94) (19). Jaffe method–based measures were converted to the spectrophotometric method values by the following equation: spectrophotometric value = 0.9435 × Jaffe method + 0.0435. Estimated glomerular filtration rate (eGFR) was calculated using the creatinine-based equation developed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) in mL/min/1.73 m2 (20). Urine protein levels were determined on a casual urine sample using an agglutination inhibition assay and reagent strip test (19) (Labstix; Ames, Elkhart, IN) and categorized as gross proteinuria when ≥0.3 g/L.
Statistical Methods
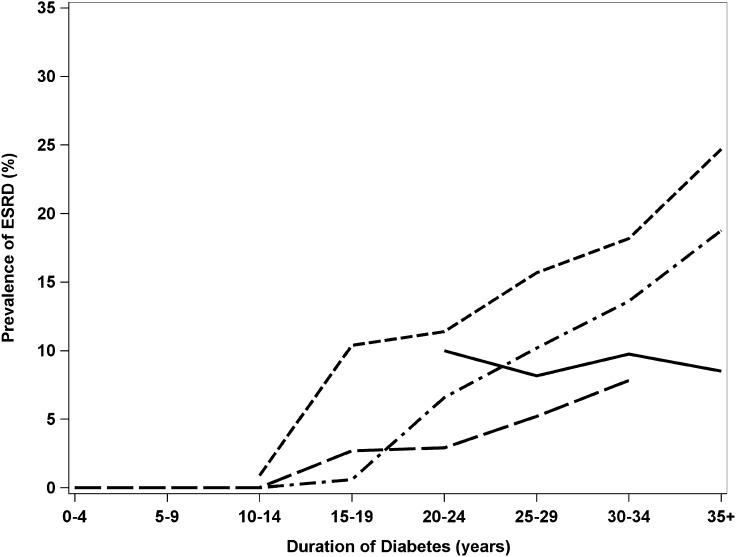
Analyses were completed using SAS version 9.3 (21). The cohort and observations were described by means, medians, and SDs (Table 1). Year of diagnosis was described by quartiles (1922–1959, 1960–1969, 1970–1974, and 1975–1980) and dichotomized at the median (1922–1969 and 1970–1980) for regression analysis. Prevalence of ESRD by duration and year of diagnosis combined data across 25 years of follow-up (Fig. 1).
Table 1.
Characteristics of the WESDR cohort at the baseline (1980–1982) and 25-year follow-up examinations (2005–2007)
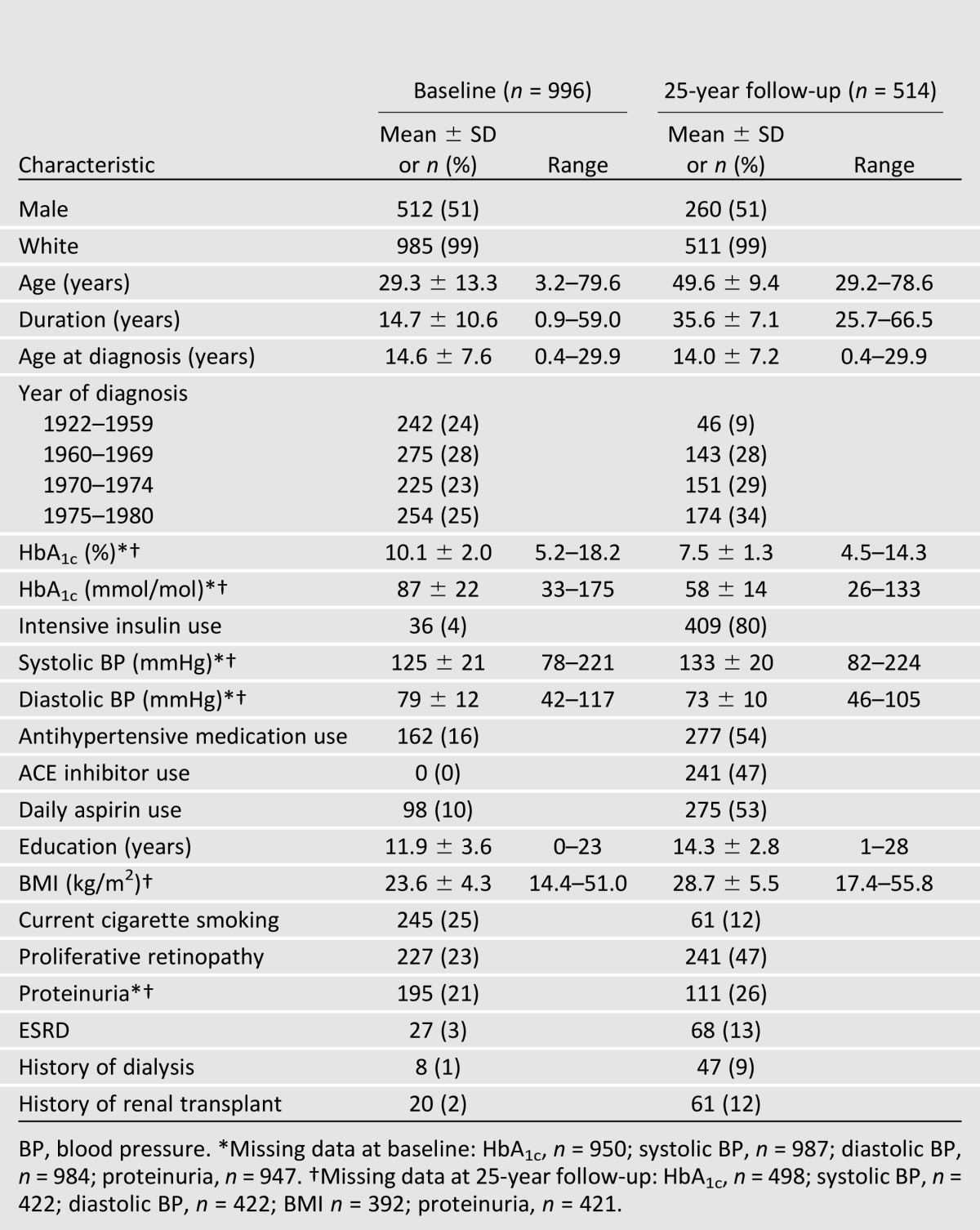
Figure 1.
Prevalence of ESRD by duration of diabetes and year of diagnosis across all WESDR examinations. Period of diagnosis:  , 1922–1959;
, 1922–1959;  , 1960–1969;
, 1960–1969;  , 1970–1974; and
, 1970–1974; and  , 1975–1980.
, 1975–1980.
Intervals were created for each successive pair of exam visits. Participants (n = 27) with ESRD at baseline were excluded from incidence analysis. An interval for a given participant was included if they were free from ESRD and there was complete data for HbA1c and blood pressure at the first visit (beginning of interval), and presence or absence of ESRD was reported at the next visit (end of the interval), with no preceding history of ESRD. First incidence of ESRD was used for analyses, and censoring occurred thereafter or upon first incidence of missing data. These steps yielded a total of 884 participants with 2,933 intervals contributing to analysis. Cumulative 25-year incidence was calculated using a modification of the Kaplan-Meier approach, accounting for censored observations and the competing risk of death (22).
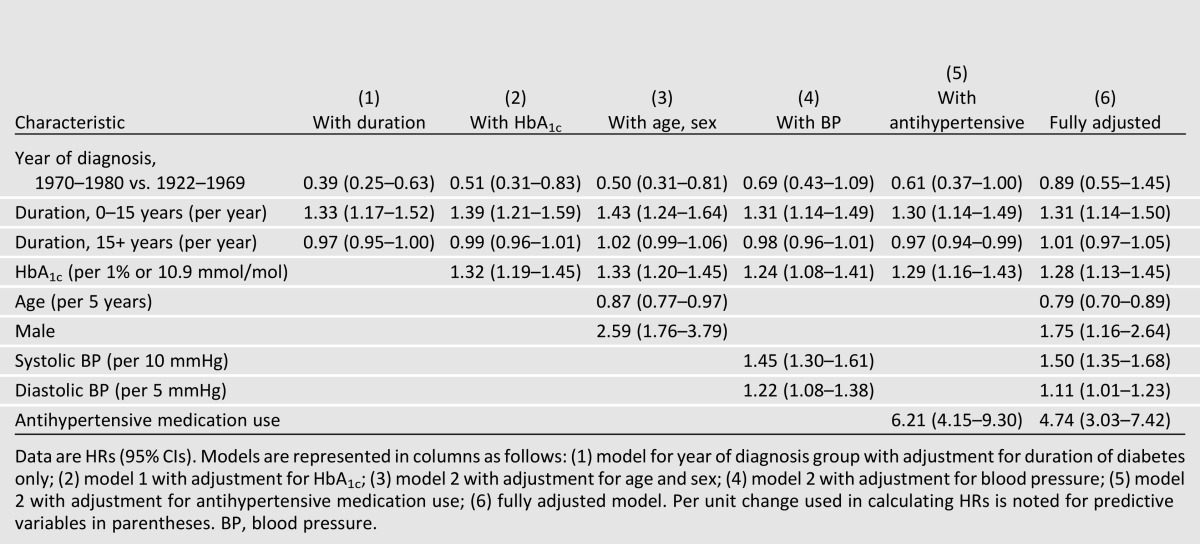
Discrete time hazard models that account for censoring due to nonattendance or death were used with time-updating covariates to estimate the hazard ratios (HRs) for incident ESRD. Piecewise linear terms were adopted to model the nonlinear association found with duration (23) with “knots” (point of curvature) at 15 years duration. Therefore models included terms representing slopes for 0–15 and 15+ years duration. Significant associations (α = 0.05) from univariate analysis were identified, including period of diagnosis. Mediation of this relationship was assessed (24) when adjusting for glycemic control (Table 2, model 2), age and sex (model 3), blood pressure (model 4), and antihypertensive medication use (model 5).
Table 2.
HRs and 95% CIs from discrete time hazard models of the effect of year of diagnosis and other risk factors on incident ESRD
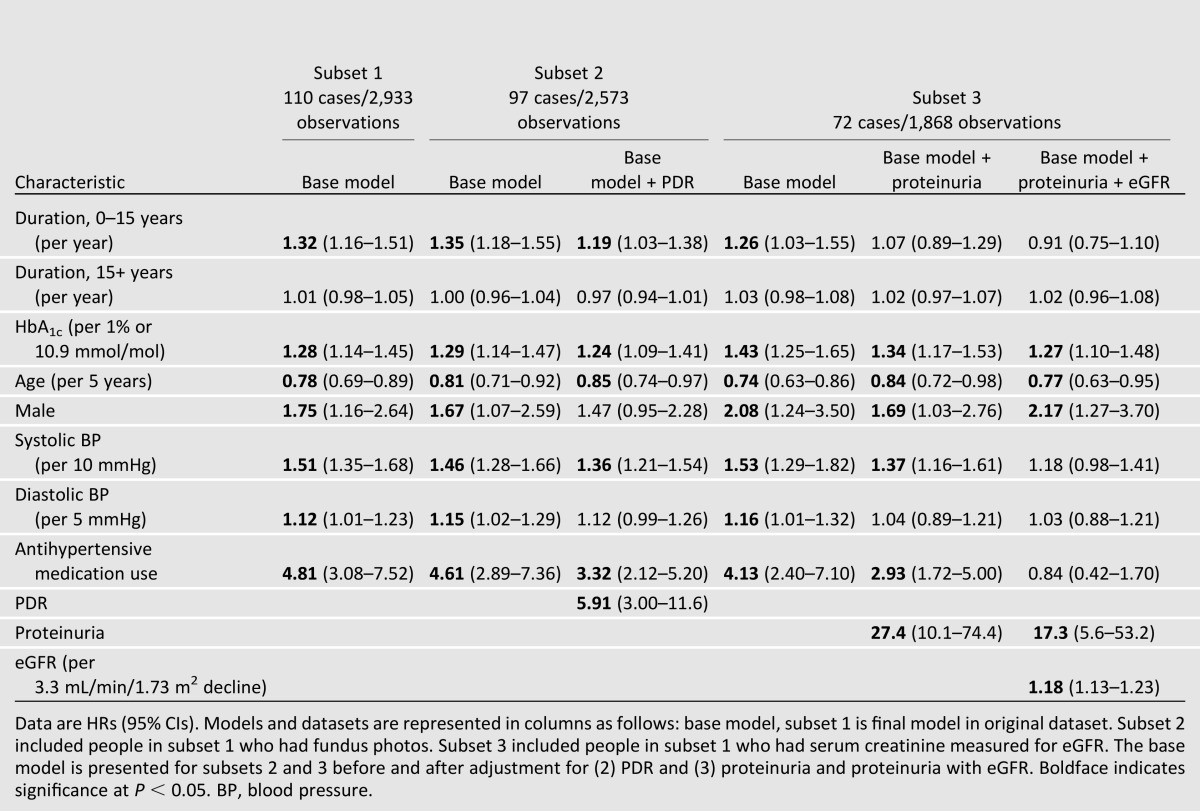
To establish the final hazard model, variables remaining significant in univariate regression and then after adjustment for duration and HbA1c were retained for a fully adjusted model. Backward selection was used to establish the most parsimonious model, with predictors significant at the α = 0.05 level (Table 3, base model). Additional subset analyses were completed for evaluating the impact of proliferative retinopathy, proteinuria, and eGFR on hazard of incident ESRD and its predictors in the most parsimonious model, as these measures were not available at all examinations.
Table 3.
HRs and 95% CIs from final discrete time hazard model and models evaluating the effect of proliferative diabetic retinopathy, proteinuria, and eGFR on risk of incident ESRD
To address possible missed cases that could have resulted from individuals who developed ESRD after their last WESDR follow-up visit, we completed sensitivity analyses modeling the hazard of incident ESRD or death due to renal disease. Death from renal disease was defined by ICD-9 (580.0–589.9) or ICD-10 (N00.0–N07.9, N17.0–N19.9, or N25.0–N27.9) codes listed as any mention on the death certificate, obtained for deaths submitted through June 2008 matched to the National Death Index.
RESULTS
Characteristics of the Study Population
Characteristics of the WESDR cohort at the baseline and 25-year follow-up examinations are shown in Table 1. At baseline, 99% of WESDR cohort members were white and 51% were male. Individuals were 3–79 years of age (mean 29) with diabetes duration of 0–59 years (mean 15), diagnosed between 1922 and 1980. Four percent of individuals used three or more daily insulin injections and none used an insulin pump. Mean HbA1c was 10.1% (87 mmol/mol). Only 16% were using an antihypertensive medication, none was using an ACE inhibitor, and 3% reported a history of renal transplant or dialysis (ESRD). At 25 years, 514 individuals participated (52% of original cohort at baseline, n = 996) and 367 were deceased (37% of baseline). Mean HbA1c was much lower than at baseline (7.5%, 58 mmol/mol), the decline likely due to the improvements in diabetes care, with 80% of participants using intensive insulin management (three or more daily insulin injections or insulin pump). The decline in HbA1c was steady, becoming slightly steeper following the results of the DCCT (25). Overall, at the 25-year follow-up, 47% had proliferative retinopathy, 53% used aspirin daily, and 54% reported taking antihypertensive medications, with the majority (87%) using an ACE inhibitor. Thirteen percent reported a history of ESRD.
Prevalence and Cumulative Incidence of ESRD
Prevalence of ESRD was negligible until 15 years of diabetes duration and then steadily increased with 5, 8, 10, 13, and 14% reporting ESRD by 15–19, 20–24, 25–29, 30–34, and 35+ years of diabetes duration, respectively. Prevalence of ESRD by period of diagnosis is shown in Fig. 1. After 15 years of diagnosis, prevalence of ESRD increased with duration in people diagnosed from 1960 to 1980, with the lowest increase in people with the most recent diagnosis. People diagnosed from 1922 to 1959 had consistent rather than increasing levels of ESRD with duration of 20+ years. If not for their greater mortality (at the 25-year follow-up, 48% of the deceased had been diagnosed prior to 1960), an increase with duration may have also been observed.
From baseline, the unadjusted cumulative 25-year incidence of ESRD was 17.9% (95% CI 14.3–21.5) in males, 10.3% (7.4–13.2) in females, and 14.2% (11.9–16.5) overall. For those diagnosed in 1970–1980, the cumulative incidence at 14, 20, and 25 years of follow-up (or ∼15–25, 20–30, and 25–35 years diabetes duration) was 5.2, 7.9, and 9.3%, respectively. At 14, 20, and 25 years of follow-up (or 35, 40, and 45 up to 65+ years diabetes duration), the cumulative incidence in those diagnosed during 1922–1969 was 13.6, 16.3, and 18.8%, respectively, consistent with the greater prevalence observed for these diagnosis periods at longer duration of diabetes.
Factors Associated With Incident ESRD
Univariate models showed diabetes duration (different slopes for 0–15 and 15+ years), sex, HbA1c, systolic and diastolic blood pressures, antihypertensive medication use, period of diagnosis (all P < 0.0001), age (P < 0.05), and ACE inhibitor use (P < 0.001) to be significantly associated with incident ESRD (data not shown). In subset analyses, presence of proteinuria, eGFR, and prevalent proliferative retinopathy were also found significantly related (all P < 0.001). The impact of race could not be tested in this primarily non-Hispanic white cohort.
The unadjusted hazard of ESRD was reduced by 70% among those diagnosed in 1970–1980 as compared with those in 1922–1969 (HR 0.29 [95% CI 0.19–0.44]). Duration (by 10%) and HbA1c (by an additional 10%) partially mediated this association (Table 2). Blood pressure and antihypertensive medication use each further attenuated the association. When fully adjusted for these and the above factors found significant in univariate analysis in the full dataset, period of diagnosis was no longer significant (HR 0.89 [0.55–1.45]). Sensitivity analyses for the hazard of incident ESRD or death due to renal disease showed similar findings (data not shown).
The most parsimonious model included diabetes duration, HbA1c, age, sex, systolic and diastolic blood pressure, and history of antihypertensive medication (Table 3, base model). A 32% increased risk for incident ESRD was found per increasing year of diabetes duration at 0–15 years (HR 1.32 per year [95% CI 1.16–1.51]). The hazard plateaued (1.01 per year [0.98–1.05]) after 15 years of duration of diabetes. Hazard of ESRD increased with increasing HbA1c (1.28 per 1% or 10.9 mmol/mol increase [1.14–1.45]) and blood pressure (1.51 per 10 mmHg increase in systolic pressure [1.35–1.68]; 1.12 per 5 mmHg increase in diastolic pressure [1.01–1.23]). Use of antihypertensive medications increased the hazard of incident ESRD nearly fivefold, and males had approximately two times the risk as compared with females. For every 5-year increase in age, the hazard for ESRD was reduced by 22% (0.78 [0.69–0.89]). Retinopathy and kidney function measures were not available at each examination; therefore, we analyzed subsets having these additional measures (Table 3). The HRs for the base model were similar in these subsets. Having proliferative retinopathy was strongly associated with increased risk (HR 5.91 [3.00–11.6]) and attenuated the association between sex and ESRD. Alternatively, proteinuria and eGFR, along with HbA1c, age, and sex, were significantly associated with incident ESRD.
CONCLUSIONS
Large, population-based studies of people with type 1 diabetes with long-term follow-up are rare, especially in the U.S. (4). Estimates for diabetic renal disease from the National Health and Nutrition Examination data primarily include people with type 2 diabetes of shorter duration (<20 years) (26). A recent report on the incidence of treatment for ESRD, using surveillance data from the U.S. Renal Data System lacked information on type and duration of diabetes as well as other important risk factors (27). The current investigation therefore sought to provide much-needed information on the prevalence and incidence of ESRD and associated risk specific to people with type 1 diabetes. Consistent with a few previous studies (5,7,8), we observed decreased prevalence and incidence of ESRD among individuals with type 1 diabetes diagnosed in the 1970s compared with prior to 1970. The Epidemiology of Diabetes Complications (EDC) Study, another large cohort of people with type 1 diabetes followed over a long period of time, reported cumulative incidence rates of 2–6% for those diagnosed after 1970 and with similar duration (7), comparable to our findings. Slightly higher cumulative incidence (7–13%) reported from older studies at slightly lower duration also supports a decrease in incidence of ESRD (28–30). Cumulative incidences through 30 years in European cohorts were even lower (3.3% in Sweden [6] and 7.8% in Finland [5]), compared with the 9.3% noted for those diagnosed during 1970–1980 in the WESDR cohort. The lower incidence could be associated with nationally organized care, especially in Sweden where a nationwide intensive diabetes management treatment program was implemented at least a decade earlier than recommendations for intensive care followed from the results of the DCCT in the U.S. (25). Notably, after these results were made available in 1993, WESDR cohort participants had had diabetes for ∼15+ years and were therefore less likely to experience these changes in care when they may have had their greatest impact. A comparison of diabetic retinopathy severity between WESDR and an incident type 1 diabetes cohort diagnosed during 1987–1992 is supportive of lower or delayed risk for severe complications in the current era of diabetes care (31). A lower cumulative incidence of diabetic nephropathy attributed to improvements in diabetes management has also been found (32), but not in all prior investigations (7).
We noted an increased risk of incident ESRD in the first 15 years of diabetes not evident at longer durations. This pattern also demonstrated by others could be due to a greater earlier risk among people most genetically susceptible, as only a subset of individuals with type 1 diabetes will develop renal disease (27,28). The risk plateau associated with greater durations of diabetes and lower risk associated with increasing age may also reflect more death at longer durations and older ages. A similar pattern with duration but loss of protection with older age was observed when modeling incidence of ESRD or death (data not shown). Because age and duration are highly correlated, we observed a positive association between age and ESRD only in univariate analyses, without adjustment for duration. The lack of adjustment for diabetes duration may have, in part, explained the increasing incidence of ESRD shown with age for some people in a recent investigation (9). Adjustment for both age and duration was found appropriate after testing for collinearity in the current analysis.
The association between period of diagnosis of diabetes and incident ESRD that we observed was partially attenuated by including duration of diabetes and glycosylated hemoglobin in the model. This is consistent with the fact that there were improvements in diabetes management strategies closely coinciding with period of diagnosis. Hyperglycemia has been associated with various stages of kidney disease (13,19,29). Glycemic control only explained a portion of the risk for ESRD, consistent with other reports (9,33,34). Here, time-updated HbA1c levels were significantly associated, indicating that continued intensive glycemic control, even in long-standing diabetes, is warranted.
Long-term aggressive antihypertensive treatment has been suggested as the primary improvement tied to the lower incidence of type 1 diabetes–related renal disease over time (35). Higher blood pressure and greater antihypertensive medication use accounted for the remaining higher risk of ESRD between periods of diagnosis in the WESDR cohort. That is, greater use of antihypertensive medications reflected greater risk, which is contrary to the protection that antihypertensive medications, especially ACE inhibitors, have been shown to have on diabetic renal disease (36). Here, antihypertensive medication use may reflect the poorer health and increased comorbidities of people requiring these medications. Although side effects of some antihypertensive medications may have impacted glycemia or compliance, confounding by indication is a more likely explanation for the finding of increased risk with treatment. The increased use of ACE inhibitors over time as well as the decreased incidence of ESRD precluded expanding our investigation of risks associated with distinct medication classes.
In agreement with some but not all studies, males were at greater risk for ESRD than females (5,6,33,34). Poorer control of modifiable risk factors, including glycemic and blood pressure levels, may have attenuated some of the excess risk (34), reported in a prior WESDR investigation of renal insufficiency through 10 years of follow-up (19). A higher competing risk of death in men was also found to minimize the sex difference in some reports (5,6). Other uncontrolled risk factors that differ between men and women might explain the difference, such as diet, lifestyle, or renal physiology. Sex hormones may also have direct effects (6). Reported ESRD risk differences between men and women could also be explained by the degree of proteinuria or treatment, where response to ACE inhibitors has been suggested to differ between the sexes (34). Interactions of specific factors with sex were not significant in the current investigation.
Adjustment for prevalent proteinuria and GFR clearly indicated the strength of associations between HbA1c, age, and sex in risk of incident ESRD and, together, attenuated the associations with blood pressure and antihypertensive medication use. Presence of proteinuria not fully attenuating these associations supports the current concept of disease progression where some individuals with proteinuria do not develop ESRD (37), yet proteinuria still showed a strong association. Associations between renal disease and proliferative diabetic retinopathy (PDR) have also been previously demonstrated (38). Weaker associations between risk of ESRD and factors also associated with PDR, including duration, sex, blood pressure, and antihypertensive medication use, would therefore be expected with adjustment for PDR.
There are several strengths in the current analysis, including the population-based nature of the cohort, excellent participation, and comprehensive long-term follow-up of WESDR participants. However, limitations do exist, including the generalizability of our findings to individuals of other ethnicities, although the cohort represented all people with type 1 diabetes receiving primary care in the region at enrollment. Further, this is a prevalent cohort with various duration history at the first examination; therefore, very early cases of ESRD could have been missed, especially for those older at baseline, which may have underestimated differences between periods of diagnosis. Time between visits could have also contributed to missed cases, and the relatively small number of incident cases may have resulted in limited power to detect some associations. Improving diabetes and kidney disease management, including greater access to renal transplants and dialysis with time (27,35), could have impacted our estimated incidence; in the current analysis, exam year was not a significant predictor of ESRD hazard. Finally, it is possible that higher mortality among those having an earlier diagnosis of type 1 diabetes impacted our findings. Although the methods chosen account for loss to follow-up due to death, we acknowledge the possibility that ESRD may have been the cause of death among some individuals not having ESRD the last time they were seen for a WESDR follow-up visit. In a sensitivity analysis modeling the hazard of incident ESRD- or renal disease–related death, we noted no change in our findings, with the period effect still attenuated by HbA1c and blood pressure.
In conclusion, this U.S. population-based report showed a lower prevalence and incidence of ESRD among those more recently diagnosed, explained by improvements in glycemic and blood pressure control over the last several decades. Even lower rates may be expected for those diagnosed during the current era of diabetes care. Intensive diabetes management, especially for glycemic control, remains important even in long-standing diabetes as potentially delaying the development of ESRD.
Article Information
Acknowledgments. The authors thank the WESDR participants and staff for their invaluable contributions.
Funding. The WESDR was supported by grant R01-EY016379 from the National Eye Institute, National Institutes of Health. T.J.L. was supported by a Mentored Postdoctoral Fellowship award from the American Diabetes Association. K.P.H. and K.E.L. received salary support and R.K. and B.E.K.K. received research and salary support from the National Institutes of Health.
Duality of Interest. R.K. serves as a consultant for Fovea and Pfizer. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. T.J.L. analyzed the data and wrote and revised the manuscript. B.E.K.K. and R.K. contributed to the discussion and reviewed and revised the manuscript. K.P.H. and K.E.L. assisted with data analysis and reviewed and revised the manuscript. T.J.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 2005;28:164–176 [DOI] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 3.Cowie CC, Port FK, Wolfe RA, Savage PJ, Moll PP, Hawthorne VM. Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. N Engl J Med 1989;321:1074–1079 [DOI] [PubMed] [Google Scholar]
- 4.Marshall SM. Diabetic nephropathy in type 1 diabetes: has the outlook improved since the 1980s? Diabetologia 2012;55:2301–2306 [DOI] [PubMed] [Google Scholar]
- 5.Finne P, Reunanen A, Stenman S, Groop P-H, Grönhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA 2005;294:1782–1787 [DOI] [PubMed] [Google Scholar]
- 6.Möllsten A, Svensson M, Waernbaum I, et al. Swedish Childhood Diabetes Study Group. Diabetes Incidence Study in Sweden. Swedish Renal Registry Cumulative risk, age at onset, and sex-specific differences for developing end-stage renal disease in young patients with type 1 diabetes: a nationwide population-based cohort study. Diabetes 2010;59:1803–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 2006;55:1463–1469 [DOI] [PubMed] [Google Scholar]
- 8.Nishimura R, Dorman JS, Bosnyak Z, Tajima N, Becker DJ, Orchard TJ, Diabetes Epidemiology Research International Mortality Study. Allegheny County Registry Incidence of ESRD and survival after renal replacement therapy in patients with type 1 diabetes: a report from the Allegheny County Registry. Am J Kidney Dis 2003;42:117–124 [DOI] [PubMed] [Google Scholar]
- 9.Rosolowsky ET, Skupien J, Smiles AM, et al. Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol 2011;22:545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BEK. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology 2008;115:1859–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol 1984;102:520–526 [DOI] [PubMed] [Google Scholar]
- 12.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol 1989;107:237–243 [DOI] [PubMed] [Google Scholar]
- 13.Klein R, Klein BEK, Moss SE, Cruickshanks KJ, Brazy PC. The 10-year incidence of renal insufficiency in people with type 1 diabetes. Diabetes Care 1999;22:743–751 [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology 1998;105:1801–1815 [DOI] [PubMed] [Google Scholar]
- 15.Klein BE, Klein R, McBride PE, et al. Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Intern Med 2004;164:1917–1924 [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Moss SE, Klein BE, Davis MD, DeMets DL. Wisconsin Epidemiology Study of Diabetes Retinopathy. XII. Relationship of C-peptide and diabetic retinopathy. Diabetes 1990;39:1445–1450 [DOI] [PubMed] [Google Scholar]
- 17.Writing Committee on Behalf of the HDFP Cooperative Group The hypertension detection and follow-up program: hypertension detection and follow-up program cooperative group. Prev Med 1976;5:207–215 [DOI] [PubMed] [Google Scholar]
- 18.Klein R, Moss S, Diabetes Control and Complications Trial Research Group A comparison of the study populations in the Diabetes Control and Complications Trial and the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Intern Med 1995;155:745–754 [PubMed] [Google Scholar]
- 19.Shankar A, Klein R, Klein BE, Moss SE. Association between glycosylated hemoglobin level and 16-year incidence of chronic kidney disease in type 1 diabetes. Exp Clin Endocrinol Diabetes 2007;115:203–206 [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SAS Institute Inc SAS/STAT 9.3 User’s Guide. Cary, North Carolina, SAS Institute Inc., 2011 [Google Scholar]
- 22.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999;18:695–706 [DOI] [PubMed] [Google Scholar]
- 23.SAS Institute Inc. Parameterizing models to test the hypotheses you want: coding indicator variables and modified continuous variables. In Proceedings of the 30th Annual SAS Users Group International Conference, Philadelphia, Pennsylvania, 2005 Cary, North Carolina, SAS Institute Inc., paper 212-30 [Google Scholar]
- 24.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173–1182 [DOI] [PubMed] [Google Scholar]
- 25.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 26.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011;305:2532–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burrows NR, Li Y, Geiss LS. Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continues to decline. Diabetes Care 2010;33:73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type I diabetes. Am J Med 1985;78:785–794 [DOI] [PubMed] [Google Scholar]
- 29.Krolewski M, Eggers PW, Warram JH. Magnitude of end-stage renal disease in IDDM: a 35 year follow-up study. Kidney Int 1996;50:2041–2046 [DOI] [PubMed] [Google Scholar]
- 30.Matsushima M, Tajima N, LaPorte RE, et al. Diabetes Epidemiology Research International (DERI) U.S.-Japan Mortality Study Group Markedly increased renal disease mortality and incidence of renal replacement therapy among IDDM patients in Japan in contrast to Allegheny County, Pennsylvania, USA. Diabetologia 1995;38:236–243 [DOI] [PubMed] [Google Scholar]
- 31.LeCaire TJ, Palta M, Klein R, Klein BEK, Cruickshanks KJ. Assessing progress in retinopathy outcomes in type 1 diabetes: comparing findings from the Wisconsin Diabetes Registry Study and the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care 2013;36:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hovind P, Tarnow L, Rossing K, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care 2003;26:1258–1264 [DOI] [PubMed] [Google Scholar]
- 33.Forsblom C, Harjutsalo V, Thorn LM, et al. FinnDiane Study Group Competing-risk analysis of ESRD and death among patients with type 1 diabetes and macroalbuminuria. J Am Soc Nephrol 2011;22:537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costacou T, Fried L, Ellis D, Orchard TJ. Sex differences in the development of kidney disease in individuals with type 1 diabetes mellitus: a contemporary analysis. Am J Kidney Dis 2011;58:565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Astrup AS, Tarnow L, Rossing P, Pietraszek L, Riis Hansen P, Parving H-H. Improved prognosis in type 1 diabetic patients with nephropathy: a prospective follow-up study. Kidney Int 2005;68:1250–1257 [DOI] [PubMed] [Google Scholar]
- 36.Hirst JA, Taylor KS, Stevens RJ, et al. The impact of renin-angiotensin-aldosterone system inhibitors on type 1 and type 2 diabetic patients with and without early diabetic nephropathy. Kidney Int 2012;81:674–683 [DOI] [PubMed] [Google Scholar]
- 37.Krolewski AS, Bonventre JV. High risk of ESRD in type 1 diabetes: new strategies are needed to retard progressive renal function decline. Semin Nephrol 2012;32:407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein R. Diabetic retinopathy and nephropathy. In The Diabetic Kidney. Totowa, NJ, Humana Press Inc., 2006, p. 473–498 [Google Scholar]