Summary
The nematode Caenorhabditis elegans has been much studied as a host for microbial infection. Some pathogens can infect its intestine [1, 2], while others attack via its external surface [1, 3–6]. Cultures of Caenorhabditis isolated from natural environments have yielded new nematode pathogens, such as microsporidia and viruses [7, 8]. We report here a novel mechanism for bacterial attack on worms, discovered during investigation of a diseased and coinfected natural isolate of Caenorhabditis from Cape Verde. Two related coryneform pathogens (genus Leucobacter) were obtained from this isolate, which had complementary effects on C. elegans and related nematodes. One pathogen, Verde1, was able to cause swimming worms to stick together irreversibly by their tails, leading to the rapid formation of aggregated “worm-stars.” Adult worms trapped in these aggregates were immobilized and subsequently died, with concomitant growth of bacteria. Trapped larval worms were sometimes able to escape from worm-stars by undergoing autotomy, separating their bodies into two parts. The other pathogen, Verde2, killed worms after rectal invasion, in a more virulent version of a previously studied infection [6]. Resistance to killing by Verde2, by means of alterations in host surface glycosylation, resulted in hypersensitivity to Verde1, revealing a trade-off in bacterial susceptibility. Conversely, a sublethal surface infection of worms with Verde1 conferred partial protection against Verde2. The formation of worm-stars by Verde1 occurred only when worms were swimming in liquid but provides a striking example of asymmetric warfare as well as a bacterial equivalent to the trapping strategies used by nematophagous fungi [4].
Highlights
-
•
Verde1 bacteria can trap swimming worms by tail adhesion and worm-star formation
-
•
Nematodes trapped in worm-stars are immobilized, killed, and used for nutrition
-
•
Trapped larval worms can escape from worm-stars by whole-body autotomy
-
•
Resistance to virulent Verde2 bacteria results in lethal hypersensitivity to Verde1
Results and Discussion
Cultures of Caenorhabditis collected from many locations have been examined for the presence of a swollen tail or “Dar” (deformed anal region) phenotype, which is the conspicuous morphological response of some rhabditid nematodes to rectal infection [6, 9]. This response was discovered in laboratory stocks fortuitously infected by a coryneform bacterium, Microbacterium nematophilum, which has been repeatedly isolated in nematode laboratories [6, 10], but never from nature. Two recent Caenorhabditis isolates were found to exhibit a Dar phenotype, which proved to carry Leucobacter infections with distinctive pathogenic effects.
One C. elegans isolate (JU1088), collected in Kakegawa, Japan, exhibited poor growth, swollen tails, and visibly adherent bacteria. A bacterial strain, CBX130, was obtained from these infected worms and was found to cause pathogenic effects similar to M. nematophilum when added to cultures of the C. elegans laboratory strain N2 (Figure 1A). 16S rRNA gene sequencing indicated that CBX130 belonged to the coryneform genus Leucobacter.
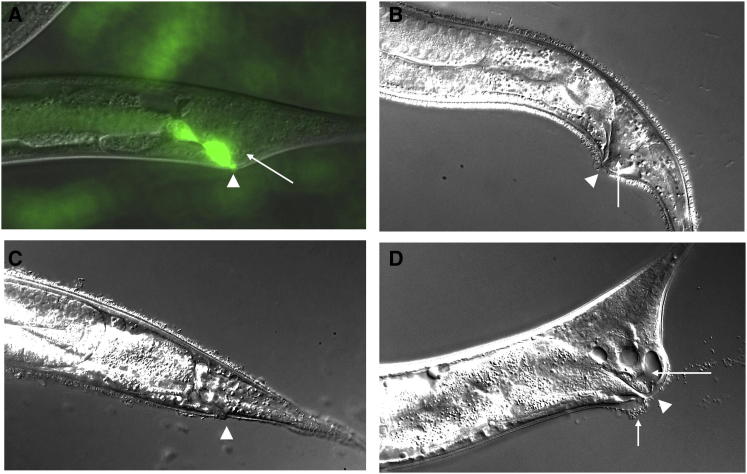
Figure 1.
Leucobacter Cells Adhere to Caenorhabditis
(A) Adult C. elegans hermaphrodite, infected with Leucobacter CBX130 and vitally stained with SYTO13 (green fluorescence). Rectal infection and tail swelling (arrow) are apparent.
(B) Tail region of strain JU1635. Note tail swelling (arrow) and extensive bacterial coating.
(C) Tail region of C. elegans infected with Verde1. Note extensive bacterial coating and absence of tail swelling.
(D) Tail region of C. elegans infected with Verde2. Note severe tail swelling (arrow) and bacteria attached only around anus (short arrow).
Arrowhead marks anal opening in (A)–(D).
A second Dar isolate (JU1635), obtained from rotting banana trunks in Cape Verde, was found to be a hermaphrodite species of Caenorhabditis (n. sp. 11) [11]. Worms in this population had swollen tails as well as a dense covering of adherent bacteria over the surface of the body (Figure 1B). A similar disease state was established in C. elegans (N2) by growing worms on bacterial lawns that had been exposed to JU1635. Infected C. elegans worms had the dense bacterial coating and swollen tails seen in the original JU1635 population.
Isolation of bacteria from the diseased C. elegans population revealed that worms carry a double infection of two distinct bacteria with contrasting pathogenic effects (Figures 1C and 1D). Two pathogenic strains were established, both typed as Leucobacter species. We refer to them here as Verde1 and Verde2; detailed phenotypic and taxonomic characterization will be presented elsewhere. Verde1 is identical in 16S RNA sequence to Leucobacter celer NAL101 [12], though distinguishable from it (see Table S1 available online), whereas Verde2 is identical in 16S RNA sequence to the Kakegawa isolate CBX130. These two bacteria had remarkably different effects on C. elegans. Both Verde1 and Verde2 were virulent pathogens, but they exerted their lethality in distinct ways.
Under standard culture conditions, Verde1 was inhibitory but not lethal to the propagation of wild-type C. elegans. Worms grown on mixed lawns of E. coli and Verde1 (∼10:1) became impaired in movement and grew more slowly (generation time increased by 27%; Table S2), but they were able to grow and reproduce. When examined microscopically, infected worms were seen to have a dense covering of tightly adherent bacteria, but no Dar phenotype (Figure 1C; Figure S1).
Exposure of worms to Verde1 bacteria in liquid had different consequences. Within 2 min after addition of a Verde1 bacterial suspension (∼108 colony-forming units [cfu]/ml) to a population of worms swimming in liquid, they began to stick to each other in their tail regions and formed star-like aggregates, which we term “worm-stars,” with dozens of worms radiating outward from a posterior zone of adhesion (Figures 2A and 2B). When the aggregates were picked out of liquid onto an agar plate, the trapped worms tried to escape from the stars, but most (>80%) of them failed (Figure 2C). Trapping had lethal consequences: after 24–48 hr incubation at 25°C, all the worms in a star were dead (Figure 3A). The worms that escaped from a star within the first hour survived for much longer, but worms that broke free at later times moved only a few body lengths from the star before dying. Worms that avoided trapping into stars could be similarly transferred from liquid onto solid media, and these showed no major ill effects and survived for many days (Figure 3A).
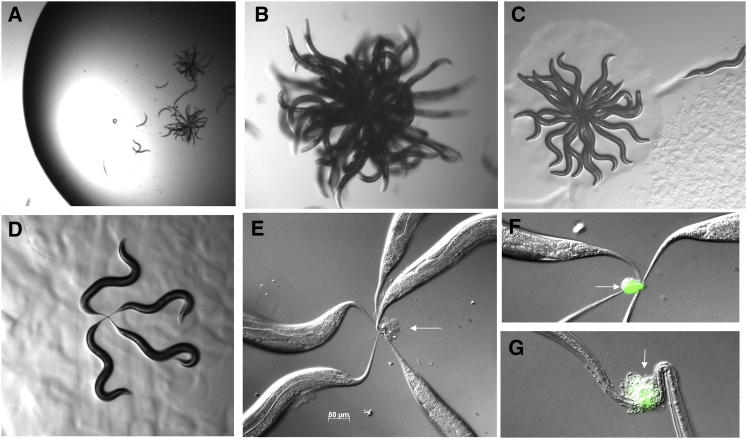
Figure 2.
Verde1 Bacteria Induce Formation of Worm-Stars
(A) Two worm-stars forming in 0.2 ml drop. Most of the worms in the drop have been captured by the stars.
(B) Close-up of a worm-star in liquid.
(C) A worm-star after transfer to a solid surface; one worm can be seen escaping.
(D) Snapshot of a quartet of worms held together by their tails.
(E) Five worms held together by a tail knot (arrow).
(F) A four-worm tail knot induced by Verde1 bacteria that had been prelabeled with SYTO13; green fluorescence (arrow) is confined to the tail-spike region.
(G) Detail of tail adhesion between two worms; fluorescent adherent bacteria can be seen (arrow).
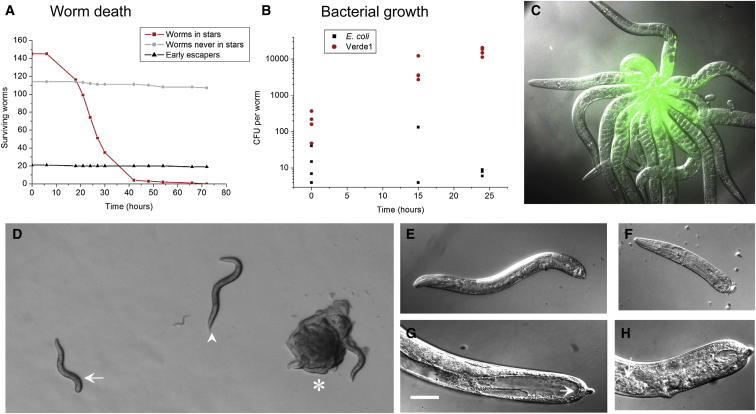
Figure 3.
Worm Killing and Escape by Autotomy
(A) Survival of worms in stars. Ten small stars were allowed to form in liquid from about 300 young adult worms and then picked to agar plates. Worms that avoided stars or that escaped in the first hour after picking were collected separately and transferred to agar plates. Plates were incubated at 25°C, and worm viability was scored over time.
(B) Bacterial counts in small worm-stars over time, on nutrient-free agarose.
(C) A 15-worm-star after 8 hr of incubation at 25°C, stained briefly with fluorescein diacetate; epifluorescence/differential interference contrast optics.
(D) Remains of an L4 worm-star (asterisk) 20 hr after formation, showing one escaper with a truncated tail spike (arrowhead) and one half-worm escaper (arrow).
(E) A 60% half-worm.
(F) A 40% half-worm.
(G) Half-worm posterior, with blind gut ending (arrow). Scale bar represents 20 μm.
(H) Half-worm posterior, with developing egg (arrow) anterior to vulva.
Death of worms in stars appeared to result primarily from destruction of permeability barriers in the tail of the worms. Loss of integrity in the posterior of worms was demonstrated by staining worm-stars with fluorescein diacetate, which could be seen diffusing into body cavities of the trapped worms from the tail forward (Figure 3C).
Stars containing few worms formed if numbers were kept low, and these were picked out and examined. Individuals were seen to be tied together by a knot formed by their interwoven tail spikes (Figure 2D–2G). Small stars sometimes disassembled, by unweaving or breakage of tail spikes, but the larger the aggregate, the more irreversible the knot. Star formation began within 2 min at high bacterial concentrations (>108 cfu/ml) but still occurred down to 105 cfu/ml, albeit more slowly. At these low concentrations, only a few thousand bacterial cells sufficed to stick worms together. Inducing the formation of worm-stars with prelabeled fluorescent Verde1 bacteria showed that the bacteria were initially preferentially attaching to worm tails (Figures 2F and 2G).
Under these liquid conditions, the bacteria were therefore waging an ingenious form of asymmetric warfare. Despite being vastly smaller than their hosts, they could immobilize substantial groups of worms, which could then presumably be degraded and used for bacterial nutrition. Cuticular damage and bacterial invasion were probably exacerbated by the worms’ continued writhing during efforts to escape, as well as tail breakage in worms that succeeded in breaking free.
Pathogenic proliferation was demonstrated by measuring the number of bacteria in worm-stars (Figure 3B). Small stars were allowed to form, washed, and sampled for total bacterial content shortly after formation or after 15 or 24 hr of incubation. Initial counts averaged ∼200 Verde1 bacteria per worm, whereas 24 hr counts averaged ∼16,000, revealing an 80-fold increase. Residual E. coli counts dropped from an average of 17 to 6 per worm over the same time, demonstrating that Verde1 dominated bacterial growth under these conditions.
Adult individuals that escaped from worm-stars often displayed broken tail spikes that had healed, consistent with wound repair [3]. Another defensive response was observed when worm-stars formed from populations of late larval (L4) worms. 24 hr after star formation, about 5% of the worms in such stars (7 of 82 worms in six stars) had escaped by undergoing a form of autotomy, whereby the worm became separated into two parts (Figures 3D–3F). The resulting anterior half-worms were able to crawl away from the degrading worm-stars. Gonads in half-worms had often undergone sufficient maturation to allow self-fertilization and progeny production (Figure 3F). Worms that had separated anterior to the vulva rarely produced progeny (1 of 11 individuals examined), but those with more posterior autotomy were usually fertile (42 of 53 individuals examined, each producing one to five progeny). Consequently, autotomy represents a viable escape strategy in terms of reproductive survival, though it was not seen in adult worms. Autotomy has not been reported previously in nematodes [13].
Verde1 was observed to form worm-stars in the original Caenorhabditis strain JU1635 and in other tested species of Caenorhabditis (Figure S2). Verde1 also induced stable star formation in nematodes from some related nematode genera, such as Oscheius tipulae and Rhabditis brassicae, but not in Pristionchus pacificus, for which only weakly bound stars and loose tangles formed. When populations of P. pacificus were mixed with C. elegans and exposed to Verde1 in liquid, stable stars containing both species were formed (Figure S2), with subsequent death of both constituents. The ability to trap and kill less susceptible species such as P. pacificus by means of mixed-species stars enlarges the potential host range of Verde1. Worm-stars were also observed to form under simulations of natural conditions, such as when rain falls on rotting fruit, a preferred habitat for C. elegans [7, 11, 14] (Figure S2).
The other Cape Verde Leucobacter, Verde2, had different effects on C. elegans. Worms grown at 20°C or below in the presence of Verde2 developed a Dar phenotype, similar to that caused by M. nematophilum and CBX130 (Figure 1D). At higher temperatures, Verde2 was more virulent. Young adult worms exposed to mixed lawns of E. coli/Verde2 at 25°C mostly died within 36 hr (Figure 4A), and larvae were almost wholly unable to survive. Rare escapers had extremely swollen tails and usually failed to reach adulthood. Verde2 is therefore a lethal pathogen for C. elegans, with effects similar to but more virulent than those of M. nematophilum.
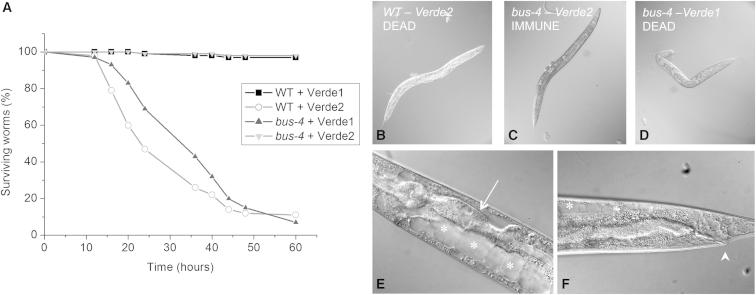
Figure 4.
Verde1 and Verde2 Exert Complementary Lethal Effects
(A) Survival of wild-type or bus-4(e2693) mutant worms on E. coli/Verde1 or E. coli/Verde2 lawns at 25°C. For each condition, 100 L4 worms were picked and examined for viability over time.
(B) Wild-type worm and (C) immune bus-4 worm after 24 hr incubation on E. coli/Verde2.
(D) bus-4 worm after 24 hr incubation on E. coli/Verde1. Note transparency, shrinkage, and collapse relative to (C).
(E and F) Higher-magnification details of wild-type worms dying after 20 hr exposure to Verde2. In (E), note the anterior gut (arrow) compressed by swollen vacuoles (asterisks) in the body cavity. Vacuoles are also visible in the tail region (F), as well as bacteria adhering to anal cuticle (arrowhead).
Adult worms killed by Verde2 exhibited an unusual rigid and swollen appearance (Figure 4B). Microscopic examination revealed that the body cavity of dying worms became filled with large vacuoles, which distorted internal organs and distended the whole body (Figures 4E and 4F). General internal lysis ensued. How the Verde2 pathogen is able to exert this effect is mysterious.
Similarity between Verde2 infection and M. nematophilum infection was seen in the response of numerous C. elegans mutants selected for altered response to M. nematophilum in previous work [15, 16]. Most of the mutants resistant to infection by M. nematophilum were also fully resistant to Verde2, and consequently they were able to grow well on E. coli/Verde2 lawns (Figure 4; Figure S3; Table S3). Among these were many mutants with altered surface glycosylation, which have been extensively described (bus and srf mutants [16–20]).
When these Verde2-resistant glycosylation mutants were exposed to Verde1, it was found that the Verde1 pathogen efficiently killed them, so populations of Bus or Srf worms were completely unable to survive on mixed E. coli/Verde1 lawns (Figure 4; Figure S3; Table S3). Death appeared to result from general cuticular destruction and loss of surface integrity, because Bus adults killed by Verde1 had a collapsed translucent appearance (Figure 4D), in contrast to the straight engorged corpses resulting from Verde2 infection of wild-type adults (Figures 4B, 4E, and 4F).
Verde1 therefore has latent potential as a virulent pathogen for C. elegans. The response of wild-type C. elegans to Verde1 infection was examined using the induction of the defensive antimicrobial peptide NLP-29 [3]. After 8 hr of exposure to Verde1, quantitative RT-PCR measurement showed that expression of nlp-29 in wild-type worms was increased by a factor of 6.8 ± 2.9. Similarly, the reporter strain IG274, which expresses GFP driven by the nlp-29 promoter [3], revealed pathogenic induction of epidermal nlp-29p::gfp expression after exposure to Verde1 (Figure S4).
The reciprocal relationship between Verde1 and Verde2 pathogenicity reveals a trade-off in susceptibility: worms cannot become resistant to Verde2 or related pathogens without becoming lethally hypersensitive to Verde1. The presence of both bacteria on the original sample of naturally infected worms, strain JU1635, raised the possibility that double infection might be better tolerated than single infections. Under some circumstances, preinfection by Verde1 was found to be protective against subsequent challenge by Verde2. This was demonstrated by briefly exposing young adult wild-type C. elegans to Verde1 in liquid conditions (for one minute, too short a time for the formation of worm-stars), followed by plating on E. coli/Verde2 lawns. After 24 hr incubation at 25°C, 73% of control worms (147 of 200) were dead, while only 43% of Verde1-treated worms (86 of 200) were dead. The tolerance of Verde2 infection in the original JU1635 sample was probably further increased by the presence of additional bacterial strains in this culture (Table S2). Verde2 did not interfere with star formation by Verde1 but rather even promoted it (Table S4).
These two pathogens, obtained from the same wild isolate of Caenorhabditis, therefore exhibited diverse effects on C. elegans. Verde1 was capable of killing worms by means of star formation under liquid conditions but was tolerated and mildly protective against Verde2 when worms were growing on solid substrates. In contrast, Verde2 caused severe rectal disease and lethality to wild-type worms, while mutant worms resistant to Verde2 by virtue of surface alteration became lethally hypersensitive to Verde1. Different killing mechanisms are used by these two pathogens, although both initially attack by means of surface adhesion.
Killing by means of worm-stars has not been previously described, but aggregation of nematodes to form “Medusa heads” or “rosettes,” resembling worm-stars, has been occasionally reported for microfilariae [21] and soil nematodes [22–24]. These cases may have involved bacterial adhesion and pathogenesis. The ability of larvae to escape by autotomy provides further evidence for the natural occurrence of worm-stars. Predatory trapping by nematophagous soil fungi, which can capture and kill nematodes by means of mechanical traps or adhesive structures, has been much studied [4, 25, 26]. The phenomenon reported here extends the trapping strategy into the bacterial world.
Experimental Procedures
General Caenorhabditis culture conditions were as described in [27].
Star Formation
Worm-stars were routinely prepared by placing a 0.1 ml drop of washed adult worms in M9 buffer on the plastic lid of a Petri dish and adding 0.05 ml of Verde1 bacteria, grown to stationary phase in LB broth and diluted to 108 cfu/ml. Stars formed more efficiently if worms were grown at low temperature (<20°C).
Assaying Bacterial Growth
Twelve individual stars of 11–20 worms were allowed to form by transferring young adult worms grown on E. coli lawns to unspread NGM plates for 30 min and then picking sets of 25 worms to 0.05 ml drops of 5 × 107 cells/ml of Verde1 in M9 buffer. After 5–10 min, each resulting worm-star was picked to 0.1 ml drops of M9 buffer to wash for 10 min and then picked to individual nutrient-free plates (buffered 1% agarose). Worms in each star were counted. After 0, 15, or 24 hr of incubation at 25°C, stars were separately picked, each to 1 ml M9 buffer in a microfuge tube, ground with sterile sand for 1 minute using a minipestle, vortexed vigorously for 1 min, and diluted for plating on LB plates. Colonies were counted after 24 hours incubation at 30°C, at which point the small Verde1 colonies were easily distinguished from residual large E. coli colonies.
Acknowledgments
This work was supported by MRC grant MR/J001309/1 to J.H., a grant from the Agence Nationale pour la Recherche (ANR 11 BSV3 01301), and a grant from Coup d’Elan de la Fondation Bettencourt-Schueller to M.-A.F. We thank Simon Spiro for assistance with nlp-29 assays, Adeline Mallet and Michalis Barkoulas for assistance with scanning electron microscopy, Hillel Schwartz for discussions, and Delia O’Rourke for comments on the manuscript. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440).
Published: October 24, 2013
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information includes four figures, four tables, and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2013.08.060.
Supplemental Information
References
- 1.Couillault C., Ewbank J.J. Diverse bacteria are pathogens of Caenorhabditis elegans. Infect. Immun. 2002;70:4705–4707. doi: 10.1128/IAI.70.8.4705-4707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pukkila-Worley R., Ausubel F.M. Immune defense mechanisms in the Caenorhabditis elegans intestinal epithelium. Curr. Opin. Immunol. 2012;24:3–9. doi: 10.1016/j.coi.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pujol N., Cypowyj S., Ziegler K., Millet A., Astrain A., Goncharov A., Jin Y., Chisholm A.D., Ewbank J.J. Distinct innate immune responses to infection and wounding in the C. elegans epidermis. Curr. Biol. 2008;18:481–489. doi: 10.1016/j.cub.2008.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barron G.L. The nematode-destroying fungi. In: Barron G.L., editor. Topics in Mycobiology, No. 1. Canadian Biological Publications Ltd.; Guelph: 1977. [Google Scholar]
- 5.Darby C., Hsu J.W., Ghori N., Falkow S. Caenorhabditis elegans: plague bacteria biofilm blocks food intake. Nature. 2002;417:243–244. doi: 10.1038/417243a. [DOI] [PubMed] [Google Scholar]
- 6.Hodgkin J., Kuwabara P.E., Corneliussen B. A novel bacterial pathogen, Microbacterium nematophilum, induces morphological change in the nematode C. elegans. Curr. Biol. 2000;10:1615–1618. doi: 10.1016/s0960-9822(00)00867-8. [DOI] [PubMed] [Google Scholar]
- 7.Troemel E.R., Félix M.A., Whiteman N.K., Barrière A., Ausubel F.M. Microsporidia are natural intracellular parasites of the nematode Caenorhabditis elegans. PLoS Biol. 2008;6:2736–2752. doi: 10.1371/journal.pbio.0060309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Félix M.A., Ashe A., Piffaretti J., Wu G., Nuez I., Bélicard T., Jiang Y., Zhao G., Franz C.J., Goldstein L.D. Natural and experimental infection of Caenorhabditis nematodes by novel viruses related to nodaviruses. PLoS Biol. 2011;9:e1000586. doi: 10.1371/journal.pbio.1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholas H.R., Hodgkin J. The ERK MAP kinase cascade mediates tail swelling and a protective response to rectal infection in C. elegans. Curr. Biol. 2004;14:1256–1261. doi: 10.1016/j.cub.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Akimkina T., Yook K., Curnock S., Hodgkin J. Genome characterization, analysis of virulence and transformation of Microbacterium nematophilum, a coryneform pathogen of the nematode Caenorhabditis elegans. FEMS Microbiol. Lett. 2006;264:145–151. doi: 10.1111/j.1574-6968.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 11.Kiontke K.C., Félix M.-A., Ailion M., Rockman M.V., Braendle C., Pénigault J.B., Fitch D.H. A phylogeny and molecular barcodes for Caenorhabditis, with numerous new species from rotting fruits. BMC Evol. Biol. 2011;11:339–357. doi: 10.1186/1471-2148-11-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin N.R., Kim M.S., Jung M.J., Roh S.W., Nam Y.D., Park E.J., Bae J.W. Leucobacter celer sp. nov., isolated from Korean fermented seafood. Int. J. Syst. Evol. Microbiol. 2011;61:2353–2357. doi: 10.1099/ijs.0.026211-0. [DOI] [PubMed] [Google Scholar]
- 13.Fleming P.A., Muller D., Bateman P.W. Leave it all behind: a taxonomic perspective of autotomy in invertebrates. Biol. Rev. Camb. Philos. Soc. 2007;82:481–510. doi: 10.1111/j.1469-185X.2007.00020.x. [DOI] [PubMed] [Google Scholar]
- 14.Barrière A., Félix M.-A. Isolation of C. elegans and related nematodes. WormBook. 2006:1–9. doi: 10.1895/wormbook.1.115.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gravato-Nobre M.J., Nicholas H.R., Nijland R., O’Rourke D., Whittington D.E., Yook K.J., Hodgkin J. Multiple genes affect sensitivity of Caenorhabditis elegans to the bacterial pathogen Microbacterium nematophilum. Genetics. 2005;171:1033–1045. doi: 10.1534/genetics.105.045716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yook K., Hodgkin J. Mos1 mutagenesis reveals a diversity of mechanisms affecting response of Caenorhabditis elegans to the bacterial pathogen Microbacterium nematophilum. Genetics. 2007;175:681–697. doi: 10.1534/genetics.106.060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gravato-Nobre M.J., Stroud D., O’Rourke D., Darby C., Hodgkin J. Glycosylation genes expressed in seam cells determine complex surface properties and bacterial adhesion to the cuticle of Caenorhabditis elegans. Genetics. 2011;187:141–155. doi: 10.1534/genetics.110.122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palaima E., Leymarie N., Stroud D., Mizanur R.M., Hodgkin J., Gravato-Nobre M.J., Costello C.E., Cipollo J.F. The Caenorhabditis elegans bus-2 mutant reveals a new class of O-glycans affecting bacterial resistance. J. Biol. Chem. 2010;285:17662–17672. doi: 10.1074/jbc.M109.065433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Partridge F.A., Tearle A.W., Gravato-Nobre M.J., Schafer W.R., Hodgkin J. The C. elegans glycosyltransferase BUS-8 has two distinct and essential roles in epidermal morphogenesis. Dev. Biol. 2008;317:549–559. doi: 10.1016/j.ydbio.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 20.Höflich J., Berninsone P., Göbel C., Gravato-Nobre M.J., Libby B.J., Darby C., Politz S.M., Hodgkin J., Hirschberg C.B., Baumeister R. Loss of srf-3-encoded nucleotide sugar transporter activity in Caenorhabditis elegans alters surface antigenicity and prevents bacterial adherence. J. Biol. Chem. 2004;279:30440–30448. doi: 10.1074/jbc.M402429200. [DOI] [PubMed] [Google Scholar]
- 21.Yoeli M. Observations of agglutination and thigmotaxis of microfilariae in bancroftian filariasis. Trans. R. Soc. Trop. Med. Hyg. 1957;51:132–136. doi: 10.1016/0035-9203(57)90057-3. [DOI] [PubMed] [Google Scholar]
- 22.Pye A.E., Burman M. Rosette formation by Heterorhabditis bacteriophora. Nematologica. 1981;27:117–119. [Google Scholar]
- 23.Sudhaus W., Hooper D.J. Rhabditis (Oscheius) guentheri sp. n., an unusual species with reduced posterior ovary, with observations on the Dolichura and Insectivora groups (Nematoda: Rhabditidae) Nematologica. 1994;40:508–533. [Google Scholar]
- 24.Stock S.P., Caicedo A.M., Calatayud P.A. Rhabditis (Oscheius) colombiana n. sp. (Nematoda: Rhabditidae), a necromenic associate of the subterranean burrower bug Cyrtomenus bergi (Hemiptera: Cydnidae) from the Cauca Valley, Columbia. Nematology. 2005;7:363–373. [Google Scholar]
- 25.Yang Y., Yang E., An Z., Liu X. Evolution of nematode-trapping cells of predatory fungi of the Orbiliaceae based on evidence from rRNA-encoding DNA and multiprotein sequences. Proc. Natl. Acad. Sci. USA. 2007;104:8379–8384. doi: 10.1073/pnas.0702770104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsueh Y.-P., Mahanti P., Schroeder F.C., Sternberg P.W. Nematode-trapping fungi eavesdrop on nematode pheromones. Curr. Biol. 2013;23:83–86. doi: 10.1016/j.cub.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.