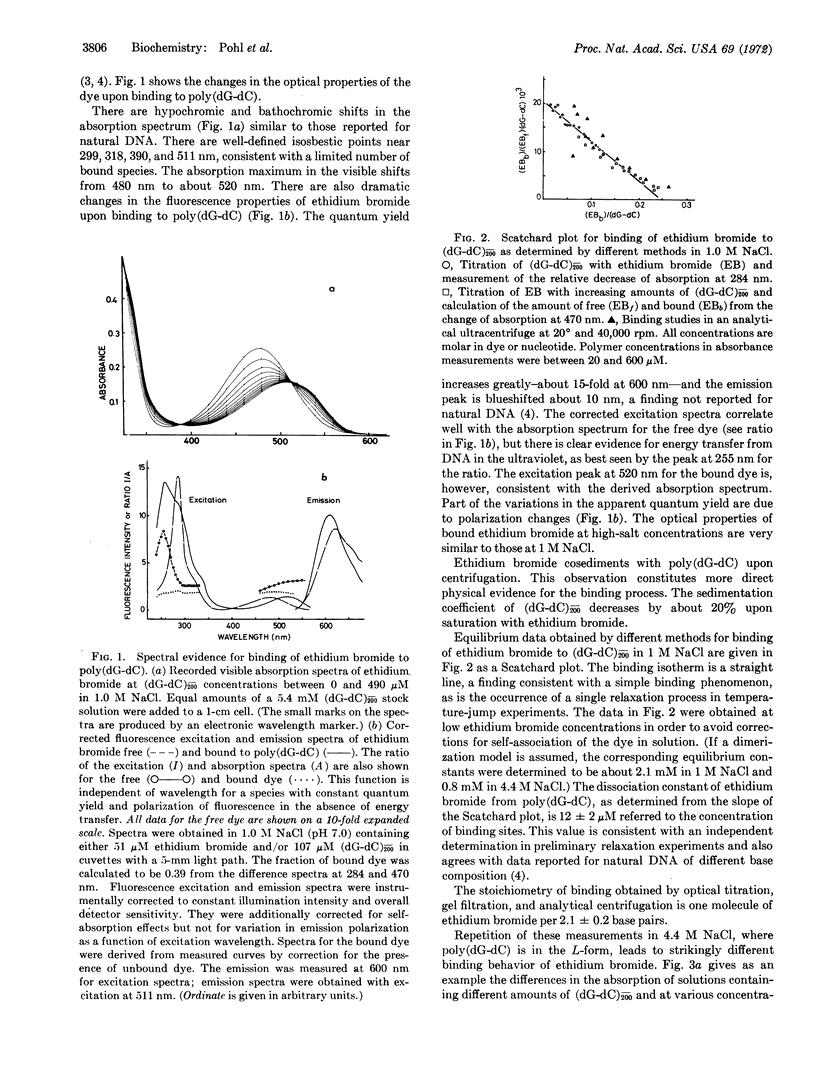
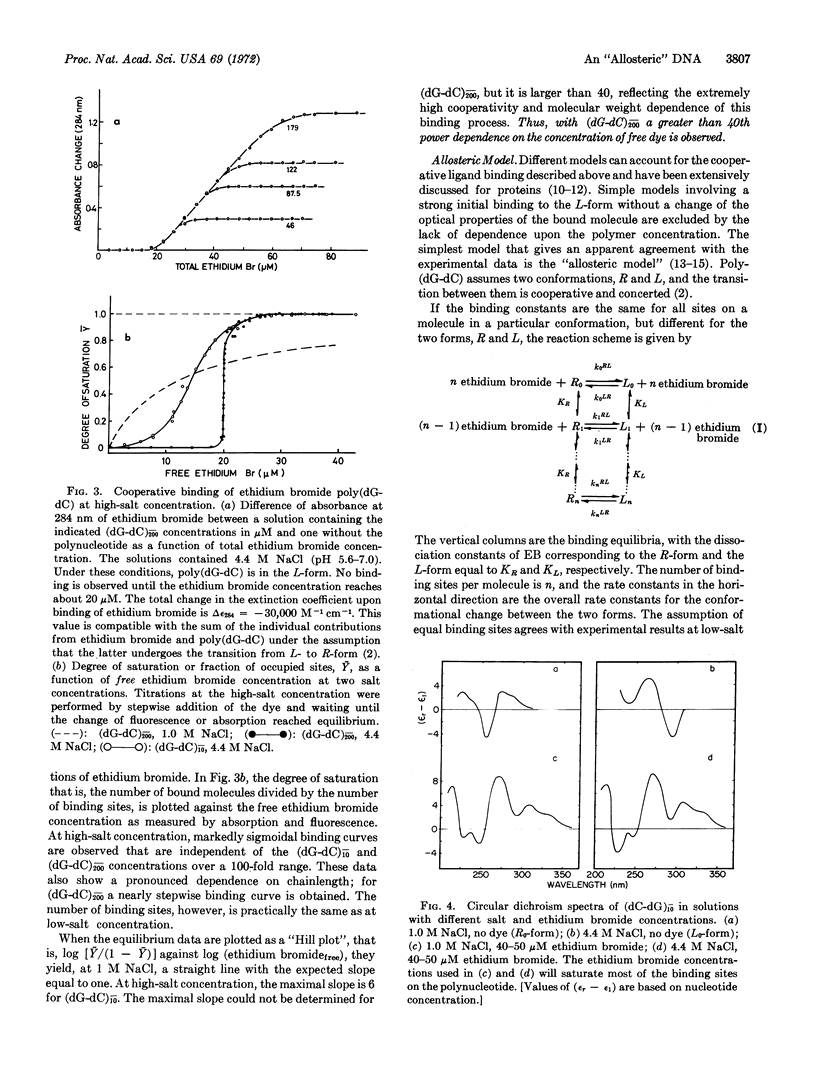
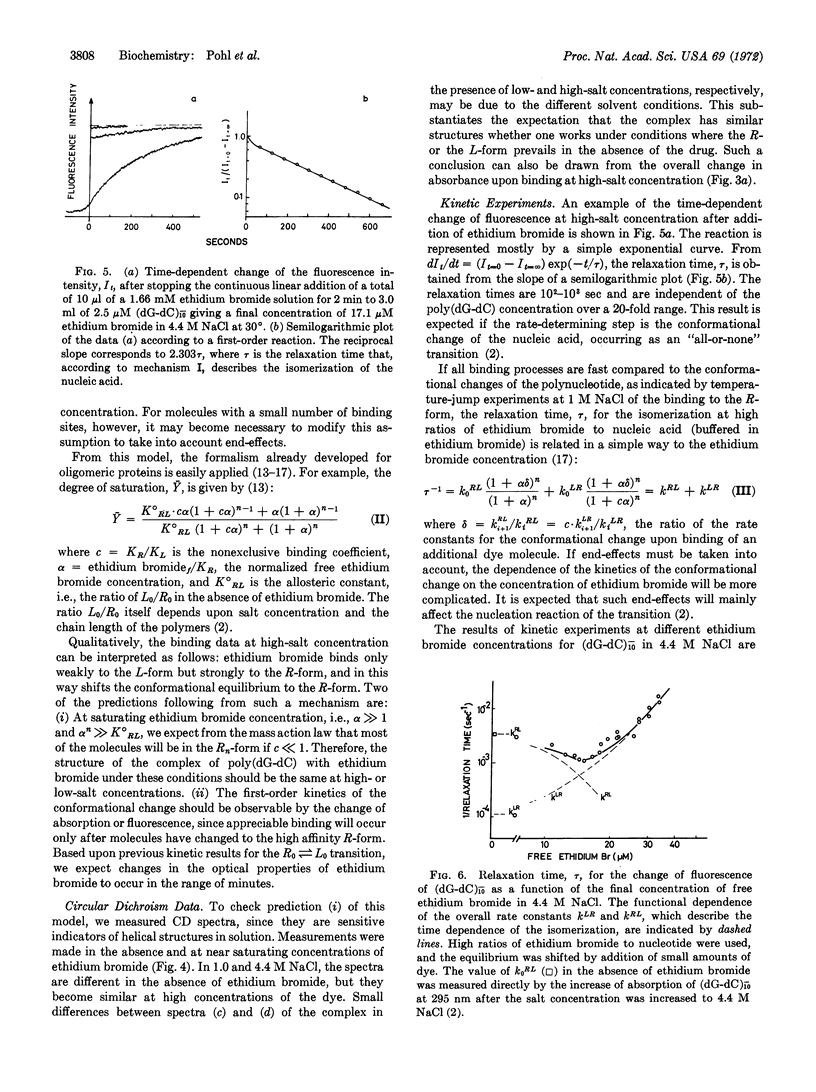
Abstract
A salt-induced cooperative conformational transition of a synthetic DNA, poly(dG-dC), is reversed by addition of ethidium bromide. Binding of the dye at high salt concentrations is highly cooperative. Circular dichroism spectra of the complex and the kinetic data support a model for this cooperative binding that is formally equivalent to the “allosteric” one proposed for oligomeric proteins by Monod et al. Thus, double-helical DNA of at least one defined sequence can undergo a cooperative conformational change in solution, with simple salts and drug molecules as antagonistic effectors. Such transitions may be involved in regulatory phenomena operating directly at the level of nucleic acid structure.
Keywords: poly(dG-dC), allosterism, drug binding, kinetics, fluorescence, circular dichroism
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer L. M., Moudrianakis E. N. Interaction of ethidium bromide with whole and selectively deproteinized deoxynucleoproteins from calf thymus. J Mol Biol. 1972 Feb 14;63(3):505–521. doi: 10.1016/0022-2836(72)90444-5. [DOI] [PubMed] [Google Scholar]
- Blangy D., Buc H., Monod J. Kinetics of the allosteric interactions of phosphofructokinase from Escherichia coli. J Mol Biol. 1968 Jan 14;31(1):13–35. doi: 10.1016/0022-2836(68)90051-x. [DOI] [PubMed] [Google Scholar]
- Caspersson T., Farber S., Foley G. E., Kudynowski J., Modest E. J., Simonsson E., Wagh U., Zech L. Chemical differentiation along metaphase chromosomes. Exp Cell Res. 1968 Jan;49(1):219–222. doi: 10.1016/0014-4827(68)90538-7. [DOI] [PubMed] [Google Scholar]
- Eigen M. New looks and outlooks on physical enzymology. Q Rev Biophys. 1968 May;1(1):3–33. doi: 10.1017/s0033583500000445. [DOI] [PubMed] [Google Scholar]
- Kirschner K., Eigen M., Bittman R., Voigt B. The binding of nicotinamide-adenine dinucleotide to yeast d-glyceraldehyde-3-phosphate dehydrogenase: temperature-jump relaxation studies on the mechanism of an allosteric enzyme. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1661–1667. doi: 10.1073/pnas.56.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- LEPECQ J. B., YOT P., PAOLETTI C. INTERACTION DU BROMHYDRATE D''ETHIDIUM (BET) AVEC LES ACIDES NUCL'EIQUES (A. N.). ETUDE SPECTROFLUORIM'ETRIQUE. C R Hebd Seances Acad Sci. 1964 Sep 7;259:1786–1789. [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Rubin M. M., Changeux J. P. On the nature of allosteric transitions: implications of non-exclusive ligand binding. J Mol Biol. 1966 Nov 14;21(2):265–274. doi: 10.1016/0022-2836(66)90097-0. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Saucier J. M., Festy B., Le Pecq J. B. The change of the torsion of the DNA helix caused by intercalation. II. Measurement of the relative change of torsion induced by various intercalating drugs. Biochimie. 1971;53(9):973–980. doi: 10.1016/s0300-9084(71)80065-2. [DOI] [PubMed] [Google Scholar]
- Waring M. J. Complex formation between ethidium bromide and nucleic acids. J Mol Biol. 1965 Aug;13(1):269–282. doi: 10.1016/s0022-2836(65)80096-1. [DOI] [PubMed] [Google Scholar]
- Weber G. Ligand binding and internal equilibria in proteins. Biochemistry. 1972 Feb 29;11(5):864–878. doi: 10.1021/bi00755a028. [DOI] [PubMed] [Google Scholar]
- Weisblum B., De Haseth P. L. Quinacrine, a chromosome stain specific for deoxyadenylate-deoxythymidylaterich regions in DNA. Proc Natl Acad Sci U S A. 1972 Mar;69(3):629–632. doi: 10.1073/pnas.69.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]



