Abstract
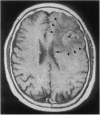
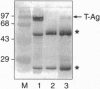
We describe molecular and clinical findings in an immunocompetent patient with an oligoastrocytoma and the concomitant presence of the human papovavirus, JC virus (JCV), which is the etiologic agent of the subacute, debilitating demyelinating disease, progressive multifocal leukoencephalopathy. Histologic review revealed a glial neoplasm consisting primarily of a moderately cellular oligodendroglioma with distinct areas of a fibrillary astrocytoma. Immunohistochemical analysis revealed nuclear staining of tumor cells with antibodies against the viral oncoprotein [tumor antigen (T antigen)], the proliferation marker (Ki67), and the cellular proliferation regulator (p53). Using primers specific to the JCV control region, PCR yielded amplified DNA that was identical to the control region of the Mad-4 strain of the virus. PCR analysis demonstrated the presence of the genome for the viral oncoprotein, T antigen, and results from primer extension studies revealed synthesis of the viral early RNA for T antigen in the tumor tissues. The presence of viral T antigen in the tumor tissue was further demonstrated by immunoblot assay. To our knowledge, this is the first report of the presence of JCV DNA, RNA, and T antigen in tissue in which viral T antigen is localized to tumor cell nuclei and suggests the possible association of JCV with some glial neoplasms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S., Rappaport J., Tada H., Kerr D., Khalili K. A nuclear protein derived from brain cells stimulates transcription of the human neurotropic virus promoter, JCVE, in vitro. J Biol Chem. 1990 Aug 15;265(23):13899–13905. [PubMed] [Google Scholar]
- Bergsagel D. J., Finegold M. J., Butel J. S., Kupsky W. J., Garcea R. L. DNA sequences similar to those of simian virus 40 in ependymomas and choroid plexus tumors of childhood. N Engl J Med. 1992 Apr 9;326(15):988–993. doi: 10.1056/NEJM199204093261504. [DOI] [PubMed] [Google Scholar]
- Bharucha V. A., Peden K. W., Tennekoon G. I. SV40 large T antigen with c-Jun down-regulates myelin P0 gene expression: a mechanism for papovaviral T antigen-mediated demyelination. Neuron. 1994 Mar;12(3):627–637. doi: 10.1016/0896-6273(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Bollag B., Chuke W. F., Frisque R. J. Hybrid genomes of the polyomaviruses JC virus, BK virus, and simian virus 40: identification of sequences important for efficient transformation. J Virol. 1989 Feb;63(2):863–872. doi: 10.1128/jvi.63.2.863-872.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaigne P., Rondot P., Escourolle R., Ribadeau dumas J. L., Cathala F., Hauw J. J. Leucoencéphalopathie multifocale progressive et "gliomes" multiples. Rev Neurol (Paris) 1974 Sep-Oct;130(9-10):379–392. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davies J. A., Hughes J. T., Oppenheimer D. R. Richardson's disease (progressive multifocal leukoencephalopathy). A study of four cases. Q J Med. 1973 Jul;42(167):481–501. [PubMed] [Google Scholar]
- Dyson N., Bernards R., Friend S. H., Gooding L. R., Hassell J. A., Major E. O., Pipas J. M., Vandyke T., Harlow E. Large T antigens of many polyomaviruses are able to form complexes with the retinoblastoma protein. J Virol. 1990 Mar;64(3):1353–1356. doi: 10.1128/jvi.64.3.1353-1356.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Buchkovich K., Whyte P., Harlow E. The cellular 107K protein that binds to adenovirus E1A also associates with the large T antigens of SV40 and JC virus. Cell. 1989 Jul 28;58(2):249–255. doi: 10.1016/0092-8674(89)90839-8. [DOI] [PubMed] [Google Scholar]
- Feigenbaum L., Khalili K., Major E., Khoury G. Regulation of the host range of human papovavirus JCV. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3695–3698. doi: 10.1073/pnas.84.11.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenbaum L., Khalili K., Major E., Khoury G. Regulation of the host range of human papovavirus JCV. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3695–3698. doi: 10.1073/pnas.84.11.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J., Bream G. L., Cannella M. T. Human polyomavirus JC virus genome. J Virol. 1984 Aug;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GiaRusso M. H., Koeppen A. H. Atypical progressive multifocal leukoencephalopathy and primary cerebral malignant lymphoma. J Neurol Sci. 1978 Feb;35(2-3):391–398. doi: 10.1016/0022-510x(78)90019-9. [DOI] [PubMed] [Google Scholar]
- Gullotta F., Masini T., Scarlato G., Kuchelmeister K. Progressive multifocal leukoencephalopathy and gliomas in a HIV-negative patient. Pathol Res Pract. 1992 Dec;188(8):964–972. doi: 10.1016/s0344-0338(11)81239-2. [DOI] [PubMed] [Google Scholar]
- Haas S., Haque N. S., Beggs A. H., Khalili K., Knobler R. L., Small J. Expression of the myelin basic protein gene in transgenic mice expressing human neurotropic virus, JCV, early protein. Virology. 1994 Jul;202(1):89–96. doi: 10.1006/viro.1994.1325. [DOI] [PubMed] [Google Scholar]
- Haggerty S., Walker D. L., Frisque R. J. JC virus-simian virus 40 genomes containing heterologous regulatory signals and chimeric early regions: identification of regions restricting transformation by JC virus. J Virol. 1989 May;63(5):2180–2190. doi: 10.1128/jvi.63.5.2180-2190.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson J. W., Schnitker B. L., Correa K. M., von Deimling A., Fassbender F., Xu H. J., Benedict W. F., Yandell D. W., Louis D. N. The retinoblastoma gene is involved in malignant progression of astrocytomas. Ann Neurol. 1994 Nov;36(5):714–721. doi: 10.1002/ana.410360505. [DOI] [PubMed] [Google Scholar]
- Kenney S., Natarajan V., Strike D., Khoury G., Salzman N. P. JC virus enhancer-promoter active in human brain cells. Science. 1984 Dec 14;226(4680):1337–1339. doi: 10.1126/science.6095453. [DOI] [PubMed] [Google Scholar]
- Khalili K., Brady J., Pipas J. M., Spence S. L., Sadofsky M., Khoury G. Carboxyl-terminal mutants of the large tumor antigen of simian virus 40: a role for the early protein late in the lytic cycle. Proc Natl Acad Sci U S A. 1988 Jan;85(2):354–358. doi: 10.1073/pnas.85.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili K., Feigenbaum L., Khoury G. Evidence for a shift in 5'-termini of early viral RNA during the lytic cycle of JC virus. Virology. 1987 Jun;158(2):469–472. doi: 10.1016/0042-6822(87)90224-8. [DOI] [PubMed] [Google Scholar]
- Khalili K., Rappaport J., Khoury G. Nuclear factors in human brain cells bind specifically to the JCV regulatory region. EMBO J. 1988 Apr;7(4):1205–1210. doi: 10.1002/j.1460-2075.1988.tb02932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F. F., Miller D. C., Koslow M., Newcomb E. W. Pathways leading to glioblastoma multiforme: a molecular analysis of genetic alterations in 65 astrocytic tumors. J Neurosurg. 1994 Sep;81(3):427–436. doi: 10.3171/jns.1994.81.3.0427. [DOI] [PubMed] [Google Scholar]
- Lednicky J. A., Garcea R. L., Bergsagel D. J., Butel J. S. Natural simian virus 40 strains are present in human choroid plexus and ependymoma tumors. Virology. 1995 Oct 1;212(2):710–717. doi: 10.1006/viro.1995.1529. [DOI] [PubMed] [Google Scholar]
- London W. T., Houff S. A., Madden D. L., Fuccillo D. A., Gravell M., Wallen W. C., Palmer A. E., Sever J. L., Padgett B. L., Walker D. L. Brain tumors in owl monkeys inoculated with a human polyomavirus (JC virus). Science. 1978 Sep 29;201(4362):1246–1249. doi: 10.1126/science.211583. [DOI] [PubMed] [Google Scholar]
- Major E. O., Amemiya K., Tornatore C. S., Houff S. A., Berger J. R. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992 Jan;5(1):49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl C. W., Frisque R. J. Characterization of cells transformed by the human polyomavirus JC virus. J Gen Virol. 1986 Aug;67(Pt 8):1733–1739. doi: 10.1099/0022-1317-67-8-1733. [DOI] [PubMed] [Google Scholar]
- Miller N. R., McKeever P. E., London W., Padgett B. L., Walker D. L., Wallen W. C. Brain tumors of owl monkeys inoculated with JC virus contain the JC virus genome. J Virol. 1984 Mar;49(3):848–856. doi: 10.1128/jvi.49.3.848-856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K., Yasui K., Kimura J., Washizu M., Yamaguchi K., Mori W. Induction of brain tumors by a newly isolated JC virus (Tokyo-1 strain). Am J Pathol. 1984 Sep;116(3):455–463. [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Eckroade R. J., Dessel B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971 Jun 19;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- RICHARDSON E. P., Jr Progressive multifocal leukoencephalopathy. N Engl J Med. 1961 Oct 26;265:815–823. doi: 10.1056/NEJM196110262651701. [DOI] [PubMed] [Google Scholar]
- Resnick J., Shenk T. Simian virus 40 agnoprotein facilitates normal nuclear location of the major capsid polypeptide and cell-to-cell spread of virus. J Virol. 1986 Dec;60(3):1098–1106. doi: 10.1128/jvi.60.3.1098-1106.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel U. Molekulare Entstehungsmechanismen menschlicher Gliome. Nervenarzt. 1993 Aug;64(8):485–493. [PubMed] [Google Scholar]
- Sima A. A., Finkelstein S. D., McLachlan D. R. Multiple malignant astrocytomas in a patient with spontaneous progressive multifocal leukoencephalopathy. Ann Neurol. 1983 Aug;14(2):183–188. doi: 10.1002/ana.410140205. [DOI] [PubMed] [Google Scholar]
- Small J. A., Scangos G. A., Cork L., Jay G., Khoury G. The early region of human papovavirus JC induces dysmyelination in transgenic mice. Cell. 1986 Jul 4;46(1):13–18. doi: 10.1016/0092-8674(86)90855-x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tada H., Lashgari M., Rappaport J., Khalili K. Cell type-specific expression of JC virus early promoter is determined by positive and negative regulation. J Virol. 1989 Jan;63(1):463–466. doi: 10.1128/jvi.63.1.463-466.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H., Rappaport J., Lashgari M., Amini S., Wong-Staal F., Khalili K. Trans-activation of the JC virus late promoter by the tat protein of type 1 human immunodeficiency virus in glial cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3479–3483. doi: 10.1073/pnas.87.9.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp B. D., Small J. A., Pulley M., Khoury G., Scangos G. A. Dysmyelination in transgenic mice containing JC virus early region. Ann Neurol. 1988 Jan;23(1):38–48. doi: 10.1002/ana.410230108. [DOI] [PubMed] [Google Scholar]
- Varakis J., ZuRhein G. M., Padgett B. L., Walker D. L. Induction of peripheral neuroblastomas in Syrian hamsters after injection as neonates with JC virus, a human polyoma virus. Cancer Res. 1978 Jun;38(6):1718–1722. [PubMed] [Google Scholar]
- Walker D. L., Padgett B. L., ZuRhein G. M., Albert A. E., Marsh R. F. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973 Aug 17;181(4100):674–676. doi: 10.1126/science.181.4100.674. [DOI] [PubMed] [Google Scholar]
- Wong A. J., Zoltick P. W., Moscatello D. K. The molecular biology and molecular genetics of astrocytic neoplasms. Semin Oncol. 1994 Apr;21(2):139–148. [PubMed] [Google Scholar]
- ZURHEIN G., CHOU S. M. PARTICLES RESEMBLING PAPOVA VIRUSES IN HUMAN CEREBRAL DEMYELINATING DISEASE. Science. 1965 Jun 11;148(3676):1477–1479. doi: 10.1126/science.148.3676.1477. [DOI] [PubMed] [Google Scholar]
- Zu Rhein G. M., Varakis J. N. Perinatal induction of medulloblastomas in Syrian golden hamsters by a human polyoma virus (JC). Natl Cancer Inst Monogr. 1979 May;(51):205–208. [PubMed] [Google Scholar]
- von Deimling A., Louis D. N., Wiestler O. D. Molecular pathways in the formation of gliomas. Glia. 1995 Nov;15(3):328–338. doi: 10.1002/glia.440150312. [DOI] [PubMed] [Google Scholar]