Abstract
Respiration is impaired by disruption of the central drive for inspiration to the diaphragm muscle (DIAm). Some function may recover involving nerve regeneration, reinnervation or neuroplasticity. A research animal model involves inducing hemiparesis of the DIAm and monitoring any recovery under different conditions. Methods to accurately track the level of functional recovery are needed. In this study, an algorithm was developed and tested to quantify the relative amount of electromyogram (EMG) activity that temporally correlated for an experimental (EXP) hemi-DIAm with its intact contralateral hemi-DIAm. An average rectified value (ARV) trace was calculated. A template was formed of the ARV trace of the intact hemi-DIAm, with higher positive values corresponding with periods of inspirations and lower negative values corresponding with quiet periods. This template was multiplied by the EXP ARV trace to reward (more positive) periods of correlating activity, and punish (more negative) periods of high activity on the EXP side that corresponded with quiet periods on the intact side. The average integrated value was the index of correlating contralateral activity (ICCA). A negative ICCA value indicated no net correlation of activity, and a positive value indicated a net correlation of activity. The algorithm was tested on rats having the conditions of control or hemi-paresis induced by denervatation (DNV), tetrodotoxin administration (TTX) or cervical spinal hemi-section (SH). Control had high positive ICCA values, and DNV had negative values. TTX maintained negative ICCA values at 3, 7 and 14 days, indicating a lack of functional recovery. SH maintained negative values at 3 and 7 days, but a subset had positive values at 14 days indicating some functional recovery.
I. Introduction
The diaphragm muscle (DIAm) is the main inspiratory muscle and is innervated by phrenic motoneurons located at the C3–C5 levels of the spinal cord. Disruption of the central drive for inspiration may result from trauma, surgery [1], cervical spinal cord injury [2] or disease (multiple sclerosis or anterior horn disease) [3]. Such disruption may lead to insufficient ventilation and the need for assisted ventilation to sustain life. In certain situations, recovery of the ability of the central drive for inspiration to induce DIAm contractions for inspiration may occur, involving nerve regeneration, reinnervation or neuroplasticity.
Research toward understanding these processes and development of potential treatments is ongoing in clinical settings [4] and in various animal models [5], including a model of permanent or transient hemi-paresis in rats [6], [7].
Electrical activity in a hemi-DIAm can be monitored by electromyography (EMG), and analyzed to determine whether the recovery of inspiratory function has begun. However, difficulties with interpreting the amplitude of an EMG signal include the influence of electrode placement, size and separation distance, and by the superposition of electrical activity from other tissues, such as cardiac muscle (electrocardiograms, ECG) or other skeletal muscles.
A method to quantify the relative level of temporal correlation of EMG activity between the two hemi-DIAm would aid interpretation. Inspiratory activity in the intact hemi-DIAm would reflect the central drive for inspiration. A typical EMG signal of an intact DIAm could be described as having a burst of EMG activity (indicating a contraction for inspiration) followed by a relatively quiet period (indicating exhalation and rest) prior to the next inspiratory burst of EMG activity. A typical EMG signal of a paralyzed hemi-DIAm could be described as having no bursts of EMG activity, but only a relatively constant level of quiet EMG signal (noise). A finding of no net correlation of activity between the contralateral hemi-DIAm would indicate continuation of hemi-paresis. Some net correlation of contralateral activity (CCA) would indicate the central drive of inspiration is resulting in simultaneous activity in both hemi-DIAm, and that recovery of function has progressed in the affected hemi-DIAm.
The purpose of this study was to develop an algorithm to determine an index of CCA (ICCA) for EMG signals simultaneously obtained from the two hemi-DIAm. The algorithm was applied to EMG recordings of rats undergoing several experimental conditions of control or hemi-paresis of DIAm function.
II. Methods
A. Animal Models
All experimental procedures with animals were approved by the Institutional Animal Care and Use Committee, and were in accordance with the American Physiological Society Animal Care Guidelines. Adult Sprague-Dawley rats were utilized for the study. For each procedure or evaluation, the rats were anesthetized via intramuscular injection of ketamine (90 mg/kg) and xylazine (10 mg/kg).
A condition of hemi-paresis was induced in some of the rats by an intervention that inhibited passage of the central drive for inspiration from passing through the right phrenic nerve by one of these methods: denervation (DNV), chronic administration of tetrodotoxin (TTX) or cervical spinal hemi-section (SH). The left side remained intact and unaltered in each case, and the central drive for inspiration passed unhindered, resulting in contractile activity in the left hemi-DIAm that sustained life. These different models of hemiparesis have been used to investigate their effects on DIAm physiology [6], [7].
For control (CTL) rats, the pathways of the central drive for inspiration to both hemi-DIAm were unaffected and retained full function for inspiration. For analysis, 56 CTL rats were utilized.
For DNV rats, the right phrenic nerve was exposed and sectioned, removing a 10 mm segment of the nerve [6]. For analysis, 11 DNV rats were utilized.
For TTX rats, a superfusion of TTX was chronically administered into a silastic cuff around the right phrenic nerve [6]. TTX blocks sodium ion channels, and thus action potentials. For analysis, 15 TTX rats were utilized after 3 days of TTX administration, 7 rats after 7 days, and 15 rats after 14 days.
For SH rats, after a cervical dorsal laminectomy, the right half of the spinal cord was sectioned at the C2 level [6]. Complete hemisection was verified by absence of inspiratory-related EMG activity in the right hemi-DIAm during eupnea in anesthetized rats immediately after the SH procedure and 3 days post-SH. For analysis, 13 SH rats were utilized 3 days following the hemisection, 6 rats after 7 days, and 23 rats after 14 days.
Following each procedure (DNV, TTX or SH), the rats were allowed to recover. Following a recovery period of 3, 7 or 14 days, each rat was again anesthetized for EMG recordings by electrodes pairs in both hemi-DIAm.
B. EMG Recordings
In each rat, electrode pairs were surgically implanted into the midcostal regions of both the right and left hemi-DIAm for EMG recordings [6], using insulated stainless steel wire (model AS631, Cooner Wire Inc., Chatsworth, CA). Insulation was stripped to expose a ~2 mm segment, implanted and secured in the DIAm, with ~3 mm distance separating the two electrode wires of each pair.
During each recording session, rats were anesthetized and each pair of externalized electrode wires was connected with instrumentation (Model 2124, DATA Inc.) for differential amplification and filtering (high pass filtered at 200 Hz). These conditioned signals were then digitally sampled at a frequency of between 0.5 and 2 kHz by a data acquisition board (National Instruments, Austin, TX) on a personal computer and stored as a computer file for later analysis. EMG activity during a period (1–2 minutes) of quiet, eupneic breathing was recorded and later analyzed.
C. Algorithm
The algorithm processed each recorded EMG trace of contralateral intact and experimental (EXP) hemi-DIAm. The intact hemi-DIAm was the unaffected side that retained functional inspiration. The EXP hemi-DIAm was the side to be tested, being either a case of CTL, DNV, TTX or SH. Both contralateral EMG tracings were recorded simultaneously.
A DC bias offset value (o) was calculated as follows.
| (1) |
where Ei is the ith EMG sample and Ei+1 would be the next EMG sample; and n is the number of samples of Ei. This value of o was calculated separately for the contralateral traces.
To minimize the effect of the absolute amplitude of the EMG signals that may be influenced by many factors, each contralateral trace was normalized by the standard deviation of a period of noise. This period of noise was selected by a user who manually viewed the EMG trace of the intact hemi-DIAm and used timing cursors to select a segment of the trace during a relatively quiet period between inspiratory bursts of EMG activity that would indicate an inspiration and between any visible ECG spikes. For all the DC offset corrected values (Ei – o) within this identified period, the standard deviation (σ) was calculated. For the same segment of time, σ was calculated separately for the contralateral traces.
A running window average was used to calculate the average rectified value (Wi) as follows.
| (2) |
where m is the number of samples in the window, specifically m = (sampling_frequency * window_duration). A window duration of 100 ms was used for this study.
Since EMG recordings of hemi-DIAm may include ECG signals that appear as spikes, an algorithm was applied to minimize any ECG spikes in the intact and experimental hemi-DIAm Wi traces.
A larger amplitude of noise in a recorded trace would be reflected in a larger minimum Wi value. To minimize the effect of this amplitude of noise on Wi, the Wi values were shifted down as follows.
| (3) |
where Si is the series of Wi values that were shifted down; and w5 is the 5th percentile value of all Wi values (typically a value close to the minimum Wi value). This value of Si was calculated separately for the contralateral traces.
A template trace (Ti) of the activity on the intact DIAm was formed based on the Si trace. First, the intact hemi-DIAm Si trace was normalized as follows.
| (4) |
where s95 is the 95th percentile value of all Si values (typically a value close to the maximum Si value); and α is constant having a value of 2 in this implementation. Next, the normalized trace Ui was shifted down such at values less than approximately half of the amplitude would be negative and the values above would be positive.
| (5) |
This formation of Ti split the signal into two parts: a positive part that would include the EMG bursts related to inspirations, and a negative part that would include the periods of relative quiet between the inspiratory bursts. A negative value with a relatively larger magnitude would indicate a quieter period of the EMG signal. A positive value with a relatively larger magnitude would indicate a more active period, such as during an inspiration.
This resulting template Ti was multiplied by the EXP hemi-DIAm trace toward determining the activity on the EXP side that correlated with activity on the intact side (Yi).
| (6) |
where Si (eq. 3) is based on the EXP side. A period of high activity on the EXP Si that correlated with high contralateral activity would result in a positive Yi value (rewarded), but a period of high activity on the EXP Si that occurred during a quiet period on the contralateral side would result in a negative Yi value (punished).
The index of Correlating Contralateral Activity (ICCA) was calculated by finding the average discrete integrated value of Yi, as follows.
| (7) |
where d is the duration of the recording in seconds, which is the quotient of the number of samples of Yi and the frequency of sampling. A negative value of ICCA would indicate that net activity in the EXP hemi-DIAm was not correlated with activity in the intact hemi-DIAm, and thus would suggest that the central drive for inspiration was not having a strong influence on the activity of the EXP hemi-DIAm. A positive value of ICCA would indicate that net activity on the EXP hemi-DIAm did correlate with activity on the intact hemi-DIAm, and thus would suggest that the central drive for inspiration was having a significant influence.
D. Implementation
The algorithm was implemented in LabView (National Instruments, Austin, TX) on a personal computer.
III. Results
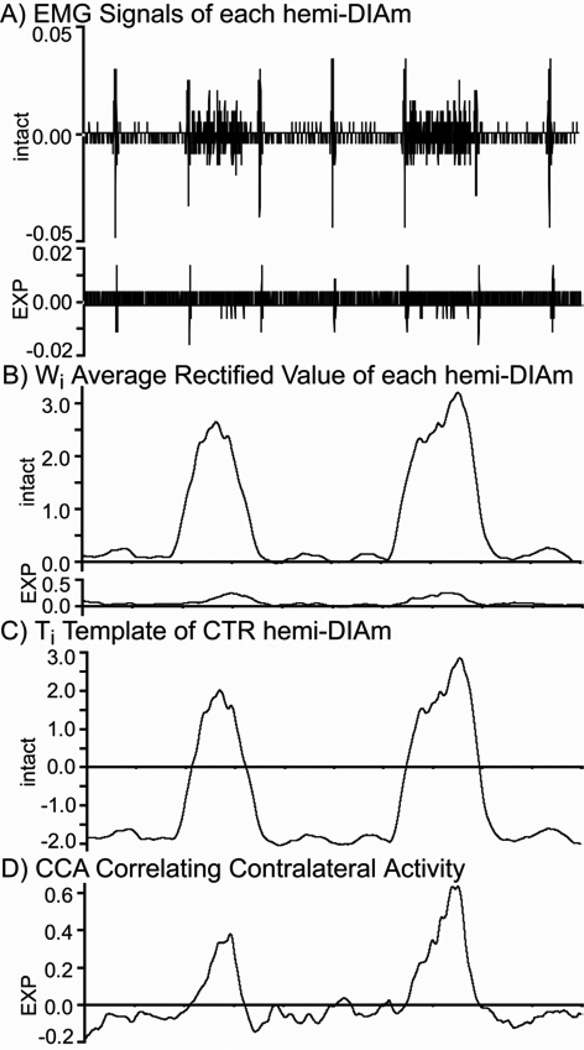
Fig. 1 shows an example of the algorithm being applied to an EMG recording for a case where a small amount inspiratory activity had returned after a 2-week period of recovery following SH. The resulting signal traces following key steps of the algorithm are shown (Fig. 1).
Fig. 1.
Example of the Algorithm being applied to a recording from the intact and EXP hemi-DIAm of a rat 2 weeks following SH, where a small amount of inspiratory activity had returned. A) shows a ~2 s period in time of the original EMG traces. B) shows the average rectified values, Wi. C) shows the template Ti based on the intact hemi-DIAm signal. D) shows the product of Ti by the Si of the EXP DIAm. The ICCA determined by the algorithm for this case was 0.014, which indicated a net correlation of contralateral activity since the value was positive.
Fig. 2 shows the values of ICCA for cases when the EXP hemi-DIAm were CTL, DNV, TTX or SH. CTL had high positive ICCA values, indicating a high correlation of EMG activity between the contralateral hemi-DIAm. Such a high correlation was expected since the pathways for both hemi-DIAm were intact. DNV had negative ICCA values (Fig. 2), indicating a lack of correlation between activity in the contralateral hemi-DIAm. Such a lack of correlation was expected since action potentials from the phrenic nerve could not reach the EXP hemi-DIAm. Likewise, TTX resulted in negative ICCA values, which were maintained through the three time points that were measured up to 14 days. This indicated that the administration of TTX was sufficient to prevent any recovery of function. The SH resulted in negative ICCA values for the initial time periods of 3 and 7 days, but by 14 days many of the ICCA values were positive, indicating that some inspiratory function was beginning to occur in a subset of the SH rats.
Fig. 2.
Index of Correlating Contralateral Activity (ICCA) for hemi-DIAm having different EXP conditions. A positive ICCA value indicated a net correlation of activity, and a negative value indicated a lack of net correlation.
IV. DISCUSSION
The algorithm and calculated ICCA values appear to have promise as a way to quantify the amount of EMG activity in one hemi-DIAm that correlates with activity in the intact contralateral hemi-DIAm.
A weakness of this algorithm is that any artifact of electrical activity that appears simultaneously on both contralateral EMG traces would contribute to a more positive ICCA value, even activity unrelated to inspirations. The ECG spikes that typically appeared on both contralateral traces are one example of a common artifact, but other sources of electrical activity unrelated to inspirations may also significantly affect the EMG signal and degrade the ability of the ICCA value to selectively differentiate correlating inspiratory activity. In the tested recordings of DIAm of anesthetized rats in this study, such common artifacts were not apparent except for the mentioned ECG spikes. An algorithm to minimize ECG spikes was utilized, but such algorithms may unintentionally affect the underlying DIAm EMG signal while attempting to remove the superimposed ECG signal.
More development and testing will be required to determine whether this algorithm would be useful in quantifying the timing and possibly also the level of recovery of inspiratory function. Such an assessment would aid research for the development of treatments to enhance the neuroplasticity for restoration of respiratory function.
Acknowledgments
The study was supported by funding from the National Institutes of Health (AR51173 and HL37680 to GCS), and the Mayo Foundation.
Contributor Information
Douglas E. Dow, Department of Electronics & Mechanical, Wentworth Institute of Technology, 550 Huntington Ave., Boston, MA 02115 USA.
Wen-Zhi Zhan, Department of Physiology & Biomedical Engineering, Mayo Clinic College of Medicine, Rochester, MN 55905 USA.
Gary C. Sieck, Department of Physiology & Biomedical Engineering, Mayo Clinic College of Medicine, Rochester, MN 55905 USA
Carlos B. Mantilla, Department of Physiology & Biomedical Engineering, Mayo Clinic College of Medicine, Rochester, MN 55905 USA
References
- 1.Tripp HF, Bolton JWR. Phrenic nerve injury following cardiac surgery: a review. Journal of Cardiac Surgery. 1998;vol. 13:218–223. doi: 10.1111/j.1540-8191.1998.tb01265.x. [DOI] [PubMed] [Google Scholar]
- 2.DiMarco AF, Onders RP, Ignagni A, Kowalski KE, Mortimer JT. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest. 2005;vol. 127:671–678. doi: 10.1378/chest.127.2.671. [DOI] [PubMed] [Google Scholar]
- 3.Grasso MG, Lubich S, Guidi L, Rinnenburger D, Paolucci S. Cerebellar deficit and respiratory impairment: a strong association in multiple sclerosis? Acta Neurologica Scandinavica. 2000;vol. 101:98–103. doi: 10.1034/j.1600-0404.2000.101002098.x. [DOI] [PubMed] [Google Scholar]
- 4.Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: an emerging paradigm shift in rehabilitation and walking recovery. Phys Ther. 2006;vol. 86(10):1406–1425. doi: 10.2522/ptj.20050212. [DOI] [PubMed] [Google Scholar]
- 5.Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;vol. 27(3):277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- 6.Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol. 1995;vol. 79(5):1640–1649. doi: 10.1152/jappl.1995.79.5.1640. [DOI] [PubMed] [Google Scholar]
- 7.Mantilla CB, Zhan WZ, Sieck GC. Neurotrophins improve neuromuscular transmission in the adult rat diaphragm. Muscle Nerve. 2004;vol. 29:381–386. doi: 10.1002/mus.10558. [DOI] [PubMed] [Google Scholar]