Abstract
Background
Studies suggest that vitamin D deficiency is a risk factor for cardiovascular disease and diabetes. Vitamin D deficiency is prevalent in HIV patients but the effect of vitamin D supplementation on cardiovascular risk in this population is unknown.
Methods
We conducted a randomized, double-blind, placebo-controlled trial among 45 HIV-infected adults in Cleveland (OH, USA) on stable antiretroviral therapy with durable virological suppression and a baseline serum 25-hydroxyvitamin D level of ≤20 ng/ml. Participants were randomized 2:1 to vitamin D3 4,000 IU daily or placebo for 12 weeks. The primary outcome was a change in flow-mediated brachial artery dilation (FMD).
Results
Baseline demographics were similar except for age (vitamin D versus placebo, mean ±SD 47 ±8 versus 40 ±10 years; P=0.009). Both groups had reduced FMD at baseline (median values 2.9% [IQR 1.6–4.8] for vitamin D versus 2.5% [IQR 1.7–6.4] for placebo; P=0.819). Despite an increase in the concentration of serum 25-hydroxyvitamin D from baseline to 12 weeks (5.0 ng/ml [IQR −0.9–7.4] versus −1.9 ng/ml [IQR −4.0–0.1] for vitamin D versus placebo, respectively; P=0.003), there was no difference in FMD change (0.55% [IQR −1.05–2.13] versus 0.29% [IQR −1.61–1.77]; P=0.748). Vitamin D supplementation was associated with a decrease in total and non-high-density lipoprotein cholesterol, and an increase in indices of insulin resistance.
Conclusions
Among HIV-infected individuals with vitamin D deficiency, supplementation with 4,000 IU vitamin D3 daily for 12 weeks modestly improved vitamin D status and cholesterol but worsened insulin resistance without change in endothelial function. The mechanisms of resistance to standard doses of vitamin D and the complex role of vitamin D in glucose metabolism in this population require further investigation.
Introduction
The role of vitamin D in maintaining calcium homeo-stasis and bone health is well-described [1]; however, mounting evidence suggests that this hormone is also important to other body systems including the heart and vasculature [2]. Large observational studies have linked vitamin D deficiency to hypertension [3], incident cardiovascular disease [4], myocardial infarction [5], cardiovascular death [6] and total mortality [7]. Despite a recent Institute of Medicine report [8] suggesting that most North Americans receive an adequate amount of vitamin D, considerable controversy persists regarding target serum 25-hydroxyvitamin D (25[OH]D) concentrations and the benefits of vitamin D supplementation. Generally, serum 25(OH)D concentrations of 30 ng/ml or more are required to maximally suppress parathyroid hormone (PTH). Some commentators have therefore proposed that concentrations <20 ng/ml are ‘deficient’ and 20–30 ng/ml are ‘insufficient’, although these definitions are not universally accepted.
Smaller studies have suggested that vitamin D supplementation might improve endothelium dependent vaso-dilation, a strong predictor of cardiovascular events [9], among patients with diabetes [10] and otherwise healthy vitamin D deficient adults [11], although a recent trial failed to confirm this finding in patients with diabetes [12]. The effect of vitamin D on vascular function could be mediated by inflammation from increased endothelial expression of nuclear factor-κB, higher concentrations of its downstream product interleukin (IL)-6 [13], and tumour necrosis factor (TNF)-induced vascular cell adhesion molecule (VCAM) and intercellular adhesion molecule (ICAM) [14].
Vitamin D deficiency is common among HIV-infected persons throughout the world, with prevalence estimates ranging widely from 29% to 87% [15]. It has been associated with faster disease progression and higher mortality [16] and greater prevalence of diabetes [17]. In two recent studies of HIV-infected subjects, low vitamin D concentrations were associated with increased carotid intima-media thickness (cIMT), a measure of subclinical atherosclerosis [18,19]. One randomized trial has demonstrated the safety and effectiveness of oral cholecalciferol (vitamin D3) in HIV-infected children [20]; how-ever, no randomized placebo-controlled trials have been conducted in adults.
The Endocrine Society has recently published guide-lines that recommend screening for vitamin D deficiency in all HIV-infected patients on antiretroviral therapy [21]. More specifically, the antiretroviral drug efavirenz (EFV) has been associated with vitamin D deficiency in recent cross-sectional studies [22,23]. Initiation of EFV lowered 25(OH)D concentrations in two prospective studies [24,25], and switching from EFV to a protease inhibitor based regimen raised 25(OH)D concentrations in the MONET trial [26]. The effect of EFV on active vitamin D supplementation is, however, unknown.
The primary objective of our study was to evaluate the effect of oral vitamin D supplementation on flow-mediated brachial artery dilation (FMD), a marker of endothelial function, among vitamin D deficient HIV-infected adults. Secondary objectives were to examine the effect of vitamin D on serum 25(OH)D concentrations, PTH, fasting lipids, and serum markers of inflammation, coagulation and insulin resistance, and to examine the effect of EFV on vitamin D supplementation.
Methods
Study design
We conducted a prospective, randomized, double-blind, placebo-controlled trial of vitamin D supplementation in 45 HIV-infected adults on stable antiretroviral therapy (ART) with durable virological suppression and a baseline 25(OH)D level ≤20 ng/ml. The study duration was 12 weeks. The primary outcome was absolute change in FMD.
The study was approved by the Institutional Review Board of the University Hospitals Case Medical Center (Cleveland, OH, USA) and written informed consent was obtained from all participants prior to enrolment.
Study population
HIV-infected adults (≥18 years of age) were recruited from a single centre in Cleveland from February to July 2010. Participants were eligible if they had a baseline serum 25(OH)D concentration of ≤20 ng/ml, HIV-1 RNA<50 copies/ml on stable ART for at least 12 weeks prior to study entry, and a willingness to refrain from additional vitamin D supplementation during the study. Exclusion criteria included current pregnancy or breast-feeding; diabetes (two fasting glucose levels >126 mg/dl or two random glucose levels >200 mg/dl); estimated glomerular filtration rate <50 ml/min/1.73 m2; any active infectious or inflammatory condition; regular use of salsalate, aspirin, non-steroidal anti-inflammatory drugs, corticosteroids, bisphosphonates or other osteoporosis treatments; daily alcohol or illicit drug use; and alanine aminotransferase or aspartate aminotransferase >2.5 × upper limit of normal within 6 months of study entry.
Treatment
Participants were randomized in a 2:1 fashion to 4,000 IU of cholecalciferol (vitamin D3; 2 × 2,000 mg capsules) or two matching placebo capsules (both from Tishcon Corporation, Westbury, NY, USA) daily for 12 weeks. All vitamin D and placebo capsules contained maltodextrin, magnesium stearate and gelatin. Randomization tables were generated by the statistician and provided to the investigational pharmacist who distributed the blinded pills on the day of enrolment and each subsequent visit. Group assignments were unblinded after all subjects completed the study. Compliance was assessed by pill count, and percent-age adherence was calculated in the following manner: ([pills dispensed - pills returned]/expected number taken) × 100. After study completion, 10 sample cholecalciferol capsules were analysed for vitamin D content (Analytical Research Laboratories, Oklahoma City, OK, USA), and all were verified to contain >2,000 IU of vitamin D3. The mean content was 136% of the stated dose.
Study evaluations
At the initial visit, relevant demographics, past medical history, medication and substance use were obtained from each participant and a targeted physical exam was conducted. Participants had blood drawn after a 12-h fast at entry and week 12. Real-time measures of glucose, insulin and lipoproteins were performed at these two time points. In addition, plasma and serum from each participant were stored at −70°C until the completion of the study. Serum samples from baseline and week 12 were sent in a blinded fashion to Emory University (Atlanta, GA, USA) for analysis of serum 25(OH)D and PTH concentrations. Total serum 25(OH)D was determined by ELISA (Immunodiagnostic Systems, Fountain Hills, AZ, USA). External quality control was ensured by participation in the external quality assessment scheme (DEQAS) and in the NIST/NIH Vitamin D Metabolites QA Program. Serum PTH was determined by ELISA (Immutopics Intl, San Clemente, CA, USA).
Stored plasma and serum samples were also tested for soluble VCAM-1 (sVCAM-1), soluble ICAM-1 (sICAM-1), high-sensitivity C-reactive protein (hs-CRP), IL-6, soluble TNF-α receptors (sTNFR-1 and sTNFR-II), d-dimer and fibrinogen. sVCAM-1, sICAM-1, IL-6, and sTNFR-I and -II were determined by quantitative sandwich ELISAs (R & D Systems, Minneapolis, MN, USA). Inter-assay variability was in the ranges 4.76–8.77%, 3.43–7.37%, 2.02–15.36%, 3.66–5.77% and 2.13–3.79%, respectively. hs-CRP and fibrinogen were determined by particle enhanced immunonepholometric assays on a BNII nephelometer (Siemens Healthcare Diagnostics, Deerfield, IL, USA). Inter-assay variability was in the range 3.01–6.46% and 3.42–7.59%, respectively. d-dimer was determined by immunoturbidometric assay on a STA-R Coagulation Analyzer (Diagnostica Stago, Parsippany, NJ, USA). Inter-assay variability ranged from 1.54% to 9.03%. All inflammation and coagulation markers were performed at the Laboratory for Clinical Biochemistry Research under the direction of Dr Russell Tracy (Department of Pathology, University of Vermont, Burlington, VT, USA). Insulin resistance was determined using the homeostasis model assessment of insulin resistance (HOMA-IR). HOMA-IR was calculated using the following formula: HOMA-IR = fasting glucose (mg/dl) × fasting insulin (µU/ml)/405) [27]. CD4+ cell counts and HIV-1 RNA concentrations were also measured as markers of HIV disease activity. A complete blood count, blood urea nitrogen (BUN), creatinine, electrolytes, calcium and a hepatic function panel were obtained at study entry and at 2, 4, 8 and 12 weeks to monitor for drug toxicity.
Endothelial function
FMD was used to evaluate endothelial function in each participant at baseline and at the 12-week follow-up visit. All brachial reactivity studies were performed by a single sonographer trained in a previously described protocol [28], using an L10-7 MHz linear array transducer and a 5 min occlusion time. Participants were fasting and had refrained from smoking and caffeine for at least 8 h prior to the exam. Semi-automated edge detection software (Medical Imaging Applications, LLC, Coralville, I A, USA]) was used to measure pre-occlusion brachial artery diameter and 60 s after release of the occlusive blood pressure cuff. The percentage of FMD was calculated as follows: (post-occlusion diameter - baseline diameter)/ baseline diameter × 100. Baseline and follow-up studies were analysed concurrently by a single experienced reader (TLC) who was blinded to study assignment.
Statistical methods
Baseline characteristics and laboratory values are described by mean and standard deviation for continuous variables following a normal distribution, by median and interquartile range (IQR) for continuous variables not following a normal distribution and by frequency and percentage for categorical variables.
The primary outcome was absolute change in FMD from baseline to follow-up. Secondary outcomes were absolute change in 25(OH)D, PTH, metabolic parameters, and serum markers of inflammation and coagulation. Within the active treatment arm, absolute changes in 25(OH)D among EFV users and non-users were compared. Because outcomes involved changes from baseline, only those participants with baseline and follow-up studies were used for the analysis. Unpaired student’s t-tests and Wilcoxon signed-rank tests were used for within-group comparisons as distributionally appropriate. Paired student’s t-tests and Wilcoxon rank-sum tests were used for between-group comparisons as distributionally appropriate. Published guide-lines suggest that a clinically relevant absolute change in FMD is 3% [29]. Assuming a standard deviation of 3%, our study had 88.5% power to detect a between-group absolute change of 3% in FMD using a two-tailed alpha of 0.05.
Post hoc, several exploratory analyses were performed. First, to understand better the relationship between change in serum 25(OH)D concentrations and change in FMD in the active treatment group, a Pearson correlation coefficient was determined. Second, to explore change in serum 25(OH)D concentrations among EFV users in the active treatment group, univariable followed by multivariable linear regression was performed. Variables of interest and those with P<0.25 were included in the multivariable model. In order to meet the assumption of normality, change in serum 25(OH)D was transformed on the natural logarithm scale. Third, to further explore the effect of vitamin D supplementation on insulin resistance, we compared the number of participants in each group who had an HOMA-IR greater than 3.0 at baseline and at follow-up. This level of HOMA-IR is generally considered clinically relevant [30], although a range of thresholds have been used in HIV.
All statistical tests were two-sided with a 0.05 significance level. Analyses were performed using SAS, version 9.2 (SAS Institute, Cary, NC, USA).
Results
Of the 71 patients who were screened, 19 were excluded because of serum 25(OH)D concentrations >20 ng/ml, one was excluded because of a random glucose level >200 mg/dl, and 6 declined consent or were lost to follow-up before study enrolment. In total, 45 patients enrolled in the study, and only one participant in the vitamin D group was lost to follow-up after randomization.
Baseline characteristics of the study participants are shown in Table 1. Participants in the vitamin D group were on average 7 years older than the placebo group. Three-quarters of all participants were men and three quarters were African-American. Both groups had similar HIV and metabolic parameters at baseline. Median duration of HAART among all participants was 7.1 years. More participants in the placebo group were taking non-nucleoside reverse transcriptase inhibitors (NNRTIs). Of those on NNRTIs, most were taking EFV (9 of 11 in the placebo group and 10 of 11 in the vitamin D group). Median adherence to study medication by pill count was high at 99% in both groups. Oral vitamin D supplementation was well-tolerated, with no grade 2 or higher adverse events or laboratory abnormalities reported. Specifically, no cases of kidney stones or hypercalcaemia were reported.
Table 1.
Baseline characteristics of the study participants
| Characteristic | Vitamin D (n=30) | Placebo (n=15) | P-value |
|---|---|---|---|
| Demographics | |||
| Age, years | 47 ±8a | 40 ±10a | 0.009 |
| Sex | 0.263 | ||
| Male, n (%) | 25 (83) | 10 (67) | |
| Female, n (%) | 5(17) | 5(33) | |
| Race | 0.814 | ||
| White, n(%) | 4(13) | 3(20) | |
| Black, n (%) | 23 (77) | 12 (80) | |
| Latino, n(%) | 2(7) | 0(0) | |
| Other, n (%) | 1(3) | 0(0) | |
| HIV parameters | |||
| Baseline CD4+ T-cell count, cells/µl | 564 (383–761)b | 608 (470–951)b | 0.271 |
| Nadir CD4+T-count, cells/µl | 127 (38–231)b | 173 (30–231)b | 0.688 |
| Baseline viral load, copies/ml | 48 (48-48)b | 48(48–111)b | 0.172 |
| Undetectable (<48 copies/ml) viral load, n (%) | 25 (83) | 10 (67) | 0.263 |
| HIV duration, years | 11 ±7a | 9 ±4a | 0.445 |
| ART duration, years | 7(4–11)b | 7 (4–8)b | 0.402 |
| Metabolic parameters | |||
| BMI, kg/m2 | 28 (25–32)b | 27 (23–31)b | 0.354 |
| Total cholesterol, mg/dl | 192 ±42a | 194 ±53a | 0.922 |
| HDL, mg/dl | 40 (36–51)b | 48 (39–56)b | 0.126 |
| Non-HDL, mg/dl | 149 ±39a | 144 ±59a | 0.733 |
| Triglycerides, mg/dl | 155 (95–243)b | 99 (58–202)b | 0.233 |
| HOMA-IR | 2.1 (1.0–3.6)b | 1.9 (1.0–2.9)b | 0.696 |
| H0MA-IR>3.0, n(%) | 10 (33) | 3(20) | 0.492 |
| Additional cardiovascular risk factors | |||
| Systolic blood pressure, mmHg | 118±14a | 119 ±12a | 0.888 |
| Diastolic blood pressure, mmHg | 79 ±9a | 81 ±9a | 0.662 |
| Smoking | 0.780 | ||
| Current, n (%) | 13 (43) | 7(47) | |
| Past, n (%) | 7(23) | 2(13) | |
| Never, n (%) | 10 (33) | 6(40) | |
| Medication use | |||
| Protease inhibitor, n (%) | 13 (43) | 5(33) | 0.519 |
| NNRTI, n(%) | 11 (37) | 11 (73) | 0.020 |
| Lipid-lowering medication, n (%) | 8(27) | 2(13) | 0.456 |
| Anti-hypertensive, n (%) | 11 (37) | 5(33) | 0.826 |
Values in bold indicate statistical significance (P<0.05).
Normally distributed data presented as mean ±sd.
Non-normally distributed data presented as median (IQR); ART, antiretroviral therapy; BMI, body mass index; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; NNRTI, non-nucleoside reverse transcriptase inhibitor.
There was no difference in the primary outcome of change in FMD (Table 2). Participants in both treatment groups had similar endothelial function at baseline and demonstrated only small non-statistically significant improvements over the course of the 12-week study. Even those with the largest increase in 25(OH)D (≥7 ng/ml; n=10) did not have a statistically significant change in FMD (P=0.125). Both groups had similar brachial artery diameters at baseline and follow-up. Furthermore, change in serum 25(OH)D and change in FMD were not correlated in the treatment group (Pearson correlation coefficient =−0.24; P=0.215).
Table 2.
Flow-madiated brachial artery dilation
| Vitamin D (n=30) | Placebo (n= 15) | P-value | |
|---|---|---|---|
| Median baseline brachial artery diameter, mm (IQR) | 4.74 (4.30–5.00) | 4.50 (3.83–5.00) | 0.493 |
| Median baseline FMD, % (IQR) | 2.87 (1.55–4.78) | 2.46(1.68–6.43) | 0.819 |
| Median 12-week follow-up brachial artery diameter, mm (IQR) | 4.75(4.21–5.07) | 4.61 (3.90–4.99) | 0.199 |
| Median 12 week follow-up FMD, % (IQR) | 3.87 (2.18–4.71) | 2.75(1.82–6.59) | 0.766 |
| Median absolute change in FMD, % (IQR) | 0.55 (−1.05–2.13) | 0.29 (−1.61–1.77) | 0.748 |
| Mean absolute change in FMD, % ±sd | 0.53 ±2.99 | 0.56 ±3.65 | NAa |
Mean difference was not tested for statistical significance given the non-normal distribution of the measurement.
FMD, flow-madiated brachial artery dilation. NA, not applicable.
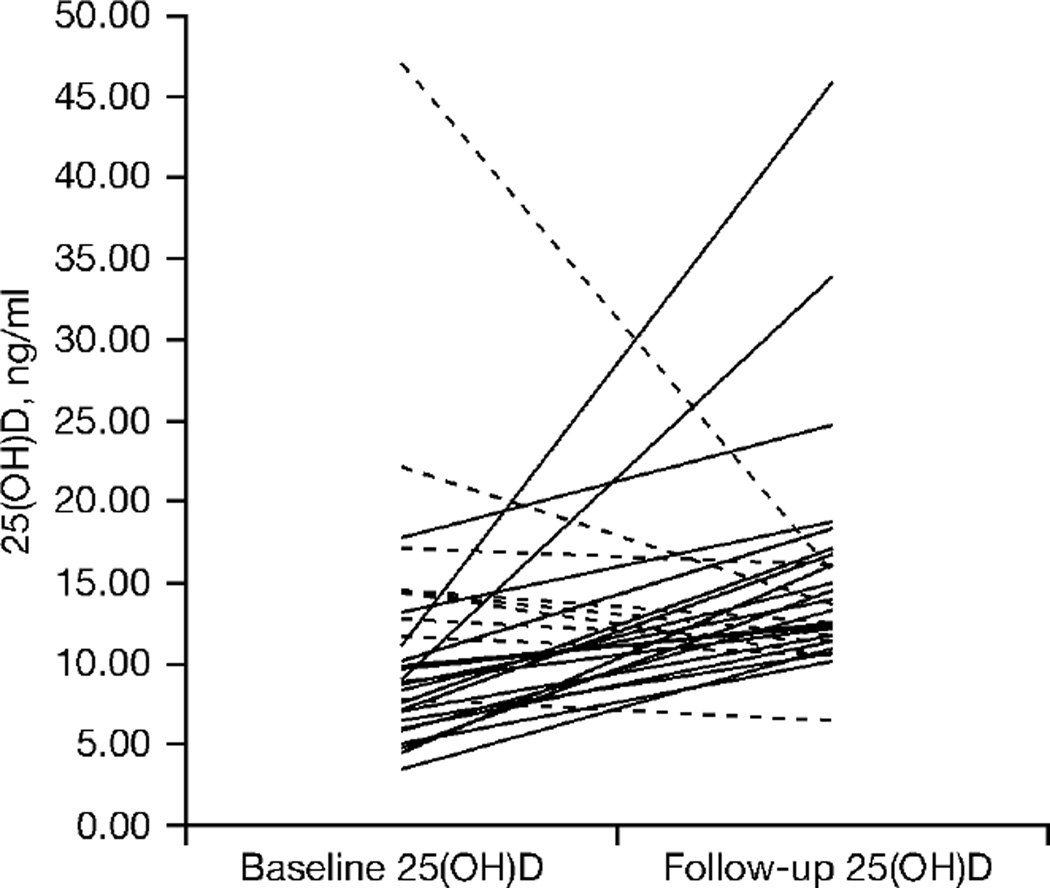
There was a statistically significant increase in serum 25(OH)D concentrations from baseline to 12 weeks in those taking vitamin D compared with those taking placebo (Table 3). Decreases in PTH were observed among participants with only slight changes in serum 25(OH)D as well as in those with a more robust response (≤5 versus ≥5 ng/ml change). The intra-individual changes are shown in Figure 1. We further compared participants in the active treatment arm who were on EFV (n=9) with those who were not on EFV (n=20). EFV use did not significantly alter the change in serum 25(OH)D concentrations (4.6 [IQR −0.9–7.0] ng/ml for EFV versus 5.15 [IQR −1.1–8.5] ng/ml for non-EFV; P=0.54). Additionally, in an exploratory multivariable analysis looking at predictors of change in serum 25(OH)D in the active treatment group, use of EFV was not independently associated. Potential confounders considered were age, race, gender, body mass index, baseline CD4+ T-cell count and month of enrolment.
Table 3.
Change in 25(OH)D, PTH, blood pressure, lipids and measures of glucose metabolism
| Vitamin D | Placebo | Between group | |||||
|---|---|---|---|---|---|---|---|
| Baseline (n=30)a | Change (n=29)a | P-valueb | Baseline (n=15)a | Change (n=15)a | P-valueb | P-valuec | |
| 25(OH)D, ng/ml | 9.0(7.1–13.1) | 5.0 (−0.9–7.4) | 0.002 | 6.2 (3.7–9.8) | −1.8 (−2.9–0.1) | 0.097 | 0.018 |
| PTH in responders, pg/ml (n=15)d | 74 (47–89) | −15 (−33–1.0) | 0.016 | 62 (44–93) | −4.0 (−21.0–4.0) | 0.165 | 0.216 |
| PTH in non-responders, pg/ml (n=14)d | 68(55–91) | −22 (−33–3.0) | 0.016 | ||||
| Systolic blood pressure, mmHg | 118(106–125) | 1.5 (−9.5–10.5) | 0.845 | 120(108–128) | 0 (−17–12) | 0.636 | 0.620 |
| Diastolic blood pressure, mmHg | 80 (72–83) | −3.0 (−4.0–4.0) | 0.498 | 80 (72–88) | −6.0 (−10.0–6.0) | 0.217 | 0.377 |
| Total cholesterol, mg/dl | 191 (157–224) | −9. 0 (−25.0–4.0) | 0.009 | 189(155–232) | −1.0 (−18–12) | 0.528 | 0.281 |
| HDL cholesterol, mg/dl | 41 (36–51) | −1.0 (−5.0–3.0) | 0.249 | 48 (39–56) | −1.0 (−7.0–3.0) | 0.237 | 0.901 |
| Non-HDL cholesterol, mg/dl | 154(115–183) | −7.0 (−21.0–8.0) | 0.022 | 146 (94–195) | −2.0 (−11–11) | 0.968 | 0.249 |
| Triglycerides, mg/dl | 156(95–243) | 20 (−19–57) | 0.051 | 99 (58–202) | 7.0 (−31–35) | 0.833 | 0.169 |
| HOMA-IR | 2.1 (1.0–3.6) | 0.8(0.3–1.9) | <0.0001 | 1.9(1.0–2.9) | 0.3 (−0.2–0.7) | 0.217 | 0.130 |
| Insulin, µlU/ml | 10(5–16) | 4 (0–6.0) | 0.0007 | 8.5(5–15) | 1.5 (−2.0–5.0) | 0.457 | 0.227 |
| Glucose, mg/dl | 84 (77–90) | 0 (−4.0–9.0) | 0.355 | 78 (69–92) | −2.0 (−13.0–7.0) | 0.572 | 0.310 |
Values in bold indicate statistical significance (P<0.05).
Date are presented as median (IQR).
P-value for within-group change.
P-value comparing D change versus placebo change; for parathyroid hormone (PTH), all vitamin D (n=29) is compared with placebo.
Response was defined as a rise in 25-hydroxyvitamin D (25[OH]D) level ≥5 ng/ml from baseline to follow-up among participants taking vitamin D. HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance.
Figure 1.
Intra-individual change in serum 25(OH)D concentrations among participants randomized to vitamin D supplementation
Dashed lines represent participants with a decline in serum 25-hydroxyvitamin D (25[OH]D) concentration and black lines represent participants with a rise in serum 25(OH)D concentration.
There were no significant differences between vitamin D and placebo groups for changes in the metabolic parameters shown in Table 3. Among those taking vitamin D, there were no changes in systolic and diastolic blood pressure from baseline to week 12. There were, however, modest but statistically significant improvements in total cholesterol and non-high-density lipoprotein (HDL) cholesterol, without a change in HDL or triglycerides. Insulin resistance as measured by HOMATR increased significantly. In addition, six participants in the vitamin D arm developed clinically relevant insulin resistance during the course of treatment, resulting in a significantly higher percentage of participants with HOMA-IR>3.0 in the vitamin D group at follow-up compared with placebo (53% versus 20%, vitamin D versus placebo; P=0.033).
Finally, the effects of vitamin D supplementation on serum markers of inflammation and coagulation are shown in Table 4. There were no significant differences between groups at baseline. There was only a slight increase in IL-6 from baseline to week 12 in the placebo group compared with the vitamin D group.
Table 4.
Serum markers of inflammation and coagulation
| Baseline | Change | |||||
|---|---|---|---|---|---|---|
| Vitamin D (n=30)a | Placebo (n=15)a | P-valueb | Vitamin D (n=29)a | Placebo (n=15)a | P-valueb | |
| slCAM-1, ng/ml | 164(101–215) | 179 (94–226) | 0.895 | −4 (−18–11) | 0(−5–21) | 0.225 |
| sVCAM-1, ng/ml | 690 (569–835) | 687 (524–793) | 0.716 | −32 (−82--13) | −36 (−61–68) | 0.298 |
| sTNFR-I, pg/ml | 1,324(1,112–1,736) | 1,188(999–1,583) | 0.224 | 32 (−80–175) | 23 (−144–212) | 0.961 |
| sTNFR-II, pg/ml | 2,667 (2,330–3,077) | 2,801 (1,783–3,673) | 0.448 | 18 (−123–270) | −1 (−187–566) | 0.804 |
| CRP, mg/dl | 1.4(0.78–3.32) | 1.25(0.99–3.10) | 0.647 | 0.21 (−0.2–0.98) | −0.03 (−1.85–0.77) | 0.287 |
| D-dimer, µg/dl | 0.14(0.09–0.31) | 0.14(0.12–0.33) | 0.453 | 0 (−0.05–0.10) | 0.02 (−0.16–0.19) | 0.853 |
| Fibrinogen, mg/dl | 356 (306–421) | 351 (305–392) | 0.376 | −21 (−28–24) | −7 (−37–87) | 0.603 |
| IL-6, pg/ml | 2.4(1.4–5.7) | 2.1 (1.0–3.2) | 0.138 | −0.1 (−2.2–0.7) | 0.5 (0.2–3.1) | 0.010 |
Values in bold indicate statistical significance (P<0.05).
Date presented as median (IQR).
P-Values for between-group difference. CRP, C-reactive protein; IL-6, interleukin-6; slCAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1; sTNFR-I and sTNFR-II, soluble tumour necrosis factor-α receptors I and II.
Discussion
To our knowledge, this is the first placebo-controlled randomized trial of vitamin D supplementation in HIV-infected adults. We found that 4,000 IU of oral vitamin D3 supplementation daily for 12 weeks did not affect the endothelial function of vitamin D deficient HIV-infected adults. However, both baseline FMD and serum 25(OH)D concentrations were quite low in this population, and most participants did not reach sufficient serum 25(OH)D concentrations. This study suggests that these patients might be resistant to the maximal daily dose of oral vitamin D supplementation recommended by the Institute of Medicine [8].
FMD is a surrogate marker of cardiovascular risk that is impaired in HIV-infected patients compared with uninfected controls [31] and improves when patients are started on combination ART [28]. In a previously published study from our laboratory of HIV-infected patients, the median FMD was 3.9%, which is comparable to other studies in HIV [32]. Thus, the median FMD of vitamin D deficient participants in our current study (2.7%) was low even for patients with HIV. The absolute cardiovascular risk associated with these low levels of FMD in the literature is dependent on the population studied and remains unknown in HIV patients. However, in a meta-analysis of 211 studies, FMD of the highest risk tertile (13.5% mean 10-year Framing-ham risk) was 4.5% ±2.1 (mean ±SD) [33], suggesting that our population of vitamin D deficient HIV-infected patients is at high risk of cardiovascular events. It is important to note that changes in brachial artery diameter can affect the FMD calculation and that this was not seen in our study.
The effect of vitamin D supplementation on endothelial function in non-HIV populations is conflicting. Two studies by the same investigators have tested vitamin D supplementation regimens in diabetics [10,12]. In an initial trial of a single oral dose of 100,000 IU of vitamin D2 (ergocalciferol) versus placebo in 34 patients, there was a greater increase in FMD from baseline to 8-week follow-up in those patients receiving vitamin D (2.35 ±3.12% versus 0.06 ±3.39%, mean ±SD, vitamin D versus placebo; P=0.048). In a larger follow-up trial of two dosing strategies (single oral doses of 100,000 or 200,000 IU vitamin D3) versus placebo in 61 patients, there was no difference in FMD at 8 and 16 weeks. Tarcin et al. [11] tested much higher doses of 300,000 IU of vitamin D3 monthly for 3 months in vitamin D deficient but otherwise healthy adults and reported an increase in FMD to levels that matched a control population. The 12-week duration of our trial was long enough to show significant changes in FMD as evidenced by the positive results obtained in two of the three previous trials [10,11]. The cumulative dose of vitamin D in the active arm of our study was 336,000 IU of vitamin D3. Our study contributes evidence that vitamin D supplementation at this dose and duration does not improve FMD in HIV-infected individuals.
Perhaps the most striking finding in our study was the small increase in the concentration of serum 25(OH)D with high dose supplementation. Indeed, only two participants in the active treatment arm achieved ‘sufficient’ 25(OH)D concentrations of >30 ng/ml. In the general population, each 100 IU of vitamin D3 supplementation increases the serum 25(OH)D concentration by 1 ng/ml [34]. In a small non-placebo-controlled study of 20 HIV-infected adults, supplementation with 2,000 IU of cholecalciferol daily increased median serum 25(OH)D from 11 ng/ml at baseline to 40 ng/ml at 12 weeks [35]. By contrast, we report a more modest response in serum 25(OH)D stores with higher doses of cholecalciferol, which we cannot account for by poor compliance (99% by pill count in the active treatment arm) or inferior product (all capsules contained >2,000 IU when tested for vitamin D content). Whether there is decreased gastrointestinal absorption of vitamin D3, increased catabolism of serum 25(OH)D or altered concentrations of vitamin D binding protein in these patients is unknown and requires future investigation.
EFV use did not seem to alter the response to vitamin D supplementation in our study, although our analysis was limited to small numbers of participants. Together with recent studies associating EFV use with vitamin D deficiency [23–26], our findings suggest the need to examine this association in a larger clinical trial of vitamin D supplementation. The mechanism of a possible interaction is incompletely understood, but might involve the induction of two hepatic enzymes: CYP3A4, a vitamin D-25 hydroxylase, and CYP24, which catabolises both 25(OH)D and 1,25(OH)D to inactive vitamin D metabolites. Other drugs are known to cause osteomalacia by this mechanism [36]. The current recommendation of the Endocrine Society is for these patients to be given two to three times more vitamin D than is typically recommended for their age group [21].
Interestingly, we observed a significant increase in insulin resistance from baseline to follow-up in the vitamin D group, although there was not a statistically significant change in HOMA-IR compared with placebo. Our findings are unexpected in light of a recent cross-sectional cohort study among HIV-infected Italian patients that associated vitamin D deficiency with 1.85-fold higher odds of diabetes [17]. Our data do, however, support the findings of a prior study [35] that cholecalciferol supplementation seems to worsen insulin resistance in vitamin D deficient HIV-infected individuals. In the non-infected population, vitamin D deficiency has been associated with insulin resistance, although whether vitamin D supplementation can improve insulin resistance is not clear [37]. Vitamin D plays a complex role in glucose homeostasis, and it is possible that vitamin D supplementation might have differing effects on insulin sensitivity in different patient populations. The positive effects of vitamin D supplementation on some markers of cardiovascular risk could be negated by the negative effect of worsening insulin resistance.
Importantly, we did not find evidence in our study that cholecalciferol affected blood pressure among normotensive participants. This is in-line with a recent meta-analysis of vitamin D trials that found significant blood pressure reductions only among populations with a mean systolic blood pressure >140 mmHg [38]. Vitamin D supplementation had a small but favourable effect on lipids in our study, which has been reported in non HIV-infected populations [39,40].
Inflammation is suspected to play a crucial role in the pathogenesis of many chronic complications of HIV, including cardiovascular disease. Markers of endothelial inflammation are correlated with HIV disease status [41] and are reduced when antiretroviral therapy is initiated [42]. Vitamin D deficiency has also been associated with higher concentrations of TNF-α but not CRP or IL-6 in otherwise healthy women [43]. In our study, inflammatory biomarkers were not changed by vitamin D supplementation. The statistically significant difference in IL-6 change between groups is more likely to be due to a chance increase in the placebo group rather than a decrease in IL-6 concentrations among those taking vitamin D. More study is needed to tease out the relative importance of specific inflammatory biomarkers in the pathophysiology of cardiovascular risk in HIV.
The principle strength of our study is the randomized, double-blind, placebo-controlled trial design. Although the study was not large, it was adequately powered to detect a clinically significant change in the primary outcome. Our trial was in-line with the recommended sample size (40–60 for a parallel group design) suggested by brachial artery reactivity testing guidelines [29]. It was not, however, powered to detected differences in all the secondary outcomes. One important limitation of our study is the inadequate response in 25(OH)D concentrations among the active treatment arm. We did not compare intramuscular dosing with oral dosing nor did we measure levels of 1,25(OH)D, vitamin D catabolites or vitamin D binding proteins, which might help to elucidate the mechanisms of vitamin D resistance in future studies. We expect this experience to inform the design of future vitamin D trials in HIV participants in regard to dosing and duration of treatment.
In summary, among HIV-infected individuals with vitamin D deficiency, supplementation with 4,000 IU vitamin D3 daily for 12 weeks modestly improved vitamin D status but did not improve FMD. This population has poor endothelial function at baseline and further studies are needed to examine whether endothelial function can be improved with longer duration of higher doses of vitamin D. Future studies should also explore the mechanisms of apparent resistance to standard doses of vitamin D supplementation and whether the benefits of vitamin D supplementation for reducing cardiovascular disease risk might be outweighed by detrimental effects such as an increase in insulin resistance.
Acknowledgements
This study was funded in part by a virology fellows grant from Bristol–Myers Squibb awarded to CTL and by a grant from the University Hospitals Harrington-McLaughlin Heart and Vascular Institute. Additional technical support was provided by the CWRU/UH Center for AIDS Research: NIH grant number P30 AI36219. The study is registered with clinicaltrials. gov number NCT01093417.
CTL has received a research grant from Bristol–Myers Squibb. COH has received a research grant from Bristol–Myers Squibb. ACR has received research grants from GlaxoSmithKline, Bristol–Myers Squibb and Cubist Pharmaceuticals. TLC serves on the DSMB of Prairie Education and Research Cooperative, has received a research grant from Baxter, Inc., and is on the speakers bureau for Sanofi-Aventis. GAM has served as a scientific advisor for Bristol–Myers Squibb, GlaxoSmithKline, Abbott, Tibotec and Gilead Sciences, has received research grants from Bristol–Myers Squibb, GlaxoSmithKline, Abbott, Merck and Gilead Sciences, and is currently serving as the DSMB Chair for a Pfizer-sponsored study.
Additional file
Additional file 1: A figure showing the study flow can be found at http://www.intmedpress.com/uploads/documents/AVT-11-OA-2241_Longenecker_Add_filel.pdf
Disclosure statement
All other authors declare no competing interests.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Lee JH, O’Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? Am Coll Cardiol. 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 3.Judd SE, Nanes MS, Ziegler TR, Wilson PW, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2008;87:136–141. doi: 10.1093/ajcn/87.1.136. [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1, 25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 7.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 8.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 10.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 11.Tarcin O, Yavuz DG, Ozben B, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–4030. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 12.Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD. The effect of different doses of vitamin D (3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2010;53:2112–2119. doi: 10.1007/s00125-010-1838-1. [DOI] [PubMed] [Google Scholar]
- 13.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–69. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinesi M, Bruni S, Stio M, Treves C. 1, 25-Dihydroxyvitamin D3 inhibits tumor necrosis factor-alpha-induced adhesion molecule expression in endothelial cells. Cell Biol Int. 2006;30:365–375. doi: 10.1016/j.cellbi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Overton ET, Yin MT. The Rapidly Evolving Research on Vitamin D Among HIV-infected Populations. Curr Infect Dis Rep. 2011;13:83–93. doi: 10.1007/s11908-010-0144-x. [DOI] [PubMed] [Google Scholar]
- 16.Mehta S, Giovannucci E, Mugusi FM, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS ONE. 2010;5:e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szep Z, Guaraldi G, Shah SS, et al. Vitamin D deficiency is associated with type 2 diabetes mellitus in HIV infection. AIDS. 2011;25:525–529. doi: 10.1097/QAD.0b013e328342fdfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross AC, Judd S, Kumari M, et al. Vitamin D is linked to carotid intima-media thickness and immune reconstitution in HIV-positive individuals. Antivir Ther. 2011;16:555–563. doi: 10.3851/IMP1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi Al, Lo JC, Mulligan K, et al. Association of vitamin D insufficiency with carotid intima-media thickness in HIV-infected persons. Clin Infect Dis. 2011;52:941–944. doi: 10.1093/cid/ciq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arpadi SM, McMahon D, Abrams EJ, et al. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics. 2009;123:el21–el26. doi: 10.1542/peds.2008-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin d deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 22.Dao CN, Patel P, Overton ET, et al. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 23.Welz T, Childs K, Ibrahim F, et al. Efavirenz is associated with severe vitamin D deficiency and increased alkaline phosphatase. AIDS. 2010;24:1923–1928. doi: 10.1097/QAD.0b013e32833c3281. [DOI] [PubMed] [Google Scholar]
- 24.Brown TT, McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D. Antivir Ther. 2010;15:425–429. doi: 10.3851/IMP1502. [DOI] [PubMed] [Google Scholar]
- 25.Wohl DA, Doroana O, Pilotto J, et al. Change in vitamin D levels smaller and risk of development of severe vitamin D deficiency lower among HIV-1-infected, treatment-naive adults receiving TMC278 compared with EFV: 48-week results from the phase III ECHO trial. 18th Conference on Retroviruses and Opportunistic Infections; 27 February–2 March 2011; Boston, MA, USA. Abstract 79LB. [Google Scholar]
- 26.Fox J, Peters B, Prakash M, Arribas J, Hill A, Moecklinghoff C. Improvement in vitamin D deficiency following antiretroviral regime change: results from the MONET Trial. AIDS Res Hum Retroviruses. 2010;26:1–4. doi: 10.1089/aid.2010.0081. [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 30.Overton ET, Grubb J, Baker J, et al. Prevalence and risk factors for insulin resistance in the study to understand the natural history of HIV (SUN study) cohort. 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention; 17–20 July 2011; Rome, Italy. Abstract TUPE13L. [Google Scholar]
- 31.Solages A, Vita JA, Thornton DJ, et al. Endothelial function in HIV-infected persons. Clin Infect Dis. 2006;42:1325–1332. doi: 10.1086/503261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hileman CO, Carman TL, Gripshover BM, et al. Salsalate is poorly tolerated and fails to improve endothelial function in virologically suppressed HIV-infected adults. AIDS. 2010;24:1958–1961. doi: 10.1097/QAD.0b013e32833c3251. [DOI] [PubMed] [Google Scholar]
- 33.Witte DR, Westerink J, de Koning EJ, van der Graaf Y, Grobbee DE, Bots ML. Is the association between flow-mediated dilation and cardiovascular risk limited to low-risk populations? J Am Coll Cardiol. 2005;45:1987–1993. doi: 10.1016/j.jacc.2005.02.073. [DOI] [PubMed] [Google Scholar]
- 34.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 35.van den Bout-van den Beukel CJ, van den Bos M, Oyen WJ, et al. The effect of cholecalciferol supplementation on vitamin D levels and insulin sensitivity is dose related in vitamin D-deficient HIV-1-infected patients. HIV Med. 2008;9:771–779. doi: 10.1111/j.1468-1293.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 36.Pascussi JM, Robert A, Nguyen M, et al. Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J Clin Invest. 2005;115:177–186. doi: 10.1172/JCI21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitri J, Muraru MD, Pittas AG. Vitamin D and type-2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005–1015. doi: 10.1038/ejcn.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. 2009;27:1948–1954. doi: 10.1097/HJH.0b013e32832f075b. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz JB. Effects of vitamin D supplementation in atorvastatin-treated patients: a new drug interaction with an unexpected consequence. Clin Pharmacol Ther. 2009;85:198–203. doi: 10.1038/clpt.2008.165. [DOI] [PubMed] [Google Scholar]
- 40.Gannagé-Yared MH, Chedid R, Khalife S, Azzi E, Zoghbi F, Halaby G. Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young Middle-Eastern population. Eur J Endocrinol. 2009;160:965–971. doi: 10.1530/EJE-08-0952. [DOI] [PubMed] [Google Scholar]
- 41.Ross AC, Armentrout R, O’Riordan MA, et al. Endothelial activation markers are linked to HIV status and are independent of antiretroviral therapy and lipoatrophy. J Acquir Immune Defic Syndr. 2008;49:499–506. doi: 10.1097/QAI.0b013e318189a794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care. 2010;33:2244–2249. doi: 10.2337/dc10-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson CA, Heffernan ME. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25 (OH) D concentrations in healthy women. J Inflamm (Lond) 2008;5:10. doi: 10.1186/1476-9255-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]