Abstract
Objective
To compare implications of Angina Pectoris (AP) and Intermittent Claudication (IC) as indicators of clinical atherosclerosis in other vascular territories.
Study Design and Setting
Prospective cohort study of cardiovascular disease (CVD) in 5,209 men and women of Framingham, MA, aged 28–62 years at enrollment in 1948–1951, who received biennial examinations during the first 36 years of follow-up. Comparative 10-year incidence of subsequent atherosclerotic CVD in participants with IC and AP relative to a reference sample free of CVD was determined.
Results
On follow-up, 95 CVD events occurred in 186 participants with IC and 206 of 413 with AP. After age, sex, and risk-factor adjustment, the proportion acquiring other CVD was 34.0% for IC and 43.4% for AP. Relative to the reference sample, those with IC had a 2.73-fold higher age and sex-adjusted 10-year hazard of CVD (95% CI 2.21, 3.38) and for AP was 3.17 (95% CI 2.73, 3.69). CVD hazard ratios remained more elevated for AP and statistically significant after standard risk factor adjustment. Risk factors accounted for more of the excess CVD risk associated with IC (34.8%) than AP (9.5%).
Conclusion
AP is as useful as IC as a hallmark of diffuse atherosclerotic CVD and an indication for comprehensive preventive measures.
Keywords: Angina pectoris, Intermittent claudication, Prognosis, Cohort study, Cardiovascular disease, Vascular damage indicator
1. Introduction
Peripheral artery disease, manifested as intermittent claudication (IC) or abnormal ankle–brachial index is an accepted marker of diffuse atherosclerotic vascular disease and increased risk for mortality, primarily from cardiovascular causes [1–9]. Angina pectoris (AP), another transient ischemic condition provoked by exertion, is regarded chiefly as a hallmark of impending myocardial infarction or a coronary fatality. For example, the Rose angina questionnaire, devised for epidemiological investigation of angina, was tested chiefly as a predictor of coronary morbidity and mortality [10–12].
We had the opportunity to examine the prognostic implications of these transient ischemic conditions, using population-based data derived from the Framingham Study between 1949 and 1990, a period during which there were few effective cardiovascular disease (CVD) therapies available or in widespread use, allowing unbiased estimates. This report compares the total atherosclerotic cardiovascular outlook of participants experiencing an initial IC or AP event, during the first 36 years of the study with a reference group of participants, free of CVD, drawn within the same calendar time frame.
2. Methods
2.1. Study sample
The Framingham Study is an ongoing, prospective cohort study of the epidemiology of CVD. From 1948 to 1953, 5,209 men and women, between the ages of 28 and 62 years, residing in Framingham, MA were enrolled and have been re-examined biennially since the study inception. Details of the original sampling have been published previously [13]. Participants with no prior history of CVD at entry were eligible for the present study. First CVD events classified as either IC or AP, occurring prior to January 1, 1980, and with no other CVD event on the same day, comprised the two exposure groups of interest. When the exact date of onset of IC or AP was unknown, the mid-point between the examination of diagnosis and the last attended examination free of symptoms was used. Participants were followed for up to 10 years for new CVD events. Participants who had not attended an examination within 2 years prior to IC or AP diagnosis were excluded from analysis (n = 46) because covariate data was taken from the examination prior to diagnosis.
The sampling scheme used to select the reference group was designed such that the distribution of age, sex, and calendar time would resemble that of the IC and AP groups. For each participant in the study, one examination was chosen, at random, from all examinations attended prior to 1990 free of CVD, to serve as the baseline for the 10-year follow-up period. If, after 10-years, the participant attended another exam, still free of CVD, a new baseline was established along with a corresponding 10-year follow-up period. This technique is the time dependent Cox regression (SAS Institute Inc. SAS/STAT User’s Guide, Version 8. Cary, NC: SAS Publishing; 1999, 2569–2656).
2.2. Diagnostic criteria for AP and IC
The clinical criteria used for the diagnosis of cardiovascular events in the Framingham Heart Study have been reported in detail [14]. A physician-administered structured questionnaire was used to determine the presence of AP and IC based on the participants’ subjective manifestations. Brief, recurrent chest discomfort of up to 15 min in duration, brought on by exertion and relieved by rest or nitroglycerin, was accepted as angina if two physicians agreed that it was present. Abnormality of the ECG, either at rest or on exercise testing, was not taken into account. Participants with angina who exhibited no other manifestations of coronary heart disease (CHD) at the current examination or any prior examination were designated as having uncomplicated AP.
The presence of IC was accepted if the interviewing physicians agreed that the participant had experienced a transient, short duration, cramping discomfort in the calf that was provoked by walking, appeared sooner when the participant walked faster or uphill, and was promptly relieved by rest [14]. Abnormalities on noninvasive testing such as ankle–brachial blood pressure testing or ultrasonography were not taken into account in the evaluation of IC.
2.3. Ascertainment of CVD outcomes and death
Participants were followed for 10 years after the onset of IC or AP for the occurrence of a cardiovascular event or death. Information regarding events was obtained from biennial examinations and hospital surveillance. CVD included CHD defined as AP, coronary insufficiency (prolonged chest pain in the presence of reversible electrocardiographic changes), myocardial infarction, or CHD death; stroke or transient ischemic attack; and congestive heart failure. CVD events were adjudicated by a panel of three senior investigators (or a panel of study neurologists for cerebrovascular disease events) using standardized criteria previously reported [15]. All deaths were reviewed and cause of death was classified by the endpoint panel as due to CHD, stroke, other CVD, cancer, other causes, or unknown cause.
2.4. Covariate measures
At each biennial examination, resting blood pressures were taken with a mercury sphygmomanometer and a 14-cm cuff on the left arm of participants and readings were recorded to the nearest even number. For the purposes of this investigation, hypertension was designated if the mean of two physician obtained blood pressures was ≥140/90 mm Hg or the participant reported taking antihypertensive medications. Serum cholesterol after exam 2 in 1952 was determined by the Abell–Kendall method. Blood glucose was measured on a casual specimen of whole blood using the Nelson method. Diabetes mellitus was diagnosed if there was a casual blood glucose level of ≥200 mg/dl or the use of insulin or oral hypoglycemic medication. Body mass index was calculated from height and weight measurements obtained from trained technicians using the formula: weight(kg)/height(m2). Current cigarette smoking was self-reported. Participants who reported regular cigarette smoking within the year prior to the examination were classified as current smokers.
2.5. Statistical analysis
The IC, AP, and reference groups were followed for up to 10 years from baseline for the occurrence of new CVD events. Endpoints of interest included the first new event, fatal or nonfatal, in each of the three types of CVD examined, CHD, congestive heart failure (CHF), and cerebral vascular accident (CVA) or transient ischemic attack, as well as the first new CVD event overall. Events in subjects in the IC group included AP while events in the AP group included IC. Subject characteristics at baseline for the IC, AP, and reference group were compared after adjustment for age and sex. For continuous variables, linear regression was used, least squares mean values were produced, and the partial F-statistic was used to test for differences between the groups. Dichotomous variables were standardized using the direct method and the Pearson chi-square was used to test for differences between the groups.
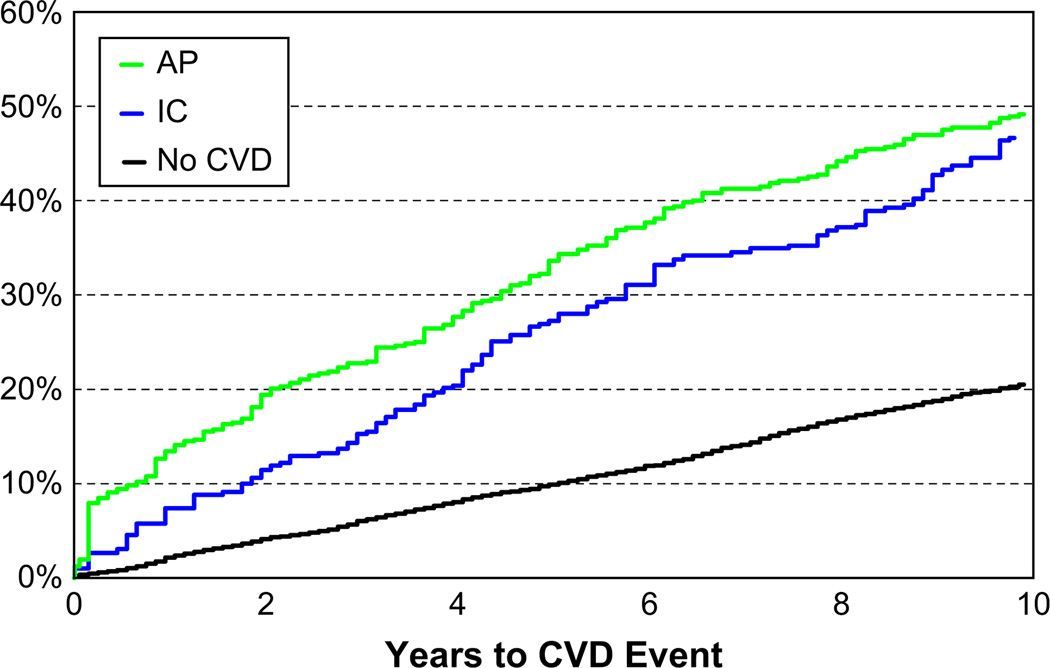
To assess differences in CVD incidence between the IC group, the AP group, and the reference group, Kaplan–Meier survival methods were used to construct incidence (1-survival) curves (Fig. 1). As indicated in Figure 1, the age- and sex-adjusted incidence of angina pectoris is greater than that for intermittent claudication throughout the follow-up. The Group Prognostic Method [16] was used to produce group-specific, predicted incidence rates adjusting for age and sex and with further adjustment for systolic blood pressure, hypertension treatment, body mass index, current cigarette smoking, diabetes, and serum total cholesterol. Cox proportional hazards regression was used to compare CVD risk in the IC group and the AP group, both relative to the reference group. Separate models were performed for men and women and then pooled.
Fig. 1.
Cardiovascular disease incidence in the Intermittent Claudication, Angina Pectoris, and Reference Group: Results of Age- and Sex-adjusted Kaplan–Meier Survival Analysis.
3. Results
Table 1 displays the baseline characteristics of the study sample. The IC and AP subjects were older and more male than the reference sample free of CVD. In addition, as expected, almost all risk factor levels were significantly (P < 0.001) higher in those with IC or AP than in the reference sample. Comparing IC subjects with those having AP, cigarette smoking prevalence was lowest in subjects with angina and BMI was lowest in IC subjects. Cigarette smoking and diabetes prevalence were substantially higher in the IC group than in the AP group. Those with AP were heavier than those with IC whose BMI was lower than that of the reference sample.
Table 1.
Baseline characteristics according to first CVD event as intermittent claudication or angina pectoris: Framingham Heart Study, 1948–1979
| Characteristic | Intermittent Claudication; n = 186 |
Angina Pectoris; n = 413 |
Reference Sample Free of CVD; n = 6,860 |
|---|---|---|---|
| Age (years) | 62.5 (8.9) | 60.8 (8.6) | 56.8 (10.8) |
| Men (%) | 56 | 48 | 43 |
| Systolic blood pressure (mm Hg)a | 146.1 (1.60) | 144.6 (1.07) | 136.9 (0.27) |
| Diastolic blood pressure (mm Hg)a | 83.1 (0.87) | 86.6 (0.59) | 82.4 (0.14) |
| Hypertension treatmenta | 25.1 | 17.5 | 11.1 |
| Total cholesterol (mg/dL)a | 248 (3.3) | 252 (2.2) | 235 (0.5) |
| Diabetes mellitusa | 16.7 | 4.5 | 3.4 |
| Cigarette smoking, currenta | 60.0 | 37.6 | 40.7 |
| Body mass index (kg/m2)a | 25.4 (0.31) | 27.2 (0.21) | 26.0 (0.05) |
All P-values for difference between the groups ≤0.001.
Age and sex-adjusted least squares means (± standard error) for continuous variables; direct age and sex-adjusted prevalence for categorical variables.
The number of men and women in the population at risk with AP was substantially larger than for IC. As a consequence, the number of other cardiovascular events over the 10-year period of follow-up was substantially larger for AP (206) than IC (95). However, the age and sex-adjusted proportion developing CVD events was about 46% compared to 20.5% in the reference group (Table 2). After multivariable risk-factor adjustment there was a greater proportion of AP victims (43.4%) having an event than observed for persons with IC (34.0%) (Table 2). This appears to be attributable to the fact that a greater fraction of the IC events (34.8%) can be ascribed to accompanying cardiovascular risk factors than for AP (9.5%).
Table 2.
Cardiovascular events according to intermittent claudication and angina pectoris status: Framingham Study cohort: men and women combined—10-year incidence rates
| Events/person years | Intermittent Claudication |
Angina Pectoris |
Reference Sample |
|---|---|---|---|
| Predicted 10-year rates | |||
| CVD | 95/1,269 | 206/2,719 | 983/45,054 |
| Unadjusted | 53.8% | 51.1% | 19.9% |
| Age and sex | 45.7% | 47.2% | 20.5% |
| Multivariable | 34.0% | 43.4% | 21.3% |
| CHD | 67/1,392 | 143/2,978 | 602/40,526 |
| Unadjusted | 39.9% | 36.1% | 13.9% |
| Age and sex | 34.9% | 34.6% | 14.2% |
| Multivariable | 25.2% | 30.8% | 14.9% |
| CVA | 38/1,517 | 42/3,446 | 419/40,441 |
| Unadjusted | 22.9% | 11.8% | 10.2% |
| Age and sex | 20.7% | 11.6% | 10.5% |
| Multivariable | 14.5% | 11.2% | 11.1% |
| CHF | 27/1,545 | 48/3,407 | 199/39,187 |
| Unadjusted | 16.0% | 13.0% | 5.1% |
| Age and sex | 14.6% | 12.8% | 5.2% |
| Multivariable | 10.8% | 12.4% | 5.5% |
| IC | 27/1,500 | 156/37,592 | |
| Unadjusted | 16.8% | 4.0% | |
| Age and sex | 16.3% | 4.0% | |
| Multivariable | 14.5% | 4.1% | |
| AP | 35/3,383 | 72/36,818 | |
| Unadjusted | 9.9% | 1.9% | |
| Age and sex | 9.8% | 1.9% | |
| Multivariable | 9.0% | 2.0% |
CHD was the most common cardiovascular hazard for both AP and IC, which adjusting for age and sex, occurred at the same 35% 10-year rate. However, as expected, on adjustment for accompanying CVD risk factors the coronary rate for AP was somewhat greater (30.8%) than for IC (25.2%). Age and sex-adjusted stroke rates appeared to be almost twice as high for IC as for AP. However, on adjustment for accompanying CVD risk factors, the 10-year stroke rates were more comparable (14.5% vs. 11.2%) but the stroke rate for AP was identical to that for the reference sample (11.1%). Contrary to expectation, the 10-year heart failure rate for IC (10.8%) was similar to that for AP (12.4%) on adjustment for accompanying CVD risk factors.
Table 2 also indicates that subjects with either one of these transient exercise-induced ischemic conditions are at about four to fivefold increased risk of developing the other. The absolute age and sex-adjusted risk of IC in subjects with AP (16.3%) is greater than the 9.8% risk of AP in subjects with IC. This greater likelihood of angina patients to develop IC than the converse persists after adjusting for associated risk factors.
Table 3 displays the age and sex-adjusted and multivariable risk-factor adjusted hazard ratios for other cardiovascular events in subjects with IC and AP. For IC, the age and sex-adjusted hazard of developing angina was greatest (4.3-fold) followed by heart failure (3.1-fold), and then coronary disease (2.7-fold) and least of all stroke (2.1-fold). This ranking order persists after multivariable risk factor adjustment. Overall, IC imposes a 2.7-fold age and sex-adjusted increased risk of other cardiovascular events, which is reduced to 1.8-fold after multivariable risk-factor adjustment.
Table 3.
CVD subsequent to intermittent claudication vs. angina pectoris as initial CVD events compared with a reference sample free of CVD at baseline, 10-year follow-up
| Sex Pooled | ||||
|---|---|---|---|---|
| Hazards Ratios (95% Confidence Intervals) | ||||
| Intermittent Claudication | Angina Pectoris | |||
| Events | Age and Sex Adjusted | Multivariate adjusteda | Age and Sex Adjusted | Multivariable Adjusteda |
| Cardiovascular disease | 2.73 (2.21, 3.38) | 1.78 (1.43, 2.22) | 3.17 (2.72, 3.69) | 2.87 (2.47, 3.35) |
| Coronary heart disease | 2.72 (2.11, 3.51) | 1.74 (1.33, 2.27) | 3.09 (2.58, 3.71) | 2.66 (2.21, 3.20) |
| Stroke | 2.13 (1.52, 2.97) | 1.36 (0.96, 1.92) | 1.12 (0.81, 1.54) | 1.02 (0.74, 1.40) |
| CHF | 3.08 (2.05, 4.61) | 2.15 (1.41, 3.29) | 2.69 (1.96, 3.69) | 2.52 (1.83, 3.48) |
| Intermittent claudication | 5.22 (3.48, 7.83) | 4.60 (3.04, 6.98) | ||
| Angina pectoris | 4.27 (2.82, 6.46) | 3.71 (2.40, 5.74) | ||
Adjusted for age, systolic blood pressure, hypertension treatment, body mass index, diabetes, current smoking, and serum total cholesterol.
For AP, the age and sex-adjusted hazard ranking is greatest for IC (5.2-fold) followed by other manifestations of coronary disease (3.1-fold), heart failure (2.7-fold), and least stroke (1.1-fold). This rank order holds after multivariable risk-factor adjustment. Overall, AP carries a 3.2-fold age and sex-adjusted hazard of other cardiovascular events, which is reduced to 2.9-fold after multivariable risk-factor adjustment.
Analysis of the multivariable hazard provides insight into the amount of influence that the associated CVD risk factors have in generating the increased propensity to other CVD. Judging by the size of the reduction in the hazard ratio from age-adjusted to multivariable-adjusted models, about 34.8% of the CVD potential of IC is attributable to the associated risk factors, whereas for AP only about 9.5%.
Table 4 provides sex specific hazard ratios for development of cardiovascular events in subjects presenting with AP and IC. For subjects with IC, the total CVD hazard ratios are greater for women than men for all outcomes, with the possible exception of stroke (not statistically significant). For AP subjects, the hazard ratios for CVD are comparable in men (hazard ratio [HR] 2.9) and women (HR 2.8). The hazard of IC in women with AP (HR 10.5) is substantially greater than for men (HR 2.7).
Table 4.
Sex-specific CVD hazard following angina vs. intermittent claudication
| Hazards Ratios (95% Confidence Intervals) | ||||
|---|---|---|---|---|
| Men | Women | |||
| Age Adjusted | Multivariable Adjusteda | Age Adjusted | Multivariable Adjusteda | |
| Intermittent claudication | ||||
| Cardiovascular disease | 2.53 (1.92, 3.30) | 1.67 (1.26, 2.22) | 3.32 (2.36, 4.67) | 2.09 (1.47, 2.99) |
| Coronary heart disease | 2.38 (1.73, 3.27) | 1.63 (1.17, 2.28) | 3.76 (2.47, 5.74) | 2.14 (1.37, 3.34) |
| Angina pectoris | 3.92 (2.32, 6.63) | 3.95 (2.27, 6.89) | 5.27 (2.70, 10.30) | 3.72 (1.81, 7.64) |
| Angina pectoris | ||||
| Cardiovascular disease | 3.14 (2.56, 3.84) | 2.93 (2.38, 3.59) | 3.24 (2.58, 4.06) | 2.81 (2.23, 3.54) |
| Coronary heart disease | 3.12 (2.48, 3.92) | 2.81 (2.23, 3.55) | 3.08 (2.27, 4.18) | 2.39 (1.75, 3.28) |
| Intermittent claudication | 3.25 (1.88, 5.63) | 2.74 (1.57, 4.79) | 11.29 (5.94,21.43) | 10.49 (5.36, 20.50) |
Events compared with a reference sample free of CVD at baseline, 10-year follow-up.
Adjusted for age, systolic blood pressure, hypertension treatment, body mass index, diabetes, current smoking, serum total cholesterol. Sex specific hazard ratios for stroke and heart failure (not shown) were not statistically significant compared to reference sample.
4. Discussion
Both AP and IC are transient symptoms of ischemic vascular disease brought on by exertion and relieved by rest. Angina is usually regarded as a hazard for development of a myocardial infarction or coronary fatality, which it clearly is. It is less often considered as an indicator of diffuse atherosclerotic disease involving other vascular territories as is now the case for IC [17–19]. The data presented indicates that angina as well as IC is a hallmark of diffuse atherosclerotic vascular disease involving the circulation to the limbs as well as the heart, imparting a two to threefold excess risk compared to the reference group. It appears that AP deserves as much attention as IC as an indicator of the need for comprehensive and more aggressive preventive measures against diffuse accelerated atherogenesis.
Atherothrombotic vascular disease is usually a diffuse condition involving the arterial circulation to the heart, brain, kidney, and periphery. Most of the risk factors that predispose to involvement of one arterial bed also apply to the others; consequently, it should be expected that having one clinical manifestation of atherosclerosis, increases the risk of developing the others [14].However, the hazard of clinical events in other vascular territories is apparently not chiefly a product of shared risk factors. Judging by the size of the reduction in the hazard ratio on adjustment for the coexistent risk factors, they only account for about 35% of the other CVD risk for IC and 9.5% of the hazard of AP. The nature of the unique effect is uncertain but it is possible that the appearance of a clinical manifestation indicates greater vulnerability to the cluster of predisposing cardiovascular risk factors. Furthermore, atherosclerosis per se begets atherothrombosis by its tendency to progress on its own.
It was previously reported from the Framingham Study that more than half of persons with IC at initial diagnosis already have coexistent atherothrombotic CVD [18,20–22]. As early as 1974, the Study also reported that the chief hazard of IC was not the loss of a limb but rather a serious cardiovascular event [23]. It was suggested that IC may be a marker for predisposition to atherothrombotic events in other vascular territories, which proved to be the case [1–3].
Vascular bruits often signify diseased arteries and it is not surprising that femoral bruits have been reported to be associated with a high (20–30%) prevalence of IC. However, these same bruits are also associated with a significantly increased prevalence of CHD and heart failure [24]. Likewise, carotid bruits, indicating vascular disease of the cerebral circulation, is not only associated with a two to threefold increased stroke risk, but also carries a two to threefold increased risk of coronary disease, IC, and heart failure. Because the peripheral vessels are more accessible to noninvasive testing for obstruction to flow, there is merit in detecting presymptomatic arterial disease, so that timely preventive measures can be implemented to protect against lethal clinical manifestations of atherothrombotic disease.
The Framingham Study has crafted multivariable risk profiles for identifying high-risk persons for development of IC and coronary disease that can be used to educate patients about modifiable risk factors for avoiding CVD [22,25]. Although this has not been done specifically for AP, the coronary risk profile can be used to identify high-risk candidates for AP. Assessment of multivariable risk of AP would indicate the need for more aggressive preventive measures to prevent ischemic vascular events in other vascular territories as well those involving the heart.
It is estimated, using Framingham Study and National Heart, Lung and Blood Institute data that the prevalence of AP in the year 2001 was 6,800,000 cases. In the national data, as in the Framingham Study, age-adjusted prevalence of angina was greater in women than men. National data also indicate that the prevalence of AP is higher in blacks and Mexican Americans [26]. Peripheral artery disease affects more than 5 million persons in the United States and is also higher in racial and ethnic minorities [4,27].
IC appears to be the most underdiagnosed and least aggressively managed clinical atherosclerotic condition [28,29]. The peripheral artery disease coalition and the NHLBI are launching a campaign to remedy this public health problem (www.padcoalition.org). The major problem the patient with IC faces is the high risk of other cardiovascular events; attention to the ischemic limb is not enough. The same applies for AP, where alleviation of myocardial ischemia is not enough. Investigation of IC in the Framingham Study has identified a number of risk factors that lead to its occurrence. These include age, sex, serum cholesterol, hypertension, cigarette smoking, diabetes, and presence of CHD [14]. They are also the standard risk factors for coronary disease and stroke. For AP, systolic blood pressure, serum cholesterol, diabetes, cigarette smoking, and hematocrit are significant independent risk factors in one or both sexes [14,30]. There is evidence supporting the efficacy of risk factor correction for reducing risk of other CVD events in patients with AP and IC [31–36].
Although lacking in sensitivity and specificity, a detailed history and physical examination are important in detecting and evaluating peripheral artery disease. The ankle–brachial index can be done to confirm the diagnosis and help stratify the risk since it correlates well with disease severity, functional symptoms, and disease progression. The ankle–brachial index also predicts CVD and stroke mortality, the greatest hazard of the condition [5–7]. Transient ischemic vascular episodes involving the circulation of the heart and limbs are often silent and a significantly compromised circulation may exist without symptoms. Noninvasive testing in a population indicates that the true prevalence of peripheral artery disease is at least five times greater than would be estimated from the reported prevalence of IC [37]. Likewise, three of every four transient ischemic cardiac episodes detected by ECG monitoring in angina are silent [38–40].
5. Limitations
The Framingham Study cohort has few blacks and other minority population subgroups, limiting generalizability of the data. The number of events in the CVD subgroups of myocardial infarction, stroke, and heart failure were too few to provide confident estimates of differences of the size of hazard ratios for AP in comparison to IC in the two sexes. The use of only a clinical assessment of transient myocardial and peripheral artery ischemia allows many patients with silent occlusive arterial disease who are also at high risk of other atherosclerotic events to go undetected. Ankle–brachial index is a feasible office procedure that could be used on asymptomatic patients with an unfavorable multivariable risk profile to detect occult peripheral artery disease needing comprehensive preventive measures; especially for those aged 50–69 with diabetes and smoking or age 70 or over [41]. Comparable simple office procedures for detecting occult myocardial ischemia in high-risk persons are currently unavailable.
6. Conclusions
Once detected, cardiovascular risk factor modification, symptomatic relief, and use of antiplatelet agents form the core of the management of both angina and IC. The major cardiovascular risk factors adversely affect all vascular territories, increasing vulnerability to multiple clinical manifestation of atherosclerosis including CHD and IC [14]. Modification of risk factors intended to prevent a particular atherosclerotic cardiovascular event should also prevent other outcomes. Optimal appraisal of the hazard, aggressiveness, and urgency for treatment is best obtained from a multivariable cardiovascular risk profile that estimates the probability of a cardiovascular event given the existing constellation of predisposing factors.
What is new?
Angina pectoris joins intermittent claudication as a robust indicator of diffuse atherosclerotic vascular involvement. Both require targeting of the underlying accelerated atherogenesis in their management in addition to protecting the limbs for claudication and the heart for angina.
Acknowledgments
This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195).
References
- 1.Bowlin SJ, Medalie JH, Flocke SA, Zyzanski SJ, Yaari S, Goldbourt U. Intermittent claudication in 8343 men and 21-year specific mortality follow-up. Ann Epidemiol. 1997;7:180–187. doi: 10.1016/s1047-2797(96)00148-2. [DOI] [PubMed] [Google Scholar]
- 2.Bainton D, Sweetnam P, Baker I, Elwood P. Peripheral vascular disease: consequence for survival and association with risk factors in the Speedwell prospective heart disease study. Br Heart J. 1994;72:128–132. doi: 10.1136/hrt.72.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith GD, Shipley MJ, Rose G. Intermittent claudication, heart disease risk factors, and mortality. The Whitehall Study. Circulation. 1990;82:1925–1931. doi: 10.1161/01.cir.82.6.1925. [DOI] [PubMed] [Google Scholar]
- 4.McDermott MM, Liu K, Criqui MH, Ruth K, Goff D, Saad MF, et al. Ankle-brachial index and subclinical cardiac and carotid disease: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 5.Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 6.O’Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the Cardiovascular Health Study. Circulation. 2006;113:388–393. doi: 10.1161/CIRCULATIONAHA.105.570903. [DOI] [PubMed] [Google Scholar]
- 7.Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelman M, Polak JF, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol. 1999;19:538–545. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, Sutton-Tyrrell K, Vogt MT, Kuller LH. Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index. JAMA. 1993;270:487–489. [PubMed] [Google Scholar]
- 9.Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 10.Lampe FC, Whincup PH, Wannamethee SG, Ebrahim S, Walker M, Shaper AG. Chest pain on questionnaire and prediction of major ischaemic heart disease, events in men. Eur Heart J. 1998;19:63–73. doi: 10.1053/euhj.1997.0729. [DOI] [PubMed] [Google Scholar]
- 11.Bulpitt CJ, Shipley MJ, Demerovic J, Ebi-Kryston KL, Markowe HL, Rose G. Predicting death from coronary heart disease using a questionnaire. Int J Epidemiol. 1990;19:899–904. doi: 10.1093/ije/19.4.899. [DOI] [PubMed] [Google Scholar]
- 12.LaCroix AZ, Guralnik JM, Curb JD, Wallace RB, Ostfeld AM, Hennekens CH. Chest pain and coronary heart disease mortality among older men and women in three communities. Circulation. 1990;81:437–446. doi: 10.1161/01.cir.81.2.437. [DOI] [PubMed] [Google Scholar]
- 13.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in the community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 14.Cupples LA, D’Agostino RB, Keily D. Bethesda, MD: US Dept. of Health and Human Services. Public Health Service, National Institutes of Health; 1987. Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements: Framingham Heart Study, 30-year follow-up. NIH Publication No. 87-2703. [Google Scholar]
- 15.Abbott RD. Bethesda, MD: National Heart, Lung and Blood Institute; 1987. The Framingham Study: an epidemiologic investigation of cardiovascular disease, section 37: the probability of developing certain cardiovascular diseases in eight years at specified values of some characteristics; p. 2004. [Google Scholar]
- 16.Chiang IM, Gelman R, Pagano M. Corrected group prognostic curves and summary statistics. J Chron Dis. 1982;35:669–674. doi: 10.1016/0021-9681(82)90019-4. [DOI] [PubMed] [Google Scholar]
- 17.Mohler ER. Peripheral arterial disease: identification and implications. Arch Intern Med. 2003;163:2306–2314. doi: 10.1001/archinte.163.19.2306. [DOI] [PubMed] [Google Scholar]
- 18.O’Riordain DS, O’Donnell JA. Realistic expectations for the patient with intermittent claudication. Br J Surg. 1991;78:861–863. doi: 10.1002/bjs.1800780728. [DOI] [PubMed] [Google Scholar]
- 19.Hagman M, Wilhelmsen L, Pennert K, Wedel H. The Multifactor Primary Prevention Trial. Gothenburg, Sweden: Factors of importance for prognosis in men with angina pectoris derived from a random population sample. [DOI] [PubMed] [Google Scholar]
- 20.Kannel WB, McGee DL. Update on some epidemiologic features of intermittent claudication. J Am Geriatr Soc. 1985;33:13–18. doi: 10.1111/j.1532-5415.1985.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 21.Mohler ER., 3rd Peripheral artery disease: identification and implications. Arch Intern Med. 2003;163:2306–2314. doi: 10.1001/archinte.163.19.2306. [DOI] [PubMed] [Google Scholar]
- 22.Murabito JM, D’Agostino RB, Silbershatz H, Wilson PW. Intermittent claudication. A risk profile from the Framingham Study. Circulation. 1997;96:44–49. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 23.Peabody CN, Kannel WB, McNamara PM. Intermittent claudication: surgical significance. Arch Surg. 1974;109:693–697. doi: 10.1001/archsurg.1974.01360050087019. [DOI] [PubMed] [Google Scholar]
- 24.Kannel WB. Atherothrombosis and cardiovascular disease. 2nd Edition. London: Lippincott Williams & Wilkins; 2005. Epidemiologic relation of cardiovascular disease among different vascular territories. [Google Scholar]
- 25.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 26.National Health and Nutrition Examination Survey NHANES III (1988–1994), CHC/NCHS. Heart Disease and Stroke Statistics-2003 Update American Heart Association [Google Scholar]
- 27.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110(6):738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 28.McDermott MM, Mehta S, Ahn H, Greenland P. Atherosclerotic risk factors are less intensively treated in patients with peripheral arterial disease than in patients with coronary artery disease. J Gen Intern Med. 1997;12(4):209–215. doi: 10.1046/j.1525-1497.1997.012004209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 30.Hagman M, Wilhelmsen L, Pennert K, Wedel H. Factors of importance for prognosis in men with angina pectoris derived from a random population sample. The multifactor primary prevention trial, Gothenberg Sweden. Am J Cardiol. 1988;61:530–535. doi: 10.1016/0002-9149(88)90759-x. [DOI] [PubMed] [Google Scholar]
- 31.Leng GC, Price JF, Jepson RG. Lipid-lowering for lower limb atherosclerosis. Cochrane Database Syst Rev. 2000:CD000123. doi: 10.1002/14651858.CD000123. [DOI] [PubMed] [Google Scholar]
- 32.Mehler PS, Coll JR, Estacio R, Esler A, Schrier RW, Hiatt WR. Intensive blood pressure control reduces risk of cardiovascular events in patents with peripheral artery disease and type 2 diabetes. Circulation. 2003;107:753–756. doi: 10.1161/01.cir.0000049640.46039.52. [DOI] [PubMed] [Google Scholar]
- 33.Ostergren J, Sleight P, Dagenais G, Danisa K, Bosch J, Davies R, et al. Impact of rampiril in patients with evidence of clinical or subclinical peripheral arterial disease. Eur Heart J. 2004;25:17–24. doi: 10.1016/j.ehj.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 34.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, rampiril on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen TR, Kjekshus J, Pyorala K, Olsson AG, Cook TJ, Musliner TA, et al. Effect of Simvastatin on ischemic signs and symptoms in the Scandinavian simvastatin survival study (4S) Am J Cardiol. 1998;81:333–335. doi: 10.1016/s0002-9149(97)00904-1. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239–1312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Vogt MT, Cauley JA, Kuller LH, Hulley SB. Prevalence and correlates of lower extremity arterial disease in elderly women. Am J Epidemiol. 1993;137:559–568. doi: 10.1093/oxfordjournals.aje.a116709. [DOI] [PubMed] [Google Scholar]
- 38.Pepine CJ. Clinical aspects of silent myocardial ischemia in patients with angina and other forms of coronary heart disease. Am J Med. 1986;80:24–34. doi: 10.1016/0002-9343(86)90449-3. [DOI] [PubMed] [Google Scholar]
- 39.Deanfield JE, Maseri A, Selwyn AP, Ribiero P, Chierchia S, Krickler S, et al. Myocardial ischemia during daily life in patients with stable angina: its relation to symptoms and heart rate changes. Lancet. 1983;2:753–758. doi: 10.1016/s0140-6736(83)92295-x. [DOI] [PubMed] [Google Scholar]
- 40.Cecchi AC, Dovellini EV, Marchi F, Pucci P, Santoro GM, Fazzini PF. Silent myocardial ischemia during ambulatory electrocardiographic monitoring in patients with effort angina. J Am Coll Cardiol. 1983;1:934–939. doi: 10.1016/s0735-1097(83)80213-7. [DOI] [PubMed] [Google Scholar]
- 41.Murabito JM, Evans JC, Larson MG, Nieto K, Levy D, Wilson PWF. The ankle-brachial index in the elderly and risk of stroke, coronary disease, and death—the Framingham study. Archives of Internal Medicine. 2003;163:1939–1943. doi: 10.1001/archinte.163.16.1939. [DOI] [PubMed] [Google Scholar]