Abstract
In the past few hundred years, science has exerted an enormous influence on the way the world appears to human observers. Despite phenomenal accomplishments of science, science nowadays faces numerous challenges that threaten its continued success. As scientific inventions become embedded within human societies, the challenges are further multiplied. In this critical review, some of the critical challenges for the field of modern chemistry are discussed, including: (a) interlinking theoretical knowledge and experimental approaches; (b) implementing the principles of sustainability at the roots of the chemical design; (c) defining science from a philosophical perspective that acknowledges both pragmatic and realistic aspects thereof; (d) instigating interdisciplinary research; (e) learning to recognize and appreciate the aesthetic aspects of scientific knowledge and methodology, and promote truly inspiring education in chemistry. In the conclusion, I recapitulate that the evolution of human knowledge inherently depends upon our ability to adopt creative problem-solving attitudes, and that challenges will always be present within the scope of scientific interests.
Keywords: Philosophy of Chemistry, Pragmatism, Reductionism, Sustainability, Systems science
1 Introduction: Why Think of Challenges?
Significant technological advances in current technologies and the potential for more revolutionary impacts in areas such as environment, electronics and medicine have fuelled major research and development efforts in chemistry, which can be considered the forefront of the molecular sciences (Future of Chemistry 2009). Or, as proclaimed by George M. Whitesides, “We are at a wonderful time for chemistry. It is, I believe, in the position of physics in the 1910s, just before quantum mechanics made the world impossibly strange, or biology in the 1950s, just before the double helix obliterated the old biology” (Whitesides 2007). Advances in analytical techniques along with tremendous strides in synthesis allow us to examine complex chemical, physical and biological phenomena, unimagined just a few decades ago, at the molecular, atomic, and even subatomic levels.
These unprecedented advances have opened up new frontiers not only in chemistry but also at the interface of chemistry and every other scientific and engineering discipline, implying the role of chemistry in understanding the material universe at practically all scales. However, as scientific and chemical research becomes more complex and interdisciplinary, it becomes ever more challenging to communicate and explain the state of the art of scientific endeavors. One of the aims of this paper is to place the latter in a bigger perspective while touching the questions and enigmas that conceal the future prospects of this field.
Sustainability of every area of human creativity depends on its potential to incessantly evolve. And vice versa: the evolution of any natural system is conditioned by its power to sustain and incessantly revitalize itself. The same reciprocity typifies the modern science. To further evolve, it has to lean onto its empirical approach, but to retain its power and successfulness in the society it has to find the way to continue to evolve.
In the past few hundred years, science has exerted an enormous influence on the way the world appears to human observers. Despite phenomenal accomplishments of science, science nowadays faces numerous challenges that threaten its continuation success. These, in addition, become multiplied in parallel with the process of incorporating scientific discoveries and ideas within the human societies. Most scientific products are thus tools that resemble double-edged swords. Depending on the users’ goals, they can be applied either for the benefit or for the detriment of humankind. This could be realized as a natural implication of the fact that each progress arises from problem-solving endeavors. Consequently, in the wake of the pathways of progress unforeseen problems are left, which will, on the other hand, present sources of novel discoveries. Whenever problems and deficiencies spread through the society, the conditions arise for both cooperative and conflicting ways of acting. Languages, sciences, arts, philosophies and religions can all be seen, in fact, as sprung out from the awareness of demerits and obscurities in human interactions with each other and with Nature (Winograd and Flores 1987).
In this work, some of the major challenges for the scientific field of physical chemistry will be discoursed. My aim has been not to redefine science and chemistry by discussing these challenges, but to open paths that are to be followed and explored in future, because they conceal the keys to the progress of these fields. The approach I have adopted is broad and systemic in nature, although I have tried to support it with as many specialist insights as possible. Physical chemistry is used as a representative example for a hard science, and conclusions reached from this point on may be valid for many, if not all, sciences and humanities. As we shall see, it is challenges that present the doors for the future progress of this and other fields of science and the fruitful branching of the tree of human knowledge.
2 Interlinking the Theoretical Knowledge and Experimental Approaches
Appropriate correlations between experimental efforts and conceptual models are of crucial importance for the evolution of hard sciences, including chemistry. However, it seems as if chemistry nowadays witnesses their serious disconnectedness (Uskokovíc 2009a). This is apparent from the fact that more and more chemists do not use any rational concepts in their quests for novel materials. The essence of their approach is reduced down to simple trial-and-error. Many of them are thus openly invited to use intuition as much as logic and know-how. In that sense, chemistry is at least partially returning to its alchemical beginnings.
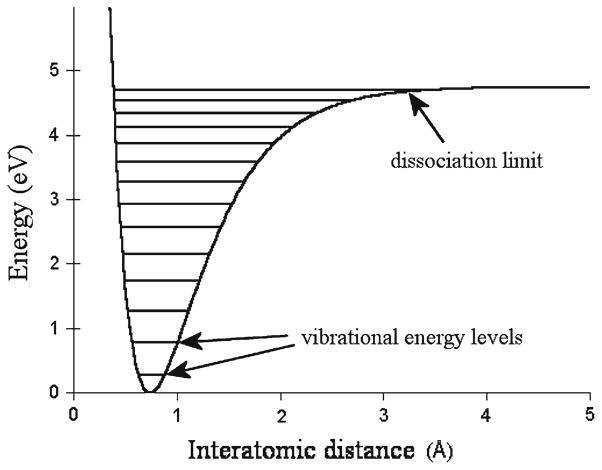
To illustrate this disconnectedness, I will refer to the example of DLVO theory, derived by Derjaguin and Landau, and Verwey and Overbeek, separately. It has been used as the basic framework for predicting and explaining the behavior of colloids since its development in the 1940s (Derjaguin and Landau 1941; Verwey et al. 1948). As a reminder, colloids are multi-phase systems in which one or more phases are dispersed in a continuous phase of different composition or state; milk, blood, ink, fog, cheese and pearls are some of the examples. It is rarely acknowledged that the development of this theory was largely supported by industrial investments driven by expectations that its concepts might improve the control of the behavior of colloids (Blume and Zemb 2002). Being aware of the benefits of feedback between applied research in the industrial domain and the basic one that has traditionally belonged to universities may ameliorate the current situation where much of industry-driven research results never reach the knowledge tree but stay within confined knowledge pockets inside companies, whereas basic discoveries, especially in developing countries, struggle to reach research spin-offs. Therefore, at the very roots of science we find the juxtaposition of practical and fundamental interests. However, from the days when this theory was born up to this date, it has never been refined to the extent that would enable applicable quantitative comparisons with experiments. Relying on its conceptual model implies the use of almost purely qualitative relationships.
The basis of DLVO theory rests on the assumption that the colloidal stability is maintained through the balance between repulsive, electrostatic double-layer forces and attractive, van der Waals forces (Fig. 1). In assuming so, the theory deploys the Gouy-Chapman-Stern concept of the double-layer of charged species that surrounds each of the dispersed particles (Ostwald 2009). Since the colloid stability depends on the properties of interfaces that separate dispersed and continuous phases (Wilde 2000), most of the effects applied in control of the colloidal behavior rest on a few simple principles. These involve modifications of the particle-medium interface by varying pH, ionic strength, particle size and chemical agents that promote steric interactions. For example, variations in pH lead to changes in the identity and density of charged species attracted around the dispersed particles. An increase in ionic strength can be used to screen Coulomb repulsion and compact the double layer of charges around each of the particles. If sufficient enough, high ionic strengths of the dispersion medium can induce phase segregation and thus produce the so-called “salting out” effect, recently invoked in attempts to elucidate the physicochemical causes of cardiovascular diseases (Uskokovíc and Matijevíc 2007; Riddick 1968; Uskokovíc 2008a,b). Reducing the size of colloidal particles may increase the stability of the system by increasing the range of Brownian motion, which overcomes the tendency of colliding particles to associate, grow in size and eventually segregate as a separate phase. Adding stabilizers, such as high molecular weight polysaccharides, in order to increase the viscosity of the system, reduce the free particle movements and diminish the rate of slow but inevitable phase segregation is regularly used for extending the shelf life of colloidal food products. This rather modest and simple conceptual model has been used by experimentalists for more than half a century, which is on one hand a sign of the model’s strength; however, owing to the fact that finer effects that are insensitive to the statistical calculus of this theory remain untouched by it signifies its imperfect character.
Fig. 1.
Potential energy, obtained as a sum of the attractive and repulsive terms from the DLVO theory, represented as a function of the interparticle distance (left). Note that thermal energy of the system, kT, needs to be significantly smaller comparing to Vmax in order for its stability to be preserved. If secondary energy minimum, Vsec, is not sufficiently higher than kT, a weak flocculation in this secondary minimum normally occurs. This curve neatly corresponds to the concept of intermolecular potential first proposed in the given form (right) by Rudjer Boškovíc in the mid-18th century. Albeit entirely qualitative, his theory is nowadays acknowledged as the cornerstone of the modern theory of atomic forces. Both theories employ the same fundamental concept: explaining the properties of matter through the interplay between attractive and repulsive forces. Reprinted with permission from Ref. Tirrell and Katz 2005
Unlike the theoretical knowledge, experimental achievements have advanced way forward since the time of the development of DLVO theory. Magnificent material structures are being obtained in laboratories worldwide by using the principles of soft chemistry, without any serious quantitative correlations applied prior to the selection of appropriate experimental conditions. Breakthroughs in the area of fine-tuned weak chemical interactions and self-assembly phenomena neatly exemplify this (Tirrell and Katz 2005; Whitesides and Grzybowski 2002; Lindoy and Atkinson 2000; Uskokovíc and Drofenik 2005). There is an impression that the design of nanostructured and biomolecular materials nowadays extends beyond the ability of the modelling and characterization tools to provide detailed analyses thereof, despite their rapid improvements (Urban 2009; Muller 2009; van Gunsteren et al. 2006, 2008; Bowker 2009; Matthiesen et al. 2009; Vlieg et al. 2007; van Embden et al. 2009). The experimental knowledge thus seem to have left the theory in this case far behind.
Nonetheless, experimental efforts in general require a conceptual framework for the systematic summary of results and logical derivation of rules that will improve the level of predictability and increase experimental efficiency. Many examples can also support the idea that modeling can anticipate results of experimental observation (Dimiduk et al. 2006; Rueda et al. 2004). Advances in density functional theory have enabled meaningful comparisons of the computational results with experiments involving catalytic reactions at surfaces (Nørskov et al. 2009). Even short simulations (nanoseconds on average) with highly simplified models of the constituents (bead-spring models of surfactants, e.g.) have provided valuable indications in attempts to clarify certain experimental notions (Rajagopalan 2001). Simulations may provide structural information that is otherwise not accessible by means of experimental analysis, such as ultra-fine structural details within colloid aggregates. Novel self-assembled structures, their dynamics and phase transitions may be anticipated by means of simulations, whereas the acceptability of approximations in theoretical approaches (such as mean-field approximation is for example) and their underlying reliability can be systematically analyzed by means of simulation-guided experiments.
Hence, it would be silly to neglect the importance of unending quests for the ways to bridge the gap between experimental observations and their sophisticated conceptual models. For, that is what the science and philosophy are all about: reconciling the world within with the world without. Both abstract and practical aspects of any science, including chemistry, have to be developed in parallel (Marcus 2009). Neglecting either of the two at the cost of exceedingly fostering the other might seriously hinder the future progress of the field.
The example of crossed molecular beam experiments applicable in molecular spectroscopy offers an insight on how theory and experiments can advance in parallel (Johnson 2009). Namely, for many decades the theory was limited to analysis of only sufficiently small molecular entities, which were, however, too miniscule to be experimentally probed. The larger systems that could be investigated by experimental means were, on the other hand, too complicated to be subjected to theoretical scrutiny. Then, the refinement of overly general tools took place, enabling the performance of quantum-state-resolved measurements on small molecules, whereas the theoretical treatments took advantage of parallel computational processing; in an incessant feedback with each other while feeding from other fields to improve the analytic efficacy, the theory and experiments have thus evolved together. A similar combined evolution of theory and experiment can be seen today in the area of probing the atomic structure of nanoscale materials using sophisticated computational and diffraction techniques (Billinge and Levin 2007).
On the other hand, we should be aware that the balance between knowledge and intuition is immanent in each act that exhibits creativity. Depriving one’s methodology of any of the two would have devastating results for one’s creativity. Therefore, I do not believe that trial-and-error in the chemical design would be overcome any time soon nor that it would be beneficial to transform experimental approaches to computational algorithms, at least in the way the industrial instigators of the development of the ultimate theory of colloidal phenomena had in mind. It is the balance between the known and the unknown that is maintained in the mindset of a productive scientist and philosopher. Just as the crystallization process requires an interface between an ordered solid phase and an amorphous liquid, the process of “crystallization” of knowledge similarly bases itself on the interplay between the known and the unknown, between the discovered and the unrevealed, between theories and perplexities, logic and intuition, order and freedom.
3 Implementing the Principles of Sustainability at the Roots of the Chemical Design
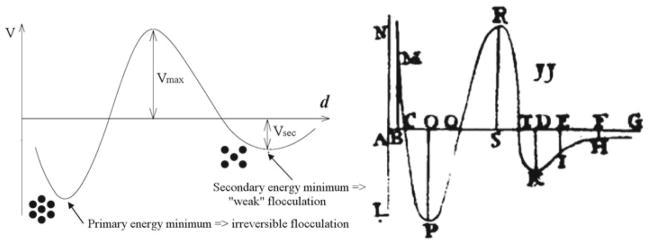
Qualities of any natural system cannot be said to end with its physical boundaries. Instead, they are determined by the interaction of the system with the physical context of its existence (Fig. 2). Energy and activity of any physical object cannot be determined by solely looking at the object itself. For example, the kinetic energy of an object in its free flight through a medium can be expressed only in terms of its relative velocity with respect to the stationary environment. Likewise, to describe any physical quality, a comparison with specific sets of standards presents a necessary precondition. Hence, instead of confining our focus to the object of our inquiry itself, we have to let our attention spread towards wide contexts of the object’s existence (Blackmore et al. 2009; Whitehead 1928). Such an enlightening shift in awareness from a tight focus on the very physical objects to their contextual interaction corresponds to a paradigmatic shift in our thinking. Just as the meaning of a line, a letter, a word or a sentence could not be evaluated until we get to grasp the larger textual wholes that they constitute, any quality that we endow the physical objects with has to be drawn with our referring to interactive frames larger than the very objects in question.
Fig. 2.
Qualities ascribed to each natural system are the product of its interaction with a given physical context. Both the physical context and the inner organization of the system are involved in the definition of its qualities. In this drawing, the lines of interaction figuring at the boundary between the system and its environment present the reflections of the system’s qualities
Let us, for example, enter the field of medicine. There, we can notice how the quality of a material applied for the restoration of damaged tissues could not be defined without a reference to the fine properties of the area of its application within the body (Lübbe et al. 1999). For example, differently sized particles are required for drug-delivery treatments of different tissues (Uskokovíc et al. 2006). Thus, disregarding the surface functionalization of the particles, extracellular maneuvers are limited to those with less than a few tens of nanometers in size (He et al. 2005), whereas micrometer-sized particles are more suitable for withstanding the flow dynamics in the circulatory system (Pankhurst et al. 2003). Furthermore, different mechanical and chemical properties of the biomaterial are employed for tackling the damages occurring at different scales. Finally, an essential feature of a biomaterial is its biocompatibility, which could not be perfectly reliably assessed without the interaction with the patient, that is, by mere in vitro studies on cell cultures. Even though it is nowadays well known that pathological states of an organism need to be explained in terms of both the effect of foreign agents and the susceptibility of the host organism, people still sit and wonder why a specific material becomes refused by one and accepted by other patients, facing a complex reflection of the subjective nature of every medical treatment and interaction with the surrounding beings and the physical world in general. Needless to say, the effective application of each biomaterial critically depends on a favorable feedback interaction between the living system and the material, during which both are most often subject to change. This observation is, of course, only an instance of a broader concept that tells us that each natural quality depends on the interaction, and that a reference to both interacting sides is required to evaluate and define any given quality.
From the most fundamental perspective of physical sciences, Heisenberg’s uncertainty principle demonstrates how an interaction with a measured system needs to take place in order for any subsequent detection of its states to occur. The nature of this interaction would, of course, be specific for each being or device, which constitutes one of the basic problems arising out of the subjectivity of experiential and natural phenomena. As a result, the way in which we pose the questions predetermines the structure of the revealed answers (Poerksen 2003). Thus, whereas based on the long tradition of objectivism we know that we partly see the world the way that it is, every now and then we should remind ourselves that the way in which we look at the world partly predetermines what we shall see Bröcker (2003). By objectivism, I mean the philosophical framework of thought in which experience is explained as essentially observer-independent and entirely defined by the nature of the physical reality. Its extreme standpoint is complementary to the one of subjectivism, which can be defined as the framework of thought in which experience is explained as thoroughly defined by the subject’s nature and attitude. And yet, the elements of subjectivity and objectivity are inextricably merged in every scientific concept. It is the subject’s biological and cognitive structure and the surrounding physical reality (which is undetectable per se and comparable to Kant’s Ding an Sich or the silent sound of one hand clapping from the famous Zen koan) that in togetherness craft the appearance of one’s experience.
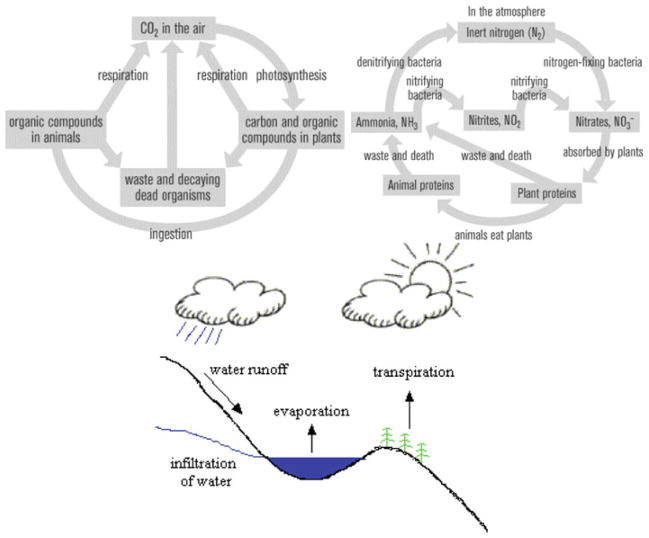
Now, many implications follow the realization that qualities of the products of our perception and judgment are not determined merely by the features of the objects studied, but also by larger existential and interpretational wholes that both the system and the observer belong to. The movement of individual water molecules in a river is not determined by their intricate structural characteristics only, but by their relationship with larger physical contexts consisting of the terrestrial and atmospheric patterns of local and global ecosystems. Likewise, physical qualities could not be defined non-contextually, that is, without taking into account a wide array of environmental factors that surround the entities attributed with specific qualities. One of the crucial consequences derivable from this observation is that ecological principles of sustainability need to become an integral part of the chemical practice (Fig. 3). In that sense, instead of the “end-of-pipe” solutions that correspond merely to detection and monitoring of the flow of toxic chemicals during their ecological journeys, the focus of ecological research should be preventively switched to qualitatively modifying the existing chemical methods in the direction of minimizing the waste production and instigating the environmental sustainability (Uskokovíc 2008c). Ecological principles based on reusing, recycling and minimizing waste would thus have to be incorporated at the very roots of the chemical industry, which belong to the designing, synthesizing and processing stages.
Fig. 3.
All atomic constituents of life—such as carbon, nitrogen and water depicted hereby—continually circle between biological, mineral, soil and atmospheric bodies of the planet. What is released as a waste by one species becomes absorbed as a nutrient by others, and industrial networks should certainly pursue the same zero-waste ideal in their future development. This is why it has been said that cities, and not rain forests, should become the mines for the “virgin” materials in the future (Gardner and Sampat 1998). However, whenever the waste is inherently non-recyclable, such as nuclear waste, or dissipates during usage with environmentally harmful consequences, as in the case of detergents, paints, chlorofluorocarbons in refrigerators and the most of industrially employed heavy metals, green alternatives at the stage of chemical synthesis should be looked for
Chemical industries have been frequently invoked as the prototypes of production sectors disconnected from the ecological principles and sustainability requirements. The fact that the contemporary chemical industries are typically gigantic businesses that rely on heavy capital and are, as such, less flexible to introduction of technological innovations, explains their inertness in attempts to adopt green methods of synthesis (Jenck et al. 2004). It is pleasing to hear that many companies and laboratories are nowadays assigning duties to sustainability analysts and specialists, but on the other hand, we should be reminded that in an ideal (utopian?) world there would be no need for experts to adjust business and research strategies to the requirements of ecological sustainability. Instead, the latter would be inherently interwoven into each aspect of the given organizations.
Science, religion and environmental activism are usually seen as disconnected as melting icebergs and household drains are in the mind of an ordinary inhabitant of the Earth. However, not a lot of imagination and knowledge is required for one to establish the links between these seemingly unrelated things. For example, accepting the fact that all knowledge is of metaphoric nature (see Sect. 4) may lead one to observe neat correlations between scientific and theological depictions (Uskokovíc 2010a). And as Albert Einstein claimed, “Physical concepts are free creations of the human mind, and are not, however it may seem, uniquely determined by the external world... The object of all science, whether natural science or psychology, is to coordinate our experiences and to bring them into a logical system”. If both sciences and theologies were defined as partly humanly derived concepts about “the way the world works”, both could be seen as pragmatic systems that help us mutually coordinate our experiences so as to enlighten each other (James 1907). Pragmatically linking the both at their roots would naturally lead to conciliation of numerous disparities that exist between them. Recognizing that aspirations to coordinate experiences with others through responsibility, love and care stand at the root of scientific, theological and common communications sets a profound base for a multidisciplinary inquiry about our place in the Universe (Uskokovíc 2009b). Today’s environmental activism rose up from the scientific achievements in understanding the ecological networks of life. By realizing that biology in particular and science in general stand at the roots of the modern environmentalism, the hostilities oriented towards the former could be naturally erased. It is also frequently forgotten that what used to be a creative and original movement of an era inevitably becomes an obsolete trend as the time passes by. The ideals of reductionism and positivism were fruitful and ahead of the times when they were first articulated, although from today’s perspective they require fundamental revisions (Laszlo 1996). Individual branches of the tree of human knowledge are ramified in the course of its evolution, but cultivating the sense of unity, reinforced by that fact that all these branches sap the underground waters from a common stem, presents an equally important challenge. Specialization and generalization, differentiation and integration, empiricism and systems science are to be fostered in parallel. Only through such a niche would the sense of the place of chemistry in a larger web of life not be lost, and the ecological principles would naturally get incorporated at the very foundations of the chemical design.
4 Defining Science from the Philosophical Perspective that Acknowledges both Pragmatic and Realistic Aspects Thereof
Let us repeat once and for all the following truism, which ought to be one of the first and the most fundamental assumptions pointed out to young scholars. Namely, there are no truths, facts or relationships about the world that are independent on the subjective nature of the observer. Hence, atoms and molecules and the entire imagery of scientific descriptions are in no way truly existing entities and events, but only explanatory models applied for the purpose of mutual coordination of human experiences (Thyssen 2003).
Map is not the territory, and name is not the thing named, as Gregory Bateson loved to say Bateson (1979a). And yet, “the thing” is, of course, not meant to be grasped in the objectivistic sense of the word, but as a metaphor of an experiential appearance. Represented by words, the latter are thus neither solely objectively existing entities, as positivistic scientific world-views hold, nor sole human inventions, as constructivist philosophies would state. They emerge as higher-order forms through the intersection of these, realistic and constructivist experiential aspects. As the subject is partly involved in defining the qualities of the perceived physical objects in accordance with his biological and cognitive predispositions and states, all seemingly objective representations of the reality should be considered only as metaphors. Human assumptions about the nature of the reality are thus reflected in the nature of scientific models as much as in the features of our experiential realities (Wittgenstein 1918). One of the major tasks of the philosophies of natural sciences is to illuminate the fact that as observers in scientific measurement, we are always partly collecting the reflections of our own experimental settings and theoretical questions that we have born in mind (Heisenberg 1979).
Now, as far as the influence of the measuring device is concerned, there is the example of atomic force microscopy (AFM), one of the most powerful techniques for the visualization of material structures on the atomic scale (Binnig et al. 1986). The device works by sliding an ultrasensitive tip across the sample surface and converting the variations in the contact pressure caused by the surface topography into an electrical signal. First of all, microscopic analyses provide images that arise as an intersection of properties and settings of the given apparatus and properties of the measured system. As such, the resulting micrographs do not present true reflections of the morphological properties of the system, but are the product of an interactive coalescence of the properties of the observer (altogether with its measuring apparatus) and the features of the observed. AFM likewise provides images that are actually convolutions of the tip shape and the surface morphology. Distortions of the tip shape are, therefore, directly reflected in the distorted appearance of the observed particles. However, it is an inescapable fact that even under ideal measurement conditions rounded tips would naturally increase sphericity of the analyzed entities, whereas the sharp ones would promote similarly sharp morphological features of the observed particles (Fig. 4).
Fig. 4.
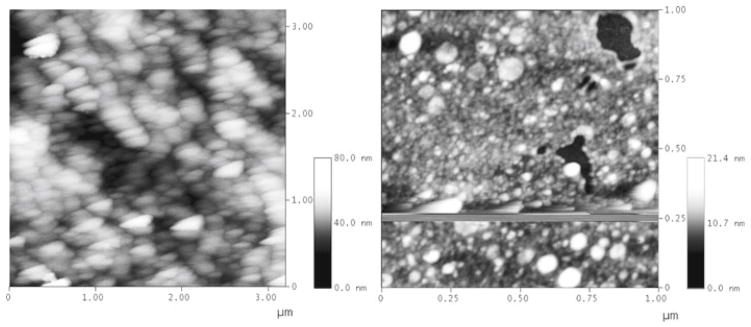
AFM micrographs of amelogenin particles appearing as triangularly shaped (left) or spherical (right), depending on the AFM tip shape. Morphology of the tip shape apparently becomes reflected in the observed morphology of amelogenin particles. Notice how below the dolphin-shaped vacuity on the right image the retraced tip starts producing similar artifacts as on the left image and eventually loses the contact with the surface. After a correction of the pressing force, the image restores its “faithful” representation of the surface morphology
In view of the effect that our abstract concepts have on the experimental settings conceived and conclusions derived thereupon, we can always refer to the fact that human expectations, intentions and inner questions present the essential drives of human acting and creativity. As Werner Heisenberg noticed, “In natural sciences, the object of research is not any more nature as such, but nature exposed to human questioning, and in that sense, man herein faces himself” (Heisenberg 1959). Thus, similar as the results of any logical inference implicitly comprise the presupposed tautologies, each scientific hypothesis or interpretation is built upon implicit beliefs or any other form of premised knowledge. Just as Albert Einstein said, “There is no inductive method which could lead to the fundamental concepts of physics. Failure to understand this fact constituted the basic philosophical error of so many investigators of the nineteenth century”. In other words, the basic propositions of scientific method cannot be experimentally derived nor proved. They are preconceived, and experimental insights can only rest on them more or less stable, but it can never be shown that these premises are true and the perfect ones. In other words, pillars of faith are verily the foundations of science.
This observation is neatly related to the nature of human perception. And as far as the latter is concerned, the world as we perceive it is also partly the result of our own constructions and partly the result of the way that it is. But these two aspects, the one of an active construction and the one of a passive detection, could not be separated, as they are being inextricably intertwined within each perceptive act.
Human eye as an organ can be invoked to evidence the entwinement of objective and subjective aspects in every perceptive act. First of all, all sensors, artificial or natural, can detect only differences (Bateson 1972). Uniform flow of any stimuli will present an imperceptible information unless it becomes modulated by either the action of the sensory organ itself or some environmental effects. For that reason, human eye possesses a set of fine strategies that provide it with the ability to detect even uniform signals from its surrounding. Scanning activity presents one, whereby saccadic palpitation presents another such visual tool that eye continuously applies in order to modulate the monotonous signals that come from the environment and render them perceptible. A few other strategies, including the redundancy of sensory data and their compression that begins at the very level of retina, to be further performed at the corresponding brain centers that internally govern the visual activity, are also included in this complex set of visual responses that help in classifying, modifying and redrawing the raw impulses of the being’s surrounding into a personal visual landscape.
Also, the amount of information that an eye can perceive at any moment is so enormous that it would induce a freezing confusion in the brain if it came to be detected in its entirety. As a result, habitual recognition and a sketchy construction of visual objects from memory are regularly carried out in advance to and aside from their perception in detail every time we notice them. Conclusively, to distinguish subjectively constructed and objectively detected aspects of the world that our mind brings forth using all the sensory data presents an impossible task, as these two are inextricably entwined.
Thus, I love to say that the world as we see it is not a single and universal world, identical for everyone, but a special and unique world of our own experience. On the other hand, neither do we live in purely solipsistic worlds, such as those depicted by desolate planet-dwellers in the story of the Little Prince (de Saint-Exupery 1946). There is a dose of realism that opens the door for a Little Prince’s journey—from planet to planet to see how the world looks from the eyes of another. It is this dynamic tension produced by the intersection and incompatibility between subjective and objective aspects of human experience that provides the drives for the social evolution of knowledge and being.
Thus, chemistry in particular and science in general could be defined from the viewpoint of an intersection of its pragmatic and realistic definitions. Whereas the former would define them as tools applied in the mutual coordination of human experiences, the latter would think of them as methods for revealing the real nature of the constituents of the physical world and their interactions. But we may know that both definitions are equally appropriate, and only when taken as complementary they may provide us with a thorough picture of what science really is.
So my message for the scholars and students of any science is the following: do not foolishly observe how atoms and molecules are entities that objectively comprise experiential appearances. Instead, mix this preconception with the idea that they are humanly derived metaphors used in the mutual coordination of experiences, and you will get the right blend of a definition.
5 Finding a Balance Between Reductionist and Holistic Methodologies
The process of human thinking is based on alternating insights during which differences between entities are discerned and those during which identicalness between entities is established. Likewise, for human knowledge to evolve in the social context, the neo-Hegelian dialectical perspective invokes similar alternate stages of diversifications and unifications. The same balance between reductionist differing and holistic uniting may be discerned as the trait of every evolving pattern in life. As far as the physical sciences are considered, building knowledge not only on the reduction to basic physical principles, but on the implementation of “inexact” qualities related to collective behavior may be said to present the way forward.
As for the need to reduce the directly observable physical qualities to more elementary and redundant ones is considered, it is apparent that slicing the world into species with fixed and contextually independent properties is required. But as far as the need for the holistic apprehension of physical qualities is concerned, we should never forget that each one of these individualized species presents an inextricable part of the whole. The wave function in the quantum mechanical conceptual apparatus (Adler and Bassi 2009), for example, implicitly refers to the states of both the system and its environment. The superposed states of a described system are, therefore, always entangled with the states of the environment which spreads infinitely in an ultimate descriptive framework (Rakovíc 2009). However, to demonstrate the holistic nature of physical qualities, we do not need to refer to quantum entanglement or biological dissipation effects, but to a simple and yet obvious aforementioned contextual conditioning of properties of natural systems. To realize that the kinetic energy of an electron can be described only by referring to its velocity with respect to a given environment or that units of any physical qualities need to be compared to specific standards requires nothing but common sense reasoning.
On one hand, chemistry has based its success on the assumptions of reductionism. With the division of the world into chemical elements, it provided an ultimate example of reductionism in human reasoning. In the mid-19th century, two main streams in the science of chemistry existed: Dalton-Davy hypothesis based on the atomistic assumption and Benjamin Brodie’s thesis according to which instead of atoms as chemical elements, there were only sequences of weight-changing operations carried out upon the spatial units. In fact, at a meeting of the Royal Chemical Society in the 1860s, the proposition that the atomic structure of matter is invalid was passed almost unanimously, with only a single voice against (Harre 2003). Thereupon, it is normal to expect that the trend of qualitative reduction would continue further down from the basic chemical notions to more “fundamental” entities and relationships. However, the problems of encountering enormous complexities that entail the transitions from quantum physical settings to the same systems described by the means of chemical parameters have so far prevented fully reliable reductions in this sense. Therefore, with the usage of numerous qualities related to collective behavior, chemistry has naturally balanced reductionism and holism. Simply saying, it has never been afraid to look at the forest instead of the individual trees, which is in many respects unlike physics that has traditionally based its explanations of the “forest” on properties of the individual trees. Analytical reasoning has thus always been typified as more physical, whereas the synthetic one in both practical and abstract terms has been often linked with the chemical approach.
The reason for this is that many chemical effects could not be explained by referring to individual atomic interactions; instead, they need to be based on cooperative effects that involve ensembles of species (Earley 2003). For example, peculiar properties of water resulting from cooperative hydrogen bonding effects, including ice floating on water and other density effects, can be described only in the frame of molecular assemblies of water. Then, the order of biological systems and other far-from-equilibrium, open chemical systems can be understood only in terms of the dynamic interaction of numerous components from which novel qualities emerge, as proposed, for example, in Prigogine’s theory of dissipative structures (Kondepudi and Prigogine 1998) and other complexity theories (Capra 2002; Gribbin 2004; Phelan 2001; Medd 2001; Richardson and Cilliers 2001). Contemporary computational protein structural analyses similarly require a compromise between local and global interactions that cooperatively determine the folding mechanisms (Kortemme and Baker 2004). For example, it is known that secondary structural elements of any given protein are rarely stable in isolation and that different amino acid sequences can fold into identical tertiary structures (Dahiyat and Mayo 1997), which all speaks in favor of the essential role of non-local interactions. This also explains why α–helices and β-hairpins with comparatively lower contact order in respect to other secondary structural motifs can be most easily determined by means of a computational structural analysis. Typically, searching for local sequence preferences from small fragments and stochastic simulations that scan the non-local ordering tendencies (such as Monte Carlo or genetic algorithms) are used in parallel to ensure the validity of the modeling approach.
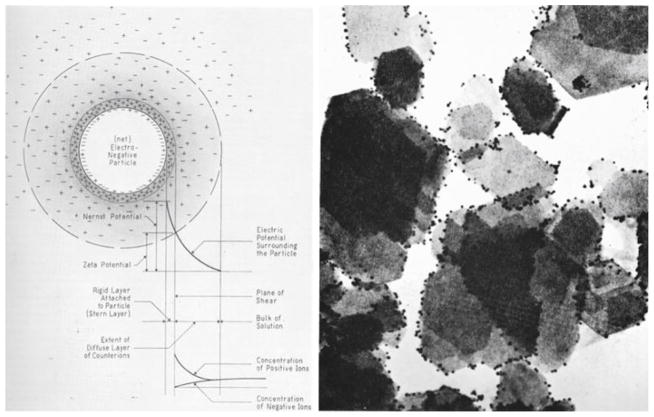
Individual and collective properties thus have to be mutually referred to in order to form the basis for reliable explanations and demystify obscure effects. To illustrate this point, we may return to the basics of DLVO theory and the corresponding pervasive use of surface charge effects. The latter are usually described through the concept of zeta-potential, being the quantity that has been used for more than a century in predicting and explaining interactions between colloidal particles (Fig. 5). However, there are many unexplained observations surrounding the classical visualization and understanding of colloid phenomena through the concept of zeta-potential, including the long-range attraction between hydrophobic surfaces (measured at separations extending over a few hundreds of nanometers) and the effect of attraction of likewise charged particles (Grier 2000; Crocker 1998). Such effects are actually quite common and cannot be described without referring to cooperative effects within comparatively large ensembles of particles. The non-intuitive attraction between likewise charged entities could not be derived from depicting the electrostatic interactions within the system composed of a few dozens of particles. But on the other hand, if only collective behavior is considered without paying attention to the characteristics of the individual particles, other unexplainable situations may result. Such is the case of electronegative gold particles adsorbed on similarly electronegative crystals. Here, however, although the latter particles were negatively charged as a whole, their edges onto which the gold particles attached turned out to be positively charged (Fig. 5). A non-intuitive observation is in this case resolved by paying more attention to morphological forms adopted by the individual entities.
Fig. 5.
The concept of zeta-potential (left) presents a standard explanatory tool in colloid chemistry, though occasional attraction between similarly charged species stands as en enigmatic phenomenon from its standpoint. Electron micrograph showing negatively charged nanosized gold particles adsorbed on electronegative plate-shaped kaolin crystals (right). Although kaolin platelets are negatively charged as a whole, their edges are electropositive and as such attract the gold particles onto them. Reprinted with permission from Ref. Uskokovíc (2009a)
6 Chemistry Giving up the Way to Physical Chemistry
The attempts to reduce chemical explanations to those of physics were only semi-successful. Most importantly, they resulted in the field of physical chemistry being born. Now, for both useful and amusing reasons, I enjoy seeing physical chemistry as a marriage of two poles: physics and chemistry. Physics is traditionally envisaged as a firm, exact and the most fundamental of all natural sciences. The laws and classical imagery of physics are based on well-defined and measurable qualities. It is, however, true that as we descend deeper, towards the quantum physical features of matter and energy, we arrive at uncertainties, probabilistic effects and the fact that the observer partly defines the states of the observed system. Wondering over the latter effect, we find ourselves in metaphysical waters. Thus we get to see another essential feature of physics: it apparently rests on firm philosophical grounds.
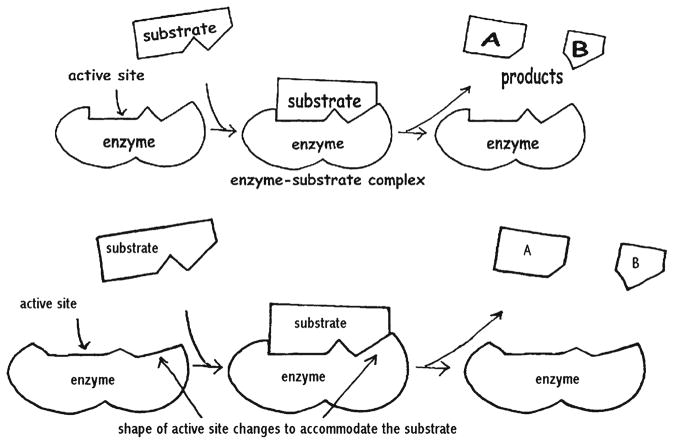
This is all so contrary to the traditional outlook of chemistry. Philosophy and chemistry have rarely been interested in each other, and the former has always been more inclined to physical situations when it came to the study of scientific questions. In fact, most weddings of chemistry and philosophy have been connected to the problem of reductionism of chemical principles to the physical ones, and providing an answer to the question whether the kinetic equations of chemistry are the result of adding ignorance to the physical description or there could not be the other way. One of the reasons is that the concepts of chemistry rely on much less quantifiable notions, including chemical potential, reactivity, surface tension, hydrophobicity and other qualities that implicitly refer to affinities, relative stabilities and tendencies, normally typified by numerous exceptions. Some critics assert that when physicists want to give a thorough explanation, they start off by providing a Hamiltonian operator of the system, whereas chemists do the same by drawing a picture. Whereas physics relies more on abstract mathematical frameworks in its explanations, chemistry is more inclined to use drawings, schemes and sometimes almost “narratives” in place of the interpretations of the analyzed phenomena. Nonetheless, it is essential to remark that the whole science, without the exception of physics and chemistry, is pervaded with metaphorical descriptions. Atomic orbitals, harmonic and anharmonic oscillators, ball-and-stick molecular models, lock-and-key mechanisms of enzymatic reactions (Fig. 6), and the representation of kinetic phenomena by the means of an undefined chemical reaction surface, present some of the models routinely used to imagine the described atomic structures and events. Chemical formulas also present only metaphors of the molecular structures of the depicted species. That these depictions can be misleading is well illustrated by the example of water (Mattingly 2003). Namely, for a long time it had been considered that the reason behind the exceptional solvency of water exists in its V -shaped structure that supposedly allowed water to approach, hook up with, and pry other species apart from each other. With the advent of quantum mechanics, however, the very notion of chemical structure in terms of a rigid form comparable to macroscopic objects was called into question. As atoms could not be said to occupy precise locations in space, the concept of geometry based on exact boundaries collapsed. Whereas relative momenta in atomic and molecular species may be preserved, their “shapes” could not be considered as real and palpable as they were once thought to be.
Fig. 6.
According to the key-and-lock metaphor of the host-guest catalytic interactions in biochemistry (scheme above), the molecular recognition effect occurs when the shape of the substrate fits the one of the enzyme. A less oversimplified model (scheme below) takes into account the conformational adjusting of the enzyme to facilitate this interaction. In some cases the substrate molecules also modify their tertiary structure as they enter the active site of catalysis, indicating that mutual changes of the interacting entities condition most, if not all, chemical modifications in Nature. It is, however, required for any more realistic depiction to refer to subtle conformational changes and physicochemical forces taking place at the atomic size scale. Note also that despite more than half a century of detailed investigations of protein structure and function, there are many open questions and a generalized theory describing the precise physical origins of enzymatic catalysis is still lacking (Gärtner 2009; Martí et al. 2004, 2008)
Objects of chemical studies normally belong to the size range between macroscopic and atomic systems. Those systems therefore resist both the averaging of quantum probabilistic effects and the application of the laws of the classical physics. Usually, these are the areas where interfacial and quantum effects meet each other and often produce peculiar effects (Uskokovíc 2008d). An important difficulty that chemistry faces in attempts to describe the behavior of systems like these is an incessant need to balance between the languages of quantum mechanics and classical physics. This explains why chemistry has ever since been permeated with uncertain, inconclusive and inexact notions, which as a result led to trial-and-error approaches in the synthetic design with all the wonderful materials that we may find on the face of the Earth today (Polanyi 1936). With the soaring complexity of systems subjected to scientific studies, the need to employ more general concepts, with the corresponding shift from strict quantitative relationships to less precise, qualitative ones, naturally arises in parallel. This explains why chemistry in its explanatory frameworks lies much closer to modern systemic analyses than physics.
Another crucial aspect of chemistry is that, according to its original definition, it presents the study of the transformation of matter. Nowadays, however, the dichotomy between physics and chemistry has taken another dimension, according to which the former is supposed to be primarily related to abstract, theoretical and computational studies, whereas the latter, although once considered a “discovery science” per excellence in terms of its research subjects that aimed at answering the fundamental questions about “how the world works” (Ball 2006a), turns out to be mainly associated with practical aspects of the science of atoms and molecules, including the development of synthetic and processing methods. In fact, whereas physical studies may be said to follow the tradition of the ancient Greek approach to the study of natural world with its engagement in experiments only in rare situations when confirmation of the validity of their abstract concepts was sought, chemical studies frequently start with loosely conceived experiments, reminiscent of light playing with Nature, which only subsequently awakens scientific curiosity and opens the door to a real research adventure. Not only were penicillin, the first electrically conducting polymer, polytetrafluoroethylene (later marketed as Teflon), hexacyanoferrate (a.k.a. Prussian Blue), the first synthetic aniline dye, sildenafil citrate (a.k.a. Viagra, which had been designed to be a hypertension-relieving drug), the method for a large-scale manufacture of blue indigo, the concept of molecular chirality and many other original ideas and inventions reached through accidental, serendipitous discoveries (Ball 2006b; Roberts 1989), but every quest for new materials inevitably comprises a dose of trial-and-error inherent to it. The corresponding attributes of changeability, flexibility, playfulness and practicality attached to the science of chemistry, and thoughtfulness, rigidity and philosophical depth emblematic of the science of physics contribute to seeing the two as reflection of the archetypical Yin-Yang opposites. In their complementary relationship, they occasionally remind me of the two main characters in the beautiful Gregory Bateson’s allegory about science and aesthetics: a brutal railroad surveyor and a ginger lady who dreams by the disused railroad tracks (Bateson 1978).
7 The Importance of Interdisciplinary Research
Complexities of the natural order at any given scale prevent the reduction of scientific qualities applicable in one complexity range to those used in others domains (Fig. 7). This is why many natural and social sciences exist, without much prospect in reducing everything down to a single “theory of everything”. Specific qualities irreducible to the qualities of its components emerge with each new level of organization of natural systems. But on the other hand, this holistic perspective does not mean that all the many fields of science are not inextricably linked. For example, if we start analyzing the bases of chemistry, we may realize that they lie in the domain of physical phenomena. If we start searching for the foundations of physics, we may come to the philosophical questions outlined, e.g., by Copenhagen interpretation of the quantum theory. Thus, we would find ourselves in the range of a philosophical discourse. But if we continue our quest for the most stable grounds to base our epistemologies on, we may find out that our reasoning is largely predetermined by our biological nature, as the theses of autopoiesis (Maturana and Varela 1987; Romesin 2002; Maturana and Poerksen 2004) and constructivism (von Glasersfeld 1995; Kordeš 2004; von Foerster and Poerksen 2002; Poerksen 2003; Waters 1999; Peschl 2001) tell us. This may clarify what Alfred North Whitehead had in mind when he stated that “physics has to be explained in terms of a general theory of the organism” (Whitehead 1925). But if we embark on the voyage of analysis of biological phenomena, we may quickly discover that they have to be described at the level of molecular biological phenomena, which can only be done by using the language of chemistry. Thus, we would make a close circle, realizing that all scientific disciplines are equally important as they all provide stable grounds to each other.
Fig. 7.

Versatile concepts used to explain the behavior of water at different scales (top). Notice how changes in the size of water aggregates instigate different scientific fields to get involved in predicting its physical transformations. Other physical contexts, such as biological, nutritional or agricultural, are tied with investigating the role and behavior of water from additional specific perspectives. The presented hierarchy of water models (bottom) in the theoretical analysis of physicochemical phenomena signifies how computational costs increase in parallel with the increase in the modeling resolution
This explains why there is an increasing emphasis placed on interdisciplinary studies of the areas at which the demarcated fields of scientific inquiry meet. Some of the most exciting scientific research indeed takes place exactly along these boundaries. But then again, we have to look after the balance between this unifying crisscrossing of boundaries and maintaining the diversity of specific scientific disciplines. For example, the concept of information is grasped differently in different scientific fields, but neither reducing this variety of comprehensions to a single universal definition nor fostering a disciplinary disconnected evolution of this concept would present an appropriate choice. Also, if we ask an economist, a physicist and a chemist why some bottles are green (Re 2003), each one of them would give us a special answer, and each one of them would be relevant under a certain context. The economist might say that it is because they are the cheapest to make, the physicist might refer to light absorbance and scattering effects, whereas the chemist would recollect the fact that green color comes from ferric ions in the glass. Instead of reducing the knowledge of all these individual professions to a single level, attempts should be made to conjoin them within fruitful complementary combinations in which everyone’s knowledge would be seen as essential.
I am convinced that the future will bring about unforeseen interdisciplinary crossings of ideas and scientific approaches. Until now, fruitful interdisciplinary studies have been limited to relatively close fields only. For example, the discovery of DNA led to the merging of chemistry and biology within the fields of biochemistry and molecular biology. The invention of quantum calculus gave rise to some of the most fruitful entwinements of physics and philosophy ever witnessed. It was a revolution for science in general when entropy, once defined through the concept of a heat engine, was described as proportional to the number of ways by which a given state of the system could be reached, and applied in a variety of fields, including cybernetics, bioinformatics and other life sciences. And such use of a new language to depict phenomena that have been described in another language should not be prevented and disvalued at any cost, particularly as we know that each scientific hypothesis does not represent a truthful reflection of the system in question, but only one of the endless number of ways to draw a convenient model that “works” in the context of coordination of human experiences. The famous Thomas Kuhn’s book on paradigm shifts in science (Kuhn 1969) awoke interests for the intersection of science and sociology. The current requirements to adopt ecological and sustainability principles in each aspect of human creativity gives rise to many “green” fields, such as green chemistry, green economy and the application of complex mathematical techniques in ecological modeling. Constructivist theories had first led to the intersection of mathematics, education and cognitive science. Later, they highlighted the idealistic observation that beliefs and preconceptions are involved in the construction of scientific representations of the physical reality and opened the space for the encounter of physics, psychology and physiology (Kitcher 1998). A parallel surge in the interest for quantum physics and oriental philosophies resulted in blending of physics and psychology (Bohm 1980; Penrose 1989), and physics and theology (Capra 1976; Zukav 1979). Breakthroughs in the branches of cybernetics dealing with the description of biological creatures and events as feedback mechanisms initiated merging of the fields of cognitive and computational sciences with the area of artificial intelligence.
Numerous recent breakthroughs in science have been initiated by innovative scientists literally shattering boundaries between scientific disciplines (Anthes 2008). The theological principle of via negativa and the philosophical argument of inaction (related to the fact that every confirmation comprises an implicit negation and every definition of a quality comprises an implicit definition of its opposite, and vice versa) have been applied in designing so-called negative databases which store information similar to the way the immune system does, that is, by excluding everything that does not match its definition of the self. The areas of theology, philosophy, neuroscience, and computer science were thus merged into one. A computer program used by surgeons to simulate the interiors of the patient’s bodies has been shown as an effective tool in visualizing and predicting star and nebula formations. This led to an exciting blending of medicine and astronomy and an implicit pointing at striking similarities between the order at microcosmic and macrocosmic scales, which previously presented a subject of many complexity science studies.
Establishing connections between diverse scientific fields is crucially dependent on freedom to describe odd and unexplainable phenomena evident in any given field. As long as scientists working in isolated fields pretend that their research proceeds flawlessly and that there is no need for assistance or advice from other scientific points of view, the opportunities for establishing a fruitful communication across scientific disciplines will be weak. A recent example shows how biologists reporting strange phenomena from nature, such as insects breathing underwater and walking on it, sparked an interest from engineers, physicists and mathematicians, who all had to offer something, leading to an eventual explanation of these odd effects using the concept of surface tension and at the same time enriching the concept of capillary behavior of water from the elementary physicochemical perspective (Denny 2008). Even the successful communication between experimentalists and theoreticians crucially depends on the freedom to report problems, misunderstandings and mistakes in given scientific fields (Cohen et al. 2008). In the end, each magnificent invention and discovery does not come “out of the blue”, without any prior research background, but presents fruits of a giant scientific tree of knowledge in which all scientists and philosophers that have ever lived ingrained some of their work. Many unsuccessful hours, days and years of scientific research are never wasted as long as they deal with roaming along uncharted territories of scientific knowledge. With their diligence, innumerable scientists have opened up the ways for the actual discoverers to follow and in most cases serendipitously arrive at some treasures along the way (Roberts 1989). By giving these results a chance to be presented to scientific audience, fertile grounds for future research are set in terms of opening the door for someone else’s later achievements to be based on edifying the missing links or exploring the sideway passages of this “failed” research. In view of that, we should always be aware that “there is more to the picture than meets the eye” when it comes to giving credit to a limited group of individuals for discoveries made.
8 Implementing the Principles of Systems Science at the Basics of Using and Understanding the Laws and Tools of any Science, Including Physics and Chemistry
Systems science is all about balancing opposites (Uskokovíc 2009c) and its insights are applicable to all natural systems, irrespective of their complexity and size. When a disciple asked Confucius if his intellectual mastery is justified by him knowing so many things about the man and the world, he replied: “No, I do not know much. All I know is one tiny thread. But that thread connects to all the others”. From the today’s perspective, we might say that Confucius was a master in the systemic knowledge. Some of the balances that systemic approach tends to establish and sustain are: periodicity/novelty, strength/flexibility, order/randomness, and integrity/differentiation.
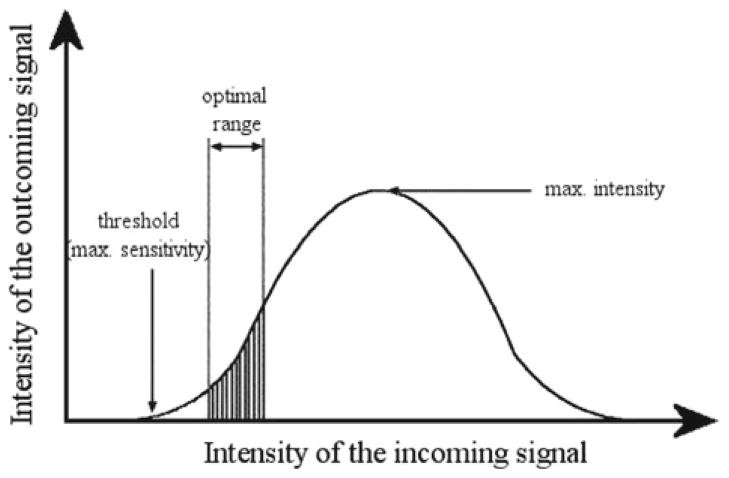
The importance of balancing various contrasting polarities in the field of chemistry also springs forth. We have seen how physics and chemistry roughly resemble analytical and synthetic intellectual approaches, respectively. A balance between the differentiation of described systems into entities and the formulation of the qualities that integrate them into interactive potentials that typify larger wholes thus presents the systemic essence of a successful scientific model. We have also seen that one of the ultimate systemic challenges of science in general is finding the balance between the two major philosophical traditions that have always lurked under its hat: realism and idealism. Another essential balance that scientific measurements implicitly maintain is the one of sensitivity and powerfulness (Fig. 8). The next important balance is the one that involves simplicity and complexity. In this sense, it is worth recalling that the properties of matter depend on the existence of wide ranges of potential interactions, and not on the simple limiting situations. To illustrate this, I will refer to the so-called lyophobic (i.e., “solvent-fearing”) and lyophilic (i.e., “solvent-loving”) colloids. Although these notions are regularly used, they are misnomers (Shaw 1992). Lyophilicity is thus usually attributed to solutions of globular proteins in their native conformations, in spite of the fact that proteins are both lyophobic and lyophilic in their molecular nature. Namely, without their inherent hydrophobicity, the protein globules would unfold and lose their native structure when dissolved in water. On the other hand, a “lyophobic dispersion” is also a misnomer since producing a colloidal suspension of an absolutely lyophobic compound is impossible. Such a compound would not allow for any wetting to occur, which would result in rapid phase segregation. The formation and stability of colloidal systems is normally associated with surface interactions between the dispersed substance and the dispersing medium that are found between the extremes of absolute lyophilicity and absolute lyophobicity. As in many real-life situations, it is not the extreme polarities, but the fuzzy spaces in-between them that should be sought after.
Fig. 8.
A typical Gaussian interrelation between the intensities of incoming and outgoing signals in a spectrometric analysis. Note that the optimum set of conditions for measurements aimed to attain the best possible resolution sacrifices both the area of maximal sensitivity and the area of maximal intensity, and instead finds the way between. Thus, among many other balances that science implicitly teaches us, the one between sensitivity and powerfulness occupies an important place in each experimental observation
However, to offer meaningful ideas and build a masterful thread of thought, a simple line, a plain division, as imperfect and general as it can be, has to be drawn at the first place. Just like Moses divided the Red Sea to continue leading his people on the predestined path, proposing a difference in an otherwise uniform field presents the way forward in diversifying our knowledge. That is what the Indo-European root for the word “science”, in fact, means: making a distinction. One should, however, always remain aware that epistemological polarizations like these are pragmatic approximations, and that real-life situations correspond to their fuzzy juxtapositions. Hence, in the Biblical story Moses divided the sea not to follow any of the seashores on its extremes, but to pursue the “middle way”.
Numerous other partially misleading polarities in regular usage can be mentioned. For example, water is frequently represented as a biphasic mixture of an icy configuration with fully formed hydrogen bonds and a liquid configuration with partly broken hydrogen bonds, even though it is known that continuous dynamic fluctuations of these bonds exist in water. Nevertheless, in many contexts it is more convenient to consider water as a continuous medium and, instead of taking into account the interactions between atomic, ionic and molecular species on a fine scale, apply the notions of water structure and the corresponding dichotomies between structure-making and structure-breaking solutes, kosmotropic and chaotropic effects, or bound and free water. The latter dichotomy is, however, considered as a slight misnomer because a continuum of states is supposed to exist between these two types of water structure in any aqueous layer in contact with the surface. Thereupon, balancing the use of continuum and discrete, static and dynamic, and scalar and vector frameworks has to be mastered, for the complexity of physicochemical effects involving aqueous environments apparently demands their mutual and complementary application. Appropriateness of the imperative proposed by Alfred North Whitehead—“seek simplicity and mistrust it”—can be, thus, illuminated.
Worthy simplifications always comprise intrinsic complexities, whereas meaningful complexities as a rule comprise elegant simplifications within. For example, Einstein’s famous mass-energy equivalence formula introduced enormous complexities in the corresponding relativistic quantum calculus, whereas the complex frameworks of quantum mechanics are based on simple philosophical arguments, including Bohr’s complementarity principle and Heisenberg’s uncertainty principle. On the other hand, oversimplifications often equal numbing generalities and as such may even border with vulgarity, whereas overly complex representations without any underlying simplicity normally present meaningless patterns. Namely, every novelty hides a stable periodic pattern within, whereas every naturally stable periodic pattern needs to produce some intricate novelties. Flaws like the aforementioned ones could easily be transcended by referring to the systemic principles of general character and being aware of the crucial importance of balancing situations.
We have already touched another essential systemic observation: namely, qualities of all natural systems are determined not by their intrinsic physical patterns only, but by the context of their interaction with the physical surrounding, including the measuring apparatus. The examples of contextual effects on atomic and molecular qualities are many (Uskokovíc 2008e). Molecular and nuclear spectroscopies offer numerous examples of how the physical context is able to change the qualities of the investigated atomic and molecular species (Barth and Zscherp 2002; Pochapsky and Pochapsky 2007; Fadini and Schnepel 1989; Fig. 9). Changes in the internal vibration of molecules can be thereby detected following modifications of the medium in which these species are dispersed. Thus, altering the dielectric constant of the medium would lead to redistribution of the dipole moments of the vibrating species, resulting in changes in the internal “music” of the investigated compound. Extending or dissociating organic molecules would lead to changes in the intensity of individual vibration modes, reflected in frequency shifts on the corresponding spectra. This is all correlated with the fact that electron “clouds” of atomic and molecular species are non-localized and sensitive to the effects of their physical surrounding.
Fig. 9.
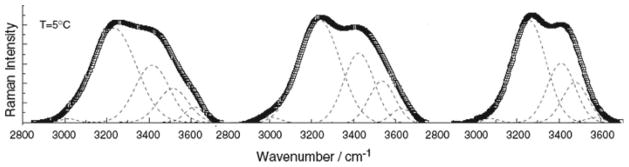
Different vibrational spectra of water in bulk conditions (left), and in droplets of 2.5 (middle) and 7.5 nanometers (right) in size. Many properties of water including diffusivity, viscosity, dielectric permittivity, polarity and acidification gradients have been shown to change depending on the size of aqueous droplets, implying that different environmental contexts trigger different physicochemical behavior of water confined to nanosized spaces, such as in biological environments. Reprinted with permission from Ref. Crupi et al. 2007
Then, there is the example of the influence of water on the structure and biological function of macromolecular species. Namely, water is getting less and less treated as a passive reaction medium that only provides the dispersed or dissolved entities with certain diffusion properties (Gun’ko et al. 2005; Vogler 1998). Due to its intricate hydrogen bonding effects, it is increasingly depicted as actively involved in biochemical interactions (Fig. 10). As a result of the tightly coupled dynamics of a protein and a solvent, they are often conceived as a single entity with a unique energy landscape (Bizzarri and Cannistraro 2002). Proteins can be considered as engaged in continuous feedback interaction during which they influence their solvation environment, but are in turn “finely tuned” and modified by the latter (Ball 2008). Even in the case of fibrous proteins, such as collagen (in which backbone hydrogen bonding between polypeptide chains in its triple-stranded structure does not present the major stabilizing force, unlike in the case of α–helices and β–sheets), additional enthalpic contributions come from water molecules that form a “scaffold” around the surface of the collagen triple helix, implying that water serves an intimate role in stabilizing this protein. Many other macromolecules, including nucleic acids, owe their structural stability to their aqueous sheaths. For example, without water screening the electrostatic repulsion between the charged phosphate groups of DNA, its helical structure would get disordered (Peyrard 2004). Flexibility of hydrogen bonds enables them to exhibit a wide range of hydration forces, and in the case of DNA, hydration has to be maintained at a precise level, so as to be neither too strong nor too weak.
Fig. 10.
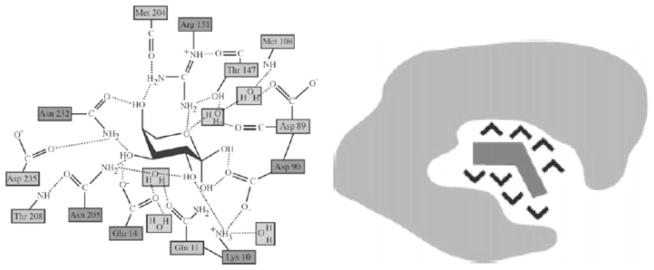
The active site of arabinose binding protein with the essential structural role of hydrogen bonds and water molecules (left), and water molecules depicted as V-shaped entities playing a similarly active role in the binding site of oligopeptide binding protein (right). Even though molecular biologists have traditionally drawn their models against uniformly colored backgrounds, the scientific understanding is nowadays getting in line with Paracelsus’ conception that “water is the matrix of the world and all of its creatures”, in which an active role of water is implicitly acknowledged. The active volume of macromolecules is thus, more and more, drawn so as to extend beyond its formal boundaries, although at the cost of limiting the timescales and conformational spaces that can be assessed in molecular dynamics simulations. As Philip Ball, a co-editor of Nature magazine, asserts, “the structure and dynamics of this hydration shell seem to feed back onto those aspects of the protein themselves so that biological function depends on a delicate interplay between what we have previously regarded as distinct entities: the molecule and its environment”. The fact that the hydrogen-bonded network in the hydration layer of a peptide molecule or water dynamics in the cellular environment are different in comparison with those of bulk water, clearly indicates a feedback, co-assembly interaction possibly within every “self-assembly” process in Nature. The question of where the system ends and where the environment begins will turn out to be increasingly crucial and harder to define as the experimental settings and human interference with physical systems become more complex and sensitive. Reprinted with permission from Ref. Whitehead (1928)
Being familiar with the art of balancing systemic polarities is vital for supporting the creativity of scientific practice. It is the destiny of the evolution of knowledge to proceed in two directions: one that streams forward to the areas of “unanswered questions”, and the other that takes steps back towards the foundations of knowledge permeated with “unquestioned answers”. The latter stand for the basic propositions and assumptions that govern our reasoning and shape the concepts we use. Today’s scientific and chemical societies witness an apparent imbalance in favor of the propagation of the former; however, turning onto philosophical and systemic aspects might once again restore the balance between doing science and understanding science. A truly comprehensive scientific education should thus not stop at the first sign of getting in touch with the philosophical and systemic foundations of the followed approaches. Instead, we should not be hesitant to promote these difficult and fundamental questions “about science”. For only pondering over the philosophical questions can help in clarifying the meaning and purpose of the scientific endeavors. And, as claimed by Albert Einstein, “the independence created by philosophical insight is the mark of distinction between a mere artisan or specialist and a real seeker after truth” (Einstein 1944).
9 Being Open to Descriptions of the Same Systems in Many Different Ways
The art of systemic thinking and the metaphoric nature of scientific representations teach us that there is a limitless number of ways to describe natural systems. The current scientific models may be evidenced as unprecedented in terms of predictability potential, but this does not mean that there could not be better and more sophisticated models behind the horizon. Science has, in fact, lately been experiencing an upsurge in the number of alternative approaches to the analysis of physical phenomena. Mathematical techniques have always provided an example of how the same outcomes can be obtained by different ways of analysis and calculation. Some alternative computational models, such as cellular automata and various forms of game theory, have been used lately with a relative success to describe and explain the evolution of natural systems (Wolfram 2004; Rasmusen 2006). Multiple models can be, thus, potentially applied in the description of identical physical phenomena. As far as the practical chemical aspects are concerned, we can notice how approximately identical material structures can be obtained by a variety of synthetic pathways. Being receptive to novel ways in which the same abstract or experimental results could be arrived at is thus essential in keeping a healthy and fair attitude towards scientific practice.
An essential systemic balance is also the one between periodicity and novelty. In fact, following the overlap between periodic and chaotic patterns within a system poses itself as an alternative to the statistical thermodynamic explanatory framework. The latter could be said to present a sophisticated systemic model used for explaining the evolution of macroscopic physical systems. However, even though it has presented an enormously useful basis for the calculus of energy states of macroscopic systems, it comprises some inherent flaws that have limited its broader application. For example, a precise line between the size of an assembly of physical entities for which the laws of thermodynamics are valid and the ones that are sufficiently small to disobey these laws could not be drawn with a precise certainty. Brownian motion and quantum fluctuations, chemical oscillatory reactions and biological systems all present the examples of local deviations from the laws of thermodynamics.
Also, these laws describe physical systems using the concept of energy. Energy, however, could not be defined per se as its thorough definition requires a reference to the overall surrounding of the system. Energy of a passing electron, for example, has meaning only if described in reference to the environment through which it passes. Activity of a proton in the solution could similarly not be defined without any reference to the structure of the solvent medium.
And not only that, but any definition of energy equally has to take into account chemical bonds and other inner interaction parameters that define stored energy. In other words, entropy of the system could not be defined by only observing the dynamic changes in the perceptible ordering, that is, without acknowledging the internal degrees of freedom. And this is where the concepts of enthalpy and internal energy are invoked. In systemic terms, this implies that one eye kept at outward patterns of the system and the other kept at its inner features need to be always well matched. For example, if we observe cultures of heart cells beating all in the same rhythm, we might start to wonder if it describes the state of high or low entropy, and the answer would depend on their degrees of freedom. As their number is large for the living entities, any such state of harmonic action would reflect low entropy. But had there been zero degrees of freedom for each cell, that would be the only possible state of the system, and the concept of entropy would become useless. The whole concept of the degrees of freedom is, however, imperfect, as any biological system apparently has incalculably large number thereof, and serious approximations have to be implemented every time their definition for a given case has to be supplied.
Similar to any other theory or worldview, statistical thermodynamics, therefore, cannot describe natural systems using their static configurations, but always has to refer to their conformational changes in time. And by actually observing patterns in time, one analyzes the switches and trends in periodic and irregular patterns. These systemic concepts upon which the modern complexity science heavily relies nowadays may thus be considered as the basic explanatory terms that stand above the fundamental concepts of “boundary” or “difference”, and right next to the concept of symmetry, frequently invoked in systemic analyses of physical models (Lipscomb 1980). Note that symmetry of translation in space leads to the law of conservation of momentum, symmetry of orientation in space to conservation of angular momentum, and symmetry in time translation to conservation of energy, as well as that late Werner Heisenberg propagated the abandonment of the concept of elementary particles, and acceptance of the concept of fundamental symmetries. However, if biological processes and logical inferences were symmetric, the evolution of life could not be explained and the whole physical reality would have presented a giant tautology, respectively. It is always in the interplay between symmetry and asymmetry that the essence of life processes is to be found. But by being a thermodynamic state property, entropic value of the system is presumed to depend only on the state of the system, and not on its history. Therefore, the irreversible and history-dependent character of biological systems implies only a partial applicability of thermodynamic concepts in their descriptions (Prigogine 1997; Ulanowicz 2004).
It is also worth noting that without a certain redundancy employed, the statistical definition of entropy as proportional to the number of ways by which a certain state could be reached (Fig. 11), could not hold. Namely, without the redundancies that include presumably identical species and their averaging at macroscopically identical energy levels, each particular state of the system would appear as unique. All of this, however, does not ruin an essential elegance of this theory. By its showing how physical systems could be represented using thoroughly different definitions of the same quantities, it still stands as a major landmark of inspiration to modern systemic worldviews.
Fig. 11.
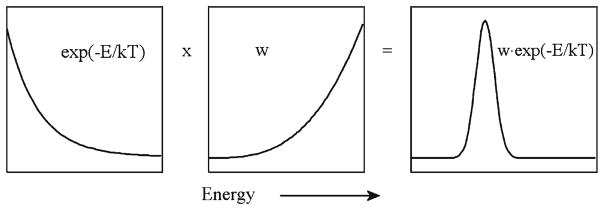
The essential concept of statistical thermodynamics is that the probability of finding the system in a given state can be represented as the product of two separate probability terms: a Boltzmann factor, according to which the higher the energy of the state, the less probable it is; and b degeneracy factor, according to which the higher the energy of the state, the more ways there is by which that state could be reached, and therefore the higher the probability of settling of the system into that state would be. As a result, the most probable state of the system does not correspond to the lowest energetic state, but there is a finite width of energy probability distribution peaking at energies above the ground level (Cooper 1999)
10 Acknowledging the Limits in Control and Chaos in Chemistry
The balance between periodicity and novelty is inherent to the contemporary complexity science and its popular branch of the theory of deterministic chaos. The latter has protruded almost every field of science; however, chemistry still seems to be battling against its penetration. Most of the instances of deterministic chaos in chemical and biochemical systems have dealt with oscillatory oxidation/reduction reactions and rhythmical intracellular (Field and Györgyi 1993) and physiological (Gleick 1987) interactions. However, the principle of deterministic chaos defined as sensitivity of the system to its initial boundary conditions that surpasses the ability of control has rarely been linked with unrepeatable and irreproducible chemical experiments. Despite this, there have been a plenty of examples reported in which minor and negligible modifications of the experimental procedures induced unexplainably large differences in the outcomes (Uskokovíc 2007a,b; Fig. 12). Such instances that display a significant sensitivity of chemical systems to the initial conditions of their evolution are highly reminiscent of the deterministically chaotic systems.
Fig. 12.
Aragonite crystals obtained: by the reaction between calcium chloride and urea at 90°C (left); by the same reaction carried out in magnetic stirring conditions (middle); by preheating the precursor solutions to 90°C and then rapidly mixing them (right). Reprinted with permission from Ref. Wang et al. 1999
Even as seemingly simple phenomenon as Mpemba effect (i.e., an observation that under certain conditions hot water freezes faster that the cold one) is still unconfirmed due to the sensitivity of the system as simple as pure water to the vessel geometry, the influence of dissolved gases, the inverse impurities/supercooling proportion, and the environment surrounding the freezing container (Ball 2006). There are also many cases in which complexity of the experimental settings exceeds the ability to control the starting conditions and the evolution of the reaction system, resulting in irreproducible outcomes. The inevitable trend of introducing more biological effects into chemical methods for synthesis and processing will eventually lead to more of unrepeatable and irreproducible experiments. The larger complexity of biochemical species implied that these problems have been so far much more troubling to biochemists than to traditional chemical engineers. But considering that the largest potential for the future technologies lies in merging of biochemical and nanomaterial processes, we can realize that the times when the problem of experimental variability will penetrate the traditional chemical fields do not seem to lie very far ahead. In the end, it is only a matter of time when the increasing complexity of the products of chemical design would reach the limits of production and usage control. Just as human beings use different and unrepeatable neural patterns even when they seemingly give identical responses to identical questions, sufficiently complex biochemical systems would similarly react in always novel ways to the designer’s impulses.
In regard of the increased entering of polypeptides and other biochemical substances in the procedures for the synthesis of functional materials, it is normal to expect that the problems of chaotic irreproducibility can only be augmented in the future. Besides, the boundary between living and non-living systems has not been precisely defined yet. Are viruses that comprise a strand of nucleic acids but no genetic expression whatsoever until they become attached to the genetic material of the host organism to be considered as biological systems or a piece of dead matter? Also, in view of the planetary ecological cycles in which atoms circle between the living species, soil, rocks, water bodies and the atmosphere, it is obvious that we can consider an inanimate stone in our hands as a matter of biological significance (Lovelock 2005). This is all in accordance with the hypothesis of Gaia proposed by James Lovelock (2000). At the end of his first book, he draws a vision for the creativity of the future by imagining the time when “the children we shall share with Gaia will peacefully co-operate with the great mammals of the ocean and use whale power to travel faster and faster in mind, as horse power once carried us over the ground”. In view of the role of proteins as the basic constituents of life, their influence by higher emergent qualities of living species, including the patterns of emotion and thought, should not be underestimated. Protein structures are significantly more complex than elementary physical systems and, as the result of their ridged free energy landscapes their ground states are not those attributed with the singular degeneracy, but quite contrary: in modeling approaches, the largest class of possible similar structures is normally selected as mostly resembling the native conformation. It is known that certain peptide sequences can adopt two dissimilar structures with approximately equal likelihood, which modifies the classical thermodynamic description of protein folding by arguing that the thermodynamic minimum in this case may not be uniquely defined (Yeates 2007). In fact, there are proteins (like α–lytic protease, e.g.) that are more stable in unfolded state than in their native conformation (Miranker 2005). Exhibiting a stable, native state with low energy barriers for conversion between many approximately isoenergetic conformations at relatively high entropy presents the basic condition for the expression of protein flexibility, which conditions molecular recognition interactions (Bochicchio and Tamburro 2002). Such a degeneracy of the native states of proteins is also essential for providing them with the self-stabilizing structural flexibility. Thus, as is also the case with intracellular reaction pathways, there always exists more than one way for a peptide to reach its native state, which is related to one of the essential characteristics of biological systems: equifinality (von Bertalanffy 1968). Proteins are subject to subtle conformational changes as they travel through the cellular environment, and this inherent sensitivity enables them to engage into molecular recognition effects. Strictly saying, due to incessant conformational fluctuations that each macromolecular structure is subject to, no protein molecule has ever existed in the same conformation twice! Consequently, their level of inertness, although far away from that exhibited by living creatures, could not be placed at the same level as the one of inorganic physical systems.
Proteins can be thus regarded as similarly positioned exactly at the boundary between living and non-living systems. Their predisposition of reversible folding/unfolding and the abilities to adopt a multitude of configurations depending on their physical surrounding simultaneously point to their unforeseen potential in the chemical synthesis and to their influence on physical factors that might lie beyond the range of the experimental control. How modifications in single amino acid residues can produce crucial differences at the level of both simple chemical reactions (Ahmad et al. 2006) and biological integrity still hardly finds any explanation at all. A single amino acid change can sometimes be enough to induce a transition into a novel folded structure (Meier et al. 2007), whereas it has been demonstrated that changes in the tertiary structure of a protein can produce a drastic effect on the secondary structure of the individual peptide chains, including the occasional transformations of α-helices to β-sheets (Minor and Kim 1996). On the other hand, substitution of a single glycine residue with alanine in a collagen molecule results first in a deformation of the helical structure of a single polypeptide chain and subsequently produces a deformation of the whole triple helix. It is known that the substitution of a single amino acid in the β-chain of hemoglobin results in sickle cell anemia, whereas a single point mutation in the amelogenin-coding gene, resulting in a single amino acid substitution, produces a severe dental enamel malformation (Bartlett and Simmer 1999). It is, however, clear that from the perspective of evolution, proteins have to be open to sequence variability, which their energy landscapes naturally promote. For example, central amino acid residues of amelogenin have been intensively varied throughout the evolution, whereas its C-terminal, which is involved in the interaction with hydroxyapatite crystals, has remained highly conserved (Paine et al. 2001), demonstrating again the vital balance between rigidity and flexibility towards change. With the human genome and many proteins being sequenced, our knowledge on the drives behind the genetic and protein expression, cellular functioning and communication are still insufficient. This is why it has been said that the past of chemistry was linked with static structures and re-actions, whereas the future belongs to dynamic inter-actions.
The systemic nature of physical qualities indicates that similar relationships occur at different scales of natural systems. En passant, this means that as we ascend from the level of molecular interactions to the domain of social and ecological ones, the realization that the evaluation of progressive ideas and their implementation is never certain dawns on us. This conclusion also holds in the domain of application of chemical substances in everyday applications. For example, although it is well evidenced that sunscreen agents containing dispersions of titania particles block ultraviolet sunrays, it has never been confirmed that they prevent skin cancer presumably caused by this high-intensity radiation (Royal Society and Royal Academy of Engineering 2004). On the other hand, an extensive usage of these cosmetic substances might have caused an increasing number of cases of measles and other illnesses related to vitamin D deficiency. It is worth noting that living systems disregard the monotonous strategy “the more, the merrier”, as only optimal states and balanced interactions pave the way to sustainability. Furthermore, biological evolution is crucially dependent on the supply of the opportunities to co-evolve with other species. Depriving living creatures from the chance to compete and, so to say, “fight” against the corrupting agents can lead to their own weakening and deterioration. For example, attacks of microorganisms on our integrity are largely responsible for the evolution and lifetime reinforcement of our immune systems, which is the essential idea behind the concept of hormesis. In a perfectly sanitary world, our immune systems would tend to collapse. The popular hygiene hypothesis, for example, states that the lack of exposure to infectious agents during the childhood increases the subsequent susceptibility to allergies. In fact, our whole bodies are symbiotic in their nature, hosting innumerable sorts of microorganisms. Correspondingly, what is good and what is bad can be evaluated only in the unapproachable context of the whole in its complete history and future. But just as the dialectical confrontations present the way to improve mutual understanding of the sides in the dialogue, the same principle of progressing only in face of the problems and challenges seems to be standing as valid within every other ontological aspect.
An observation that naturally entails the idea that uncertainties present the source of new knowledge is that every form of perfection is only a perfect imperfection. To illustrate this, we can return to the field of biomaterials and biomedical engineering. In the medicine of restorative hard tissues, for many years it has been considered that the stronger the material used as an in vivo substitute for the damaged tissue, the better. However, if a biomaterial with elastic properties identical to those of the natural bone is applied, the implanted cells would not do any work at all to proliferate and “instill life” therein; eventually, the probability for its rejection by the body would be high. Therefore, a biomaterial has to remain slightly imperfect in order for the host cells to proliferate within and additionally reinforce both the material and the preexisting tissue structure. This brings us to what I call the ultimate systemic balance: the balance of balanced and unbalanced states.
Thus we arrive at the final systemic balance mentioned in this work, and it is the one between order and freedom. Namely, it is a popular misconception that the smaller the entropy of the system, the better. However, in a state of zero entropy, there would be no internal predispositions for the evolution of the system, and it would become constrained to inert and perfectly predictable behavior. Every sustainable evolution, that is, the one that opens space for new progressive options and directions, inherently feeds on the entropy. It is well known that parameters of order and sources of randomness have to be specified prior to each computer simulation, which implicitly reflects the nature of the evolution of human knowledge, inevitably based on the encounters between order and freedom. Ross Ashby, one of the fathers of the science of cybernetics, thus proclaimed: “Only randomness can give rise to novelties” (Ashby 1956), whereby Henry David Thoreau observed what nowadays presents an ecological truism: “In wilderness is the preservation of the world” (Thoreau 1994). A certain level of disorder within the evolving system should be thus carefully cultivated, lest the very order of the system might get disrupted.
11 Nanosciences and Nanotechnologies: Time to be so Small
The most recent example of intensive confrontations of pro and con arguments in the scientific R&D domain is given by nanomaterials and nanotechnologies (Uskokovíc 2007c). More than any other technology in the past, it has become faced with innumerable threats and controversies (Richman and Hutchison 2009). For example, their proponents state that they present an inescapable line of progress for the modern science and technology, while their opponents refer to numerous undesirable but uninvestigated health effects that could result from their premature release into the environment. In order to ensure the prosperous development of this field, both sides would need to be tackled simultaneously. Effects of these new technologies at relatively short-term, toxicological levels, and broader and long-term, ecological sustainability levels, would thus have to be assessed in parallel with their development (Barnard 2009). As far as the short-term evaluations are considered, we should bear in mind that different contexts can trigger different responses of the same physical structures. This is neatly exemplified by the case of thalidomide. Even though this drug had passed all the safety studies on animals, it later proved to be extremely harmful when used by humans. These novel materials should also be analyzed within a large variety of contexts, including scientific, ecological, social and economic ones. The example of genetically modified foods is instructive in this sense (Tuxill 1998). Namely, their critical effect on humans comes not through digesting or getting in direct contact with, but through the long-term disruption of the genetic diversity of the environment. Therefore, a thorough analysis of the benefits and dangers of a new technology, such as genetic engineering, should not end up with scientific studies, but has to be extended to social, political and economic aspects. When it comes to implementation of a new pervasive technology in a globalized society, it seems that many, if not all, scientific disciplines need to be asked for their opinions.
The current interest in physical phenomena at the nanoscale presents a natural result of the shift in the controlling abilities from macroscopic and microscopic objects and processes to the nanoscopic ones (Fig. 13). Today’s nano-hype can be thus seen as a natural consequence of the gradual refinement of the resolution of human interference with the physical substrate of their experience. Gregory Bateson defined information as the difference that makes a difference (Bateson 1972), and correspondingly we can think of the evolution of our planet in terms of multiplying the amount of boundaries that are “readable” and that matter. With every new day, the amount of these meaningful boundaries increases, and more and more they find their way to ever smaller size scales. Hence, the interests of science are naturally getting focused on the physical phenomena on an ultra-fine scale, and it is estimated that 2 million scientists will be working in the field of nanoscience by 2015 (Greenberg 2009). Thereupon, a huge challenge for the modern physics and chemistry is making noise-to-signal transformations by developing more sensitive experimental setups for measurement and control. For, what the measurement “problem” springing out of Heisenberg’s uncertainty principle teaches us is that we need to be small and sensitive in order to see things on small scale.
Fig. 13.
Ever since Isaac Newton wrote that “I do not know what I may appear to the world, but to myself I seem to have been only like a boy playing on the seashore, and diverting myself in now and then finding a smoother pebble or a prettier shell than ordinary, whilst the great ocean of truth lay all undiscovered before me” (Brewster 1885), the metaphor of the coast of solid knowledge and the ocean of unknown meeting at the seashore of human mind has been one of the most beautiful ones in depicting the quest for knowledge. The current interest for nanoscale physical phenomena presents a natural consequence of the continually improving scientific mastery of “playing” with the planetary matter at ever smaller size scales. This is why a boy facing downward while being immersed in the beauty and secret meanings of small and negligible seashore pebbles may present a symbol of the curiosity that drives the voyage towards new discoveries in the field of nanosciences and nanotechnologies
Henceforth, the challenge that the involvement of physics and chemistry in the progress of the field of nanotechnologies bears is that science has to revive the virtue of being small in all of its aspects. As the scientific inquiry descends down to increasingly smaller size scales, it is crucial to learn to cede the place of big and gigantic to humble, patient, careful and small. The field of nanosciences and nanotechnologies can thus present an entrance to the story of life in which one would be able to show to the world how through smallness and humbleness a true mightiness and greatness can be attained.
Through this example of analogical understanding we arrive at one of the most important tasks for the contemporary science: providing not only pragmatic metaphors that can be utilized for the sake of improving comfortable living, but metaphors that will be absorbed into human consciousness as the sources for the beautification of spirit. If we take a peer at the planet Earth from a cosmic perspective in time, and through the lens of the current scientific knowledge of its history, we would readily recognize the trend of an exponential multiplication of meaningful boundaries on its inhabited surface. Simple extrapolation of this trend into future would provide us with visions of a continuous evolution of both the landscapes of the Earth and the landscapes of human mind. Thus, we can only imagine how wonderful and inspiring some future days on our planet will be, both in terms of enlightened experience and an organized flow of information throughout the planetary matter. For these two aspects of evolution—spiritual and informational—seem to have been proceeding in parallel ever since. The rational clarity of human mind has developed together with richness of the informational content of our physical environment, and they are both captured in an unforeseen and endless evolution towards ever more inspiring emanations. And by cultivating these visionary attitudes, we can realize how our egotistic nature diminishes on the account of the growth of an altruistic and, so to say, cosmic consciousness. So we realize how scientific knowledge can help us transcend cognitive attitudes cocooned within solipsistic ways of thinking about the physical reality and learn how to appreciate beauty of the evolving world as more important and greater than oneself. Science has ever since implicitly taught humbleness, and with its future descriptions of the world, although clarified with acknowledging their proper philosophical background (that is, accepting scientific descriptions as partly subjective and partly objective; partly constructivist and partly realistic; partly tautological and partly empirical; partly pragmatic and partly l’art pour l’art), this aesthetic tradition should be expected to abide. Hereby we come to the final challenge for the modern science, which is learning to recognize, appreciate and utilize the aesthetic character of its knowledge and method.
12 Beauty of the Chemical Fields of Scientific Study
By defining chemistry and science from the perspectives which lie somewhere between the ones connected with revealing the secrets of the real organization of Nature and the ones tied with clarifying the order in the domain of human experiences, we can adopt the middle way and deduce that chemistry and science are languages for both the coordination of human experiences and communicating with the secrets of Nature, and as such have the purpose of enlightening both the domain of human cognition and the face of the planet. The products of science in terms of its discoveries concerning either the fundamental order of life and the Universe or practical applications tend to beautify the landscapes of human experiences.
One of the greatest tasks that will underlie the prospects of the future science is learning how to bring its meaning and beauty to ordinary people. For, initiating the sense of wonder in them presents the first creative sparkle that might induce endless streams of enlightening insights and discoveries. Learning how to present one’s ideas in both rationally rigorous and imaginatively inspiring ways presents an enormously important challenge for the future scientists (Bateson 1979a). Peter Medawar held the opinion that “scientists should not be ashamed to admit, as many of them apparently are ashamed to admit, that hypotheses appear in their minds along uncharted by-ways of thought; that they are imaginative and inspirational in character; that they are indeed adventures of the mind” (Medawar 1963). In that sense, we should keep in mind that metaphoric thinking in terms of finding parallels between the scientifically investigated relationships and those that daily experiences abound with have led the whole tradition of the most fruitful scientists and philosophers to novel ideas (Harrison and Treagust 2005). Although scientists are often skeptical with regard to overly relying on metaphors in drawing inferences, it is the statement of fact that the greatest scientists have looked for metaphorical inspiration in the natural world in solving numerous scientific problems and puzzles. Aside from the standard examples of Archimedes discovering the buoyancy principle while plunging in a bathtub, Einstein arriving at the principle of relativity while moving in a tram away from the Bern clock tower (Kaku 2004), Newton having an apple fall on his head to instigate him to build up the law of gravity, and Harold Rosen designing the stable trajectory of geostationary satellites after the way quarterbacks spin the passing ball in his most beloved sport (Rosen 2008), a particularly fascinating example is the one of Nikola Tesla’s discovery of the principle behind the work of alternate current motor. It was in Budapest, while having a walk through the city park with a friend who was meant to alleviate Tesla’s strange mental occurrences with a healthy exercise. Although this idea had been sprouting in Tesla’s mind for a long time, he simply could not fit the pieces of the puzzle yet (Cheney 2001). At one moment, Tesla glimpsed the setting Sun which reminded him of the verses from Goethe’s Faust that he then started reciting: “The glow retreats, done in the day of toil; it yonder hastes, new fields of life exploring; ah, that no wing can lift me from the soil, upon its track to follow, follow soaring!” With envisioning the Sun descending and himself ascending to reach it, “the idea came like a flash of lightning, and in an instant the truth was revealed”, in Tesla’s own words. “See my motor here; watch me reverse it”, Tesla happily exclaimed, while drawing a diagram of the AC motor with a broken branch in the dust.
The way the model of the atom has evolved over time offers another example of how everyday observations may become reflected in scientific visions and images which would still be supported by the rigorous rules of logic and math. The model of the atom that Ernest Rutherford proposed in 1911 and Niels Bohr subsequently elaborated into a more complex, quantum model was a revolution in the world of science in terms of showing how magnificently large may find its reflections in minutely small. In this case, it was the very Sun that was imagined as residing in a single atom. It was the time when a mess of concurrent models was used to explain the atomic behavior – the cubic model, the plum-pudding model, and the Saturn rings model. And yet, the Bohr-Rutherford model clearly invoked the metaphor of the Sun as it depicted electrons as circling around the nucleus just as the planets circle around the Sun. The only difference was in the nature of forces that supported this incessant movement. Whereas in case of the solar system it is the balance of centrifugal force that tends to drive planets away from the Sun and gravity that tends to bring them closer to it that is responsible for the planetary revolutions, in case of the atom gravity was hypothetically replaced by the electrostatic attraction. However, an enigma faced by the atomic physicists of those times was how come the electrons stay in stable orbits when the calculated balancing of the forces predicted their slow descending into the sun, that is, the center of the atom. To explain that, Erwin Schroedinger picked on the major improvement that Bohr introduced compared to Rutherford’s model, i.e., the quantization the atomic energy levels, and represented them as different harmonic modes of vibration of a guitar string. His blunt idea was to depict the atom as a source of music. And so, after many theoretical attempts he succeeded and in 1926 the Schroedinger’s equation was born. Two years earlier, Louis de Broglie showed how the ancient idea of atom as a solid particle inevitably fails and replaced it by the wave-particle dualism. With representing the atom as a particle and a wave at the same time, our ability to imagine and comprehend things had been stretched to the very limits. What is important to notice from this brief revisit of the way the model of the atom evolved over time, from plum-pudding to the Saturn rings to the Sun to the music and beyond, is that scientific pictures continuously evolve in feedback with human imagination, the one that incessantly feeds on perceptive impressions and transforms them onto the level of scientific relationships. In such a way, it becomes quite clear that Nature and human mind in togetherness draw sketches of science. A clear implication of such a stance is that sensitivity in recognizing signs that Nature strews around us, certainly being promoted through learning to appreciate the works of art, is a crucial feature of productive scientific thinking. Science and arts are thus fundamentally entwined. No wonder then that some of the most productive scientists in the history of humanity were inclined to arts. Albert Einstein, Louis de Broglie, Richard Feynman, Ernst Mach, Max Planck, Ilya Prigogine, William Lipscomb, Gerald Edelman, Werner Heisenberg, Wilhelm Ostwald, J. H. Van’t Hoff and many others were musicians, Albert Michelson and Walter Thirring were composers, Marie Curie, Otto Hahn, Erwin Schroedinger, Walther Nernst, Roald Hoffmann and Jožef Stefan were all poets, Alexander Fleming, Edward Wilson, Louis Paster, Santiago Ramón y Cajal, Lawrence and William Bragg were painters, Robert Holley and Roger Sperry were sculptors, and so on and on (Hollingsworth 2007). Certainly, the landscapes of human thinking would be illuminated by the light of human spirit should we understand the pervasion of metaphors and the importance of cultivating an artistic view of the world for the sake of maintaining high levels of scientific creativity and vice versa, that is, should we understand the importance of scientific discipline and diligence for the thriving of the technological and advanced communicational bases through which human minds and artistic pieces are shaped into ever more beautiful expressions and forms. By developing sensitivity to notice beauty in relationships other than scientific ones, one simultaneously develops capabilities to perform scientific tasks in magnificent ways (Uskokovíc 2009d). It is by looking at the world and its small and neglected details with a whole lot of genuine imaginativeness that we will find similar suns around us and place them in the center of the Universe of human imagination and make them spin our thoughts in unforeseen beautiful directions which would endow ourselves and the planet with ever more diverse, amusing and profound outlooks.
And yet, although the core of creative thinking is based on the balanced usage of logical and analogical thinking, we should be aware that, although unavoidable, metaphors could be deeply misleading. Logic that spreads horizontally has its strict rules and obvious limits, ending with some “visible horizons”, whereby analogies and metaphors, spreading vertically, do not have any limits, as infinite number of relationships could be projected onto any given link. A virtuous choice of metaphors is thus crucial because we apparently have an infinite choice thereof in front of us for describing any given event. And it is this choice of metaphors that demarcates a genius from a schizophrenic, and a simpleton from a graceful mind. In particular, we can be reminded of a lineage of systemic philosophers, spanning from Leonardo da Vinci to Johann Wolfgang von Goethe to Gregory Bateson, and so forth. It seemed impossible for each one of them to patiently isolate creative interests in the sole fields of science, arts, philosophy or narration. They were all combining, crisscrossing and mutually influencing all these fundamental areas of human inquiry and creativity. For example, Leonardo da Vinci, a profound trans-disciplinary thinker ahead of his time, in his treatises frequently jumped from one topic to another, so that instead of sifting the topical ideas or revolving around a specific theme, his thoughts simply flowed in seemingly unconstrained ways (Capra 2007). He would draw a picture to illustrate one thing, and instead of continuing the discourse, the drawing would remind him of another relationship he would then start analyzing, arriving to novel ideas in the further course. And such seems to be the nature of all the systemic philosophers, the minds of whom are abundant with the flashes of metaphoric correlations.
As we see, every relationship and description that we find in scientific textbooks presents a metaphor that could provide us with an endless stream of meanings applicable at various other levels of our experience. Take, for example, the metaphor of an anharmonic oscillator depicted by the Morse curve, shown in Fig. 14. If conveyed to the domain of human-to-human interactions, it may be understood as a pointer to the most favorable attitude that ought to be adopted in social relationships. The nature of the interaction of physical entities in this case does not let them either permanently merge or derail too far. Instead, they are forced to preserve their own integrity and yet “dance” with others. When we shift our attention to non-equilibrium systems, we may notice how this “dance” begins to flow with the sense of a global and dynamic coherence, whereas the same balance between autonomous and connected behavior of individual entities, described by Kauffmann’s law of optimal connectivity, seems to be preserved (Kelly 1994). Such a dynamic equilibrium between individuality and wholeness may be understood as a metaphoric pointer to the balance between self-responsible inwardness and expressive compassionateness in social relationships, implicitly pointed out by Erich Fromm as the key to the art of loving (Fromm 1956). Oscillations like the ones depicted by the Morse curve are inherent to all atomic aggregates, including molecules, gases, liquids, crystals and stellar plasmas, whereby the quantum calculus predicts that even at the absolute zero a minimal, but finite oscillation mode would exist. These all-pervasive “back-and-forth” movements by which physical entities approach and derail from each other present the only mechanism for creating and sustaining the “music” of life. Hence, as the blind spot effect instructs us, the appropriate way of deepening our knowledge about natural systems is not to permanently maintain the same cognitive stance in relation thereto, but to periodically conjoin with them and distance from them. Each minute scientific description, in fact, opens the room for seeing its endless meanings by copying its relationships onto some other levels of organization of life and Nature. It is in this process of metaphoric comprehension where the true talent for beautifying science comes forth.
Fig. 14.
Morse curve representing the potential energy of an anharmonic oscillator as a function of distance between the oscillating entities (the scale herein corresponds to atoms in a hydrogen molecule). Had the shape of this curve been symmetrical (i.e. harmonic oscillator), numerous effects, including the finite thermal conductance of solid bodies, thermal expansion, molecular dissociation and the appearance of combination bands and overtones, could not have been explained
Thus, scientific metaphors are shaping our own metaphors through which we understand life, whereas, as noted previously, these relationships from the domain of common and palpable natural events are used to shape the scientific metaphors. Thus we come to a closed circle, which clearly indicates what was already known: through scientific models humans are not only revealing the secrets of Nature, but the secrets of their own being and mind as well. Scientific models may be thus understood as the stories made through an adventurous dialogue between human mind and Nature. And in that dialogue, both mind and Nature are apparently evolving, as can be evidenced by cognitive and planetary landscapes getting enriched in parallel in the course of the development of the human civilization.
However, it seems to be a remarkable challenge to succeed in finding the balance between scientific rigorousness and inspiring aesthetics that would be neither charlatanic from the scientific point of view nor pathetic from an artistic stance. The attempts to wed these two inherent aspects of human creativity have so far resulted mostly either in the reduction of aesthetic elements of science and arts to technical jargon and the usage of mathematical approaches and the concept of symmetry and its derivatives, such as the golden ratio, to explain the aesthetic secrets of artistic compositions, or in popular works in which the scientific method is presented with the aim to educate and inspire, although most frequently with a little of artistic value. Whereas the former, overly scientific approach disregards the need to instill the ideas with an aesthetically pleasing sense at each level of their expression, the latter disregards the need to adopt the basic principles that typify an artistic masterpiece: congenial overall structure, originality and an underlying passion to beautify, inspire and sanctify the world. These features are what scientific method ought to be integrated with, owing to their profound potential influence on scientific creativity. For in the end, the reference to presupposed laws in thinking and experience is together with normally deeply subconscious aesthetic principles used in the course of selection, analysis and synthesis of experiential observables. Thus, although in a subtle manner, aesthetic and ethical criteria are responsible for scientific creativity as much as intelligence and training in the given field are.
The parallel focusing of intellect and emotions is common to enlightening scientific insights and artistic impressions, whereas a commonality between scientific and artistic attributes of our mind also pervades the moments of abstract creativity. “Hardware” of our cognitive apparatuses corresponds to our innate biological predispositions, but altogether with the “software” in terms of learned knowledge and values it guides our decisions in life and science. Without a great desire to succeed in our earthly mission by exhibiting creativity for the sake of enlightening others, I believe that no truly valuable works in either science or arts could have been made. It is this invisible link between human values and scientific creativity that may be posed as one of the truly inspiring links between the two fundamental aspects of human cognition. Hereupon, we can notify how social milieus have been reflected in the implicit nature of scientific discoveries. For example, it is no wonder that Greek philosophy and arts had flourished side by side, and that the awakening of ancient aesthetic values during the Renaissance gave impetus to the rise of the scientific method. Quantum theory was developed in Germany at the turn of the 20th Century, during the times when radical artistic approaches that were discarding the established conventions thrived. Both Bohr and Heisenberg recognized the influence of Eastern philosophical thought on the epistemological foundations of quantum theory, suggesting that much more than pure scientific observation had been involved in forging this and, quite probably, every other scientific theory. Then, Big Bang theory with its abandonment of the idea of static Cosmos came from the cultural background of values shifting towards change, dynamism and action. The current interests in biomimicry as one of the frontiers in modern chemistry seem to be developing in parallel with the immense shift of our contemporary culture away from the scientific and technological design that raises man at the pedestal of superiority in relationship with Nature towards the one of ecological awareness, sustainability and eco-friendliness, wherein the attitudes of respect and preservation of the biological foundations of human civilization are implicitly nurtured.
On the other hand, scientific imagery also leaves traces on cognitive predispositions and trends in thinking. To illustrate this, I will refer to an impression that scientists working in life sciences oftentimes seem to be pervaded with more liveliness than materials scientists. The reason may be that they naturally reflect the lively and dynamic, or the static and inanimate subjects of their studies, respectively. Henceforth, just like the proper understanding of a painting or a musical piece requires the knowledge of the context in which they were created, the same idea can be applied for scientific ideas as well. Stripped from the social context from which they originated and to which they will eventually return bringing new understanding and implementation in terms of pragmatic tools, scientific theories and judgments lose some of their aesthetic clarity. However, similar as the most valuable artistic pieces possess a “cosmic” character insusceptible to the passage of time or the shifts in social trends, the most significant and influential scientific conceptions refer to the permanently actual questions concerning the nature of physical reality.
Be that as it may, as we deepen the inextricable relationship between knowledge and beauty, the superficial view that scientific inquiry is coldly rational, and artistic inquiry is heatedly emotional could not stand anymore. But there is no recipe for reaching and maintaining this balance. Sometimes we need to trust our own insights in accordance with Paul Dirac’s principle of beauty in science (“It is more important to have beauty in one’s equations than to have them fit the experiments” (Dirac 1963)), and keep on believing in our ideas despite them not fitting the experiments, whereas sometimes it is smarter to conform our conceptions to experience or tools that we have in our hands, similar to the way Heisenberg let his ideas flow along a mathematical stream and as such developed the principle of uncertainty (Yang 1980). This balance reflects the general systemic struggle between adaptation and evolution that is evidenced to take place in the domains of biological evolution, ontogenetic development and the constructivist/objectivistic cognitive synchrony alike. If we define science as “imaginative, observational and critical love of nature” (Hartsthorne 1980), essentially being “the search for the hidden beauty of the world”, it can be naturally recognized as the most profound form of art, that is, the one of an active dialogue between the epistemological depths of human mind, permeated with ethical and aesthetical qualities as much as with the power of imagination and logical weaving of thoughts, and the wondrous hidden treasures of Nature.
The last one of these major challenges for the modern chemistry, that is, the need to be simultaneously rational and inspirational in our scientific quests and presentations, brings us, in a way, back to its very beginnings: to the mastery of creative and inspiring education. In order to promote a successful education, we need to awake awe and wonder in young scholars as much as to teach them the rules of analysis existent within the given science. Also, a praiseworthy education certainly needs to balance the incentives to generalize and specialize. Without the latter, young scientists would learn how to swim carelessly in the sea of knowledge, but they would never be able to dive for the pearls at the seafloor and bring them back to the social daylight. But the former aspect, which is inherently linked to the aforementioned systemic knowledge, is inextricably linked with realizing the purpose of the entire field of chemistry and each one of its studies in a wider context wherein these small deeds would be endowed with great value. Thus we learn to see greatness in smallness and mightiness in humbleness. The arrogant and pretentious attitudes that increasingly typify participants in the modern scientific arena could be thus transformed into approaches that entwine a childish joy and austere wisdom that the human tradition behind our back is undoubtedly abundant with. In the end, we may be guided by the words that Fyodor Dostoyevsky famously proclaimed through his character of Prince Myshkin: “Beauty will save the world” (Dostoyevsky 2003). Dealing with science without being aware of its inherent beauty can be as devastating for the scientific creativity as being immersed in the aesthetic forms without being aware of their underlying rationality. In the end, as much as it is a great challenge to realize and employ the underlying streams of artistic creativity behind virtuous scientific conduct, it is essential for the contemporary artists to accept that their fecundity can rest in a long term only upon a developed intellectual framework for quality assessment and analysis. To avoid the extremes of robotized, inert and programmable intellect and infertile, schizophrenic fancy, knowledge and beauty ought to be always heading hand-in-hand.
13 Conclusion
Throughout this work, the following major challenges for the modern chemistry have been outlined: (i) interlinking theoretical knowledge and experimental approaches; (ii) implementing the principles of sustainability at the roots of the chemical design; (iii) defining science from a philosophical perspective that acknowledges both pragmatic and realistic aspects thereof; (iv) finding a balance between reductionist and holistic methodologies; (v) promoting the proliferation of physical chemistry; (vi) instigating interdisciplinary research; (vii) implementing the principles of systems science as the basis of scientific practice; (viii) accepting multiple and equally relevant descriptions of identical systems; (ix) acknowledging the uncertainty and limits within experiments; (x) developing appropriate R&D models in the area of nanosciences and nanotechnologies; (xi) learning to recognize and appreciate the aesthetic aspects of scientific knowledge and methodology, and promote truly inspiring education in chemistry. In concert with the aforementioned interconnectedness of all sciences, individual challenges outlined hereby can also be recognized as inextricably related. Therefore, promoting an advance in one of the described fields of interest would inevitably be significant for all the others.
The final question is how far we’d be able to advance in tackling these challenges. Some of them, e.g., interlinking the theoretical knowledge and experimental approaches, will have equally belonged to future and past, whereas some others, e.g., implementing the principles of sustainability at the roots of the chemical design, have yet to capture a thorough attention of the chemical society. One thing is, however, certain: challenges, big or small, will always be present within the scope of scientific interest, because the evolution of human knowledge inherently depends on them.
However, there has always existed a temptation to turn the unresolved situations into ones with fixed conclusions, to lock the doors of the natural inquiry that spurs our creativity, and to promote algorithms of conduct that would independently of the human imagination and wonder drive the development of our science and behavior. Science and human reasoning have ever since been tempted to oversimplify the relationships that comprise the physical reality. Models of natural systems are inevitably imperfect, and there is not a single scientific framework of analysis of natural phenomena that does not fail under specific conditions. In the end, the greater the human knowledge, the more questions would arise out of it. As such, it is always a big challenge arising in front of a scientific mind to live with ambiguities and enigmas. The solution is certainly not to cut them down to overly simple models, but to acknowledge beautifulness and evolutionary drive that arise at the boundary between an inquiring mind and the horizons of mystery. Otherwise, this natural grace of the scientific mind would be overlapped with the layers of ignorant and arrogant attitudes. So to say, whenever we oversimplify Nature, we should be sure that Nature is going to oversimplify us in terms of degrading our intellectual susceptibility to many inspiring wonders that the reality abounds with.
Each scientific research should, therefore, acquire the balance of disciplined and adventurous attitudes. Responsibility and diligence that tie us to approach the old and actual scientific traditions with awe and respect on one side, and a thirst for knowledge that makes us send our ships away from the safe harbors of the standardized science and to the unforeseen and uncharted territories on the other side ought to be entwined in a well-balanced scientific practice. If the former aspect prevails over imagination and freedoms, robotized attitudes of the intellect will come to pervade the scientific society, and the inertness and creative passivity will take over. On the other hand, if freedoms instigated exceed the respectful and responsible scientific practice, anarchism and irrationality will take over. However, it may be said that the former diagnosis seems to better fit most of the scientific societies worldwide. In that context, students nowadays ought to be primarily reminded that each scientific research should present an adventure in the relationship between human mind and Nature. It should be a quest for the treasures of knowledge, and a mind on this path needs to fully and faithfully reflect this pioneering epistemological nature. The personality and attitude adopted by a fruitful scientist need to be adventurous in each of their facets, from posing questions about the deepest existential meanings to contemplating about creative and inspirational conduct of behavior and thought.
Thus, the final challenge is that of being certain in uncertainties. This is neatly described in the words of Richard Feynman: “Western civilization, it seems to me, stands by two great heritages. One is the scientific spirit of adventure—the adventure into the unknown, an unknown that must be recognized as unknown in order to be explored... To summarize it: humility of the intellect. The other great heritage is Christian ethics—the basis of action on love, the brotherhood of all men, the value of the individual, the humility of the spirit… It was a struggle to be permitted to doubt, to be unsure. And I do not want us to forget the importance of the struggle and, by default, to let the thing fall away. I feel a responsibility as a scientist who knows the great value of a satisfactory philosophy of ignorance, and the progress made possible by such a philosophy, progress which is the fruit of freedom of thought. I feel a responsibility to proclaim the value of this freedom and to teach that doubt is not to be feared, but that it is to be welcomed as the possibility of a new potential for human beings. If you know that you are not sure, you have a chance to improve the situation. I want to demand this freedom for future generations” (Feynman 1998). Invoking beauty in being unsure and doubtful presents a true challenge for the current generation (Uskokovíc 2009e). For in the end, what science teaches us is not fulfillment in the act of finding, but beauty awakened in the moments of searching.
Biography
Author Biography
Vuk Uskokovíc a native of Belgrade, Serbia, holds degrees in Physical Chemistry (BS/MS), Materials Science (MSc), and Nanosciences and Nanotechnologies (PhS). He is currently at the University of California, San Francisco, where he is involved in the research of biomimetic formation of biomineralized tissues. He is a former member of Jožef Stefan Institute, Ljubljana, where he developed methods for the synthesis of magnetic nanoparticles applicable in the fields of electronics and biomedicine, and of the Center for Advanced Materials Processing of Clarkson University in Potsdam, NY, where he investigated the mechanisms for the precipitation of cholesterol. Besides his dedication to scientific research in the area of physical chemistry, he has published studies in the fields of cognitive science, ecology, philosophy, theology, arts and social science. Contact: Division of Biomaterials and Bioengineering, Department of Preventive and Restorative Dental Sciences, University of California, 707 Parnassus Avenue, San Francisco, CA 94143, USA.
References
- Adler SL, Bassi A. Is quantum theory exact? Science. 2009;235:275. doi: 10.1126/science.1176858. [DOI] [PubMed] [Google Scholar]
- Ahmad G, Dickerson MB, Church BC, Cai Y, Jones SE, Naik RR, King JS, Summers CJ, Kröger N, Sandhage KH. Rapid, room-temperature formation of crystalline calcium molybdate phosphor microparticles via peptide-induced precipitation. Advanced Materials. 2006;18:1759–1763. [Google Scholar]
- Anthes E. The Re-Envisionaries. Seed. 2008 Jul-Aug:76–84.
- Ashby RW. An introduction to cybernetics. London: Chapman & Hall; 1956. [Google Scholar]
- Ball P. Chemistry and power in recent American fiction. HYLE—International Journal for Philosophy of Chemistry. 2006a;12(1):45–66. [Google Scholar]
- Ball P. Chancing upon chemical wonders. Chemistry World. 2006b Jun32 [Google Scholar]
- Ball P. Does Hot water freeze first? Physics World. 2006 Apr19 [Google Scholar]
- Ball P. Water as an active constituent in cell biology. Chemical Reviews. 2008;108(1):74–108. doi: 10.1021/cr068037a. [DOI] [PubMed] [Google Scholar]
- Barnard AS. Computational strategies for predicting the potential risks associated with nanotechnology. Nanoscale. 2009;1:89–95. doi: 10.1039/b9nr00154a. [DOI] [PubMed] [Google Scholar]
- Barth A, Zscherp C. What vibrations tell us about proteins. Quarterly Review of Biophysics. 2002;35(4):369–430. doi: 10.1017/s0033583502003815. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Simmer JP. Proteinases in developing dental enamel. Critical Reviews in Oral Biology and Medicine. 1999;10(4):425–441. doi: 10.1177/10454411990100040101. [DOI] [PubMed] [Google Scholar]
- Bateson G. Steps to an ecology of mind. Chicago, IL: University of Chicago Press; 1972. [Google Scholar]
- Bateson G. Allegory. CoEvolution Quarterly. 1978 Spring:44–46. [Google Scholar]
- Bateson G. Mind and nature: A necessary unity. Cresskill, NJ: Hampton Press; 1979a. [Google Scholar]
- Bateson G. Time is out of joint. Memorandum for the board of regents of the University of California. In: Bateson G, editor. Mind and nature a necessary unity. Cresskill, NJ: Hampton Press; 1979b. [Google Scholar]
- Billinge SL, Levin I. The problem with determining atomic structure at the nanoscale. Science. 2007;316:561–565. doi: 10.1126/science.1135080. [DOI] [PubMed] [Google Scholar]
- Binnig G, Quate CF, Gerber Ch. Atomic force microscope. Physical Review Letters. 1986;56:930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Bizzarri AR, Cannistraro S. Molecular dynamics in water at the protein-solvent interface. Journal of Physical Chemistry B. 2002;106(26):6617–6633. [Google Scholar]
- Blackmore JT, Itagaki R, Tanaka S, editors. Ernst Mach’s Philosophy (Pro and Con) Sentinel Open Press; Orlando, FL: 2009. [Google Scholar]
- Blume A, Zemb Th. Self-assembly: Weak molecular forces at work for building mesoscopic architectures. Current Opinion in Colloid and Interface Science. 2002;7:66–68. [Google Scholar]
- Bochicchio B, Tamburro AM. Polyproline II structure in proteins: Identification by chiroptical spectroscopies, stability, and functions. Chirality. 2002;14:782–792. doi: 10.1002/chir.10153. [DOI] [PubMed] [Google Scholar]
- Bohm D. Wholeness and the implicate order. London: Ark Paperbacks; 1980. [Google Scholar]
- Bowker M. A prospective: Surface science and catalysis at the nano scale. Surface Science. 2009;603:2359–2362. [Google Scholar]
- Brewster D. Memoirs of the life, writings, and discoveries of Sir Isaac Newton. Boston, MA: Adamant; 1885. [Google Scholar]
- Bröcker M. Between the lines: The part-of-the-world position of heinz von foerster. Cybernetics and Human Knowing. 2003;10(2):51–65. [Google Scholar]
- Capra F. Complexity and life. Emergence. 2002;4(1/2):15–33. [Google Scholar]
- Capra F. The science of Leonardo: Inside the mind of the great genius of the renaissance. New York, NY: Doubleday; 2007. [Google Scholar]
- Capra F. The tao of physics. Belgrade: Opus; 1976. [Google Scholar]
- Cheney M. Tesla: Man out of time. London: Simon and Schuster; 2001. pp. 43–44. [Google Scholar]
- Cohen AJ, Mori-Sanchez P, Yang W. Insights into current limitations of density functional theory. Science. 2008;321(5890):792–794. doi: 10.1126/science.1158722. [DOI] [PubMed] [Google Scholar]
- Cooper A. Thermodynamics of protein folding and stability. In: Allen Geoffrey., editor. Protein: A comprehensive treatise. Vol. 2. JAI Press; Stamford, CN: 1999. pp. 217–270. [Google Scholar]
- Crocker JC. Interactions and Dynamics in Charge-Stabilized Colloid. MRS Bulletin. 1998;23:24–31. [Google Scholar]
- Crupi V, Interdonato S, Longo F, Majolino D, Migliardo P, Venuti V. A new insight in the hydrogen bonding structures of nanoconfined water: A Raman study. Journal of Raman Spectroscopy. 2007;39(2):244–249. [Google Scholar]
- Dahiyat BI, Mayo SL. De Novo protein design: Fully automated sequence selection. Science. 1997;278(5335):82–87. doi: 10.1126/science.278.5335.82. [DOI] [PubMed] [Google Scholar]
- de Saint-Exupery A. The little prince. Zagreb: Mladost; 1946. [Google Scholar]
- Denny MW. The intrigue of the interface. Science. 2008;320(5878):886. doi: 10.1126/science.1158189. [DOI] [PubMed] [Google Scholar]
- Derjaguin BV, Landau L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solution of electrolytes. Acta Physicochimica (URSS) 1941;14(6):633–662. [Google Scholar]
- Dimiduk DM, Woodward C, LeSar R, Uchic MD. Scale-free intermittent flow in crystal plasticity. Science. 2006;312(5777):1188–1190. doi: 10.1126/science.1123889. [DOI] [PubMed] [Google Scholar]
- Dirac P. The evolution of physicist’s picture of nature. Scientific American. 1963;208(5) [Google Scholar]
- Dostoyevsky FM. The idiot. London, UK: Vintage; 2003. [Google Scholar]
- Earley JE., Sr Varieties of properties: An alternative distinction among qualities. Annals of the New York Academy of Sciences. 2003;988:80–89. doi: 10.1111/j.1749-6632.2003.tb06087.x. [DOI] [PubMed] [Google Scholar]
- Einstein A. a letter to R. A. Thornton. 1944 Dec 7; retrieved from http://www.kleinerskorner.com/arch.2005.08.html.
- Fadini A, Schnepel F-M. Vibrational spectroscopy: Methods and applications. New York, NY: John Wiley & Sons; 1989. [Google Scholar]
- Feynman RP. The meaning of It all: Thoughts of a citizen scientist. Reading, MA: Helix Books/Addison-Wesley; 1998. [Google Scholar]
- Field R, Györgyi L, editors. Chaos in chemistry and biochemistry. Singapore: World Scientific; 1993. [Google Scholar]
- Fromm E. The art of loving. Zagreb: Naprijed; 1956. [Google Scholar]
- Future of Chemistry. Nature Chemistry. 2009;1:5–15. [Google Scholar]
- Gardner G, Sampat P. Mind over matter: Recasting the role of materials in our lives. W. W. Norton and Co; New York: 1998. Worldwatch Paper 144, WorldWatch Institute. [Google Scholar]
- Gärtner W. Enzyme catalysis reilluminated. Angewandte Chemie International Edition. 2009;48:2–4. doi: 10.1002/anie.200900840. [DOI] [PubMed] [Google Scholar]
- Gleick J. Chaos: Making a new science. London, UK: Heinemann; 1987. [Google Scholar]
- Greenberg A. Integrating Nanoscience into the classroom: Perspectives on nanoscience education projects. ACS Nano. 2009;3(4):762–769. doi: 10.1021/nn900335r. [DOI] [PubMed] [Google Scholar]
- Gribbin J. Deep simplicity: Chaos, complexity and the emergence of life. London: Penguin; 2004. [Google Scholar]
- Grier DG. When like-charges attract: Interactions and dynamics in charge-stabilized colloidal suspensions. Journal of Physics: Condensed Matter. 2000;12:A85–A94. [Google Scholar]
- Gun’ko VM, Turov VV, Bogatyrev VM, Zarko VI, Leboda R, Goncharuk EV, Novza AA, Turov AV, Chuiko AA. Unusual properties of water at hydrophilic/hydrophobic interfaces. Advances in Colloid and Interface Science. 2005;118:125–172. doi: 10.1016/j.cis.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Harre R. Structural explanation in chemistry and its evolving forms. Annals of the New York Academy of Sciences. 2003;988:1–15. doi: 10.1111/j.1749-6632.2003.tb06080.x. [DOI] [PubMed] [Google Scholar]
- Harrison AG, Treagust DF. Teaching and learning with analogies: Friend or foe? In: Aubusson PJ, Harrison AG, Ritchie SM, editors. Metaphor and analogy in science education. Dordrecht: Springer; 2005. [Google Scholar]
- Hartsthorne C. Science as the search for the hidden beauty of the world. In: Curtin DW, editor. The aesthetic dimension of science. New York, NY: Philosophical Library; 1980. [Google Scholar]
- He YP, Wang SQ, Li CR, Miao YM, Wu ZY, Zou BS. Synthesis and characterization of functionalized silica-coated Fe3O4superparamagnetic nanocrystals for biological applications. Journal of Physics D. 2005;38:1342–1350. [Google Scholar]
- Heisenberg W. Physics and philosophy. London: George Allen and Unwin Edition; 1959. [Google Scholar]
- Heisenberg W. Philosophical problems of quantum physics. Woodbridge, CT: Ox Bow Press; 1979. [Google Scholar]
- Hollingsworth RJ. High cognitive complexity and the making of major scientific discoveries. In: Sales A, Fournier M, editors. Knowledge, communication, and creativity. London, UK: Sage; 2007. pp. 129–155. [Google Scholar]
- James W. Pragmatism: A new name for some old ways of thinking. New York: Longman Green and Co; 1907. [Google Scholar]
- Jenck JF, Agterberg F, Droescher MJ. Products and processes for a sustainable chemical industry: A review of achievements and prospects. Green Chemistry. 2004;6:544–556. [Google Scholar]
- Johnson MA. Experiment and theory in harmony. Nature Chemistry. 2009;1:8–9. doi: 10.1038/nchem.145. [DOI] [PubMed] [Google Scholar]
- Kaku M. Einstein’s cosmos: How lbert Einstein’s vision transformed our understanding of space and time. New York, NY: W. W. Norton & Co; 2004. [Google Scholar]
- Kelly K. Out of control: The new biology of machines, social systems and the economic world. Reading, MA: Perseus Books; 1994. [Google Scholar]
- Kitcher P. A plea for science studies. In: Koertge N, editor. A House built on sand: Exposing postmodernist myths about science. Oxford, UK: Oxford University Press; 1998. [Google Scholar]
- Kondepudi DK, Prigogine I. Modern thermodynamics: From heat engines to dissipative structures. New York, NY: John Wiley & Sons; 1998. [Google Scholar]
- Kordeš U. From truth to trust. Ljubljana: Studia Humanitatis; 2004. [Google Scholar]
- Kortemme T, Baker D. Computational design of protein–protein interactions. Current Opinion in Chemical Biology. 2004;8(1):91–97. doi: 10.1016/j.cbpa.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Kuhn T. The structure of scientific revolutions. Belgrade: Nolit; 1969. [Google Scholar]
- Laszlo E. The systems view of the world: A holistic vision for our time. Cresskill, NJ: Hampton Press; 1996. [Google Scholar]
- Lindoy LF, Atkinson IM. Self-assembly in supramolecular systems. Cambridge: Royal Society of Chemistry; 2000. [Google Scholar]
- Lipscomb WN. Aesthetic aspects of acience. In: Curtin DW, editor. The aesthetic dimension of science. New York, NY: Philosophical Library; 1980. [Google Scholar]
- Lovelock J. Gaia: A new look at life on earth. Oxford, UK: Oxford University Press; 2000. [Google Scholar]
- Lovelock J. Gaia: Medicine for an ailing planet. London: Gaia Books; 2005. [Google Scholar]
- Lübbe AS, Bergemann C, Brock J, McClure DG. Physiological aspects in magnetic drug-targeting. Journal of Magnetism and Magnetic Materials. 1999;194:149–155. [Google Scholar]
- Malescio G. Intermolecular potentials—Past, present, future. Nature Materials. 2003;2:501–503. doi: 10.1038/nmat949. [DOI] [PubMed] [Google Scholar]
- Marcus RA. Interaction between experiments, analytical theories, and computation. Journal of Physical Chemistry C. 2009;113:14598–14608. [Google Scholar]
- Martí S, Roca M, Andrés J, Moliner V, Silla E, Tuñón I, Bertrán J. Theoretical Insights in Enzyme Catalysis. Chemical Society Review. 2004;33:98–107. doi: 10.1039/b301875j. [DOI] [PubMed] [Google Scholar]
- Martí S, Andrés J, Moliner V, Silla E, Tuñón I, Bertrán J. Computational design of biological catalysts. Chemical Society Reviews. 2008;371:2634–2843. doi: 10.1039/b710705f. [DOI] [PubMed] [Google Scholar]
- Matthiesen J, Wendt S, Hansen JØ, Madsen GKH, Lira E, Galliker P, Vestergaard EK, Schaub R, Lægsgaard E, Hammer B, Besenbacher F. Observation of all the intermediate steps of a chemical reaction on an oxide surface by scanning tunneling microscopy. ACS Nano. 2009;3:517. doi: 10.1021/nn8008245. [DOI] [PubMed] [Google Scholar]
- Mattingly J. Gauge theory and chemical structure. Annals of the New York Academy of Sciences. 2003;988:193–202. doi: 10.1111/j.1749-6632.2003.tb06098.x. [DOI] [PubMed] [Google Scholar]
- Maturana HR, Poerksen B. Varieties of objectivity. Cybernetics and Human 7Knowing. 2004;11(4):63–71. [Google Scholar]
- Maturana H, Varela F. The tree of knowledge: The biological roots of human understanding. Boston, MA: Shambhala; 1987. [Google Scholar]
- Medawar P. Is the scientific paper a fraud? The Listener. 1963 Sep 12;:377–378. [Google Scholar]
- Medd W. What is complexity science? Toward an ‘ecology of ignorance’. Emergence. 2001;3(1):43–60. [Google Scholar]
- Meier S, Jensen PR, David CN, Chapman J, Holstein TW, Grzesiak S, Özbek S. Continuous Molecular evolution of protein domain structures by single amino acid changes. Current Biology. 2007;17:173–178. doi: 10.1016/j.cub.2006.10.063. [DOI] [PubMed] [Google Scholar]
- Minor DL, Jr, Kim PS. Context-dependent secondary structure formation of a designed protein sequence. Nature. 1996;380:730–734. doi: 10.1038/380730a0. [DOI] [PubMed] [Google Scholar]
- Miranker AD. Fibres hinge on swapped domains. Nature. 2005;437(8):197–198. doi: 10.1038/437197a. [DOI] [PubMed] [Google Scholar]
- Muller DA. Structure and bonding at the atomic scale by scanning transmission electron microscopy. Nature Materials. 2009;8:263–267. doi: 10.1038/nmat2380. [DOI] [PubMed] [Google Scholar]
- Nørskov JK, Bligaard T, Rossmeisl J, Christensen CH. Towards the computational design of solid catalysts. Nature Chemistry. 2009;1:37–46. doi: 10.1038/nchem.121. [DOI] [PubMed] [Google Scholar]
- Ostwald CWW. An Introduction to theoretical and applied colloid chemistry. LLC: BiblioBazaar; 2009. [Google Scholar]
- Paine ML, White SN, Luo W, Fong H, Sarikaya M, Snead ML. Regulated gene expression dictates enamel structure and tooth function. Matrix Biology. 2001;20:273–292. doi: 10.1016/s0945-053x(01)00153-6. [DOI] [PubMed] [Google Scholar]
- Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of Magnetic Nanoparticles in Biomedicine. Journal of Physics D. 2003;36:R167–R181. [Google Scholar]
- Penrose R. The emperor’s new mind: Concerning computers, minds, and the laws of physics. Oxford, UK: Oxford University Press; 1989. [Google Scholar]
- Peschl MF. Constructivism, cognition, and science—An investigation of its links and possible shortcomings. Foundations of Science. 2001;6(1–3):125–161. [Google Scholar]
- Peyrard M. Nonlinear dynamics and statistical physics of DNA. Nonlinearity. 2004;17:R1–R40. [Google Scholar]
- Phelan SE. What is complexity science, really? Emergence. 2001;3(1):120–136. [Google Scholar]
- Pochapsky TC, Pochapsky SS. NMR for physical and biological scientists. New York, NY: Taylor & Francis; 2007. [Google Scholar]
- Poerksen B. At each and every moment, I can decide who I am: Heinz von Foerster on the Observer, dialogic life, and a constructivist philosophy of distinctions. Cybernetics & Human Knowing. 2003;10(3–4):9–26. [Google Scholar]
- Polanyi M. The value of the inexact. Philosophy of Science. 1936;13:233–234. [Google Scholar]
- Prigogine I. The end of certainty. Columbus, OH: Free Press; 1997. [Google Scholar]
- Rajagopalan R. Simulations of self-assembling systems. Current Opinion in Colloid and Interface Science. 2001;6:357–365. [Google Scholar]
- Rakovíc D. Integrative biophysics, quantum medicine and quantum-holographic informatics: Psychosomatic-cognitive implications. Belgrade: IASC & IEFPG; 2009. [Google Scholar]
- Rasmusen E. Games and information. New York, NY: Wiley; 2006. [Google Scholar]
- Re GD. Reaction mechanisms and chemical explanation. Annals of the New York Academy of Sciences. 2003;988:133–140. doi: 10.1111/j.1749-6632.2003.tb06092.x. [DOI] [PubMed] [Google Scholar]
- Richardson K, Cilliers P. What is complexity science? A view from different directions. Emergence. 2001;3(1):5–23. [Google Scholar]
- Richman EK, Hutchison JE. The nanomaterial characterization bottleneck. ACS Nano. 2009;3:2441–2446. doi: 10.1021/nn901112p. [DOI] [PubMed] [Google Scholar]
- Riddick TM. Control of colloid stability through zeta potential. Wynnewood: Livingston Publishing; 1968. [Google Scholar]
- Roberts RM. Serendipity: Accidental discoveries on science. New York, NY: Wiley; 1989. [Google Scholar]
- Romesin HM. Autopoiesis, structural coupling and cognition: A history of these and other notions in the biology of cognition. Cybernetics and Human Knowing. 2002;9(3–4):5–34. [Google Scholar]
- Rosen B. Spin me to the moon. 2008 retrieved from http://www.benrosen.com/files/87fe7b185f0be2a0fab10ff2a498a4f0-25.html.
- Royal Society and Royal Academy of Engineering. Nanoscience and Nanotechnologies: Opportunities and Uncertainties. London, UK: 2004. retrieved from http://www.nanotec.org.uk/finalreport.htm. [Google Scholar]
- Rueda M, Cubero E, Laughton CA, Orozco M. Exploring the counterion atmosphere around dna: What can be learned from molecular dynamics simulations? Biophysical Journal. 2004;87(2):800–811. doi: 10.1529/biophysj.104.040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DJ. Introduction to colloid and surface chemistry. Oxford, UK: Butterworth Heinemann; 1992. [Google Scholar]
- Thoreau HD. Walking. New York: HarperCollins; 1994. [Google Scholar]
- Thyssen Ole. TRUTH IS WAR: Conversations with Heinz von Foerster. Cybernetics and Human Knowing. 2003;10(3–4):179–181. [Google Scholar]
- Tirrell MV, Katz A, editors. Self-assembly in materials synthesis. MRS Bulletin. 2005;30 [Google Scholar]
- Tuxill J. Losing strands in the web of life: Vertebrate declines and the conservation of biological diversity. New York: W. W. Norton and Co; 1998. Worldwatch Paper 141 WorldWatch Institute. [Google Scholar]
- Ulanowicz Robert E. On the nature of ecodynamics. Ecological Complexity. 2004;1:341–354. [Google Scholar]
- Urban KW. Is science prepared for atomic- resolution electron microscopy? Nature Materials. 2009;8:260–262. doi: 10.1038/nmat2407. [DOI] [PubMed] [Google Scholar]
- Uskokovíc V. Theoretical and practical aspects of colloid science and self-assembly phenomena revisited. Reviews in Chemical Engineering. 2007a;23(5):301–372. [Google Scholar]
- Uskokovíc V. Questions and challenges for the upcoming trends in practical colloid science. In: Scarpetti Emelio A., editor. Progress in colloid and surface science research. Hauppauge, NY: Nova Science Publishers; 2007b. [Google Scholar]
- Uskokovíc V. Nanotechnologies: What we do not know. Technology in Society. 2007c;29(1):43–61. [Google Scholar]
- Uskokovíc V. Insights into morphological nature of precipitation of cholesterol. Steriods. 2008a;73:356–369. doi: 10.1016/j.steroids.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Uskokovíc V. Surface charge effects involved in the control of stability of sols comprising uniform cholesterol particles. Materials and Manufacturing Processes. 2008b;23(6):620–623. [Google Scholar]
- Uskokovíc V. Of sustainability, elephants and prefab sprouts. International Journal of Sustainable Society. 2008c;1(1):85–102. [Google Scholar]
- Uskokovíc V. Nanomaterials and nanotechnologies: Approaching the crest of this big wave. Current Nanoscience. 2008d;4:119–129. [Google Scholar]
- Uskokovíc V. Isn’t self-assembly a misnomer? multi-disciplinary arguments in favor of co-assembly. Advances in Colloid and Interface Science. 2008e;141(1–2):37–47. doi: 10.1016/j.cis.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Uskokovíc V. Trends in Practical colloid science. Hauppauge, NY: Nova Science Publishers; 2009a. [Google Scholar]
- Uskokovíc V. On science of metaphors and the nature of systemic reasoning. World Futures: Journal of General Evolution. 2009b;65:241–269. [Google Scholar]
- Uskokovíc V. On the relational character of mind and nature. Res Cogitans: Journal of Philosophy. 2009c;6(1):286–400. [Google Scholar]
- Uskokovíc V. A collection of micrographs: Where science and art meet. Technoetic Arts: A Journal of Speculative Research. 2009d doi: 10.1386/tear.7.3.231/1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uskokovíc V. On the light doves and learning on mistakes. Axiomathes: An International Journal in Ontology and Cognitive Systems. 2009e;19:17–50. [Google Scholar]
- Uskokovíc V. The metaphorical model: The bridge between science and religion. Journal for Interdisciplinary Research on Religion and Science. 2010a;6:11–34. [Google Scholar]
- Uskokovíc V. A collection of micrographs: Where science and art meet. Technoetic Arts: A Journal of Speculative Research. 2010b;7(3):231–248. doi: 10.1386/tear.7.3.231/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uskokovíc V, Drofenik M. Synthesis of materials within reverse micelles. Surface Review and Letters. 2005;12(2):239–277. [Google Scholar]
- Uskokovíc V, Matijevíc E. Uniform particles of pure and silica coated cholesterol. Journal of Colloid and Interface Science. 2007;315(2):500–511. doi: 10.1016/j.jcis.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Uskokovíc V, Košak A, Drofenik M. Preparation of silica-coated lanthanum-strontium manganite particles with designable curie point, for application in hyperthermia treatments. International Journal of Applied Ceramic Technology. 2006;3(2):134–143. [Google Scholar]
- van Embden J, Sader JE, Davidson M, Mulvaney P. Evolution of colloidal nanocrystals: Theory and modeling of their nucleation and growth. Journal of Physical Chemistry C. 2009;113:16342–16355. [Google Scholar]
- van Gunsteren WF, Bakowies D, Baron R, Chandrasekhar I, Christen M, Daura X, Gee P, Geerke DP, Glattli A, Hunenberger PH, Kastenholz MA, Oostenbrink C, Schenk M, Trzesniak D, van der Vegt NFA, Yu HB. Biomolecular modeling: Goals, problems, perspectives. Angewandte Chemie International Edition English. 2006;45:4064–4092. doi: 10.1002/anie.200502655. [DOI] [PubMed] [Google Scholar]
- van Gunsteren WF, Dolenc J, Mark AE. Molecular simulation as an aid to experimentalists. Current Opinion in Structural Biology. 2008;18:149–153. doi: 10.1016/j.sbi.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Verwey EJW, Overbeek JTG, Van Ness K, editors. Theory of the stability of lyophobic colloids—The interactions of soil particles having an electrical double layer. Amsterdam: Elsevier; 1948. [Google Scholar]
- Vlieg E, Deij M, Kaminski D, Meekes H, van Enckevort W. Towards an atomic-Scale understanding of crystal growth in solution. Faraday Discussions. 2007;136:57–69. doi: 10.1039/b618566p. discussion 107–123. [DOI] [PubMed] [Google Scholar]
- Vogler EA. Structure and reactivity of water at biomaterial surfaces. Advances in Colloid and Interface Science. 1998;74:69–117. doi: 10.1016/s0001-8686(97)00040-7. [DOI] [PubMed] [Google Scholar]
- von Bertalanffy L. General system theory: Foundations, development, applications. New York, NY: George Braziller; 1968. [Google Scholar]
- von Foerster H, Poerksen B. The metaphysics of ethics: A conversation. Cybernetics and Human Knowing. 2002;9(3–4):149–157. [Google Scholar]
- von Glasersfeld E. Radical constructivism: A way of knowing and learning. London, UK: RoutledgeFalmer; 1995. [Google Scholar]
- Wang L, Sondi I, Matijevíc E. Preparation of uniform needle-like aragonite particles by homogeneous precipitation. Journal of Colloid and Interface Science. 1999;218:545–553. doi: 10.1006/jcis.1999.6463. [DOI] [PubMed] [Google Scholar]
- Waters C. Invitation to dance—A conversation with Heinz von Foerster. Cybernetics and Human Knowing. 1999;6(4):81–84. [Google Scholar]
- Whitehead AN. Science and the modern world. New York, NY: Mentor; 1925. [Google Scholar]
- Whitehead AN. Process and reality. Columbus, OH: Free Press; 1928. [Google Scholar]
- Whitesides GM. Revolutions in Chemistry. Chemical & Engineering News. 2007 Mar 26;:12–17. [Google Scholar]
- Whitesides G, Grzybowski B. Self-assembly at all scales. Science. 2002;295:2418–2421. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- Wilde PJ. Interfaces: Their role in foam and emulsion behaviour. Current Opinion in Colloid and Interface Science. 2000;5:176–181. [Google Scholar]
- Winograd T, Flores F. Understanding computers and cognition: A new foundations for design. Norwood, NJ: Ablex Publishing Corporation; 1987. [Google Scholar]
- Wittgenstein L. Tractatus logico-philosophicus. London: Routledge; 1918. [Google Scholar]
- Wolfram S. A new kind of science. Champaign, IL: Wolfram Media; 2004. [Google Scholar]
- Yang CN. Beauty and theoretical physics. In: Curtin DW, editor. The aesthetic dimension of science. New York, NY: Philosophical Library; 1980. [Google Scholar]
- Yeates TO. Protein structures: Evolutionary bridges to new folds. Current Biology. 2007;17(2):R48–R50. doi: 10.1016/j.cub.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Zukav G. The Dancing Wu Li Masters: An overview of the new physics. London, UK: Random House; 1979. [Google Scholar]