Abstract
Background
Inadvertent removal of parathyroid glands is a challenge in endocrine operations. There is a critical need for a diagnostic tool that provides sensitive, real-time parathyroid detection during procedures. We have developed an intraoperative technique using near-infrared (NIR) fluorescence for in vivo, real-time detection of the parathyroid regardless of its pathologic state.
Methods
NIR fluorescence was measured intraoperatively from 45 patients undergoing parathyroidectomy and thyroidectomy. Spectra were measured from the parathyroid and surrounding neck tissues during the operation with the use of a portable, probe-based fluorescence system at 785-nm excitation. Accuracy was evaluated by comparison with histology or visual recognition by the surgeon.
Results
NIR fluorescence detected the parathyroid in 100% of patients. Parathyroid fluorescence was stronger (1.2–18 times) than that of the thyroid with peak fluorescence at 822 nm. Surrounding tissues showed no auto-fluorescence. Disease state did not affect the ability to discriminate parathyroid glands but may account for signal variability.
Conclusions
NIR fluorescence spectroscopy can detect intraoperatively the parathyroid regardless of tissue pathology. The signal may be caused by calcium-sensing receptors present in the parathyroid. The signal strength and consistency indicates the simplicity and effectiveness of this method. Its implementation may limit operative time, decrease costs, and improve operative success rates.
Thyroid and parathyroid diseases require thyroidectomy and parathyroidectomy to remove both benign and malignant glands in more than 80,000 patients per year in the United States.1 These procedures require careful resection of the diseased gland(s) while preserving healthy tissues in the neck. Problems arise because of the small size and variable position of the parathyroid glands, making them difficult to distinguish from thyroid and surrounding tissues in the neck. Accidental removal or injury of the parathyroid glands during thyroidectomy can cause long-term issues with calcium regulation as the result of hypoparathyroidism and subsequent hypocalcemia. Incomplete removal of or inability to identify hypersecreting parathyroid tissue during parathyroidectomy may require reoperation as the result of persisting hyperparathyroidism. Surgeons must rely on visual inspection to distinguish tissues, which can be subjective and inconclusive. In many cases, patients fail to achieve postoperative normocalcemia because of the infrequency of cases and limited experience of the individual surgeon.1,2
Localization and identification of parathyroid glands has been attempted by a variety of techniques, such as preoperative ultrasound, Sestamibi scintigraphy, computed tomography (CT), magnetic resonance imaging, and intraoperative intact parathyroid hormone assay.1 These existing methods for identifying parathyroid glands are limited in their applicability and sensitivity, rendering them inadequate to prevent surgical complications.3 Most rely on preoperative identification of diseased parathyroid glands either by size or uptake of radiotracer, limiting their intraoperative utility. Cost, especially for CT scanning and magnetic resonance imaging, remains prohibitive for routine use. The turnaround time for intact parathyroid hormone monitoring with state-of-the-art assays still runs at least 9 minutes, not taking into account the travel time of the sample to the assay machine. The sensitivity of Sestamibi and preoperative ultrasound is only 47–61% as reported in most studies.4,5 Therefore, there remains a need for a way to accurately identify parathyroid tissue intraoperatively.
Fluorescence spectroscopy has been used in several other applications for the identification of tissues. It has been used to detect and diagnose many diseases such as brain tumors,6 skin conditions,7 and esophageal disease8 as well as evaluate surgical margins after resections for breast cancer.9 We have previously conducted a pilot study using NIR fluorescence spectroscopy to detect the parathyroid during surgical procedures.10 NIR wavelengths are attractive in biomedical applications due to their increased penetration depth and decreased scattering and absorption in tissues relative to ultraviolet and visible wavelengths.11 Furthermore, the NIR region is considered the optical window because there are no naturally occurring fluorophores known to emit peak fluorescence at NIR wavelengths. This makes the NIR region optimal for medical applications.12
We have developed an optical method to detect the parathyroid from other tissues in neck in a way that overcomes limitations of surgeon-based identification and current localization techniques. Using NIR fluorescence spectroscopy, we hypothesize that we can provide sensitive, real-time information to the surgeon about the location of the parathyroid regardless of disease state.
Patients and Methods
Patients with primary thyroid or parathyroid disease undergoing parathyroidectomy or thyroidectomy at the Vanderbilt Endocrine Surgery Center were considered for this study. The operating endocrine surgeon determined the eligibility of each patient by evaluating their overall health in a preoperative assessment at the Vanderbilt Clinic. Under the approval of the Institutional Review board, written consent was obtained for a total of 45 patients between the ages of 18 and 99, regardless of sex or race.
Fluorescence measurement
For optical detection of the parathyroid gland during surgery, we used a portable fluorescence spectroscopy system designed for clinical use shown in Fig 1. The system is composed of a near-infrared (NIR) diode laser (U-type; IPS, Monmouth Junction, NJ) coupling 785-nm light through a 7-around-1 sterilized fiber optic probe. Tissue was irradiated with 80 mW of light in a 400-μm spot size. Fluorescence was detected by a spectrometer (S2000-FL; Ocean Optics, Dundelin, FL) via the collection fibers. Inline filtering at the tip of the fiber optic and at the port of the spectrometer ensured that no visible or laser light interfered with the fluorescence signal. Fluorescence measurements were controlled by a laptop computer running custom software in Lab-View (National Instruments, Austin, TX).
Fig 1.
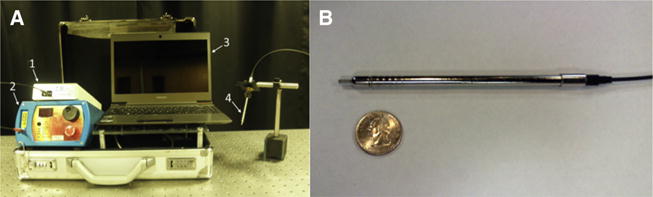
(A) NIR fluorescence spectroscopy system showing a (1) spectrometer, (2) 785-nm diode laser, (3) laptop computer, and (4) hand-held fiber optic probe. (B) Fiberoptic probe next to a quarter to show scale.
Measurements were collected in the operating room after the parathyroid and thyroid were exposed. The surgeon placed the pencil-sized fiberoptic probe onto multiple tissue sites while fluorescence spectra were measured. Spectra from the parathyroid and thyroid were measured for all patients, as well as fat, muscle, lymph, and trachea, depending on accessibility. All measurements were made with the overhead lights turned off and the operating lights directed away from the patient. Background measurements were collected with the laser off. Six spectra were collected from each tissue site with an integration time of 300 ms per spectra. The confidence level (high, medium, or low) of the surgeon in visually identifying each tissue type was recorded. Because visual identification is the technique used most commonly for intraoperative parathyroid detection, it was used as a point of comparison to assess the performance of this technology. Tissue sites with a low operative confidence level were excluded from analysis unless there was available histology from that tissue. Histology was compared with the measured fluorescence spectra and used as the gold standard for parathyroid detection. It was not feasible to collect parathyroid gland histology from every patient because tissue determined to be healthy is not removed during the operation.
Data processing and statistical analysis
Processing and analysis of the NIR spectra were performed postoperatively. In MATLAB software (Mathworks, Inc, Natick, MA), the spectra for each tissue site were averaged and processed for noise smoothing and background subtraction. Day-to-day variations in the system were corrected by performing spectral calibration with a neon argon lamp. The wavelength-dependent response of the system was corrected using a National Institute of Standards and Technology–calibrated tungsten lamp. After data processing, the spectra were normalized to the maximum fluorescence intensity of the thyroid to account for intensity variability across the patient population. The peak parathyroid to thyroid fluorescence intensity ratio (P/T ratio) was calculated for each patient. A P/T ratio equal to or less than one indicates that NIR fluorescence spectroscopy is incapable of distinguishing between tissue types.
The normalized spectra were categorized according to the following disease states: benign thyroid disease, malignant thyroid disease, hyperthyroidism, and hyperparathyroidism. The data was analyzed using a 2-sample t-test to examine the differences in the peak parathyroid and thyroid autofluorescence intensities.
Results
The goal of this study was to determine whether NIR fluorescence spectroscopy could distinguish the parathyroid gland from the thyroid and all other tissues in the neck during surgery, regardless of disease type Fluorescence spectra of these tissues were obtained from 45 patients. Of these patients, 14 (31%) underwent thyroidectomy for benign thyroid disease, 6 (13%) underwent thyroidectomy for malignant disease, 9 (20%) underwent thyroidectomy for hyperthyroidism, and 16 (36%) underwent parathyroidectomy for primary or secondary hyperparathyroidism. Three patients fell in two disease categories.
For all patients, the fluorescence intensity of the parathyroid was greater than all other tissues regardless of disease state. The thyroid exhibited a weaker fluorescence signal than the parathyroid but consistently stronger than that of muscle, fat, and surrounding tissues, which showed no fluorescence. Using a 2-sample t-test at a 99.9% significance level, the parathyroid fluorescence intensity was found to be greater than thyroid fluorescence with a P value of 5.91 × 10−14. A typical spectrum collected during surgery shows a fluorescence peak at about 822 nm for the thyroid and the parathyroid (Fig 2). The spectral shape of the parathyroid and thyroid curves shows similarity when normalized to their respective peaks. The P/T ratio ranged from 1.2 to 18 for patients in all disease categories as shown in Fig 3. A one-way analysis of variance test was performed to determine whether the observed autofluorescence signal was affected by disease state. Results showed no difference in P/T ratio across disease types (P < .01).
Fig 2.
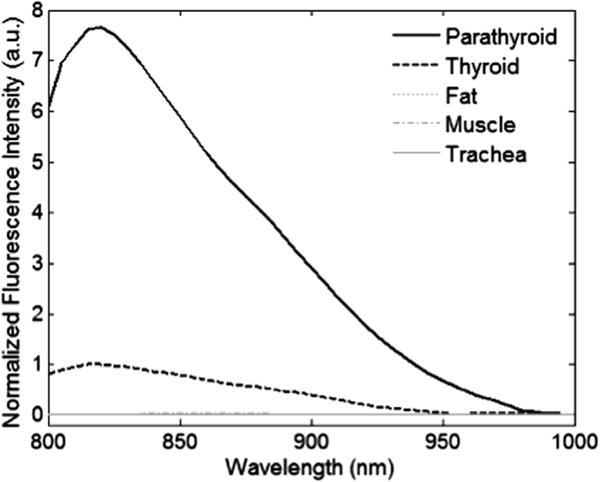
Typical normalized NIR spectra. Each spectrum is taken as the average of six measurements at the site of investigation. The parathyroid signal is significantly stronger than the anything else in the neck. It is 7.5 times greater than the thyroid peak intensity.
Fig 3.
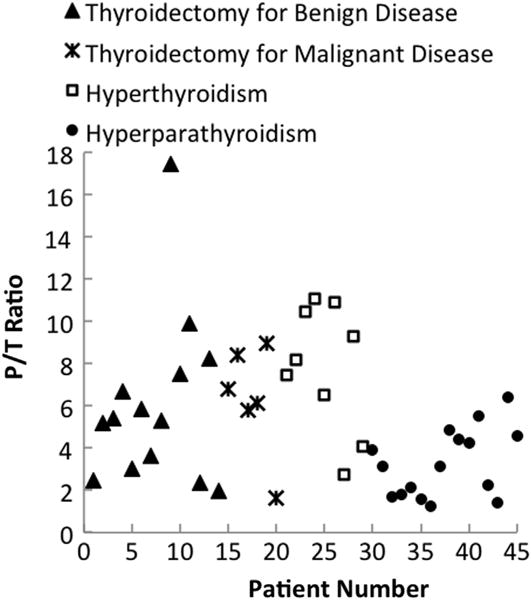
The ratio of peak parathyroid to thyroid fluorescence intensity ranges from 1.2 to 18 for all disease types. Patients are broken down by disease type to show variation in parathyroid autofluorescence. *Indicates patient also underwent parathyroidectomy for hyperparathyroidism.
Histologic validation of the parathyroid gland was available for 22 of the 45 patients. In these patients, spectral measurements were collected before resection. Tissue histology confirmed the finding that parathyroid tissue exhibits stronger fluorescence than thyroid tissue. Table shows NIR fluorescence spectroscopy can identify tissue type with greater accuracy than visual inspection by the surgeon. This is evident in cases 3, 5, and 18, in which visual assessment of the surgeon incorrectly identified tissues which NIR fluorescence spectroscopy correctly identified compared with histology. With 22 patients who have histology to confirm the tissue identity determined from spectral measurements, the power of this study is 99.89% for detecting a 5% difference in NIR auto-fluorescence between thyroid and parathyroid tissue. However, more patients must be measured in each disease category before further conclusions can be drawn on the source of the variability across patients as is relates to disease state.
Table. Tissue identification using fluorescence spectroscopy compared with visual inspection and histology.
| Case | Surgeon assessment (by visual assessment) | Confidence | P/T ratio | Histology |
|---|---|---|---|---|
| 1 | Parathyroid | High | 1.63 | Parathyroid |
| 2 | Parathyroid | Medium | 3.969 | Parathyroid |
| 3 | Parathyroid | High | 7.9 | Parathyroid |
| Parathyroid | Low/Medium | 0.23 | Benign fatty tissue | |
| 4 | Parathyroid | High | 11.3 | Parathyroid |
| 5 | Unknown | Low | 3.63 | Parathyroid |
| 6 | Parathyroid | Medium/High | 3.12 | Parathyroid |
| 7 | Parathyroid | Medium/High | 4.55 | Parathyroid |
| 8 | Parathyroid | Medium | 1.82 | Parathyroid |
| 9 | Parathyroid | High | 3.64 | Parathyroid |
| 10 | Parathyroid | High | 1.57 | Parathyroid |
| 11 | Parathyroid | High | 8.95 | Parathyroid |
| 12 | Parathyroid | Medium | 1.2 | Parathyroid |
| 13 | Parathyroid | High | 3.12 | Parathyroid |
| 14 | Parathyroid | High | 4.82 | Parathyroid |
| 15 | Parathyroid | High | 4.41 | Parathyroid |
| 16 | Parathyroid | High | 4.28 | Parathyroid |
| 17 | Parathyroid | High | 5.51 | Parathyroid |
| 18 | Parathyroid | High | 0.88 | Thyroid |
| Parathyroid | High | 2.69 | Parathyroid | |
| 19 | Parathyroid | High | 1.98 | Parathyroid |
| 20 | Parathyroid | High | 4.55 | Parathyroid |
| 21 | Parathyroid | High | 1.36 | Parathyroid |
| 22 | Parathyroid | High | 6.40 | Parathyroid |
In cases 1–22, near-infrared spectroscopy results are confirmed by tissue histology. In cases 3, 5, and 18, visual inspection by the surgeon incorrectly identified the tissue type.
P/T ratio, Parathyroid to thyroid fluorescence intensity ratio.
The parathyroid was accurately detected with NIR fluorescence spectroscopy in 100% of patients. When the detection threshold is set to a P/T ratio of 1.2 the sensitivity is 1.0 and the specificity is 1.0. Peak sensitivity and specificity for common parathyroid detection modalities are 60% and 81% for CT,13 47% and 87% for ultrasound,4 and 61% and 84% for Sestamibi scintigraphy.5 NIR fluorescence spectroscopy was able to detect parathyroid tissue with higher sensitivity and specificity than all other modalities.
Discussion
We present a novel tool that uses the intrinsic NIR autofluorescence to detect parathyroid tissue and guide surgeons in real-time during endocrine surgeries. The results reported here show that NIR fluorescence spectroscopy is capable of reliable and repeatable detection of the parathyroid gland in patients undergoing thyroidectomy and parathyroidectomy regardless of the disease state. For all patients, parathyroid fluorescence intensity is greater than thyroid fluorescence for all patients. Fat, muscle, lymph node, and other surrounding neck tissues do not exhibit any fluorescence, allowing surgeons to distinguish parathyroid glands from fat or other tissues. When normalized to their respective peaks, the spectra from the parathyroid and thyroid exhibit similarity in their spectral line shape. Both tissue types show only one emission peak at 822 nm upon excitation with 785 nm light, indicating that the autofluorescence is a result of a single, naturally occurring fluorophore present in both the parathyroid and thyroid.
The identity of the fluorophore is presently unknown, and there are no reports of biological fluorophores with peak fluorescence around 822 nm. Our leading hypothesis is that the NIR autofluorescence in the parathyroid is caused by the calcium-sensing receptor (CaSR). CaSRs are involved in controlling synthesis and secretion of PTH and calcitonin. The greatest levels of CaSR expression are found in parathyroid cells, and smaller concentrations are found in the C-cells of the thyroid but nowhere else in the muscle, fat, or lymph of the neck region. This provides a fluorophore that is present in high concentrations in parathyroid tissue and low concentrations in thyroid tissue making CaSR a highly probable candidate for the observed fluorescence. Cell culture studies must be done to determine the mechanism of the parathyroid autofluorescence.
The variability in the parathyroid to thyroid fluorescence intensity ratio (P/T ratio) ranges from 1.2 to 18. There are many factors that may contribute to the interpatient variability in the P/T ratio, the most prominent being patient disease state. Insufficient visual diagnosis may also contribute to variability in the P/T ratio across patients. Six patients demonstrated a P/T ratio between 1 and 2. Although the degree of variability in the ratio needs to be better understood and could be potentially related to the interpatient variability or fat overlaying the parathyroid during measurement, even the 20% difference was found to be statistically significant (P = 5.91 × 10−14) and reproducible.
We have shown that NIR fluorescence spectroscopy of the parathyroid can improve upon the visual inspection of the surgeon during endocrine procedures. On its own, visual diagnosis of the parathyroid is highly subjective and depends heavily on the experience and frequency in which the surgeon performs these procedures. Our analysis of 22 tissue samples identified on the basis of either visual diagnosis or NIR fluorescence spectroscopy and compared with histology confirm visual inspection is insufficient as a stand-alone detection method. In 3 of the 22 cases shown in Table, the tissue was misdiagnosed by the surgeon and properly diagnosed by NIR fluorescence spectroscopy. Case 3 shows an example of the surgeon improperly labeling fat tissue as parathyroid tissue. Case 5 shows an example of the surgeon unable to identify a sample of tissue as the parathyroid gland. In case 18, the surgeon misidentifies thyroid as parathyroid tissue. If these cases went uncorrected, the error could result in persistent hyperparathyroidism and the necessity of reoperation because of inadequate removal of all abnormal tissue at the time of initial operation. Also, during thyroid resections, inaccurate identification of parathyroid tissue could lead to inadvertent resection of one or more parathyroid glands leading to potential hypoparathyroidism and permanent dependence on calcium salts and vitamin D analogs.
NIR fluorescence spectroscopy can achieve a sensitivity and specificity of 100% when the detection threshold is set to a P/T ratio > 1. This result shows higher accuracy in parathyroid detection than preoperative imaging techniques such as CT, ultrasonography, and Sestamibi scintigraphy.1,14 Unlike Sestamibi scintigraphy, NIR autofluorescence can distinguish both normal and abnormal functioning parathyroid glands. Current intraoperative detection methods such as frozen section analysis and needle aspiration with parathyroid hormone analysis do not offer the time, cost, and safety benefits of NIR fluorescence spectroscopy. Frozen section analysis represents great cost to the patient and long waiting times associated with pathological processing. This method also risks damaging/destroying the parathyroid when tissue is removed for analysis. Needle aspiration with parathyroid hormone assay allows for accurate identification of the parathyroid gland, but the false-negative rate is as high as 13%.15,16 Alternatively, NIR fluorescence spectroscopy is capable of identifying the parathyroid in 30–40 seconds without damage to the tissue. The briefcase-sized spectroscopy system is associated with a one-time cost of approximately $8,000. Because the tissue discrimination can be automated yielding an answer in real time and data acquisition can be performed by clinical personnel, a trained technician is not required and will not add to the cost of this device. Because the system is fully reusable, the upfront cost can be defrayed over its lifetime. It has the added benefits of being portable and requiring minimal set-up time (3 minutes) in the operating room. With further investigations of this technology, the added steps of turning off room lights and diverting overheads lights may be able to be bypassed as well.
The parathyroid detection tool proposed here is unique because it relies on intrinsic fluorescence in the NIR region, excluding the need for exogenous chemicals. Fluorescence detection modalities operating on the autofluorescence of biological tissue are commonly used clinically for applications such as breast tumors,17 gliomas,18 and cervical precancers.19 However, many of these tools use ultraviolet or visible light because biological fluorophores typical exhibit peak autofluorescence in this range. The NIR region is considered an ideal optical window because it provides a deep penetration depth in tissue and there are few known biological fluorophores that emit at these wavelengths.
This method may have some limitations. Though NIR light at 785 nm penetrates several millimeters deep in tissue, the tissue must be exposed for the optical probe to deliver light and collect the signal. If the other tissue such as fat is overlaying the parathyroid, the fluorescence signal will be affected. However, this issue can be addressed by altering the collection geometry of the probe to allow for subsurface parathyroid gland and adenoma detection. Second, the probe-based system provides point measurements in tissue. This means surgeons are unable to scan the surgical field and receive spatial information about the location of the parathyroid. However, the fiberoptic probe has the advantage of allowing the surgeon to reach under layers of tissue to obtain a measurement. Visual inspection is necessary to provide guidance to the point of measurement. The development of an imaging device could improve the diagnostic capacity of this technology by allowing the surgeon to view the entire operative field in two dimensions. Work is being done to develop such an imaging system that would provide intuitive information to the surgeons to detect the glowing parathyroid gland(s) in real-time.
Conclusions
This study presents a novel tool using NIR autofluorescence spectroscopy to detect the parathyroid gland in real time during endocrine surgeries. We have shown its ability to accurately detect the parathyroid regardless of disease state with greater sensitivity and specificity than other imaging modalities including visual diagnosis. The mechanism of the autofluorescence is still unknown, but this technique shows promise as an intraoperative tool for improving success of parathyroidectomies and thyroidectomies.
Acknowledgments
Supported by NCI 1R25CA136440-01 and NIH R41 EB015291.
Biographies
Discussion
Dr Mark S. Cohen (Ann Arbor, MI): Have you looked at any differences in detection between hypercellular parathyroid and normal parathyroid glands? Also, I noticed on your scatterplot that when you looked at patients with hyperparathyroidism, a lot of the PT ratios were in the lower range, some of which were under 2. Do you have any idea why that was a little different than with thyroid disease states?
Dr James Broome: We looked across all disease; therefore, the variety of these cases were thyroid cases in which we exposed the parathyroid gland and then took the opportunity to take a measurement from it. So we don't really see a lot of difference between hyperparathyroidism and just normal parathyroid states. When you do look, it sort of seems to be lower overall; but, statistically, it didn't show any difference at this point. Parathyroid adenomas are known to have down-regulated/less expression of the calcium sensing receptor, which may explain the observed trend.
Now, the power analysis of the study would say that we ought to be able to detect a difference in parathyroid to thyroid, but we certainly need more patients across the disease categories to try to get an idea of what, if any, difference there is in those different categories. Unfortunately, if we don't know what the underlying mechanism of this autofluorescence is, it's a little hard to predict the differences.
Dr Brian D. Saunders (Hershey, PA): Were there any detectable differences in the autofluorescence should the parathyroid incidentally become devascularized? Were you able to detect if a parathyroid was ischemic and needed to be transplanted?
Dr James Broome: Unfortunately no. We have taken ex vivo measurements. A lot of the initial studies were done on parathyroid tissue that had been stored for other reasons. And you get the same sort of autofluorescence and the same increase in signal from the parathyroid to the thyroid. So whatever this underlying mechanism is, I don't think it's related to the viability of the tissue. There still needs to be a competent surgeon examining the parathyroid gland and trying to decide whether it's viable. But the current method of dropping the tissue in question in a cup of saline and seeing if it floats or sinks, I think this is a little more accurate than that method. And that's a lot of the idea behind this technology.
Dr Gregory W. Randolph (Boston, MA): Did you notice that the moistness of the overlying field or blood staining affected this in any way?
Dr James Broome: We didn't notice any overall interference. Moisture, fluid, or blood does not impede NIR light. Now, the near infrared laser will penetrate a couple of centimeters or more. And what you're really limited by is any sort of fatty tissue overlying the gland that causes optical scattering. You can still detect autofluorescence, but it's a little less pinpoint or focused.
Dr Gregory W. Randolph (Boston, MA): And then, can you give us a sense of this laser power? What sort of level of laser is it in terms of how we can anticipate it may injure tissue or not injure tissue in terms of its strength?
Dr James Broome: It's relatively low. You don't want to look at the laser directly with your eye, no different than any other kind of laser pointer. But 80 milliwatts is not a very high power overall. And to be honest, as significant as the intensity ratio is between parathyroid to thyroid, we could probably and probably are going to plan on doing some studies on turning down the power of the laser even more. But at 80 milliwatts, it shouldn't cause any damage and we haven't noticed it to cause any damage to tissues at this point.
Dr A. Bergenfelz (Lund, Sweden): NIR fluorescence has been used also to identify lymph nodes, especially within the concept of sentinel node. Did you identify any lymph nodes, and especially did you measure anything from them? And what was then the ratio between the parathyroid and the lymph nodes?
Dr James Broome: So we looked at lymph nodes and just like muscle, trachea, and other tissues in the neck, lymph nodes don't have any inherent autofluorescence themselves. One of the things we thought is if you get a signal with thyroid or parathyroid, we have looked into is it possible then to do NIR fluorescence to identify pathologic or malignant involvement of lymph nodes? If it's driven by the calcium-sensing receptor, then perhaps medullary thyroid cancer would be a good modality to pick this up. But we don't have enough medullary patients at this time that we've had a chance to evaluate that. But just normal or regular lymphadenectomy should have no near infrared fluorescence signal at this point.
Dr Haggi Mazeh (Jerusalem, Israel): Have you considered comparing it head to head with gamma probe? I think it would be interesting to look at the PT ratio in comparison to the gamma probe reading and see if it correlates.
Dr James Broome: We haven't just as of yet. I don't personally use the gamma probe during surgery. I would be interested to see in a comparison between the two, and then, is this any easier to detect the background signal you're going get from thyroid versus parathyroid that is seen with a gamma probe versus NIR autofluorescence and draw a comparison that way. I think it would be an interesting study.
References
- 1.Mohebati A, Shaha AR. Imaging techniques in parathyroid surgery for primary hyperparathyroidism. Am J Otolaryngol. 2012;33:457–68. doi: 10.1016/j.amjoto.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malmaeus J, Granberg PO, Holvorsen J, Akerstrom G, Johansson H. Parathyroid surgery in Scandanavia. Acta Chir Scand. 1988;33:1364–7. [PubMed] [Google Scholar]
- 3.Prosst RL, Gahlen J, Schnuelle P, Post S, Willeke F. Fluorescence-guided minimally invasive parathyroidectomy: a novel surgical therapy for secondary hyperparathyroidism. Am J Kidney Dis. 2006;48:327–31. doi: 10.1053/j.ajkd.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Patel CN, Salahudeen HM, Lansdown M, Scarsbrook AF. Clinical utility of ultrasound and 99mTc Sestamibi SPECT/CT for preoperative localization of parathyroid adenoma in patients with primary hyperparathyroidism. Clin Radiol. 2010;65:278–87. doi: 10.1016/j.crad.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Nichols KJ, Tomas MB, Tronco GG, Palestro CJ. Sestamibi parathyroid scintigraphy in multigland disease. Am J Otolaryngol. 2012;33:43–50. doi: 10.1097/MNM.0b013e32834bfeb1. [DOI] [PubMed] [Google Scholar]
- 6.Butte PV, Mamelak AN, Nuno M, Bannykh SI, Black KL, Marcu L. Fluorescence lifetime spectroscopy for guided therapy of brain tumors. Neuorimage. 2011;54:125–35. doi: 10.1016/j.neuroimage.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemos MC. Fluorescence spectroscopy as a tool to detect and evaluate glucocorticoid-induced skin atrophy. Lasers Med Sci. 2012;27:1059. doi: 10.1007/s10103-011-1045-4. [DOI] [PubMed] [Google Scholar]
- 8.DaCosta RS, Wilson BC. Spectroscopy and fluorescence in esophageal disease. Best Pract Res Clin Gastroenterol. 2006;20:41–57. doi: 10.1016/j.bpg.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Keller MD, Majumder SK, Kelley MC, Meszoely IM, Boulos FI, Olivares GM, et al. Autofluorescence and diffuse reflectance spectroscopy and spectral imaging for breast surgical margin analysis. Lasers Surg Med. 2010;42:15–23. doi: 10.1002/lsm.20865. [DOI] [PubMed] [Google Scholar]
- 10.Paras C, Keller M, White L, Phay J, Mahadevan-Jansen A. Near-infrared auto-fluorescence for the detection of parathyroid glands. J Biomed Optics. 2011;16:067012. doi: 10.1117/1.3583571. [DOI] [PubMed] [Google Scholar]
- 11.Mahadevan-Jansen A, Mitchell MF, Ramanujam N, Malpica A, Thomsen S, Utzinger U, et al. Near-infrared Raman spectroscopy for in vitro detection of cervical pre-cancers. Photochem Photobiol. 1998;68:123–32. doi: 10.1562/0031-8655(1998)068<0123:nirsfv>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Umezawa K, Nakamura Y, Makino H, Citterio D, Suzuki K. Bright, color-tunable fluorescent dyes in the visible-nearinfrared region. J Am Chem Soc. 2008;130:1550. doi: 10.1021/ja077756j. [DOI] [PubMed] [Google Scholar]
- 13.Zald PB, Hamilton BE, Larsen ML, Cohen JI. The role of computed tomography for localization of parathyroid adenomas. Laryngoscope. 2008;118:1405–10. doi: 10.1097/MLG.0b013e318177098c. [DOI] [PubMed] [Google Scholar]
- 14.Hunter GJ, Schellingerhout D, Vu TH, Perrier ND, Hamberg LM. Accuracy of four-dimensional CT for the localization of abnormal parathyroid glands in patients with primary hyperparathyroidism. Radiology. 2012;264:789–95. doi: 10.1148/radiol.12110852. [DOI] [PubMed] [Google Scholar]
- 15.Rose J, Guerrero MA. Management of primary hyperparathyroidism. Ward L, editor. Thyroid and parathyroid diseases – new insights into some old and some new issues. Available from: http://www.intechopen.com/books/thyroid-and-parathyroid-diseases-new-insights-into-some-old-and-some-new-issues/-management-of-primary-hyperparathyroidism-past-present-and-future-
- 16.Bancos I, Grant CS, Nadeem S, Stan MN, Reading CC, Sebo TJ, et al. Risks and benefits of parathyroid fine-needle aspiration with parathyroid hormone washout. Endocr Pract. 2012;18:441–9. doi: 10.4158/EP11148.OR. [DOI] [PubMed] [Google Scholar]
- 17.Gupta KP, Majumder K, Uppal A. Breast cancer diagnosis using N2 laser excited autofluorescence spectroscopy. Lasers Surg Med. 1997;22:417–22. doi: 10.1002/(sici)1096-9101(1997)21:5<417::aid-lsm2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 18.Toms S, Lin W, Weil RJ, Mahlon J, Jansen ED, Mahadevan-Jansen A. Intraoperative optical spectroscopy identifies infiltrating glioma margins with high sensitivity. Neurosurgery. 2005;57:382–91. doi: 10.1227/01.neu.000176855.39826.2d. [DOI] [PubMed] [Google Scholar]
- 19.Ramanujam N, Mitchell MF, Mahadevan-Jansen A, Thomsen SL, Staerkel G, Malpica A, et al. Cervical precancer detection using a multivariate statistical algorithm based on laser-induced fluorescence spectra at multiple excitation wavelengths. Photochem Photobiol. 1996;64:720–35. doi: 10.1111/j.1751-1097.1996.tb03130.x. [DOI] [PubMed] [Google Scholar]


