Abstract
While genetic and environmental factors have been shown to control visually-guided eye growth and influence myopia development, investigations into the intersection of these two factors in controlling refractive development have been limited by the lack of a genetically modifiable animal model. Technological advances have now made it possible to assess refractive state and ocular biometry in the small mouse eye and therefore to exploit the many genetic mouse mutants to investigate mechanisms of visually-guided eye growth. This review considers the benefits and challenges of studying refractive development in mice, compares the results of refractive error and ocular biometry from wild-type strains and genetic models in normal laboratory visual environments or with disrupted visual input, and discusses some of the remaining challenges in interpreting data from the mouse to validate and standardize methods between labs.
Keywords: refractive development, myopia, emmetropia, mouse, axial length, form deprivation
During normal refractive development, ocular growth is regulated such that the optical power matches the axial length of the eye so that a focused image falls on the retina, a state referred to as emmetropia. In animals the eye length does not match the optical power at birth, being either too short (hyperopia) or too long [myopia; see (Wallman and Winawer, 2004) for review]. The eye grows to match the optical power over the course of weeks, months, or years, depending on the species. Observations in humans and other animals have shown that this is an active process that requires visual input. For unknown reasons, abnormal refractive errors most commonly result in myopia and the prevalence of myopia is increasing and reaching alarming proportions in many developed societies. In the US, the prevalence of myopia increased from 25 to 41.6% between 1972 and 2004 (Vitale et al., 2009). In Asian countries, myopia has been reported as high as 88% (Edwards and Lam, 2004, Lam et al., 2004) or even 96% (Jung et al., 2012). A first step in understanding the increasing prevalence is to deduce the mechanisms controlling refractive development and eye growth. Many elegant studies in animals and humans have demonstrated that both genes and environment influence these processes. However, the exact mechanisms remain unknown. In this review, we examine the potential of the mouse to inform and advance our understanding of the mechanisms controlling refractive development. The mouse has been unparalleled as a vertebrate experimental model to probe and manipulate genes in disease and development. Here we examine the potential benefits of the mouse for refractive development research, the development of special techniques and methods to evaluate refractive errors and ocular biometry in the small mouse eye, current findings that have been reported, how these results compare to those from other animals, and challenges in interpreting data from mouse models.
1. Insights into refractive development from animal models
Experiments in animals over the last 35 years have provided important insights into the mechanisms that control refractive development. Several reviews have described emmetropization and potential mechanisms of eye growth (Charman, 2011, Saw et al., 2000, Siegwart and Norton, 2011, Stone and Khurana, 2010, Wallman and Winawer, 2004). While efforts to understand refractive development have been reported over the last century [reviewed in (Young, 1967)] and some clues were present that visual input might influence post-natal eye growth (Chiu et al., 1975), the finding that unilateral suturing of the eye lids in neonatal primates induced myopia (Wiesel and Raviola, 1977) broadened the interest in studying the biological mechanisms of normal refractive development and ametropias. The relatively translucent neonatal lid blocked form vision, but allowed light into the eye. These experiments have been replicated with form depriving diffuser goggles to induce myopia in primates (Troilo and Judge, 1993, Wiesel and Raviola, 1977), chickens (Wallman et al., 1978), tree shrews (Sherman et al., 1977), guinea pigs (McFadden et al., 2004), fish (Shen et al., 2005), and mice (Schaeffel et al., 2004). Additionally, defocusing the eye’s image with plus or minus lenses has been shown to induce growth at the proper rate during development to compensate for the induced error [see (Edwards, 1996) for review]. Based on results from experiments in which the optic nerve is severed or hemi-diffusers or lenses are applied, mechanisms controlling refractive development appear to be localized chiefly to the retina and not strictly controlled by higher order visual centers (Wallman and Winawer, 2004). Numerous studies have investigated neuromodulators, genes, and other retinal pathways that may be involved in signaling eye growth (Wallman and Winawer, 2004). It appears that refractive development is driven by visual input to the retina. While it is unknown how the retina detects image blur, the choroid thickness can be altered, thereby moving the retinal plane and providing important clues about the direction of defocus (Nickla and Wallman, 2010). Retinal growth signals may pass through the retinal pigment epithelium (Rymer and Wildsoet, 2005), and the retinal pigment epithelium may facilitate the delivery of growth signals to the sclera (Rada et al., 2006).
The mammals that have been used most commonly to study refractive development include monkeys, cats, tree shrews, marmosets and guinea pigs (Edwards, 1996). The eyes of these animals all respond more slowly to deprivation or defocus than the chicken, another commonly studied vertebrate; but the mammalian eyes more closely resemble the human eye in regards to ocular and retinal structure. The greatest limitation of these mammalian models is the difficulty of probing specific gene defects that might be influencing eye growth.
The mouse offers several advantages compared to the other animals in studying refractive development, as reviewed previously (Schaeffel, 2008a, 2010):
The mouse genome can be exploited to probe complex signaling pathways and retinal circuits.
Both genes and environment can be manipulated in the same animal.
While it lacks a fovea, the mouse retina otherwise resembles the human retina structurally and has been used to model and develop treatments for several blinding retinal diseases.
The gestational period of the mouse is quite short and the litter sizes are fairly large which make it an excellent experimental model for primary hypothesis testing.
However, the mouse also has some limitations. The foremost is the very small eye size. This creates a number of challenges to accurately measure eye size changes and refraction, as outlined below. The mouse eye has a rudimentary ciliary muscle and is not thought to have lenticular accommodation (Tansley, 1965). In addition, the mouse is nocturnal, does not have a fovea and has poor visual acuity. In fact, the visual resolution of the mouse using optokinetic tracking is estimated to be 0.4 cycles/degree (c/d) (Prusky et al., 2004); far below that of a chicken (8.0 c/d (Schmid and Wildsoet, 1997), primate (15 c/d) (Regal et al., 1976) or man (20 c/d). The depth of field of the mouse eye has been reported to >20 dioptres (D) (Remtulla and Hallett, 1985) and also 5-8 D (de la Cera et al., 2006). Such a large depth of field also suggests that the mouse eye would not be sensitive to out-of focus images within this range, but the data below indicate that this is not the case. There is evidence that the visual sensitivity of the mouse retina is similar to that of the human peripheral retina (Naarendorp et al., 2010). The development of myopia appears to be slow and of smaller magnitude in the mouse compared to chicken, but similar to other mammalian models. Despite these limitations, the mouse eye responds to form deprivation and lens defocus, indicating it does have emmetropization mechanisms that may be shared with other animals. In addition, these responses suggest that the quality of the visual image may not be the only visual parameter driving refractive development.
2. Utilizing mice for myopia studies
2.1 Measuring optical properties
Due to the small size of the mouse eye, measuring refractive error and ocular parameters requires much precision to achieve biologically meaningful accuracy (Schaeffel, 2008b). According to the schematic model of the mouse eye developed by Dr. Frank Schaeffel, 5.4-6.5 μm change in axial length is needed for one diopter change in optical power (Schmucker and Schaeffel, 2004b). In humans and chickens, one diopter change corresponds to 0.34 or 0.06 mm (Schaeffel and Howland, 1988), respectively - changes that are easily assessed with generally available methods such as ultrasounography or frozen sections. However, the mouse eye requires instruments with much greater precision.
The dimensions of the mouse eye have been most successfully measured with low or partial coherence interferometry which allows high accuracy and, after the initial cost of the instrument, is relatively inexpensive for on-going use (Schmucker and Schaeffel, 2004a). The precision of axial length measurements is 21 ± 45 μm (Park et al., 2012). This technique allows for rapid measurements, even in non-anesthetized mice (Park et al., 2012); however, only the most prominent peak originating at the retinal pigment epithelium/choroidal interface is consistently visible and not reflections from the other ocular surfaces. Thus, low coherence interferometry only provides axial length measurements (Table I). Scanning with low coherence interferometry in two dimensions creates optical coherence tomography (OCT) and the ability to measure multiple ocular dimensions (corneal thickness, anterior chamber depth, lens thickness, posterior chamber depth, axial length, and retinal thickness). Four OCT methods have been applied to eye measurements in mice: 1) Real-time OCT controlled by a stepper-motor that advances the focal plane through the eye and allows the dimensions of each ocular parameter to be measured (Zhou et al., 2008b)(Table I). These measurements take about 1.3 minutes each and had an average standard deviation of 13 μm for axial length between consecutive days. 2) A 1310 nm spectral domain OCT (SD-OCT; Bioptigen, Inc., Morrisville, NC) captures the entire mouse globe quickly in one frame using a mirror artifact to see both the front and back of the eye overlaid on the same image (Figure 1) (Park et al., 2012). While the 1310nm OCT has less resolution than the typical 940nm instruments used for detailed examination of the retina, the larger imaging window provides a clear image of each ocular surface. The 1310 nm OCT had a standard deviation of 10 μm on repeated measurements. These OCT measurements have excellent intra-class correlation of 0.92 with partial coherence interferometry (Table I) (Park et al., 2012). 3) Recently, depth-enhanced swept source OCT was developed to capture biometric data from the entire mouse eye with 512 depth scans in 18.3 seconds (Wang et al., 2011b). This instrument was reported to have reproducibility of axial length within approximately 16 μm. 4) Alternatively, a single-shot SD-OCT has also been developed to measure ocular parameters in the mouse eye, capturing over 2000 depth scans in 85 msec (Jiang et al., 2012). This instrument is reported to have axial resolution of 4.5 μm.
Table I.
Comparison of axial parameters in the mouse eye using different instruments at 1 month or approximately 2 months of age. LCI: Low coherence interferometry
| Instrument | Age (d) | Strain | Axial length (μm) | Lens thickness (μm) | Vitreous Chamber depth (μm) | Reference |
|---|---|---|---|---|---|---|
| 1 month of age | ||||||
| Histological sections | 30 | C57BL/6J | 3256 ±138 | (Tejedor and de la Villa, 2003) | ||
| Photomicrograph | 28 | C57BL/6J | 3042 ±82 | (Qian et al., 2009) | ||
| LCI | 29 | C57BL/6J | 3160 ±100 | (Schmucker and Schaeffel, 2004a) | ||
| LCI | 28 | C57BL/6J | 3190 ±10 | (Schippert et al., 2007) | ||
| LCI | 30 | C57BL/6J | 3066 ±7 | (Wisard et al., 2011) | ||
| LCI | 28 | BALB/cJ | 2931 ±8 | (Barathi et al., 2008) | ||
| OCT with stepper motor | 29 | C57BL/6J | 3003.3 ±44.1 | 1558.7 ±18.0 | 707.4 ±21.4 | (Zhou et al., 2008b) |
| OCT with stepper motor | 28 | C57BL/6J | 2943 ±17 | 1544 ±8 | 697 ±7 | (Zhou et al., 2010b) |
| OCT with stepper motor | 28 | C57BL/6J | 2932.4 ±45.7 | 719.6 ±41.5 | (Zhou et al., 2010a) | |
| Single shot SD-OCT | 28 | C57BL/6J | 2972.39 ±33.21 | 1622.11 ±10.72 | 705.53 ±25.06 | (Jiang et al., 2012) |
| A-scan ultrasonography | 28 | C57BL/6J | 2820 ±20 | (Yu et al., 2011) | ||
| MRI | 32 | C57BL/6J | 3100±9 | 1727±17 | 954 ±11 | (Tkatchenko et al., 2010a) |
| 2 months of age | ||||||
| Laser micrometer | 60 | C57BL/6J | 3231 ±69 | (Wisard et al., 2010) | ||
| LCI | 58 | C57BL/6J | 3262 ±42 | (Park et al., 2012) | ||
| 1310 nm SD-OCT | 58 | C57BL/6J | 3264 ±47 | (Park et al., 2012) | ||
| Custom OCT | 60-90 | C57BL/6J | 3089 ±45 | 1771 ±23 | 586 ±31 | (Chou et al., 2011) |
| Custom OCT | 60-90 | DBA/2J | 3069 ±43 | 1906 ±29 | 606 ±37 | (Chou et al., 2011) |
| Swept source OCT | 56 | MF1 albino | 3255 ±100 | (Wang et al., 2011b) | ||
Figure 1.
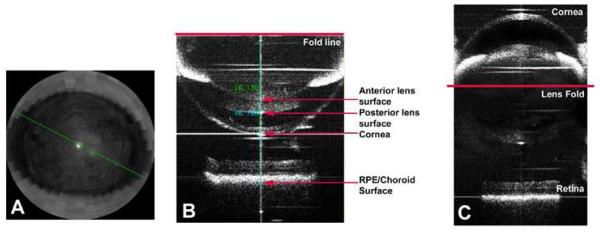
Images of the mouse eye taken with a 1310 nm OCT. A) A view of the cornea in “radial” mode for alignment of the first Purkinje image in the center of the pupil. B) A typical image of the mouse eye which takes advantage of the “mirror artifact” to superimpose the anterior and posterior portions of the eye in the same imaging frame. The different ocular structures are indicated that allow for axial length, corneal thickness, lens thickness, anterior and posterior chamber depth. Axial length is calculated by adding the caliper distance from the lens fold to the anterior corneal surface and from the lens fold to the RPE/choroid interface. C) An “unfolded” image of the mouse created in an imaging program to assist with visualization of the folded image in B. From (Park et al., 2012), with permission from Wolters Kluwer Health.
Other instruments used in mice to obtain high resolution ocular dimensions include the laser micrometer and magnetic resonance imaging (MRI). The laser micrometer has the highest resolution of 0.7679 μm (Wisard et al., 2010). While this approach closely matches the values obtained with OCT (Wisard et al., 2011), its disadvantage is that the measurements are ex vivo and include only external ocular dimensions (axial length, equatorial diameter, etc). MRI has a resolution of 23.4 μm (Tkatchenko et al., 2009). Similar to the OCT image, each ocular parameter is clearly visible and measurable. The disadvantages include the high cost an MRI instrument dedicated to animal research, accessibility of the MRI, the length of time for each scan (>35 minutes; (Tkatchenko et al., 2010a)) and the cost associated with scan time.
The refractive error of the mouse eye is generally reported as being hyperopic when measured retinoscopically (Table II)(Pardue et al., 2008, Schaeffel et al., 2004). Hyperopia in small animals is often attributed to the small eye artifact in which the retinoscopic reflex originates from the inner limiting membrane instead of the outer limiting membrane, creating the perception of a shorter eye (Glickstein and Millodot, 1970). Studies in rats have demonstrated that refractive values obtained by retinoscopy are very similar to those found with the visually evoked potential, suggesting that the retinoscopic reflex is located in the outer retina (Mutti et al., 1997). Furthermore, calculation of refractive error from a paraxial schematic eye model also could not account for the small eye artifact in the mouse (Schmucker and Schaeffel, 2004b). Supporting the small eye artifact, measurements of refractive error using Shack-Hartmann wavefront sensor spots, in which the wavefront sensor beacon can be focused on different layers of the retina, showed that the refractive error in the mouse eye was ~22D hyperopic when measured on the inner retina while -7D myopic when measured from the outer retina and that refractive error was dependent on the wavelength of light (Geng et al., 2011). Using this technique Geng et al. conclude that “true refraction” of the mouse eye is actually myopic. Thus, it is not clear if hyperopic refractions in the mouse reflect the true refractive value of the eye.
Table II.
Refractive error measurements reported in mice under normal laboratory visual conditions. The photorefractor is a similar model across all labs (Schaeffel, 2008b).
| Strain | Age (d) | Refractive error (D) | Instrument | Reference |
|---|---|---|---|---|
| C57BL/6J | 27 | 5.5 ±1.75 | Photorefractor | (Schaeffel et al., 2004) |
| C57BL/6J | 28 | 8.6 ±0.05 | Photorefractor | (Schippert et al., 2007) |
| C57BL/6J | 28 | 6.38 ±0.28 | Photorefractor | (Pardue et al., 2008) |
| C57BL/6J | 29 | −0.10 ±4.42 | Photorefractor | (Zhou et al., 2008a) |
| C57BL/6J | 32 | −0.5 ±1.5 | Photorefractor | (Tkatchenko et al., 2010a) |
| C57BL/6J | 28 | 0.0 ±1.5 | Photorefractor | (Zhou et al., 2010b) |
| C57BL/6J | 28 | −0.68 ±4.51 | Photorefractor | (Zhou et al., 2010a) |
| C57BL/6J | 30 | 5.94 ±1.09 | Photorefractor | (Wisard et al., 2011) |
| C57BL/6J | 28 | 1.83 ±1.46 | Photorefractor | (Yu et al., 2011) |
| C57BL/6J | 30 | 13.68 ±2.04 | Streak retinoscope | (Tejedor and de la Villa, 2003) |
| C57BL/6J | 28 | 10.10 ±1.97 | Streak retinoscope | (Qian et al., 2009) |
| BALB/cJ | 28 | 9.983 ±0.161 | Streak retinoscope | (Barathi et al., 2008) |
| DBA/2 | 27 | 4.55 ±2.95 | Streak retinoscope | (Schaeffel et al., 2004) |
While it is possible to measure the refractive state of the murine eye using streak retinoscopy (Barathi et al., 2008, Qian et al., 2009, Tejedor and de la Villa, 2003)(unpublished data), the high hyperopic refractions with retinoscopy measurements make seeing the very small retinoscopic reflex particularly difficult. Thus, most studies have used an automated eccentric infrared photorefractor to measure the mouse eye that was originally modified for the murine eye by Dr. Frank Schaeffel (Schaeffel et al., 2004). The standard deviation of refractive measurements recorded across several animals using the photorefractor is 2.97D (Schaeffel et al., 2004). By detecting the 1st Purkinje image centered on the pupil, the instrument captures refractions at 30Hz. While several investigators have used this photorefractor to measure refractive errors in the mouse, it is notable that the values are not uniform across labs (Table II). Some differences may be attributed to different techniques in handling the mice such as making recordings with or without anesthesia (Tkatchenko and Tkatchenko, 2010), although some labs find no differences with anesthesia (Pardue et al., 2008); and some perform refractions while awake mice are only lightly restrained (Schaeffel et al., 2004) or completely immobilized on a restraining platform (Tkatchenko et al., 2010b). Other sources of between-lab differences may include how each system is calibrated with trial lenses, the brightness settings of the camera, the pupil size or the exact alignment of the mouse eye with the camera along the optic axis. For instance, if alignment includes the optic nerve head, the refractions may be more myopic (Schaeffel, 2008a, b). Until the source of refractive error variability is discovered, it seems wise to use refractive error measurements to make relative comparisons within experiments while using a single instrument, but not between two different labs.
2.2 Creating form deprivation or defocus
Fitting the mouse eye with form depriving goggles or defocusing optical lenses provides the opportunity to manipulate environment/visual experience and explore the outcome under a myriad of genotypes/genetic paradigms. The small size of the mouse and its rapid growth make the attachment of goggles or lens during early development a challenge. The first report of form deprivation in mice used lid suture (Tejedor and de la Villa, 2003). However, this approach appeared to cause corneal flattening in this and other animal studies (McBrien and Norton, 1992, Tejedor and de la Villa, 2003, Troilo and Judge, 1993). Other approaches have included using Velcro rings to attach a form-depriving goggle or defocusing lens to the fur (Barathi et al., 2008, Faulkner et al., 2007, Qian et al., 2009, Schaeffel et al., 2004) or attaching the goggle or lens directly to the fur using sutures (Tkatchenko et al., 2010b). For treatment compliance, these approaches need to include an Elizabethan collar (Faulkner et al., 2007, Schaeffel et al., 2004), clipping the nails (Tkatchenko et al., 2010b), or wrapping the paws (Qian et al., 2009) so that the mice do not remove the goggles. Since ocular health can decline with the use of a glued goggle and Elizabethan collar, a head pedestal was been adapted to manipulate visual input in mice (Faulkner et al., 2007)(Figure 2). This approach has been used successfully in tree shrews for over 18 years (Siegwart and Norton, 1994) and involves attaching a head pedestal to the skull which receives a goggle/lens frame when the mice are 28 days of age (Faulkner et al., 2007). A small balance bar is placed on the opposite side of the head to assist in securing the apparatus. While this approach requires a large investment of time for each mouse, ocular health is much improved in the mice (Faulkner et al., 2007). The frame can readily hold either a diffuser goggle or a lens in place.
Figure 2.
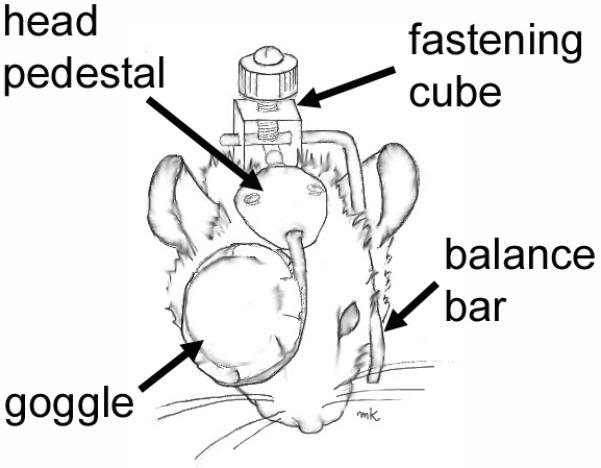
Sketch of the head-mounted goggle for mouse form deprivation. A head pedestal is attached directly to the skull which accepts a frame and diffuser goggle that covers the right eye. A balance bar rests on the opposite side of the head to help secure the fastening cube. A similar apparatus can be used for applying a spectacle lens. [modified from (Faulkner et al., 2007), with permission from Elsevier]
3. Current findings from mouse studies
3.1 Emmetropization in mice
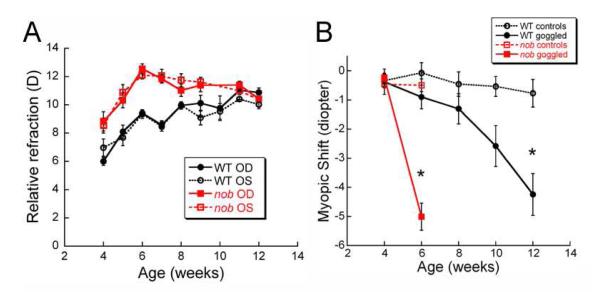
A number of studies have examined the normal refractive development of C57BL/6J mice, a common pigmented strain used in vision research and for generating transgenic models. In general, most studies have found that C57BL/6J mice have hyperopic refractive errors (Table II) that become relatively more hyperopic until about 5-6 weeks of age when they stabilize (Pardue et al., 2008, Schaeffel et al., 2004, Schippert et al., 2007, Schmucker and Schaeffel, 2004b, Wisard et al., 2011, Yu et al., 2011, Zhou et al., 2010a, Zhou et al., 2010b, Zhou et al., 2008a)(Figure 3A). DBA/2 mice are the only other strain in which refraction has been studied across age in eyes with normal visual input (see Table II), but measurements were often confounded by the development of cataracts at an early age (Schaeffel et al., 2004). While the refractive values appear to stabilize, axial length and other ocular dimensions increase continuously with age in most strains [different strains: (Puk et al., 2006); C57BL/6J (Chou et al., 2011, Schaeffel et al., 2004, Schippert et al., 2007, Schmucker and Schaeffel, 2004b, Tkatchenko et al., 2010a, Wisard et al., 2011, Yu et al., 2011, Zhou et al., 2010a, Zhou et al., 2008a); BALB/c (Barathi et al., 2008)]. These data suggest that the visual input during the first 6 weeks of age does influence refractive state and results in a type of visually-driven emmetropia in the mouse strains studied. The continuing eye growth suggests that non-visually driven eye growth continues throughout the length of the studies. However, DBA/2J mice are a known exception to these generalizations because their axial length, lens thickness and anterior chamber depth growth appear to plateau after 8-12 weeks (Chou et al., 2011). Whether this growth pattern relates to the pigmentary-type of glaucoma these mice develop at later ages or some other strain feature is not known. While these trends can be seen in the data reported from the various labs, the refractive error and ocular parameter values tend to be quite variable (Table I and II). Only some of this variability may be attributed to differences in methods and/or techniques, as described above, and other variance may be due to strain differences.
Figure 3.
A) Refractive error values across age in C57BL/6J wild-type (WT) and nob mice. Both strains became more hyperopic until about 6 weeks of age when C57BL/6J WT mice plateau and nob mice shifted towards less hyperopia. Nob mice refractions were significantly more hyperopic than WT mice from 4 to 10 weeks of age (repeated measures ANOVA, p<0.001). B) With the application of diffuser goggles over the right eye, nob mice developed ~ 5D myopic shift in 2 weeks compared to the contralateral eye, while C57BL/6J WT mice took 8 weeks to develop a similar magnitude shift. * p<0.001 [Modified from (Pardue et al., 2008), with permission from Association for Research in Vision and Ophthalmology].
3.2 Comparisons of mouse strains
The first report of differences in murine eye size compared the eye weight, lens weight and retinal ganglion cell counts in 50 mouse strains (Zhou and Williams, 1999b). While this study compared all mice at only one age (all mice were normalized using multiple linear regression to a female C57BL/6J mouse at postnatal day 75), it clearly indicated that there are differences in eye size of different mouse strains. This has been further confirmed by other studies that have compared C57BL/6J with DBA/2J (Chou et al., 2011, Schaeffel et al., 2004) and several other pigmented (C3H, 129S2/Sv) and albino strains (BALB/c, CD-1)(Puk et al., 2006). For instance, these studies have found that DBA/2J mice have smaller anterior chambers (Puk et al., 2006) or larger overall axial lengths (Chou et al., 2011). However, it should be noted that, in DBA/J2 mice, these anatomical features may be consequences of the glaucoma syndrome and not indicative of emmetropization mechanisms in these mice(Howell et al., 2008, Morrison et al., 2011). While these studies have not found consistent results, it is clear that differences in eye size between strains do exist. While strain differences may be due to benign genetic differences, some genetic changes result directly in abnormalities. For instance, DBA/2J mice have a pigment disturbance that leads to glaucoma (John et al., 1998), and albino mice are known to have abnormal optic track decussation among other developmental abnormalities (Searle, 1990). Thus, when comparing different genotypes, it is imperative that both the homozygous mutants and wild-type mice are on the same background strain to isolate the effects of the gene of interest, versus other unknown strain effects. Awareness of the genetic effect of the other functions and structure of the eye is also important in understanding whether growth effects are secondary to underlying eye disease.
3.3 Alterations in visual environments on murine refractive development
In order to determine if the small mouse eye is an appropriate mammalian model to examine the influence of visual manipulations, the effects of form deprivation and lens defocus have been tested. In response to form deprivation using diffusers, C57BL/6J mice developed relative myopic shifts of 3-6 D after 1 to 6 weeks (Barathi et al., 2008, Pardue et al., 2008, Qian et al., 2009, Schaeffel et al., 2004, Schmucker and Schaeffel, 2004a) (Table 3). The response of the mouse eye to diffusers was similar to guinea pigs, which responded with 4-6 D myopic shift in 11 to 14 days (Howlett and McFadden, 2006, Lu et al., 2006). Compared to tree shrews that developed a 9D myopia shift in 11 days (Siegwart and Norton, 2001), the myopic response to form deprivation in mice was comparable temporally, but smaller in magnitude. Compared to primates that developed a 4 D myopic shift in 115 days (Qiao-Grider et al., 2004), the mouse developed form deprivation much faster but with similar magnitude. It should be noted at an even larger magnitude myopic shift has been obtained when mice are housed under constant light (Tkatchenko et al., 2010b), but it is unclear if constant light provides a visual environment that permits naturally-driven emmetropization. Application of -10 or -25D lens defocus induced relative myopic refractions in the mouse. A -10 D lens fitted over the mouse eye induced a refractive shift of -13 D in 46 days (Barathi et al., 2008) under a _12:12 hr light:dark cycle and a -25 lens induced 15 D myopic shift in 21 days under constant light (Tkatchenko et al., 2010b). These values compare to 10-14 days for 3-5D myopic shift with a -4D spectacle lens in guinea pigs (Howlett and McFadden, 2009, Lu et al., 2009), 11 days for 5D myopic shift with -5D spectacle lens in tree shrews (Siegwart and Norton, 2005), 100 days for a 2-5 D myopic shift with a -3 or -6 D spectacle lens in primates (Hung et al., 1995). While the temporal susceptibility of the mouse eye to lens defocus has not yet been reported, these results indicate that the mouse eye responds to minus lens wear in a similar direction and magnitude as other mammalian models.
Table III.
Reported shifts in refraction and axial length with deprivation of form vision. Form deprivation was produced by a diffuser goggle unless otherwise noted.
| Strain | Visual disruption | Change in refractive error (D) | Change in Axial length (μm) | Axial length (μm )/D | Reference |
|---|---|---|---|---|---|
| C57BL/6J | Form deprivation | −8.84 | 79 | 8.9 | (Qian et al., 2009) |
| C57BL/6J | Form deprivation | −2.02* | 38 | 18 | (Schmucker and Schaeffel, 2004a) |
| C57BL/6J | Form deprivation | −3.85 | NS* | (Schaeffel et al., 2004) | |
| C57BL/6J | Form deprivation | −6.16 | 50 | 8 | (Zhou et al., 2012) |
| C57BL/6J | Form deprivation | −8.72 | 46 | 5 | (Yu et al., 2011) |
| BALB/c | Form deprivation (lid suture) | −6.14 | 229 | 37 | (Barathi et al., 2008) |
| BALB/c | −10D lens defocus | −13.03 | 367 | 28 | (Barathi et al., 2008) |
| C57BL/6J | Form deprivation (lid suture) | −6.33 | 252 | 39 | (Tejedor and de la Villa, 2003) |
| C57BL/6J | 2 Hz Flickering light | −4.85 | 24 | 5 | (Yu et al., 2011) |
| C57BL/6J | Prolonged light | −2.4 | 63.6 | 26.5 | (Zhou et al., 2010a) |
Difference between the visually manipulated and control eyes were not significantly different.
Other alterations in visual input included exposing mice to prolonged daily light periods at 12 days of age (Zhou et al., 2010a) or flickering light at 28 days of age (Yu et al., 2011). Under these conditions, the murine eye became relatively myopic, with a similar effect as that found with form deprivation (Yu et al., 2011) (Table 3). This is an opposite effect to what has been found in chickens; constant light induced a hyperopic shift (Li et al., 1995, Stone et al., 1995) and flicker had no effect on refractive development (Crewther et al., 2006). In addition, one group found mice to have a greater response to form deprivation or lens defocus starting at 21 days of age with exposure to constant light (Tkatchenko et al., 2010b).
While these studies have shown consistent shifts in the mouse model toward more myopic refractions with form deprivation, lens defocus, prolonged light exposure, or flicker, the differences in ocular parameters between treatment groups has varied. Based on other animal models of myopia, it would be expected that myopic refractions would be associated with longer axial length. While some studies have reported such a correlation in mice (Barathi et al., 2008, Qian et al., 2009, Tejedor and de la Villa, 2003, Yu et al., 2011, Zhou et al., 2010a, Zhou et al., 2012), others have found the axial length measurements to be variable and not so clearly correlated with refractive errors (Schaeffel et al., 2004, Schmucker and Schaeffel, 2004a) (Table 3). Additionally, even when the expected longer axial lengths are found with a shift toward myopia in the mouse, the values do not match that of 5-6 μm change in axial length for each diopter change in refractive error as predicted by the schematic eye (Schmucker and Schaeffel, 2004b); however, a similar shift has been reported for refractive error and vitreous chamber depth (Tkatchenko et al., 2010b). The reasons for these discrepancies are not clear. Results suggest that the mouse may not respond consistently with axial myopia, as reported in other myopia models. While the instruments used to measure axial length in mice have high resolution, the margin of error is still rather large relative to the physiologically pertinent ocular dimensions, with standard deviation of in vivo measurements ranging from 7 to 100 μm (see Table 1). Another possibility is that some of the assumptions made in the paraxial model eye are not accurate for the small mouse eye, such as the curvature or refractive index values for each component. Due to the small size of the mouse eye, the accuracy of the measurement of each optical component is critical and even a small inaccuracy could result in a large error in applying the optical modeling.
3.4 Probing gene defects in murine refractive development
One of the most useful aspects of using murine models is testing refractive development in strains with specific genetic mutations. Mice with specific genetic features can be used either to test the involvement of specific genes or to assess complex pharmacological signaling pathways not readily amenable to other methods. A number of studies have indicated changes in eye size due to various mutations that affect normal eye development; including larger eyes in mice with defects in the genes encoding the early growth response protein-1 (Egr-1), the mammalian orthologue to ZENK (Schippert et al., 2007); interphotoreceptor retinoid-binding protein (IRBP; (Wisard et al., 2011) and adenosine A2A receptor (Zhou et al., 2010b); and smaller eyes in mice missing retinoic acid receptors (Zhou et al., 2001). Of these studies, some investigated genes that have been independently implicated in controlling refractive development. For instance, Egr-1 is a transcription factor that has been implicated in regulating eye growth such that ocular growth is suppressed with upregulation of Egr-1 and enhanced with downregulation of Egr-1 (Bitzer and Schaeffel, 2002). Thus, the Egr-1-/- mouse was predicted to have relative myopia, as reported (Schippert et al., 2007). Others models have been more serendipitous discoveries, such as the influence of IRBP on excessive eye growth in early development (Wisard et al., 2011). While these studies have not yet identified definitive pathways that control refractive development, they do suggest that a number of different genes are responsible for normal eye size development. It is likely that some of these genes will impact visually-driven emmetropization, while others signal non-visual eye growth. With the increasing numbers of mice being examined for refractive development, it is likely that many new genes, proteins, and signaling pathways will be revealed that influence eye growth. The challenges will be to distinguish genes that are important for visually-driven refractive development versus non-visually guided eye growth, to properly evaluate the mouse models for eye size changes and to relate experimental findings to the clinical conditions.
Another advantage of the mouse is probing changes in genes or proteins that potentially alter refractive development. For instance, quantitative trait loci (QTL) analysis has revealed Eye 1 and Eye 2 as potential gene candidates controlling normal variation in eye size (Zhou and Williams, 1999a). These studies took advantage of backcrossing two unrelated strains to screen for genes that control specific traits. Similarly, QTLs for heritability of ocular component dimensions can be examined using OCT measurements from first generation progeny (MF1) (Wang et al., 2011b). Additionally, gene expression has been examined using gene arrays in the Egr-1-/- mouse (Schippert et al., 2009) and after image contrast changes (Brand et al., 2007). Egr-1, VIP and Shh mRNA and Egr-1 protein levels have been examined after exposure to different lighting conditions or form deprivation (Brand et al., 2005). These studies suggest that Egr-1 is upregulated between shifts from dark to light (Brand et al., 2005) or altered contrast levels (Brand et al., 2007). In contrast, glucagon expression was not measurably changed in the mouse retina after changes in visual experience (Mathis and Schaeffel, 2006). Together, these studies further support the hypothesis that Egr-1 is involved in controlling refractive eye growth. Further studies to evaluate changes in sclera gene expression have examined mice during postnatal development (Lim et al., 2012). The expression of specific genes, such as muscarinic receptors, have been compared between mouse and human eyes (Barathi et al., 2009) and evaluated after form deprivation with and without atropine treatment (Barathi and Beuerman, 2011). These studies have confirmed the presence of muscarinic receptors in both mouse and human sclera and provide evidence that atropine treatment may reduce myopia progression by regulating gene expression of muscarinic receptors. Probing gene expression in the mouse sclera in combination with form deprivation has also implicated the frequency of methylation of cytosine-phosphate-guanine sites in the collagen 1A1 promoter (Zhou et al., 2012). Finally, comparisons of lens proteins in mice exposed to form deprivation or flickering light indicate reduced alpha-A crystalline levels with flickering light (Li et al., 2012). Together these studies provide examples of how mice can be used to investigate genes and proteins in several structures of the eye under normal or visually disrupted conditions in relationship to refractive development.
3.5 Altering both genes and visual environment in a mouse model
Perhaps the most promising aspect of the mouse model is the ability to examine the effects of a specific gene defect on visually-driven refractive development under conditions of normal and altered visual input – that is, studying potential gene/environment interactions. A number of studies have now examined separately the effects of a gene defect on normal eye growth or the effects of visual input manipulations in wild-type (WT) strains, but the interactions of genes with visual input are only now beginning to be evaluated in mouse models of refractive development. For example, the nob mouse has a mutation in nyx that encodes the protein nyctalopin, located on the post-synaptic side of the photoreceptor to ON bipolar cells synapse (Morgans et al., 2006); this mutation causes a non-functional ON pathway. Patients with the complete form of congenital stationary night blindness share this same mutation and have high myopia (Bech-Hansen et al., 2000, Miyake et al., 1986, Pusch et al., 2000). Under normal laboratory environmental conditions, the loss of nyx causes only slightly more hyperopic refractions in nob mice compared to WT. However, with form deprivation, nob mice develop myopic shifts much more rapidly than WT mice (2 weeks versus 4-6 weeks; Figure 3B)(Pardue et al., 2008). Thus, the absence of ON pathway function appears to accelerate the susceptibility to myopia from blurred visual input. Additionally, dopamine and DOPAC levels were significantly decreased in nob mice at 12 weeks of age, potentially implicating endogenous dopamine levels in the increased susceptibility of form deprivation in nob mice (Pardue et al., 2008). Previously investigations in chickens with sawtooth illumination to selectively stimulate the ON and OFF pathways or drugs to block these pathways have reported diminished responses to blur or defocus (Crewther and Crewther, 2002, Crewther et al., 1996), while pharmacological inhibition of the ON and OFF pathways revealed potential selective roles for the different pathways in the response to negative or positive defocus (Crewther and Crewther, 2003). Further studies are needed to examine how ON and OFF pathways may be affecting eye growth. Nonetheless, these results demonstrate the potential of mouse studies to examine the interaction of genes and environment in ways that may impact the understanding of clinical myopia mechanisms.
4. Challenges of using murine models for eye size studies
While mice are a well-established model in a number of research areas, their use in refractive development and myopia is just beginning. In the early stage of establishing a model, methods need to be validated and standardized within and across labs. The preceding summary raises a number of challenges in using mice to study refractive development. The first issue is the small eye artifact. There is currently inconsistency between studies on the refractive value of the mouse eye (Table II) or even if the small eye artifact exists (Glickstein and Millodot, 1970, Mutti et al., 1997, Schmucker and Schaeffel, 2004b). The tree shrew also has a small eye with hyperopic refractions; and in this model, the small eye artifact was addressed by subtracting the small eye artifact from the reported refractions (Gao et al., 2011). Further studies are needed to investigate the refractive state of the mouse eye using different methods. One approach would be to improve the accuracy of the schematic eye model. Calculating the schematic eye requires radii of curvature, axial distance and refractive indices. The current schematic eye models rely on refractive indices taken from a single publication (Remtulla and Hallett, 1985) in which measurements were obtained from frozen sections, the Abbe refractometer and an interference microscope. Values for radii of curvature have been taken from frozen sections which may have sustained sectioning artifacts or have insufficient resolution, as discussed above for the small mouse eye (Schmucker and Schaeffel, 2004b). In addition, refractive index measurements on small mouse ocular structures may not be accurate using these instruments. Schematic eye calculations would indicate that the refractive index of the mouse lens increases with age from 21 to 100 days (Schmucker and Schaeffel, 2004b). In addition, these same refractive indices are being used with low coherence interferometry and OCT to calculate the geometrical linear distances from the optical linear distances. Thus, the OCT measurements may even be affected by inaccuracies in the refractive index values of each ocular component, especially since the lens occupies a large proportion of the mouse eye. Some studies have used a single average refractive index value for the entire eye (1.433) when calculating geometrical distances (Schmucker and Schaeffel, 2004a), while others have used refractive index values for each optical structure (Zhou et al., 2008a). Comparing axial length measurements from 1310 nm OCT images of C57BL/6J mouse eyes between 28 and 102 days of age, using these two methods, showed that axial length measurements could differ by 2-5%, differences that could correspond to 11-30 D of estimated optical power (Pardue, unpublished data). Further assessments of such details and how they might impact the relationship of ocular refraction to axial length might reconcile the reported measurement discrepancies and clarify the potential role of the small eye artifact in the mouse eye.
Another challenge is the differences in eye size between strains. There are many differences between mouse strains, including body weight, body temperature, blood pH, temperament, melatonin levels, etc. (Guo et al., 2012, Ingram and Corfman, 1980, Yilmazer-Hanke, 2008). Thus, it is not surprising that the different strains would have different ocular parameters (Zhou and Williams, 1999b). While a comprehensive comparison of mouse refractions has not been reported, the baseline refractions in Table II would suggest that refractions may vary between strains; a somewhat surprising finding since the requirement for “good” vision is universal and might predict similar refractive errors across these mostly inbred strains (a breeding strategy that produces within-strain genetic and phenotypic similarities). Reasons for reported strain differences could include measurement issues discussed above, as well as refractive indices of the ocular lens that may not be the same in all strains, the large focal depth of the mouse eye, which may make a few diopters difference irrelevant to “good” vision, and the potential that known or unknown gene defects could produce increased sensitivity to changes in the visual environment. Differences in refractive errors between strains will need to be assessed carefully to determine if these changes can be attributed to potential gene defects that cause phenotypical differences, frank ocular disease (DBA/2J with glaucoma) or modifiers of ocular growth like Eye 1 and Eye 2.
Furthermore, the use of homozygous mutants may produce other phenotypic differences that influence eye size measurements. For instance, the overall body size of many homozygous mutant mice is smaller than their wild-type counterparts. A search of the Jackson Laboratories website revealed 70 mice that had both abnormal body size and either known retinal degeneration or eye defects. It is well known that human eye size is highly correlated with height (Wang et al., 2011a, Yin et al., 2012) and head dimensions (Larsen, 1979). Body weight, body length and head width have also been found to predict 50% of the variation in chicken eye parameters (Prashar et al., 2009). In previous refractive development studies, normalizing for body size has not been needed when wild-type chickens of the same breed or monkeys of the same species were used. One recent example of the importance of considering the relative body size to eye size occurs in the retinopathy, globe enlarged (RGE) chicken which has overall smaller body size, but eyes that are proportionally larger compared to wild-type chickens (Ritchey et al., 2012). Thus, reporting raw values between the wild-type and RGE chickens would leave the impression of a smaller eye in the RGE. However, when percent change in axial length is compared, the difference in eye size is significantly larger for RGE chickens (Ritchey et al., 2012). As the evaluation of eye size in mutant mouse models becomes more frequent, special attention needs to be given to overall body size and the normalization of values by comparing baseline values or examining relative differences between strains or eyes.
5. Opportunities for future directions
It is an exciting time in the field of refractive development research. The advances in high resolution diagnostic instruments are now allowing the use of genetic models in mice to investigate the mechanisms controlling eye growth. Genes controlling specific receptors, synapses, neurotransmitters, and cell types in different structures of the eye such as the retina, RPE, choroid, and sclera can be selectively probed to evaluate their influence in normal refractive development as well as under altered visual conditions. In addition, retinal mutations in mice might provide an opportunity to study the link between refractive abnormalities and retinal disease, such as retinal degenerations (Laties and Stone, 1991, Sieving and Fishman, 1978), cone-rod dystrophy (Pras et al., 2009, Smith et al., 2007), or congenital stationary night blindness (Miyake et al., 1986, Pardue et al., 2008). The power of the mouse lies in probing genetic and environmental interactions that are not accessible in other species. Evaluating mice with specific deletions of candidate genes that signal eye growth provides a new level of testing for each candidate gene that has not been available with other animal models. However, evaluating these genes only under normal laboratory conditions may miss important interactions between genes and environments. Thus, studies need to investigate not only form deprivation and lens defocus, but also changes in light, photoperiod, chemical toxins and other environmental parameters which have also been implicated as potential modulators of refractive development.
Highlights.
Mice have advantages and challenges for elucidating mechanisms of refractive development.
Studying refractive development in mice requires special equipment and methods.
The influence of genetic/environmental factors on myopia can be studied in mice.
Acknowledgements
The authors acknowledge Dr. Gregor Schmid for calculating the ocular biometry with different refractive indices and the following sources of financial support: NIH grants R01- EY016435 (MTP), R01-EY018838 (RAS), R01-EY004864 (PMI), P30 EY001583 (U PA), P30- EY006360 (Emory); the Paul and Evanina Bell Mackall Foundation Trust (RAS), Research to Prevent Blindness (RAS, Emory), Rehabilitation Research and Development Service, Department of Veterans Affairs Research Career Scientist Award (MTP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barathi VA, Beuerman RW. Molecular mechanisms of muscarinic receptors in mouse scleral fibroblasts: Prior to and after induction of experimental myopia with atropine treatment. Mol Vis. 2011;17:680–92. [PMC free article] [PubMed] [Google Scholar]
- Barathi VA, Boopathi VG, Yap EP, Beuerman RW. Two models of experimental myopia in the mouse. Vision Res. 2008;48:904–16. doi: 10.1016/j.visres.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Barathi VA, Weon SR, Beuerman RW. Expression of muscarinic receptors in human and mouse sclera and their role in the regulation of scleral fibroblasts proliferation. Mol Vis. 2009;15:1277–93. [PMC free article] [PubMed] [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Sparkes RL, Koop B, Birch DG, Bergen AA, Prinsen CF, Polomeno RC, Gal A, Drack AV, Musarella MA, Jacobson SG, Young RS, Weleber RG. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet. 2000;26:319–23. doi: 10.1038/81619. [DOI] [PubMed] [Google Scholar]
- Bitzer M, Schaeffel F. Defocus-induced changes in ZENK expression in the chicken retina. Invest Ophthalmol Vis Sci. 2002;43:246–52. [PubMed] [Google Scholar]
- Brand C, Burkhardt E, Schaeffel F, Choi JW, Feldkaemper MP. Regulation of Egr-1, VIP, and Shh mRNA and Egr-1 protein in the mouse retina by light and image quality. Mol Vis. 2005;11:309–20. [PubMed] [Google Scholar]
- Brand C, Schaeffel F, Feldkaemper MP. A microarray analysis of retinal transcripts that are controlled by image contrast in mice. Mol Vis. 2007;13:920–32. [PMC free article] [PubMed] [Google Scholar]
- Charman WN. Keeping the world in focus: how might this be achieved? Optom Vis Sci. 2011;88:373–6. doi: 10.1097/OPX.0b013e31820b052b. [DOI] [PubMed] [Google Scholar]
- Chiu PSL, Lauber JKL, Kinnear A. Dimensional and physiological lesions in the chick eye as influenced by the light environment. Proc Soc Exp Biol Med. 1975;148:1223–1228. doi: 10.3181/00379727-148-38721. [DOI] [PubMed] [Google Scholar]
- Chou TH, Kocaoglu OP, Borja D, Ruggeri M, Uhlhorn SR, Manns F, Porciatti V. Postnatal elongation of eye size in DBA/2J mice compared with C57BL/6J mice: in vivo analysis with whole-eye OCT. Invest Ophthalmol Vis Sci. 2011;52:3604–12. doi: 10.1167/iovs.10-6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crewther DP, Crewther SG. Refractive compensation to optical defocus depends on the temporal profile of luminance modulation of the environment. Neuroreport. 2002;13:1029–32. doi: 10.1097/00001756-200206120-00010. [DOI] [PubMed] [Google Scholar]
- Crewther DP, Crewther SG, Xie RZ. Changes in eye growth produced by drugs which affect retinal ON or OFF responses to light. J Ocul Pharmacol Ther. 1996;12:193–208. doi: 10.1089/jop.1996.12.193. [DOI] [PubMed] [Google Scholar]
- Crewther SG, Barutchu A, Murphy MJ, Crewther DP. Low frequency temporal modulation of light promotes a myopic shift in refractive compensation to all spectacle lenses. Exp Eye Res. 2006;83:322–8. doi: 10.1016/j.exer.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Crewther SG, Crewther DP. Inhibition of retinal ON/OFF systems differentially affects refractive compensation to defocus. Neuroreport. 2003;14:1233–7. doi: 10.1097/00001756-200307010-00009. [DOI] [PubMed] [Google Scholar]
- de la Cera EG, Rodriguez G, Llorente L, Schaeffel F, Marcos S. Optical aberrations in the mouse eye. Vision Res. 2006;46:2546–53. doi: 10.1016/j.visres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Edwards MH. Animal models of myopia. A review. Acta Ophthalmol Scand. 1996;74:213–9. doi: 10.1111/j.1600-0420.1996.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Edwards MH, Lam CS. The epidemiology of myopia in Hong Kong. Ann Acad Med Singapore. 2004;33:34–8. [PubMed] [Google Scholar]
- Faulkner AE, Kim KK, Iuvone PM, Pardue MT. Head-mounted goggles for murine form deprivation myopia. Journal of Neuroscience Methods. 2007;161:96–100. doi: 10.1016/j.jneumeth.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Gao H, Frost MR, Siegwart JT, Jr., Norton TT. Patterns of mRNA and protein expression during minus-lens compensation and recovery in tree shrew sclera. Mol Vis. 2011;17:903–19. [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Schery LA, Sharma R, Dubra A, Ahmad K, Libby RT, Williams DR. Optical properties of the mouse eye. Biomed Opt Express. 2011;2:717–38. doi: 10.1364/BOE.2.000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168:605–6. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- Guo Y, Flaherty MP, Wu WJ, Tan W, Zhu X, Li Q, Bolli R. Genetic background, gender, age, body temperature, and arterial blood pH have a major impact on myocardial infarct size in the mouse and need to be carefully measured and/or taken into account: results of a comprehensive analysis of determinants of infarct size in 1,074 mice. Basic Res Cardiol. 2012;107:288. doi: 10.1007/s00395-012-0288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Libby RT, John SW. Mouse genetic models: an ideal system for understanding glaucomatous neurodegeneration and neuroprotection. Prog Brain Res. 2008;173:303–21. doi: 10.1016/S0079-6123(08)01122-9. [DOI] [PubMed] [Google Scholar]
- Howlett MH, McFadden SA. Form-deprivation myopia in the guinea pig (Cavia porcellus) Vision Res. 2006;46:267–83. doi: 10.1016/j.visres.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49:219–27. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995;1:761–5. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Corfman TP. An overview of neurobiological comparisons in mouse strains. Neurosci Biobehav Rev. 1980;4:421–35. doi: 10.1016/0149-7634(80)90032-9. [DOI] [PubMed] [Google Scholar]
- Jiang M, Wu PC, Fini ME, Tsai CL, Itakura T, Zhang X, Jiao S. Single-shot dimension measurements of the mouse eye using SD-OCT. Ophthalmic Surg Lasers Imaging. 2012;43:252–6. doi: 10.3928/15428877-20120308-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John SW, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–62. [PubMed] [Google Scholar]
- Jung SK, Lee JH, Kakizaki H, Jee D. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in seoul, South Korea. Invest Ophthalmol Vis Sci. 2012;53:5579–83. doi: 10.1167/iovs.12-10106. [DOI] [PubMed] [Google Scholar]
- Lam CS, Goldschmidt E, Edwards MH. Prevalence of myopia in local and international schools in Hong Kong. Optom Vis Sci. 2004;81:317–22. doi: 10.1097/01.opx.0000134905.98403.18. [DOI] [PubMed] [Google Scholar]
- Larsen JS. Axial length of the emmetropic eye and its relation to the head size. Acta Ophthalmol (Copenh) 1979;57:76–83. doi: 10.1111/j.1755-3768.1979.tb06662.x. [DOI] [PubMed] [Google Scholar]
- Laties AM, Stone RA. Ametropia in retinal disorders. In: Anderson RE, Hollyfield JG, LaVail M, editors. Retinal Degenerations. CRC Press; Boca Raton: 1991. pp. 383–390. [Google Scholar]
- Li S, Wu J, Ding H, Liao A, He H, Stell WK, Zhong X. Flicker downregulates the content of crystallin proteins in form-deprived C57BL/6 mouse retina. Exp Eye Res. 2012;101:1–8. doi: 10.1016/j.exer.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Li T, Troilo D, Glasser A, Howland HC. Constant light produces severe corneal flattening and hyperopia in chickens. Vision Res. 1995;35:1203–9. doi: 10.1016/0042-6989(94)00231-a. [DOI] [PubMed] [Google Scholar]
- Lim W, Kwan JL, Goh LK, Beuerman RW, Barathi VA. Evaluation of gene expression profiles and pathways underlying postnatal development in mouse sclera. Mol Vis. 2012;18:1436–48. [PMC free article] [PubMed] [Google Scholar]
- Lu F, Zhou X, Jiang L, Fu Y, Lai X, Xie R, Qu J. Axial myopia induced by hyperopic defocus in guinea pigs: A detailed assessment on susceptibility and recovery. Exp Eye Res. 2009;89:101–8. doi: 10.1016/j.exer.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Lu F, Zhou X, Zhao H, Wang R, Jia D, Jiang L, Xie R, Qu J. Axial myopia induced by a monocularly-deprived facemask in guinea pigs: A non-invasive and effective model. Exp Eye Res. 2006;82:628–36. doi: 10.1016/j.exer.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Mathis U, Schaeffel F. Glucagon-related peptides in the mouse retina and the effects of deprivation of form vision. Graefes Arch Clin Exp Ophthalmol. 2006 doi: 10.1007/s00417-006-0282-x. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Norton TT. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri) Vision Res. 1992;32:843–52. doi: 10.1016/0042-6989(92)90027-g. [DOI] [PubMed] [Google Scholar]
- McFadden SA, Howlett MH, Mertz JR. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res. 2004;44:643–53. doi: 10.1016/j.visres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Yagasaki K, Horiguchi M, Kawase Y, Kanda T. Congenital stationary night blindness with negative electroretinogram. A new classification. Arch Ophthalmol. 1986;104:1013–20. doi: 10.1001/archopht.1986.01050190071042. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Ren G, Akileswaran L. Localization of nyctalopin in the mammalian retina. Eur J Neurosci. 2006;23:1163–71. doi: 10.1111/j.1460-9568.2006.04647.x. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Cepurna Ying, Guo WO, Johnson EC. Pathophysiology of human glaucomatous optic nerve damage: insights from rodent models of glaucoma. Exp Eye Res. 2011;93:156–64. doi: 10.1016/j.exer.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti DO, Ver Hoeve JN, Zadnik K, Murphy CJ. The artifact of retinoscopy revisited: comparison of refractive error measured by retinoscopy and visual evoked potential in the rat. Optom Vis Sci. 1997;74:483–8. doi: 10.1097/00006324-199707000-00014. [DOI] [PubMed] [Google Scholar]
- Naarendorp F, Esdaille TM, Banden SM, Andrews-Labenski J, Gross OP, Pugh EN., Jr. Dark light, rod saturation, and the absolute and incremental sensitivity of mouse cone vision. J Neurosci. 2010;30:12495–507. doi: 10.1523/JNEUROSCI.2186-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–68. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue MT, Faulkner AE, Fernandes A, Yin H, Schaeffel F, Williams RW, Pozdeyev N, Iuvone PM. High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Invest Ophthalmol Vis Sci. 2008;49:706–12. doi: 10.1167/iovs.07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Qazi Y, Tan C, Jabbar SB, Cao Y, Schmid G, Pardue MT. Assessment of axial length measurements in mouse eyes. Optom Vis Sci. 2012;89:296–303. doi: 10.1097/OPX.0b013e31824529e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pras E, Abu A, Rotenstreich Y, Avni I, Reish O, Morad Y, Reznik-Wolf H. Cone-rod dystrophy and a frameshift mutation in the PROM1 gene. Mol Vis. 2009;15:1709–16. [PMC free article] [PubMed] [Google Scholar]
- Prashar A, Hocking PM, Erichsen JT, Fan Q, Saw SM, Guggenheim JA. Common determinants of body size and eye size in chickens from an advanced intercross line. Exp Eye Res. 2009;89:42–8. doi: 10.1016/j.exer.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–6. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- Puk O, Dalke C, Favor J, de Angelis MH, Graw J. Variations of eye size parameters among different strains of mice. Mamm Genome. 2006;17:851–7. doi: 10.1007/s00335-006-0019-5. [DOI] [PubMed] [Google Scholar]
- Pusch CM, Zeitz C, Brandau O, Pesch K, Achatz H, Feil S, Scharfe C, Maurer J, Jacobi FK, Pinckers A, Andreasson S, Hardcastle A, Wissinger B, Berger W, Meindl A. The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nat Genet. 2000;26:324–7. doi: 10.1038/81627. [DOI] [PubMed] [Google Scholar]
- Qian YS, Chu RY, Hu M, Hoffman MR. Sonic hedgehog expression and its role in form-deprivation myopia in mice. Curr Eye Res. 2009;34:623–35. doi: 10.1080/02713680903003492. [DOI] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL., 3rd Recovery from form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:3361–72. doi: 10.1167/iovs.04-0080. [DOI] [PubMed] [Google Scholar]
- Rada JA, Shelton S, Norton TT. The sclera and myopia. Exp Eye Res. 2006;82:185–200. doi: 10.1016/j.exer.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Regal DM, Boothe R, Teller DY, Sackett GP. Visual acuity and visual responsiveness in dark-reared monkeys (Macaca nemestrina) Vision Res. 1976;16:523–30. doi: 10.1016/0042-6989(76)90034-1. [DOI] [PubMed] [Google Scholar]
- Remtulla S, Hallett PE. A schematic eye for the mouse, and comparisons with the rat. Vision Res. 1985;25:21–31. doi: 10.1016/0042-6989(85)90076-8. [DOI] [PubMed] [Google Scholar]
- Ritchey ER, Zelinka C, Tang J, Liu J, Code KA, Petersen-Jones S, Fischer AJ. Vision-guided ocular growth in a mutant chicken model with diminished visual acuity. Exp Eye Res. 2012;102:59–69. doi: 10.1016/j.exer.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymer J, Wildsoet CF. The role of the retinal pigment epithelium in eye growth regulation and myopia: a review. Vis Neurosci. 2005;22:251–61. doi: 10.1017/S0952523805223015. [DOI] [PubMed] [Google Scholar]
- Saw SM, Chua WH, Wu HM, Yap E, Chia KS, Stone RA. Myopia: gene-environment interaction. Ann Acad Med Singapore. 2000;29:290–7. [PubMed] [Google Scholar]
- Schaeffel F. The mouse eye as a model for myopia, and optics of the eye. In: Chalupa LM, Willaims RW, editors. Eye, Retina and Visual System of the Mouse. The MIT Press; Cambridge: 2008a. pp. 73–85. [Google Scholar]
- Schaeffel F. Test systems for measuring ocular parameters and visual function in mice. Front Biosci. 2008b;13:4904–11. doi: 10.2741/3049. [DOI] [PubMed] [Google Scholar]
- Schaeffel F. The mouse model of myopia. In: Beuerman RW, Saw SM, Tan DTH, Wong TY, editors. Myopia: Animal Models to Clinical Trials. World Scientific; New Jersey: 2010. pp. 303–330. [Google Scholar]
- Schaeffel F, Burkhardt E, Howland HC, Williams RW. Measurement of refractive state and deprivation myopia in two strains of mice. Optom Vis Sci. 2004;81:99–110. doi: 10.1097/00006324-200402000-00008. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Howland HC. Visual optics in normal and ametropic chickens. Clinical Vision Science. 1988;3:83–98. [Google Scholar]
- Schippert R, Burkhardt E, Feldkaemper M, Schaeffel F. Relative axial myopia in Egr-1 (ZENK) knockout mice. Invest Ophthalmol Vis Sci. 2007;48:11–7. doi: 10.1167/iovs.06-0851. [DOI] [PubMed] [Google Scholar]
- Schippert R, Schaeffel F, Feldkaemper MP. Microarray analysis of retinal gene expression in Egr-1 knockout mice. Mol Vis. 2009;15:2720–39. [PMC free article] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. Contrast and spatial-frequency requirements for emmetropization in chicks. Vision Res. 1997;37:2011–21. doi: 10.1016/s0042-6989(97)00014-x. [DOI] [PubMed] [Google Scholar]
- Schmucker C, Schaeffel F. In vivo biometry in the mouse eye with low coherence interferometry. Vision Res. 2004a;44:2445–56. doi: 10.1016/j.visres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Schmucker C, Schaeffel F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vision Res. 2004b;44:1857–67. doi: 10.1016/j.visres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Searle AG. Comparative genetics of albinism. Ophthalmic Paediatr Genet. 1990;11:159–64. doi: 10.3109/13816819009020974. [DOI] [PubMed] [Google Scholar]
- Shen W, Vijayan M, Sivak JG. Inducing form-deprivation myopia in fish. Invest Ophthalmol Vis Sci. 2005;46:1797–803. doi: 10.1167/iovs.04-1318. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Res. 1977;124:154–7. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr., Norton TT. Goggles for controlling the visual environment of small animals. Lab Anim Sci. 1994;44:292–4. [PubMed] [Google Scholar]
- Siegwart JT, Jr., Norton TT. Steady state mRNA levels in tree shrew sclera with form-deprivation myopia and during recovery. Invest Ophthalmol Vis Sci. 2001;42:1153–1159. [PubMed] [Google Scholar]
- Siegwart JT, Jr., Norton TT. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2005;46:3484–92. doi: 10.1167/iovs.05-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegwart JT, Jr., Norton TT. Perspective: how might emmetropization and genetic factors produce myopia in normal eyes? Optom Vis Sci. 2011;88:E365–72. doi: 10.1097/OPX.0b013e31820b053d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieving PA, Fishman GA. Refractive errors of retinitis pigmentosa patients. Br J Ophthalmol. 1978;62:163–7. doi: 10.1136/bjo.62.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M, Whittock N, Searle A, Croft M, Brewer C, Cole M. Phenotype of autosomal dominant cone-rod dystrophy due to the R838C mutation of the GUCY2D gene encoding retinal guanylate cyclase-1. Eye (Lond) 2007;21:1220–5. doi: 10.1038/sj.eye.6702612. [DOI] [PubMed] [Google Scholar]
- Stone RA, Khurana TS. Gene profiling in experimental models of eye growth: clues to myopia pathogenesis. Vision Res. 2010;50:2322–33. doi: 10.1016/j.visres.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Lin T, Desai D, Capehart C. Photoperiod, early post-natal eye growth, and visual deprivation. Vision Res. 1995;35:1195–202. doi: 10.1016/0042-6989(94)00232-b. [DOI] [PubMed] [Google Scholar]
- Tansley K. Vision in Vertebrates. Chapman and Hall; London: 1965. [Google Scholar]
- Tejedor J, de la Villa P. Refractive changes induced by form deprivation in the mouse eye. Invest Ophthalmol Vis Sci. 2003;44:32–6. doi: 10.1167/iovs.01-1171. [DOI] [PubMed] [Google Scholar]
- Tkatchenko TV, Shen Y, Tkatchenko AV. Analysis of postnatal eye development in the mouse with high-resolution small animal magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2009;51:21–7. doi: 10.1167/iovs.08-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkatchenko TV, Shen Y, Tkatchenko AV. Analysis of postnatal eye development in the mouse with high-resolution small animal magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2010a;51:21–7. doi: 10.1167/iovs.08-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkatchenko TV, Shen Y, Tkatchenko AV. Mouse experimental myopia has features of primate myopia. Invest Ophthalmol Vis Sci. 2010b;51:1297–303. doi: 10.1167/iovs.09-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkatchenko TV, Tkatchenko AV. Ketamine-xylazine anesthesia causes hyperopic refractive shift in mice. J Neurosci Methods. 2010;193:67–71. doi: 10.1016/j.jneumeth.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus) Vision Res. 1993;33:1311–24. doi: 10.1016/0042-6989(93)90039-y. [DOI] [PubMed] [Google Scholar]
- Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004. Arch Ophthalmol. 2009;127:1632–9. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201:1249–51. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–68. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Wang D, Ding X, Liu B, Zhang J, He M. Longitudinal changes of axial length and height are associated and concomitant in children. Invest Ophthalmol Vis Sci. 2011a;52:7949–53. doi: 10.1167/iovs.11-7684. [DOI] [PubMed] [Google Scholar]
- Wang L, Povazay B, Chen YP, Hofer B, Drexler W, Guggenheim JA. Heritability of ocular component dimensions in mice phenotyped using depth-enhanced swept source optical coherence tomography. Exp Eye Res. 2011b;93:482–90. doi: 10.1016/j.exer.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–8. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- Wisard J, Chrenek MA, Wright C, Dalal N, Pardue MT, Boatright JH, Nickerson JM. Non-contact measurement of linear external dimensions of the mouse eye. J Neurosci Methods. 2010;187:156–66. doi: 10.1016/j.jneumeth.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisard J, Faulkner A, Chrenek MA, Waxweiler T, Waxweiler W, Donmoyer C, Liou GI, Craft CM, Schmid GF, Boatright JH, Pardue MT, Nickerson JM. Exaggerated eye growth in IRBP-deficient mice in early development. Invest Ophthalmol Vis Sci. 2011;52:5804–11. doi: 10.1167/iovs.10-7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmazer-Hanke DM. Morphological correlates of emotional and cognitive behaviour: insights from studies on inbred and outbred rodent strains and their crosses. Behav Pharmacol. 2008;19:403–34. doi: 10.1097/FBP.0b013e32830dc0de. [DOI] [PubMed] [Google Scholar]
- Yin G, Wang YX, Zheng ZY, Yang H, Xu L, Jonas JB. Ocular Axial Length and Its Associations in Chinese: The Beijing Eye Study. PLoS One. 2012;7:e43172. doi: 10.1371/journal.pone.0043172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young FA. Animal experimentation and research in refractive state. In: Hirsch MJ, editor. Synposis of the Refractive State of the Eye: A Symposium. Burgess Publishing Company; St. Paul: 1967. pp. 26–38. [Google Scholar]
- Yu Y, Chen H, Tuo J, Zhu Y. Effects of flickering light on refraction and changes in eye axial length of C57BL/6 mice. Ophthalmic Res. 2011;46:80–7. doi: 10.1159/000323179. [DOI] [PubMed] [Google Scholar]
- Zhou G, Strom RC, Giguere V, Williams RW. Modulation of retinal cell populations and eye size in retinoic acid receptor knockout mice. Mol Vis. 2001;7:253–60. [PubMed] [Google Scholar]
- Zhou G, Williams RW. Eye1 and Eye2: gene loci that modulate eye size, lens weight, and retinal area in the mouse. Invest Ophthalmol Vis Sci. 1999a;40:817–25. [PubMed] [Google Scholar]
- Zhou G, Williams RW. Mouse models for the analysis of myopia: an analysis of variation in eye size of adult mice. Optom Vis Sci. 1999b;76:408–18. doi: 10.1097/00006324-199906000-00021. [DOI] [PubMed] [Google Scholar]
- Zhou X, An J, Wu X, Lu R, Huang Q, Xie R, Jiang L, Qu J. Relative axial myopia induced by prolonged light exposure in C57BL/6 mice. Photochem Photobiol. 2010a;86:131–7. doi: 10.1111/j.1751-1097.2009.00637.x. [DOI] [PubMed] [Google Scholar]
- Zhou X, Huang Q, An J, Lu R, Qin X, Jiang L, Li Y, Wang J, Chen J, Qu J. Genetic deletion of the adenosine A2A receptor confers postnatal development of relative myopia in mice. Invest Ophthalmol Vis Sci. 2010b;51:4362–70. doi: 10.1167/iovs.09-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Ji F, An J, Zhao F, Shi F, Huang F, Li Y, Jiao S, Yan D, Chen X, Chen J, Qu J. Experimental murine myopia induces collagen type Ialpha1 (COL1A1) DNA methylation and altered COL1A1 messenger RNA expression in sclera. Mol Vis. 2012;18:1312–24. [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Shen M, Xie J, Wang J, Jiang L, Pan M, Qu J, Lu F. The development of the refractive status and ocular growth in C57BL/6 mice. Invest Ophthalmol Vis Sci. 2008a;49:5208–14. doi: 10.1167/iovs.07-1545. [DOI] [PubMed] [Google Scholar]
- Zhou X, Xie J, Shen M, Wang J, Jiang L, Qu J, Lu F. Biometric measurement of the mouse eye using optical coherence tomography with focal plane advancement. Vision Res. 2008b;48:1137–43. doi: 10.1016/j.visres.2008.01.030. [DOI] [PubMed] [Google Scholar]