Abstract
Background
The proliferation of cardiomyocytes is highly restricted after postnatal maturation, limiting heart regeneration. Elucidation of the regulatory machineries for the proliferation and growth arrest of cardiomyocytes is imperative. Chemical biology is efficient to dissect molecular mechanisms of various cellular events and often provide therapeutic potentials. We have been investigating cardiovascular differentiation with pluripotent stem cells (PSCs). The combination of stem cell and chemical biology can provide novel approaches to investigate the molecular mechanisms and manipulation of cardiomyocyte proliferation.
Methods and Results
To identify chemicals that regulate cardiomyocyte proliferation, we performed a screening of a defined chemical library based on proliferation of mouse PSC-derived cardiomyocytes and identified 4 chemical compound groups - inhibitors of glycogen synthase kinase-3 (GSK3), p38 mitogen-activated protein kinase (MAPK) and Ca2+/calmodulin-dependent protein kinase II (CaMKII), and activators of extracellular signal-regulated kinase (ERK). Several appropriate combinations of chemicals synergistically enhanced proliferation of cardiomyocytes derived from both mouse and human PSCs, notably up to a 14-fold increase in mouse cardiomyocytes. We also examined the effects of identified chemicals on cardiomyocytes in various developmental stages and species. Whereas ERK activators and CaMKII inhibitors showed proliferative effects only on cardiomyocytes in early developmental stages, GSK3 and p38 MAPK inhibitors substantially and synergistically induced reentry and progression of cell cycle in not only neonatal but also adult cardiomyocytes.
Conclusions
Our approach successfully uncovered novel molecular targets and mechanisms controlling cardiomyocyte proliferation in distinct developmental stages and offered PSC-derived cardiomyocytes as a potent tool to explore chemical-based cardiac regenerative strategies.
Keywords: cardiomyocyte, embryonic stem cell, proliferation, small molecules
Life-threatening heart diseases, such as myocardial infarction and heart failure, are major causes of death in developed countries. Due to the almost non-existent cardiomyocyte turnover in the human heart after birth, recovery of cardiac function after heart disease is insufficient.1–3 The low levels of proliferation and regeneration ability of cardiomyocytes must be overcome to effectively treat these diseases. Although numerous causes of postnatal cell cycle arrest were extensively investigated, such as balances of cyclins, cyclin dependent kinases (CDKs) and CDK inhibitors4, growth factors5–9, transcription factors10–14 and micro RNA15, heart regenerative medicine has not been effective. One of the limitations is lack of efficient methods for manipulating multiple factors simultaneously.
We hypothesized that a chemical biological approach would be a suitable answer to this problem. Compared to conventional genetic methods, chemical-biological approaches for exploring key biological mechanisms have many advantages, enabling temporal control, rapid inhibition or activation, and regulation of functionally overlapping targets.16,17 Moreover, chemicals can function across similar species and can be directly applied as therapeutic drugs. Thus, identifying novel chemicals would be an efficient approach to elucidate novel mechanisms regulating cardiomyocyte proliferation and finally employ cardiac regeneration as a therapeutic strategy. Nevertheless, no efficient chemical screening platform for cardiomyocyte proliferation has been explored to date. Recent advances in imaging and analyzing have led to novel approaches to analyze numerous samples automatically. These cell-based and imaging-based methods to screen are called high-content screening (HCS), providing various information on cellular phenotype including cell division.18
Pluripotent stem cells (PSCs), including both embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) have great potentials for therapeutic purpose and for drug discovery as they can give rise to any cell types, including cardiomyocytes.19–23 We have been investigating cardiovascular cell differentiation and regeneration with the use of PSCs, and have established efficient methods for cardiac differentiation from mouse and human PSCs.24–28 Here we combined our stem cell technology and chemical-biology with HCS to identify chemicals inducing cardiomyocyte proliferation. We successfully identified several chemicals with distinct molecular targets and confirmed their proliferative effects on cardiomyocytes from mouse PSCs. We further demonstrated that the chemical-induced effects on cardiomyocytes from different stages of maturation - embryos, neonates and adults. This study provides novel understanding for molecular machineries and would offer efficient ways to regulate cardiomyocyte proliferation with chemicals.
Methods
Reagents and Antibodies
The SCADS inhibitor kit containing approximately 280 well-established kinase inhibitors29,30 was a gift from the Screening Committee of Anticancer Drugs supported by a Grant-in-Aid for Scientific Research on Priority Area ‘Cancer’ from The Ministry of Education, Culture, Sports, Science and Technology, Japan. See Supplemental Methods for reagents and antibodies.
High-Content Screening
The screening process is summarized in Figure S1 and S2. FACS-purified mouse ESC-derived cardiomyocytes (mESCMs) were plated on 0.1% gelatin-coated 96-well plates at 500 cells per well. Cells were treated with chemicals from SCADS inhibitor kit (0.2μM–5μM) for 5 days, followed by fixation and staining with anti-α-myosin heavy chain (αMHC) antibody and DAPI. A DAPI-positive spot in an αMHC-positive area was counted as one nucleus (Figure S2c). αMHC-positive nuclei in four low magnification fields, covering approximately 60% of a single well of 96-well plates, were counted using HCS system ImageXpress (Molecular Devices, Sunnyvale, CA, USA) and image processing software MetaXpress (Molecular Devices).
Statistics
All experiments were repeated at least three times, except the first screen that was repeated twice. Values were reported as mean ± SD and were analyzed by Mann-Whitney test (for two-group comparison), or by Dunn’s test (for multiple comparison) using a statistics software, GraphPad Prism (GraphPad Software, Inc., CA, USA). Values of p < 0.05 were considered to be statistically significant.
See Supplemental Methods for mouse ESC and human ESC/iPSC culture and differentiation, cardiomyocyte isolation, immunostaining, cell-cycle analysis by flow cytometry, Western blotting, gene knockdown, electrophysiological study, and quantitative reverse transcriptional polymerase chain reaction (qPCR).
Results
Chemical Library Screening for Cardiomyocyte Proliferation in mESCMs
A mouse ESC line carrying αMHC promoter-driven enhanced green fluorescent protein (αMHC-EGFP)25 was used to prepare purified early differentiated cardiomyocytes (Figure S1), with which we evaluated the chemical-induced effects on cardiomyocyte proliferation. mESCMs appear at 3–4 days after Flk1-positive mesoderm cells were cultured on OP9 stroma cells as we previously reported (Flkd3 to d4; Figure S1).25,27 Within a few days after the cells differentiated to cardiomyocytes, they ceased their proliferation similar to cardiomyocytes in vivo. To screen chemicals that exhibit a direct pro-proliferative effect on mESCMs, we sorted, purified and re-cultured αMHC-EGFP-positive mESCMs at Flkd6 (Figure S2b–d). For the primary screen, we performed HCS to directly count the number of cardiomyocyte nuclei (Figure S2c). Purified mESCMs were re-plated on 96-well plate with treatment of each chemical from the SCADS inhibitor kit in three concentrations (0.2, 1, 5 μM). Five days after treatment (Flkd6+5), the average number of mESCM nuclei was 35.6 ± 17.5 (cells per field, n = 35) in the control condition. Seven chemicals increased mESCM nucleus number more than mean + 2SD of control (red spots in Figure 1a and Table S1). Two Ca2+/calmodulin-dependent kinase II (CaMKII) inhibitors increased the number of mESCM nuclei more than mean + 1SD of control (blue spots in Figure 1a and Table S1).
Figure 1.
Three Chemicals were Identified With Chemical Library Screening for mESCM Proliferation. (a) Primary screen with high content screening for nucleus numbers in αMHC-positive cells (mESCMs). Mean nucleus numbers at Flkd6+5 treated with approximately 280 chemicals at three concentrations (● 5μM, ■ lμM, and ▲ 0.2μM, n = 2). Seven chemicals (red symbols) increased mESCMs more than mean + 2SD of control (control: n = 35). Two CaMKII inhibitors (blue symbols) increased mESCM more than mean + 1SD. (b) Secondary screen for the seven chemicals calculating actual cardiomyocyte numbers with flow cytometry. Abbreviation: Aminoglutethimide, AGT and Glibenclamide, Gli. Concentration: AGT 5μM, BIO 1μM, NU6102 5μM, HA14-1 1μM, SU1498 5μM, Gli 5μM and KN93 5μM. *, p < 0.05, **, p < 0.01 vs. DMSO treatment (Dunn’s test for multiple comparisons, n = 3–11). (c) Chemicals increased mESCM numbers during Flkd6+2 to Flkd6+5. p value: Mann-Whitney test, n = 3. (d) Captured images from time-lapse video recording during Flkd6+0.5 (0:00) to 4.5 (96:00).
Because more than 80% of murine mature cardiomyocytes in adults are reported to have two nuclei1, 31, an increase in the number of cardiomyocyte nuclei may not directly reflect the actual increase in the number of cardiomyocytes. To evaluate the effects of the chemicals on the actual cardiomyocyte number, we performed a secondary screen with seven small molecules of independent kinase targets among the nine candidate chemicals (Table S1). We purified mESCMs, re-cultured with chemicals for 5 days, and calculated the actual cardiomyocyte number by cell counting and flow cytometry for αMHC-EGFP (Figure S2d). Three out of the seven chemicals significantly increased mESCM cell number (Figure 1b). BIO (GSK3 inhibitor, 1 μM), SU1498 (Flk1 inhibitor, 5 μM), and KN93 (CaMKII inhibitor, 5 μM) increased cardiomyocyte number to 3.4 ± 1.4, 2.5 ± 0.6, and 2.2 ± 0.4 times over control, respectively.
Next, we compared mESCM number between Flkd6+2 and Flkd6+5 to confirm whether these chemicals could actually increase cardiomyocyte proliferation and whether they did not simply improve the re-plating viability of mESCMs. Whereas re-plated mESCMs showed no increase in cell number with DMSO alone, all three chemicals significantly increased mESCM number during Flkd6+2 to Flkd6+5 (Figure 1c). We further confirmed cardiomyocyte proliferation with time-lapse video recording (Figure 1d, Supplemental Movie 1–4). Whereas a control cardiomyocyte (Movie S1) ceased proliferation and just underwent hypertrophic change, cardiomyocytes treated with these chemicals caused cell division and proliferation (Movie S2–4). These data clearly show that the three chemicals (BIO, SU1498 and KN93) enhance mESCM proliferation and increase in mESCM number accompanying actual cell division but not binucleation.
Induction of Cell Cycle Progression in Cardiomyocytes by Chemicals
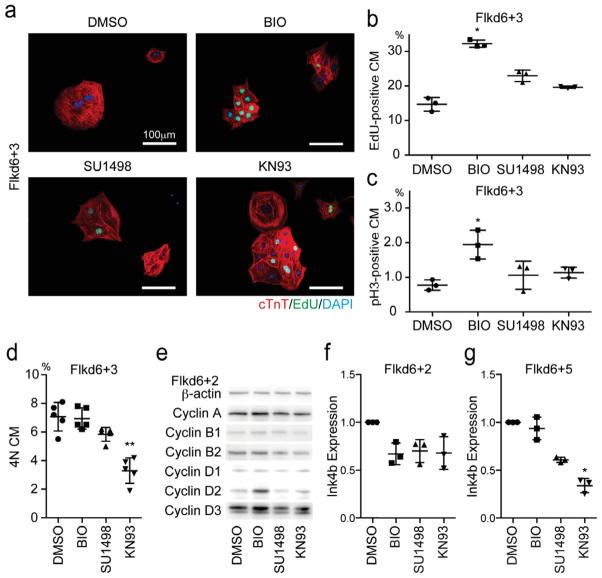
Next, we examined changes in mESCM cell cycle. The ratio of S phase in mESCMs, as estimated with 3 hour pulse-labeled EdU incorporation, and the ratio of M phase in mESCMs, as estimated by immunostaining of Phosphorylated Histone H3 (pH3) at Flkd6+3 were increased more than 2.2 times by BIO treatment and increased approximately 1.5–2 times by SU1498 and KN93 (Figure 2a–c). The ratio of 4N cardiomyocytes was examined as doubled DNA contents (4N) and EdU-negative cells in cardiac Troponin T (cTnT)-positive population by flow cytometry. 4N cardiomyocytes, consisting of cells in G2/M phase and cells with binuclei, were not increased by any of chemical treatment but reduced by KN93 treatment compared with DMSO treatment, suggesting binuclear cardiomyocytes were not induced by these chemicals (Figure 2d). Western blotting for cyclins showed that BIO but not SU1498 or KN93 treatment increased Cyclin D2 and Cyclin D3 (Figure 2e) as reported.32 On the other hand, a CDK inhibitor, Ink4b expression was downregulated 30% by treatment of each chemical at Flkd6+2 (Figure 2f). Downregulation of Ink4b was sustained until Flkd6+5 in SU1498 or KN93 treatment (Figure 2g). These results indicate that these chemicals actually induce cell cycle progression in cardiomyocytes by regulating Cyclin/CDK activity.
Figure 2.
Cell Cycle in mESCM was Enhanced by Identified Chemicals. (a) Immunostaining of purified mESCMs for cardiac troponin T (red), EdU (Green), and DAPI (Blue) at Flkd6+3. Scale bar = 100μm (b–c) Quantification of mESCMs positive for cell cycle markers (b) S phase (EdU-positive) and (c) M phase (phospho histone H3: pH3-positive) at Flkd6+3. *, p < 0.05 vs. DMSO group (Dunn’s test, n = 3). (d) Quantification of 4N (Binuclear (2×2N) and G2/M) mESCMs by flow cytometry. **, p < 0.01 vs. DMSO group (Dunn’s test, n = 5). (e) Western blotting for cyclins at Flkd6+2. BIO treatment increased Cyclin Ds. (f–g) qRT-PCR for Ink4b, a CDK inhibitor at Flkd6+2 (f) and Flkd6+5 (g). The expression of Ink4b was suppressed by all three chemicals at Flkd6+2 and more than 60% by KN93 at Flkd6+5. Relative expression levels compared to DMSO treatment group were shown. *, p < 0.05 and **, p < 0.01 vs. DMSO group (Dunn’s test, n = 3).
Molecular Targets of the Chemicals
Next we confirmed the molecular targets of these three chemicals. Addition of CHIR99021, another GSK3-specific inhibitor (Figure 3a), or Wnt3a (data not shown) increased cardiomyocyte numbers similar to BIO, indicating that inhibition of GSK3 enhanced mESCM proliferation as reported in rat cardiomyocytes.32
Figure 3.
Identified Chemicals were GSK3 Inhibitor, ERK Activator and CaMKII Inhibitor. (a) Effects of GSK3 inhibitors, BIO and CHIR99021 (CHIR) (Mann-Whitney test, n = 3) on mESCM numbers. (b) Western blotting for Phosphorylated ERK (phos-ERK) and total ERK at Flkd6+2. SU1498 treatment increased phos-ERK. (c) mESCM cell number at Flkd6+5. SU1498 (SU)-elicited mESCM proliferation was attenuated by a MEK inhibitor (PD98059, PD) treatment. A Raf activator (ZM336372, ZM) treatment also increased mESCM number. *, p < 0.05 vs. control group (Dunn’s test, n = 3–9). (d) Effects of CaMKII inhibitors, KN93 and KN62, on mESCM numbers at Flkd6+5 (Mann-Whitney test, n = 3).
SU1498, first reported as a tyrosine kinase inhibitor of vascular endothelial growth factor receptor 2 (VEGFR2; also designated as Flk1)33, enhanced cardiomyocyte proliferation even though Flk1 is not expressed in mESCMs25, suggesting that SU1498 exerted its effect through targets other than Flk1. SU1498 was reported to cause accumulation of phosphorylated extracellular signal-regulated kinase (ERK) via inhibiting phosphatase binding to ERK.34 We performed Western Blot analysis to show if SU1498 cause accumulation of phosphorylated ERK in mESCMs. Phosphorylation of ERK was increased only when cells were treated by SU1498 but not with BIO or KN93 (Figure 3b). Then we determined whether SU1498-elicited mESCM proliferation is mediated by ERK signaling. Treatment with a MAPK/ERK kinase (MEK) inhibitor PD98059 (PD) that inhibits ERK phosphorylation abolished the increase in SU1498-induced mESCMs (Figure 3c). In addition, ZM336372 (ZM), an activator of Raf1/ERK signaling, similarly increased mESCM number (Figure 3c). Furthermore, an ERK activating growth factor, NRG1β, also increased mESCM numbers by approximately 1.5 times (data not shown) as reported.9,35 Taken together, these data indicate that SU1498 increase cardiomyocyte proliferation through activation of Raf-MEK-ERK signal cascade.7,36
CaMKII was reported to be involved in cardiomyocyte hypertrophy, but little is known about its role in cardiomyocyte proliferation. Two distinct CaMKII inhibitors, KN93 and KN62, similarly increased cardiomyocyte numbers (Figure 3d), suggesting that CaMKII is a novel regulator of cardiomyocyte proliferation. We investigated the role of CaMKII in cardiomyocyte proliferation. CaMKII is a family protein coded by the Camk2a, Camk2b, Camk2d and Camk2g isoform genes. Among them, Camk2a, Camk2d and Camk2g mRNA but not Camk2b mRNA expressions were detected in purified mESCM at Flkd6 (data not shown). We evaluated the effect of specific small interfering RNA (siRNA) against each CaMKII isoform to identify the main isoform(s) that inhibit mESCM proliferation. Specific knockdown of Camk2d and 2g, the major CaMKII isoforms in cardiomyocytes37, by siRNA increased mESCM cell numbers by 50% (Figure S3a–b). Conversely, knockdown of Camk2a reciprocally decreased mESCM numbers (Figure S3b). These data suggest that KN93 and KN62 increase mESCM by inhibiting CaMKIId/g. Knockdown of Camk2d but not Camk2g by siRNA resulted in 50% reduction of Ink4b expression similar to KN93 treatment (Figure S3c). Furthermore, reduction of Ink4b with siRNA significantly increased mESCM numbers (Figure S3d–e). These data suggest that CaMKIId negatively regulates mESCM proliferation through Ink4b.
Our results, thus, show that these chemicals affect cardiomyocyte proliferation through distinct pathways–BIO/GSK3, SU1498/ERK, and KNs/CaMKII.
Combinatory Effects of Chemicals on mESCM Proliferation
Distinct molecular targets of these chemicals prompted us to examine combinations of inhibitors to maximize their proliferative effects. We first examined combinations of two inhibitors. BIO+SU1498 (SU) and BIO+KN93 (KN) significantly increased mESCM proliferation (Figure 4a). Unexpectedly, a combination of SU+KN cancelled their individual effects. Additionally a combination of all three inhibitors, BIO+SU+KN, neither showed any effect on cardiomyocyte proliferation (Figure 4a), suggesting that SU1498 and KN93 could be conflicting in the cardiomyocyte proliferation machinery. A p38 MAPK inhibitor SB203580 (p38i) is known to increase neonatal or adult cardiomyocyte proliferation.38 In fact, p38i (1 μM) significantly increased the mESCM number by approximately twofold also in our experimental system (Figure 4b). Therefore, we added p38i to each combination, and found that BIO+SU+p38i and BIO+KN+p38i increased in mESCM numbers up to 6–7 times more than control until the mESCMs reached confluency (data not shown). To allow more robust proliferation, we next tested mESCM culture at a lower cell density (5.0×104 / well (6-well plate)). In this condition, the BIO+SU+p38i combination increased mESCM up to 14-fold (Figure 4c). We also examined cell cycle of mESCMs treated with each combination (Figure 4d). Both combinations (BIO+SU+p38i and BIO+KN+p38i) significantly increased EdU (3hr pulse) incorporation to approximately 2.5 and 4 times more than DMSO treatment at Flkd6+3 and Flkd6+5, respectively (Figure 4d). These combinations also significantly increased pH3 in mESCMs up to approximately 2.7 and 2.5 times more than DMSO treatment at Flkd6+3 and Flkd6+5, respectively (Figure 4d). We also evaluated 4N fraction (G2/M and binucleated cardiomyocytes) by flow cytometry, but there were no significant differences (data not shown). Thus, we successfully demonstrated that optimal combinations of the chemicals effectively induced substantial cardiomyocyte proliferation. We, collectively, named these seven chemicals (BIO, CHIR99021, SU1498, ZM336372, KN93, KN62 and p38i) as cardiomyocyte proliferative chemicals (CPCs).
Figure 4.
Combination of Chemicals Synergistically Enhanced mESCM Proliferation. (a) Combinatory effects of chemicals on mESCM cell number (Dunn’s test: *, p < 0.05 and **, p < 0.01, vs. DMSO treated group, n = 5) (b) Effects of a p38 inhibitor (p38i, SB203580). p value: Mann-Whitney test (n = 6) (c) Combinatory effects of chemicals on mESCM cell number in low cell density culture (Dunn’s test: ***, p < 0.001, n = 5, vs. DMSO control). Note that BIO+SU+p38i treatment increased mESCM number 14-fold compared to DMSO treatment. (d) Immunostaining and quantification of cell cycle markers. Cardiac troponin T (cTnT, red), EdU (Green), phospho-histone H3 (pH3: white), and DAPI (Blue). Scale bar = 100 μm.
We further confirmed cardiomyocyte features after proliferation. All αMHC-EGFP-positive cells used in our study were also positive for cTnT (Figure S1). These cells were spontaneously beating before and after the chemical treatments. Proliferated cardiomyocytes with combined CPC treatments (BIO+SU1498+p38i and BIO+KN93+p38i) showed similar cardiomyocyte-like action potentials (Figure S4a). The main parameters of action potentials, maximum diastolic potential (MDP), maximum rate of rise of the action potential (dv/dt) and action potential duration (ADP50), were not different among control and CPC-treated mESCMs (Figure S4b). Cardiac myosins (Myh6, Myh7, Myl2 and Myl7), mature cardiac markers, were increased more than two-eight times during robust proliferation with combined CPCs (BIO+SU1498/KN93+p38i) from Flkd6 to Flkd6+5 (Figure S4c). The data demonstrated that CPCs enhanced proliferation of cardiomyocytes with no apparent bias in cardiomyocyte features.
Developmental Stage Specific Effects of Chemicals on Embryonic, Neonatal and Adult Cardiomyocytes
Next, we examined the effects of CPCs on cardiomyocytes in various developmental stages, such as embryos, neonates and adults.
Cardiomyocytes isolated from the hearts of E9.5 mice, which is in comparable differentiation stage to mESCMs39, were cultured and treated with each CPC or with the combinations for 3–5 days (E9.5+3 and E9.5+5, respectively). Each CPC treatment increased the S phases and M phases in embryonic cardiomyocytes at E9.5+3 (Figure S5a). Combined CPC treatments (BIO+p38i+SU/KN) increased S phase to approximately 4.5 times and M phase to 3 times more than control at E9.5+3 (Figure S5a). Combined CPC treatments rapidly increased embryonic cardiomyocyte nuclei number up to 8.5 times more than control at E9.5+5 (Figure S5). These data indicated that CPCs efficiently proliferate embryonic cardiomyocytes in a similar manner to those in mESCMs.
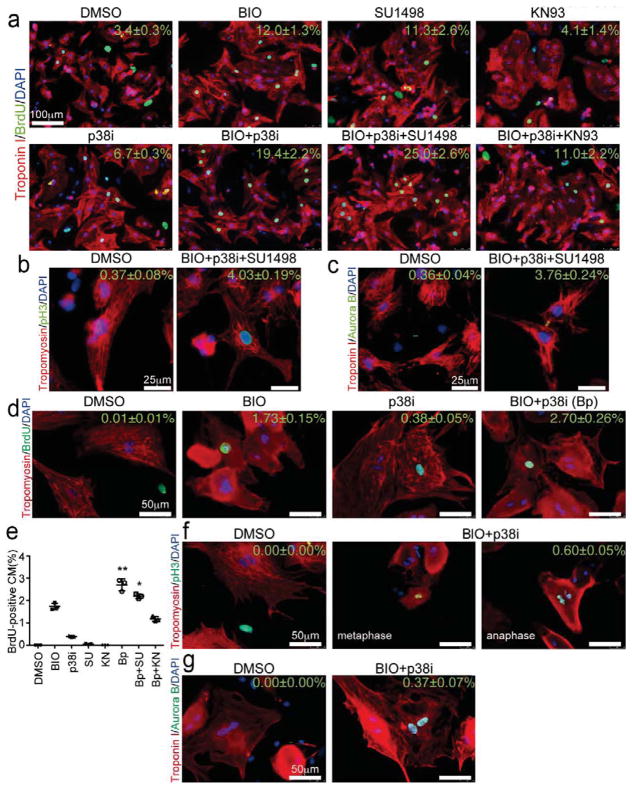
Neonatal (P3) rat ventricular cardiomyocytes (NRVCs) were isolated and cultured with CPCs for 2 days. As a baseline, DMSO-treated neonatal cardiomyocytes rarely proliferated (BrdU-positive cells, 3.4±0.3%; pH3-positive cells, 0.37±0.08%; Aurora B-positive cells, 0.36±0.04%). BIO, SU1498 or p38i alone increased in number of BrdU-positive cardiomyocytes by 2–3.5 times (Figure 5a). In contrast to mESCMs and embryonic cardiomyocytes, KN93 failed to increase BrdU incorporation and further progression of the cell cycle in NRVCs (BrdU-positive cells, 4.1±1.4%, Figure 5a). Combination of BIO+p38i synergistically increased BrdU incorporation and BIO+SU+p38i further enhanced the BrdU incorporation (19.4±2.2% and 25.0±2.6%, respectively. p < 0.05 vs. control. Figure 5a). In BIO+p38i and BIO+p38i+SU1498 condition, the cell cycle progression to M phase (pH3-positive, 3.70±0.20% and 4.03±0.19%, respectively. Figure 5b) and cell division (Aurora B-positive, 3.47±0.21% and 3.76±0.24%, respectively. Figure 5c) were significantly increased compared to the baseline.
Figure 5.
Identified Chemicals Induced Cardiomyocytes Proliferation with Developmental Stage Specific Manner. (a–c) Immunostaining of neonatal rat cardiomyocytes for cell cycle analysis at day 2 after chemical treatment. (a) Troponin I (red), BrdU (green) and DAPI (blue). Scale bar = 100 μm (b) Tropomyosin (red), pH3 (green) and DAPI (blue). Scale bar = 25 μm (c) Troponin I (red), Arurora B (green) and DAPI (blue). Scale bar = 25 μm (d–g) Immunostaining of adult rat cardiomyocytes for cell cycle markers at day 6 after chemical treatment. (d) Tropomyosin (red), BrdU (green) and DAPI (blue). (e) Quantification of BrdU-positive cardiomyocytes. (Dunn’s test: *, p < 0.05 and **, p < 0.01, vs. DMSO treated group, n = 3.) (f) Tropomyosin (red), pH3 (green) and DAPI (blue). (g) Troponin I (red), Aurora B (green) and DAPI (blue).
Adult rat cardiomyocytes were isolated and cultured with CPCs for 6 days. Less than 0.01% of adult cardiomyocytes were positive for BrdU and none of them were pH3-positive at the baseline (DMSO treatment). BIO or p38i treatment alone evoked cell cycle reentry in adult cardiomyocytes, that is, BrdU-positive and pH3-positive cells were increased (Figure 5d). Combination of BIO and p38i synergistically and significantly enhanced the cell cycle progression markers in adult cardiomyocytes compared to baseline (BrdU-positive cells, 2.7±0.3%; pH3-positive cells, 0.60±0.05%; Aurora B-positive cells, 0.37±0.07%) (Figure 5d–g). pH3-positive metaphase and anaphase cardiomyocytes were found in BIO+p38i condition (Figure 5f). In contrast, SU1498 and KN93 failed to enhance the effects of BIO+p38i (Figure 5d and e).
These findings suggest that GSK3 and p38 MAPK are broadly and cooperatively regulating various cardiomyocytes including in adults whereas ERK and CaMKII play a novel developmental stage-specific function.
Effects of Chemicals on Cardiomyocytes from Human iPSCs and ESCs
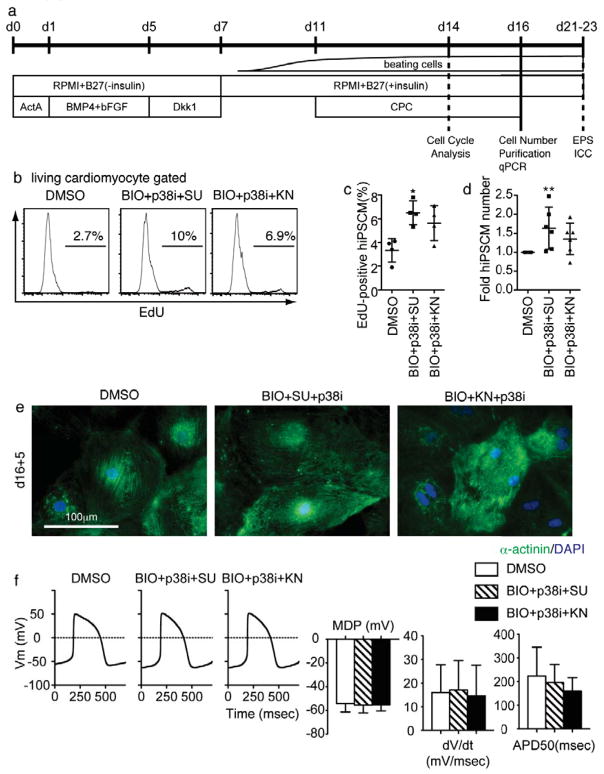
Finally, we evaluated the effects of CPCs on human iPSC- and ESC-derived cardiomyocytes. A human iPSC line 201B6 is one of the most characterized four-factor (Oct3/4, Sox2, c-myc and Klf4)-iPSCs for differentiation properties, including cardiac differentiation.28,40 Cardiomyocytes (hiPSCMs) were induced with a two-dimensional, defined, serum-free condition-based cardiomyocyte differentiation method (Figure 6a).22,28 Spontaneously-beating hiPSCMs appeared around d8–10 after Activin A treatment. At day 11, CPC treatment was started. The cell cycle was evaluated by flow cytometry at day 14, and the cardiomyocyte number was calculated at day 16. In agreement with the results from mESCMs, BIO+p38i+SU significantly increased EdU incorporation in hiPSCMs at d14 (Figure 6b–c) and hiPSCM number at d16 (Figure 6d). BIO+p38i+KN had tendencies to increase EdU incorporation and hiPSCM number (Figure 6b–d). Similarly, BIO+p38i+SU increased BrdU incorporation in hESC-derived cardiomyocytes by approximately 1.5 times (data not shown). We purified hiPSCMs with a recently identified cell-surface marker for hiPSCMs, VCAM128, and evaluated structural and functional features of CPC-expanded hiPSCMs. CPC-expanded hiPSCMs showed clear sarcomere formation (Figure 6e) and action potentials (Figure 6f), similar to those in control hiPSCMs. These data indicate that CPCs also successfully work in human cardiomyocytes.
Figure 6.
Effects of Chemicals on Human iPSCs-Derived Cardiomyocytes. (a) Differentiation and experimental scheme for human iPSC study. Differentiation of human iPSCs was induced for 11 days. Cells were then treated by CPCs. (b) Representative plot of EdU in cardiomyocytes at d14. (c) EdU-positive ratio in cTnT-positive cell at d14 after induction. Dunn’s test: *, p < 0.05, vs. DMSO treated group, n = 3.) (d) cTnT-positive cell number at d16. Dunn’s test: *, p < 0.05, vs. DMSO treated group, n = 6.) (e) Immunostaining for α-actinin at d16+5-7. Scale bar = 100μm. (f) Representative action potential of purified VCAM1-positive cells and graphs for action potential parameters at d16+5. MDP, mean diastolic potential. dV/dt, maximum rate of depolarization. APD50, action potential duration. (i) Immuno staining for α-actinin at d16+5-7. No significant difference vs. control (n = 17).
Discussion
In the present study, we screened a chemical library with a HCS for effects on the proliferation of mESCMs and succeeded in identifying several distinct chemicals promoting cardiomyocyte proliferation. These findings uncovered common and developmental stage-specific machineries controlling cardiomyocyte proliferation. They also would provide broad and efficient ways of manipulating cardiomyocyte number from embryos to adults and in mouse and human.
We identified chemicals regulating four distinct signaling pathways, namely GSK3 (BIO and CHIR99021), ERK (SU1498 and ZM336372), CaMKII (KN93 and KN62) and p38 MAPK (SB203580) (Fig. 7). Consistent with previous reports32,38, GSK3 and p38 MAPK signal cascades negatively regulated cardiomyocyte proliferation and inhibitors of these kinases enhanced rodent and human cardiomyocyte proliferation. This is the first report to show synergistic effects of combinatorial inhibition of GSK3 and p38 MAPK, especially on adult cardiomyocyte proliferation.
Figure 7.
Scheme of Regulatory Machinery of Cardiomyocyte Proliferation. Four distinct signaling pathways, GSK3, p38 MAPK, and CaMKII signaling negatively, and ERK signaling positively, regulate cardiomyocyte proliferation. Inhibitors for GSK3 (BIO and CHIR), p38 MAPK (SB203580), and CaMKII (KN93 and KN62), and activators for ERK (SU1498 and ZM336372) can be used to induce cardiomyocyte proliferation. A combinatory regulation of these signaling efficiently enhances each effect. We named these chemicals as cardiomyocyte proliferating chemicals (CPCs). CPCs show developmental stage-specific effects. CaMKII inhibitors act only on embryonic and PSC-derived cardiomyocytes. ERK activators still work on neonatal cardiomyocytes but not in adult cardiomyocytes. GSK3 and p38 MAPK inhibitors synergistically enhance cardiomyocyte proliferation broadly even in adults.
In contrast to GSK3 and p38 MAPK inhibitors, ERK activators and CaMKII inhibitors have not been reported to be involved in cardiomyocyte proliferation. Raf-MEK-ERK signal cascade positively regulates the proliferation of cardiomyocytes as a downstream target of growth factors.7,8 Furthermore, phospho-ERK is evident in E8.0 heart, which depends on the FGF receptor.41 SU1498 enhances ERK phosphorylation through inhibiting phosphatase (Figure 3b).34 Phosphatases, such as dual-specificity phosphatase 6 (Dusp6), negatively regulate ERK phosphorylation.42 Dusp6 null mice showed enhanced ERK phosphorylation and increased cardiomyocyte proliferation during embryonic development and in the early postnatal period.36 Dusp6 is a possible candidate for a SU1498 target. A Raf activator, ZM336372, also showed a similar pro-proliferative effect. Collectively, all of these results indicate that activation of Raf-MEK-ERK pathway could be a potent molecular target to induce cardiomyocyte proliferation. CaMKII has been reported to regulate cardiac hypertrophy in the later stages of development or in adults.43,44 Here we showed that in an earlier developmental stage corresponding to E9.5 mouse embryos, CaMKII could inhibit cardiomyocyte proliferation. We confirmed phosphorylation of CaMKII in early mouse embryos (E9.5 and E13.5) and found that CaMKII phosphorylation was drastically increased between E9.5 and E13.5 (Figure S6). CaMKII phosphorylation was more evident in trabecular layer cardiomyocytes than in compact layer counterparts at E13.5. This timing of CaMKII activation in trabecular cardiomyocytes is in accordance with the period when the proliferative ability of trabecular layer cardiomyocytes is reduced13, suggesting that CaMKII at this differentiation stage could be involved in growth arrest of early cardiomyocytes. CaMKII inhibitors lost its proliferative effect on postnatal and adult cardiomyocytes, suggesting that the growth inhibitory effect of CaMKII has an early developmental stage-specific effect, and switching of its effect to hypertrophy would occur in the later stage. Currently, the precise molecular mechanisms for the developmental stage-specific CaMKII effect are unknown, but this finding is a clue to the precise understanding of CaMKII functions in cardiomyocytes.
Though ERK activators and CaMKII inhibitors did not show sufficient proliferative effects in adult cardiomyocytes, our finding of an effective chemical cocktail for cardiomyocyte proliferation can offer a therapeutic potential for cardiac regeneration. In diseased hearts, cardiomyocytes express embryonic genes45, and small fraction of cardiomyocytes is de-differentiated and proliferating3 or newly differentiated from progenitor cells46, indicating that these cardiomyocytes in diseased hearts are in a more immature stage. Hence, not only will the combination of GSK inhibitors and p38i but also our effective cocktails including three chemicals exert their potent pro-proliferative effects in diseased hearts, facilitating cardiac regeneration.
Recently, PSC-derived cells have drawn considerable interest for use in drug screening, disease modeling20,21,47 and for regenerative medicine as cell therapies48 are highlighted. In addition, PSC-derived cells can provide an experimental system for studying cellular physiology especially in cell types difficult to obtain from adults. With the use of our PSC systems, it is likely that other more effective chemicals would be identified from other libraries. Thus, our novel screening system for cardiomyocyte proliferation combining stem cell technology and chemical biology could be a potent tool for investigating efficient molecules and largely contribute to future cardiac regenerative medicine, especially through regeneration with drugs.
Supplementary Material
Supplemental Movie 1. ESCMs were recorded during Flkd6+0.5-4.5 with DMSO.
Supplemental Movie 2. ESCMs were recorded during Flkd6+0.5-4.5 with BIO.
Supplemental Movie 3. ESCMs were recorded during Flkd6+0.5-4.5 with SU1498.
Supplemental Movie 4. ESCMs were recorded during Flkd6+0.5-4.5 with KN93.
Supplemental Figure 1: Scheme of cardiomyocyte differentiation from mouse ESCs.
A mouse ESC line EMG7 carrying αMHC-GFP is cultured with LIF for maintenance. Withdrawal of LIF and exposure to MEM alpha supplemented with 10% serum induces differentiation of EMG7 on gelatin-coat dish. At 108–110 hours after induction, EMG7 cells express Flk1 mesoderm marker. Flk1 positive EMG7 cells are cultured on OP9 feeder cells with Cyclosporin-A (Wako, Japan) to induce cardiomyocyte differentiation. Three to four days after Flk1-positive cell culture (Flkd3-4), self-beating cells appear, which express αMHC-GFP and cardiac troponin T.
Supplemental Figure 2: Scheme of screening
(a) Study design for screening cardiomyocyte proliferating chemicals (b) For screening chemicals, αMHC-GFP positive mouse embryonic stem cell-derived cardiomyocyte (mESCMs) were sorted at Flkd6. (c) As a primary screen, a high content screening was performed to identify chemicals that increased the nucleus number of ESCMs. “m”: cardiomyocytes. “nm”: non-myocytes. Inset: Binuclei cardimyocytes (counted as two). (d) As a secondary screen, actual cardiomyocyte number was evaluated with flow cytometry. Chemicals that significantly increased the number of cardiomyocytes were further investigated in cell cycle analysis, molecular target validation and combination assay. Chemicals were also tested whether they could increase cardiomyocyte number from various sources – mouse embryonic heart, rat neonatal and adult heart and human pluripotent stem cells.
Supplemental Figure 3: CaMKIId was responsible for mESCM proliferation and Ink4b regulation.
(a–c) Effects of Camk2 isoform-specific siRNA at Flkd6+5 (a) Specific reduction of target gene expressions by Camk2 isoform-specific siRNAs (n = 3). (b) Effects of siRNAs on mESCM number. Knockdown of Camk2d and Camk2g increased mESCM cell number by 1.5 folds (n = 6). (c) Effects of siRNAs on Ink4b expression. Knockdown of Camk2d reduced Ink4b expression by 50% (n = 3). (d–e) Effects of Ink4b-specific siRNA on Ink4b expression (d) and mESCM cell number (e) at Flkd6+5 (Mann-Whitney test, n = 5).
Supplemental Figure 4: Characters of expanded ESCMs
(a) Representative action potentials of control (DMSO) and expanded (BIO+SU+p38i and BIO+KN+p38i) ESCMs at Flkd6+5+4. Vm, membrane potential. (b) Parameters of action potentials. MDP; maximum diastolic potential, dV/dt;, APD50; action potential duration. No significant differences were observed. (n = 6–13, Dunn’s test) (c) Myosin expression level in each mRNA from Flkd6-ESCM and Flkd6+5 ESCM treated with DMSO, BIO+SU+p38i or BIO+KN93+p38i. ESCMs were resorted at Flkd6+5. All myosin genes tested were increased at Flkd6+5 compared to Flkd6. (n = 3, Dunn’s test, *, p < 0.05 vs. Flkd6-GFP+ ESCMs)
Supplemental Figure 5: Effects of chemicals on cardiomyocytes derived from E9.5 mouse embryonic hearts
(a–d) Immunostaining for cardiac troponin T (red), EdU (Green), phospho-histone H3 (pH3: white), and DAPI (Blue) at E9.5+3, and E9.5+5. Scale bar = 100μm. at (a) E9.5+3 and (b) E9.5+5. (c, d) Cardiomyocyte cell numbers with combined treatment of chemicals at E9.5+3, and +5, respectively (n = 3, Dunn’s test, *, p < 0.05, **, p < 0.01 vs. DMSO treated group). Note that in optimal combination (BIO+SU+p38i or BIO+KN+p38i), cardiomyocyte numbers reached eight times more than those in DMSO control condition at E9.5+5.
Supplemental Figure 6: Phosphorylation of CaMKII in developing heart.
Phosphorylated-CaMKII (phospho-CaMKII, Green), cardiac tropobin-T (cTnT, Red), and DAPI (Blue). Scale bar = 100μm. CaMKII activation became evident in the embryonic heart from E9.5 to E13.5. At E13.5, CaMKII activation was more evident in trabecular layer than compact layer, inversely reflecting cardiomyocyte proliferating ability.
Supplementary Table 1: Hit Chemicals in 1st HCS
Supplementary Table 2: qRT-PCR primers
Supplemental Table 3: siRNA pools for Camk2 isoforms
Acknowledgments
We thank Prof. Shinya Yamanaka for the generous gift of human iPS cells (201B6). We thank the Screening Committee of Anticancer Drugs supported by Grant-in-Aid for Scientific Research on Priority Area ‘Cancer’ from The Ministry of Education, Culture, Sports, Science and Technology for the generous gift of The SCADS inhibitor kit. We also thank Mr. Yukihiro Furuyama (Molecular Devices Japan, Tokyo, Japan) for technical assistant for high content imaging, and Drs. Meiko Takahashi and Mark Ranek for critical reading of the manuscript.
Funding Sources: This work was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan, the Ministry of Health, Labour and Welfare, the Project for Realization of Regenerative Medicine, and Japan Science and Technology Agency. H.U. and H.F. are supported by the Japan Society for the Promotion of Science.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kajstura J, Gurusamy N, Ogórek B, Goichberg P, Clavo-Rondon C, Hosoda T, et al. Myocyte turnover in the aging human heart. Circ Res. 2010;107:1374–1386. doi: 10.1161/CIRCRESAHA.110.231498. [DOI] [PubMed] [Google Scholar]
- 3.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bicknell KA, Coxon CH, Brooks G. Can the cardiomyocyte cell cycle be reprogrammed? J Mol Cell Cardiol. 2007;42:706–721. doi: 10.1016/j.yjmcc.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Engel FB, Hsieh PCH, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci USA. 2006;103:15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimoji K, Yuasa S, Onizuka T, Hattori F, Tanaka T, Hara M, et al. G-csf promotes the proliferation of developing cardiomyocytes in vivo and in derivation from escs and ipscs. Cell Stem Cell. 2010;6:227–237. doi: 10.1016/j.stem.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, et al. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kühn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 9.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/erbb4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 10.Singh MK, Li Y, Cobb RM, Zhou D, Lu MM, Epstein JA, et al. Gata4 and gata5 cooperatively regulate cardiac myocyte proliferation in mice. J Biol Chem. 2010;285:1765–1772. doi: 10.1074/jbc.M109.038539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trivedi CM, Lu MM, Wang Q, Epstein JA. Transgenic overexpression of hdac3 in the heart produces increased postnatal cardiac myocyte proliferation but does not induce hypertrophy. J Biol Chem. 2008;283:26484–26489. doi: 10.1074/jbc.M803686200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campa VM, Gutiérrez-Lanza R, Cerignoli F, Díaz-Trelles R, Nelson B, Tsuji T, et al. Notch activates cell cycle reentry and progression in quiescent cardiomyocytes. J Cell Biol. 2008;183:129–141. doi: 10.1083/jcb.200806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toyoda M, Shirato H, Nakajima K, Kojima M, Takahashi M, Kubota M, et al. Jumonji downregulates cardiac cell proliferation by repressing cyclin d1 expression. Dev Cell. 2003;5:85–97. doi: 10.1016/s1534-5807(03)00189-8. [DOI] [PubMed] [Google Scholar]
- 14.Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, et al. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497:249–253. doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Shi Y, Ding S. A chemical approach to stem-cell biology and regenerative medicine. Nature. 2008;453:338–344. doi: 10.1038/nature07042. [DOI] [PubMed] [Google Scholar]
- 17.Nakao Y, Narazaki G, Hoshino T, Maeda S, Yoshida M, Maejima H, et al. Evaluation of antiangiogenic activity of azumamides by the in vitro vascular organization model using mouse induced pluripotent stem (ips) cells. Bioorg Med Chem Lett. 2008;18:2982–2984. doi: 10.1016/j.bmcl.2008.03.053. [DOI] [PubMed] [Google Scholar]
- 18.Zanella F, Lorens JB, Link W. High content screening: Seeing is believing. Trends Biotechnol. 2010;28:237–245. doi: 10.1016/j.tibtech.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida Y, Yamanaka S. Recent stem cell advances: Induced pluripotent stem cells for disease modeling and stem cell-based regeneration. Circulation. 2010;122:80–87. doi: 10.1161/CIRCULATIONAHA.109.881433. [DOI] [PubMed] [Google Scholar]
- 20.Egawa N, Kitaoka S, Tsukita K, Naitoh M, Takahashi K, Yamamoto T, et al. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci Transl Med. 2012;4:145ra104. doi: 10.1126/scitranslmed.3004052. [DOI] [PubMed] [Google Scholar]
- 21.Kondo T, Asai M, Tsukita K, Kutoku Y, Ohsawa Y, Sunada Y, et al. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell. 2013;12:487–496. doi: 10.1016/j.stem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita JK. Es and ips cell research for cardiovascular regeneration. Experimental cell research. 2010;316:2555–2559. doi: 10.1016/j.yexcr.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita JK, Takano M, Hiraoka-Kanie M, Shimazu C, Peishi Y, Yanagi K, et al. Prospective identification of cardiac progenitors by a novel single cell-based cardiomyocyte induction. FASEB J. 2005;19:1534–1536. doi: 10.1096/fj.04-3540fje. [DOI] [PubMed] [Google Scholar]
- 26.Narazaki G, Uosaki H, Teranishi M, Okita K, Kim B, Matsuoka S, et al. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- 27.Yan P, Nagasawa A, Uosaki H, Sugimoto A, Yamamizu K, Teranishi M, et al. Cyclosporin-A potently induces highly cardiogenic progenitors from embryonic stem cells. Biochem Biophys Res Commun. 2009;379:115–120. doi: 10.1016/j.bbrc.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Uosaki H, Fukushima H, Takeuchi A, Matsuoka S, Nakatsuji N, Yamanaka S, et al. Efficient and Scalable Purification of Cardiomyocytes from Human Embryonic and Induced Pluripotent Stem Cells by VCAM1 Surface Expression. PLoS ONE. 2011;6:e23657. doi: 10.1371/journal.pone.0023657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawada M, Inoue H, Masuda T, Ikeda D. Insulin-like growth factor i secreted from prostate stromal cells mediates tumor-stromal cell interactions of prostate cancer. Cancer Res. 2006;66:4419–4425. doi: 10.1158/0008-5472.CAN-05-4239. [DOI] [PubMed] [Google Scholar]
- 30.Tsuchiya A, Tashiro E, Yoshida M, Imoto M. Involvement of protein phosphatase 2a nuclear accumulation and subsequent inactivation of activator protein-1 in leptomycin b-inhibited cyclin d1 expression. Oncogene. 2007;26:1522–1532. doi: 10.1038/sj.onc.1209962. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, Yue S, Chen X, Kubin T, Braun T. Regulation of cardiomyocyte polyploidy and multinucleation by cycling1. Circ Res. 2010;106:1498–1506. doi: 10.1161/CIRCRESAHA.109.211888. [DOI] [PubMed] [Google Scholar]
- 32.Tseng AS, Engel FB, Keating MT. The gsk-3 inhibitor bio promotes proliferation in mammalian cardiomyocytes. Chem Biol. 2006;13:957–963. doi: 10.1016/j.chembiol.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Strawn LM, McMahon G, App H, Schreck R, Kuchler WR, Longhi MP, et al. Flk-1 as a target for tumor growth inhibition. Cancer Res. 1996;56:3540–3545. [PubMed] [Google Scholar]
- 34.Boguslawski G, McGlynn PW, Harvey KA, Kovala AT. Su1498, an inhibitor of vascular endothelial growth factor receptor 2, causes accumulation of phosphorylated erk kinases and inhibits their activity in vivo and in vitro. J Biol Chem. 2004;279:5716–5724. doi: 10.1074/jbc.M308625200. [DOI] [PubMed] [Google Scholar]
- 35.Zhu WZ, Xie Y, Moyes KW, Gold JD, Askari B, Laflamme MA. Neuregulin/erbb signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res. 2010;107:776–786. doi: 10.1161/CIRCRESAHA.110.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maillet M, Purcell NH, Sargent MA, York A, Bueno OF, Molkentin JD. Dusp6 (mkp3) null mice show enhanced erk1/2 phosphorylation at baseline and increased myocyte proliferation in the heart affecting disease susceptibility. J Biol Chem. 2008;283:31246–31255. doi: 10.1074/jbc.M806085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase ii mrnas. J Biol Chem. 1989;264:17907–17912. [PubMed] [Google Scholar]
- 38.Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, et al. P38 map kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanagi K, Takano M, Narazaki G, Uosaki H, Hoshino T, Ishii T, et al. Hyperpolarization-activated cyclic nucleotide-gated channels and T-type calcium channels confer automaticity of embryonic stem cell-derived cardiomyocytes. Stem Cells. 2007;25:2712–2719. doi: 10.1634/stemcells.2006-0388. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Corson LB, Yamanaka Y, Lai KM, Rossant J. Spatial and temporal patterns of erk signaling during mouse embryogenesis. Development. 2003;130:4527–4537. doi: 10.1242/dev.00669. [DOI] [PubMed] [Google Scholar]
- 42.Jeffrey KL, Camps M, Rommel C, Mackay CR. Targeting dual-specificity phosphatases: Manipulating map kinase signalling and immune responses. Nat Rev Drug discov. 2007;6:391–403. doi: 10.1038/nrd2289. [DOI] [PubMed] [Google Scholar]
- 43.Zhu W, Zou Y, Shiojima I, Kudoh S, Aikawa R, Hayashi D, et al. Ca2+/calmodulin-dependent kinase ii and calcineurin play critical roles in endothelin-1-induced cardiomyocyte hypertrophy. J Biol Chem. 2000;275:15239–15245. doi: 10.1074/jbc.275.20.15239. [DOI] [PubMed] [Google Scholar]
- 44.Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, et al. The delta isoform of cam kinase ii is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci USA. 2009;106:2342–2347. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuwahara K, Nishikimi T, Nakao K. Transcriptional regulation of the fetal cardiac gene program. J Pharmacol Sci. 2012;119:198–203. doi: 10.1254/jphs.12r04cp. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh PCH, Segers VFM, Davis ME, MacGillivray C, Gannon J, Molkentin JD, et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flügel L, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 48.Masumoto H, Matsuo T, Yamamizu K, Uosaki H, Narazaki G, Katayama S, et al. Pluripotent stem cell-engineered cell sheets reassembled with defined cardiovascular populations ameliorate reduction in infarct heart function through cardiomyocyte-mediated neovascularization. Stem Cells. 2012;30:1196–1205. doi: 10.1002/stem.1089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Movie 1. ESCMs were recorded during Flkd6+0.5-4.5 with DMSO.
Supplemental Movie 2. ESCMs were recorded during Flkd6+0.5-4.5 with BIO.
Supplemental Movie 3. ESCMs were recorded during Flkd6+0.5-4.5 with SU1498.
Supplemental Movie 4. ESCMs were recorded during Flkd6+0.5-4.5 with KN93.
Supplemental Figure 1: Scheme of cardiomyocyte differentiation from mouse ESCs.
A mouse ESC line EMG7 carrying αMHC-GFP is cultured with LIF for maintenance. Withdrawal of LIF and exposure to MEM alpha supplemented with 10% serum induces differentiation of EMG7 on gelatin-coat dish. At 108–110 hours after induction, EMG7 cells express Flk1 mesoderm marker. Flk1 positive EMG7 cells are cultured on OP9 feeder cells with Cyclosporin-A (Wako, Japan) to induce cardiomyocyte differentiation. Three to four days after Flk1-positive cell culture (Flkd3-4), self-beating cells appear, which express αMHC-GFP and cardiac troponin T.
Supplemental Figure 2: Scheme of screening
(a) Study design for screening cardiomyocyte proliferating chemicals (b) For screening chemicals, αMHC-GFP positive mouse embryonic stem cell-derived cardiomyocyte (mESCMs) were sorted at Flkd6. (c) As a primary screen, a high content screening was performed to identify chemicals that increased the nucleus number of ESCMs. “m”: cardiomyocytes. “nm”: non-myocytes. Inset: Binuclei cardimyocytes (counted as two). (d) As a secondary screen, actual cardiomyocyte number was evaluated with flow cytometry. Chemicals that significantly increased the number of cardiomyocytes were further investigated in cell cycle analysis, molecular target validation and combination assay. Chemicals were also tested whether they could increase cardiomyocyte number from various sources – mouse embryonic heart, rat neonatal and adult heart and human pluripotent stem cells.
Supplemental Figure 3: CaMKIId was responsible for mESCM proliferation and Ink4b regulation.
(a–c) Effects of Camk2 isoform-specific siRNA at Flkd6+5 (a) Specific reduction of target gene expressions by Camk2 isoform-specific siRNAs (n = 3). (b) Effects of siRNAs on mESCM number. Knockdown of Camk2d and Camk2g increased mESCM cell number by 1.5 folds (n = 6). (c) Effects of siRNAs on Ink4b expression. Knockdown of Camk2d reduced Ink4b expression by 50% (n = 3). (d–e) Effects of Ink4b-specific siRNA on Ink4b expression (d) and mESCM cell number (e) at Flkd6+5 (Mann-Whitney test, n = 5).
Supplemental Figure 4: Characters of expanded ESCMs
(a) Representative action potentials of control (DMSO) and expanded (BIO+SU+p38i and BIO+KN+p38i) ESCMs at Flkd6+5+4. Vm, membrane potential. (b) Parameters of action potentials. MDP; maximum diastolic potential, dV/dt;, APD50; action potential duration. No significant differences were observed. (n = 6–13, Dunn’s test) (c) Myosin expression level in each mRNA from Flkd6-ESCM and Flkd6+5 ESCM treated with DMSO, BIO+SU+p38i or BIO+KN93+p38i. ESCMs were resorted at Flkd6+5. All myosin genes tested were increased at Flkd6+5 compared to Flkd6. (n = 3, Dunn’s test, *, p < 0.05 vs. Flkd6-GFP+ ESCMs)
Supplemental Figure 5: Effects of chemicals on cardiomyocytes derived from E9.5 mouse embryonic hearts
(a–d) Immunostaining for cardiac troponin T (red), EdU (Green), phospho-histone H3 (pH3: white), and DAPI (Blue) at E9.5+3, and E9.5+5. Scale bar = 100μm. at (a) E9.5+3 and (b) E9.5+5. (c, d) Cardiomyocyte cell numbers with combined treatment of chemicals at E9.5+3, and +5, respectively (n = 3, Dunn’s test, *, p < 0.05, **, p < 0.01 vs. DMSO treated group). Note that in optimal combination (BIO+SU+p38i or BIO+KN+p38i), cardiomyocyte numbers reached eight times more than those in DMSO control condition at E9.5+5.
Supplemental Figure 6: Phosphorylation of CaMKII in developing heart.
Phosphorylated-CaMKII (phospho-CaMKII, Green), cardiac tropobin-T (cTnT, Red), and DAPI (Blue). Scale bar = 100μm. CaMKII activation became evident in the embryonic heart from E9.5 to E13.5. At E13.5, CaMKII activation was more evident in trabecular layer than compact layer, inversely reflecting cardiomyocyte proliferating ability.
Supplementary Table 1: Hit Chemicals in 1st HCS
Supplementary Table 2: qRT-PCR primers
Supplemental Table 3: siRNA pools for Camk2 isoforms