Abstract
Voluntary action control requires selection of appropriate responses and stopping of inappropriate responses. Selection and stopping are often investigated separately, but they appear to recruit similar brain regions, including the pre-supplementary motor area (preSMA) and inferior frontal gyrus. We therefore examined the evidence for overlap of selection and stopping using two approaches: a meta-analysis of existing studies of selection and stopping, and a novel within-subject fMRI study in which action selection and a stop signal task were combined factorially. The novel fMRI study also permitted us to investigate hypotheses regarding a common mechanism for selection and stopping. The preSMA was identified by both methods as common to selection and stopping. However, stopping a selected action did not recruit preSMA more than stopping a specified action, nor did stop signal reaction times differ significantly across the two conditions. These findings suggest that the preSMA supports both action selection and stopping, but the two processes may not require access to a common inhibition mechanism. Instead, the preSMA might represent information about potential actions that is used in both action selection and stopping in order to resolve conflict between competing available responses.
Abbreviations: FDRc, false discovery rate cluster corrected; fMRI, functional magnetic resonance imaging; preSMA, pre-supplementary motor area; RT, reaction time; SSRT, stop signal reaction time
Keywords: Action selection, Action stopping, Inferior frontal gyrus, Inhibition, Pre-supplementary motor area, Stop signal task
Highlights
-
•
GingerALE meta-analysis shows overlap of action selection and stopping in preSMA.
-
•
Within-subjects fMRI study confirms overlap using same stimuli and subjects.
-
•
Although selection and stopping overlap, there is no behavioural interaction.
-
•
The preSMA may support the representation of competing action options.
Introduction
Successful goal-directed behaviour requires the selection of an appropriate response for the task at hand. The intentional selection of a response from several alternatives is a hallmark of voluntary action, and can take the form of choosing what action to perform, when to act, or whether to act at all (Brass and Haggard, 2008). The cognitive neuroscience of “what” type action selection has a long history, but has been described using a range of terminology, including “willed action” (Frith et al., 1991, Jahanshahi and Frith, 1998), “free selection” (Deiber et al., 1996, Lau et al., 2006) and “action selection” (Hughes et al., 2010, Rowe et al., 2010). This type of action decision has been consistently associated with a characteristic set of fronto-parietal activations in neuroimaging (Frith et al., 1991, Hughes et al., 2010, Jahanshahi and Frith, 1998, Rowe et al., 2010), including the inferior and middle frontal gyri, the pre-supplementary motor area (preSMA), premotor cortex, and the inferior parietal cortex, regions resembling the “multiple-demand” network (Duncan, 2010).
In addition to the selection of actions, goal-directed behaviour may also require the stopping of inappropriate responses. The indication that an action is inappropriate may come before a response is triggered, by the prevailing rules or context, or it may be concurrent with prepotent cues for action, as in Go/NoGo tasks. Stop cues may also occur shortly after the action has been triggered, indicating the need to cancel the action while it is being selected or prepared. This “cancellation” type of stopping is exemplified by the stop signal task (Logan et al., 1984, Verbruggen and Logan, 2008), in which a cue to respond is followed after a short delay (typically 200–300 ms) by an instruction to cancel the action.
Intriguingly, similar patterns of activation have been reported from neuroimaging studies of action selection and stopping tasks. Regions associated with successful stopping on stop signal tasks include the inferior frontal gyrus, preSMA, and inferior parietal cortex (Aron et al., 2007, Chikazoe et al., 2009, Swick et al., 2011). These observations raise the possibility that action selection and stopping are supported by similar brain regions, perhaps even by similar neural populations within these regions (Jasinska, 2013, Mostofsky and Simmonds, 2008). However, the overlap of selection and stopping in prefrontal cortex, the preSMA, and inferior parietal cortex.
We examined the anatomical relationship between selection and stopping of voluntary action using two methods. First, we conducted a meta-analysis of action selection and stopping studies to examine overlap in activation during these two tasks across studies. This method has the advantage of pooling results from a very large number of individuals. However, such a meta-analysis is based on different groups of subjects performing either action selection or stopping tasks, and has limited anatomical precision. Moreover, the particular task requirements vary across tasks and studies.
To complement the meta-analytic approach, we developed a novel task that combined selection with stopping. Using this task and event-related fMRI, we examined the anatomical relationship between selection and stopping in the same subjects, affording improved anatomical resolution over that available in meta-analytic approaches, and also matching of task requirements and stimuli across selection and stopping trials.
An anatomical overlap of selection and stopping raises a further question, of why the two aspects of voluntary action control show similar regional activations. There are several possible explanations for overlapping cortical activations for selection and stopping. Our novel fMRI task, in which selection and stopping were combined factorially, provided an opportunity to explore these alternative explanations: by examining the neural and behavioural interaction between selection and stopping, and the activations arising when subjects must stop an action they have selected themselves.
We sought to test a general hypothesis regarding anatomical overlap of selection and stopping: that both cancellation of an action that is in preparation (Aron and Poldrack, 2006), and selection between possible actions, require inhibition. For example, action selection or action reprogramming may require either the inhibition of current valid alternative options, or the inhibition of previous actions in a sequence (Duque et al., 2013, Macoveanu et al., 2013, Mars et al., 2007, Neubert et al., 2010, Rowe et al., 2010, Zhang et al., 2012). It is possible, therefore, that both action selection and stopping utilise inhibition (see also Jasinska, 2013, Mostofsky and Simmonds, 2008).
We identified three possible outcomes from our combined task in relation to this hypothesis: (1) if action selection and stopping both engage a common inhibitory mechanism, then performing action selection and stopping simultaneously, or in close temporal proximity within the same trial, would be expected to affect stopping performance. A beneficial effect on stopping efficiency (i.e. shorter SSRT with less BOLD activation) would be seen if selection is mediated in part by inhibition of alternative responses, such that stopping could be “primed” by performing selection within the same trial (Scherbaum et al., 2011). (2) A cost effect on stopping efficiency (i.e. longer SSRT, with more BOLD activation) would be seen if selection and stopping share a common inhibitory mechanism with limited resources, resulting in a cognitive “bottleneck” (Pashler, 1994) when subjects have to both select and stop within the same trial. (3) There might be no difference in the SSRT and BOLD activation, even when selection and stopping proceed simultaneously, or within close temporal proximity. This would suggest that selection and stopping might operate on common action representations but not require a shared inhibitory mechanism (cf. Yamaguchi et al., 2012).
Materials and methods
GingerALE meta-analysis
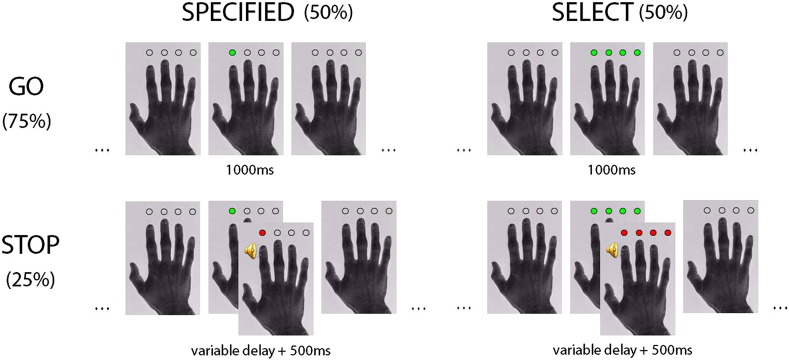
The action selection studies in our meta-analysis were included according to specific criteria, as the concepts and definition of “action selection” can vary between studies and researchers. Here, we define action selection as a decision about what action to perform, selecting from a range of alternatives, and contrasted against the performance of similar single actions specified by the experimenter (see Fig. 1). Alternative concepts of “action selection” were not included, such as when to perform an action specified by the experimenter, whether to execute a given action at all, or combinations of these action decisions (see Brass and Haggard, 2008).
Fig. 1.
The combined selection and stopping task. Trials can be either “specified” or “select”. On specified trials, subjects press the button indicated by a green circle above the corresponding finger. On select trials, subjects can choose which finger movement to make from four equally valid alternatives. 75% of trials are go trials, in which a signal to stop never occurs and subjects execute the movement. 25% of trials are stop trials, in which after a short variable presentation of the green go cue, an auditory signal (1000 Hz tone) and visual cue (change in colour of circle(s) to red) indicates subjects should withhold their response.
The action stopping studies in our meta-analysis were selected according to specific criteria. Only studies that used a stop signal task were included, in which the stop cue is presented after the go cue (Go/NoGo tasks were excluded). Additionally, we included only contrasts of “correct stop” trials versus “go” trials. Stop signals in either the auditory or visual modality were permitted (the stop signal in our combined fMRI study comprised both an auditory and visual cue).
Within these selection and stopping studies, additional criteria were required for inclusion: (1) manual response, as opposed to a saccade; (2) healthy adult subjects (if a clinical study, separate results must be reported for the control group); (3) whole-brain analysis with report of MNI or Talairach coordinates; and (4) if separate studies used the same data from a single group of subjects (e.g. Aron and Poldrack, 2006, Aron et al., 2007) the data is included in the meta-analysis only once. Both fMRI and PET studies meeting these criteria were included, as some of the earliest action selection studies used PET as the imaging modality. Three PET studies were included in the action selection meta-analysis, each of which used H2O PET.
The large majority of the selection and stopping studies included in the meta-analyses used right-handed subjects, although three studies did not specify subject handedness (Boehler et al., 2012, Jahfari et al., 2011, Lenartowicz et al., 2011), and one study included a single left-handed subject with a group of 18 right-handed subjects (van der Meer et al., 2011). In 53% of the selection and 44% of the stopping studies, subjects were required to make actions with their right hand only, whilst in the remainder of the studies, subjects were required to make actions with either their left or right hand from trial to trial (or this information was not reported). See Table 1 for details of all the included studies, and the Supplementary Material for study references.
Table 1.
Studies included in the action selection and stop signal GingerALE meta-analyses. R = right-handed actions, L = left-handed actions, B = both right- and left-handed actions, NS = not specified, A = auditory stop cue, V = visual stop cue. See Supplementary Material for study references.
| Task | First author | Year | Contrast | Response hand | fMRI/PET | Stop cue | N subjects | Foci |
|---|---|---|---|---|---|---|---|---|
| Selection | Beudel | 2009 | Finger selection, free versus fixed | R | fMRI | 16 | 5 | |
| Button selection, free versus fixed | R | 16 | 10 | |||||
| Deiber | 1991 | Random versus fixed | R | PET | 8 | 11 | ||
| Deiber | 1996 | Free vs. full | R | PET | 13 | 9 | ||
| Francois-Brosseau | 2009 | Self initiated movements vs externally triggered, left hand | L | fMRI | 14 | 16 | ||
| Self initiated movements vs externally triggered, right hand | R | 14 | 13 | |||||
| Frith | 1991 | Internally generated response versus routine response | R | PET | 6 | 3 | ||
| Gerardin | 2004 | Select vs prepare, right hand | R | fMRI | 9 | 6 | ||
| Select vs prepare, left hand | L | 9 | 8 | |||||
| Hoffstaedter | 2013 | Timed > no choice | B | fMRI | 35 | 11 | ||
| Hyder | 1997 | Random minus repeat | R | fMRI | 16 | 11 | ||
| Krieghoff | 2009 | Action selection: internal > external | NS | fMRI | 14 | 7 | ||
| Lau | 2004 | Free versus routine | NS | fMRI | 12 | 19 | ||
| Free versus specified | NS | 12 | 6 | |||||
| Lau | 2006 | Free vs. compatible | NS | fMRI | 13 | 2 | ||
| Free* vs. compatible* | NS | 13 | 3 | |||||
| Mueller | 2007 | Internally vs. externally selected actions | R | fMRI | 15 | 6 | ||
| Rowe | 2005 | Free selection versus externally specified (action tasks only) | R | fMRI | 12 | 3 | ||
| Rowe | 2008 | Action-selection vs. action specification (experiment 1) | R | fMRI | 20 | 18 | ||
| Rowe | 2010 | Chosen > specified | R | fMRI | 20 | 20 | ||
| van Eimeren | 2006 | Selection > no-selection | B | fMRI | 12 | 14 | ||
| Full-selection > no-selection | B | 12 | 15 | |||||
| Restricted-selection > no-selection | B | 12 | 14 | |||||
| van Oostende | 1997 | Self-fix, group analysis | NS | fMRI | 6 | 1 | ||
| Stopping | Aron | 2007 | Critical stopinhibit vs. critical go | R | fMRI | A | 15 | 37 |
| Boecker | 2011 | Stopinhibit-go | R | fMRI | A | 15 | 13 | |
| Boehler | 2010 | Successful stop (stop relevant blocks) > go (stop relevant blocks) | R | fMRI | V | 15 | 30 | |
| Cai | 2009 | Successful stop-go (color task) | B | fMRI | V | 12 | 8 | |
| Successful stop-go (orientation task) | B | 12 | 14 | |||||
| Successful stop-go (colour) AND Successful stop-go (orientation) | B | 12 | 3 | |||||
| Cai | 2011 | Successful stop > go | NS | fMRI | V | 23 | 21 | |
| Chevrier | 2007 | Successful stop phases (successful inhibition-go) | B | fMRI | V | 14 | 3 | |
| Chikazoe | 2009 | (Correct) stop versus uncertain-go | R | fMRI | V | 22 | 57 | |
| Cummins | 2011 | Successful inhibition-go | NS | fMRI | V | 50 | 5 | |
| Hughes | 2012 | (Correct) stops > implicit-baseline (go trials), controls | B | fMRI | A | 10 | 5 | |
| Jahfari | 2011 | Successful stop > go | B | fMRI | A | 20 | 7 | |
| Lenartowicz | 2011 | Go/stop-stop (correct) > go/stop-go | R | fMRI | A | 23 | 7 | |
| Marco-Pallares | 2008 | Inhibited trials vs. correct responses | B | fMRI | V | 10 | 10 | |
| Sharp | 2010 | Correct stop trials (StC) versus correct go trials (GoC) | B | fMRI | V | 26 | 10 | |
| van der Meer | 2011 | Stop (correct) > go | NS | fMRI | V | 19 | 13 | |
| Xue | 2008 | Stopinhibit-go, manual task | R | fMRI | A | 15 | 13 | |
| Zheng | 2008 | Successful inhibition minus go, stop-signal task | R | fMRI | V | 18 | 10 | |
Studies meeting these criteria were identified using PubMed searches with the following search terms: “action selection”, “response selection”, “movement selection”, “free selection”, “willed action”, and “self movement” (selection); “stop signal” (stopping). The large number of search terms used to identify action selection studies reflects the wide range of terminology historically applied to describe this concept. In March 2012, a total of 17 studies, containing 24 appropriate contrasts, were identified for selection, and 16 studies, containing 19 appropriate contrasts, were identified for stopping. These studies reported a total of 231 foci for selection (10 outside the brain mask used in the meta-analysis process) and 268 foci for stopping (11 outside the brain mask).
We used the BrainMap GingerALE tool, version 2.3 (www.brainmap.org) to perform two separate meta-analyses of selection and stopping studies, and then to perform a conjunction of these two ALE meta-analyses (selection AND stopping). An ALE (Activation Likelihood Estimation) analysis uses the peak coordinates of activations from multiple published studies to identify regions associated with performance of a cognitive task across the multiple studies, giving a large total number of subjects and enhanced power (Eickhoff et al., 2012, Turkeltaub et al., 2012).
For the two separate meta-analyses of selection and stopping, any studies reporting Talairach coordinates instead of MNI were converted to MNI space using the Talairach to MNI transform as implemented in GingerALE. In the GingerALE analysis, we then applied the following options in addition to the default settings: non-additive ALE method (Turkeltaub et al., 2012); output cluster minimum volume, 100 mm3; and FDR p < 0.05. The resulting thresholded ALE map was viewed in MRIcroN (http://www.mccauslandcenter.sc.edu/mricro) and the anatomical labelling of foci facilitated by the Anatomy toolbox (Eickhoff et al., 2005) in SPM8 (www.fil.ion.ucl.ac.uk/spm).
To perform a GingerALE conjunction (action selection AND stopping), we selected the “contrast studies” option in GingerALE, with the previously generated selection and stopping ALE maps as inputs. The same options used in the previous selection and stopping ALEs were applied and permutations testing carried out with 10,000 iterations. A threshold of FDR p < 0.05 was applied to the conjunction image.
Combined selection and stopping fMRI study
Subjects and task
21 healthy right-handed subjects were recruited from the MRC Cognition and Brain Sciences Unit volunteer panel. Four subjects showed poor behavioral performance, and were excluded before further analysis (3 subjects showed high “go” trial omission error rates: 41–49%, close to 2 standard deviations beyond the mean go omission rate of the 21 subjects of 11%; 1 subject showed low stop accuracy: 35%, close to 2 standard deviations beyond the mean stop accuracy of 47%). The data from 17 subjects (age = 20–38, mean age = 28, 12 males) were analysed further. The study was approved by the local Research Ethics Committee, and all subjects gave informed written consent in accordance with the Declaration of Helsinki.
Subjects underwent fMRI during a combined selection and stopping task (Fig. 1). Subjects were presented with a picture of a right hand with four circles, one above each finger. A change in colour of the circles from the grey background colour to green indicated a movement should be initiated (a right hand button press on a magnetic resonance-compatible four button box). There were two trial types: “specified” or “select”. On a “specified” trial, a single circle changed to green above the corresponding finger the subject should press with. On a “select” trial, all four circles changed to green, and subjects chose which finger to press with. On “select” trials, subjects were asked to “make a fresh response on each trial, using any of the four buttons, regardless of what you have done before”, comparable to previous studies using the same action selection paradigm (Hughes et al., 2010, Rowe et al., 2010). During both specified and select trials the green circle movement cues were presented for 1000 ms, during which time subjects were required to respond. If subjects failed to make a response, a negative feedback cue was superimposed on the hand picture for 500 ms post-trial. An inter-trial interval varying in length between 2000 and 8000 ms comprised continuous presentation of the hand picture with no change in the grey background color of the dots. The stimulus-onset asynchrony varied between 3000 and 9000 ms (mean: 4500 ms), resulting in greater design efficiency.
Subjects first completed 20 go-specified and 20 go-select trials in a preliminary section, during which they were advised that no stopping would be required. This provided an estimate of their reaction times, and familiarised subjects with performance in the scanner. The action selection task was then combined with a stop signal task (Logan et al., 1984). On 25% of specified and 25% of select trials, after a short variable delay, the green “go” cue changed to a red “stop” cue in conjunction with an auditory stop signal (a 1000 Hz tone 100 ms in duration), indicating the subjects should withhold their response. Subjects were instructed not to anticipate the stop signal, and that they should prepare a movement on each trial. If subjects incorrectly made a response on these stop trials, a red negative feedback cue was superimposed on the hand picture for 500 ms post-trial. This increased attention to task performance and maintained motivation over a 50-minute task performance period. The initial starting delay between the “go” and “stop” cues was set at the subject's reaction time from the preliminary go-trial only section, minus 225 ms (defined on the basis of pilot studies in healthy young adults). Two initial starting delays were defined, one for specified, and one for select trials, as we hypothesised stopping might function differently on the two trial types (mean initial specified starting delay: 351 ms; mean initial select starting delay: 401 ms). Staircase tracking algorithms modified the delay between go and stop cues on a trial-by-trial basis to maintain overall successful stopping at 50%. We used three parallel algorithms, maintaining stopping probability at 30%, 50%, and 70%, for both specified and select trials respectively (total of six trackers). The use of three tracking algorithms (with high, medium and low stopping probability rates) reduces the tendency for subjects to strategically slow their responses on stop signal tasks. Subjects completed 432 go trials (216 specified, 216 select) and 144 stop trials (72 specified, 72 select), during continuous imaging, but divided into 6 blocks of equal length with a rest break of 20 s between blocks. Trial order was fully randomised.
The stop signal reaction time (SSRT) measures the efficiency of response inhibition, with lower SSRTs indicative of less time required to inhibit a response. Subjects' SSRTs were calculated according to the “integration method” described by Logan et al. (1984): all go trials with a response were ranked according to reaction time in descending order (fastest to slowest). If n is the probability of incorrectly responding on stop trials, and t the number of go trials in rank order of reaction time, the SSRT is calculated as the difference between the reaction time of the (n*t)th trial and the subject's mean stop signal delay. The integration method is a commonly used algorithm for estimation of SSRT throughout the stop signal literature, and is particularly beneficial when successful stopping does not perfectly converge on 50% (Boehler et al., 2012). SSRTs calculated according to the integration method are reliable across runs and across sessions (Congdon et al., 2012). We assessed whether SSRT differed between stop-specified and stop-select trials with a repeated measures t-test (SPSS 19.0, IBM).
Previous stop signal studies have observed a “carry-over” inhibitory effect from a stop trial to a subsequent go trial, such that reaction times on n + 1 go trials are longer than when not preceded by a stop trial (Rieger and Gauggel, 1999, Verbruggen et al., 2008). We assessed the impact of preceding trial type (go or stop) on subsequent n + 1 go trial reaction times using a repeated measures analysis of variance with factors of preceding trial (go or stop) and action type (selected or specified) (SPSS 19.0, IBM).
Functional MRI acquisition and pre-processing
1450 BOLD-sensitive T2*-weighted echo-planar images were acquired on a Siemens Trio 3 T (repetition time = 2000 ms, echo time = 30 ms, 32 descending oblique slices 3 mm thick with 0.75 mm slice gap, in-plane resolution 3 × 3 mm). The first five images were discarded to allow for steady-state magnetisation. A high resolution MPRAGE structural scan of each subject was acquired for subsequent registration purposes (repetition time = 2250 ms, echo time = 2.99 ms, 1 × 1 × 1 mm3 resolution). fMRI pre-processing and statistical modelling used SPM8 in matlab version 7, with Automatic Analysis scripts (https://github.com/rhodricusack/automaticanalysis). Images were converted from dicom to nifti format, re-aligned to the mean image and sinc interpolated in time to correct for slice timing during volume acquisition. The MPRAGE was co-registered to the mean echo planar image, using normalised mutual information. The MPRAGE was iteratively segmented and normalised to the SPM MNI152 structural template. The resulting normalisation parameters were then applied to all the re-aligned and slice-time corrected echo planar images. The normalised images were smoothed with a Gaussian kernel of full-width half-maximum 8 mm.
Functional MRI modelling and statistics
First level analysis for each subject used an event-related general linear model, with covariates indicating the onset of each trial. Events were modelled with a duration of 500 ms after trial onset, as this time-point captures both selection and stopping processes. Trials were convolved with the default SPM canonical hemodynamic response function, and defined in the design matrix using 8 regressors: preliminary go-select and go-specified trials; main task go-select, go-specified, stop-specified-correct, stop-specified-incorrect, stop-select-correct, and stop-select-incorrect trials. Any go trials on which an error was made (for example, not responding within the 1000 ms presentation of the go cue, or pressing with the wrong finger on a specified trial) were modelled separately. Six nuisance regressors modelled subject movement in translations and rotations. Contrast images of interest were calculated from the main task for the following: go-specified, go-select, stop-specified-correct, and stop-select-correct.
For the second-level analysis, a flexible factorial design was used, with “task” as the first factor, and “subject” as a second factor. Contrast images using parameter estimates from correct go trials were included in the second level models (whilst incorrect trials were not included). In keeping with much of the previous stop signal literature (e.g. Cai and Leung, 2009, Chikazoe et al., 2009, Jahfari et al., 2011, Lenartowicz et al., 2011, Sharp et al., 2010), to examine stopping we used correct stop trials (response successfully withheld). We examined the following second-level contrasts of interest, across the whole-brain: (1) action selection (go-select > go-specified), equivalent to the “select > specified” contrasts entered into the “selection” meta-analysis; (2) stopping of specified actions (stop-specified-correct > go-specified), equivalent to the “correct stop > go” contrasts entered into the “stopping” meta-analysis; (3) stopping of selected actions (stop-select-correct > go-select); (4) the interaction between action selection and stopping ± [(stop-specified-correct > go-specified) > (stop-select-correct > go-select)]; and (5) the conjunction between action selection and stopping (go-select > go-specified AND stop-specified-correct > go-specified).
The resulting statistic images for the first three contrasts (action selection and stopping) were thresholded at p < 0.05 cluster-based FDR (with p < 0.001 voxel level) (Chumbley and Friston, 2009). The interaction and conjunction contrasts were threshholded for whole brain analysis at the liberal exploratory threshold of p < 0.001 uncorrected, and then corrected for multiple comparisons within a small volume correction mask based on the preSMA peak focus in the meta-analysis conjunction (action selection AND stopping). For the small volume correction mask, we took the y and z co-ordinates from the location of the peak preSMA ALE statistic in the meta-analysis conjunction (y = 18, z = 48), and to be sensitive to both left and right hemisphere preSMA, set the x co-ordinate to 0. Then, in SPM we generated a 12 mm sphere centred on this co-ordinate (0, 18, 48) and using this mask, applied a small volume correction for multiple comparisons correction to both the interaction and conjunction contrasts.
Results
GingerALE meta-analysis
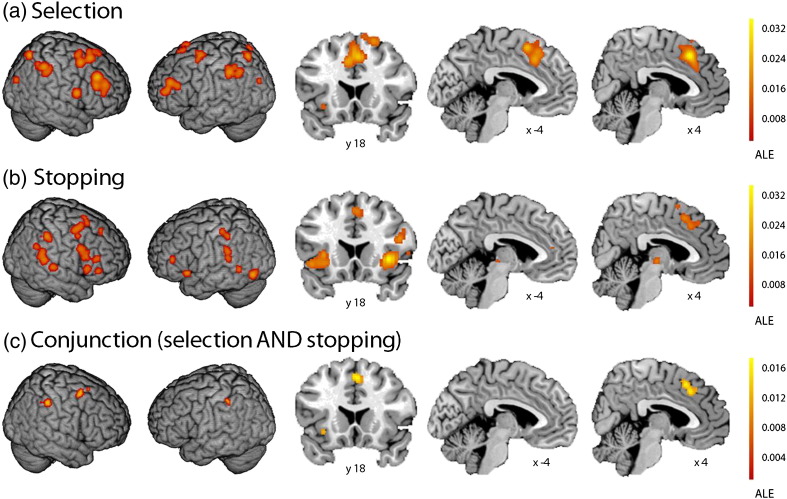
The action selection GingerALE confirmed a pattern of fronto-parietal activity associated with action selection (“select” > “specified”), namely: bilateral preSMA, bilateral middle frontal gyrus, the right inferior frontal gyrus (pars opercularis), bilateral premotor cortex, bilateral inferior parietal cortex, and bilateral visual cortices (see Fig. 2a and Supplementary Table 1).
Fig. 2.
GingerALE meta-analysis. (a) Action selection (select > specified) is associated with bilateral preSMA, bilateral middle frontal gyrus, right inferior frontal gyrus, bilateral premotor cortex, and bilateral inferior parietal cortex; (b) action stopping (correct stop > go) is associated with right preSMA, right inferior frontal gyrus (inferior frontal junction, pars opercularis, pars triangularis), left inferior frontal gyrus (pars triangularis), bilateral anterior insula, right premotor cortex, and bilateral inferior parietal cortex; (c) conjunction of action selection and stopping (select > specified) AND (correct stop > go) shows the right preSMA, right premotor cortex, and bilateral inferior parietal cortex as common to both processes. (a), (b), and (c) all shown at FDR p < 0.05; the colour bar represents the ALE statistic, which increases in significance from bottom (red) to top (yellow).
The action stopping GingerALE showed that successful stopping (“correct stop” > “go”) has been associated with the right preSMA, right inferior frontal gyrus (inferior frontal junction, pars opercularis, pars triangularis), left inferior frontal gyrus (pars triangularis), bilateral anterior insula, right premotor cortex, bilateral inferior parietal cortex, and bilateral auditory and left visual cortices (Fig. 2b, Supplementary Table 1).
In a conjunction meta-analysis, we asked which regions of the brain were associated with both action selection and stopping across the 17 action selection and 16 stop signal studies. The GingerALE conjunction identified the right preSMA, right premotor cortex, and bilateral inferior parietal cortex as regions common to both action selection and stopping (Fig. 2c, Supplementary Table 1). No regions within the inferior frontal gyrus were common to selection and stopping, suggesting that the inferior frontal foci identified in the separate action selection and stopping meta-analyses did not overlap, despite falling within the broader area described as inferior frontal gyrus.
Combined selection and stopping fMRI study
Behaviour
94% of go trials were correct. Subjects made omission errors on 3% of go trials (reaction time > 1000 ms on both go-specified and go-select trials), and incorrect finger presses on 3% (e.g. index finger pressed in response to a middle finger cue on go-specified trials), attributed to fatigue. Subjects' reaction time (RT) on go trials during the combined task was shorter on go-specified than go-select trials (t(16) = 5.52, p < 0.001, mean specified = 607 ms, mean select = 634 ms).
On the stop trials, the tracking algorithms converged stop accuracy (i.e. response withheld) at 48% on stop-specified and 46% on stop-select trials (no significant difference between conditions; t(16) = -1.81, p = 0.09).
Behavioural indices of stopping performance were similar on stop-specified and stop-select trials. The stop signal reaction time (SSRT; indexing stop efficiency) was not significantly different on stop-specified and stop-select trials (t(16) = -0.59, p = 0.57, mean specified SSRT = 297 ms, mean select SSRT = 292 ms) (see Table 2).
Table 2.
Behavioural performance on the combined selection and stopping task. Mean (range).
| Specified | Select | Statistic p-value | |
|---|---|---|---|
| Preliminary action selection task | |||
| Go reaction time (ms) | 576 (415:701) | 626 (462:770) | p < 0.001a |
| Combined action selection/stop signal task | |||
| Go reaction time (ms) | 607 (502:715) | 634 (528:743) | p < 0.001a |
| Stop signal reaction time (SSRT; ms) | 297 (245:380) | 292 (211:375) | p = 0.57a |
| Stop signal delay (SSD; ms) | 316 (196:480) | 357 (204:546) | p = 0.002a |
| Stop accuracy (%) | 48 (42:54) | 46 (36:54) | p = 0.09a |
| Go reaction time after stop trial (ms) | 642 (521:785) | 664 (535:806) | p = 0.89b |
t-test.
F-test.
We analysed the “carry-over” effect (Rieger and Gauggel, 1999, Verbruggen et al., 2008) of a preceding stop trial on subsequent go trial RTs. The carry-over effect was not significantly different between stop-specified/go-specified and stop-select/go-select trials (interaction F(1,16) = 0.021, p = 0.89, mean specified go RT = 642 ms, mean select go RT = 664 ms), although there was as expected an overall effect of a preceding stop trial on go RTs (F(1,16) = 6.59, p = 0.01, mean go RT after stop trial = 653 ms, mean go RT after go trial = 605 ms).
fMRI results
Action selection
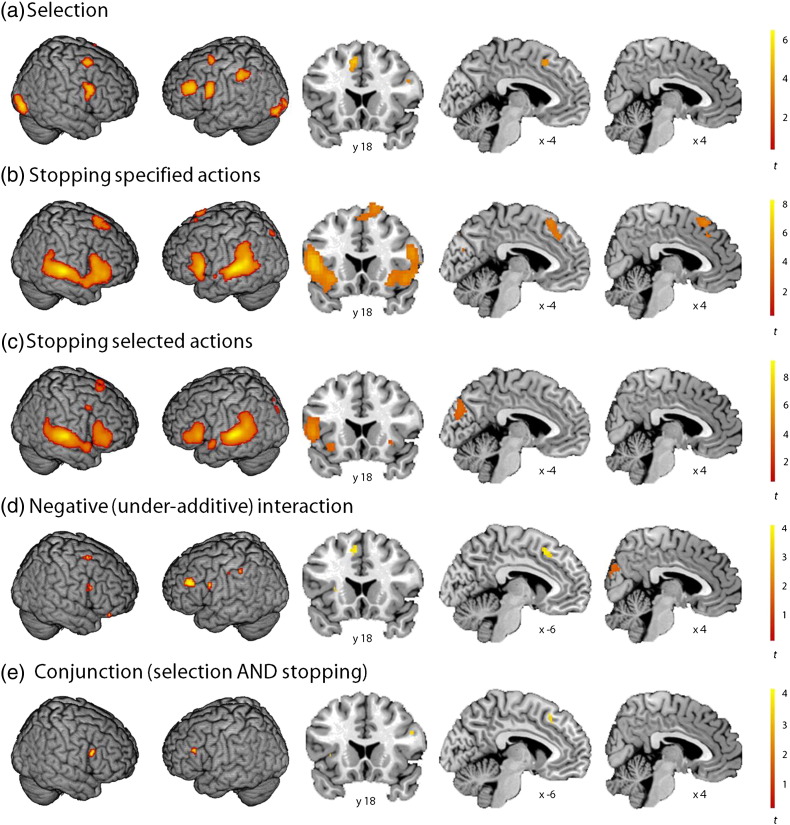
We used the same contrasts to analyse action selection to those entered into the “action selection” meta-analysis. Action selection (go-select > go-specified) revealed a network of fronto-parietal activity similar to that identified by the GingerALE meta-analysis, and our previous studies of action selection (Hughes et al., 2010, Hughes et al., 2013, Rowe et al., 2010), including the left preSMA, bilateral inferior frontal gyrus (inferior frontal junction), left middle frontal gyrus (extending into the inferior frontal sulcus), bilateral premotor cortex, left inferior parietal cortex, bilateral visual cortices, and the left cerebellum (p < 0.05 FDRc; Fig. 3a, Supplementary Table 2). There was a tendency for activations to be left lateralised: in the meta-analysis, action selection was also associated with right preSMA, right middle frontal gyrus, and right inferior parietal cortex, but these right sided regions were not observed at corrected thresholds in the current study. The left lateralisation observed here may be related to the task requirement for subjects to make right-handed actions.
Fig. 3.
fMRI results from the combined selection and stopping task. (a) Selection (go-select > go-specified); (b) stopping specified actions (stop-specified-correct > go-specified); (c) stopping selected actions (stop-select-correct > go-select); (d) negative (under-additive) interaction [(stop-select-correct > go-select) > (stop-specified-correct > go-specified)]; (e) conjunction (go-select > go-specified AND stop-specified-correct > go-specified). (a–c) are thresholded at p < 0.05 FDRc. (d–e) are illustrated at p < 0.001 uncorrected, but the preSMA foci are significant (p < 0.05 FWE) within a priori small volume correction masks based on the preSMA peak focus in the meta-analysis conjunction (see Materials and methods).
Stopping specified actions
The contrast “stopping specified actions” (stop-specified-correct > go-specified) is equivalent to the contrasts entered into the “action stopping” meta-analysis (as all the stop signal studies included in the meta-analysis required subjects to stop actions specified by the experimenter). The “stopping specified actions” contrast showed activations corresponding closely to the pattern identified by the GingerALE of stop signal tasks. This contrast was associated with bilateral preSMA, right inferior frontal gyrus (inferior frontal junction, pars opercularis, pars triangularis), left inferior frontal gyrus (pars opercularis, pars triangularis), left insula, left inferior parietal cortex, and bilateral auditory and left visual cortices (p < 0.05 FDRc; Fig. 3b, Supplementary Table 2).
Stopping selected actions
A novel contrast, representing the stopping of responses that subjects had chosen themselves (stop-select-correct > go-select), showed activation in a subset of regions observed when stopping a specified action, including bilateral inferior frontal gyrus (pars triangularis), bilateral insula, and bilateral auditory and visual cortices (p < 0.05 FDRc; Fig. 3c, Supplementary Table 2). Interestingly, regions often observed when stopping a specified action, such as bilateral preSMA, right inferior frontal gyrus (inferior frontal junction, pars opercularis), bilateral premotor cortex and bilateral inferior parietal cortex (see Fig. 2b), were not reliably more engaged when stopping a selected action, compared to making a selected action. These findings do not support the notion that stopping a selected action makes heavier demands on a common mechanism underlying selection and stopping.
To test the null hypothesis of whether stopping a selected action (as opposed to stopping a specified action) was associated with an increase in demand for neural resources (for example, due to a cognitive “bottleneck”), we examined the contrast for a negative interaction. The interaction [(stop-specified-correct > go-specified) > (stop-select-correct > go-select)] revealed very similar levels of activation associated with stopping a selected action and stopping a specified action in the left preSMA (p < 0.05 FWE, correcting for multiple comparisons within a small volume mask based on the preSMA focus in the meta-analysis conjunction). At a more liberal exploratory threshold (p < 0.001 uncorrected) weaker negative interactions were seen in bilateral inferior frontal gyrus (inferior frontal junction), left middle frontal gyrus (extending into the inferior frontal sulcus), right premotor cortex, and left inferior parietal cortex (Fig. 3d, Supplementary Table 2).
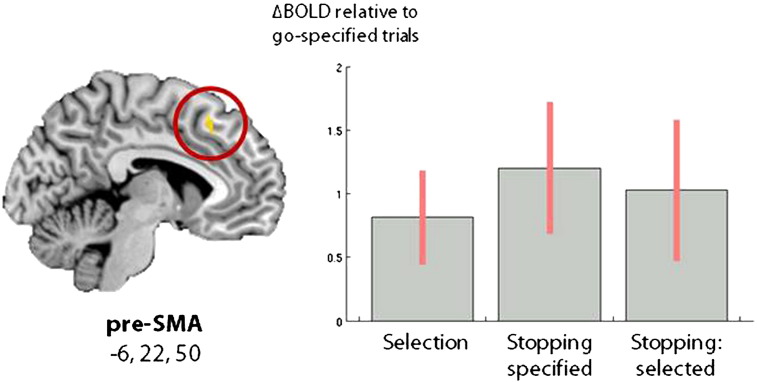
In other words, when stopping a selected action, the preSMA activity did not represent the sum of ‘selection’ and ‘stopping’ activations observed when these tasks had been performed individually (see BOLD effect sizes in Fig. 4).
Fig. 4.
The combined selection and stopping fMRI task reveals a significant negative interaction in the preSMA: BOLD effect sizes at the preSMA peak co-ordinate identified by the conjunction, for action selection (go-select > go-specified), stopping of specified actions (stop-specified-correct > go-specified), and stopping of selected actions (stop-select-correct > go-specified). Each condition is shown contrasted against the go-specified baseline.
Action selection and stopping
The conjunction analysis, identifying regions associated with both action selection and stopping (go-select > go-specified AND stop-specified-correct > go-specified), revealed conjoint activations in the left preSMA, right inferior frontal gyrus (inferior frontal junction), left inferior frontal sulcus and left insula, at a liberal exploratory threshold (p < 0.001 uncorrected, Fig. 3e, Supplementary Table 2). Using a region of interest based on the preSMA cluster identified by the meta-analysis conjunction, the conjoint activation of selection and stopping in the preSMA was significant at p < 0.05 FWE (Supplementary Table 2).
The conjunction analysis indicates that at the resolution of fMRI, and with the degree of spatial consistency across a group of subjects in a second level voxel-wise analysis, action selection and stopping are co-localised in these regions. At the specified thresholds, we did not observe co-localisation of action selection and stopping activations in the premotor cortex or inferior parietal cortex, regions which were identified in the meta-analysis conjunction. The right inferior frontal gyrus (inferior frontal junction) was identified by the combined fMRI study's conjunction analysis, but not the meta-analysis conjunction (above). Thus, the preSMA was the only region to be associated with both action selection and stopping in both the meta-analysis and within-subjects combined fMRI study.
Discussion
Action selection and stopping are two elements of voluntary action control for successful goal-directed behaviour. Here we investigated selection and stopping together, to determine the potential similarities and interactions between them. Our aims were first to establish to what degree selection and stopping activations overlap; and second, to investigate why selection and stopping might show similar regional activations, with particular reference to the hypothesis that both selection and stopping utilise inhibition (also see Jasinska, 2013, Mostofsky and Simmonds, 2008).
Overlap of selection and stopping
Similar patterns of fronto-parietal activation have been reported in separate neuroimaging studies of selection and stopping (Aron et al., 2007, Chikazoe et al., 2009, Hughes et al., 2010, Rowe et al., 2010, Swick et al., 2011). We approached the question of anatomical overlap of selection and stopping activations using two approaches, in order to take advantage of the relative benefits of each method: a meta-analysis of thirty-three selection and stopping studies, and a novel within-subjects fMRI experiment that combined action selection with the stop signal paradigm. Interestingly, using these two methods combined, we observed a more limited degree of overlap across selection and stopping than had been implied by a casual overview of existing separate studies. The preSMA was the only region associated with selection and stopping in both the meta-analysis and the within-subjects fMRI task. The meta-analysis also identified the premotor cortex and inferior parietal cortex as common to action selection and stopping (across subject groups). Our within-subjects fMRI results also identified the right inferior frontal gyrus (in the vicinity of the inferior frontal junction) as common to both selection and stopping within the same subject group, although this was not identified by the meta-analysis conjunction.
The meta-analysis conjunction identified the right preSMA as common to both selection and stopping, but the within-subjects fMRI study conjunction identified a peak in left preSMA. This may be related to the fact that different studies included in the meta-analysis had varying manual requirements: some studies used right-handed selection and stopping tasks, whilst others used bimanual tasks. In contrast, our fMRI experiment required only right-handed actions, leading to the left-hemispheric dominance of preSMA activations common to both selection and stopping. Moreover, the preSMA cluster identified in the meta-analysis conjunction crossed the midline (see Fig. 2). There may be functional differences for selection and stopping between the left and right preSMA regions, but the existing data do not provide strong evidence for lateralisation.
The preSMA and lateral prefrontal cortex in selection and stopping
Having confirmed a significant degree of anatomical overlap of selection and stopping activations, we review the proposed roles for the preSMA and the inferior frontal gyrus in selection and stopping. We next consider why selection and stopping might show similar regional activations.
In both our meta-analysis and fMRI experiment, we found that action selection (go-select > go-specified) was associated with the preSMA, and lateral prefrontal cortex (middle and inferior frontal gyri). Despite the common co-activation of these regions in action selection tasks, a functional dissociation has been proposed between medial and lateral prefrontal regions for action selection. The preSMA has been associated with voluntary action generation, whilst lateral prefrontal cortex is also associated with monitoring recent action history, or implementation of task strategies in a sequence of action decisions (Cunnington et al., 2005, Hoffstaedter et al., 2013, Lau et al., 2004, Passingham et al., 2010, Rowe et al., 2000, Rowe et al., 2010, Zhang et al., 2012). When several equally appropriate response options are available for voluntary action decisions, the preSMA may resolve conflict between those options (Duque et al., 2013, Rushworth, 2008). Such a situation arises either when there are no differential outcome values associated with the available responses, or when the value representations of the options, encoded by anterior cingulate cortex (ACC), provide conflicting information about the optimal choice. One mechanism by which conflict resolution in action selection could proceed in the preSMA is via a competitive race between representations of the available responses (Rowe et al., 2010, Zhang et al., 2012).
In both our meta-analysis and fMRI experiment, we found that action stopping (stop-specified-correct > go-specified) was associated with the preSMA, and lateral prefrontal cortex (inferior frontal gyri). The role of the inferior frontal gyrus sub-regions (inferior frontal junction, pars opercularis, pars triangularis) in action stopping remains controversial (Levy and Wagner, 2011). Some studies suggested that the inferior frontal gyrus is critical for top–down inhibitory suppression of actions (Aron and Poldrack, 2006, Aron et al., 2004), whilst others have suggested that the inferior frontal gyrus (Hampshire et al., 2010, Sharp et al., 2010) and inferior frontal junction (Cai and Leung, 2011) are associated with detection of a salient and behaviorally relevant target, such as stimuli relevant to stopping.
With a large corpus of stop signal and Go/NoGo imaging studies, it is clear that activations labelled as “inferior frontal gyrus” cover a variety of cortical locations including the anterior insula, inferior frontal junction, pars opercularis, pars triangularis, and even pars orbitalis (Aron and Poldrack, 2006, Cai and Leung, 2011, Chikazoe et al., 2009, Hampshire et al., 2010, Macoveanu et al., 2013, Sharp et al., 2010). The presence of significant functional heterogeneity across these areas is suggested by anatomical differences in cytoarchitecture, receptor-architecture, and diffusion-based tractography (Amunts and Zilles, 2012, Amunts et al., 1999, Amunts et al., 2010, Anwander et al., 2007). Our meta-analysis of stop signal studies identified several regions associated with stopping, within or adjacent to the “right inferior frontal gyrus”: specifically, the anterior insula, inferior frontal junction, inferior pars opercularis, and two clusters on pars triangularis, one dorsal and one ventral. In our fMRI experiment, the activations related to stopping a specified action extend from the right inferior frontal junction to right pars triangularis. Stopping a selected action (contrasted against executing such a chosen action) activated primarily pars triangularis. Together, these results are consistent with the hypothesis that separate regions within or close to the inferior frontal gyrus may perform different roles in action stopping.
In contrast to the inferior frontal gyrus, the preSMA shows activation during stop signal tasks even when controlling for the effect of a salient task-relevant cue (Sharp et al., 2010). This suggests there may be a direct role for the preSMA in implementing inhibition to stop a motor response.
Selection and stopping for voluntary action: the role of preSMA
In both the meta-analysis and within-subjects fMRI conjunctions, the preSMA was associated with both selection and stopping. The inferior frontal gyrus was observed in the within-subjects fMRI conjunction, but not the meta-analysis conjunction. While this discrepancy may be type II error, it is also possible that the within-subject design is more sensitive and anatomically precise than a between groups meta-analysis conjunction, especially in a region with fine grained functional heterogeneity, such as the inferior frontal gyrus. At present, the discrepancy across the two approaches calls for caution in interpreting the significance of co-localisation in the inferior frontal gyrus, and further replication is required. Here, we focus on the role of the preSMA, as it was identified by both analysis methods.
There are several potential explanations for common preSMA activation during selection and stopping. We proposed that a common cognitive process with a shared mechanism for selection and stopping might be inhibitory in nature: for example, action selection could include the inhibition of alternative actions, or the inhibition of previous actions in a sequence of action decisions (Rowe et al., 2010, Zhang et al., 2012), whilst action stopping might require the inhibition of an action currently in preparation (Aron and Poldrack, 2006). The behavioural data and fMRI interaction provided tests of the hypothesis of a common inhibition mechanism for selection and stopping. We identified three possible outcomes: (1) performing selection within close temporal proximity to stopping could “prime” the inhibition mechanism, with the result that stopping is more efficient, i.e. shorter SSRTs, and less BOLD activation (Scherbaum et al., 2011); (2) sharing of cognitive resources could result in an inhibition “bottleneck” (Pashler, 1994), with the result that stopping is less efficient, i.e. longer SSRTs, and more BOLD activation; (3) selection and stopping might not require shared access to the same inhibitory process (cf. Yamaguchi et al., 2012), resulting in no difference in either SSRT or BOLD activation when selection and stopping proceed simultaneously (or within close temporal proximity).
The behavioural data from the fMRI study were consistent with the third outcome: subjects' SSRTs were closely matched when subjects were required to stop actions they had selected on stop-select trials (SSRT = 292 ms), and when they were required to stop actions specified by the experimenter on stop-specified trials (SSRT = 297 ms). Since a difference may not be manifest in the stop trial itself, but in the consequences of stopping for subsequent trials, we also measured the “carry-over” effect in stop signal tasks. We measured the impact of prior stopping on reaction time in subsequent go trials, to test whether the reaction times on n + 1 go trials were longer than when not preceded by a stop trial (Rieger and Gauggel, 1999, Verbruggen et al., 2008). We found that the inhibitory “carry-over” effect on n + 1 go trial reaction times was the same regardless of whether the prior stop trial was specified (go RT = 641 ms) or selected (go RT = 642 ms). Thus, there was neither a significant behavioural inhibition cost, nor inhibition benefit, to stopping and selection within close temporal proximity (within-trial). In a recent behavioural study by Yamaguchi et al. (2012), SSRTs were found to be the same in both a standard stop signal task and a dual-task paradigm in which subjects were required to stop one response whilst executing another. Together, these SSRT findings suggest that an inhibitory process in stopping a response may not be subject to a dual-task bottleneck.
Examining the fMRI data, we found a significant interaction between selection and stopping, indicating a significant under-additive effect (Fig. 3d). This contrast tested the null hypothesis of whether stopping a selected action (as opposed to stopping a specified action) was associated with an increase in BOLD activation. The interaction contrast revealed significantly less activation than would be expected from simple addition of selection and stopping effects: there were very similar levels of activation associated with stopping a selected action and stopping a specified action in the left preSMA (Figs. 3d, 4).
Together, these behavioural and fMRI results do not provide strong evidence that action selection and stopping share a common inhibitory mechanism in the preSMA. However, the failure to reject the null hypothesis does not rule this possibility out. Several alternative interpretations of the data warrant discussion. It is possible that both priming (Scherbaum et al., 2011) and a “bottleneck” (Pashler, 1994) operate on select-stop trials, with the net result that any priming benefit to stopping, from performing selection within the same trial, is negated by selection and stopping being unable to proceed simultaneously. We note that on a given stop-select trial, we do not know how close in time action selection is to completion when the stop signal is received. This interpretation implies that selection and stopping are rapid, sequential processes within a stop-select trial, rather than concurrent. If that were the case, then our task alone does not resolve the question of whether such a shared inhibitory mechanism would be required simultaneously, or simply in close temporal proximity, between selection and stopping. However, a recent TMS study suggests that during action decisions, the selection among response alternatives does not complete before preparation of the chosen response (Klein-Flugge and Bestmann, 2012), providing evidence that an inhibitory mechanism to suppress alternative action representations could still be required within the same trial while a chosen action is prepared, overlapping in time with reception of a stop signal.
Another possibility underlying the co-localisation of selection and stopping to the preSMA, without a behavioural interaction, is that the medial frontal cortex monitors or detects response conflict and the need for cognitive control (Botvinick et al., 2004). Detecting response conflict on stop-select trials might not require additional resources compared to the detection of response conflict on selection or stopping trials alone. The preSMA region we identify as associated with selection and stopping is dorsal to the anterior cingulate cortex, a different medial frontal region which is more commonly associated with detection or monitoring of conflict (Botvinick et al., 2004, Lau et al., 2006, Sheth et al., 2012). However, the functional localisation of conflict detection on the medial wall has been controversial and is still unresolved, with some evidence for a role of the preSMA as well as the anterior cingulate (Ridderinkhof et al., 2004). Once response conflict has been detected, if a response is required, conflict resolution should follow. Conflict resolution, as opposed to detection, has also been proposed as a function of the preSMA (Nachev et al., 2005, Rushworth, 2008).
Conflict resolution is consistent with the co-localisation of selection and stopping to the preSMA: resolving conflict between competing available responses (Duque et al., 2013, Rowe et al., 2010, Zhang et al., 2012), or conflict between a stop and go response (Logan et al., 1984). The results of our fMRI study suggest that resolution of conflict on stop-select trials was comparable to the resolution of conflict on selection or stopping trials alone, and could be based on neuronal representations of multiple actions in motor association areas, including the preSMA (Cisek and Kalaska, 2010, Cunnington et al., 2005). Both selection and stopping make use of, or access, such neuronal representations. Simultaneous access to different action representations would enable effective stopping on select trials without a further increase in BOLD response.
We propose that the preSMA supports the representation of competing potential actions, for both selection and stopping (cf. Cisek and Kalaska, 2010, Cunnington et al., 2005, Nachev et al., 2007, Zhang et al., 2012). Signals from other regions may provide input to the preSMA to influence the representation of competing response options, resolving conflict between competing responses in selection trials, and between stop and go response in stop trials. For example, information on the outcomes of responses from the anterior cingulate cortex may influence action representations in the preSMA in action selection trials (Rushworth, 2008, Rushworth et al., 2004) while “stop” signals from the inferior frontal cortex in response to behaviorally relevant salient stimuli may influence action representations in the preSMA in stopping trials (Cai and Leung, 2011, Hampshire et al., 2010, Sharp et al., 2010, Verbruggen et al., 2010). The behavioural and fMRI results from our combined selection and stopping task are consistent with this model. The preSMA thereby supports both action selection and stopping by modulating representations of actions, when selection and stopping occur separately, or in close temporal proximity — such as on stop-select trials in our combined task. The stopping of selected actions is relevant to many real-world settings, when changes must rapidly be made to our chosen actions in response to environmental cues (Filevich et al., 2012).
Limitations of the present study
There are several limitations to the current study. Some relate to the different results observed using the meta-analysis and within-subjects fMRI experiment. There are several potential reasons why the conjunction results were not identical across the two approaches. The first may just be type II error. However, the different results may also relate to several key differences between the methods. The GingerALE meta-analysis software (Turkeltaub et al., 2012) is a commonly used tool for analysing results from many separate neuroimaging studies (e.g. Levy and Wagner, 2011, Swick et al., 2011). The advantages of a meta-analysis include an increase in the number of subjects, and thereby statistical power to detect an effect, above and beyond what is possible in a typical fMRI study. However, there is anatomical variance across subjects and across studies, limiting the interpretation of foci co-localisation from different tasks. There is greater smoothing of the underlying signal with GingerALE than in our fMRI study, reducing spatial resolution in the meta-analysis results. The anatomical accuracy of the meta-analysis foci is also limited by the inclusion of studies using PET, which has lower anatomical precision than fMRI. In addition, across the meta-analysis and within-subjects fMRI study, there will have been subtle differences in stimuli presentation or required motor response, potentially contributing to different findings across the two methods. The within-subjects selection and stopping experiment allowed us to address some of the issues of anatomical accuracy, but has lower power than the meta-analysis.
With regard to our second question of why selection and stopping might show similar regional activations, the null behavioural result and significant negative fMRI interaction (under-additive activation) require caution in inference: however small the absolute effect size, one cannot infer that there was no behavioral effect at all. Further work is therefore required to characterise the mechanisms underlying the relationship between selection and stopping in the preSMA, including perhaps the combination of brain imaging with precisely timed perturbations such as TMS.
Imaging the subthalamic nucleus is of particular relevance to stop signal tasks (Aron and Poldrack, 2006, Aron et al., 2007, Frank et al., 2007), as the preSMA is proposed to influence the subthalamic nucleus during response inhibition (Cai and Leung, 2011, Forstmann et al., 2012, Sharp et al., 2010). However, the subthalamic nucleus is a small oblique ovoid structure, which may be poorly visualised in a whole-brain imaging with relatively large voxels and spatial smoothing. Higher resolution imaging and probabilistic mapping may be more sensitive to the subthalamic nucleus in future studies (Forstmann et al., 2012). In addition, higher resolution imaging may be of benefit in applying analysis techniques such as multi-voxel pattern analysis (MVPA, Kriegeskorte et al., 2008) to disentangle separate neuronal populations within brain regions. Finally, our analysis is restricted to regional activations. Future studies of effective connectivity would be of interest in order to quantify the interactions within the networks for selection and stopping, including the interactions between inferior frontal gyrus and preSMA (cf. Duann et al., 2009, Zandbelt et al., 2013), and building on structure–function correlations for stopping efficiency emerging from the analysis of white matter tract structure (Coxon et al., 2012, Forstmann et al., 2012).
Conclusion
In both a meta-analysis and a combined selection and stopping fMRI task, we found that selection and stopping co-localise to the preSMA. However, activation of the preSMA was similar whether stopping specified actions, stopping selected actions, or executing selected actions. Moreover, the stop signal reaction times were similar whether subjects stopped specified or selected actions. We suggest that the preSMA supports the representation of competing responses, in such a way that does not require shared access to a single inhibitory mechanism, in order to resolve conflict between action representations.
Funding
This work was supported by the Medical Research Council (MC-A060-5PQ30 and a Doctoral Training Award to CLR), the Wellcome Trust (088324 to JBR, LEH), the James F McDonnell Foundation, the Bill and Melinda Gates Foundation Scholarship (CW) and the NIHR Cambridge Biomedical Research Centre.
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References to studies included in the meta-analysis, meta-analysis clusters and coordinates, and action selection/stop signal task fMRI peak coordinates, can be found in the Supplementary Material. Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neuroimage.2013.10.012.
Appendix A. Supplementary data
Supplementary material 1.
Supplementary material 2.
References
- Amunts K., Zilles K. Architecture and organizational principles of Broca's region. Trends Cogn. Sci. 2012;16:418–426. doi: 10.1016/j.tics.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Amunts K., Schleicher A., Burgel U., Mohlberg H., Uylings H.B., Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J. Comp. Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Amunts K., Lenzen M., Friederici A.D., Schleicher A., Morosan P., Palomero-Gallagher N., Zilles K. Broca's region: novel organizational principles and multiple receptor mapping. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwander A., Tittgemeyer M., von Cramon D.Y., Friederici A.D., Knosche T.R. Connectivity-based parcellation of Broca's area. Cereb. Cortex. 2007;17:816–825. doi: 10.1093/cercor/bhk034. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Poldrack R.A. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Behrens T.E., Smith S., Frank M.J., Poldrack R.A. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J. Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler C.N., Appelbaum L.G., Krebs R.M., Hopf J.M., Woldorff M.G. The influence of different Stop-signal response time estimation procedures on behavior–behavior and brain–behavior correlations. Behav. Brain Res. 2012;229:123–130. doi: 10.1016/j.bbr.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M.M., Cohen J.D., Carter C.S. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brass M., Haggard P. The what, when, whether model of intentional action. Neuroscientist. 2008;14:319–325. doi: 10.1177/1073858408317417. [DOI] [PubMed] [Google Scholar]
- Cai W., Leung H.C. Cortical activity during manual response inhibition guided by color and orientation cues. Brain Res. 2009;1261:20–28. doi: 10.1016/j.brainres.2008.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W., Leung H.C. Rule-guided executive control of response inhibition: functional topography of the inferior frontal cortex. PLoS One. 2011;6:e20840. doi: 10.1371/journal.pone.0020840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J., Jimura K., Hirose S., Yamashita K., Miyashita Y., Konishi S. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J. Neurosci. 2009;29:15870–15877. doi: 10.1523/JNEUROSCI.3645-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbley J.R., Friston K.J. False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage. 2009;44:62–70. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Cisek P., Kalaska J.F. Neural mechanisms for interacting with a world full of action choices. Annu. Rev. Neurosci. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- Congdon E., Mumford J.A., Cohen J.R., Galvan A., Canli T., Poldrack R.A. Measurement and reliability of response inhibition. Front. Psychol. 2012;3:37. doi: 10.3389/fpsyg.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon J.P., Van Impe A., Wenderoth N., Swinnen S.P. Aging and inhibitory control of action: cortico-subthalamic connection strength predicts stopping performance. J. Neurosci. 2012;32:8401–8412. doi: 10.1523/JNEUROSCI.6360-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington R., Windischberger C., Moser E. Premovement activity of the pre-supplementary motor area and the readiness for action: studies of time-resolved event-related functional MRI. Hum. Mov. Sci. 2005;24:644–656. doi: 10.1016/j.humov.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Deiber M.P., Ibanez V., Sadato N., Hallett M. Cerebral structures participating in motor preparation in humans: a positron emission tomography study. J. Neurophysiol. 1996;75:233–247. doi: 10.1152/jn.1996.75.1.233. [DOI] [PubMed] [Google Scholar]
- Duann J.R., Ide J.S., Luo X., Li C.S. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J. Neurosci. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn. Sci. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Duque J., Olivier E., Rushworth M. Top-down inhibitory control exerted by the medial frontal cortex during action selection under conflict. J. Cogn. Neurosci. 2013;25:1634–1648. doi: 10.1162/jocn_a_00421. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K., Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Bzdok D., Laird A.R., Kurth F., Fox P.T. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filevich E., Kuhn S., Haggard P. Intentional inhibition in human action: the power of ‘no’. Neurosci. Biobehav. Rev. 2012;36:1107–1118. doi: 10.1016/j.neubiorev.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Forstmann B.U., Keuken M.C., Jahfari S., Bazin P.L., Neumann J., Schafer A., Anwander A., Turner R. Cortico-subthalamic white matter tract strength predicts interindividual efficacy in stopping a motor response. Neuroimage. 2012;60:370–375. doi: 10.1016/j.neuroimage.2011.12.044. [DOI] [PubMed] [Google Scholar]
- Frank M.J., Samanta J., Moustafa A.A., Sherman S.J. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- Frith C.D., Friston K., Liddle P.F., Frackowiak R.S. Willed action and the prefrontal cortex in man: a study with PET. Proc. R. Soc. Lond. B Biol. Sci. 1991;244:241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- Hampshire A., Chamberlain S.R., Monti M.M., Duncan J., Owen A.M. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F., Grefkes C., Zilles K., Eickhoff S.B. The “what” and “when” of self-initiated movements. Cereb. Cortex. 2013;23:520–530. doi: 10.1093/cercor/bhr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes L.E., Barker R.A., Owen A.M., Rowe J.B. Parkinson's disease and healthy aging: independent and interacting effects on action selection. Hum. Brain Mapp. 2010;31:1886–1899. doi: 10.1002/hbm.20979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes L.E., Altena E., Barker R.A., Rowe J.B. Perseveration and choice in Parkinson's disease: the impact of progressive frontostriatal dysfunction on action decisions. Cereb. Cortex. 2013;23:1572–1581. doi: 10.1093/cercor/bhs144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M., Frith C.D. Willed action and its impairments. Cogn. Neuropsychol. 1998;15:483–533. doi: 10.1080/026432998381005. [DOI] [PubMed] [Google Scholar]
- Jahfari S., Waldorp L., van den Wildenberg W.P., Scholte H.S., Ridderinkhof K.R., Forstmann B.U. Effective connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. J. Neurosci. 2011;31:6891–6899. doi: 10.1523/JNEUROSCI.5253-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska A.J. Automatic inhibition and habitual control: alternative views in neuroscience research on response inhibition and inhibitory control. Front. Behav. Neurosci. 2013;7:25. doi: 10.3389/fnbeh.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Flugge M.C., Bestmann S. Time-dependent changes in human corticospinal excitability reveal value-based competition for action during decision processing. J. Neurosci. 2012;32:8373–8382. doi: 10.1523/JNEUROSCI.0270-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N., Mur M., Bandettini P. Representational similarity analysis — connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2008;2:4. doi: 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau H.C., Rogers R.D., Ramnani N., Passingham R.E. Willed action and attention to the selection of action. Neuroimage. 2004;21:1407–1415. doi: 10.1016/j.neuroimage.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Lau H., Rogers R.D., Passingham R.E. Dissociating response selection and conflict in the medial frontal surface. Neuroimage. 2006;29:446–451. doi: 10.1016/j.neuroimage.2005.07.050. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A., Verbruggen F., Logan G.D., Poldrack R.A. Inhibition-related activation in the right inferior frontal gyrus in the absence of inhibitory cues. J. Cogn. Neurosci. 2011;23:3388–3399. doi: 10.1162/jocn_a_00031. [DOI] [PubMed] [Google Scholar]
- Levy B.J., Wagner A.D. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann. N. Y. Acad. Sci. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan G.D., Cowan W.B., Davis K.A. On the ability to inhibit simple and choice reaction time responses: a model and a method. J. Exp. Psychol. Hum. Percept. Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Macoveanu J., Hornboll B., Elliott R., Erritzoe D., Paulson O.B., Siebner H., Knudsen G.M., Rowe J.B. Serotonin 2A receptors, citalopram and tryptophan-depletion: a multimodal imaging study of their interactions during response inhibition. Neuropsychopharmacology. 2013;38:996–1005. doi: 10.1038/npp.2012.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars R.B., Piekema C., Coles M.G., Hulstijn W., Toni I. On the programming and reprogramming of actions. Cereb. Cortex. 2007;17:2972–2979. doi: 10.1093/cercor/bhm022. [DOI] [PubMed] [Google Scholar]
- Mostofsky S.H., Simmonds D.J. Response inhibition and response selection: two sides of the same coin. J. Cogn. Neurosci. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Nachev P., Rees G., Parton A., Kennard C., Husain M. Volition and conflict in human medial frontal cortex. Curr. Biol. 2005;15:122–128. doi: 10.1016/j.cub.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P., Wydell H., O'Neill K., Husain M., Kennard C. The role of the pre-supplementary motor area in the control of action. Neuroimage. 2007;36(Suppl. 2):T155–T163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert F.X., Mars R.B., Buch E.R., Olivier E., Rushworth M.F. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13240–13245. doi: 10.1073/pnas.1000674107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H. Dual-task interference in simple tasks: data and theory. Psychol. Bull. 1994;116:220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- Passingham R.E., Bengtsson S.L., Lau H.C. Medial frontal cortex: from self-generated action to reflection on one's own performance. Trends Cogn. Sci. 2010;14:16–21. doi: 10.1016/j.tics.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Ullsperger M., Crone E.A., Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rieger M., Gauggel S. Inhibitory after-effects in the stop signal paradigm. Br. J. Psychol. 1999;90:509–518. [Google Scholar]
- Rowe J.B., Toni I., Josephs O., Frackowiak R.S., Passingham R.E. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Rowe J.B., Hughes L., Nimmo-Smith I. Action selection: a race model for selected and non-selected actions distinguishes the contribution of premotor and prefrontal areas. Neuroimage. 2010;51:888–896. doi: 10.1016/j.neuroimage.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth M.F. Intention, choice, and the medial frontal cortex. Ann. N. Y. Acad. Sci. 2008;1124:181–207. doi: 10.1196/annals.1440.014. [DOI] [PubMed] [Google Scholar]
- Rushworth M.F., Walton M.E., Kennerley S.W., Bannerman D.M. Action sets and decisions in the medial frontal cortex. Trends Cogn. Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Scherbaum S., Fischer R., Dshemuchadse M., Goschke T. The dynamics of cognitive control: evidence for within-trial conflict adaptation from frequency-tagged EEG. Psychophysiology. 2011;48:591–600. doi: 10.1111/j.1469-8986.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- Sharp D.J., Bonnelle V., De Boissezon X., Beckmann C.F., James S.G., Patel M.C., Mehta M.A. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth S.A., Mian M.K., Patel S.R., Asaad W.F., Williams Z.M., Dougherty D.D., Bush G., Eskandar E.N. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 2012;488:218–221. doi: 10.1038/nature11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Turkeltaub P.E., Eickhoff S.B., Laird A.R., Fox M., Wiener M., Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L., Groenewold N.A., Nolen W.A., Pijnenborg M., Aleman A. Inhibit yourself and understand the other: neural basis of distinct processes underlying Theory of Mind. Neuroimage. 2011;56:2364–2374. doi: 10.1016/j.neuroimage.2011.03.053. [DOI] [PubMed] [Google Scholar]
- Verbruggen F., Logan G.D. Response inhibition in the stop-signal paradigm. Trends Cogn. Sci. 2008;12:418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F., Logan G.D., Liefooghe B., Vandierendonck A. Short-term after effects of response inhibition: repetition priming or between-trial control adjustments? J. Exp. Psychol. Hum. Percept. Perform. 2008;34:413–426. doi: 10.1037/0096-1523.34.2.413. [DOI] [PubMed] [Google Scholar]
- Verbruggen F., Aron A.R., Stevens M.A., Chambers C.D. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13966–13971. doi: 10.1073/pnas.1001957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Logan G.D., Bissett P.G. Stopping while going! Response inhibition does not suffer dual-task interference. J. Exp. Psychol. Hum. Percept. Perform. 2012;38:123–134. doi: 10.1037/a0023918. [DOI] [PubMed] [Google Scholar]
- Zandbelt B.B., Bloemendaal M., Hoogendam J.M., Kahn R.S., Vink M. Transcranial magnetic stimulation and functional MRI reveal cortical and subcortical interactions during stop-signal response inhibition. J. Cogn. Neurosci. 2013;25:157–174. doi: 10.1162/jocn_a_00309. [DOI] [PubMed] [Google Scholar]
- Zhang J., Hughes L.E., Rowe J.B. Selection and inhibition mechanisms for human voluntary action decisions. Neuroimage. 2012;63:392–402. doi: 10.1016/j.neuroimage.2012.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1.
Supplementary material 2.