Abstract
Objective:
To assess the incidence and complications of pre-septal (pre-SC) and post-septal (post-SC) cellulitis over 10 years. Pre-SC and post-SC are also known as periorbital and orbital cellulitis, respectively.
Methods:
Retrospective analysis of CT scans. Data included the presence of pre-SC and post-SC, paranasal sinus disease (PNS) and complications.
Results:
Among 125 patients scanned for these suspected diagnoses, 67 had both pre-SC and post-SC, 37 had pre-SC and 4 had post-SC; there were 17 normal scans. 110 patients had PNS. 68/71 (96%) patients with post-SC had PNS. Post-SC complications included orbital and/or subperiosteal abscess (50/71: 30 medial orbital, 10 superomedial, 3 lateral, 2 anteromedial, 2 inferomedial, 1 superior, 1 anterosuperior and 1 not specified), cavernous sinus thrombosis (CST) (1), superior ophthalmic vein (SOV) thrombosis (4) and subdural frontal empyema (2); 1 patient had SOV and CST and subdural empyema.
Conclusion:
71/125 (57%) patients had post-SC. 50/125 (40%) patients imaged for pre-SC/post-SC had orbital abscess; 44/50 (88%) of these involved the medial orbit. Patients can develop solely superior or inferior abscesses that are difficult to identify by axial imaging alone, hence coronal reformatted imaging is essential. 5/125 (4%) patients developed major complications (SOV/CST/empyema), hence imaging review of the head and cavernous sinus region is essential. A diagnosis of post-SC on CT should alert the radiologist because this diagnosis can be associated with an increased incidence (5/71, 7%) of complications.
Advances in knowledge:
We recommend that all patients with a suspected diagnosis of post-SC should undergo CT scan (post-contrast orbits and post-contrast head, with multiplanar reformats and a careful review of the SOV and the cavernous sinus). Particular attention should be paid to exclude intracranial complications including subdural empyema and cerebral abscess. As soon as a diagnosis of post-SC is made, in addition to informing the referring clinical team, urgent opinion should be sought from ear, nose and throat (ENT), neurology and ophthalmology with a view to urgently drain of the paranasal sinuses`.
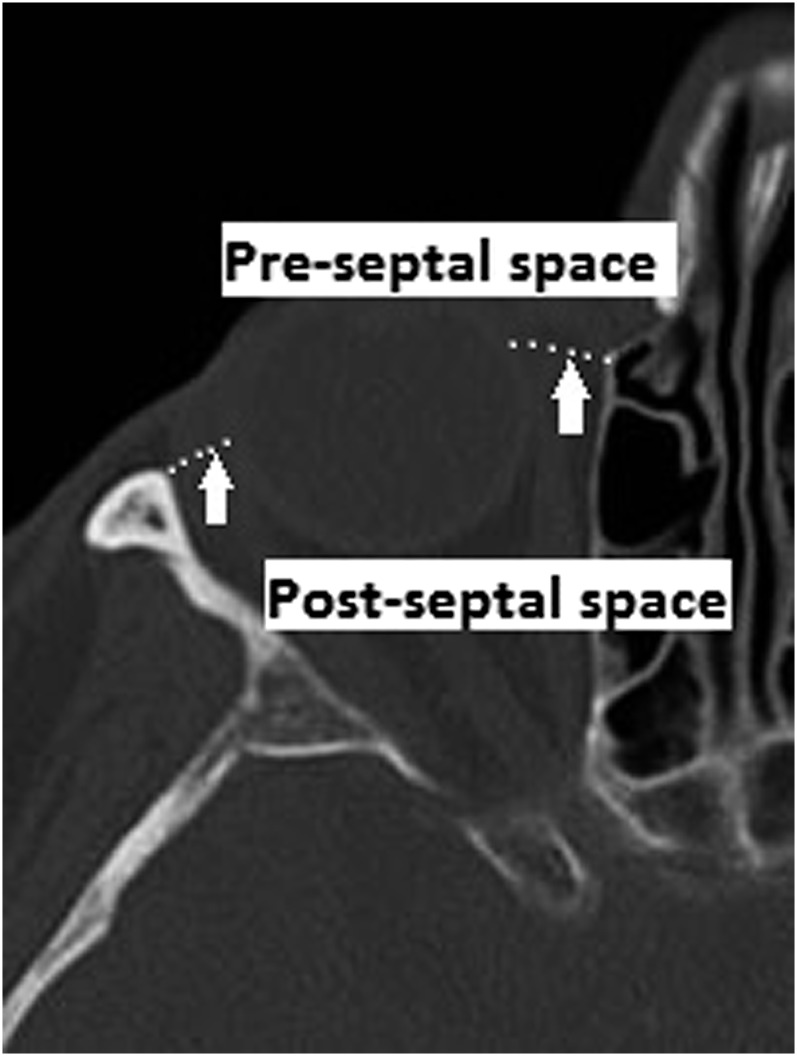
Bacterial orbital cellulitis is an uncommon condition that previously was associated with severe complications. It is classified as pre-septal cellulitis (pre-SC) and post-septal cellulitis (post-SC) based on the anatomical landmark, the orbital septum (Figure 1). Pre-SC (periorbital cellulitis) is an infection of the eyelid and surrounding skin anterior to the orbital septum and is usually caused by local trauma or arises from an infective origin of the skin and adnexae of the eyelid (Figure 2). Post-SC (orbital cellulitis) is an infection of the orbital tissues posterior to the orbital septum (Figure 3) and is usually a result of paranasal sinus disease (PNS), particularly of the ethmoidal sinus. The ethmoidal sinus is separated from the orbit by only the thin medial orbital wall (the lamina papyracea) (Figure 4).
Figure 1.
Diagram showing the position of the orbital septum (white arrows) and pre-septal and post-septal spaces.
Figure 2.
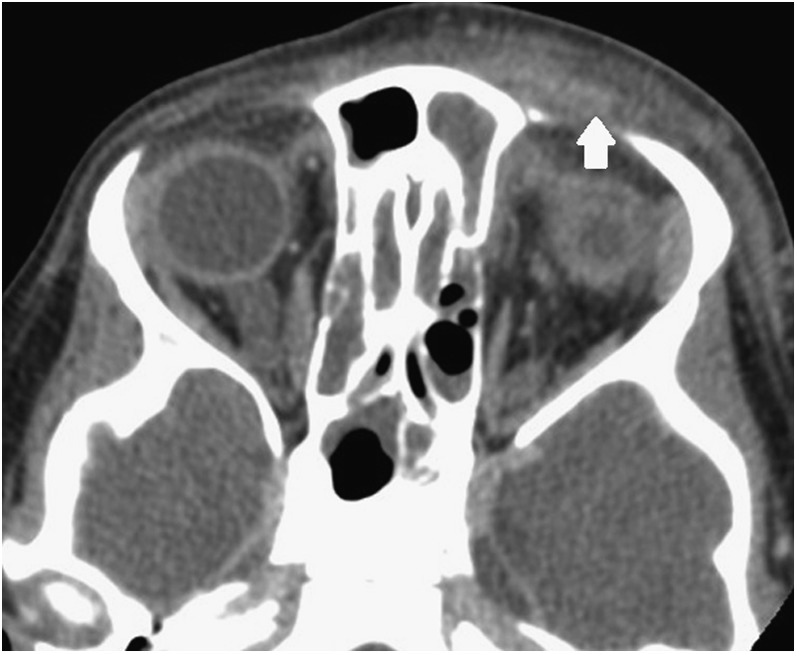
Axial CT showing left pre-septal cellulitis (white arrow).
Figure 3.
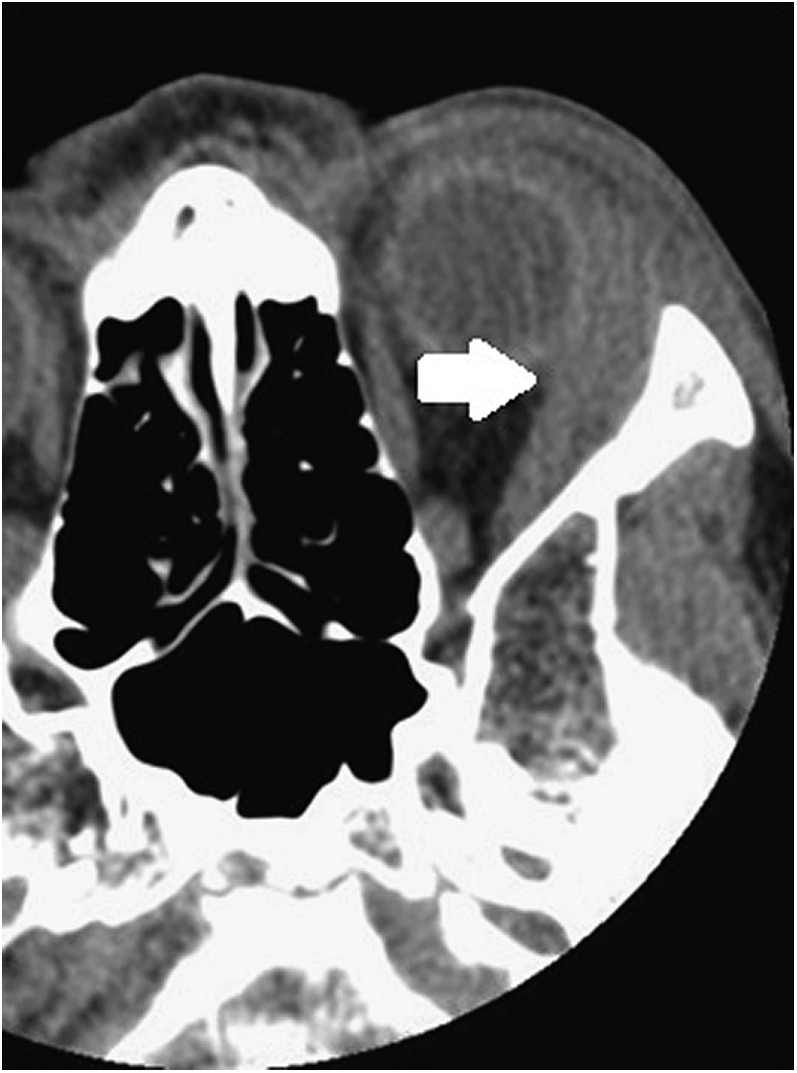
Axial CT showing left post-septal cellulitis (white arrow).
Figure 4.
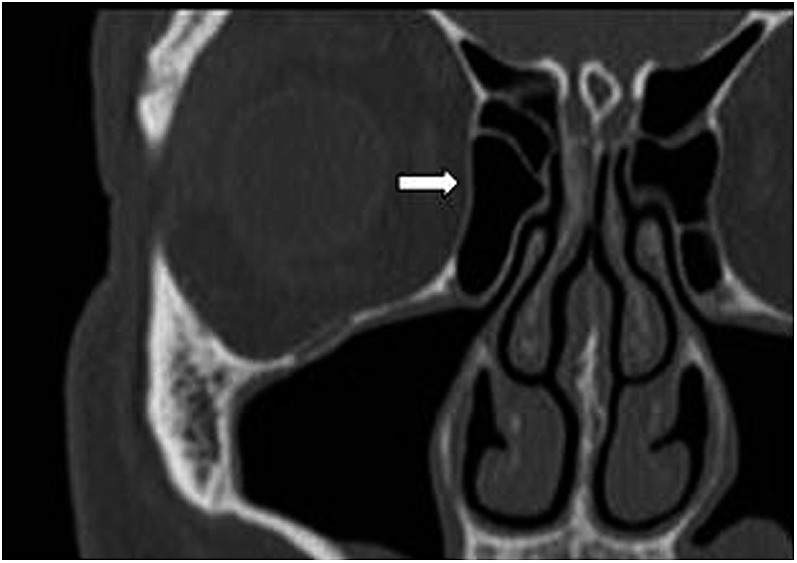
Coronal CT showing the right lamina papyracea (white arrow).
Inflammation in the post-septal space can result in high intraorbital pressure and is associated with central retinal artery/vein occlusion or with damage to the optic nerve, potentially leading to loss of vision. Differentiation between the two, prompt diagnosis and early management are essential when these conditions are suspected, to avoid potentially catastrophic sequelae including loss of vision and intracranial complications such as venous thrombosis and empyema. Blindness occurs in approximately 1% of patients with orbital cellulitis.1 Chandler et al2 have categorized orbital infection into five categories (Table 1); this classification is used as an indicator of disease severity.
Table 1.
Classification of orbital disease according to Chandler et al2
| Class | Category | Description |
|---|---|---|
| 1 | Pre-septal disease | Eyelid swelling without proptosis, ophthalmoplegia or loss of vision |
| 2 | Orbital cellulitis | Inflammation of the orbital fat, proptosis, restricted eye movements, colour desaturation |
| 3 | Subperiosteal abscess | Pus collection elevating the periosteum off the bony orbit |
| 4 | Orbital abscess | Pus collection within the orbit |
| 5 | Cavernous sinus thrombosis |
Pre-SC and post-SC can be difficult to differentiate clinically3 and, therefore, imaging is often required for confirmation of diagnosis and to evaluate for complications of post-SC.
Objectives
The aims of this study were to analyse the relative incidence of pre-SC and post-SC in the paediatric age group, the rate of complications and the use of CT in the diagnosis of this condition.
METHODS
This was a retrospective review of patients who had CT scans of their orbits for suspected pre-SC and post-SC, in a tertiary-level paediatric hospital in the UK, over a 10-year period. A record of all patients who had CT scans of their orbits was obtained from the radiology database. Only patients with suspected pre-SC and post-SC were included in the study. Patients who had scans for trauma, tumour diagnosis (or follow-up) or for any other reason were excluded. CT reports were analysed for all included patients.
Data collected included the age of patients at the time of imaging, date of scan, the presence of pre-SC and post-SC (or both), PNS, complications including orbital and/or subperiosteal abscess (including location), involvement of the globe, osteomyelitis, superior ophthalmic vein (SOV) thrombosis, cavernous sinus thrombosis (CST) and intracranial abscess/empyema. The CT protocols were analysed with regard to views obtained (axial, coronal and sagittal), the use of image reformats, the use of intravenous contrast and whether the brain was scanned.
RESULTS
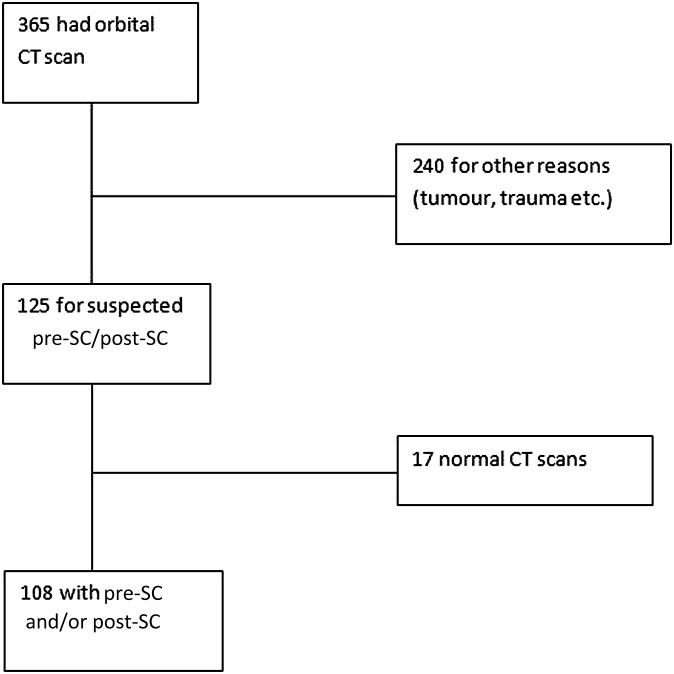
365 patients had CT scans of the orbits during the study period. Among these, 125 patients were scanned for suspected pre-SC and post-SC (Figure 5). The age of the patients ranged from 0 to 15 years (mean age, 6 years). 108/125 patients were diagnosed with pre-SC and/or post-SC.
Figure 5.
Recruitment flowchart.
Pre- and post-contrast scans were obtained in 87 patients. 32 patients had only post-contrast scans, whereas 6 patients had only pre-contrast scans. Pre-contrast axial images were obtained in 46 patients, axial images with coronal reformats in 34, and axial images with coronal and sagittal reformats in 13. The respective numbers for post-contrast views were 63, 43 and 13. CT of the brain (all post-contrast) was performed in 84 patients.
67 patients had pre-SC and post-SC. 37 had solely pre-SC and 4 had solely post-SC. 17 patients had a normal scan with neither pre-SC nor post-SC.
110/125 patients had PNS; the majority had ethmoidal sinus disease on the affected side. 50 patients had orbital and/or subperiosteal abscess. Among these, 30 were medial (Figure 6), 10 were superomedial, 3 were lateral, 2 each were anteromedial and inferomedial, and 1 each was superior (Figure 7) and anterosuperior (1 not specified).
Figure 6.
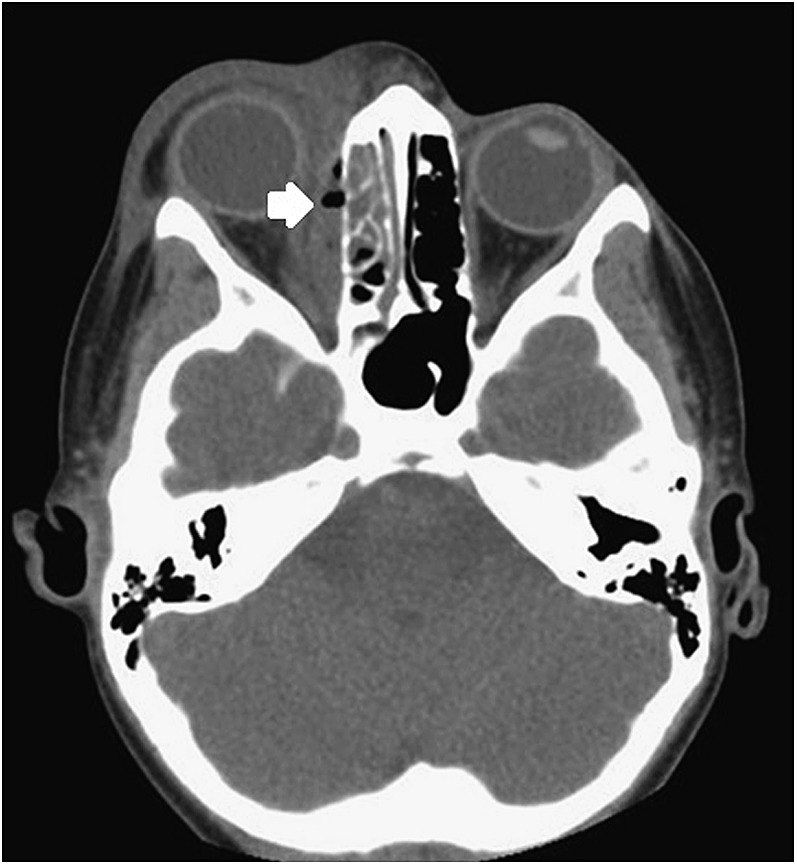
Axial CT showing a medial orbital abscess with air locules on the right (white arrow) and inflammation of the right ethmoidal sinus.
Figure 7.
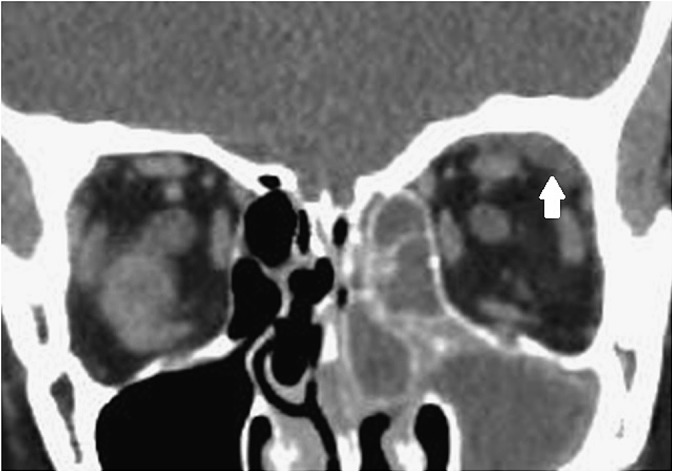
Coronal CT showing superior orbital abscess on the left (white arrow) and inflammation of the right ethmoidal and maxillary sinuses.
Four patients had SOV thrombosis, two patients had frontal subdural empyema (Figure 8) and one patient had CST (Figure 9) (this patient had SOV thrombosis and subdural empyema). No other complications were recorded in the reports during the period of this study.
Figure 8.
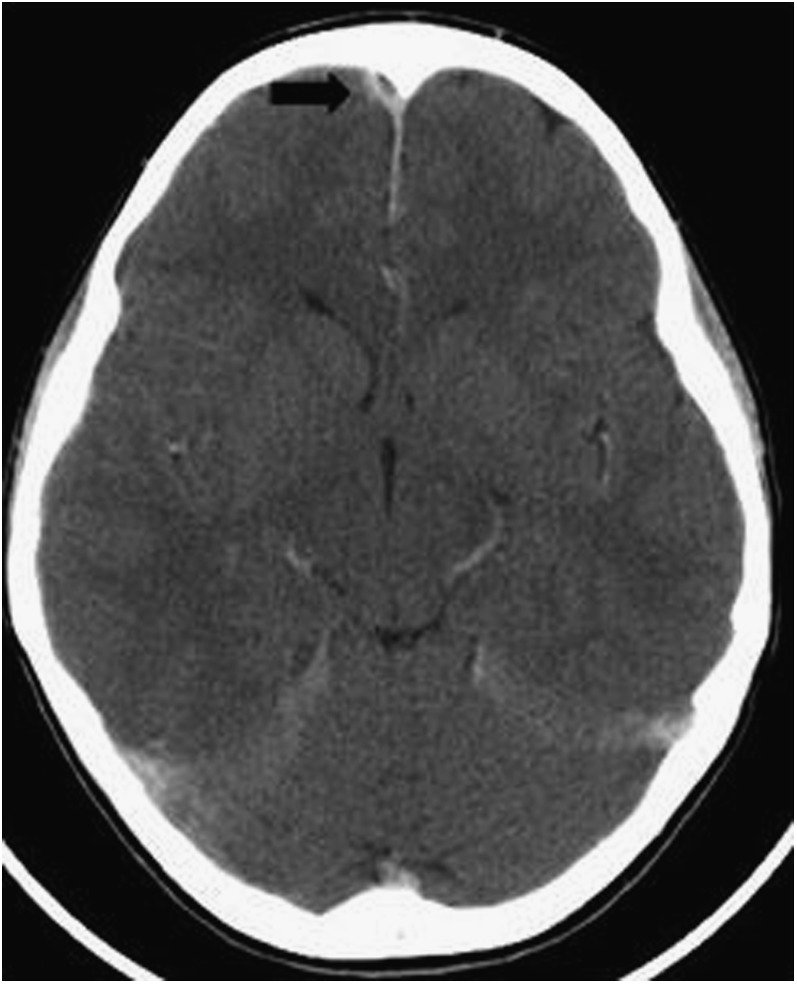
Axial post-contrast CT of the brain showing a small right frontal subdural empyema (black arrow).
Figure 9.
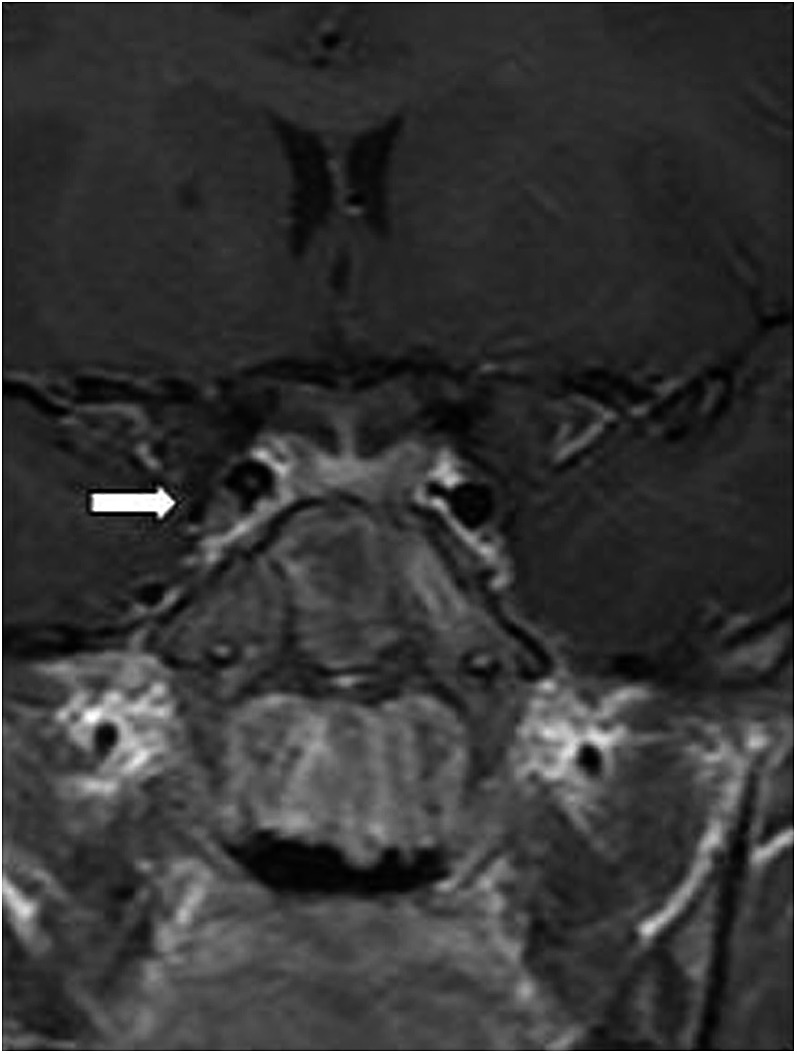
Coronal T1 weighted MRI showing a right cavernous sinus thrombosis (white arrow).
Pre-contrast studies added no extra information to the post-contrast studies. Post-contrast studies allowed easier delineation of phlegmon from intraorbital abscess. Similarly, intracranial subdural empyema was easier to visualize on post-contrast CT imaging. Post-contrast studies also added value by allowing diagnosis of the ophthalmic vein or CST that could not be appreciated with pre-contrast studies.
DISCUSSION
108 patients were diagnosed with either pre-SC or post-SC during our 10-year study period. In a similar review over 4 years, Ferguson and McNab4 identified 52 patients with post-SC with the highest incidence in the paediatric age group (<16 years). No major complications (e.g. intracranial spread or visual problems) were recorded in the paediatric population in their study.
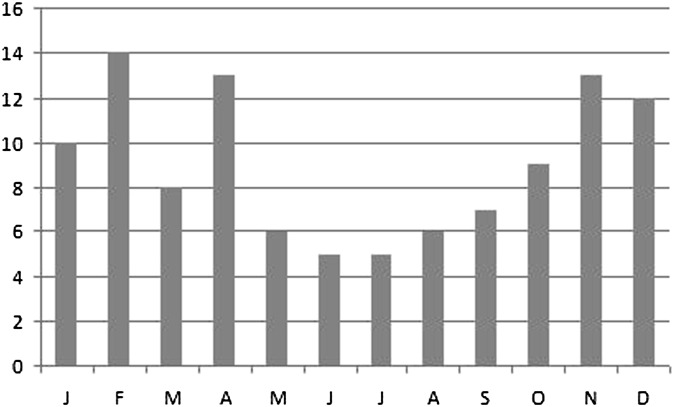
This study confirms the strong association of PNS with post-SC. 68/71 (96%) patients with post-SC had PNS. This is the most likely explanation for the bimodal seasonal distribution of cases reported by Ferguson and McNab, with peaks occurring in late summer and late winter/early spring. In our population, we noted peak incidence during winter and spring (Figure 10).
Figure 10.
Seasonal variation in the number of cases. J, January; F, February; M, March; A, April; M, May; first J, June; second J, July; A, August; S, September; O, October; N, November; D, December.
50 (46%) patients had orbital or subperiosteal abscess. There was a predisposition for the medial orbit (44 among the 50 were medial, anteromedial, superomedial or inferomedial). This is because of the close proximity of the ethmoidal sinuses to the medial wall of the orbit with only the thin lamina papyracea separating the two. However, coronal imaging is essential, as a small proportion of patients have collections localized to the superior or inferior orbit only, which could be difficult to identify on axial imaging alone. Multiplanar imaging in our study became easier with the installation of a multislice CT scanner roughly half way through the study recruitment period.
5 (5%) patients in our study developed major complications. One patient developed CST, SOV thrombosis and subdural empyema. Three patients had SOV thrombosis, and one patient had an isolated subdural empyema. All these patients had post-SC, making the complication rate for patients with post-SC 5/71 (7%). Therefore, head CT is recommended in all patients with post-SC, with careful evaluation of the SOV and cavernous sinus. The SOV communicates directly with the cavernous sinus (there are no valves between the two structures) and this facilitates the direct extension of thrombus. Despite modern antibiotic and anticoagulation therapy CST remains a condition with significant morbidity and potential mortality.
We observed variation in scanning protocols among radiologists. Some did not routinely do pre-contrast views. There was limited use of reformatted images during the early years, whereas in the latter years, most radiologists performed reformats in at least two planes, namely axial and coronal. We did not see any benefit of routine pre-contrast CT, i.e all pathology seen on pre-contrast views was observed on post-contrast views, and hence pre-contrast CT seems to be redundant. Discarding pre-contrast views also has the added advantage of dose reduction in the paediatric population, a population that is more susceptible to the harmful effects of radiation. Post-contrast imaging allowed easier identification of not only intraorbital abscess and subdural empyema but also of SOV and CST that were difficult to identify on pre-contrast scans.
Howe and Jones5 recommend a CT scan in selected patients: if full evaluation of the eye is not possible because of gross oedema, gross proptosis, ophthalmoplegia, deteriorating visual acuity or colour vision, bilateral periorbital oedema or central symptoms or signs. On the other hand, Givner6 proposed that if bedside examination cannot comfortably rule out orbital cellulitis (or if orbital cellulitis is suspected), an orbital CT scan with contrast should be obtained. We would agree with the latter imaging policy.
Rudloe et al7 have identified predictors (proptosis and/or pain or limitation of extraocular movements) for intraorbital or intracranial abscess among children who present with signs or symptoms of periorbital infection. Other features (high neutrophil count, the absence of infectious conjunctivitis, periorbital oedema, age greater than 3 years and previous antibiotic therapy) can identify patients who do not have such predictors but do have significant risk of disease. These predictors could be used to better target patients for CT.
According to a study by Kapur et al,8 MRI should be considered for cases in which there is clinical or CT-based suspicion for intracranial complications (e.g. subdural empyema or CST). This is supported by our study in which intracranial complications were confirmed using MRI.
In another article,9 the same team noted that diffusion-weighted imaging (DWI) improved diagnostic confidence in nearly all cases of orbital abscess when used in conjunction with contrast-enhanced imaging. DWI also confirmed abscess in a majority of cases without contrast-enhanced imaging, which may be of particular use when contrast material is contraindicated; this, however, is slightly irrelevant in a paediatric population where the main contraindications to contrast would be allergy/anaphylaxis rather than nephropathy. We would recommend DWI of the head and orbit with post-contrast imaging of the head (T1 weighted) and orbit (T1 with fat saturation) in patients being investigated for post-SC and its complications.
Baring and Hilmi3 suggested that all patients with suspected pre-SC/post-SC should be commenced on broad-spectrum intravenous antibiotics and nasal decongestants. We advise that an urgent ear, nose and throat (ENT) opinion should be obtained with a view to endoscopic drainage of the affected sinuses. Patients should be assessed for visual symptoms, including visual acuity and eye movements, and also for neurological symptoms that might indicate intracranial spread, and an urgent ophthalmological and neurosurgical opinion should be sought as required.
CT with contrast remains the optimal imaging study for orbital inflammation. We have shown that coronal imaging of the orbit is essential, particularly as in a small proportion of children post-SC collections are localized to the superior or inferior orbit. Post-contrast imaging of the whole head is also essential in children with post-SC because, in a small proportion of children, subdural empyemas may occur. In a child with orbital cellulitis, prompt diagnosis and treatment is essential in obtaining the best outcome.
RECOMMENDATIONS
(1) All patients with a suspected diagnosis of post-SC should undergo CT scan. We recommend post-contrast orbit scans and post-contrast head scans, with multiplanar reformats and a careful review of the SOV and the cavernous sinus. Particular attention should also be paid to exclude intracranial complications, including subdural empyema and cerebral abscess. (2) If a diagnosis of SOV thrombosis is made, the patient should proceed to MRI to evaluate for CST. If there is any concern about intracranial spread of infection, MRI should be performed to include DWI and post-gadolinium T1 imaging. (3) As soon as a diagnosis of post-SC is made, in addition to informing the referring clinical team, urgent opinion should be sought from the ENT team with a view to draining the sinuses. Neurology and ophthalmology opinions should also be sought early. The management of this condition requires a multidisciplinary approach, in which the radiologist plays a vital role, to ensure the best outcome for the patient.
REFERENCES
- 1.Fisher RG, Boyce TG. Moffet's pediatric infectious diseases: a problem-oriented approach. Philadelphia, PA: Lippincott Williams & Wilkins; 2005, p.102. [Google Scholar]
- 2.Chandler JR, Langenbrunner DJ, Stevens ER. The pathogenesis of orbital complications in acute sinusitis. Laryngoscope 1970; 80: 1414-28. 10.1288/00005537-197009000-00007 [DOI] [PubMed] [Google Scholar]
- 3.Baring DE, Hilmi OJ. An evidence based review of periorbital cellulitis. Clin Otolaryngol 2011; 36: 57-64. 10.1111/j.1749-4486.2011.02258.x [DOI] [PubMed] [Google Scholar]
- 4.Ferguson MP, McNab AA. Current treatment and outcome in orbital cellulitis. Aust N Z J Ophthalmol 1999; 27: 375-9. [DOI] [PubMed] [Google Scholar]
- 5.Howe L, Jones NS. Guidelines for the management of periorbital cellulitis/abscess. Clin Otolaryngol Allied Sci 2004; 29: 725-8. 10.1111/j.1365-2273.2004.00889.x [DOI] [PubMed] [Google Scholar]
- 6.Givner LB. Periorbital versus orbital cellulitis. Pediatr Infect Dis J 2002; 21: 1157-8. 10.1097/01.inf.0000041790.79459.80 [DOI] [PubMed] [Google Scholar]
- 7.Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics 2010; 125: e719-26. 10.1542/peds.2009-1709 [DOI] [PubMed] [Google Scholar]
- 8.Kapur R, Sepahdari AR, Mafee MF, Putterman AM, Aakalu V, Wendel LJ, et al. MR imaging of orbital inflammatory syndrome, orbital cellulitis, and orbital lymphoid lesions: the role of diffusion-weighted imaging. AJNR Am J Neuroradiol 2009; 30: 64-70. 10.3174/ajnr.A1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sepahdari AR, Aakalu VK, Kapur R, Michals EA, Saran N, French A, et al. MRI of orbital cellulitis and orbital abscess: the role of diffusion-weighted imaging. AJR Am J Roentgenol 2009; 193: W244-50. 10.2214/AJR.08.1838 [DOI] [PubMed] [Google Scholar]