Abstract
Objective:
This study retrospectively evaluated the efficacy and toxicity of particle therapy using carbon ions or protons for primary sacral chordomas.
Methods:
We evaluated 23 patients with primary sacral chordoma treated with carbon ion therapy (CIT) or proton therapy (PT) between July 2005 and June 2011 at the Hyogo Ion Beam Medical Center, Hyogo, Japan. The median patient age was 72 years. 14 patients were treated with 70.4 Gy equivalents (GyE) in 16 fractions and 9 were treated with 70.4 GyE in 32 fractions. CIT was used for 16 patients, and PT was used for 7 patients.
Results:
The median follow-up period was 38 months. At 3 years, local control (LC), overall survival (OS) and progression-free survival (PFS) for all patients were 94%, 83% and 68%, respectively. The log-rank test revealed that male sex was significantly related to better PFS ( p = 0.029). No other factors, including dose fractionation and ion type, were significant for LC, OS or PFS. In nine patients, ≥Grade 3 acute dermatitis was observed, and ≥Grade 3 late toxicities were observed in nine patients. The 32-fraction protocol reduced severe toxicities in both the acute and late phases compared with the 16-fraction protocol.
Conclusion:
Particle therapy for patients with sacral chordoma showed favourable LC and OS. Severe toxicities were successfully reduced by modifying the dose fractionation and treatment planning in the later treatment era. Thus, this therapeutic modality should be considered useful and safe.
Advances in knowledge:
This is the first study including both CIT and PT for sacral chordomas.
Chordomas are rare bone tumours that arise from remnants of the notochord.1–5 They constitute 1–4% of all primary malignant bone tumours, and sacral chordomas account for >50% of all chordomas.1,2,6 Chordomas grow slowly1–3 and show fewer metastases than other bone and soft-tissue tumours,4,7–10 and mortality is almost invariably because of local disease progression.4,9 These tumours are difficult to treat because they tend to progress extensively to anatomical locations in proximity to the sacral nerve plexus, cauda equina and bowel and because the sacrum is important for the patient's mobility. The long symptom duration prior to diagnosis, the delay in diagnosis and the large tumour volume may contribute to the relatively poor prognosis of these tumours.6 The conventional treatment for chordomas includes surgical resection with adequate margins or complete radical resection, which achieves longer local control (LC), survival and disease-free status than subtotal resection.4,6,8,11–19 However, complete resection is rarely possible because tumours grow to large sizes before symptoms appear.4,7,20 Thus, regardless of the low-grade malignancy, LC is poor8,10,13,17 and chordomas show low sensitivity to chemotherapy1,2,4,6,13,16,17,21 and conventional photon radiotherapy.4,6,10,19
Particle therapy, such as carbon ion therapy (CIT) and proton therapy (PT), can deliver high-dose radiation to tumours while minimizing the dose delivered to organs at risk because of its precise dose distribution compared with conventional photon therapy.22–32 Particle beams possess a physical characteristic called the Bragg peak, which emits a relatively low dose near the body surface and releases the maximum energy just before it stops in the depth. Recently, several investigators have reported on the efficacy of CIT for sacral chordomas, describing high LC rates and low toxicities.7,16,20 Some reports demonstrating the usefulness of PT for sacral chordomas have also been published.10,33–35
The Hyogo Ion Beam Medical Center (HIBMC) is the world's first institution capable of applying both CIT and PT, and we have treated sacral chordoma patients with both types of ion beam therapy for years. In this study, we retrospectively evaluated the efficacy and toxicity of particle therapy using carbon ions or protons for primary sacral chordomas at HIBMC.
METHODS AND MATERIALS
Patients
The patient eligibility criteria were as follows: (1) histologically confirmed primary sacral chordoma without metastases; (2) no previous radiotherapy; (3) Eastern Cooperative Oncology Group performance status ≤3; (4) adequate organ function (such as heart, lung, liver and kidney functions good enough for radiotherapy); (5) no active concomitant malignancy; and (6) written informed consent. A total of 23 eligible patients with sacral chordomas were enrolled in this study between July 2005 and June 2011. The patient characteristics are summarised in Table 1. 17 patients (71%) with tumours adjacent to the intestines (usually <1 cm) underwent surgical spacer placement prior to particle therapy to generate a sufficient distance between the tumour and intestines. The details of surgical spacer placement have been previously published.36–38
Table 1.
Patient characteristics
| Characteristic | n = 23 |
|---|---|
| Age (years), median (range) | 72 (35–84) |
| Sex | |
| Male | 15 (65%) |
| Female | 8 (35%) |
| ECOG PS | |
| 0 | 2 (9%) |
| 1 | 17 (74%) |
| 2 | 3 (13%) |
| 3 | 1 (4%) |
| Tumour volume (ml), median (range) | 264 (94–1037) |
| Spacer placement | |
| (+) | 17 (74%) |
| (−) | 6 (26%) |
| Dose fractionation | |
| 70.4 GyE/16 Fr | 14 (61%) |
| Carbon ion/proton | 12/2 |
| 70.4 GyE/32 Fr | 9 (39%) |
| Carbon ion/proton | 4/5 |
| Ion type | |
| Carbon ion | 16 (70%) |
| Proton | 7 (30%) |
ECOG, Eastern Cooperative Oncology Group; GyE, Gy equivalents; PS, performance status.
Treatment
Ion beam treatment plans were developed using a CT-based three-dimensional treatment planning system —FOCUS-M [Computerized Medical Systems Inc. (CMS), St Louis, MO, and Mitsubishi Electric Corporation, Tokyo, Japan] until April 2008 and Xio-M (CMS and Mitsubishi Electric Corporation) from May 2008. Each patient was immobilized with a custom-made thermoplastic cast in the prone position, and CT (with 2-mm slice thickness) and MRI (5-mm slice thickness) were performed. The gross tumour volume (GTV) was defined as the primary tumour volume determined by CT-MRI fusion images. The clinical target volume (CTV) comprised the addition of a 5-mm basic margin to the GTV. The planning target volume was defined as the CTV plus a set-up margin (5 mm) and an internal margin (1 mm) under the respiratory gating system for all patients. We took the CT images at inhalation and exhalation phases for every patient, and we found the amount of respiratory motion was <1 mm in all directions. We, however, added a respiratory gating margin (internal margin) of 1 mm to all patients for the safety.
During the early treatment era, we used a protocol of 70.4 Gy equivalents (GyE)* in 16 fractions (4.4 GyE per fraction) imported from an experienced institution, the National Institute of Radiological Sciences (Chiba, Japan).7 In the later treatment era, we employed a protocol of 70.4 GyE in 32 fractions (2.2 GyE per fraction), which is less toxic to normal tissues, although 70.4 GyE in 16 fractions was also used for cases meeting the dose constraints for organs at risk (OARs), as described below. The dose constraints for the small bowel, large bowel, rectum and spinal cord (cauda equina was not included) consisted of Dmax (maximum dose) ≤ 43 GyE, Dmax ≤ 47 GyE, (volume receiving ≥ 35 GyE) ≤ semiperimeter and Dmax ≤ 42 GyE, respectively, for the 16-fraction protocol and of Dmax ≤ 52 GyE, Dmax ≤ 57 GyE [V65 (volume receiving ≥ 65 GyE) ≤ 17% and V40 ≤ 35%] and Dmax ≤ 48 GyE, respectively, for the 32-fraction protocol. For the skin dose, few data were available for the early treatment era, although Dmax ≤ 56 GyE was set for the 16-fraction protocol during the later treatment era, with no specific constraint for the 32-fraction protocol. The policy for selecting the beam type was based on the dose distribution. A better beam type for each patient was selected after discussion by several radiation oncologists. A representative case is shown in Figure 1.
Figure 1.
Comparison of the carbon ion (a) and proton (b) treatment plans for a patient with sacral chordoma who underwent surgical spacer placement prior to particle therapy. The solid and dashed curves represent the carbon ion plan and proton plan, respectively, in the dose-volume histogram (DVH) (c). Proton treatment was selected in this patient in terms of the skin dose.
The patients were treated with carbon ion or proton beams. A respiratory gating system was used to irradiate the beam during the exhalation phase. The patient set-up was performed daily by subtracting the two sets of orthogonal digital radiographs before irradiation. The translation and rotation of the patient detected by the positioning system were compensated for by adjusting the treatment couch. The set-up was continued until the bony landmarks on the digitally reconstructed radiographs corresponded to within 1 mm.
Statistical analysis
After particle therapy, the patients were observed at 3-month intervals during the first to third year and at 6-month intervals during the fourth year and thereafter. Regular follow-up studies included a physical examination, diagnostic imaging (CT and/or MRI) and blood tests. Local recurrence was defined as the radiographical enlargement of the primary tumour. Toxicities were evaluated using the Common Terminology Criteria for Adverse Events v. 3.0.39 Biologically effective dose at α/β = 10 GyE (BED10) and 3 GyE (BED3) of the maximum skin dose (MSD) for each patient were calculated using a linear quadratic model40 to compare skin toxicities of the 16- and 32-fraction protocols in the acute and late phases, respectively. The BEDn is given by the following formula: BEDn (GyEn) = [total dose (GyE)] × {[1 + daily dose (GyE)]/n (GyE)}.
The follow-up time was calculated from the initial date of radiotherapy. Continuous variables are presented as medians and ranges, and categorical variables are presented as frequencies and proportions. Comparisons of the follow-up period and the BED of the MSD between the 16- and 32-fraction protocols were made using the Mann–Whitney U-test. The LC, overall survival (OS) and progression-free survival (PFS) curves were estimated using the Kaplan–Meier method and were compared using the log-rank test. All p-values are two-sided, and a p < 0.05 was considered statistically significant. Statistical analyses were performed using PASW® Statistics 18 (IBM Corporation, Armonk, NY).
RESULTS
Local control and survival
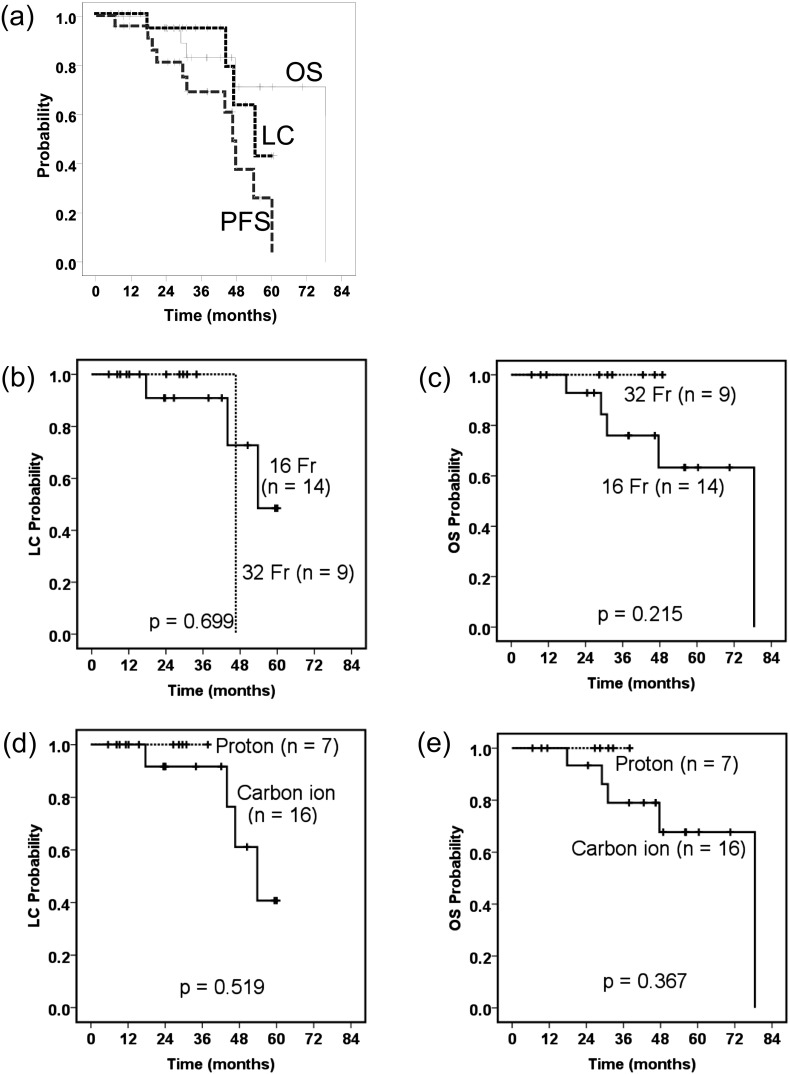
The median follow-up period was 38 months (range, 7–78 months) for all patients. There was a significant difference in the duration of follow-up period between the 16-fraction protocol (median, 42 months; range, 17–78 months) and the 32-fraction protocol (median, 31 months; range, 7–49 months) (p = 0.044). LC, OS and PFS for all patients were 94% [95% confidence interval (CI): 83–100%], 83% (95% CI: 66–100%) and 68% (95% CI: 47–90%) at 3 years, respectively (Figure 2).
Figure 2.
Local control (LC), overall survival (OS) and progression-free survival were determined using the Kaplan–Meier method for all patients (a). Comparisons were made between the 16-fraction cases and the 32-fraction cases in terms of LC (b) and OS (c), and comparisons were made between the carbon ion cases and the proton cases in terms of LC (d) and OS (e).
Of the 23 patients studied, 4 (17%) presented local recurrence. One patient experienced recurrence within the GTV (18 months after particle therapy) and three others developed marginal recurrence (44, 47 and 54 months). Six patients (26%) experienced distant metastases. Tumour dissemination occurred in three patients (13%), and metastases to the lung, bone and pelvic lymph node occurred in one patient (4%).
The log-rank test revealed that male sex was associated with significantly better PFS (p = 0.029), and smaller tumour volume (<400 ml) had a tendency to be related to better OS (p = 0.052) (Table 2). No other factors, including dose fractionation and ion type, were significantly associated with LC, OS or PFS.
Table 2.
Log-rank test
| Variable | Group (n) |
p-value |
||
|---|---|---|---|---|
| LC | OS | PFS | ||
| Age (years) | <70 (10) | 0.898 | 0.781 | 0.668 |
| ≥70 (13) | ||||
| Sex | Male (15) | 0.544 | 0.486 | 0.029 |
| Female (8) | ||||
| ECOG PS | 0–1 (19) | 0.162 | 0.133 | 0.482 |
| 2–3 (4) | ||||
| Tumour volume | <400 ml (15) | 0.272 | 0.052 | 0.541 |
| ≥400 ml (8) | ||||
| Spacer placement | (+) (17) | 0.384 | 0.781 | 0.245 |
| (−) (6) | ||||
| Dose fractionation | 70.4 GyE/16 Fr (14) | 0.699 | 0.215 | 0.607 |
| 70.4 GyE/32 Fr (9) | ||||
| Ion type | Carbon ion (16) | 0.519 | 0.367 | 0.614 |
| Proton (7) | ||||
ECOG, Eastern Cooperative Oncology Group; F, fractions; GyE, Gy equivalents; LC, local control; OS, overall survival; PFS, progression-free survival; PS, performance status.
Toxicity
With respect to Grade 3 or greater acute reactions, Grades 3 and 4 dermatitis were observed in 8 (35%) patients and 1 (4%) patient, respectively. However, all patients completed their planned radiotherapy and recovered from their reactions. With respect to late toxicities, Grade 3 or greater events were observed in nine patients (39%). The most frequent event was dermatitis [Grade 4 in five patients (22%)], followed by neuropathies, including motor disorder, sensory disorder/pain and urinary retention [Grade 3 in four patients (17%)]. Two patients' symptoms were complicated by Grade 4 dermatitis and Grade 3 myositis. The acute and late toxicities of treatments are summarised in Table 3, particularly with respect to the comparison between the 2 treatment protocols; most of the events (8/9 for acute; 9/11 for late) occurred in patients treated with 70.4 GyE in 16 fractions. The analysis of the skin dose showed that the medians of the MSDs in patients treated with the 16-fraction protocol were 98.9 GyE10 (range, 60.8–106.5 GyE10) and 169.4 GyE3 (range, 104.2–182.4 GyE3), whereas the medians of the MSDs in patients treated with the 32-fraction protocol were 77.3 GyE10 (range, 60.1–85.9 GyE10) and 109.8 GyE3 (range, 85.4–122 GyE3). Statistically significant differences were found between the 16- and 32-fraction protocols in both BED10 (p = 0.003) and BED3 (p < 0.001). Acute Grade 3 and Grade 4 dermatitis occurred in patients with MSDs of 71–106.5 (median, 98.9) GyE10 (eight patients) and 101.4 GyE10 (one patient), respectively. Late Grade 4 dermatitis occurred in patients with MSDs of 165–182.4 (median, 173.7) GyE3 (five patients). For Grade 4 dermatitis patients, wound care for skin ulcers (e.g. debridement, skin flap plastic surgery, wound bed preparation, moist wound healing and hyperbaric oxygen therapy) was required.
Table 3.
Adverse events (≥Grade 3; Common Terminology Criteria for Adverse Events. v. 3.0)
| Toxicities of treatments | Total (n = 23) | 70.4 GyE/16 Fr (n = 14) | 70.4 GyE/32 Fr (n = 9) |
|---|---|---|---|
| Acute dermatitis | |||
| > Grade 3 | 8 (35%) | 7 (50%) | 1 (11%) |
| Grade 4 | 1 (4%) | 1 (7%) | – |
| Late dermatitis | |||
| Grade 4 | 5 (22%)a | 5 (36%)a | – |
| Late myositis | |||
| Grade 3 | 2 (9%)a | 2 (14%)a | – |
| Late neuropathyb | |||
| Grade 3 | 4 (17%) | 2 (14%) | 2 (22%) |
GyE, Gy equivalents.
Two patients had Grade 4 dermatitis and Grade 3 myositis.
Neuropathy includes motor disorder, sensory disorder/pain, and urinary retention.
DISCUSSION
In this retrospective study, particle therapy using carbon ions or protons for sacral chordomas showed favourable LC and OS, although the follow-up periods were relatively short. Although definitive surgery is still widely accepted as the standard care,6,12,13,18 several reports on high-technology radiotherapy such as particle therapy, including CIT7,16,20,41 and PT,10,33–35 and intensity-modulated radiotherapy (IMRT)15 have been published recently. Table 4 outlines a comparison of treatment modalities based on the available literature. Because all of these studies were retrospective and patient characteristics (such as tumour size and post-operative status) vary, a precise comparison of these studies would be difficult. However, treatment protocols involving CIT or PT seemed to attain better outcomes than other protocols. Nishida et al16 reported a comparison between surgery and CIT with respect to efficacy and toxicity at their institutions, and the results demonstrated that CIT provided a high LC rate and preservation of urinary–anorectal function compared with surgery. Zabel-du Bois et al15 proposed that particle therapy may improve the long-term LC and OS rates compared with photon radiotherapy, based on their IMRT experience. Moreover, particle therapy could be beneficial in secondary cancer risk compared with IMRT. Yoon et al42 reported that secondary cancer risk in PT was significantly lower than in IMRT because the average secondary doses were generally lower for PT than for IMRT.
Table 4.
Comparison with other studies
| Study | Year | No. patients | Treatment | Median f/u | LC | OS |
|---|---|---|---|---|---|---|
| Fuchs et al6 | 2005 | 52 | Surgery (±post-operative photon) | 94 months | 59% (5 years)a | 74% (5 years) |
| Park et al33 | 2006 | 27 | Proton/photon (±prior surgery) | 78/59 monthsb | 72% (5 years) | 83% (5 years) |
| Ruggieri et al18 | 2010 | 56 | Surgery | 114 months | 65% (5 years) | 97% (5 years) |
| Zabel-du Bois15 | 2010 | 34 | IMRT (±prior surgery) | 54 months | 27% (5 years) | 70% (5 years) |
| Imai et al20 | 2011 | 95 | Carbon ion | 42 months | 88% (5 years) | 86% (5 years) |
| Staab et al10 | 2011 | 40c | Proton (+prior surgery) | 43 months | 62% (5 years) | 80% (5 years) |
| Present study | 2013 | 23 | Carbon ion or proton | 38 months | 94% (3 years) | 83% (3 years) |
f/u, follow-up; IMRT, intensity-modulated radiotherapy; LC, local control; OS, overall survival.
Recurrence-free rate.
Median f/u periods of primary and recurrent chordoma patients were 78 and 59 months, respectively.
Extracranial chordomas, including 11 sacral chordomas.
Initially, we employed 70.4 GyE in 16 fractions (4.4 GyE per fraction), and little attention was paid to the skin dose; as a consequence, we observed severe acute and late skin morbidities in some patients. Because sacral chordomas usually grow just beneath the skin, it is difficult to reduce the skin dose while maintaining the coverage for the target volume. Therefore, we subsequently employed 70.4 GyE in 32 fractions (2.2 GyE per fraction), which is close to conventional fractionation, to mainly reduce the occurrence of late toxicity. As shown in Table 3, we successfully reduced severe toxicities in both the acute and late phases, with the exception of late neuropathy, although there was a significant difference in the duration of the follow-up period between the 16- and 32-fraction protocols. With respect to the skin dose, the 32-fraction protocol significantly reduced the MSD in both BED10 and BED3 compared with the 16-fraction protocol. The dose constraints for the peripheral nerve remain poorly defined. DeLaney et al34 reported that three sacral chordoma patients treated with proton/photon therapy consisting of 77.4 GyE (1.8 GyE per fraction) developed neural injuries (sacral neuropathy in two patients and erectile dysfunction in one patient), while no neural injuries occurred at doses ≤70.2 GyE. Imai et al20 reported that a length of >10 cm and a total dose of >70 GyE (4.4–4.6 GyE per fraction) may represent thresholds for sciatic nerve injury. The same group (Nishida et al16) reported that CIT (range, 54.0–73.6 GyE; median, 70.4 GyE) did not result in late neurological toxicity. Forming a conclusion from these results is difficult because of differences in fraction size, but the fact that even 70.4 GyE in 32 fractions caused neuropathy in the present study represents a new finding and provides useful information for other particle facilities. With respect to antitumour effects, 70.4 GyE in 32 fractions (86 GyE10) may be suboptimal as Park et al33 recommended 77.4 GyE in 43 fractions (91 GyE10) for unresected chordomas (70.4 GyE in 16 fractions is 101 GyE10). However, there was no significant difference between the 16- and 32-fraction protocols in terms of LC, OS or PFS in the present study (Figure 2 and Table 2), although there was a significant difference in the duration of the follow-up period between the 16- and 32-fraction protocols, as mentioned previously. Therefore, we used 70.4 GyE in 32 fractions as a standard. However, greater patient accumulation and longer follow-up periods are warranted in future studies.
Univariate analysis using the log-rank test revealed that male chordoma patients had significantly better PFS (p = 0.029; Table 2). Thieblemont et al21 also reported that PFS, but not OS, was significantly longer in male than in female chordoma patients. In another study by Staab et al,10 male patients showed significantly better OS than female patients in a series of extracranial chordoma cases. As discussed in a review article by Halperin,43 several hypotheses have been proposed to explain the possible influence of sex on the treatment outcome for chordomas. For example, sex hormone receptors may represent an influential factor in adults with chordomas, and genetic factors may also play a role in the clinical outcome. Smaller tumour volumes (<400 ml) showed a tendency for better OS, although this difference did not reach statistical significance (p = 0.052; Table 2), and other reports have also found a relationship between tumour size and clinical outcome.6,10,35 In this study, ion type did not significantly affect LC, OS or PFS (Figure 2 and Table 2). In particular, the finding that the LC of PT was comparable to that of CIT might be surprising for some people because the carbon ion beam has a higher relative biological effectiveness and should be more effective for those chordomas considered to be radioresistant. Clearly, it is difficult to make accurate conclusions from these limited data, but the comparison of CIT and PT is of great interest in the particle therapy field. Therefore, we plan to recruit and observe patients for this type of future study.
The use of spacer placement was demonstrated in this study. Although most patients (17 patients, 74%) required surgical spacer placement before particle therapy because of the location of the tumour in relation to the intestines, gastrointestinal toxicities were not observed. Moreover, appreciable complications after spacer placement did not occur, and we plan to analyse the usefulness of spacer placement in another article.
This study had certain limitations. First, this study is retrospective, and the statistical power is low. However, other published studies have also been retrospective, and performing a prospective study is difficult because of the rarity of this disease. Second, the number of patients was small (23 patients), which was also because of the rarity of the disease, and only a few reports6,18,20 have included >50 patients. Third, the follow-up period was relatively short (median, 38 months). Chordomas are slow-growing tumours, and local recurrence can be observed even 5 years after particle therapy.10,20,33 Regarding the second and third issues, we aim to continue recruiting and observing patients and to report these follow-up data. Fourth, no conclusions about the clinical merit of PT or CIT can be drawn from this study. Dosimetric comparisons with IMRT would be required; however, it is impossible for us because IMRT is not available at our facilities.
CONCLUSIONS
Particle therapy using carbon ions or protons for patients with sacral chordoma showed favourable LC and OS. Although severe dermatitis was observed in the earlier treatment era, we successfully reduced these severe toxicities by modifying the dose fractionation and treatment planning in the later treatment era. Thus, this therapeutic modality should be considered useful and safe. Moreover, univariate analysis using the log-rank test revealed that male sex was significantly associated with better PFS in this retrospective study. However, the recruitment of greater numbers of patients with a longer follow-up period is warranted.
Footnotes
The biological effects of both CIT and PT at HIBMC were evaluated in vitro and in vivo. The relative biological effectiveness (RBE) value for carbon ion ranged between 2 and 3.7 (depending on the depth of the spread-out Bragg peaks), and the RBE for proton irradiation was 1.1. Because all tissues are assumed to have almost the same RBE for carbon ions or protons, doses expressed in GyE are directly comparable to photon doses.
REFERENCES
- 1.Sundaresan N. Chordomas. Clin Orthop Relat Res 1986; 204: 135-42. [PubMed] [Google Scholar]
- 2.Sundaresan N, Galicich JH, Chu FC, Huvos AG. Spinal chordomas. J Neurosurg 1979; 50: 312-19. 10.3171/jns.1979.50.3.0312 [DOI] [PubMed] [Google Scholar]
- 3.Mindell ER. Chordoma. J Bone Joint Surg Am 1981; 63: 501-5. [PubMed] [Google Scholar]
- 4.Catton C, O'Sullivan B, Bell R, Laperriere N, Cummings B, Fornasier V, et al. Chordoma: long-term follow-up after radical photon irradiation. Radiother Oncol 1996; 41: 67-72. [DOI] [PubMed] [Google Scholar]
- 5.Cummings BJ, Hodson DI, Bush RS. Chordoma: the results of megavoltage radiation therapy. Int J Radiat Oncol Biol Phys 1983; 9: 633-42. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH. Operative management of sacral chordoma. J Bone Joint Surg Am 2005; 87: 2211-16. 10.2106/JBJS.D.02693 [DOI] [PubMed] [Google Scholar]
- 7.Imai R, Kamada T, Tsuji H, Yanagi T, Baba M, Miyamoto T, et al. Carbon ion radiotherapy for unresectable sacral chordomas. Clin Cancer Res 2004; 10: 5741-6. 10.1158/1078-0432.CCR-04-0301 [DOI] [PubMed] [Google Scholar]
- 8.Cheng EY, Ozerdemoglu RA, Transfeldt EE, Thompson RCJr. Lumbosacral chordoma. Prognostic factors and treatment. Spine (Phila Pa 1976) 1999; 24: 1639-45. [DOI] [PubMed] [Google Scholar]
- 9.Schoenthaler R, Castro JR, Petti PL, Baken-Brown K, Phillips TL. Charged particle irradiation of sacral chordomas. Int J Radiat Oncol Biol Phys 1993; 26: 291-8. [DOI] [PubMed] [Google Scholar]
- 10.Staab A, Rutz HP, Ares C, Timmermann B, Schneider R, Bolsi A, et al. Spot-scanning-based proton therapy for extracranial chordoma. Int J Radiat Oncol Biol Phys 2011; 81: e489-96. 10.1016/j.ijrobp.2011.02.018 [DOI] [PubMed] [Google Scholar]
- 11.York JE, Kaczaraj A, Abi-Said D, Fuller GN, Skibber JM, Janjan NA, et al. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery 1999; 44: 74-9. [DOI] [PubMed] [Google Scholar]
- 12.Ozaki T, Hillmann A, Winkelmann W. Surgical treatment of sacrococcygeal chordoma. J Surg Oncol 1997; 64: 274-9. [DOI] [PubMed] [Google Scholar]
- 13.Yonemoto T, Tatezaki S, Takenouchi T, Ishii T, Satoh T, Moriya H. The surgical management of sacrococcygeal chordoma. Cancer 1999; 85: 878-83. [PubMed] [Google Scholar]
- 14.Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer 2000; 88: 2122-34. [DOI] [PubMed] [Google Scholar]
- 15.Zabel-du Bois A, Nikoghosyan A, Schwahofer A, Huber P, Schlegel W, Debus J, et al. Intensity modulated radiotherapy in the management of sacral chordoma in primary versus recurrent disease. Radiother Oncol 2010; 97: 408-12. 10.1016/j.radonc.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 16.Nishida Y, Kamada T, Imai R, Tsukushi S, Yamada Y, Sugiura H, et al. Clinical outcome of sacral chordoma with carbon ion radiotherapy compared with surgery. Int J Radiat Oncol Biol Phys 2011; 79: 110-16. 10.1016/j.ijrobp.2009.10.051 [DOI] [PubMed] [Google Scholar]
- 17.Prabhakaran PS, Misra S, Kannan V, Chandrashekar M, Vijayakumar M, Veerendrakumar KV, et al. Sacral chordomas: a 10-year study. Australas Radiol 1998; 42: 42-6. [DOI] [PubMed] [Google Scholar]
- 18.Ruggieri P, Angelini A, Ussia G, Montalti M, Mercuri M. Surgical margins and local control in resection of sacral chordomas. Clin Orthop Relat Res 2010; 468: 2939-47. 10.1007/s11999-010-1472-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moojen WA, Vleggeert-Lankamp CL, Krol AD, Dijkstra SP. Long-term results: adjuvant radiotherapy in en bloc resection of sacrococcygeal chordoma is advisable. Spine (Phila Pa 1976) 2011; 36: E656-61. 10.1097/BRS.0b013e3181f8d1f3 [DOI] [PubMed] [Google Scholar]
- 20.Imai R, Kamada T, Sugahara S, Tsuji H, Tsujii H. Carbon ion radiotherapy for sacral chordoma. Br J Radiol 2011; 84: S48-54. 10.1259/bjr/13783281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thieblemont C, Biron P, Rocher F, Bouhour D, Bobin JY, Gerard JP, et al. Prognostic factors in chordoma: role of postoperative radiotherapy. Eur J Cancer 1995; 31A: 2255-9. [DOI] [PubMed] [Google Scholar]
- 22.Kagawa K, Murakami M, Hishikawa Y, Abe M, Akagi T, Yanou T, et al. Preclinical biological assessment of proton and carbon ion beams at Hyogo Ion Beam Medical Center. Int J Radiat Oncol Biol Phys 2002; 54: 928-38. [DOI] [PubMed] [Google Scholar]
- 23.Demizu Y, Kagawa K, Ejima Y, Nishimura H, Sasaki R, Soejima T, et al. Cell biological basis for combination radiotherapy using heavy-ion beams and high-energy X-rays. Radiother Oncol 2004; 71: 207-11. 10.1016/j.radonc.2004.03.008 [DOI] [PubMed] [Google Scholar]
- 24.Hishikawa Y, Oda Y, Mayahara H, Kawaguchi A, Kagawa K, Murakami M, et al. Status of the clinical work at Hyogo. Radiother Oncol 2004; 73(Suppl. 2): S38-40. [DOI] [PubMed] [Google Scholar]
- 25.Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol 2007; 25: 953-64. 10.1200/JCO.2006.09.7816 [DOI] [PubMed] [Google Scholar]
- 26.Nishimura H, Ogino T, Kawashima M, Nihei K, Arahira S, Onozawa M, et al. Proton-beam therapy for olfactory neuroblastoma. Int J Radiat Oncol Biol Phys 2007; 68: 758-62. 10.1016/j.ijrobp.2006.12.071 [DOI] [PubMed] [Google Scholar]
- 27.Mayahara H, Murakami M, Kagawa K, Kawaguchi A, Oda Y, Miyawaki D, et al. Acute morbidity of proton therapy for prostate cancer: the Hyogo Ion Beam Medical Center experience. Int J Radiat Oncol Biol Phys 2007; 69: 434-43. 10.1016/j.ijrobp.2007.03.009 [DOI] [PubMed] [Google Scholar]
- 28.Demizu Y, Murakami M, Miyawaki D, Niwa Y, Akagi T, Sasaki R, et al. Analysis of vision loss caused by radiation-induced optic neuropathy after particle therapy for head-and-neck and skull-base tumors adjacent to optic nerves. Int J Radiat Oncol Biol Phys 2009; 75: 1487-92. [DOI] [PubMed] [Google Scholar]
- 29.Murakami M, Hishikawa Y. Current status and future of particle radiotherapy at the Hyogo Ion Beam Medical Center. [In Japanese.] Gan To Kagaku Ryoho 2009; 36: 1791-4. [PubMed] [Google Scholar]
- 30.Miyawaki D, Murakami M, Demizu Y, Sasaki R, Niwa Y, Terashima K, et al. Brain injury after proton therapy or carbon ion therapy for head-and-neck cancer and skull base tumors. Int J Radiat Oncol Biol Phys 2009; 75: 378-84. 10.1016/j.ijrobp.2008.12.092 [DOI] [PubMed] [Google Scholar]
- 31.Terashima K, Demizu Y, Hashimoto N, Jin D, Mima M, Fujii O, et al. A phase I/II study of gemcitabine-concurrent proton radiotherapy for locally advanced pancreatic cancer without distant metastasis. Radiother Oncol 2012; 103: 25-31. 10.1016/j.radonc.2011.12.029 [DOI] [PubMed] [Google Scholar]
- 32.Iwata H, Murakami M, Demizu Y, Miyawaki D, Terashima K, Niwa Y, et al. High-dose proton therapy and carbon-ion therapy for stage I nonsmall cell lung cancer. Cancer 2010; 116: 2476-85. 10.1002/cncr.24998 [DOI] [PubMed] [Google Scholar]
- 33.Park L, Delaney TF, Liebsch NJ, Hornicek FJ, Goldberg S, Mankin H, et al. Sacral chordomas: impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int J Radiat Oncol Biol Phys 2006; 65: 1514-21. 10.1016/j.ijrobp.2006.02.059 [DOI] [PubMed] [Google Scholar]
- 34.DeLaney TF, Liebsch NJ, Pedlow FX, Adams J, Dean S, Yeap BY, et al. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys 2009; 74: 732-9. 10.1016/j.ijrobp.2008.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutz HP, Weber DC, Sugahara S, Timmermann B, Lomax AJ, Bolsi A, et al. Extracranial chordoma: outcome in patients treated with function-preserving surgery followed by spot-scanning proton beam irradiation. Int J Radiat Oncol Biol Phys 2007; 67: 512-20. 10.1016/j.ijrobp.2006.08.052 [DOI] [PubMed] [Google Scholar]
- 36.Fukumoto T, Komatsu S, Hori Y, Murakami M, Hishikawa Y, Ku Y. Particle beam radiotherapy with a surgical spacer placement for advanced abdominal leiomyosarcoma results in a significant clinical benefit. J Surg Oncol 2010; 101: 97-9. 10.1002/jso.21417 [DOI] [PubMed] [Google Scholar]
- 37.Komatsu S, Hori Y, Fukumoto T, Murakami M, Hishikawa Y, Ku Y. Surgical spacer placement and proton radiotherapy for unresectable hepatocellular carcinoma. World J Gastroenterol 2010; 16: 1800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi M, Fukumoto T, Kusunoki N, Tsuchida S, Kido M, Takebe A, et al. Particle beam radiotherapy with a surgical spacer placement for unresectable sacral chordoma. [In Japanese.]. Gan To Kagaku Ryoho 2010; 37: 2804-6. [PubMed] [Google Scholar]
- 39.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003; 13: 176-81. 10.1016/S1053-4296(03)00031-6 [DOI] [PubMed] [Google Scholar]
- 40.Thames HD, Bentzen SM, Turesson I, Overgaard M, Van den Bogaert W. Time-dose factors in radiotherapy: a review of the human data. Radiother Oncol 1990; 19: 219-35. [DOI] [PubMed] [Google Scholar]
- 41.Kamada T, Tsujii H, Tsuji H, Yanagi T, Mizoe JE, Miyamoto T, et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol 2002; 20: 4466-71. [DOI] [PubMed] [Google Scholar]
- 42.Yoon M, Ahn SH, Kim J, Shin DH, Park SY, Lee SB, et al. Radiation-induced cancers from modern radiotherapy techniques: intensity-modulated radiotherapy versus proton therapy. Int J Radiat Oncol Biol Phys 2010; 77: 1477-85. 10.1016/j.ijrobp.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 43.Halperin EC. Why is female sex an independent predictor of shortened overall survival after proton/photon radiation therapy for skull base chordomas? Int J Radiat Oncol Biol Phys 1997; 38: 225-30. [DOI] [PubMed] [Google Scholar]