Summary
Inhibitory microcircuits are wired with a precision that underlies their complex regulatory roles in neural information processing. In the spinal cord, one specialized class of GABAergic interneurons (GABApre) mediates presynaptic inhibitory control of sensory-motor synapses. The synaptic targeting of these GABAergic neurons exhibits an absolute dependence on proprioceptive sensory terminals, yet the molecular underpinnings of this specialized axoaxonic organization remain unclear. Here, we show that sensory expression of an NB2 (Contactin5)/Caspr4 coreceptor complex, together with spinal interneuron expression of NrCAM/CHL1, directs the high-density accumulation of GABAergic boutons on sensory terminals. Moreover, genetic elimination of NB2 results in a disproportionate stripping of inhibitory boutons from high-density GABApre-sensory synapses, suggesting that the preterminal axons of GABApre neurons compete for access to individual sensory terminals. Our findings define a recognition complex that contributes to the assembly and organization of a specialized GABAergic microcircuit.
Highlights
-
•
Sensory Ig/Caspr4 complex directs inhibitory synapse formation in mouse spinal cord
-
•
Eliminating NB2 results in a reduced number of GABApre-sensory synapses
-
•
Quantitative modeling suggests competition for formation of axoaxonic synapses
-
•
Role for a contactin/Caspr complex in central synapse formation
The mechanisms that confer synaptic specificity in mammalian circuits remain unclear. Ashrafi et al. describe a selective role for contactin-Caspr recognition in directing the formation of a presynaptic inhibitory microcircuit in the developing mouse spinal cord.
Introduction
In many regions of the mammalian CNS, inhibitory microcircuits are wired with high precision, fine-tuning synaptic input and modulating neural output (Stepanyants et al., 2004). The assembly of functional inhibitory microcircuits can be considered in several independent steps: the selection of membrane subdomains on specific neuronal targets, the assignment of appropriate synaptic innervation densities, and the regulation of transmitter phenotype and level (Williams et al., 2010). How these diverse cellular processes are orchestrated at individual synapses within defined CNS microcircuits remains unclear.
One informative instance of the subcellular targeting of inhibitory synapses is found in primary sensory systems, where sensory terminals serve both as presynaptic structures that innervate recipient CNS neurons and as the postsynaptic target of local inhibitory interneurons at axoaxonic synapses (Rudomin, 2009). Such axoaxonic arrangements provide an anatomical substrate for selective filtering of sensory information (Rudomin and Schmidt, 1999). In the ventral spinal cord, the central terminals of proprioceptive sensory neurons are studded with numerous synaptic boutons that derive from a discrete set of GABAergic inhibitory interneurons, termed GABApre neurons (Betley et al., 2009, Hughes et al., 2005). This set of spinal inhibitory interneurons can be distinguished by expression of the GABA synthetic enzyme glutamic acid decarboxylase-2 (GAD2/GAD65) (Betley et al., 2009, Hughes et al., 2005), an essential determinant of sustained GABA release (Tian et al., 1999). High-level expression of GAD65 in GABApre neurons is directed by a sensory source of brain-derived neurotrophic factor (BDNF) (Betley et al., 2009). Moreover, sensory terminals in the ventral spinal cord represent the sole target of GABApre neurons (Betley et al., 2009), implying stringent recognition specificity in the assembly and organization of this specialized inhibitory microcircuit.
The molecular mediators of stringent axoaxonic specificity have remained unclear, however. In this study, we used mouse molecular genetic approaches to show that the sensory expression of the immunoglobulin (Ig) superfamily protein NB2 (Contactin5) and the contactin-associated protein Caspr4 are required to establish high-density studding of GABApre boutons on proprioceptive sensory terminals. In a complementary manner, two members of the L1 Ig family, CHL1 and NrCAM, are expressed by GABApre neurons and their function is required for the formation of high-density GABApre synapses with sensory terminals. Our findings pinpoint a molecular recognition system that helps to direct the formation of presynaptic inhibitory synapses.
Results
NB2 Expression by Proprioceptive Sensory Neurons
To define potential GABApre recognition molecules expressed by sensory neurons we screened 45 transcripts encoding Ig domain-containing proteins for expression in dorsal root ganglia (DRG) and spinal cord at postnatal days (p)5 to p6—the period at which GABApre axons form contacts with proprioceptive sensory terminals (Table S1 available online) (Betley et al., 2009). To explore the idea that incoming GABApre axons recognize receptors on sensory but not motor neurons, we focused our attention on transcripts expressed selectively by proprioceptive sensory neurons.
This expression screen identified four transcripts, Contactin (Cntn) 3, Cntn5, Kirrel, and Kirrel-3, each of which was expressed by DRG neurons but not motor neurons. Only two of these, Kirrel-3 and Cntn5 were expressed by proprioceptors, as revealed by coexpression of Parvalbumin (Pv) (Table S1) (Arber et al., 2000). Analysis of Kirrel-3 mutant mice (Prince et al., 2013) did not reveal a GABApre targeting phenotype (unpublished observations), leading us to focus on the potential role of the contactin family (Shimoda and Watanabe, 2009).
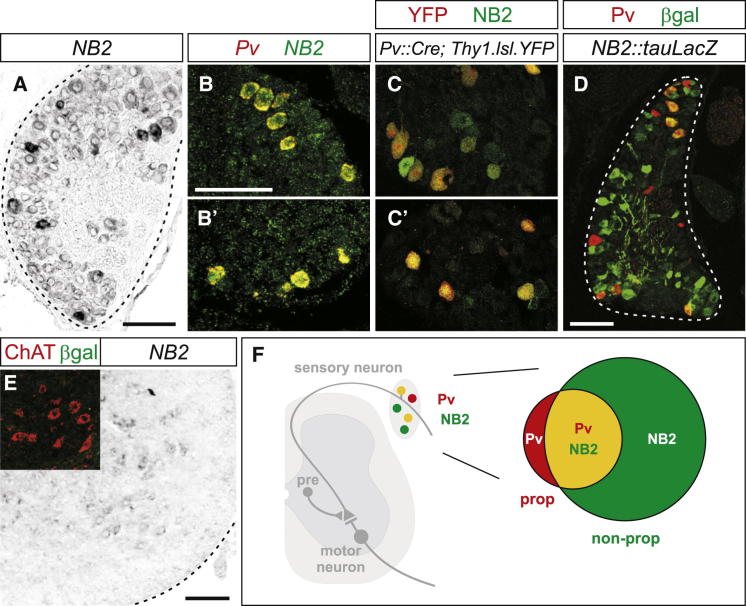
We found that five of the six contactins, Cntn1, TAG-1 (Cntn2), BIG-1 (Cntn3), BIG-2 (Cntn4), and NB2 (Cntn5) (Furley et al., 1990, Gennarini et al., 1989, Ogawa et al., 1996, Yoshihara et al., 1994, Yoshihara et al., 1995) were expressed by DRG neurons (Figure 1A; Table S1). Of these, Cntn1, TAG-1, and BIG-2, were also expressed by motor neurons and, based on our design constraints were therefore excluded from further analysis (Table S1). We failed to detect overlap in BIG-1 and Pv expression (data not shown), whereas Pv exhibited extensive overlap with NB2 transcript and protein (Figures 1B–1C′). In addition, analysis of βgal expression in NB2::tauLacZ mice (Li et al., 2003) revealed overlap in βgal expression and Pv-positive (PvON) proprioceptors, as well as expression in a subset of Pv-negative (PvOFF) sensory neurons (Figures 1D and 1F). In spinal cord, we found that neither endogenous NB2, nor βgal were expressed by motor neurons, marked by choline acetyltransferase (ChAT) expression (Figure 1E; for full spinal cord views and NB2 probe specificity see Figures S1A–S1D). These data establish that NB2 is expressed by proprioceptive sensory but not motor neurons.
Figure 1.
NB2 Expression in Sensory Neurons
(A) NB2 transcript expression in p6 DRG neurons. Scale bar represents 100 μm.
(B and B′) PvON (red) proprioceptive sensory neurons express NB2 (green) in p6 DRG neurons. Scale bar represents 100 μm.
(C and C′) YFPON (red) proprioceptive sensory neurons in p6 Pv::Cre; Thy1.lsl.YFP mice express NB2 (green). In Pv::Cre; Thy1.lsl.YFP mice, YFP is expressed in PvON proprioceptors (Hippenmeyer et al., 2005).
(D) Approximately 70% of PvON (red) proprioceptive sensory neurons express βgal (green) in p7 NB2::tauLacZ mice (n = 3 L4 DRG, three mice). Scale bar represents 100 μm.
(E) In situ hybridization against NB2 reveals no expression in motor neurons. In NB2::tauLacZ mice, ChATON (red) motor neurons in the ventral spinal cord do not express βgal (green; shown in inset). Scale bar represents 100 μm.
(F) In the mouse spinal cord, PvON proprioceptive sensory neurons form monosynaptic contacts onto motor neurons. GABApre interneurons modulate sensory input onto motor neurons through presynaptic inhibition of sensory afferent terminals. NB2 is expressed in a subset of proprioceptive (prop, PvON) and nonproprioceptive (nonprop, PvOFF) sensory neurons. Venn diagram shows fraction of PvON/NB2ON neurons as a function of PvON/NB2OFF (prop) and PvOFF/NB2ON (nonprop) cells.
Sensory NB2 Expression Ensures a High Density of GABApre Bouton Contacts
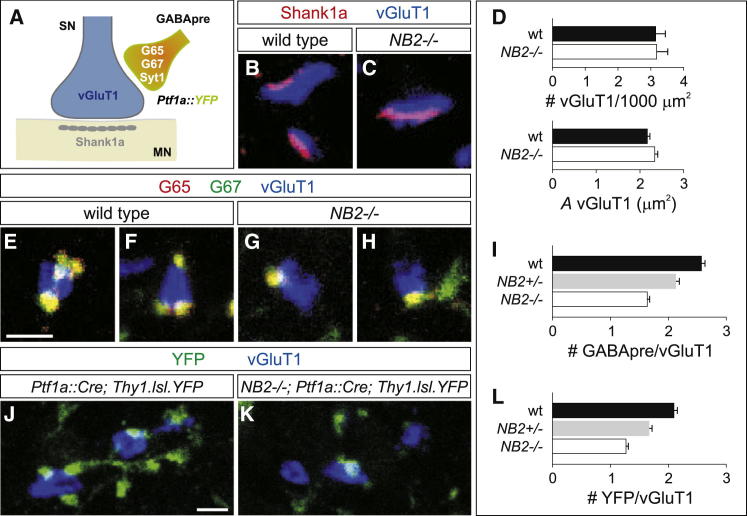
To test the involvement of NB2 in the formation of GABApre-sensory contacts, we assessed synaptic organization in NB2 heterozygous and homozygous null mutant mice (Li et al., 2003). NB2 mutants survive, breed normally, and did not exhibit obvious locomotor abnormalities (Li et al., 2003). The number of PvON proprioceptive neurons in lumbar DRG was similar in wild-type and NB2 mutant mice analyzed at p7 (Figures S2A and S2B; data not shown). Moreover, density of proprioceptive sensory axons and the number and size of vGluT1ON proprioceptive sensory terminals in the ventrolateral spinal cord, as well as their alignment with postsynaptic Shank1a protein expression was similar in p21 wild-type and NB2 null mice (Figures 2A–2D; data not shown) (Betley et al., 2009). Thus, the differentiation of sensory-motor synapses appears unaffected by the loss of NB2 function.
Figure 2.
NB2 Promotes High-Density GABApre Bouton Contacts
(A) Sensory neuron (SN) terminals express vGluT1 and are apposed to Shank1a on motor neurons (MN). GABApre boutons express GAD65 (G65), GAD67 (G67) and Syt1, and can be genetically labeled using mice in which Ptf1a::Cre drives expression of Thy1.lsl.YFP (abbreviated as Ptf1a::YFP).
(B and C) vGluT1ON (blue) sensory terminals are apposed to Shank1a (red) in the ventral spinal cord of p21 wild-type (B) and NB2 mutant mice (C).
(D) Similar number of vGluT1ON sensory terminals per 1,000 μm2 area in the ventral spinal cord of p21 NB2 mutants as compared to wild-type mice (wild-type: 3.14 ± 0.29, three mice; NB2−/−: 3.17 ± 0.34, three mice; t test, p = 0.95). Also, the size (A) of vGluT1ON sensory terminals in wild-type and NB2 mutant mice does not change (wild-type: 2.17 ± 0.06 μm2, n = 327 terminals, three mice; NB2−/−: 2.34 ± 0.07 μm2, n = 320 terminals, three mice; t test, p = 0.06).
(E–H) Fewer G65ON (red)/G67ON (green) GABApre boutons contact vGluT1ON (blue) sensory afferent terminals in p21 NB2 mutants (G and H) as compared to wild-type mice (E and F). Scale bar represents 2 μm.
(I) Compiled average number of G65ON/G67ON and Syt1ON/G67ON GABApre boutons on vGluT1ON sensory terminals is reduced by 17% and 36% in NB2 heterozygous and homozygous mutant mice respectively (wild-type: 2.57 ± 0.06, n = 769 boutons, four mice; NB2+/−: 2.13 ± 0.06, n = 796 boutons, four mice; NB2−/−: 1.64 ± 0.04, n = 1193 boutons, six mice; ANOVA, p < 0.0001).
(J and K) YFPON (green) GABApre terminals on vGluT1ON (blue) sensory afferent terminals in Ptf1a::Cre; Thy1.lsl.YFP (J) and NB2−/−; Ptf1a::Cre; Thy1.lsl.YFP mice (K). In Ptf1a::Cre; Thy1.lsl.YFP mice, YFP is expressed in GABApre interneurons (Betley et al., 2009). Scale bar represents 2 μm.
(L) Twenty-one percent and 40% fewer YFPON terminals form in NB2+/−; Ptf1a::Cre; Thy1.lsl.YFP and NB2−/−; Ptf1a::Cre; Thy1.lsl.YFP mice, respectively (wild-type: 2.10 ± 0.06, n = 840 boutons, three mice; NB2+/−: 1.67 ± 0.06, n = 886 boutons, three mice; NB2−/−: 1.27 ± 0.04, n = 1,161 boutons, four mice; ANOVA, p < 0.0001).
All data reported as mean ± SEM. See also Figure S2.
We next examined whether NB2 expression is required for the organization of GABApre boutons on sensory terminals. For this analysis, we monitored the expression of two selective GABApre bouton markers, the GABA-synthetic enzyme GAD65, and the vesicle-associated protein Synaptotagmin1 (Syt1), both in the context of expression of the general GABAergic inhibitory marker GAD67 (Figure 2A) (Betley et al., 2009). Thus, the coincident expression of GAD65 or Syt1 with GAD67 provides a secure molecular definition of GABApre boutons.
In the ventral spinal cord of p21 NB2 mutant mice we detected a 36% reduction in the number of GAD65ON/GAD67ON boutons in contact with vGluT1ON sensory terminals (ANOVA, p < 0.0001) (Figures 2E–2I) and a 37% reduction in the number of sensory-associated Syt1ON/GAD67ON boutons (ANOVA, p < 0.0001) (Figure 2I). We did not observe an increase in the number of sensory terminal-associated GAD67ON boutons that expressed either GAD65, or Syt1 alone, indicating there is a coordinate loss of these two defining GABApre bouton markers (data not shown). In addition, in NB2 heterozygous mice we detected a 17% decrease in the number of GAD65ON/GAD67ON and Syt1ON/GAD67ON sensory-associated boutons (ANOVA, p < 0.0001) (Figure 2I), implying a dosage-dependence on NB2 expression level. Thus, sensory neuron expression of NB2 is required for the expression of defining GABApre bouton markers.
We next examined whether the coordinate loss of GABApre synaptic markers actually signifies the absence of GABApre boutons themselves. To assess this issue, we took advantage of the fact that GABApre neurons can be marked by lineage tracing on the basis of the Ptf1a transcriptional character of their progenitors (Betley et al., 2009, Glasgow et al., 2005). Ptf1a::Cre; Thy1.lsl.YFP-directed fluorescent protein (YFP) (Buffelli et al., 2003, Kawaguchi et al., 2002) was expressed in ∼70% of GAD65ON GABApre terminals and was largely excluded from GABApost terminals that form contacts on motor neurons (Betley et al., 2009). The detection of some YFPOFF/GAD65ON GABApre boutons is likely a consequence of the mosaic nature of reporter expression driven by the Thy1.lsl.YFP allele (Betley et al., 2009). In mice marked by Ptf1a::Cre; Thy1.lsl.YFP expression, we found that the number of YFPON GABApre boutons in contact with vGluT1ON sensory terminals was reduced by 40% in NB2 null mutants and by 21% in heterozygous NB2 mice (ANOVA, p < 0.0001) (Figures 2J–2L). Thus, the absence of GAD65 and Syt1 in NB2 mutant mice appears to reflect the loss of GABApre boutons from sensory terminals and not simply the absence of marker expression.
We also determined whether the reduction in GABApre bouton density on sensory terminals in NB2 mutants is accompanied by the appearance of ectopic contacts on nonsensory targets. The synaptic localization of GAD65 is dependent on local sensory terminal-derived BDNF signaling (Betley et al., 2009), leading us to monitor the impact of NB2 inactivation on the expression of the other defining GABApre marker, Syt1, in YFPON boutons. In p21 wild-type mice, we found that 91% of YFPON/Syt1ON boutons were associated with vGluT1ON sensory terminals. We detected a similarly high incidence of YFPON/Syt1ON boutons associated with sensory terminals in NB2 mutants (data not shown). We suspect that the few YFPON/Syt1ON processes that are separated from vGluT1ON sensory terminals reflect a degree of vesicle accumulation in interterminal axonal domains. Together, these data support the idea that the loss of GABApre boutons from sensory terminals is not accompanied by the appearance of additional GABApre synapses with other neuronal targets, suggesting that sensory NB2 acts to promote the early elaboration of presynaptic boutons.
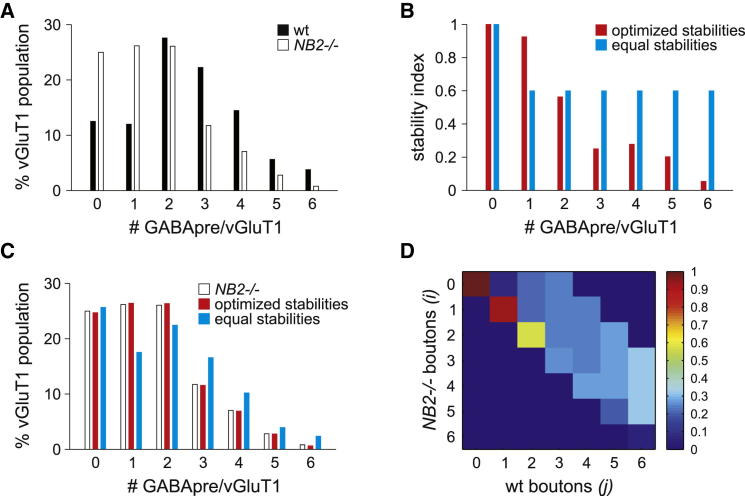
We next considered whether the decrease in GABApre bouton packing density is spread evenly over the entire population of proprioceptive terminals, or reflects a preferential depletion from a smaller subset. Strikingly, in wild-type mice, the number of GABApre boutons in contact with individual sensory terminals varied from zero to ten, with a mean density of approximately three boutons/sensory terminal (Figure 3A) (Betley et al., 2009). In NB2 mutants, we observed a clear reduction in the incidence of sensory terminals that possessed three or more GABApre boutons and in addition observed a doubling in the number of sensory terminals that lacked any associated GABApre boutons (Figure 3A). These observations suggest that inhibitory boutons are lost from sensory terminals that receive inputs across the spectrum of GABApre bouton packing densities.
Figure 3.
Enhanced Impact of NB2 Loss on High-Density GABApre-Sensory Synapses
(A) Wild-type (black) and NB2 mutant (white) GABApre bouton density frequency histograms. Bars represent the percent of vGluT1ON sensory afferent terminals that receive zero to six GABApre boutons in wild-type and NB2 mutant mice.
(B) Probabilities of GABApre bouton maintenance in the NB2 mutant using a quantitative model. In the equal stabilities model (blue), the probability of GABApre bouton maintenance was held constant at 0.6. In the optimized stabilities model (red), these probabilities were adjusted with respect to the experimentally observed GABApre bouton density distribution for the NB2 mutant, revealing lower probabilities of GABApre bouton maintenance among high bouton-density GABApre synapses (see Supplemental Experimental Procedures).
(C) GABApre bouton density distributions for NB2 mutant mice (white), and equal (blue) and optimized (red) probabilities of GABApre bouton maintenance (see Supplemental Experimental Procedures). Applying equal stabilities across all GABApre synapses resulted in an overestimation of high bouton-density GABApre synapses. Optimal fitting of the experimental data required the reduction of GABApre bouton maintenance among high bouton-density GABApre synapses.
(D) Optimized transition matrix for the NB2 mutant. Each element (i, j) of the matrix is the probability of having i GABApre boutons on a sensory afferent terminal in the NB2 mutant given j GABApre boutons in wild-type mice (see Supplemental Experimental Procedures).
We also examined whether the impact of NB2 varies as a function of GABApre bouton density. In NB2 mutant mice, we observed a disproportionally large reduction in GABApre bouton number at the high end of the wild-type distribution range (those with four to six boutons/sensory terminal) (Figure 3A). To provide further insight into the question of whether high-density bouton arrangements are more sensitive to the loss of NB2, we modeled the impact of a uniformly applied 40% decrease in GABApre bouton number, comparing predicted and experimentally-derived bouton packing data (see Supplemental Experimental Procedures). We found that with the assumption of a uniformly-applied bouton loss, independent of starting packing density, there was a marked overestimation in the number of retained high bouton-density GABApre synapses in NB2 mutants (Figures 3B and 3C). In contrast, a quantitative model that optimized the likelihood of retaining GABApre boutons in NB2 mutants revealed that high bouton-density GABApre synapses are more vulnerable to the loss of NB2 (Figures 3B–3D). These modeling studies support the view that NB2 loss disproportionally strips GABApre bouton synapses from sensory terminals that exhibit a high bouton-packing density (Figure 6B).
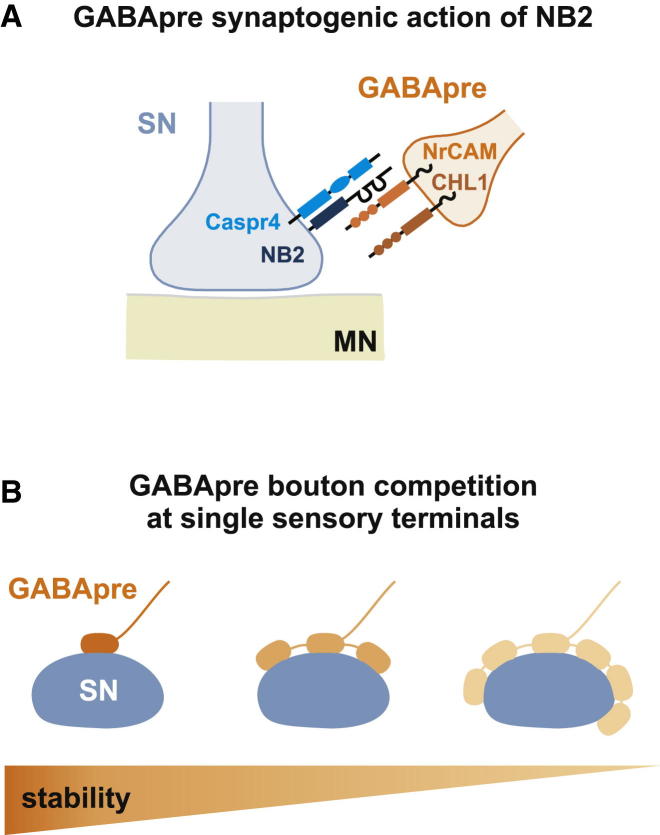
Figure 6.
An NB2/Caspr4 Adhesion Complex Regulates GABApre Synaptic Organization
(A) Sensory (SN)-derived NB2 and Caspr4 act together with GABApre-derived NrCAM and CHL1 as an adhesive complex in the formation of GABApre-sensory terminals.
(B) GABApre boutons compete for sensory terminal space. Sensory neuron (SN) terminals with larger GABApre bouton density show lower GABApre stability compared to sensory terminals with few GABApre boutons. Fading color of GABApre boutons indicates relative stability of individual GABApre boutons on sensory terminals.
Sensory Caspr4 Serves as an NB2 Coreceptor for GABApre Boutons
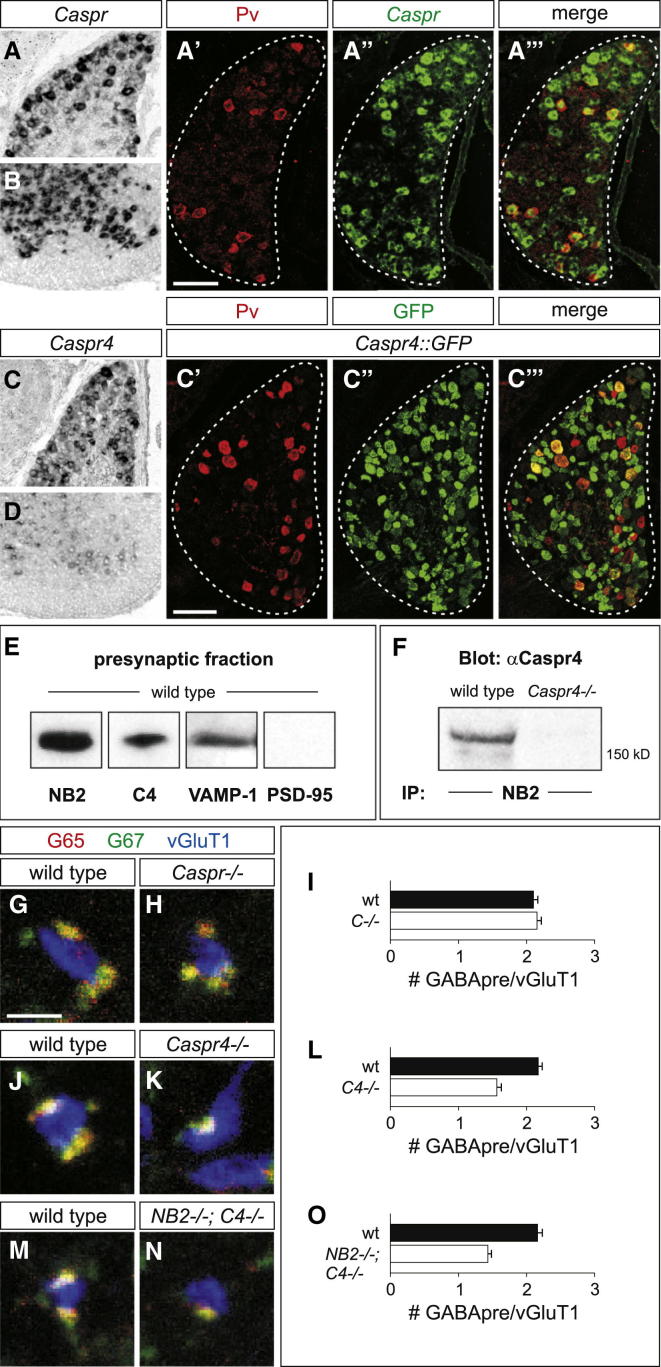
In nodes of Ranvier, the specialized localization of ion channels depends on the interaction of contactin proteins with transmembrane Caspr coreceptors (Poliak and Peles, 2003). We therefore analyzed whether Caspr proteins might function together with NB2 in the assembly of GABApre synapses on sensory terminals. Analysis of the expression of the five Caspr genes, Caspr (Cntnap1) to Caspr5 (Cntnap5) (Peles et al., 1997, Poliak et al., 2003, Spiegel et al., 2002), in p5 to p7 DRG and spinal cord revealed that Caspr, Caspr2, and Caspr4 were expressed by proprioceptive sensory neurons (Figures 4A–4A″′ and 4C–4C″′; data not shown). Moreover, in NB2::tauLacZ; Caspr4::GFP mice, we detected overlap of GFP and βgal in numerous PvON sensory neurons (Figures S3M–S3R), suggesting that many proprioceptive sensory neurons express both Caspr4 and NB2. Caspr and Caspr2 were also expressed at high levels by motor neurons, whereas Caspr4 was expressed at much lower levels in motor neurons (Figures 4B and 4D; for full spinal cord views of Caspr and Caspr4 as well as Caspr4 probe specificity see Figures S3A–S3C; data not shown).
Figure 4.
Caspr4 in Proprioceptive Sensory Neurons Serves as an NB2 Coreceptor
(A and B) Caspr expression in p5 DRG (A) and motor neurons (B). PvON (red) proprioceptive DRG neurons express Caspr (green) at p6 (A′–A″′). Scale bar represents 100 μm.
(C and D) Caspr4 is expressed at high levels in p5 DRG (C), but only weakly present in motor neurons (D). Fifty-three percent of PvON (red) proprioceptive DRG neurons express GFP (green) in p7 Caspr4::GFP mice (C–C″′; n = 3 L4 DRG, three mice). Scale bar represents 100 μm.
(E) Presynaptic preparations were made from p6 to p7 mouse spinal cords. The presynaptic protein, VAMP-1, a synaptic vesicle-associated protein, is expressed in this fraction, whereas the postsynaptic protein, PSD-95 is excluded. Using this preparation, we found that NB2 and Caspr4 are expressed presynaptically in the mouse spinal cord. Molecular weights are as follows: Caspr4 = 190 kDa; NB2 = 130 kDa; PSD-95 = ∼100 kDa; VAMP-1 = 17 kDa.
(F) Brain lysates from wild-type and Caspr4 mutant mice were immunoprecipitated using an NB2 antibody. Blotting with Caspr4 revealed an interaction between Caspr4 and NB2 that was absent in Caspr4 mutant brains.
(G–I) Normal numbers of G65ON (red)/G67ON (green) and Syt1ON/G67ON boutons per vGluT1ON (blue) sensory terminals in p21 wild-type (G) and Caspr mutant mice (H and I; wild-type: 2.10 ± 0.07, n = 563 boutons, three mice; Caspr−/−: 2.15 ± 0.06, n = 541 boutons, three mice; t test, p = 0.56). Scale bar represents 2 μm in (G), (H), (J), (K), (M), and (N).
(J and K) A 28% reduction (mean) in number of G65ON (red)/G67ON (green) and Syt1ON/G67ON GABApre boutons per vGluT1ON (blue) sensory terminals in Caspr4 mutants (K) as compared to wild-type mice (J and L; wild-type: 2.17 ± 0.06, n = 750 boutons, three mice; Caspr4−/−: 1.56 ± 0.07, n = 569 boutons, three mice; t test, p < 0.0001). Similar results were obtained for a second Caspr4 mutant mouse line (data not shown).
(M–O) A 34% reduction (mean) in the number of G65ON (red)/G67ON (green) and Syt1ON/G67ON GABApre boutons per vGluT1ON (blue) sensory terminals in NB2; Caspr4 double mutant mice (N) as compared to wild-type mice (M and O; wild-type: 2.16 ± 0.07, n = 367 boutons, two mice; NB2−/−; Caspr4−/−: 1.43 ± 0.05, n = 534 boutons, three mice; t test, p < 0.0001).
All data reported as mean ± SEM. See also Figure S3.
We attempted to localize NB2 and Caspr4 protein expression at sensory-motor synapses in the ventral spinal cord. Analysis of aldehyde, ethanol, and methanol-fixed and fresh-frozen sections of p6 and p21 spinal cords, however, failed to reveal NB2/Caspr4 immunoreactivity at sensory afferent terminals, even under conditions of antigen retrieval. To address synaptic localization of NB2 and Caspr4 biochemically, we isolated the presynaptic fraction of synaptosomal preparations from p6 to p7 spinal cord (Phillips et al., 2001). As controls, we detected the presynaptic protein marker, VAMP-1 but not the postsynaptic protein marker PSD-95 in such preparations (Figure 4E). In addition, we detected NB2 and Caspr4 protein expression in this presynaptic fraction (Figure 4E), providing biochemical evidence that both proteins are expressed in nerve terminals in the postnatal spinal cord. One potential explanation for the lack of synaptic protein immunoreactivity in histological sections is that NB2 and Caspr4 form a protein complex in which the antigen epitope is masked or otherwise occluded (Fritschy et al., 1998).
We next determined whether Caspr4 interacts with NB2 in brain tissue. We probed the interaction of Caspr4 with NB2 in brain lysates by immunoprecipitation, revealing that Caspr4 forms a complex with NB2 in wild-type, but not in Caspr4 mutant, brain tissue (Figure 4F). In addition, Caspr4 and NB2 associate in transfected cells in vitro (Figures S3S and S3T). Together, these data provide evidence that NB2 and Caspr4 form an interaction complex in neural tissue.
Based on these findings, we explored whether loss of Caspr4, like that of NB2, prevents high bouton-packing. We found that the overall trajectory of PvON proprioceptive axons was similar in p7 Caspr4 mutants as compared to wild-type mice (Figures S3D and S3E). In addition, the number and size of vGluT1ON sensory terminals, as well as their alignment with motor neuron Shank1a plaques was similar in wild-type and Caspr4 mutant mice (Figures S3F–S3K). Analysis of p21 Caspr4 mutants revealed that the number of GAD65ON/GAD67ON GABApre boutons on sensory terminals was reduced by 39% (t test, p < 0.0001) (Figures 4J–4L and S3L). In contrast, analysis of Caspr (Gollan et al., 2003) and Caspr2 (Poliak et al., 2003) mutants revealed no change in the density of GABApre boutons (Figures 4G–4I; data not shown), indicating the specificity of Caspr4 function.
If both NB2 and Caspr4 act in the same pathway, we might anticipate a similar severity of phenotype when ablating one or both components of this putative receptor complex. We therefore analyzed GABApre bouton formation in mice in which both NB2 and Caspr4 genes were inactivated. In NB2; Caspr4 double mutant mice, we detected a 34% reduction in GAD65ON/GAD67ON and Syt1ON/GAD67ON GABApre boutons in contact with vGluT1ON sensory terminals (t test, p < 0.0001) (Figures 4M–4O), a reduction similar in extent to that observed in NB2 and Caspr4 single mutant mice. These genetic data support the view that NB2 and Caspr4 act as coreceptors on sensory terminals to direct the formation of GABApre bouton synapses.
A Role for L1 Ig Proteins in GABApre Recognition of Sensory Terminals
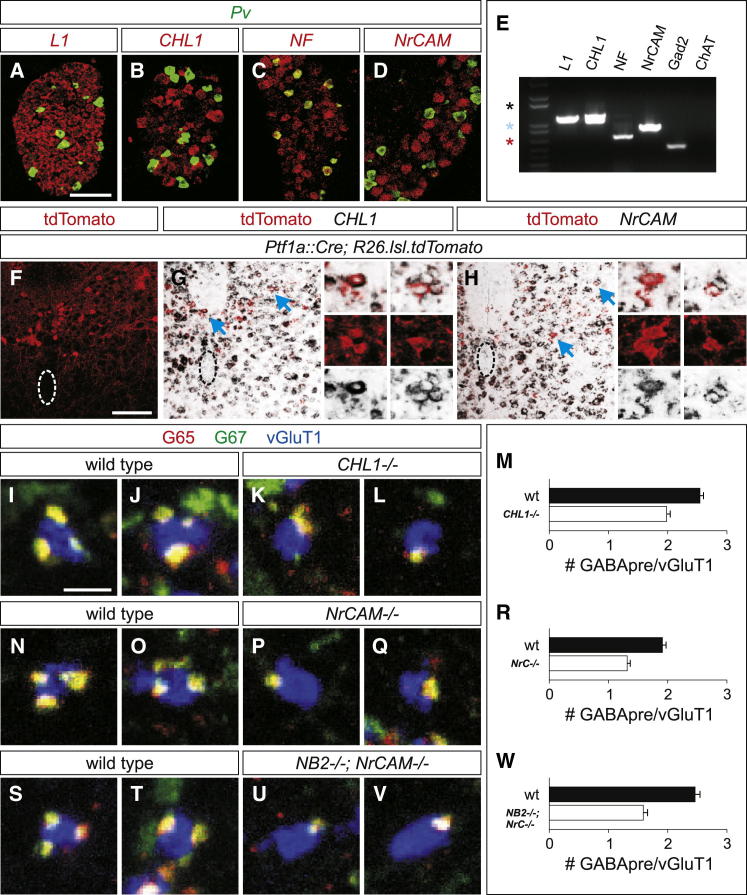
Certain Schwann cell-axonal interactions in myelinated nerves are mediated by binding of contactins to various members of the L1 Ig superfamily (Poliak and Peles, 2003, Shimoda and Watanabe, 2009). We therefore examined whether any of the four L1 family members—L1, close homolog of L1 (CHL1), neurofascin (NF), and NrCAM—might function in GABApre neurons as ligands for sensory terminal-expressed NB2/Caspr4.
We monitored expression of L1, CHL1, NF, and NrCAM in wild-type p5 to p7 DRG and spinal cord and found broad transcript expression by many spinal cord and DRG neurons (Figures 5A–5D; for full spinal cord views see Figures S4A–S4D). In situ hybridization histochemistry revealed that neither L1, CHL1, nor NrCAM were expressed by PvON proprioceptive sensory neurons (Figures 5A, 5B and 5D). To assess expression of L1 members in Ptf1a-derived dI4 interneurons that include the GABApre set we FACS-isolated YFPON interneurons from the spinal cord of p0 Ptf1a::Cre; Rosa26.lsl.YFP mice (Betley et al., 2009, Srinivas et al., 2001). All four L1 family transcripts were expressed by YFPON neurons, as detected in a RT-PCR analysis (Figure 5E). Moreover, we detected NrCAM and CHL1 expression in tdTomatoON cells in the intermediate region of Ptf1a::Cre; Rosa26.lsl.tdTomato mice (Figures 5F–5H and S4E), the assigned location of GABApre neuronal cell bodies (Betley et al., 2009, Hughes et al., 2005).
Figure 5.
A Role for L1 Family Members in GABApre Recognition of Sensory Terminals
(A–D) Assessing mRNA expression of L1 family members in p6 DRG using double fluorescent in situ hybridization. PvON (green) proprioceptive sensory neurons express NF (red, C). Pv does not colabel with L1 (red, A), CHL1 (red, B), or NrCAM (red, D). Based on its expression in proprioceptive sensory neurons, we excluded NF as a trans-acting candidate for NB2/Caspr4. Scale bar represents 100 μm.
(E) RT-PCR of L1 family member transcripts using p0 Ptf1a::Cre; Rosa26.lsl.YFP cDNA. cDNA was generated from FACS-sorted YFPON cells from Ptf1a::Cre; Rosa26.lsl.YFP lumbar spinal cords. Gad2 (GAD65) serves as a positive control because it is known to be expressed in YFPON neurons of Ptf1a::Cre; Thy1.lsl.YFP mice (Betley et al., 2009). ChAT is not expressed in Ptf1a-derived YFPON interneurons and thus serves as a negative control. Stars: 1,600 bp (black); 1,000 bp (blue); 650 bp (red).
(F) tdTomatoON (red) cells in the intermediate spinal cord of p7 Ptf1a::Cre; Rosa26.lsl.tdTomato mice (dotted outline indicates central canal). Scale bar represents 100 μm.
(G and H) CHL1 (G) and NrCAM (H) coexpression with tdTomatoON neurons in the intermediate zone of p7 Ptf1a::Cre; Rosa26.lsl.tdTomato spinal cords (dotted outline indicates central canal). Small images show magnifications of individual colabeled neurons indicated with blue arrows.
(I–M) Mean number of GABApre synapses is reduced by 22% in CHL1 mutants (K–M) as compared to wild-type mice (I, J, and M; wild-type: 2.54 ± 0.06, n = 579 boutons, three mice; CHL1−/−: 1.98 ± 0.06, n = 538 boutons, three mice; t test, p < 0.0001). Scale bar represents 2 μm in (I)-(L), (N)-(Q), and (S)-(V).
(N–R) vGluT1ON (blue) sensory terminals in NrCAM mutant mice (P–R) receive 31% fewer GAD65 (G65)ON (red)/GAD67 (G67)ON (green) and Syt1ON/G67ON GABApre boutons as compared to wild-type mice (N, O, and R; wild-type: 1.91 ± 0.06, n = 522 boutons, three mice; NrCAM−/−: 1.31 ± 0.05, n = 538 boutons, three mice; t test, p < 0.0001).
(S–W) Analysis of NB2−/−; NrCAM−/− double mutants (U–W) showed a mean 36% reduction in the number of G65ON (red)/G67ON (green) and Syt1ON/GAD67ON GABApre boutons located on vGluT1ON (blue) sensory terminals as compared to wild-type mice (S, T, and W; wild-type: 2.46 ± 0.08, n = 362 boutons, two mice; NB2−/−; NrCAM−/−: 1.59 ± 0.07, n = 379 boutons, two mice; t test, p < 0.0001).
All data reported as mean ± SEM. See also Figure S4.
To assess the functional role of L1 family members, we analyzed GABApre bouton contacts with sensory terminals in L1, CHL1, and NrCAM mutant mice (Dahme et al., 1997, Montag-Sallaz et al., 2002, Sakurai et al., 2001). The organization of proprioceptive sensory terminals was similar in wild-type, L1, CHL1, and NrCAM mutants (Figures S4H, S4I, S4L, S4M, and S4S). Moreover, in these three L1 family mutants, vGluT1ON sensory terminals on motor neurons remained juxtaposed to the postsynaptic motor neuron marker Shank1a (Figures S4F, S4G, S4J, and S4K). Analysis of the density of GABApre boutons on vGluT1ON sensory terminals revealed a 31% reduction in NrCAM mutants (t test, p < 0.0001) (Figures 5N–5R and S4N), a 22% reduction in CHL1 mutants (t test, p < 0.0001) (Figures 5I–5M), but no change in L1 mutants (t test, p = 0.65) (Figures S4O–S4R and S4T). Thus, elimination of NrCAM and CHL1 from GABApre interneurons decreases GABApre bouton density on sensory terminals, consistent with a role for these two L1 family members in the organization of GABApre synapses.
To ask whether NrCAM functions in the same recognition process as NB2, we generated mice in which both NB2 and NrCAM were deleted. NB2; NrCAM double mutant analysis showed a 36% reduction in the number of GABApre boutons that form on vGluT1ON sensory terminals (t test, p < 0.0001) (Figures 5S–5W). This observation supports the idea that GABApre-derived NrCAM and sensory-derived NB2 form a ligand-receptor pair that directs high-density GABApre bouton formation with sensory afferent terminals (Figure 6A).
Discussion
Axoaxonic synapses are a specialized feature of primary sensory circuits in the mammalian CNS, providing a structural substrate for the selective filtering of afferent information. Moreover, inactivation of GABApre neurons in mice results in a profound disruption in skilled motor behavior (A. Fink and T.M.J., personal communication), emphasizing the specialized developmental, anatomical, and functional properties of this inhibitory synapse. Our analysis of the developmental organization of presynaptic contacts with proprioceptive sensory terminals indicates that the formation of this axoaxonic arrangement has its basis in an immunoglobulin recognition complex mediated, in part, by interactions between sensory NB2/Caspr4 and interneuron NrCAM/CHL1 that promote or stabilize contacts between the axons of GABApre interneurons and the terminals of sensory afferents (Figure 6A). Restricted expression of the L1 family protein NF underlies the focal accumulation of GABAergic synapses at the axon initial segment of Purkinje neurons (Ango et al., 2004), suggesting a more general function of L1 family proteins in synaptic organization. Thus neuronal Ig proteins may have conserved roles in defining spatial domains of synaptogenesis in the mammalian CNS, in addition to their well-established functions in defining specific membrane domains at and around the nodes of Ranvier (Feinberg et al., 2010, Gollan et al., 2003, Poliak et al., 2003, Sherman et al., 2005, Traka et al., 2003).
Inactivation of NB2/Caspr4 and CHL1/NrCAM proteins (either as single mutants or in combination as double mutants) elicits only a partial reduction in the number of GABApre boutons on sensory terminals, indicating that other recognition systems function together with this set of Ig proteins. One plausible idea is that related Ig proteins serve overlapping functions in instructing presynaptic contacts on sensory terminals. Indeed, Cntn1 and TAG-1 are also expressed by proprioceptive sensory neurons, although the function of their known interacting partners, Caspr and Caspr2, is not required for GABApre bouton packing, at least when Caspr proteins are inactivated individually (Figure 4; data not shown). We note that NB2 is expressed in cutaneous sensory neurons in the DRG (Figure 1F), and thus could have a general role in mediating presynaptic inhibition onto other sensory afferents. Moreover, other recent studies have implicated contactins in synaptic assembly in the chick retina (Yamagata and Sanes, 2012), indicating a more general synaptogenic function for this set of recognition proteins.
Our quantitative studies are consistent with the idea that depletion of sensory terminal NB2 expression covaries with presynaptic packing density: sensory terminals with the greatest density of GABApre boutons appear most sensitive to loss of NB2. We speculate that GABApre boutons normally establish axoaxonic contacts with their target sensory terminals under conditions of competition. The rarity of axoaxonic synaptic arrangements characterized by higher numbers of GABApre boutons presumably reflects the limited availability of sensory terminal target membrane. In essence, our findings suggest the operation of a competitive program of GABApre bouton stabilization, exerted at the level of individual sensory terminals (Figure 6B).
In many regions of the CNS, inputs to individual neurons are pruned extensively through competitive mechanisms to achieve a final, functionally-appropriate, innervation density (Buffelli et al., 2003, Kwon et al., 2012). In the peripheral nervous system, the geometry of postsynaptic dendritic domains of ciliary ganglion neurons defines the number and spacing of their synaptic inputs (Hume and Purves, 1981). We observe a 10-fold variation in the density of GABApre bouton packing between individual sensory terminals, which may reflect functional heterogeneity in the local organization of presynaptic inhibitory circuits (Quevedo et al., 1997, Walmsley et al., 1987).
Our findings on the role of neuronal Ig proteins in establishing the packing density of inhibitory synapses complements and extends studies on the transcriptional control of inhibitory synapse number. Most notably, the transcription factor NPAS4 has been shown to regulate the density of inhibitory synapses in the mammalian CNS (Lin et al., 2008). Future studies may help to define how transcriptional mechanisms and Ig-based recognition conspire to establish the final density of inhibitory synapses in defined circuits within the mammalian CNS.
Experimental Procedures
Mouse Strains
The following mouse strains were used in this study (lsl designates a loxP.STOP.loxP cassette): Caspr (Gollan et al., 2003), Caspr2 (Poliak et al., 2003), Caspr4 (GFP knockin line where GFP-pA followed by PGK-Neo-pA is knocked in immediately following the methionine start codon in the Caspr4 gene; T. Karayannis, E. Au, E. Peles and G. Fishell, personal communication; requests for this mutant should be addressed to E. Peles), CHL1 (Montag-Sallaz et al., 2002), Kirrel-3 (Prince et al., 2013), L1 (Dahme et al., 1997), NB2 (tauLacZ knockin line) (Li et al., 2003), NrCAM (Sakurai et al., 2001), Ptf1a::Cre (Kawaguchi et al., 2002), Pv::Cre (Hippenmeyer et al., 2005), Rosa26.lsl.YFP (Srinivas et al., 2001), Rosa26.lsl.tdTomato (Jackson, Ai14) (Madisen et al., 2010), and Thy1.lsl.YFP (line 15) (Buffelli et al., 2003). Experiments conform to the regulatory standards of the Institutional Animal Care and Use Committee of Memorial Sloan-Kettering Cancer Center.
Candidate Screen
We identified genes coding for candidate receptors by searching the National Center for Biotechnology Information (NCBI) for transcripts in the mouse genome that were predicted to code an extracellular Ig domain and either a transmembrane domain and internal PDZ binding motif or a GPI anchor to the membrane. We performed in situ hybridization analysis on p5 to p6 mouse spinal cord and DRG tissue with probes designed to anneal to these transcripts. Candidates that showed high level of expression in sensory neurons and not motor neurons were further assessed for expression specifically in proprioceptive sensory neurons by performing double in situ hybridizations with the proprioceptive marker gene Parvalbumin (Pv).
Histochemistry
In situ and double fluorescent in situ hybridization histochemistry on 12 μm thick cryostat sections was performed as described previously (Arber et al., 1999, Price et al., 2002). In situ hybridization histochemistry combined with antibody staining was performed as described in Ashrafi et al. (2012). tdTomato detection in combination with in situ hybridization was performed with additional TSA amplification (Perkin-Elmer) of the RFP antibody. Antisense and sense in situ probes were generated from mouse e12.5/p6 spinal cord, DRG, and brain cDNAs using PCR amplification. Probes ranged in length from ∼600 to 1,300 bp. CHL1 antisense probe was generated from a full-length mouse clone (ThermoFisher MMM1013-211694136).
Immunohistochemistry
Immunohistochemistry on 12 μm thick cryostat sections of lumbar level (L) 4 to 5 spinal cord was performed as previously described (Betley et al., 2009). Rabbit anti-βgal (gift from J. Sanes) (Gray and Sanes, 1991), rabbit anti-ChAT (generously provided by S.B.-M. and T.M.J., unpublished data), rabbit anti-GAD65 1:50,000 (Betley et al., 2009), mouse anti-GAD67 1:10,000 (Millipore), rabbit anti-GAD67 1:10,000 (Betley et al., 2009), chicken anti-GFP 1:1,000 (Millipore), sheep anti-GFP 1:1,000 (Molecular Probes), rat anti-NB2 (1A6) 1:4 (Shimoda et al., 2012), chicken anti-Pv 1:10,000 (generously provided by S.B.-M. and T.M.J., unpublished data), rabbit anti-RFP (Rockland), rabbit anti-Shank1a 1:64,000 (Betley et al., 2009), rabbit anti-Shank1a 1:1,000 (Millipore), mouse anti-Syt1 1:100 (ASV48, Developmental Studies Hybridoma Bank), and guinea pig anti-vGluT1 1:32,000 (Betley et al., 2009).
Synaptic Quantification
Synaptic quantifications were performed using Leica LAS software plug-in (Version 2.3.1 build 5194) on z stacks (0.5 μm optical sections) obtained on a Leica TCS SP5 confocal. At least three animals per genotype were analyzed and ∼100 vGluT1ON terminals were counted per animal. Differences between wild-type and mutant mice were assessed using t test (when comparing two groups) or ANOVA (when comparing three groups) (significant at p < 0.05). Data are reported as mean ± SEM.
Quantitative Modeling
The probability of GABApre bouton maintenance on individual sensory afferent terminals was estimated using wild-type and NB2 mutant GABApre-density data distributions. The underlying set of conditional probability mass functions was parameterized, and these parameters were interpreted as GABApre bouton synaptic stabilities in the context of loss of NB2. Parameters were optimized using a constrained linear least-squares approach (Supplemental Experimental Procedures).
Synaptosomal Preparation and Immunoprecipitation
Synaptosomal membranes were prepared using Syn-Per (Thermofisher/Pierce) and pelleted at 15,000 × g for 20 min. The presynaptic fraction was isolated as described in Phillips et al. (2001). The nonsynaptic proteins were extracted from the pellet using a low pH buffer. The pellet was resuspended in Tris pH 8.0, 1% TX-100, 1 mM CaCl2, 1 mM MgCl2 and protease inhibitors, and incubated on ice for 20 min to extract the presynaptic proteins. The insoluble fraction was pelleted at 40, 000 × g for 20 min.
Expression plasmids containing Caspr family cDNAs were described previously (Peles et al., 1997, Poliak et al., 1999, Spiegel et al., 2002). NB2-myc cDNA was prepared by inserting a myc-tag sequence after the signal sequence of rat NB2 by PCR (the first 18 amino acids were removed and the sequence was ligated 54 bp from ATG codon). Transient expression in HEK293T cells, preparation of brain and cell lysates, immunoprecipitation, and western blot analysis was performed as described previously (Gollan et al., 2003).
The following antibodies were used for biochemical experiments: rat anti-NB2 (1A6) (Shimoda et al., 2012), rat anti-NB2 (1B10) (Toyoshima et al., 2009, Shimoda et al., 2012), rabbit anti-Caspr (Peles et al., 1997), rabbit anti-Caspr2 (Poliak et al., 1999), rabbit anti-Caspr3 (Spiegel et al., 2002), rabbit anti-Caspr4 (Spiegel et al., 2002), rabbit anti-Caspr5 (antibody was made by immunizing rabbits with a GST-fusion protein containing the intracellular domain of human Caspr5), mouse anti-myc (clone 9E10; Roche), rabbit anti-VAMP-1 (SYSY), and mouse anti-PSD-95 (Thermo Scientific).
Fluorescence-Activated Cell Sorting and RT-PCR
To isolate putative GABApre neurons, YFPON cells from p0 Ptf1a::Cre; Rosa26.lsl.YFP mice were purified using fluorescence-activated cell sorting (FACS). Briefly, spinal cords were dissociated using Papain dissociation kit (Worthington) and sorted based on YFP fluorescence. RNA was then isolated using the Absolutely RNA Nanoprep Kit (Agilent) and cDNA was generated from these cells using WT-Pico Ovation Amplification Kit (NuGEN). RT-PCR was performed on cDNA generated from purified RNA using the following primers: ChAT (forward primer (FP): TCAGGGCAGCCTCTCTGTAT, reverse primer (RP): ATGTTGTCCACCCGACCTTC), CHL1 (FP: AGGACAGCGAAACTCTGGAA, RP: TCGTGTTCTGCATTTTGAGC), GAD2 (FP: AAAATCTCTTGGGCCCTTTC, RP: CCGGAGTCTCCATAGAGCAG), L1 (FP: CAAAGTCCAGGCAGTGAACA, RP: CTGTACTCGCCGAAGGTCTC), NF (FP: ACCTGGAGACCATCAACCTG, RP: TCAGGCAAGGGAATAGATGG), NrCAM (FP: AATCCAGTGTGAGGCCAAAG, RP: GAAAGCACGAGGTTTTGAGG).
Author Contributions
S.A., J.N.B., J.D.C., S.B.-M., V.B., and J.A.K. performed experiments. S.A., J.N.B., J.D.C., E.P., T.M.J., and J.A.K. designed the study and interpreted results. E.P., S.B.-M., Y.S., and K.W. provided reagents. S.A., J.N.B., J.D.C., T.M.J., and J.A.K. wrote the paper.
Acknowledgments
We thank J. Sanes for generously providing antibodies, T. Sakurai for experimental help and helpful discussions, and N. Balaskas, A. Fink, S. Poliak, and S.-H. Shi for comments on the manuscript. We are grateful to K. Kridsada for technical assistance, D. Ng and J. Zhang for critical assistance with in situ hybridization, J. Bikoff for providing Ptf1a-derived cDNA, I. Horresh for checking antibodies to Caspr5, A. Todd for assistance with synaptic staining, D. Montag for CHL1 mutant tissue, and T. Cutforth for providing Kirrel-3 mutant mice. We thank Y. Zhang and J. Salzer for breeding Caspr mutant mice, S. Markx and J. Gogos for breeding Caspr2 mutant mice, T. Karayannis, E. Au, and G. Fishell for breeding Caspr4 mutant mice, D. Felsenfeld for breeding L1 mutant mice, and T. Sakurai and C. Mason for breeding NrCAM mutant mice. This work was supported by a National Institutes of Health (NIH) predoctoral training grant (527975) and a Columbia University Neuroscience Fellowship (J.N.B.), a Medical Scientist Training Program (MSTP) grant from the National Institute of General Medical Sciences of the NIH under award number T32GM007739 to the Weill Cornell/Rockefeller/Sloan-Kettering Tri-Institutional MD-PhD Program (J.D.C.), a Grant-in-Aid for Scientific Research (B) (#18300120) from the Japan Society for the Promotion of Science (Y.S. and K.W.), NIH grant NS50220 and the Israel Science Foundation (E.P.), HHMI, Project ALS, The Wellcome Trust, EU Framework Program 7, and NIH RO1 NS33245 (T.M.J.), and by Memorial Sloan-Kettering start-up funds, a Whitehall Foundation Research Grant and a Louis V. Gerstner, Jr. Young Investigators Award (J.A.K.). The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Published: January 8, 2014
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information includes Supplemental Experimental Procedures, four figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2013.10.060.
Contributor Information
Thomas M. Jessell, Email: tmj1@columbia.edu.
Julia A. Kaltschmidt, Email: kaltschj@mskcc.org.
Supplemental Information
References
- Ango F., di Cristo G., Higashiyama H., Bennett V., Wu P., Huang Z.J. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at Purkinje axon initial segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Arber S., Han B., Mendelsohn M., Smith M., Jessell T.M., Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- Arber S., Ladle D.R., Lin J.H., Frank E., Jessell T.M. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- Ashrafi S., Lalancette-Hebert M., Friese A., Sigrist M., Arber S., Shneider N.A., Kaltschmidt J.A. Wnt7A identifies embryonic gamma-motor neurons and reveals early postnatal dependence of gamma-motor neurons on a muscle spindle-derived signal. J. Neurosci. 2012;32:8725–8731. doi: 10.1523/JNEUROSCI.1160-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley J.N., Wright C.V., Kawaguchi Y., Erdélyi F., Szabó G., Jessell T.M., Kaltschmidt J.A. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell. 2009;139:161–174. doi: 10.1016/j.cell.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffelli M., Burgess R.W., Feng G., Lobe C.G., Lichtman J.W., Sanes J.R. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- Dahme M., Bartsch U., Martini R., Anliker B., Schachner M., Mantei N. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat. Genet. 1997;17:346–349. doi: 10.1038/ng1197-346. [DOI] [PubMed] [Google Scholar]
- Feinberg K., Eshed-Eisenbach Y., Frechter S., Amor V., Salomon D., Sabanay H., Dupree J.L., Grumet M., Brophy P.J., Shrager P., Peles E. A glial signal consisting of gliomedin and NrCAM clusters axonal Na+ channels during the formation of nodes of Ranvier. Neuron. 2010;65:490–502. doi: 10.1016/j.neuron.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy J.M., Weinmann O., Wenzel A., Benke D. Synapse-specific localization of NMDA and GABA(A) receptor subunits revealed by antigen-retrieval immunohistochemistry. J. Comp. Neurol. 1998;390:194–210. [PubMed] [Google Scholar]
- Furley A.J., Morton S.B., Manalo D., Karagogeos D., Dodd J., Jessell T.M. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell. 1990;61:157–170. doi: 10.1016/0092-8674(90)90223-2. [DOI] [PubMed] [Google Scholar]
- Gennarini G., Cibelli G., Rougon G., Mattei M.G., Goridis C. The mouse neuronal cell surface protein F3: a phosphatidylinositol-anchored member of the immunoglobulin superfamily related to chicken contactin. J. Cell Biol. 1989;109:775–788. doi: 10.1083/jcb.109.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow S.M., Henke R.M., Macdonald R.J., Wright C.V., Johnson J.E. Ptf1a determines GABAergic over glutamatergic neuronal cell fate in the spinal cord dorsal horn. Development. 2005;132:5461–5469. doi: 10.1242/dev.02167. [DOI] [PubMed] [Google Scholar]
- Gollan L., Salomon D., Salzer J.L., Peles E. Caspr regulates the processing of contactin and inhibits its binding to neurofascin. J. Cell Biol. 2003;163:1213–1218. doi: 10.1083/jcb.200309147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G.E., Sanes J.R. Migratory paths and phenotypic choices of clonally related cells in the avian optic tectum. Neuron. 1991;6:211–225. doi: 10.1016/0896-6273(91)90357-6. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S., Vrieseling E., Sigrist M., Portmann T., Laengle C., Ladle D.R., Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D.I., Mackie M., Nagy G.G., Riddell J.S., Maxwell D.J., Szabó G., Erdélyi F., Veress G., Szucs P., Antal M., Todd A.J. P boutons in lamina IX of the rodent spinal cord express high levels of glutamic acid decarboxylase-65 and originate from cells in deep medial dorsal horn. Proc. Natl. Acad. Sci. USA. 2005;102:9038–9043. doi: 10.1073/pnas.0503646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume R.I., Purves D. Geometry of neonatal neurones and the regulation of synapse elimination. Nature. 1981;293:469–471. doi: 10.1038/293469a0. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Cooper B., Gannon M., Ray M., MacDonald R.J., Wright C.V. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat. Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Kwon H.B., Kozorovitskiy Y., Oh W.J., Peixoto R.T., Akhtar N., Saulnier J.L., Gu C., Sabatini B.L. Neuroligin-1-dependent competition regulates cortical synaptogenesis and synapse number. Nat. Neurosci. 2012;15:1667–1674. doi: 10.1038/nn.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Takeda Y., Niki H., Ogawa J., Kobayashi S., Kai N., Akasaka K., Asano M., Sudo K., Iwakura Y., Watanabe K. Aberrant responses to acoustic stimuli in mice deficient for neural recognition molecule NB-2. Eur. J. Neurosci. 2003;17:929–936. doi: 10.1046/j.1460-9568.2003.02514.x. [DOI] [PubMed] [Google Scholar]
- Lin Y., Bloodgood B.L., Hauser J.L., Lapan A.D., Koon A.C., Kim T.K., Hu L.S., Malik A.N., Greenberg M.E. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag-Sallaz M., Schachner M., Montag D. Misguided axonal projections, neural cell adhesion molecule 180 mRNA upregulation, and altered behavior in mice deficient for the close homolog of L1. Mol. Cell. Biol. 2002;22:7967–7981. doi: 10.1128/MCB.22.22.7967-7981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa J., Kaneko H., Masuda T., Nagata S., Hosoya H., Watanabe K. Novel neural adhesion molecules in the Contactin/F3 subgroup of the immunoglobulin superfamily: isolation and characterization of cDNAs from rat brain. Neurosci. Lett. 1996;218:173–176. doi: 10.1016/s0304-3940(96)13156-6. [DOI] [PubMed] [Google Scholar]
- Peles E., Nativ M., Lustig M., Grumet M., Schilling J., Martinez R., Plowman G.D., Schlessinger J. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO J. 1997;16:978–988. doi: 10.1093/emboj/16.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G.R., Huang J.K., Wang Y., Tanaka H., Shapiro L., Zhang W., Shan W.S., Arndt K., Frank M., Gordon R.E. The presynaptic particle web: ultrastructure, composition, dissolution, and reconstitution. Neuron. 2001;32:63–77. doi: 10.1016/s0896-6273(01)00450-0. [DOI] [PubMed] [Google Scholar]
- Poliak S., Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat. Rev. Neurosci. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- Poliak S., Gollan L., Martinez R., Custer A., Einheber S., Salzer J.L., Trimmer J.S., Shrager P., Peles E. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 1999;24:1037–1047. doi: 10.1016/s0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- Poliak S., Salomon D., Elhanany H., Sabanay H., Kiernan B., Pevny L., Stewart C.L., Xu X., Chiu S.Y., Shrager P. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J. Cell Biol. 2003;162:1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S.R., De Marco Garcia N.V., Ranscht B., Jessell T.M. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell. 2002;109:205–216. doi: 10.1016/s0092-8674(02)00695-5. [DOI] [PubMed] [Google Scholar]
- Prince J.E.A., Brignal A.C., Cutforth T., Shen K., Cloutier J.F. Kirrel3 is required for the coalescence of vomeronasal sensory neuron. Development. 2013;140:2398–2408. doi: 10.1242/dev.087262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo J., Eguibar J.R., Lomeli J., Rudomin P. Patterns of connectivity of spinal interneurons with single muscle afferents. Exp. Brain Res. 1997;115:387–402. doi: 10.1007/pl00005709. [DOI] [PubMed] [Google Scholar]
- Rudomin P. In search of lost presynaptic inhibition. Exp. Brain Res. 2009;196:139–151. doi: 10.1007/s00221-009-1758-9. [DOI] [PubMed] [Google Scholar]
- Rudomin P., Schmidt R.F. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp. Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Lustig M., Babiarz J., Furley A.J., Tait S., Brophy P.J., Brown S.A., Brown L.Y., Mason C.A., Grumet M. Overlapping functions of the cell adhesion molecules Nr-CAM and L1 in cerebellar granule cell development. J. Cell Biol. 2001;154:1259–1273. doi: 10.1083/jcb.200104122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman D.L., Tait S., Melrose S., Johnson R., Zonta B., Court F.A., Macklin W.B., Meek S., Smith A.J., Cottrell D.F., Brophy P.J. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 2005;48:737–742. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Shimoda Y., Watanabe K. Contactins: emerging key roles in the development and function of the nervous system. Cell Adhes. Migr. 2009;3:64–70. doi: 10.4161/cam.3.1.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda Y., Koseki F., Itoh M., Toyoshima M., Watanabe K. A cis-complex of NB-2/contactin-5 with amyloid precursor-like protein 1 is localized on the presynaptic membrane. Neurosci. Lett. 2012;510:148–153. doi: 10.1016/j.neulet.2012.01.026. [DOI] [PubMed] [Google Scholar]
- Spiegel I., Salomon D., Erne B., Schaeren-Wiemers N., Peles E. Caspr3 and caspr4, two novel members of the Caspr family are expressed in the nervous system and interact with PDZ domains. Mol. Cell. Neurosci. 2002;20:283–297. doi: 10.1006/mcne.2002.1110. [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanyants A., Tamás G., Chklovskii D.B. Class-specific features of neuronal wiring. Neuron. 2004;43:251–259. doi: 10.1016/j.neuron.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Tian N., Petersen C., Kash S., Baekkeskov S., Copenhagen D., Nicoll R. The role of the synthetic enzyme GAD65 in the control of neuronal gamma-aminobutyric acid release. Proc. Natl. Acad. Sci. USA. 1999;96:12911–12916. doi: 10.1073/pnas.96.22.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima M., Sakurai K., Shimazaki K., Takeda Y., Shimoda Y., Watanabe K. Deficiency of neural recognition molecule NB-2 affects the development of glutamatergic auditory pathways from the ventral cochlear nucleus to the superior olivary complex in mouse. Dev. Biol. 2009;336:192–200. doi: 10.1016/j.ydbio.2009.09.043. [DOI] [PubMed] [Google Scholar]
- Traka M., Goutebroze L., Denisenko N., Bessa M., Nifli A., Havaki S., Iwakura Y., Fukamauchi F., Watanabe K., Soliven B. Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J. Cell Biol. 2003;162:1161–1172. doi: 10.1083/jcb.200305078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B., Wieniawa-Narkiewicz E., Nicol M.J. Ultrastructural evidence related to presynaptic inhibition of primary muscle afferents in Clarke’s column of the cat. J. Neurosci. 1987;7:236–243. doi: 10.1523/JNEUROSCI.07-01-00236.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.E., de Wit J., Ghosh A. Molecular mechanisms of synaptic specificity in developing neural circuits. Neuron. 2010;68:9–18. doi: 10.1016/j.neuron.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M., Sanes J.R. Expanding the Ig superfamily code for laminar specificity in retina: expression and role of contactins. J. Neurosci. 2012;32:14402–14414. doi: 10.1523/JNEUROSCI.3193-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y., Kawasaki M., Tani A., Tamada A., Nagata S., Kagamiyama H., Mori K. BIG-1: a new TAG-1/F3-related member of the immunoglobulin superfamily with neurite outgrowth-promoting activity. Neuron. 1994;13:415–426. doi: 10.1016/0896-6273(94)90357-3. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y., Kawasaki M., Tamada A., Nagata S., Kagamiyama H., Mori K. Overlapping and differential expression of BIG-2, BIG-1, TAG-1, and F3: four members of an axon-associated cell adhesion molecule subgroup of the immunoglobulin superfamily. J. Neurobiol. 1995;28:51–69. doi: 10.1002/neu.480280106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.