Abstract
Experimental modulation of capsule size is an important technique for the study of the virulence of the encapsulated pathogen Cryptococcus neoformans. In this paper, we summarize the techniques available for experimental modulation of capsule size in this yeast and describe improved methods to induce capsule size changes. The response of the yeast to the various stimuli is highly dependent on the cryptococcal strain. A high CO2 atmosphere and a low iron concentration have been used classically to increase capsule size. Unfortunately, these stimuli are not reliable for inducing capsular enlargement in all strains. Recently we have identified new and simpler conditions for inducing capsule enlargement that consistently elicited this effect. Specifically, we noted that mammalian serum or diluted Sabouraud broth in MOPS buffer pH 7.3 efficiently induced capsule growth. Media that slowed the growth rate of the yeast correlated with an increase in capsule size. Finally, we summarize the most commonly used media that induce capsule growth in C. neoformans.
Keywords: Cryptococcus neoformans, Infection, Virulence
Introduction
Microbial capsules are structures found surrounding the cell body that confer particular characteristics to encapsulated organisms. In the case of the pathogenic fungus Cryptococcus neoformans, the capsule is an important virulence factor (see review in (1)). The C. neoformans capsule is composed of at least two polysaccharides glucuronoxylomannan and galactoxylomannan, and a much smaller proportion of mannoprotein (see review in (2)). This polysaccharide capsule has a large number of effects on the host, such as complement activation and depletion, Ab unresponsiveness, inhibition of leukocyte migration and inhibition of phagocytosis (3-9).
One of the characteristics of cryptococcal cells is great variation in capsule size depending on the environmental conditions. During in vitro culture conditions (10) the size of the capsule is usually small, although capsular enlargement can be induced by several factors, including high CO2 and low iron (11, 12). During mammalian infection, the size of the capsule increases dramatically (13-16), and it is thought that this phenomenon is necessary for cryptococcal pathogenesis (11). Hence, modulation of capsule size is an important subject of investigation for the cryptococcal field. To date, the mechanism of capsule growth remains poorly characterized.
The purpose of this paper is to review the different ways of manipulating capsule size and to highlight the options and limitations available to cryptococcal investigators who plan to manipulate the size of a cryptococcal strain. In particularly, we will review the great variability in the behavior of strains with different genetic backgrounds and the heterogeneity in the response in the same strain. We also suggest an experimental roadmap to identify the proper conditions to induce capsule size of a particular C. neoformans strain.
Materials and Methods
Yeast strain and growth conditions
The serotype A C. neoformans strain H99 was kindly provided by Dr. John Perfect (Durham, NC) and used for capsule growth experiments. The yeast cells were grown overnight in 10 ml of acid Sabouraud medium (DifcoTM, Catalogue number 238130, Sparks, MD) at 30oC with moderate shaking (150 r.p.m), collected in the logarithmic phase of growth by centrifugation (5 minutes at 1200 r.p.m. at room temperature), washed twice with PBS (137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8.5 mM Na2HPO4) and incubated in the capsule growth inducing media overnight at 37oC. The cells were placed in six-wells polystyrene plates at a cell density of 5 x 106-107 cells/ml in the designated media (listed in Table 1) and capsule induction was performed for 18-24 hours.
India Ink staining and light microscopy
To observe and measure the size of the capsule, 10 mL of a cell suspension were mixed with an India Ink drop (Becton Dickinson, NJ, Cat. Number 261194) and observed in an Olympus AX70 microscope. Pictures were taken with a QImaging Retiga 1300 digital camera using the QCapture Suite V2.46 software (QImaging, Burnaby BC, Canada), and processed with Adobe Photoshop 7.0 for windows (San Jose, CA). At least five different fields were randomly chosen and photographed, and 40 to 60 cells were analyzed.
Measurement of capsule volume
To calculate the capsule volume, the diameter of the whole cell and the cell body were each measured with Adobe Photoshop 7.0 and capsule volume was defined as the difference between the volume of the whole cell (yeast cell + capsule) and the volume of the cell body (as limited by the cell wall). Volumes were calculated using the equation for volume of a sphere as (4π/3)(D/2)3. Between 15 and 40 cells were measured for each determination. For statistical analysis, t-test was used with Unistat 5.5 software for Excel.
Results and Discussion
Capsule size in the ecological niches of C. neoformans
C. neoformans is a pathogenic yeast that is acquired from the environment during mammalian infection. This organism can be isolated from several environmental niches and is commonly found in pigeon excreta, soils, and some trees (see review in (1)). For environmental isolates, the size of the capsule is uniformly small. During in vitro growth conditions in the media commonly used in research laboratories (e.g. Sabouraud medium), the size of the capsule is usually small (10). This is in contrast with the situation found during infection whereby cells with very large capsules are commonly found in tissue (13-16). When cells infect the host, there is a rapid increase in capsule size (17). This observation suggests the importance of the environmental conditions in determining the size of the cryptococcal capsule. In the following sections, we summarize and discuss some factors that influence capsule size and their interplay in laboratory conditions (see Fig. 1 for a summary).
Fig. 1. Fig. 1: Scheme of the factors that influence capsule size in C. neoformans.
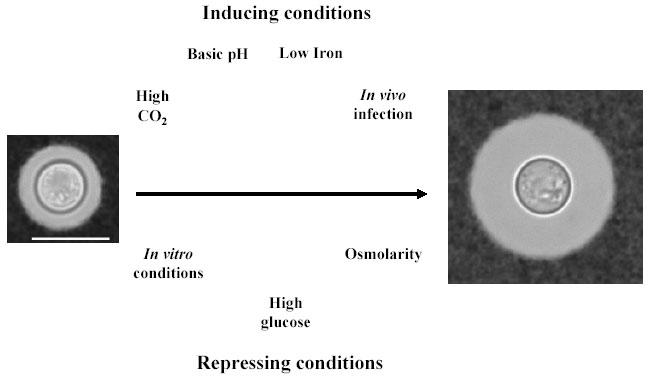
The diagram summarizes the main factors involved in the modulation of capsule size in C. neoformans. In the upper part we represent factors that increase capsule size, and in the lower part, factors that inhibit capsule growth. Bar on left panel denotes 10 microns and applies also for panel on the right.
Classical stimuli used to increase capsule size
The size of the capsule of C. neoformans in most laboratory media is small, and ranges between 2-4 microns in transversal length, without considering the distance between the cell wall. Similarly, organisms recovered from the environment usually have small capsules. In the late fifties, Littman formulated a mineral medium in which the growth of the organisms was accompanied by capsule enlargement (10). The composition of this medium was based on the nutrients that the yeast was likely to have available in physiological fluids, such as the cerebrospinal fluid. That study highlighted the importance of certain nutrients in determining the size of the capsule, and identified several amino acids, vitamins, and carbon sources that contributed to this process. However, this method has not been extensively used to induce capsule size, possibly because it is not successful with all strains. Several decades later, Granger et al. identified CO2 as an inducing factor for capsule growth (11). That study used strain H99. It showed, when incubated in DMEM medium in an atmosphere rich in CO2, induced capsule growth. This observation had potentially important physiological implications because it identified a product of mammalian respiration as a stimulus for capsule growth. Consequently, CO2 is one of the most commonly used stimuli to manipulate capsule size. However, not all strains responded to CO2 with increased capsule growth (18). In 1993, Vartivarian et al. observed that a low iron concentration also induced capsule growth (12). This medium (known as LIM for limited iron medium) has been extensively used in the literature to induce capsule size. More recently, we described mammalian serum as a potent activator of capsule growth (18).
Other factors that affect capsule size
Capsule growth is affected by a variety of factors, such as pH, osmolarity, carbon source, nutrient concentration and temperature. Alkaline conditions facilitate capsule growth (11, 18-21), although a basic pH is not sufficient to mediate this effect. Increasing the pH of Sabouraud medium does not enhance capsule growth (18). High osmolarity may block capsule growth since high glucose concentrations inhibit capsule growth (21), and although this effect does not apply to any solute (22), sodium chloride produces the same effect (21, 22). Finally, temperature seems to have an effect on the size of the cell. This can affect the size of the capsule (the diameter of the cell is used in deriving the size of the capsule). However, temperature alone does not affect the relative size of the capsule (18, 23).
Problems with the reproducibility
One of the problems of the cryptococcal field is that strain capsule size and the enlargement phenomenon is poorly reproducible from laboratory to laboratory. Consequently, this effect has not been studied extensively despite its association with virulence. Heterogeneity in the response presumably reflects the genetic background, and also the complexity in the interplay between the different inducing factors. For example, both CO2 and serum are very effective as inducing factors, but only in specific media (18). Neither serum nor CO2 are able to induce capsule growth in media that is rich in nutrients, like Sabouraud media, and they require that the yeast is placed in either DME or PBS, respectively, to induce capsule growth (18). This implies that capsule growth is a highly complex phenomenon that is not determined by a unique factor.
There is great variation between different strains (inter-strain heterogeneity) in the response to the different stimuli used for inducing capsule growth (18). Stimuli that produce capsule enlargement in some strains do not produce it in others. This variability adds more complexity to this phenomenon. For example, the capsular growth induction by CO2 or iron may differ from strain-to-strain (12, 18). The reason for this inter-strain heterogeneity is not known, but most probably it is due to genetic differences between the strains. Consequently, for a given strain one must evaluate several media empirically to ascertain the best conditions for achieving capsule growth. Furthermore, certain strains demonstrate intra-strain heterogeneity in the response such that all cells may not respond in the same manner (11, 17-18). For instance, it has been reported that when strain H99 is in the lung, there is a high heterogeneity in the size of the cells and capsules of the yeast (17). Similar heterogeneity was also reported for strain 24067 in vitro (18). In the case of strain 24067, the heterogeneity is probably due to a different behavior of the buds that emerge during the incubation in the inducing medium (18). The mechanisms responsible for intra- and inter-strain heterogeneity are not known, but it is likely that an explanation includes genetic differences combined with growth differences for individual cells in the medium used (18). Alternatively, intra-strain heterogeneity could reflect a phenotypic-switching phenomenon that is manifested at the cellular level.
New media able to induce capsule size
During our studies of C. neoformans biology, we serendipitously discovered that yeast cells of strain H99 placed in a Sabouraud medium diluted 10 times with H2O developed large capsules (Fig. 2). That result suggested that capsule enlargement was related to the cell growth rate. In addition, we observed that when Sabouraud medium was diluted, not in water, but in buffer with basic pH, such as MOPS, HEPES or PBS, the increase in capsule size was more noticeable (Fig. 2). Moreover, increasing the pH of diluted Sabouraud in water by addition of some drops of NaOH enhanced the efficiency of the process (Fig. 2).
Fig. 2. Fig. 2: Capsule growth in different media containing diluted Sabouraud.
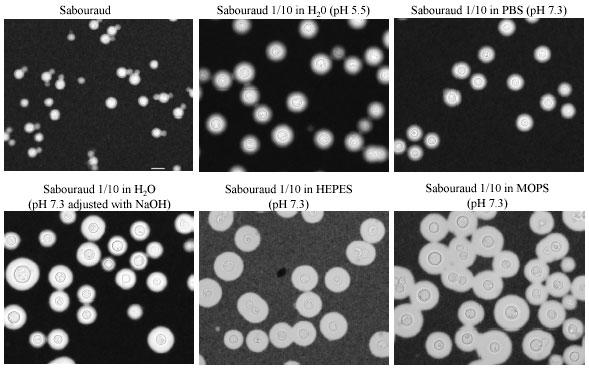
H99 cells were grown in Sabouraud medium, collected in the logarithmic phase of growth, washed and transferred to 2 ml of media indicated in each case. In some cases, a few drops of NaOH were used to manipulate the pH, while in other experiments MOPS or HEPES buffer were used. The cells were incubated overnight at 37ºC as described in material and methods. Bar on first panel denotes 10 microns and applies also for the rest of the panels.
The effect was observed by six hours and appeared complete by 24 h. Longer incubations did not significantly change the size of the capsule. Among the media evaluated, the biggest capsules were observed when pH was buffered with 50 mM MOPS pH 7.3, and was associated with noticeably slower growth (data not shown). These results provide new experimental conditions to increase capsule size in the absence of serum or CO2 incubators. Comparison of the efficiency of diluted Sabouraud in MOPS buffer, with CO2 and serum to previously established methods revealed that our methods were more consistent in promoting capsule growth (Fig. 3).
Fig. 3. Fig. 3: Comparison of capsule growth on diluted Sabouraud, serum and CO2.
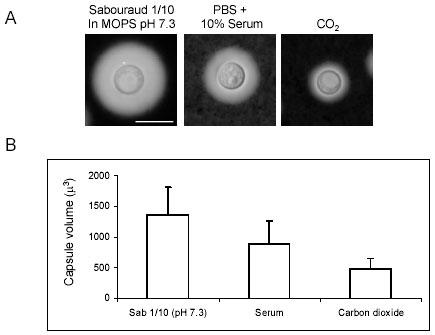
H99 cells were grown in Sabouraud overnight and then transferred to the following media: Diluted Sabouraud (1/10) in 50 mM MOPS buffer (pH 7.3), or 10% FBS (in PBS) or in DME in the presence of an atmosphere of 10% CO2. The cells were incubated in these media overnight at 37ºC. A) Picture of a representative cell in an India Ink suspension. The bar in the first panel denotes 10 microns, and this scale applies to the rest of pictures. B) Measurement of the capsule size for the samples described above. The size of the capsule was expressed as the total capsule volume. P value for all comparisons was below 0.001.
Capsule size during infection
The increase of capsule size during infection is believed to be biologically significant because of the virulence-enhancing properties of capsular polysaccharide. It is thought that this increase in capsule size is necessary for the virulence of the yeast since mutants that are unable to do this are not as virulent as wild type strains (11, 24-25). This increase in capsule size is noticeable after several hours of infection (17). It is interesting that after several days of infection, the increase in capsule size is accompanied by an increase in the size of both the cell and the capsule, which results in giant forms of the yeast, whose role in virulence is unknown (17). Finally, the increase in capsule size is dependent on the organ of infection, with capsules being larger in the lung than in the brain (16). This reflects the importance of the environment on the size of the capsule. With regard to organic substances, both coagulase plasma (26) and mammalian sera (18) have been reported to be effective in inducing capsule growth.
Physiological meaning of the factors that induce capsule growth
We have described several new stimuli for capsule growth. However, the fact that this phenomenon occurs only in certain conditions raises the question of what factors are specifically responsible for the effect. In the case of CO2 induction, capsule growth appears to resemble the in vivo situation that the yeast cells encounter in the lung, which is the first organ infected after inhalation of the infectious particles. Limited iron medium is another stimulus that can be used to induce capsule growth. The mechanism by which iron limitation induces capsule growth remains unknown. Iron regulates the production of some virulence factors in bacteria (27, 28), and regulation of Fe concentration in physiological fluids by the host could affect the virulence of these organisms. It has been suggested that if iron uptake is mediated through the capsule, an increase in capsule size would be a response to transport iron more efficiently, but the same authors argued that this was unlikely since acapsular mutants grow normally during iron limitation conditions (12). Serum is known to contain iron-binding chelators and the effect in serum could reflect lack of iron, which in turn would affect growth. However, serum-induced capsule growth was not inhibited by addition of iron to the medium (18), indicating that the inducing signal is different. Since C. neoformans in a mammalian host would encounter serum components in the course of infection, the phenomenon of serum-induced capsular growth may reflect the stimulus that induces capsule size during pathogenesis. Concerning the other media described in this paper, such as diluted Sabouraud medium in buffers with basic pH, we believe that there is a decrease in the growth rate of the yeast, which could trigger capsule growth, possibly as a consequence of a stress response. This result, if validated by subsequent experimental work, would link capsule growth to cell growth. Consistent with this hypothesis is the fact that capsule growth is enhanced by an alkaline pH, a condition in which fungal cells grow more slowly. Finally, and related to the in vivo situation, it has been shown that in the lung, the size of the capsule is bigger than in the brain (16). Although the cause of this phenomenon is not known, the inflammatory response in much higher in the lung than in the brain (1), which may slow the growth of the yeast in the lung and is also consistent the view that capsule growth is correlated with slower replication. In addition, brain tissue may contain more iron (16), which could promote faster growth. However, it is important to stress that the concept that capsule growth is inversely proportional to growth rate is currently only a hypothesis.
Acknowledgments
This work was supported by the following NIH grants: HL59842, AI33142 and AI33774. The authors have no conflicts of interest to declare related to this publication.
Appendix
Protocols
Technical hints to check your favorite strain for capsule growth
In this section we briefly propose a pragmatic scheme to identify the conditions that induce capsule growth for an individual strain (see Table 1), and highlight the advantages and disadvantages of each approach. These suggestions must take into consideration that the efficiency of the process is highly strain dependent, and the most effective protocol may differ from strain to strain, as already reported (18). To induce capsule growth, we suggest first to grow the yeast in liquid Sabouraud medium overnight, washed with sterile PBS, and inoculated in 6-wells plates with 2 ml of each media at a cell density of 5 x 106-107 cells/ml. In our hands, the best results were obtained when capsule growth was induced at 37oC, although incubation at room temperature and 30oC could also increase capsule size.
Diluted Sabouraud. One of the most efficient methods for capsule induction is to incubate the yeast in diluted Sabouraud broth in H2O. For increasing the efficiency of these methods one can dilute the Sabouraud medium 10 times in either PBS, MOPS 50 mM pH 7.3 or HEPES 50 mM pH 7.3. This method is highly efficient at both 30 and 37oC and will produce good results in both shaking flasks and culture plates. In our hands this was the most efficient method for inducing capsule growth. The fact that neither serum, CO2 nor complex media are required makes this a convenient and powerful new tool to induce capsule size in future studies.
Serum. The effect of serum is highly dependent on the medium in which it is diluted. In our hands PBS worked very well as the diluent to induce capsule growth. Dilution in other media, such as Sabouraud was not effective. It has been reported that a 10% concentration is optimal, but higher concentrations can be used (18). Different mammalian sera can induce this effect, but rat and fetal bovine sera seemed to be more effective. When serum is used, best results were observed when the culture plates were incubated without agitation at 37oC.
CO 2 . Incubation of the yeast in a rich atmosphere in CO2 can be used to induce capsule growth. However, induction of capsule growth by CO2 was observed only when the cells were incubated in DMEM or RPMI media. It some reports, the medium was supplemented with 22 mM NaHCO3, but the conditions when this was required were not established. Consequently, the requirement for NaHCO3 needs to be established empirically. Five and 10% concentrations of CO2 have been reported to be effective. In general, the use of a routine laboratory tissue culture CO2 incubator for supplying this atmosphere makes agitation impractical. However, incubation without agitation is highly efficient in inducing capsule growth. The requirement of special equipment to supply an enriched CO2 atmosphere may limit the use of this method.
Limited iron medium (LIM). A low iron concentration is one of the most common stimuli used to induce capsule growth. Its effect is enhanced by combination with the CO2 stimulus (12). In Table 1, we have included the composition of the medium as originally described (12), although other investigators have modified the composition of this medium (29). Complete depletion of iron is achieved by treatment of some of its components with the ion exchange resin Chelex-100 (BioRad). Iron depletion can be also performed by addition of 56 μM ethylenediaminedi(ο-hydroxylphenyl acetic acid) (EDDA). Since LIM is a complex medium, in many occasions it is convenient to use a control in which iron is added. For iron repletion, one can add 100 μM Fe(III) hydroxyethylenediaminetriacetate (FeHEDTA). Although this method has been largely used in the past, the concern that iron is not completely eliminated from the medium might introduce uncertainty on its effectiveness when the capsule is not induced.
Table 1. Methods to obtain .
C. neoformans cells with big capsule
| Inducing factor | Composition and protocol |
| Diluted Sabouraud | Dilute Sabouraud (DifcoTM 238230) 1/10 in H2O, or PBS, or 50 mM HEPES (Gibco, 15630-080) or MOPS (Sigma, Cat. No M-9381) pH 7.3. Incubate at 37oC. |
| CO2 | DME (Cellgro by Mediatech Inc., 10-013-CM) or RPMI (Cellgro by Mediatech Inc.,MT 10-040-CM). Inoculate and incubate in CO2 incubators (5 or 10% CO2). If necessary, add 22 mM NaHCO3 (Fisher Scientific, S233-500). Incubate at 37oC. |
| LIM | LIM (pH 7.4) as described originally (12), contains per liter (all products from Sigma Aldrich), 5 g of glucose (G5767), 400 mg of K2HPO4 (P3786), 5 g of asparagine (A0884), 250 mg of CaCl2 · 2H2O (C3881), 0.4 mg of thiamine (T4625), 5 mg of CuSO4 (C2857), 2 mg of ZnSO4 · 7H2O (Z4750), 0.01 mg of MnCl2 · 4H2O (M3634), 80 mg of MgSO4 · 7H2O (M5921), 0.46 mg of Na2MoO4 (M1003) and 0.057 mg of boric acid (B0394). Incubate yeast at room temperature. |
| Serum | 10% fetal bovine serum (Gemini BioProducts, Woodland, CA, A37606V) in PBS. Incubate at 37oC. |
References
- Casadevall A, Perfect JR. Cryptococcus neoformans (Press A, Ed.), ASM Press, Washington DC, 1998.
- Cherniak R, Sundstrom JB. Polysaccharide antigens of the capsule of Cryptocccus neoformans. Infect Immun. 1994;62:1507–1512. doi: 10.1128/iai.62.5.1507-1512.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macher AM, Bennett JE, Gadek JE, Frank MM. Complement depletion in cryptococcal sepsis. J Immunol. 1978;120:1686–1690. [PubMed] [Google Scholar]
- Murphy JW, Cozad GC. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972;5:896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR, Gulley WF, Cazin J Jr. Immune response to Cryptococcus neoformans soluble polysaccharide: immunological unresponsiveness. Infect Immun. 1977;18:701–707. doi: 10.1128/iai.18.3.701-707.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong ZM, Murphy JW. Effects of the two varieties of Cryptococcus neoformans cells and culture filtrate antigens on neutrophil locomotion. Infect Immun. 1995;63:2632–2644. doi: 10.1128/iai.63.7.2632-2644.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR, Pfrommer GS, Guerlain AS, Highison BA, Highison GJ. Strain variation in phagocytosis of Cryptococcus neoformans: dissociation of susceptibility to phagocytosis from activation and binding of opsonic fragments of C3. Infect Immun. 1988;56:2794–2800. doi: 10.1128/iai.56.11.2794-2800.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolaños B, Mitchell TG. Killing of Cryptococcus neoformans by rat alveolar macrophages. J Med Vet Mycol. 1989;27:219–228. [PubMed] [Google Scholar]
- Zaragoza O, Taborda CP, Casadevall A. The efficacy of complement-mediated phagocytosis of Cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3-mediated interactions. Euro J Immnunol. 2003;33:1957–1967. doi: 10.1002/eji.200323848. [DOI] [PubMed] [Google Scholar]
- Littman M. Capsule synthesis by Cryptococcus neoformans. Trans NY Acad Sci. 1958;20:623–648. doi: 10.1111/j.2164-0947.1958.tb00625.x. [DOI] [PubMed] [Google Scholar]
- Granger DL, Perfect JR, Durack DT. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest. 1985;76:508–516. doi: 10.1172/JCI112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartivarian SE, Anaissie EJ, Cowart RE, Sprigg HA, Tingler MJ, Jacobson ES. Regulation of cryptococcal capsular polysaccharide by iron. J Infect Dis. 1993;167:186–190. doi: 10.1093/infdis/167.1.186. [DOI] [PubMed] [Google Scholar]
- Bergman F. Studies on capsule synthesis of Cryptococcus neoformans. Sabouraudia. 1965;4:23–31. [PubMed] [Google Scholar]
- Cruickshank JG, Cavill R, Jelbert M. Cryptococcus neoformans of unusual morphology. Appl Microbiol. 1973;25:309–312. doi: 10.1128/am.25.2.309-312.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love GL, Boyd GD, Greer DL. Large Cryptococcus neoformans isolated from brain abscess. J Clin Microbiol. 1985;22:1068–1070. doi: 10.1128/jcm.22.6.1068-1070.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Feldmesser M, Cammer M, Casadevall A. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect Immun. 1998;66:5027–5030. doi: 10.1128/iai.66.10.5027-5030.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmesser M, Kress Y, Casadevall A. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology. 2001;147:2355–2365. doi: 10.1099/00221287-147-8-2355. [DOI] [PubMed] [Google Scholar]
- Zaragoza O, Fries BC, Casadevall A. Induction of capsule growth in Cryptococcus neoformans by mammalian serum and CO(2). Infect Immun. 2003;71:6155–6164. doi: 10.1128/IAI.71.11.6155-6164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp C, Ruiz A, Bulmer GS. Culture of Cryptococcus neoformans in the nonencapsulated state. Mycopathologia. 1981;76:129–131. doi: 10.1007/BF00437192. [DOI] [PubMed] [Google Scholar]
- Farhi F, Bulmer GS, Tacker JR. Cryptococcus neoformans: The not-so-encapsulated yeast. Infect Immun. 1970;1:526–531. doi: 10.1128/iai.1.6.526-531.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra MA, Friedman L, Murphy JW. Capsule size of Cryptococcus neoformans: control and relationship to virulence. Infect Immun. 1977;16:129–135. doi: 10.1128/iai.16.1.129-135.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson ES, Tingler MJ, Quynn PL. Effect of hypertonic solutes upon the polysaccharide capsule in Cryptococcus neoformans. Mycoses. 1989;32:14–23. doi: 10.1111/j.1439-0507.1989.tb02163.x. [DOI] [PubMed] [Google Scholar]
- Jacobson ES, Compton GM. Discordant regulation of phenoloxidase and capsular polysaccharide in Cryptococcus neoformans. J Med Vet Mycol. 1996;34:289–291. doi: 10.1080/02681219680000491. [DOI] [PubMed] [Google Scholar]
- D'Souza CA, Alspaugh JA, Yue C, Harashima T, Cox GM, Perfect JR, Heitman J. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol Cell Biol. 2001;21:3179–3191. doi: 10.1128/MCB.21.9.3179-3191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh JA, Pukkila-Worley R, Harashima T, Cavallo LM, Funnell D, Cox GM, Perfect JR, Kronstad JW, Heitman J. Adenylyl cyclase functions downstream of the Galpha protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot Cell. 2002;1:75–84. doi: 10.1128/EC.1.1.75-84.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anna EJ. Rapid in vitro capsule production by cryptococci. Am J Med Technol. 1979;45:585–588. [PubMed] [Google Scholar]
- Weinstein DL, Holmes RK, O'Brien AD. Effects of iron and temperature on Shiga-like toxin I production by Escherichia coli. Infect Immun. 1988;56:106–111. doi: 10.1128/iai.56.1.106-111.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorn MJ, Iglewski BH, Ives SK, Sadoff JC, Vasil ML. Effect of iron on yields of exotoxin A in cultures of Pseudomonas aeruginosa PA-103. Infect Immun. 1978;19:785–791. doi: 10.1128/iai.19.3.785-791.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykus KJ, Jacobson ES. Genetic and physiologic characterization of ferric/cupric reductase constitutive mutants of Cryptococcus neoformans. Infect Immun. 1999;67:2357–2365. doi: 10.1128/iai.67.5.2357-2365.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


