Abstract
Background
Myocardial siderosis is the most common cause of death in patients with beta thalassemia major(TM). This study aimed at investigating the occurrence, prevalence and severity of cardiac iron overload in a young Chinese population with beta TM.
Methods and Results
We analyzed T2* cardiac magnetic resonance (CMR), left ventricular ejection fraction (LVEF) and serum ferritin (SF) in 201 beta TM patients. The median age was 9 years old. Patients received an average of 13 units of blood per year. The median SF level was 4536 ng/ml and 165 patients (82.1%) had SF>2500 ng/ml. Myocardial iron overload was detected in 68 patients (33.8%) and severe myocardial iron overload was detected in 26 patients (12.6%). Twenty-two patients ≤10 years old had myocardial iron overload, three of whom were only 6 years old. No myocardial iron overload was detected under the age of 6 years. Median LVEF was 64% (measured by CMR in 175 patients). Five of 6 patients with a LVEF<56% and 8 of 10 patients with cardiac disease had myocardial iron overload.
Conclusions
The TM patients under follow-up at this regional centre in China patients are younger than other reported cohorts, more poorly-chelated, and have a high burden of iron overload. Myocardial siderosis occurred in patients younger than previously reported, and was strongly associated with impaired LVEF and cardiac disease. For such poorly-chelated TM patients, our data shows that the first assessment of cardiac T2* should be performed as early as 6 years old.
Introduction
Myocardial siderosis remains the most common cause of death in patients with beta thalassemia major (TM) [1]. In affluent countries such as the UK, there has been a dramatic improvement in survival since the introduction of cardiovascular magnetic resonance(CMR) for assessment of myocardial iron [2], but iron induced cardiomyopathy still accounts for the majority of all deaths [3]. Early detection of myocardial iron overload is important because survival is only 50% once overt heart failure is manifest [4] and patients remain asymptomatic until late in the course of development of iron overload cardiomyopathy. Usefully, noninvasive CMR can quantify iron overload in different organs such as the heart and liver even before the development of symptoms [5], [6]. In this regard, CMR relaxometry T2* is being increasingly used worldwide for monitoring transfusion-dependent TM [7], [8].
The guidelines for the clinical management of thalassemia edited by Thalassemia international federation (TIF) recommended that the first assessment of cardiac T2* would be done at puberty for the well-chelated patients who received chelation therapy early and regularly [9], but there is not a specific guideline for poorly-chelated patients. Most TM patients in developing countries including China are poorly-chelated and many of them die in childhood or early adolescence due to the lack of access to specialist care [10], [11]. For poorly-chelated patients, there is limited data on when the myocardial siderosis occurs and when the CMR T2* screening should be initiated. To answer these questions, we designed this study to determine the age when cardiac iron overload occurs and the prevalence of abnormal cardiac MRI T2* and myocardial function on a large young TM population in mainland China.
Materials and Methods
Study Population
We studied 201 TM patients (192 children and 9 adults) from November 2010 to January 2013. The median age was 9 (4–25) years old. All patients were transfusion-dependent and required transfusions from early childhood. Thirty-five patients (17.4%) received splenectomy. Five patients (2.5%) were well-chelated and 196 patients (97.5%) were poorly-chelated. Patients were considered to be well-chelated if the following criteria were met [12], [13]: (i) initiate treatment after first 10–20 transfusions or serum ferritin (SF) level above 1000 ng/ml; (ii) chelators dose: Deferoxamine (DFO) is 20–40 mg/kg/day in children and 50–60 mg/kg/day in adults, at least 5 days a week. Deferiprone (DFP) is 75 mg/kg/day. Deferasirox (DFX) is 20–40 mg/kg/day; (iii) good compliance with chelation therapy, defined as a≥90% adherence to the instructions given by the hematologists. The study was performed at the first affiliated hospital of Guangxi Medical University. Restrospective review of medical records, coded exchange of clinical data, SF levels and MRI examininations were authorized by the local institute. All of patients provided their written informed consent to participate in this study. Adults (≥18) finished the informed consent by themselves. And for minors (<18), the informed consents were obtained from the guardians on the behalf of the minors participants involved in this study. The study was approved by the Medical Ethics Committee of First Affiliated Hospital of Guangxi Medical University (the approval number: NO. 2008(KY-002).
Serum Ferritin
Laboratory blood tests were performed 2 weeks after the last blood transfusion, followed by MRI evaluations with a 1-month interval in all patients. Measurements were carried out by an Electrochemiluminescence immunoassay (COBASE E 601, Roch, USA).
MRI Protocols
Patients were scanned with a 1.5T MRI scanner (Siemens Avanto, Siemens Medical Systems, Erlangen, Germany) with the combination of body-matrix and spine-matrix surface coils. To ensure quality of the work, the local staff involved in this study (technicians, radiologists, and hematologists) received intensive onsite training from CMR specialists of Royal Brompton Hospital (London, UK). Each scan included the measurement of heart T2* and left ventricular ejection fraction (LVEF) using previously published techniques [14], [15], [16]. For T2* imaging, a single short axis mid-ventricular slice was acquired at eight echo times (2.97–21.68 ms) using a gradient-echo sequence within a single breath-hold. For myocardial T2* measurement, a homogeneous full thickness region of interest was chosen in the septum and signal intensities at lengthening echo times analysed using a truncation model [17]. LVEF were determined from steady-state free precession cines using CMR tools (Cardiovascular Imaging Solutions, London, UK).
Statistical Analysis
Data were expressed as mean± one standard deviation, or median (range) as appropriate. Mann Whitney U test or t test were used for comparison of continuous variables between groups. The significance of the correlation between parameters was assessed using Spearman rank as the data was not normally distributed. Categorical data were compared using the chi-square test. Multivariate analysis used multiple linear regression analyses. Statistical analyses were carried out using SPSS Statistics 13.0 (SPSS Inc., USA). A p value less than 0.05 was considered statistically significant, and all p values were two sided.
Results
Patients
The main characteristics of these patients along with the main results of the study were summarized in Table 1.
Table 1. The main characteristics of Chinese TM patients along with the main results of the study.
| Patients(n = 201) | |
| Male [n (%)] | 125(62.2) |
| Ethnicity | |
| Han | 161 |
| Other | 40 |
| Median age (range),years | 9(4–25) |
| Age group, years | |
| <6 [n (%)] | 14(7) |
| 6–<10 [n (%)] | 89(44.2) |
| 10–<15 [n (%)] | 79(39.3) |
| 15–<20 [n (%)] | 14(7) |
| 20–<25 [n (%)] | 5(2.5) |
| Previous chelation therapy, [n (%)] | |
| DFO | 42(20.9) |
| DFP | 25(12.4) |
| DFO+DFPa | 95(47.3) |
| DFX | 8(4) |
| DFO+DFXb | 20(9.9) |
| None | 11(5.5) |
| Mean ± SD number of transfusions | 116.3±91.4 |
| Median SF ng/mL (range) | 4536(524.7–23,640) |
| SF category [n (%)] | |
| <2500 ng/mL | 36(17.9) |
| ≥2500 ng/mL | 165(82.1) |
| cardiac T2*, ms | 25.5±12.5 |
| T2* category [n (%)] | |
| <10 ms | 26(12.9) |
| 10–20 ms | 42(20.9) |
| >20 ms | 133(66.2) |
| LVEF, [%] | 64.8±5.6 |
| LVEF category [n] | 175 |
| <56% [n (%)] | 6(3.4) |
| ≥56% [n (%)] | 169(96.6) |
DFO,deferoxamine; DFP, deferiprone; DFX, deferasirox; SD, standard deviation; SF, serum ferritin; LVEF, left ventricular ejection fraction.
a: patients received both DFO and DFP as prior chelation therapies, but these may not have been in combination.
b: patients received both DFO and deferasirox as prior chelation therapies, but these may not have been in combination.
Serum Ferritin
The median SF level of the patients was 4536 (525–23,640) ng/ml. The SF was >2500 ng/ml in 165 patients (82.1%). There was a significant correlation between SF and the total transfusion units (r = 0.175, p = 0.013). A similar result was found in the correlation between SF and age (r = 0.144, p = 0.042).
Cardiac T2*
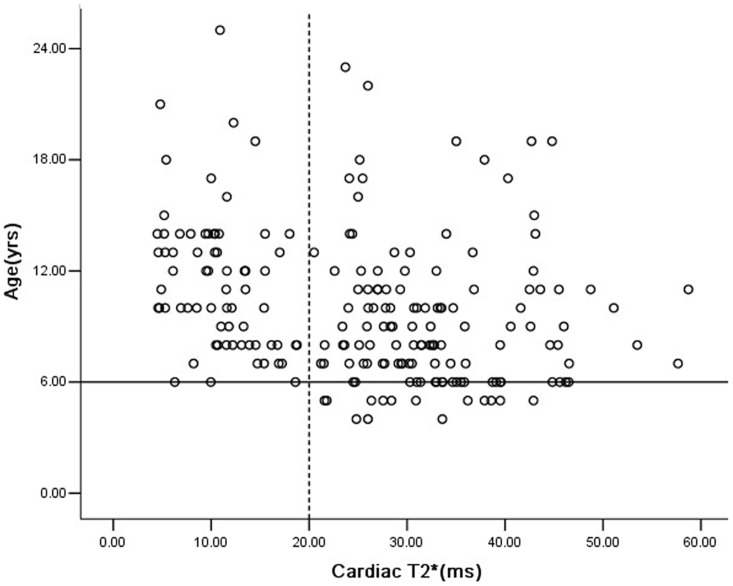
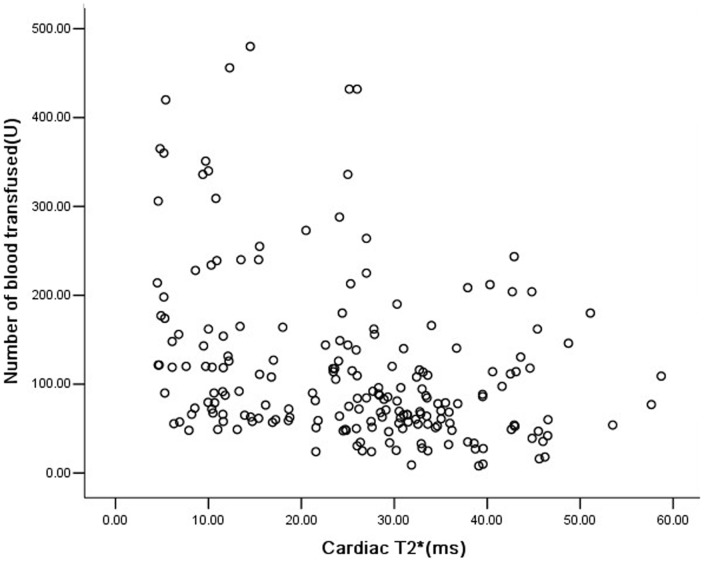
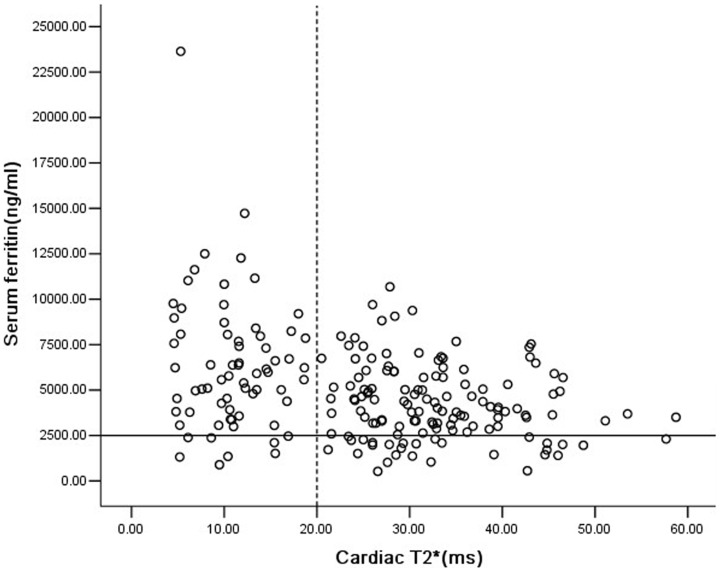
The mean cardiac T2* value was 25.5±12.5 ms (4.5–58.7 ms) for all 201 patients. Cardiac T2*≤20 ms were detected in 68 patients (33.8%), and cardiac T2*<10 ms were detected in 26 patients (12.6%). For patients ≤10 years old (n = 113), 32 patients (28.3%) had cardiac T2*≤20 ms, 3 of whom were only 6 years old. Fourteen patients of 5 years of age and younger were scanned, all with normal cardiac T2*>20 ms (Table 2). The five well-chelated patients all had cardiac T2*>20 ms. The mean cardiac T2* and incidence of cardiac T2*<10 ms in 161 Han ethnicity patients was not significantly different with 40 other ethnicity patients (25.8±12.7 vs 23.6±11.9, p = 0.311;33.5% vs 37.5%,p = 0.637).Cardiac T2* was negatively associated with age (Fig. 1, r = −0.328, p = 0.000) and the total units transfused (Fig. 2, r = −0.360, p = 0.000), respectively. A similar result was found in the correlation between cardiac T2* and SF (Fig. 3, r = −0.319, p = 0.000). Multivariate analysis confirmed the negative correlation between cardiac T2* and total units transfused (β = −0.297, P = 0.006), and SF (β = −0.234, P = 0.000), but the non-correlation between cardiac T2* and age (β = −0.081, P = 0.421).
Table 2. T2* information of patients divided into five-year age groups.
| T2* category | Age groups, n (%) | ||||
| ≤5 y | 6–10 y | 11–15 y | 16–20 y | >20 y | |
| >20 ms | 14(100) | 81(71.7) | 27(48.2) | 9(64.3) | 2(50) |
| 10–20 ms | 0 | 23(20.3) | 14(25.0) | 4(28.6) | 1(25) |
| <10 ms | 0 | 9(8.0) | 15(26.8) | 1(7.1) | 1(25) |
Figure 1. Correlation between cardiac T2* and age in Chinese TM patients.
The dotted line represents cardiac T2* of 20 ms. The solid line represents age of 6 years old. Cardiac T2* was negatively associated with age (r = −0.328, p = 0.000) in these patients.
Figure 2. Correlation between cardiac T2* and total units of blood transfused in Chinese TM patients.
Cardiac T2* was negatively associated with units of transfused blood(r = −0.360, p = 0.000).
Figure 3. Correlation between cardiac T2* and serum ferritin(SF) values in Chinese TM patients.
Cardiac T2* was negatively associated with SF (r = −0.319, p = 0.000). The dotted line represents reference range for cardiac T2* of 20 ms, and the solid line represents SF of 2500 ng/ml.
Left Ventricle Ejection Fraction (LVEF)
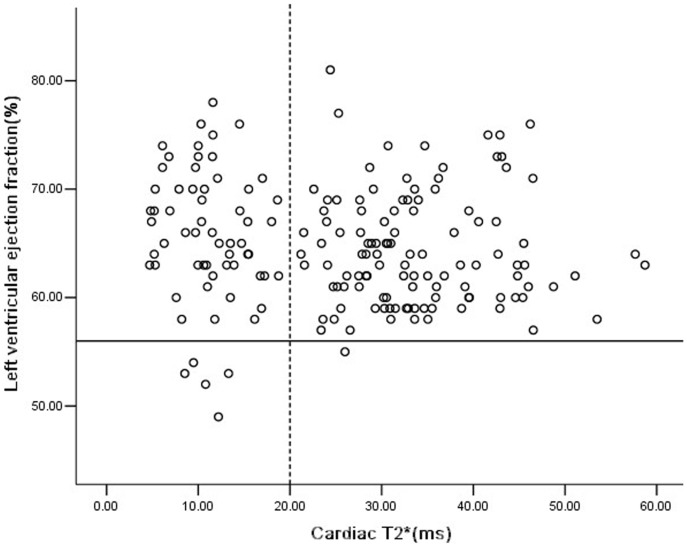
LVEF was measured by CMR in 175 patients and the median of LVEF was 64% (49%–81%). Twenty-six young patients failed to measure LVEF because they could not comply sufficiently with breath-hold instructions. Six patients (3.4%) had LVEF<56% [6]. The median LVEF of 62 patients with cardiac iron overload (T2*<20 ms) was not significantly different from those 113 patients without cardiac iron overload (T2*>20 ms) (65.5% vs 63%, p = 0.146). LVEF<56% was seen in 5 patients (8.1%) with cardiac iron overload, comparing with only 1 (0.9%) without cardiac iron overload (p = 0.021. Three patients with LVEF<56% were under 10 years old. No correlation was found between LVEF and SF (r = 0.111, p = 0.145) or LVEF and cardiac T2* (r = −0.147, p = 0.052) in these 175 patients (Fig. 4).
Figure 4. Correlation between cardiac T2* and left ventricular ejection fraction (LVEF) in Chinese TM patients.
No correlation was found between cardiac T2* and LVEF (r = −0.147, p = 0.052). The solid line presents reference range (56%) for LVEF. The dotted line represents reference range for cardiac T2* of 20 ms.
Cardiac Disease
A patient requiring cardiac medications, or with a LVEF<56%, or with persistent arrhythmia [18], [6] was considered to have a cardiac disease. Ten patients (5%) had cardiac disease, 3 with heart failure hospitalization, 1 with junctional rhythm, and 6 with impaired LV systolic function (LVEF<56%). Among them, 8 patients had cardiac iron overload; of the two patients with normal cardiac iron, one had myocarditis and the other junctional rhythm disturbance. None of the well-chelated patients had cardiac involvement. Four patients with cardiac involvement were under 10 years old. Three heart failure patients had cardiac iron overload but LVEF>56% (63%, 68% and 68%). The patient with junctional rhythm had both a normal cardiac T2* and a normal LVEF.
Discussion
Iron induced cardiomyopathy can be reversed if aggressive chelation begins early [19], [20]. Thus, early detection of myocardial iron deposition is imperative to prevent overt heart failure. In non-chelated patients receiving regular transfusions, cardiomegaly develops by the age of 10 years and heart failure by the age of 16 years [21]. The duration of life after the onset of failure was less than 3 months in over half of the patients. Clinical diagnosis is often delayed due to the typically late onset of symptoms. Iron overload in TM occurs due to a combination of repeated blood transfusions and excessive gastrointestinal absorption. Little is known about the natural history of iron deposition in the heart. Previous studies suggested that myocardial deposition takes place after a minimum of 75 blood transfusions [22], [23]. Assessment of myocardial iron is essential clinically but conventional noninvasive techniques are less than ideal. Endomyocardial biopsy is invasive and not reliable because myocardial iron deposition is not homogeneous [24], [25]. Even the combination of SF, liver iron concentration and ventricular function can only detect heart failure at a late stage [26], [27], [28]. Usefully, cardiac T2* can be used to monitor cardiac iron overload non-invasively, reproducibly and accurately. However, cardiac T2* data on children younger than 10 years is rather limited. Wood et al recently reported from a cohort of 77 TM patients in Italy and US that no cardiac iron was observed under the age of 9.5 years [29]. In another earlier report, it was suggested that cardiac iron overload occurred only after at least 13 years of chronic transfusion therapy [18]. These studies however, were based on well-chelated patients. Fernandes et al recently reported a single patient with cardiac iron overload at the age of 7 years but this study was limited to only 23 patients with TM and other forms of anemia [30]. In our study, the vast majority of patients (97.5%) had not received a good standard of chelation therapy. Cardiac iron overload was found in patients under 10 years old with severe cardiac iron overload in a proportion, associated with impaired LVEF and cardiac symptoms. We detected severe myocardial siderosis in patients as young as 6 years old.
The median age of our TM patients was 9 (4–25) years old. The median SF level was 4536 ng/ml and 165 patients (82.1%) had SF>2500 ng/ml. The data confirmed our previous finding that Chinese TM patients were younger and had greater burden of iron overload than those in western countries [31]. Myocardial iron overload was detected in 33.8% of our patients. This value was lower than that reported in west countries, where cardiac T2*<20 ms were seen in 32%–65% of adults with TM [32], [33], [16]. One explanation was that our patients were much younger and most of them were children, but it is also notable that the average yearly units transfused was only 13 per patient. SF was positive associated with age. Cardiac T2* was negatively associated with age and with SF. This finding was in contrast to other reports showing no relation between cardiac T2* and SF in patients chelated from a young age with a variety of different regimes, in which the varying regimes may have resulted in the loss of relationship between cardiac T2* and SF. For instance it is recognized that DFO results in preferential chelation from the liver whereas Deferiprone may be more effective for chelation of cardiac iron. The younger age and lower levels of chelation therapy may account for the discrepancy between our and previous studies [34], [35], [33].
To our knowledge, this was the first cardiac T2* study on a large and poorly-chelated and modestly transfused TM population. The results may help develop guidelines to the clinical assessment and management of iron overload in TM patients in developing countries. It is estimated that more than 300,000 children are born annually with severe inherited disorders of hemoglobin and approximately 80% of these births occur in low- or middle-income countries [36], [37]. Over the next 20 years approximately 100,000 cases of Hb E/βthalassaemia will be added to the Thailand population and 20,000 beta TM babies will be born each year in China [38], [39]. It is a global public health challenge particularly in developing countries. In low- or middle-income countries, many TM children receive inadequate iron chelating therapy and many of them die as teenagers due to the effects of iron overload [10]. Bejaoui M et al studied 391 TM patients in Tunisia. They found that the mean age of death was 10.48 years with heart failure as the major cause of death [40]. A disturbing study of 106 TM patients in Baise City (Guangxi,China), showed that 85 patients (80%) died before the age of 5 years [11]. At present, TM patients older than 30 years old are seldom found in mainland China. The current life expectancy of TM patients is unclear. While progress has been made for the control and genetic screening for thalassemia trait over the last decade in China, health care for patients suffering with TM in China continues to pose a great challenge to the public health services. Many factors, including the lack of disease specific knowledge, shortage of blood, limited availability of chelators, lack of specialist physicians and the limited resources within the health insurance system pose challenges to optimum care. Although health insurance coverage has increased dramatically over the last decade in China, from 15% in 2000 to 96% in 2011, the financial protection remains insufficient [41]. Although DFO has been used for over 40 years in Western countries, it has only been available in China since 1998. DEP and DFX were introduced to China in 2003 and 2010, respectively. Further, CMR T2* was first introduced to China in 2010 and only a small number of TM patients have benefitted from this technique. In brief, developing countries lag far behind developed countries in the control and management of thalassemia. It is therefore vital that international health agencies and governments of countries where these diseases are common start to develop the partnerships between rich and low-income countries and establish specific guidelines towards the most economic and effective approach to control and manage thalassemia.
In conclusion, we present the findings of a large cohort of Chinese TM children and adolescents. The vast majority of patients are poorly-chelated and as a result of relative chelation naivety, clear associations can still be demonstrated between cardiac iron stores and age and serum ferritin. Myocardial siderosis is strongly associated with impaired LVEF and heart failure. Our results show the effects of poor chelation of young patients and that the first assessment of cardiac T2* should take place as early as 6 years of age.
Funding Statement
This work was supported by research grants from National Natural Science Foundations of China (Grant No. 30860307; No. 81160175) and Guangxi Natural Science Foundations (Grant No. 2011GXNSFD018033). Dr.. He receives support from Wellcome Trust, and Higher Education Funding for England (HEFCE) and British Heart Foundation (BHF) (FS/08/26225). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Modell B, Khan M, Darlison M (2000) Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet 355: 2051–2052. [DOI] [PubMed] [Google Scholar]
- 2. Modell B, Khan M, Darlison M, Westwood MA, Ingram D, et al. (2008) Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson 10: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, et al. (2004) Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 89: 1187–1193. [PubMed] [Google Scholar]
- 4. Kremastinos DT, Tsetsos GA, Tsiapras DP, Karavolias GK, Ladis VA, et al. (2001) Heart failure in beta thalassemia: a 5-year follow-up study. Am J Med 111: 349–354. [DOI] [PubMed] [Google Scholar]
- 5. Brittenham GM, Badman DG (2003) Noninvasive measurement of iron: report of an NIDDK workshop. Blood 101: 15–19. [DOI] [PubMed] [Google Scholar]
- 6. Kirk P, Roughton M, Porter JB, Walker JM, Tanner MA, et al. (2009) Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation 120: 1961–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson LJ, Wonke B, Prescott E, Holden S, Walker JM, et al. (2002) Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta-thalassaemia. Lancet 360: 516–520. [DOI] [PubMed] [Google Scholar]
- 8. Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, et al. (2007) A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation 115: 1876–1884. [DOI] [PubMed] [Google Scholar]
- 9.Guidelines for clinical management of thalassaemia 2ND Revised Edition (2008) pp. 86.
- 10. Angastiniotis M, Modell B (1998) Global epidemiology of hemoglobin disorders. Ann N Y Acad Sci 850: 251–269. [DOI] [PubMed] [Google Scholar]
- 11. Pan HF, Long GF, Li Q, Feng YN, Lei ZY, et al. (2007) Current status of thalassemia in minority populations in Guangxi, China. Clin Genet 71: 419–426. [DOI] [PubMed] [Google Scholar]
- 12. Aessopos A, Farmakis D, Hatziliami A, Fragodimitri C, Karabatsos F, et al. (2004) Cardiac status in well-treated patients with thalassemia major. Eur J Haematol 73: 359–366. [DOI] [PubMed] [Google Scholar]
- 13.Guidelines for clinical management of thalassaemia 2ND Revised Edition (2008) pp. 61–63.
- 14. Westwood M, Anderson LJ, Firmin DN, Gatehouse PD, Charrier CC, et al. (2003) A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. J Magn Reson Imaging 18: 33–39. [DOI] [PubMed] [Google Scholar]
- 15. Maceira AM, Prasad SK, Khan M, Pennell DJ (2006) Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 8: 417–426. [DOI] [PubMed] [Google Scholar]
- 16. Alpendurada F, Carpenter JP, Deac M, Kirk P, Walker JM, et al. (2010) Relation of myocardial T2* to right ventricular function in thalassaemia major. Eur Heart J 31: 1648–1654. [DOI] [PubMed] [Google Scholar]
- 17. He T, Zhang J, Carpenter JP, Feng Y, Smith GC, et al. (2013) Automated truncation method for myocardial T2* measurement in thalassemia. J Magn Reson Imaging 37: 479–483. [DOI] [PubMed] [Google Scholar]
- 18. Wood JC, Tyszka JM, Carson S, Nelson MD, Coates TD (2004) Myocardial iron loading in transfusion-dependent thalassemia and sickle cell disease. Blood 103: 1934–1936. [DOI] [PubMed] [Google Scholar]
- 19. Aldouri MA, Wonke B, Hoffbrand AV, Flynn DM, Ward SE, et al. (1990) High incidence of cardiomyopathy in beta-thalassaemia patients receiving regular transfusion and iron chelation: reversal by intensified chelation. Acta Haematol 84: 113–117. [DOI] [PubMed] [Google Scholar]
- 20. Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, et al. (2008) Combined chelation therapy in thalassemia major for the treatment of severe myocardial siderosis with left ventricular dysfunction. J Cardiovasc Magn Reson 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Engle MAEM, Ch S (1964) Late cardiac complictions of chronic, severe, refractory anemia with hemochromatosis. Circulation 30: 698–705. [DOI] [PubMed] [Google Scholar]
- 22. Wood JC (2008) Cardiac iron across different transfusion-dependent diseases. Blood Rev 22 Suppl 2: S14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tziomalos K, Perifanis V (2010) Liver iron content determination by magnetic resonance imaging. World J Gastroenterol 16: 1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fitchett DH, Coltart DJ, Littler WA, Leyland MJ, Trueman T, et al. (1980) Cardiac involvement in secondary haemochromatosis: a catheter biopsy study and analysis of myocardium. Cardiovasc Res 14: 719–724. [DOI] [PubMed] [Google Scholar]
- 25. Barosi G, Arbustini E, Gavazzi A, Grasso M, Pucci A (1989) Myocardial iron grading by endomyocardial biopsy. A clinico-pathologic study on iron overloaded patients. Eur J Haematol 42: 382–388. [DOI] [PubMed] [Google Scholar]
- 26. Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, et al. (1994) Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med 331: 567–573. [DOI] [PubMed] [Google Scholar]
- 27. Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, et al. (1994) Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med 331: 574–578. [DOI] [PubMed] [Google Scholar]
- 28. Davis BA, O'Sullivan C, Jarritt PH, Porter JB (2004) Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood 104: 263–269. [DOI] [PubMed] [Google Scholar]
- 29. Wood JC, Origa R, Agus A, Matta G, Coates TD, Galanello R (2008) Onset of cardiac iron loading in pediatric patients with thalassemia major. Haematologica 93: 917–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fernandes JL, Fabron A Jr, Verissimo M (2009) Early cardiac iron overload in children with transfusion-dependent anemias. Haematologica 94: 1776–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lai Yong-rong, Li Chun-fu, Huang Shao-liang, Qi-Li, Shen Zhi-xiang, et al. (2013) Efficacy of deferasirox for the treatment of iron overload in Chinese thalassemia major patients: results from a prospective, open-label, multicenter clinical trial. Transfus Med 2013 Dec;23 6: 389–96 doi: 10.1111/tme.12077. Epub 2013 Oct 23 [DOI] [PubMed] [Google Scholar]
- 32. Tanner MA, Galanello R, Dessi C, Westwood MA, Smith GC, et al. (2006) Myocardial iron loading in patients with thalassemia major on deferoxamine chelation. J Cardiovasc Magn Reson 8: 543–547. [DOI] [PubMed] [Google Scholar]
- 33. Positano V, Pepe A, Santarelli MF, Ramazzotti A, Meloni A, et al. (2009) Multislice multiecho T2* cardiac magnetic resonance for the detection of heterogeneous myocardial iron distribution in thalassaemia patients. NMR Biomed 22: 707–715. [DOI] [PubMed] [Google Scholar]
- 34. Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, et al. (2001) Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 22: 2171–2179. [DOI] [PubMed] [Google Scholar]
- 35. Leung AW, Chu WC, Lam WW, Lee V, Li CK (2009) Magnetic resonance imaging assessment of cardiac and liver iron load in transfusion dependent patients. Pediatr Blood Cancer 53: 1054–1059. [DOI] [PubMed] [Google Scholar]
- 36.Christianson A HCP, Modell B (2006) March of Dimes Global Report on Birth Defects. New York, NY: March of Dimes Birth Defects Foundation. [Google Scholar]
- 37. Modell B, Darlison M (2008) Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ 86: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weatherall DJ, Clegg JB (1996) Thalassemia–a global public health problem. Nat Med 2: 847–849. [DOI] [PubMed] [Google Scholar]
- 39.Weatherall DJ, Clegg JB (2001) The thalassaemia syndromes. Oxford, Blackwell Science. [Google Scholar]
- 40. Bejaoui M, Guirat N (2013) Beta thalassemia major in a developing country: epidemiological, clinical and evolutionary aspects. Mediterr J Hematol Infect Dis 5: e2013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meng Q, Xu L, Zhang Y, Qian J, Cai M, et al. (2012) Trends in access to health services and financial protection in China between 2003 and 2011: a cross-sectional study. Lancet 379: 805–814. [DOI] [PubMed] [Google Scholar]