Summary
We describe a second primase in human cells, PrimPol, which has the ability to start DNA chains with deoxynucleotides unlike regular primases, which use exclusively ribonucleotides. Moreover, PrimPol is also a DNA polymerase tailored to bypass the most common oxidative lesions in DNA, such as abasic sites and 8-oxoguanine. Subcellular fractionation and immunodetection studies indicated that PrimPol is present in both nuclear and mitochondrial DNA compartments. PrimPol activity is detectable in mitochondrial lysates from human and mouse cells but is absent from mitochondria derived from PRIMPOL knockout mice. PRIMPOL gene silencing or ablation in human and mouse cells impaired mitochondrial DNA replication. On the basis of the synergy observed with replicative DNA polymerases Polγ and Polε, PrimPol is proposed to facilitate replication fork progression by acting as a translesion DNA polymerase or as a specific DNA primase reinitiating downstream of lesions that block synthesis during both mitochondrial and nuclear DNA replication.
Graphical Abstract
Highlights
-
•
PrimPol is a second human primase that is able to start DNA chains with dNTPs
-
•
Identification of PrimPol as a DNA polymerase that tolerates AP sites and 8oxoG lesions
-
•
PrimPol is located at both DNA compartments, mitochondria, and the nucleus
-
•
PRIMPOL gene silencing affects mtDNA replication
Introduction
DNA polymerases make and repair DNA, thereby controlling both the stability and variability of genetic information. Whereas both replication and repair employ DNA polymerases that maximize faithful DNA synthesis, other tasks such as lesion bypass come at the price of lower fidelity and are performed by the so-called “sloppy” DNA polymerases (Goodman and Tippin, 2000). In humans, 16 different DNA-synthesizing enzymes are dedicated to replication, repair, damage tolerance, and variability of nuclear DNA (reviewed in Hübscher et al., 2002). In stark contrast, only one DNA polymerase has been described in mitochondria (Bolden et al., 1977), DNA polymerase gamma (Polγ), which has long been presumed to mediate every DNA synthesis reaction related to the replication and repair of mitochondrial DNA (mtDNA).
None of the known 17 human DNA polymerases can initiate DNA synthesis, and, because RNA polymerases can polymerize ribonucleotides from a single nucleoside 5′-triphosphate (NTP), RNA is widely used to prime DNA synthesis. In most cases, such RNA primers are produced by specialized polymerases or “primases” (Frick and Richardson, 2001). Primases can be divided in two evolutionarily unrelated families: DnaG-like (bacteria) and AEP-like (archaea and eukaryotes) (Aravind et al., 1998, Iyer et al., 2005). The human primase operating at nuclear DNA is a heterodimer (Pri1+ Pri2) wherein Pri1 is the small catalytic subunit belonging to the AEP family. During nuclear DNA replication, the primase interacts with Polα, forming a very stable complex (Polα-primase) that triggers the initiation of DNA synthesis. The few primers made at ori sequences are further elongated by the leading-strand DNA polymerase (Polε); conversely, other primers are synthesized repeatedly, much like those used to initiate the synthesis of Okazaki fragments in the antiparallel lagging strand.
Exceptionally, RNA primers can be generated by the transcription activity of RNA polymerases, as in plasmid ColE1 (Itoh and Tomizawa, 1980) and during the replication of mtDNA (Chang and Clayton, 1985, Fusté et al., 2010). The replication of mtDNA (a small circle of about 17 kb in human cells) is a complex process involving multiple mechanisms (Holt and Reyes, 2012). At the replication fork, the mitochondrial DNA helicase TWINKLE opens the duplex, and the mitochondrial single-stranded binding protein (mtSSB) stabilizes the unwound DNA (Falkenberg et al., 2007), although the extent of coating of the lagging-strand template with mtSSB may be minimal because of the incorporation of processed transcripts (Reyes et al., 2013). Mitochondrial RNA polymerase (POLRMT) primes at one major initiation site on each strand, oriH and oriL (Fusté et al., 2010 and the references therein), and primer elongation is performed by Polγ (Falkenberg et al., 2007). However, minor replication products of coupled leading- and lagging-strand mtDNA synthesis (Holt et al., 2000) would need frequent priming on the lagging strand. As long ago as 1985, a primase activity distinct from POLRMT was detected in human mitochondria, although the protein responsible has not yet been identified (Wong and Clayton, 1985).
Here, ccdc111, a putative primase based on in silico predictions (Iyer et al., 2005), is shown to have both DNA primase and DNA polymerase activities (and, thus, is renamed PrimPol) and a striking capacity to tolerate DNA damage. PrimPol was shown to be present at both nuclear and mitochondrial DNA compartments but is absent in mitochondria derived from a mouse PrimPol knockout (KO) model. RNAi experiments in human cells and analysis of PRIMPOL KO mouse embryonic fibroblasts (MEFs) indicated that PrimPol plays a key role in mtDNA synthesis. A plausible hypothesis is that this primase/polymerase facilitates replication restart after programmed or damage-induced fork arrest.
Results
CCDC111, an Ancient Eukaryotic Gene Predicted to Encode a Primase
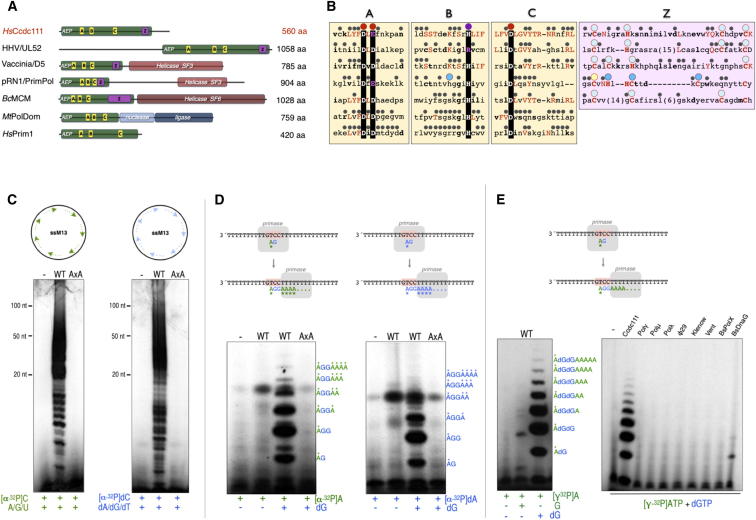
Human gene CCDC111 (also known as FLJ33167), which is located on chromosome 4q35.1 and codes for coiled coil domain-containing (ccdc111) protein, was predicted to be a member of the AEP family (Iyer et al., 2005). ccdc111 contains the active-site motifs of AEP primases and a putative Zn finger similar to that of herpesvirus UL52 primase and other AEP-like enzymes (Figures 1A and 1B; Figure S1 available online).
Figure 1.
Human ccdc111 Is a Unique RNA/DNA Primase
(A) Modular organization of various AEP-like enzymes. A conserved AEP domain (green bar) contains the three conserved regions A, B, and C, which form the primase active site. Hsccdc111 contains, in addition to regions A, B, and C, a Zn-finger-containing region also present in the viral, plasmidic, and bacterial specimens. Additionally, some APE enzymes contain additional activities and domains as helicases (red bars) or a combination of nuclease and ligase (blue bars). Hsccdc111, human ccdc111 protein; HHV/UL52, herpes virus UL52 primase; Vaccinia/D5, vaccinia virus primase; pRN1/PrimPol, plasmid pRN1 ORF904 from S. islandicus; BcMCM, PrimPol helicase from Bacillus cereus; MtPolDom, LigD polymerization domain from Mycobacterium tuberculosis; and HsPrim1, small catalytic subunit of the human RNA primase. See also Figure S1.
(B) Conserved regions in proteins having and AEP-related domain. Large dots indicate metal ligands (red), nucleotide ligands (purple), and demonstrated (cyan) or potential (light blue) cysteine residues forming a Zn finger. Small dots indicate invariant residues in each group of AEP-related enzymes. Residues that are either conserved or identical to those in ccdc111 are indicated in bold or red letters, respectively. See also Figure S1 for a more extensive alignment.
(C) Primer synthesis on M13 ssDNA using either NTPs or dNTPs. A scheme of the primase reaction is shown. WT ccdc111 (but not mutant AxA) synthesized de novo RNA (left) or DNA (right) primers on the M13 ssDNA circular template in the presence of activating Mn2+ ions. See also Figures S2 and S3.
(D) Priming at an ori-containing oligonucleotide. The schematic shows the different (initiation and elongation) products synthesized by a primase that recognizes the central GTCC sequence as a starting ori, providing the indicated combinations of NTPs (in green) and dNTPs (in blue). The primase products obtained with ccdc111 were very similar when either ATP (left) or dATP (right) were provided as 5′ nucleotide (in addition to dGTP, provided as a common 3′nucleotide). See also Figures S2 and S3.
(E) The same primase assay as in (D) except that primase products were labeled with [γ-32P]ATP as the 5′ nucleotide. dGTP was a much better alternative than GTP as 3′ nucleotide (left). In these preferred conditions, the formation of the ribo-deoxy dimer (and further elongation products) is a hallmark of PrimPol, which cannot be obtained with other DNA polymerases or conventional primases (right).
Iterative BLAST searches with human ccdc111 and putative homologs indicated the presence of ccdc111 in a broad range of unicellular and multicellular eukaryotes, including animals, plants, unicellular microalgae, protists, choanoflagellates, fungi, and picoeukaryotes such as Ostreococcus (the smallest known free-living eukaryote with a single chloroplast and a single mitochondrion). Alignment of putative ccdc111 homologs (Figure S1) revealed 14 conserved regions, including the Zn finger domain and the motifs A, B, and C shared with AEP primases, nonhomologous end-joining primases, PrimPols, and Herpes and poxvirus-like primases (Figures 1A, 1B, and S1). All members of the ccdc111 family have the motif A variant DxE (Figures 1B and S1) instead of the DxD motif present in most members of the AEP superfamily. The DxE variant is also found in the PrimPol of plasmid pRN1 from Sulfolobus islandicus, which has both primase and DNA polymerase activities (Lipps et al., 2003).
Ccdc111 Is an Active Primase that Can Initiate Nucleic Acid Synthesis with dNTPs
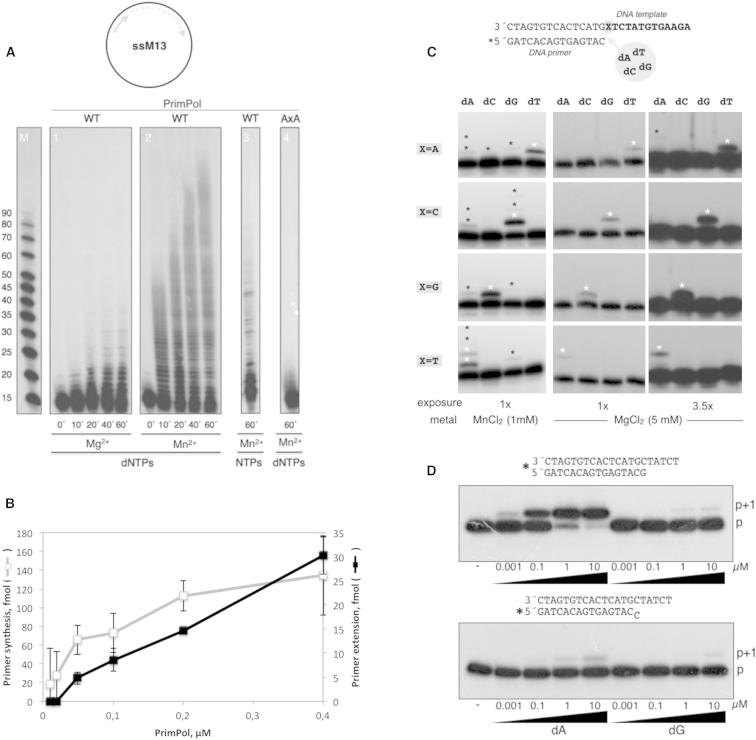
In agreement with in silico predictions (Iyer et al., 2005), purified human ccdc111 (Figure S2A) displayed RNA primase activity on a single-stranded circular DNA template (M13 ssDNA) (Figure 1C, left). Interestingly, the protein was dependent on manganese for RNA synthesis, yielding remarkably long primers (>100 nt) that were not generated in the presence of magnesium (Figures S2B and S3A, left). Strikingly, a similar primase activity was observed when NTPs were replaced by deoxynucleoside 5′-triphosphates (dNTPs) (Figures 1C and S3A, right). In general, primases make RNA primers, but the AEP-related primases of archea and some bacteria are exceptions to this rule (Lao-Sirieix et al., 2005, Sánchez-Berrondo et al., 2012) and use dNTPs as valid substrates for priming. This unusual RNA and DNA primase activity is not present in Escherichia coli and was demonstrated to be inherent to human ccdc111 by ablating the activity with a double mutation (AxA) in the two potential metal ligands (Asp114 and Glu116, motif DxE) at the predicted active site (Figure 1C), which was in agreement with enzymatic and structural studies of the PrimPol from plasmid pRN1 (Lipps et al., 2004).
Next, we evaluated the primase activity on a template oligonucleotide in which a potential recognition sequence (GTCC) was flanked by thymine residues (Cavanaugh and Kuchta, 2009). Such a tract of pyrimidines is the preferred template context for several viral, prokaryotic, and eukaryotic RNA primases (Frick and Richardson, 2001). Again, Mn2+-activated synthesis of DNA primers and their extensive elongation were observed with purified human ccdc111 (Figure S2C). Ccdc111 primase recognized the 3′ GTCC-5′ sequence, preferentially forming initiating dinucleotides 5′ A-dG-3′ or 5′ dA-dG-3′ (Figures 1D and 1E). There was no difference in the efficiency of initiation when providing either a ribo- or a deoxynucleotide at the 5′position (Figure 1D). Again, mutant AxA was inactive in this assay, supporting the intrinsic nature of the primase activity attributable to ccdc111 protein. In respect to the second nucleotide, ccdc111 displayed a marked preference for a dNTP (Figure 1E, left). Mn2+-activated formation of a ribo-deoxy-initiating dimer (occurring at Mn2+ concentrations as low as 50 μM; Figure S3B) is a hallmark of ccdc111, given that several other DNA synthesizing enzymes were unable to catalyze this reaction, and a conventional primase such as Bacillus subtilis DnaG displayed only a modest activity (Figure 1E, right).
Ccdc111 Primase Is also a DNA-Directed DNA Polymerase
Unlike eukaryotic primases, archeal primases are able to carry out the initiation and extension of both RNA and DNA chains of up to 1 or 7 kb, respectively (Lao-Sirieix and Bell, 2004, Lao-Sirieix et al., 2005, Chemnitz Galal et al., 2012). Hence, these enzymes are both primases and polymerases, or PrimPols (Lipps et al., 2003). Therefore, we tested whether, in addition to its DNA and RNA primase activity, ccdc111 possessed DNA-dependent polymerase activity. ccdc111 was able to extend a DNA primer hybridized to M13 ssDNA by polymerizing dNTPs (Figure 2A). Again, Mn2+ was the preferred metal (Figure 2A, compare panels 1 and 2), allowing the nonprocessive elongation of DNA chains up to 5 kb (data not shown). The enzyme also utilized NTPs, albeit with a much lower efficiency (Figure 2A, panel 3). As with the primase activity, the DNA polymerase activity was abolished in the AxA mutant (Figure 2A, panel 4). Hence, the primase and polymerase activities of ccdc111 (compared in Figure 2B as a function of enzyme concentration) appear to share the same active site. Thus, in light of its identified activities, we propose that ccdc111 should be renamed PrimPol.
Figure 2.
ccdc111 Is a DNA-Dependent DNA Polymerase
(A) Elongation of a 17-mer primer annealed to M13 circular ssDNA in the presence of either 5 mM MgCl2 or 1 mM MnCl2 as metal activators. WT ccdc111 (but not mutant AxA) extended the primer producing elongation intermediates characteristic of distributive DNA synthesis. NTPs were worse substrates for polymerization by ccdc111.
(B) Compared efficiency of primase and polymerase activities of PrimPol. The primase assay, using the 29-mer GTCC oligo (5′-T15CCTGT10-3′) as template, and the DNA polymerase assay, using a template/primer oligonucleotide, were carried out as described in the Experimental Procedures. Data are represented as mean ± SD (n = 3). See also Figure S3.
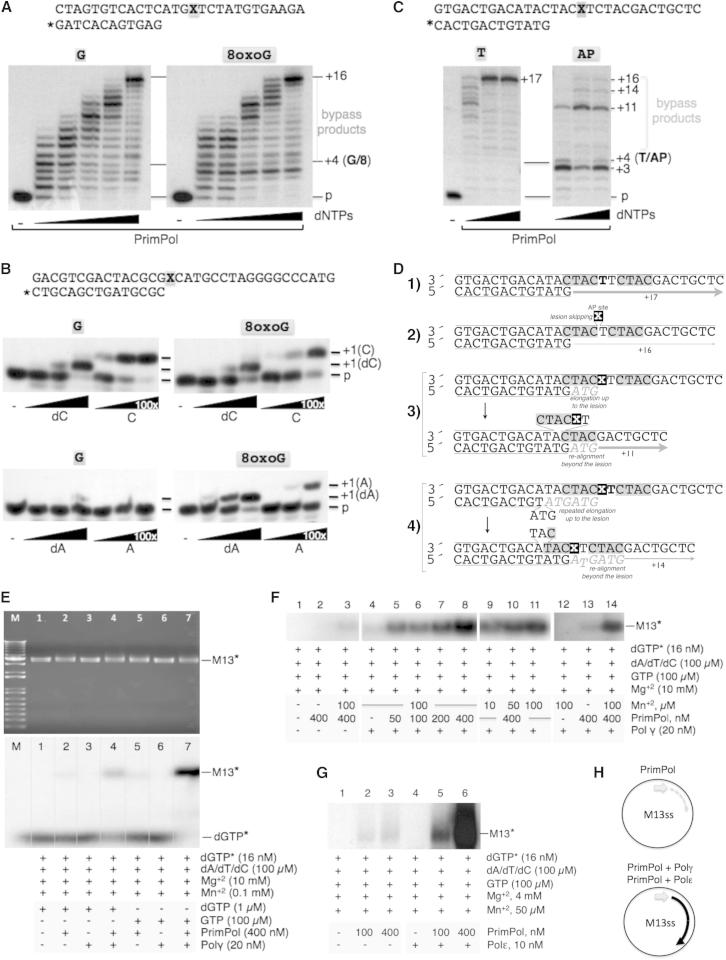
(C) Matched versus mismatched nucleotide insertion at the four template bases. The four template/primer structures used, differing in the first template base (X), are indicated. Nucleotide insertion on each template/primer was analyzed in the presence of each individual dNTP. PrimPol preferentially inserted the complementary nucleotide dictated by the first available templating base. The insertion of some errors (indicated with asterisks) can be detected.
(D) Polymerization assays were carried out with either a matched (top) or mismatched (middle) template/primer and the indicated concentrations of a correct (dA) or wrong (dG) nucleotide. After incubation, +1 extension of the 5′-labeled (∗) strand was analyzed by denaturing gel and autoradiography.
PrimPol efficiently catalyzed DNA polymerization within a wide range of Mn+2 concentrations (Figure S3C). Even at the lowest Mn+2 concentration tested (10 μM), PrimPol was more efficient than using a physiological concentration of Mg+2 (10 mM). Thus, Mn+2 is a more than 1,000-fold better metal activator than Mg2+, suggesting that it is likely to be the physiological cofactor of PrimPol. A similar behavior has been described for human Polλ (Blanca et al., 2003). Independent of the metal cation, PrimPol behaved as a DNA-instructed DNA polymerase, preferentially inserting the complementary nucleotide dictated by the first available templating base (Figure 2C). PrimPol provided a 1,000-fold bias toward correct Watson-Crick base pairs when extending a correctly-paired primer terminus (Figure 2D, top). PrimPol could not excise a mismatched primer (Figure 2D, bottom), demonstrating the absence of a proofreading 3′-5′ exonuclease activity. This is in agreement with the lack of ExoI, ExoII, and ExoIII consensus motifs, which form an evolutionarily conserved 3′-5′exonuclease active site in several DNA polymerase families (Bernad et al., 1989). However, the extension of a mismatched primer terminus by PrimPol is about 1,000-fold less efficient than the extension of a correctly paired primer terminus (Figure 2D).
PrimPol Is Located in Both Compartments with DNA, Mitochondria, and the Nucleus
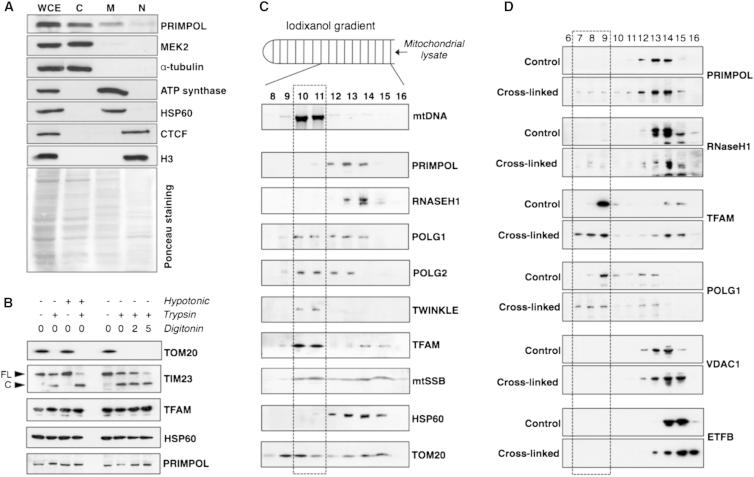
Given that PrimPol is a DNA primase/polymerase (Figures 1C–1E, 2, S2, and S3), it should logically operate in the nucleus and/or mitochondrion. Biochemical fractionation of HeLa cells revealed that PrimPol was distributed between the cytosol (47%), mitochondria (34%), and nuclear (19%) compartments (Figure 3A). In order to determine whether the mitochondrial-associated PrimPol was inside the organelles, mitochondria from human embryonic kidney (HEK) 293T cells were treated with trypsin and exposed to hypotonic buffer or digitonin (Figure 3B). As expected, a mitochondrial protein located in the outer membrane (TOM20) proved sensitive to trypsin, as did the portion of an inner membrane protein facing the intermembrane space (TIM23) after rupturing of the outer membrane (Figure 3B), whereas matrix proteins (TFAM and HSP60) and PrimPol were resistant to trypsin in all cases (Figure 3B). Hence, the fraction of PrimPol associated with mitochondria is inferred to be in the matrix.
Figure 3.
PrimPol Localizes at Both DNA Compartments, Mitochondria, and Nucleus
(A) 5 × 106 HeLa cells were fractionated (see the Experimental Procedures) in order to estimate the distribution of PrimPol between the different subcellular compartments. The distribution, calculated as an average of three independent experiments, was 47% cytosolic (C), 34% mitochondrial (M), and 19% nuclear (N). WCE, whole-cell extract. Mek2 and tubulin are shown as controls for the cytosolic fraction, Hsp60 and ATP synthase are shown as controls for the mitochondrial fraction, and CTCF and H3 are shown as controls for the nuclear fraction.
(B) Mitochondria isolated from HEK 293T cells were treated with hypotonic buffer or increasing concentrations of digitonin (2 or 5 ×10 mg/ml) followed or not by tripsin digestion. The amount of PrimPol remaining after each treatment was determined by immunoblot. TOM20 and TIM23 were used as outer and inner membrane markers, whereas TFAM and HSP60 were used as matrix markers.
(C) Mitochondria isolated from HEK 293T cells were subjected to lysis and fractionation on an iodixanol gradient (IG). DNA and protein were recovered from the different fractions and hybridized to a DNA probe specific for human mtDNA or antibodies against PrimPol and proteins involved in mtDNA maintenance, respectively.
(D) Mitochondria isolated from HEK 293T cells were crosslinked with formaldehyde or left untreated and subjected to lysis and fractionation on an IG. Proteins were recovered from the different fractions and hybridized to antibodies against PrimPol, proteins involved in mtDNA maintenance (RHaseH1, TFAM, and POLG1), and controls for nonspecific crosslinking (VDAC1 and ETFB).
Mitochondrial PrimPol-Containing Fractions Show Primase Activity
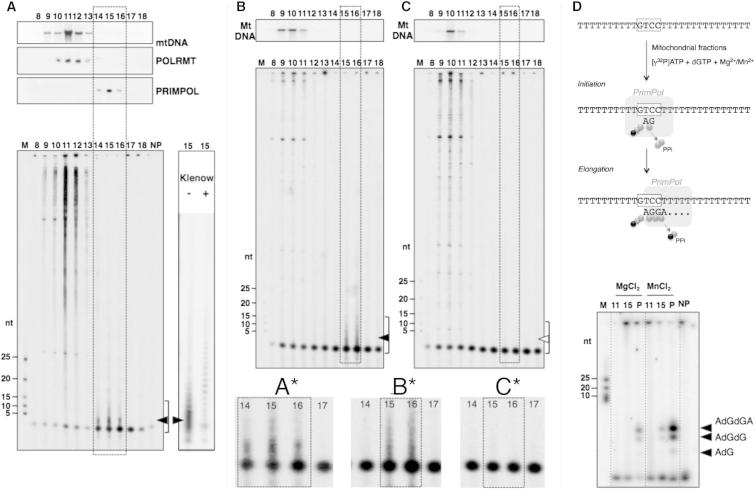
Fractionation of detergent-solubilized mitochondria of HEK 293T cells on an iodixanol gradient (IG) separated the majority of PrimPol and RNASE-H1 from mtDNA and mtDNA maintenance proteins such as TWINKLE and TFAM (Figure 3C). Formaldehyde crosslinking increased the amount of PrimPol and RNASE-H1 cofractionating with mtDNA but not other mitochondrial proteins, such as VDAC1 or ETFB (Figure 3D). These data suggest that PrimPol, like RNASE-H1, interacts with mtDNA transiently and that this interaction may be disrupted by the conditions used to lyse mitochondria. Importantly, in the absence of crosslinking, IG sedimentation completely separated PrimPol from the mitochondrial RNA polymerase POLRMT (Figure 4A), thereby enabling the activities of the two enzymes to be assessed independently. When the different fractions from the IGs were subjected to a DNA primase assay with the use of oligo dT25 as template, those fractions enriched in mtDNA (10–12) yielded a mixed pattern of products (Figure 4A) that can be attributed to the activities of Polγ alone or in conjunction with POLRMT, terminal transferase(s), and poly-A polymerase; in contrast, fractions enriched in PrimPol (14–16) displayed a precise pattern of oligonucleotide products of ∼2–12 nt (Figure 4A, filled arrowhead, see also the zoomed-in area in Figure 4A∗), whose relative abundance correlated with the amount of protein detected by immunoblotting and that could be further extended by Klenow DNA polymerase (Figure 4A, right).
Figure 4.
PrimPol-Enriched Fractions from Mitochondria Have Primase Activity Similar to the Purified Protein
(A) Mitochondria isolated from HEK 293T cells were treated with trypsin prior to lysis and fractionation on an IG. Primase assays were carried out from each sample with oligo dT25 as template, and the resulting products were resolved in 7M urea-containing 8% polyacrylamide gels. Filled arrowheads indicate specific products detected in PrimPol fractions. The presence of mtDNA was confirmed by southern blot, whereas PrimPol and POLRMT were detected by immunoblotting.
(B and C) Mitochondria isolated from PrimPol +/+ (B) or PrimPol −/− (C) MEFs (see also Figure S4) were fractionated in IG, and primase assays were carried out as in (A). Filled or empty arrowheads indicate specific products detected in PrimPol fractions or their absence, respectively. Zoom-in regions (A∗, B∗, and C∗) contain PrimPol specific products from (A), (B), and (C), respectively.
(D) PrimPol-specific labeling of de novo initiation products with oligo d(T20GTCCT36) as a template. Selected mtDNA-, POLRMT- (11), and PrimPol-enriched (15) IG fractions were assayed in parallel to recombinant purified PrimPol (P) and no-protein control (NP) in the conditions described in Figure S2E. The resulting products were resolved in 8M urea-containing 20% polyacrylamide gels and visualized by autoradiography.
To establish that PrimPol was responsible for the observed DNA primase activity, we subjected mitochondrial fractions from MEFS possessing (+/+) or lacking (−/−) the PRIMPOL gene (Figure S4) to the DNA primase assay. In PRIMPOL+/+ MEF mitochondria, short DNA products of ∼2–12 nt were detected in gradient fractions 15 and 16 (Figure 4B); i.e., the portion of the gradient where human PrimPol migrates (Figure 4A). No such products were detected in mitochondria from PRIMPOL−/− cells (Figure 4C; empty arrowhead, compare the zoomed-in areas in Figures 4B∗ and 4C∗).
In addition, selected mitochondrial fractions from HEK 293T cells were tested for the PrimPol-specific primase activity with oligo d(T20GTCCT36) as a template, ATP, and dGTP (see the scheme in Figure 4D). Fraction 15 of the IG (where PrimPol is concentrated) displayed the same initiation specificity and preference for Mn2+ as purified PrimPol, yielding the same products in the same ratios (Figure 4D), whereas IG fraction 11 (containing Polγ and POLRMT) lacked such an activity.
PrimPol Tolerates 8oxoG and Abasic Sites in DNA
By virtue of its primase activity, PrimPol might facilitate DNA replication by restarting DNA synthesis downstream of pauses or fork arrest due to damaged DNA, as has been shown to occur in bacteria, yeast, and human cells (Heller and Marians, 2006, Lopes et al., 2006, Callegari and Kelly, 2006, Elvers et al., 2011). On the other hand, the distributive pattern of DNA polymerization and the lack of a proofreading exonuclease suggest that PrimPol might also perform translesion synthesis (TLS) in order to facilitate elongation across a damaged template. Considering its presence in mitochondria, TLS by PrimPol was tested on synthetic templates containing either an oxidized form of guanine (8-oxoguanine or 8oxoG) or an abasic (AP) site, given that these are the most frequent forms of damage occurring in mtDNA as a consequence of oxidative stress (Berquist and Wilson, 2012).
As a control, and as previously documented (Graziewicz et al., 2007), Polγ dealt poorly with an 8oxoG lesion in the template (Figure S5A). In contrast, PrimPol gave rise to similar elongation products with both damaged and undamaged templates (Figure 5A), indicating that it was not hindered by the presence of the 8oxoG lesion. By using an oligonucleotide whose primer terminus was juxtaposed to the lesion (standing start conditions), the efficiency and fidelity of nucleotide insertion opposite the 8oxoG site was studied (Figure 5B). In agreement with an efficient TLS capacity, the error-free insertion of dC opposite 8oxoG by PrimPol paralleled that opposite an undamaged dG template, and the misinsertion of dA opposite 8oxoG was slightly more efficient (1.5-fold). Interestingly, PrimPol could also use cytidine 5′-triphosphate (CTP) or ATP to bypass 8oxoG, although, in this case, the error-free insertion (Figure 5C) was 10-fold more efficient than the error-prone one (Figure 5A).
Figure 5.
PrimPol Tolerates 8oxoG and Abasic Sites in DNA and Functionally Cooperates with Polγ and Polε
(A) Running start DNA polymerization by human PrimPol on a control template (left) or on a template containing an 8oxoG lesion (right). dNTPs were provided at 0.05, 0.1, 0.5, 1, or 10 μM. The position of the lesion in the template strand is marked (X) in the top schematic as well as in the autoradiogram. Human PrimPol elongated the damaged template with the same efficiency as in the case of the undamaged template. See also Figure S5 for a comparison to Polγ.
(B) Standing start DNA polymerization by human PrimPol on a control template (left) or on a template containing an 8oxoG lesion (right). The error-free insertion of either dCTP or CTP and the error-prone insertion of either dATP or ATP are compared at the indicated concentrations. The mobility of the +1 extended (opposite the lesion) primers is indicated.
(C) Running start DNA polymerization assay on a template containing an AP site. dNTPs were provided at 1, 10, and 100 μM. Human PrimPol bypassed a significant proportion of the templates containing the AP site (right) but generated incomplete bypass products. See also Figure S5 for a comparison with Polγ.
(D) Schematics summarizing the origin and relative abundance of different bypass products shown in (C): (1) efficient copy of the normal template (+17), (2) polymerization + skipping of the AP nucleotide (+16), (3) elongation up to the lesion + realignment beyond the lesion (+11), and (4) elongation and primer slippage before the lesion + realignment beyond the lesion (+14).
(E) Synergy between human PrimPol and human Polγ. Using circular M13 ssDNA as template and the indicated amounts of both enzymes (see the Experimental Procedures), the RNA/DNA primers made by PrimPol were readily extended by Polγ. The PrimPol-Polγ synergistic effect was maximal when GTP was provided in order to maximize the primase activity of PrimPol.
(F) Synergy with Polγ allowed the detection of PrimPol action at much lower enzyme (50 nM) and manganese (10 μm) concentrations.
(G) Synergy between human PrimPol and human Polε. The DNA primers made by PrimPol on M13 ssDNA were efficiently extended by Polγ.
(D) Schematics of the functional coupling between PrimPol and the replicative DNA polymerases Polγ and Polε.
Mitochondrial Polγ was unable to bypass AP sites (Figure S5B), given that its 3′-5′ exonuclease favors primer degradation near the site of the lesion. Conversely, human PrimPol efficiently polymerized across the AP site (indicated with an X in Figure 5C), although the accumulation of the +3 elongation product indicated some delay at this point. PrimPol continued polymerization beyond the AP site in ∼80% of the molecules, which contrasts with the null bypass observed with Polγ. Interestingly, the size of the longest elongation product (+16) was 1 nt shorter than that obtained on an equivalent template with no damage (+17), suggesting that PrimPol does not insert a nucleotide opposite the AP site but skips the lesion, copying the next template base available (Figures 5C and 5D). Moreover, the size of the main (+11) and other minor elongated products (+14) suggested that primer extension was occurring by a different mechanism. Strikingly, the AP site was flanked by a 4 nt (CTAC) repeat (gray boxes in Figure 5D). Given the difficulty to “copy” the AP site, the microhomology provides an opportunity for a partially extended (+3) primer terminus to be realigned ahead of the lesion. Further elongation of the relocated primer terminus would explain the most abundant +11 product obtained (see Figure 5D for additional details). This peculiar capacity of PrimPol to bypass a lesion in the template probably reflects its features as a primase (avidity for ssDNA regions and flexibility to accept very limited primers [even a single nucleotide]). Thus, the marked ability of PrimPol to bypass oxidative stress DNA lesions suggests that its DNA polymerase activity may have an important role in DNA damage tolerance during the replication of mtDNA.
The DNA Primers Synthesized by PrimPol Can Be Utilized by Polγ and Polε
In a first approach, we measured deoxynucleotide polymerization using circular M13 ssDNA as a template, an assay which strictly requires a primase activity in order to allow the start of DNA synthesis (Figure 5E); by only providing PrimPol, some incorporation was observed (Figure 5E, lanes 2 and 5), but incorporation was not observed when Polγ was independently provided (Figure 5E, lanes 3 and 6). The simultaneous presence of both enzymes had a synergistic effect (Figure 5E, lanes 4 and 7), particularly when a physiological concentration of GTP (100 μM) was provided in order to maximize the primase activity of PrimPol (Figure 5E, lane 7). Moreover, under these optimized conditions (Figure 5F), much lower amounts of PrimPol (50 nM; Figure 5E, lane 5) or a very low concentration of manganese ions (10 μM; Figure 5E, lane 9) were sufficient to detect the synergistic effect of PrimPol and Polγ, strongly suggesting their functional coupling. Very similar results were obtained when the synergy of PrimPol and Polε was evaluated (Figure 5G). These experiments demonstrate that, during restart of DNA synthesis, the DNA primers made by PrimPol (and most likely its TLS products) can be efficiently extended by the mitochondrial and nuclear replicative polymerases Polγ and Polε, respectively (Figure 5H).
PRIMPOL Silencing or Ablation Affects Synthesis of mtDNA
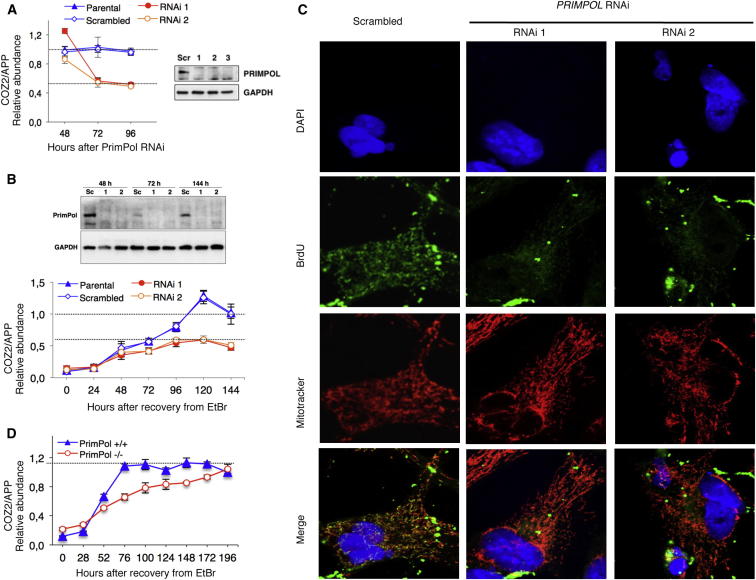
The presence of PrimPol in mitochondria suggested that it might play a role in mtDNA replication and maintenance; therefore, we investigated the effect of gene-silencing PRIMPOL on mtDNA metabolism. PRIMPOL small interfering RNA (siRNA) in human cells caused mtDNA levels to decrease to half those of controls (Figure 6A), similar to Twinkle siRNA (Tyynismaa et al., 2004), and prevented the recovery of mtDNA copy number after transient drug-induced mtDNA depletion (Figure 6B). Both results suggest that mtDNA replication is impaired when there is an abrupt loss of PrimPol. We also analyzed mtDNA replication intermediates, given that the perturbation of DNA replication in mitochondria is typically associated with changes in their abundance and properties (Holt et al., 2000, Yasukawa et al., 2005, Wanrooij et al., 2007). Although RNA-containing intermediates (Yasukawa et al., 2006) increased after 48 hr of PRIMPOL siRNA, they returned to normal levels at later time points (Figures S6A and S6B). Because mtDNA copy number was decreasing between 48 and 96 hr of PRIMPOL gene silencing, we inferred that replication was not progressing normally. To determine the extent of mtDNA replication after 72 hr of PRIMPOL gene silencing, we analyzed BrdU incorporation into mtDNA in double-stranded RNA (dsRNA)-transfected cells after preincubation with aphidicolin, an inhibitor of nuclear DNA replication. Almost all the control cells (96%) incorporated BrdU into mtDNA after 24 hr of labeling, whereas the figure was 13% for PRIMPOL-depleted cells (p < 0.0001) (Figure 6C). Hence, PRIMPOL gene silencing in human cultured cells caused a profound arrest of mtDNA synthesis. Notwithstanding these results, the contribution of PrimPol to mtDNA maintenance is redundant, given that it proved possible to derive a viable PRIMPOL−/− mouse (Figure S4). Although the mouse has adapted to the absence of PrimPol, mtDNA replication was adversely affected in cells derived from the PrimPol KO mouse, given that the rate of restoring mtDNA copy number after drug-induced transient depletion was 2.3-fold lower than in mouse cells with PrimPol (Figure 6D).
Figure 6.
PRIMPOL Gene Silencing or Ablation Perturbs mtDNA Replication
(A) HEK 293T cells were transfected with PRIMPOL-specific dsRNAs (see the Experimental Procedures), and DNA was harvested daily from 48 to 96 hr after transfection. Controls were untransfected parental and scrambled dsRNA transfected HEK 293T cells. mtDNA copy number was assayed by quantitative PCR (qPCR; see the Experimental Procedures). Data are represented as mean ± SEM. Proteins from cells harvested 72 hr after transfection were immunoblotted for specific antibodies against PrimPol and GAPDH as controls (right). See also Figure S6.
(B) HEK 293T cells were depleted of mtDNA to 10% of normal by exposure to 100 ng/ml EtBr for 72 hr. After 24 hr of depletion, they were transfected with scrambled or two PRIMPOL-specific dsRNAs (RNAi 1 and RNAi 2), and DNA was harvested daily from 0 to 192 hr after removal of the EtBr. mtDNA copy number was assayed by qPCR (see the Experimental Procedures). Data are represented as mean ± SEM. Protein was also harvested at 48, 72, and 144 hr after the removal of the EtBr. PrimPol and GAPDH (reference protein) were detected by immunoblotting with specific antibodies.
(C) HOS cells, transfected with scrambled or two PRIMPOL-specific dsRNAs (RNAi 1 and RNAi 2) for 72 hr, were incubated with BrdU for 24 hr in the presence of aphidicolin. After fixation, cells were incubated with DAPI (blue), an antibody against BrdU (green), and MitoTracker (red) and examined by confocal microscopy.
(D) PRIMPOL+/+ and PRIMPOL−/− MEFs (see also Figure S4) were depleted of mtDNA to 10%–20% of normal by exposure to 200 ng/ml EtBr for 172 hr. DNA was harvested daily from 0 to 196 hr after the removal of the EtBr. mtDNA copy number was assayed by qPCR (see the Experimental Procedures). Data are represented as mean ± SEM.
Discussion
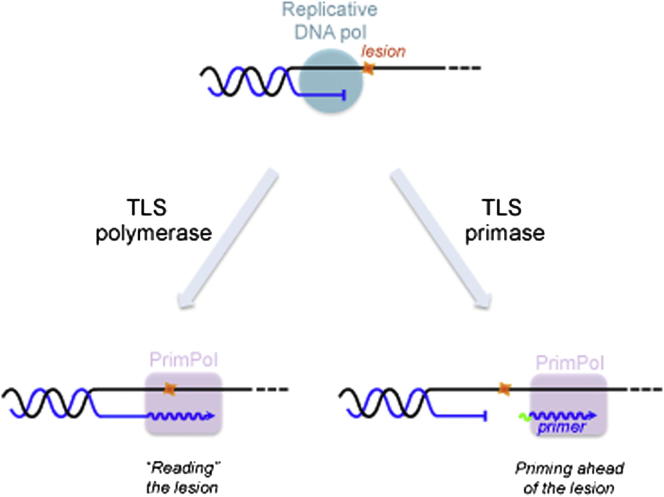
Human ccdc111 (or PrimPol) possesses DNA and RNA primase and DNA-dependent DNA and RNA polymerase activities (Figures 1, 2, S2, and S3), demonstrating its functional relationship to archeal PrimPol enzymes and its role as a unique human enzyme capable of de novo DNA synthesis solely with dNTPs. Another remarkable feature of PrimPol is its capacity to tolerate lesions such as 8oxoG and AP sites in DNA. Some PrimPol is present in the nucleus, but a larger fraction is located inside mitochondria. PRIMPOL gene silencing in human cultured cells has multiple adverse affects on mtDNA, and mouse cells lacking PrimPol display inefficient mtDNA replication. However, given that a mouse lacking the gene is viable, PRIMPOL is not essential for mtDNA maintenance.
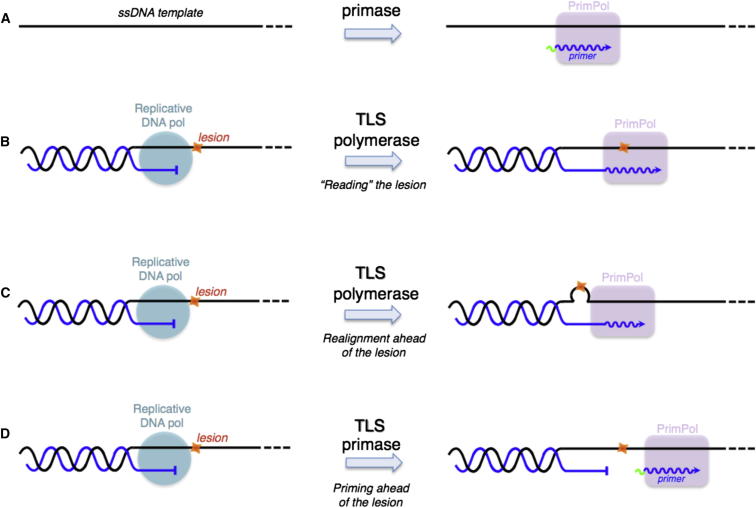
POLRMT, and not PrimPol, is believed to provide the primers for leading-strand mtDNA synthesis at the heavy strand origin of replication in the major noncoding region and at the major site of second strand mtDNA synthesis known as OriL (Falkenberg et al., 2007), and this is compatible with PrimPol being a nonessential gene. Instead, PrimPol might participate in priming at disparate sites when mtDNA replication pauses or stalls due to damaged DNA. In respect of damaged DNA, PrimPol, as a TLS DNA polymerase (Figure 7B), could assist Polγ in “reading” template lesions (e.g., 8oxoG), and it has the potential to bypass unreadable lesions (e.g., AP sites) by realigning the stalled primer terminus to an alternative template position ahead of the lesion (Figure 7C). Alternatively or additionally, PrimPol could exploit both its DNA primase and DNA polymerase activities in order to facilitate replication fork progression by acting as a specific TLS primase in order to reinitiate replication at pause sites or downstream of lesions that block continuous synthesis by replicative DNA polymerases (Figure 7D). PrimPol would be very well suited for this task, given that using deoxynucleotides for priming would leave little or no RNA to be further processed. Hence, the properties and behavior of PrimPol fit well with the proposed roles in mtDNA metabolism. Finally, PrimPol might contribute to multipriming events (Figure 7A) occurring on the lagging strand during coupled leading- and lagging-strand mtDNA replication (Holt et al., 2000).
Figure 7.
Alternative Functions of PrimPol in DNA Replication
(A) PrimPol can act as a “conventional” primase but is able to start DNA synthesis on its own.
(B) PrimPol can act as a “classical” TLS polymerase, reading lesions as 8oxoG.
(C) PrimPol can realign the stalled primer terminus ahead of the lesion, acting as a “pseudo” TLS polymerase.
(D) PrimPol can reinitiate DNA synthesis ahead of the lesion, by means of its DNA primase activity, at the single-stranded region generated by continued helix opening after the replicative DNA polymerase got stalled at a lesion. Such a “TLS primase” activity would allow replication fork progression, but a lesion-containing gap would be left behind for later repair.
It has only recently been recognized that leading-strand DNA synthesis is not always continuous. Genotoxic damage has been linked to discontinuities in leading-strand synthesis that entail skipping the obstacle, resuming fork progression, and leaving a gap behind (Langston and O’Donnell, 2006, Lopes et al., 2006, Heller and Marians, 2006, Yeeles and Marians, 2011). Daughter-strand gap formation after DNA damage induction has been detected in yeast and mammals, suggesting that repriming is a universal mechanism for bypassing DNA lesions (Meneghini, 1976, Prakash, 1981). In this regard, we believe that PrimPol's primase may play a direct role in replication restart during nuclear DNA replication, further supported by the functional coupling shown here between PrimPol and human Polε. We have recently found that PrimPol is required for repriming synthesis at stalled replication forks in the nucleus and its downregulation in cells produced persistent replication stress and genome instability (S.M., S. Rodríguez-Acebes, M.I.M.-J., S.G.-G., E.S.C., L.B., and J.M. unpublished data). These findings give additional support to our hypothesis of a similar function for PrimPol in mitochondria, given that replicative stress is constitutive during mtDNA replication, most likely due to a highly oxidative environment.
In summary, we identify and characterize a DNA primase/polymerase in human cells endowed with the unique ability to initiate synthesis using dNTPs and a remarkable capacity for translesion synthesis. Our initial analysis of its function in mitochondria suggests that PrimPol plays a key role in DNA metabolism, pointing to a greater level of complexity in mtDNA replication than previously recognized and implying that Polγ is not the sole DNA polymerase in mammalian mitochondria. The presence of PrimPol in human mitochondria suggests that it represents an ancestral cellular response to genotoxic insults, including AP sites and oxidized bases, contributing to tolerance of the environmental damage inside mitochondria. Now, the challenge is to tease out its precise contribution to DNA damage tolerance in the two DNA-containing compartments of the cell.
Experimental Procedures
Mice and Treatments
The generation of PRIMPOL−/− mice is described in detail in the Supplemental Information. All experiments with mice were performed according to Spanish and European regulations for the use and treatment of experimental animals and with the approval of the ethics committee of the Centro de Biología Molecular Severo Ochoa.
Primase Assays on M13 ssDNA and Specific Oligonucleotide Templates
The reaction (20 μl) was carried out in buffer R (50 mM Tris-HCl [pH 7.5], 75 mM NaCl, 1 mM MnCl2, 1 mM dithiothreitol, 2.5% glycerol, and 0.1mg/ml BSA) and [α-32P]CTP (16 nM; 3,000 Ci/mmol) + ATP, GTP, and UTP (100 μM) or [α-32P]dCTP (16 nM; 3,000 Ci/mmol) + dATP, dGTP, and dTTP (100 μM) in the presence of PrimPol (400 nM) wild-type (WT) or mutant AxA. M13 ssDNA (10 ng/μl) was used as a template. Various concentrations of MgCl2 or MnCl2 were used to define the range of metal activation. After 60 min at 30°C, reactions were stopped by the addition of formamide loading buffer (10 mM EDTA, 95% v/v formamide, and 0.3% w/v xylen-cyanol) and loaded in 8 M urea-containing 20% polyacrylamide sequencing gels. After electrophoresis, de novo synthesized polynucleotides were detected by autoradiography.
To test the productive coupling of PrimPol with Polγ and Polε, a similar assay was conducted in the presence of both MgCl2 and MnCl2 along with 16 nM [α-32P]dGTP and 100 μM dA, dT, and dCTP. When indicated, the nucleotide mix was supplemented with 1 μM dGTP or 100 μM GTP. Human Polγ was kindly provided by Maria Falkenberg’s lab (Gothenborg University). Human Polε was kindly provided by Zachary F. Pursell (Tulane University School of Medicine). After incubation for 30 min at 37°C, de novo synthesized products (priming + elongation), associated to the M13 ssDNA template, were detected by autoradiography after electrophoresis on neutral agarose gels.
As an alternative to M13 DNA, 60-mer oligonucleotide 5′-T36CCTGT20-3′ or a 29-mer 5′-T15CCTGT10-3′, both containing a putative herpes virus priming initiation site (Cavanaugh and Kuchta, 2009), were used as templates. A detailed protocol is available in the Supplemental Information.
DNA Polymerase Assays on Primed M13 ssDNA and Specific Template/Primer Molecules
A 5′ [32P]-labeled oligonucleotide primer (5′-GTTTTCCCAGTCACGAC-3′) was hybridized to M13 ssDNA. The reaction mixture (20 μl) contained buffer R, 1 mM MnCl2 or 5 mM MgCl2, 1.5 nM primed M13 ssDNA, 1 μM dNTP or NTPs, and 400 nM purified PrimPol, WT, or AxA mutant. After the indicated incubation time at 30°C, reactions were stopped and analyzed as described above.
When indicated, alternative templates were prepared by hybridization of a 5′[32P]-labeled 15-mer oligonucleotide primer (5′-GATCACAGTGAGTAC-3′) to a 28-mer oligonucleotide template (5′-AGAAGTGTATCT(X)GTACTCACTGTGATC-3′ where X stands for any of the four bases). The reaction mixture (20 μl) contained buffer R, 2.5 nM [γ-32P]-labeled template/primer DNA, the specified dNTP at the concentration indicated, and purified human PrimPol (200 nM). When indicated, PrimPol was used at different concentrations. Also when indicated, various concentrations of either MgCl2 or MnCl2 were used to define the range of metal activation. After 60 min of incubation at 30°C, reactions were stopped and analyzed as described above. Elongation of a 3′ terminally mismatched primer (16-mer, 5′-GATCACAGTGAGTACC-3′) hybridized to a 22-mer template (5′-TCTATCGTACTCACTGTGATC-3′) was evaluated under the same conditions.
Translesion DNA Synthesis Assays
For “running start” experiments, a 5′ [32P]-labeled 12-mer oligonucleotide (5′-GATCACAGTGAG-3′) was hybridized to either the control (undamaged) 28-mer oligonucleotide (5′-AGAAGTGTATCTGGTACTCACTGTGATC-3′) template or an oligonucleotide template of the same sequence with the G in 13th position (bold) substituted with 8oxoG (obtained from Eurogentec). For “standing start” experiments designed to evaluate the nucleotide inserted opposite 8oxoG, a 5′[32P]-labeled 15-mer oligonucleotide (5′-CTGCAGCTGATGCGC-3′) was hybridized to either the control (undamaged) 34-mer oligonucleotide (5′-GTACCCGGGGATCCGTACGGCGCATCAGCTGCAG-3′) template or an oligonucleotide template of the same sequence with the G in 19th position (bold) substituted with 8oxoG (Eurogentec). Alternatively, a 5′ [32P]-labeled 13-mer oligonucleotide primer (5′-CACTGACTGTATG-3′) was hybridized to either control (undamaged) 30-mer oligonucleotide template (5′-CTCGTCAGCATCTTCATCATACAGTCAGTG-3′) or damaged templates (30- or 31-mer) containing an AP site (5′-CTCGTCAGCATCT(AP)CATCATACAGTCAGTG-3′). The synthetic AP nucleotide was obtained from LinkTech and used to customize oligonucleotide synthesis. The reaction mixture (20 μl) contained buffer R, 2.5 nM template/primer DNA, dNTPs or NTPs at the indicated concentration, and either purified human PrimPol (200 nM) or human Polγ (20 nM, kindly provided by Maria Falkenberg’s lab). In the latter case, the reaction was tested in the presence of 10 mM MgCl2. After incubation for either 60 min at 30°C (PrimPol) or 15 min at 37°C (Polγ), primer extension products were analyzed as described above.
Subcellular Fractionation of HeLa Cells
HeLa cells were lysed and fractionated with a MitoSciences Cell Fractionation Kit (ab109719), which allowed the isolation of mitochondrial, cytoplasmic, and nuclear fractions. Two different markers were used to assess the purity of each fraction: cytoplasm (MEK2 and α-tubulin), mitochondria (ATP synthase and Hsp60), and nucleus (CTCF and histone H3). The amount of PrimPol in each compartment was estimated with quantitative immunoblotting.
Trypsin Digestion and IG Fractionation of Mitochondria
Subcellular fractionation of HEK 293T cells was performed as described previously (Reyes et al., 2011), and sucrose-gradient purified mitochondria were left untreated or subjected to hypotonic or digitonin treatment (Ohsato et al., 2002) followed by incubation with 100 μg/ml trypsin (30 min at room temperature [RT]) and heat inactivation of trypsin at 95°C for 5 min in 0.1% SDS. For IG, sucrose-gradient-purified mitochondria (2 mg/ml) were treated with 100 μg/ml of trypsin at RT for 30 min. After repeated washing, mitochondria were lysed with 0.4% n-Dodecyl-ß-maltoside and centrifuged (10 min at 1,000 × gmax); the supernatant was loaded on a 20%–42.5% IG and centrifuged at 100,000 × gmax for 14 hr. In some cases, mitochondria were crosslinked with 1% formaldehyde for 30 min at RT prior to lysis. When indicated, HEK 293T cells were treated with 100 ng/ml ethidium bromide for 72 hr or exposed to UV light (20 J/m2) and allowed to recover for 18 hr prior to mitochondrial extraction and further IG fractionation. Nucleic acid was extracted from a portion of each fraction of the gradient and after southern blotting and hybridized to a radiolabeled probe. The remainder of each fraction was used for immunoblotting and primase assays. A complete list of the antibodies used in this study can be found in the Supplemental Information.
siRNA-Mediated Depletion of PrimPol and mtDNA Copy Number Estimation
For siRNA experiments, HEK 293T and human osteosarcoma (HOS) cells were transfected with Lipofectamine RNAiMAX (Invitrogen) and 10 nM Stealth RNA as described previously (Reyes et al., 2011). Stealth RNA sequences are indicated in the Supplemental Information. Qiagen AllStars negative control siRNA was used. Mitochondrial DNA copy number was estimated by comparing the abundance of the mitochondrial COX2 gene to that of the nuclear APP gene, as described previously (Tyynismaa et al., 2004).
BrdU Labeling of Mitochondrial DNA
Parental or PRIMPOL-silenced HOS cells were incubated with 20 μM aphidicolin for 3 hr in order to block nuclear DNA replication before the addition of BrdU (1:1,000; Roche). After 24 hr, BrdU-labeled cells were stained with 60 nM MitoTracker Orange (Invitrogen), fixed, and sequentially incubated with anti-BrdU and anti-mouse IgG fluorescein according to the manufacturer’s indications (Roche). Slides were mounted in DABCO (Air Products and Chemicals) supplemented with DAPI (200 ng/ml) and visualized in a Zeiss LSM 510 confocal microscope. Images were acquired with a 63× oil immersion objective and processed in Adobe Photoshop.
Author Contributions
A.R. and M.I.M.-J. contributed equally to this work.
Acknowledgments
We would like to thank John Diffley for his suggestion that PrimPol could be a mitochondrial enzyme and Toño Enriquez and Miguel García-Díaz for critical reading of the manuscript. L.B. and J.M. are funded by the Spanish Ministry of Economy and Competitiveness (BFU2009-10085, BFU2010-21467, BFU2012-37969, and CSD2007-00015) and la Comunidad de Madrid (S2011/BMD-2361). A.R. and I.J.H. are funded by the UK Medical Research Council. S.G.-G. was the recipient of a fellowship from the Spanish Ministry of Economy and Competitiveness.
Published: October 24, 2013
Footnotes
Supplemental Information contains Supplemental Experimental Procedures and six figures and can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2013.09.025.
Supplemental Information
References
- Aravind L., Leipe D.D., Koonin E.V. Toprim—a conserved catalytic domain in type IA and II topoisomerases, DnaG-type primases, OLD family nucleases and RecR proteins. Nucleic Acids Res. 1998;26:4205–4213. doi: 10.1093/nar/26.18.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernad A., Blanco L., Lázaro J.M., Martín G., Salas M. A conserved 3′----5′ exonuclease active site in prokaryotic and eukaryotic DNA polymerases. Cell. 1989;59:219–228. doi: 10.1016/0092-8674(89)90883-0. [DOI] [PubMed] [Google Scholar]
- Berquist B.R., Wilson D.M., 3rd Pathways for repairing and tolerating the spectrum of oxidative DNA lesions. Cancer Lett. 2012;327:61–72. doi: 10.1016/j.canlet.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanca G., Shevelev I., Ramadan K., Villani G., Spadari S., Hübscher U., Maga G. Human DNA polymerase lambda diverged in evolution from DNA polymerase beta toward specific Mn(++) dependence: a kinetic and thermodynamic study. Biochemistry. 2003;42:7467–7476. doi: 10.1021/bi034198m. [DOI] [PubMed] [Google Scholar]
- Bolden A., Noy G.P., Weissbach A. DNA polymerase of mitochondria is a gamma-polymerase. J. Biol. Chem. 1977;252:3351–3356. [PubMed] [Google Scholar]
- Callegari A.J., Kelly T.J. UV irradiation induces a postreplication DNA damage checkpoint. Proc. Natl. Acad. Sci. USA. 2006;103:15877–15882. doi: 10.1073/pnas.0607343103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh N.A., Kuchta R.D. Initiation of new DNA strands by the herpes simplex virus-1 primase-helicase complex and either herpes DNA polymerase or human DNA polymerase alpha. J. Biol. Chem. 2009;284:1523–1532. doi: 10.1074/jbc.M805476200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D.D., Clayton D.A. Priming of human mitochondrial DNA replication occurs at the light-strand promoter. Proc. Natl. Acad. Sci. USA. 1985;82:351–355. doi: 10.1073/pnas.82.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemnitz Galal W., Pan M., Kelman Z., Hurwitz J. Characterization of DNA primase complex isolated from the archaeon, Thermococcus kodakaraensis. J. Biol. Chem. 2012;287:16209–16219. doi: 10.1074/jbc.M111.338145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvers I., Johansson F., Groth P., Erixon K., Helleday T. UV stalled replication forks restart by re-priming in human fibroblasts. Nucleic Acids Res. 2011;39:7049–7057. doi: 10.1093/nar/gkr420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg M., Larsson N.G., Gustafsson C.M. DNA replication and transcription in mammalian mitochondria. Annu. Rev. Biochem. 2007;76:679–699. doi: 10.1146/annurev.biochem.76.060305.152028. [DOI] [PubMed] [Google Scholar]
- Frick D.N., Richardson C.C. DNA primases. Annu. Rev. Biochem. 2001;70:39–80. doi: 10.1146/annurev.biochem.70.1.39. [DOI] [PubMed] [Google Scholar]
- Fusté J.M., Wanrooij S., Jemt E., Granycome C.E., Cluett T.J., Shi Y., Atanassova N., Holt I.J., Gustafsson C.M., Falkenberg M. Mitochondrial RNA polymerase is needed for activation of the origin of light-strand DNA replication. Mol. Cell. 2010;37:67–78. doi: 10.1016/j.molcel.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Goodman M.F., Tippin B. Sloppier copier DNA polymerases involved in genome repair. Curr. Opin. Genet. Dev. 2000;10:162–168. doi: 10.1016/s0959-437x(00)00057-5. [DOI] [PubMed] [Google Scholar]
- Graziewicz M.A., Bienstock R.J., Copeland W.C. The DNA polymerase gamma Y955C disease variant associated with PEO and parkinsonism mediates the incorporation and translesion synthesis opposite 7,8-dihydro-8-oxo-2′-deoxyguanosine. Hum. Mol. Genet. 2007;16:2729–2739. doi: 10.1093/hmg/ddm227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller R.C., Marians K.J. Replication fork reactivation downstream of a blocked nascent leading strand. Nature. 2006;439:557–562. doi: 10.1038/nature04329. [DOI] [PubMed] [Google Scholar]
- Holt I.J., Reyes A. Human mitochondrial DNA replication. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt I.J., Lorimer H.E., Jacobs H.T. Coupled leading- and lagging-strand synthesis of mammalian mitochondrial DNA. Cell. 2000;100:515–524. doi: 10.1016/s0092-8674(00)80688-1. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Maga G., Spadari S. Eukaryotic DNA polymerases. Annu. Rev. Biochem. 2002;71:133–163. doi: 10.1146/annurev.biochem.71.090501.150041. [DOI] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. Formation of an RNA primer for initiation of replication of ColE1 DNA by ribonuclease H. Proc. Natl. Acad. Sci. USA. 1980;77:2450–2454. doi: 10.1073/pnas.77.5.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer L.M., Koonin E.V., Leipe D.D., Aravind L. Origin and evolution of the archaeo-eukaryotic primase superfamily and related palm-domain proteins: structural insights and new members. Nucleic Acids Res. 2005;33:3875–3896. doi: 10.1093/nar/gki702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston L.D., O’Donnell M. DNA replication: keep moving and don’t mind the gap. Mol. Cell. 2006;23:155–160. doi: 10.1016/j.molcel.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Lao-Sirieix S.H., Bell S.D. The heterodimeric primase of the hyperthermophilic archaeon Sulfolobus solfataricus possesses DNA and RNA primase, polymerase and 3′-terminal nucleotidyl transferase activities. J. Mol. Biol. 2004;344:1251–1263. doi: 10.1016/j.jmb.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Lao-Sirieix S.H., Pellegrini L., Bell S.D. The promiscuous primase. Trends Genet. 2005;21:568–572. doi: 10.1016/j.tig.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Lipps G., Röther S., Hart C., Krauss G. A novel type of replicative enzyme harbouring ATPase, primase and DNA polymerase activity. EMBO J. 2003;22:2516–2525. doi: 10.1093/emboj/cdg246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipps G., Weinzierl A.O., von Scheven G., Buchen C., Cramer P. Structure of a bifunctional DNA primase-polymerase. Nat. Struct. Mol. Biol. 2004;11:157–162. doi: 10.1038/nsmb723. [DOI] [PubMed] [Google Scholar]
- Lopes M., Foiani M., Sogo J.M. Multiple mechanisms control chromosome integrity after replication fork uncoupling and restart at irreparable UV lesions. Mol. Cell. 2006;21:15–27. doi: 10.1016/j.molcel.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Meneghini R. Gaps in DNA synthesized by ultraviolet light-irradiated WI38 human cells. Biochim. Biophys. Acta. 1976;425:419–427. doi: 10.1016/0005-2787(76)90006-x. [DOI] [PubMed] [Google Scholar]
- Ohsato T., Ishihara N., Muta T., Umeda S., Ikeda S., Mihara K., Hamasaki N., Kang D. Mammalian mitochondrial endonuclease G. Digestion of R-loops and localization in intermembrane space. Eur. J. Biochem. 2002;269:5765–5770. doi: 10.1046/j.1432-1033.2002.03238.x. [DOI] [PubMed] [Google Scholar]
- Prakash L. Characterization of postreplication repair in Saccharomyces cerevisiae and effects of rad6, rad18, rev3 and rad52 mutations. Mol. Gen. Genet. 1981;184:471–478. doi: 10.1007/BF00352525. [DOI] [PubMed] [Google Scholar]
- Reyes A., He J., Mao C.C., Bailey L.J., Di Re M., Sembongi H., Kazak L., Dzionek K., Holmes J.B., Cluett T.J. Actin and myosin contribute to mammalian mitochondrial DNA maintenance. Nucleic Acids Res. 2011;39:5098–5108. doi: 10.1093/nar/gkr052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A., Kazak L., Wood S.R., Yasukawa T., Jacobs H.T., Holt I.J. Mitochondrial DNA replication proceeds via a ‘bootlace’ mechanism involving the incorporation of processed transcripts. Nucleic Acids Res. 2013;41:5837–5850. doi: 10.1093/nar/gkt196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Berrondo J., Mesa P., Ibarra A., Martínez-Jiménez M.I., Blanco L., Méndez J., Boskovic J., Montoya G. Molecular architecture of a multifunctional MCM complex. Nucleic Acids Res. 2012;40:1366–1380. doi: 10.1093/nar/gkr831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyynismaa H., Sembongi H., Bokori-Brown M., Granycome C., Ashley N., Poulton J., Jalanko A., Spelbrink J.N., Holt I.J., Suomalainen A. Twinkle helicase is essential for mtDNA maintenance and regulates mtDNA copy number. Hum. Mol. Genet. 2004;13:3219–3227. doi: 10.1093/hmg/ddh342. [DOI] [PubMed] [Google Scholar]
- Wanrooij S., Goffart S., Pohjoismäki J.L., Yasukawa T., Spelbrink J.N. Expression of catalytic mutants of the mtDNA helicase Twinkle and polymerase POLG causes distinct replication stalling phenotypes. Nucleic Acids Res. 2007;35:3238–3251. doi: 10.1093/nar/gkm215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T.W., Clayton D.A. Isolation and characterization of a DNA primase from human mitochondria. J. Biol. Chem. 1985;260:11530–11535. [PubMed] [Google Scholar]
- Yasukawa T., Yang M.Y., Jacobs H.T., Holt I.J. A bidirectional origin of replication maps to the major noncoding region of human mitochondrial DNA. Mol. Cell. 2005;18:651–662. doi: 10.1016/j.molcel.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Yasukawa T., Reyes A., Cluett T.J., Yang M.Y., Bowmaker M., Jacobs H.T., Holt I.J. Replication of vertebrate mitochondrial DNA entails transient ribonucleotide incorporation throughout the lagging strand. EMBO J. 2006;25:5358–5371. doi: 10.1038/sj.emboj.7601392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeeles J.T.P., Marians K.J. The Escherichia coli replisome is inherently DNA damage tolerant. Science. 2011;334:235–238. doi: 10.1126/science.1209111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.