Abstract
To assess the role of genes required for skin organogenesis, tissue regeneration and homeostasis, we have established in vitro skin equivalents composed of primary cells or cell lines, respectively. In these organotypic cocultures keratinocytes generate a normal epidermis irrespective of the species and tissue origin of fibroblasts. The combination of cells derived from mouse and human tissues facilitates the identification of the origin of compounds involved in epidermal tissue reconstitution and thus the precise analysis of growth regulatory mechanisms.
Keywords: Cocultures, Keratinocytes, Tissue engineering
Introduction
In skin, epithelial cells develop an orderly structured and well-organized epithelium consisting of basal, spinous, granular, and cornified strata. Their development during embryogenesis, regeneration in wound healing, as well as the maintenance of the homoeostasis of the epidermis depends on epithelial interactions with the underlying connective tissue, the dermis. The continuous process involving keratinocyte proliferation and terminal differentiation is mainly regulated by mesenchymal influences (1). To examine cellular growth, signalling processes and cell-cell interactions, culture systems have been developed combining the two major cell types of skin, keratinocytes and fibroblasts. In simple two-dimensional feeder-layer cocultures combining postmitotic dermal fibroblasts (feeder cells) and epidermal keratinocytes, properly stratified epithelia are not formed. Only in advanced three-dimensional in vitro systems keratinocytes develop well-ordered epithelia, thus offering an opportunity to analyse the cellular mechanisms of tissue formation, such as cell-cell interactions, the regulation of proliferation and differentiation as well as the reepithelialisation process after wounding (2). To reconstruct skin in vitro several organ-like culture systems have been established (3-6). In so called organotypic cocultures, epidermal keratinocytes grow air-exposed on a matrix of native collagen type I, extracted from rat tail tendon or calf skin. To become functional dermal equivalents, the collagen gels contain viable fibroblasts which reorganize this matrix by producing extracellular matrix components comparable to the wound situation (7, 8). The cultures are nourished by diffusion from below, optionally in serum containing medium or in defined medium (9).
The characterisation of paracrine acting factors produced by either fibroblasts or keratinocytes is difficult in a homologous skin model containing human cells in both compartments. On the other hand, in a heterologous skin equivalent harbouring cell types of different species, the source of the respective gene products can be easily and unequivocally distinguished and assigned to the specific cell types (10).
Those in vitro skin equivalents are generally composed of freshly isolated cells from human skin specimens. However, more preferable tools for large scale examinations - either the molecular analysis of regulatory processes or pharmaco-toxicological studies - are skin equivalents composed of cell lines, which are easier to handle and to standardize. In our experiments, we chiefly utilize the spontaneously transformed human keratinocyte cell line HaCaT derived from normal human trunk skin (11). Despite the altered karyotype and unlimited growth potential, HaCaT cells are not tumorigenic and form an orderly structured and differentiated epidermis with essentially all structural and functional features after transplantation onto athymic nude mice (11-13). In organotypic cocultures in culture medium supplemented with TGF-α HaCaT cells form well organised and differentiated epithelia in the presence of human or mouse fibroblasts, respectively (14).
Materials and Methods
Cell culture
Human dermal fibroblasts (HDF) were derived from adult skin by trypsinization as described (8, 9). HDF obtained from outgrowth of explant cultures, were grown in DMEM (Dulbecco’s modified Eagle's medium; Bio Whittaker) supplemented with 10% fetal calf serum (FCS), and cells from passages 4 to 8 were used. Mouse embryonic fibroblasts (MEF) were isolated from mouse embryos (day 9.5; 19), immortalised according to the 3T3 protocol (15), and grown in DMEM (Bio Whittaker) supplemented with 10% FCS (16, 17).
Normal epidermal keratinocytes (NEK) derived from adult skin (8, 9) were plated on X-irradiated fibroblast feeder cells (HDF, 70 Gy; MEF, 20 Gy) in FAD medium (DMEM:Ham’s F-12 / 3:1) with 100 U/ml penicillin, 100 µg/ml streptomycin and supplemented with 5% FCS, 5 µg/ml insulin, 1 ng/ml recombinant human EGF, 10-10 M cholera toxin, 10-4 M adenine, and 0.4 µg/ml hydrocortisone (Sigma) as described (8).
Cells of the immortalized human skin keratinocyte cell line HaCaT (11) were subcultivated with a split ratio of 1:10 every 10 days in DMEM with 10% FCS.
Organotypic cocultures
Lyophilized Collagen type I was resolubilised at the desired concentration (2-4 mg/ml; dry weight) in 0.1% acetic acid and kept at 4°C. (Suppliers e.g. (a) Vitrogen-100, bovine dermal collagen (type I) (Collagen Corp., Palo Alto, California); (b) Type I collagen, calf skin (Cerard, Lyon, France); (c) Type I collagen, rat tail tendons (SIGMA); (d) Type I collagen, calf skin (IBFB, Leipzig, Germany). The ice cold collagen solution (80% of total volume, 4 mg/ml) was mixed with Hanks salt 10x with phenol red (10% of total volume) and adjusted to pH 7.4 by adding about 80 µl of 2 M NaOH per 12 ml collagen mixture while gently stirring on ice to avoid air bubbles and premature gelation. The fibroblast number necessary for the desired concentration in the gel (1x105-5x105 per ml) was resuspended in FCS (10% of total volume) and added to the gel solution on ice under cautious stirring. With cooled pipettes aliquots of the collagen gel mixture were poured into filter inserts (2.5 ml/ filter insert; one insert per well of a 6-well plate) (Falcon cell culture inserts (3.0 µm pores) and Biocoat 6-fold Deep Well Plates from Becton Dickinson; see also 9) (Fig. 1). For gelation, the collagen solution in filter inserts is incubated for 1 h at 37°C in a humified incubator. Thereafter, glass rings (inner diameter 18 mm, wall thickness 2 mm, 80 mm high) were placed on the gel and gently pushed down by mild pressure with forceps in order to confine the area for epithelial cell growth. The gels with the glass rings are placed for 1 h at 37°C in a humified incubator. The excess liquid pressed out of the gel was gently and carefully aspirated. Eventually, the gels were equilibrated by complete immersion in culture medium for 24 h.
Fig. 1. Schematic illustration of the organotypic culture system.
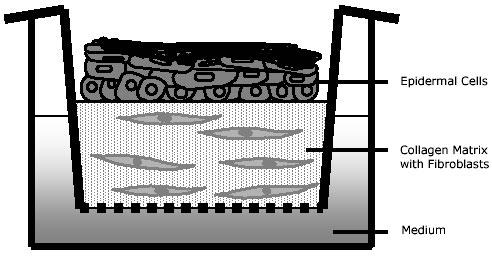
Keratinocytes grow air exposed on a fibroblast containing collagen gel. The filter inserts are in contact with the culture medium.
Thereafter keratinocytes were plated inside the glass ring (after removing the medium) at a density of 1x106 in 1 ml FAD medium for each 2.5 cm insert. They attach within 12-24 h and form a nearly confluent layer on top of the collagen gel. 24-30 h after seeding the glass rings were removed, thereby avoiding any mechanical distortion of the epithelial cell sheet.
Culture medium was changed, and, by lowering the medium level to the lower part of the gels, the cultures were raised to the air-liquid interphase thus restricting nourishment to diffusion from below. This air-lift procedure is defined as the start of the culture time of organotypic cocultures. Organotypic cocultures with primary keratinocytes were grown in FAD-Medium or DMEM with 10% FCS and 50 µm ascorbic acid, those with HaCaT cells were supplemented with 2 ng/ml TGF-α. Medium was changed every 2-3 days. Depending on the purpose of the respective study, alternative media might be used, such as serum free formulations or media with specific supplementations such as inhibitors, neutralizing antibodies, growth factors, et cetera (described in detail in 1-2, 8-9).
Analysis
For histological or immunohistochemical analysis, organotypic cocultures were fixed in formaldehyde (3.7%) for at least 24h. Thereafter each culture was covered by a drop of hand-warm agar (2%) to prevent dislodgement of the epithelium during further preparation, and then the whole specimen was processed for paraffin embedding following protocols for routine histology. Alternatively, for cryosectioning specimens were embedded in Tissue Tek-OTC-compound and subsequently snap frozen in liquid nitrogen vapour.
The most important quality parameter of epidermal tissue reconstitution is its morphologic appearance. In addition , tissue maturation is readily evaluated by in situ-analytical techniques on frozen or fixed tissue sections determinig expression (in situ-hybridization) and distribution of specific epidermal differentiation products (immunohistochemistry) such as involucrin, keratin 1/10, transglutaminase, filaggrin, loricrin (2, 4, 9-10). Usually, the formation of a regular epidermal tissue architecture is paralleled by typical, in vivo-like expression patterns of differentiation products and the development of a stratum corneum.
Results and Discussion
For generating organotypic cocultures, epidermal keratinocytes were plated onto the upper surface of collagen gels containing embedded fibroblasts, where they attached rapidly and formed confluent layers within 1-2 days. Subsequently, these keratinocytes reconstituted an epithelial tissue architecture resembling native epidermis and expressing characteristic epidermal differentiation markers (18-19, 1, 9). In the absence of fibroblasts, only thin epithelia developed with rapid loss of proliferation within 2 to 3 days (20). However, the addition of fibroblasts into the collagen matrix - either proliferative or postmitotic cells at a density of at least 2x104/ml – caused a sustained keratinocyte growth accompanied by regular differentiation. It could be demonstrated by means of this in vitro-system, that the cocultured fibroblasts produce growth factors which are essential for epidermal morphogenesis (8, 20). In general, using normal human epidermal keratinocytes (1x106 per culture, 3x105 per cm2) and human dermal fibroblasts (2.5-5x105 per culture, 1-2x105 per cm2), multilayered and differentiated epithelia had formed after 7-10 days comprising all characteristic epidermal layers including an orthokeratotic stratum corneum. Beside the dependency on fibroblasts, variations in epithelial quality can be observed due to the interindividual variability of primary epithelial cells (different donors, body sites, ages) and differences in the isolation procedures as well as culture conditions (21).
Depending on the aim of the studies different fibroblast numbers or genotypes have been used. For example, heterologous skin equivalents consisting of mouse embryonic fibroblasts (MEF) and human keratinocytes are an excellent tool to identify the source of paracrine factors contributing in dermal-epidermal interactions (22-24). In both, homologous as well as heterologous organotypic cocultures, a typical epidermal tissue is formed within one week suggesting that responsible factors are effective also beyond species barriers (Fig. 2a and b).
Fig. 2. Epithelial architecture of organotypic cocultures of different epithelial and mesenchymal cell types.
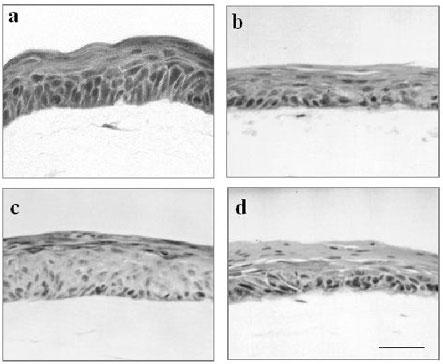
Epidermal tissue morphology of 7 day organotypic cocultures of human keratinocytes with human dermal fibroblasts (a) and human keratinocytes with mouse embryonic wild type fibroblasts (b). HaCaT cells formed epithelia on human dermal fibroblasts (c) and mouse embryonic wild type fibroblasts (d). (H and E staining; same magnification; bar: 100 µm).
In coculture with mouse fibroblasts, growth of human keratinocytes is clearly delayed resulting after 7 days in thinner epithelia as compared to cocultures with human fibroblasts. This is indicative for a reduced efficacy of these cells in supporting the growth of human keratinocytes. Nevertheless, this can be compensated by a prolonged culture time of 10 to 14 days. However, heterologous skin equivalent models have the unique advantage, that genetically modified mouse cells can be exploited such as embryonic fibroblasts from knock-out or transgenic mice. In recent studies we have analyzed the distinct effects of different embryonic mouse fibroblast lines on epidermal morphogenesis of human keratinocytes, thus establishing a sensitive and efficient read out system for fibroblast derived mediators (10, 16, 25).
To further standardise the in vitro skin equivalent model and to avoid donor-dependent variations, the epidermal cell line HaCaT has been used instead of primary keratinocytes. The HaCaT cell line is a spontaneously transformed keratinocyte line (11) with sustained genetic alterations indicative of transformed but not tumorigenic cells (26). When grown on plastic the HaCaT cells show continuous proliferation, independence of feeder-cells, and a rather typical epithelial morphology with expression of a large panel of keratins (12-13, 26). When transplanted onto the back of nude mice, HaCaT cells regenerate and differentiate to well structured epithelia expressing the characteristic epidermal markers (12). On the other hand, formation of multilayered epithelia by HaCaT cells in organotypic cocultures is delayed and require an increased number of fibroblasts (13). This was found to be due to certain deficiencies of HaCaT cells in the production of IL-1 as well as a low expression of the receptors for KGF and GM-CSF what can be compensated by application of TGF-α (for details see 14). In organotypic cocultures of HaCaT cells either with human or mouse fibroblasts well structured and differentiated stratified epithelia developed, when supplemented with TGF-α , although the stratum corneum remained parakeratotic indicating incomplete keratinization (Fig. 2c and d). Clearly resembling normal human keratinocytes, cocultures of HaCaT cells with mouse mesenchymal cells show a diminished stratification within 7 days. In conclusion, their reproducible production and standardized quality renders HaCaT organotypic cocultures an excellent tool for large scale examinations of mechanisms regulating skin reepithelialization and homoeostasis in a tissue-type context.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG Fu 91/4-1). The authors have no conflicts of interest related to this publication.
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- EGF
epidermal growth factor
- FCS
fetal calf serum
- HDF
human dermal fibroblasts
- MEF
mouse embryonic fibroblasts
- NEK
normal epidermal keratinocytes
- TGF-α
transforming growth factor alpha
Appendix
Protocols
(A) Dermal equivalents of organotypic skin cocultures
The collagen is resolubilised at the desired concentration (2-4 mg/ml; dry weight) in 0.1% acetic acid and kept at 4°C.
Ice cold collagen solution (80% of total volume, 4 mg/ml) is mixed with 10x Hanks buffer (10% of total volume) and adjusted to pH 7.4 by adding 80 µl 2N NaOH per 12 ml while gently stirring on ice (i.e. for 6 gels 12 ml collagen + 1.5 ml 10x Hanks buffer).
The fibroblast number (1x105-5x105 per ml) is resuspended in FCS (10% of total volume) or, in case of cell-free gels, just FCS (10% of total volume) is mixed with the gel solution on ice under cautious stirring. (i.e. for 6 gels 1.5 ml FCS).
With cooled pipettes aliquots of the complete collagen solution are poured into filter inserts (2.5 ml per filter insert).
For gelation, the filter inserts are placed in a 6-deep-well plate and incubated for 1 h at 37°C in a humidified incubator.
Glass rings corresponding to the diameter of the filter inserts (inner diameter 18 mm, wall thickness 2 mm, 80 mm height) are placed on the gel and gently pushed down to delineate the area for seeding the keratinocytes.
Gels with the glass rings are placed for 1 h at 37°C in a humidified incubator.
The gels are equilibrated by complete immersing in culture medium for 24 h.
(B) Epithelia of organotypic skin cocultures
Epithelial cells, e.g. human skin keratinocytes, (1x106 in 1 ml FAD medium for a 2.5 cm insert) are plated inside the glass ring (after removing the medium).
24-30 h after seeding the keratinocyte cell layer is rinsed with 1 ml medium, and the glass rings are removed.
Culture medium is changed, and, by lowering the medium level to the lower part of the collagen gel, the cultures are raised to the air-liquid interphase.
Medium is changed every 2-3 day.
References
- Fusenig NE. Epithelial-mesenchymal interactions regulate keratinocyte growth and differentiation in vitro. In I Leigh, B Watt, F Lane (eds.) The Keratinocyte Handbook. Cambridge University Press; 1994, pp 71-94.
- Garlick JA, Taichman LB. Fate of human keratinocytes during reepithelialization in an organotypic culture model. Lab Invest. 1994;70:916–924. [PubMed] [Google Scholar]
- Bell E, Ehrlich HP, Buttler DJ, Nakatsuji T. Living tissue formed in vivo and accepted as skin-equivalent tissue of full thickness. Science. 1981;211:1052–1054. doi: 10.1126/science.7008197. [DOI] [PubMed] [Google Scholar]
- Pruniéras M, Régnier M, Fougère S, Woodley D. Keratinocytes synthesize basal-lamina proteins in culture. J Invest Dermatol. 1983;81:74–81. doi: 10.1111/1523-1747.ep12540736. [DOI] [PubMed] [Google Scholar]
- Fusenig NE, Breitkreutz D, Dzarlieva RI, Boukamp P, Bohnert A, Tilgen W. Growth and differentiation of transformed keratinocytes from mouse and human skin in vitro and in vivo. J Invest Dermatol. 1983;81:168–175. doi: 10.1111/1523-1747.ep12541032. [DOI] [PubMed] [Google Scholar]
- Fusenig NE. Cell interaction and epithelial differentiation. In R.I. Freshney (ed.). Culture of epithelial cells. New York: Wiley-Liss Inc; 1992, pp 25-57.
- Coulomb B, Dubertret L, Merrill C, Touraine R, Bell E. The collagen lattice: A model for studying epidermalization in vitro. Br J Dermatol. 1984;114:91–101. [Google Scholar]
- Smola H, Thiekötter G, Fusenig NE. Mutual induction of growth factor gene expression by epidermal-dermal cell interaction. J Cell Biol. 1993;122:417–429. doi: 10.1083/jcb.122.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark HJ, Baur M, Breitkreutz D, Mirancea N, Fusenig NE. Organotypic keratinocyte cocultures in defined medium with regular epidermal morphogenesis and differentiation. J Invest Dermatol. 1999;112:681–691. doi: 10.1046/j.1523-1747.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- Maas-Szabowski N, Szabowski A, Stark HJ, Andrecht S, Kolbus A, Schorpp-Kistner M, Angel P, Fusenig NE. Organotypic cocultures with genetically modified mouse fibroblasts as a tool to dissect molecular mechanisms regulating keratinocyte growth and differentiation. J Invest Dermatol. 2001;116:816–820. doi: 10.1046/j.1523-1747.2001.01349.x. [DOI] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitkreutz D, Stark HJ, Mirancea N, Tomakidi P, Steinbauer H, Fusenig NE. Integrin and basement membrane normalization in mouse grafts of human keratinocytes – implications for epithelial homeostasis. Differentiation. 1997;61:195–209. doi: 10.1046/j.1432-0436.1997.6130195.x. [DOI] [PubMed] [Google Scholar]
- Breitkreutz D, Schoop VM, Mirancea N, Baur M, Stark HJ, Fusenig NE. Epidermal differentiation and basement membrane formation by HaCaT cells in surface transplants. Eur J Cell Biol. 1998;75:273–286. doi: 10.1016/S0171-9335(98)80123-4. [DOI] [PubMed] [Google Scholar]
- Maas-Szabowski N, Stärker A, Fusenig NE. Epidermal tissue regeneration and stromal interaction in HaCaT cells is initiated by TGF-α. 116. 2003;J:2937–2948. doi: 10.1242/jcs.00474. [DOI] [PubMed] [Google Scholar]
- Todaro GJ, Green H. High frequency of SV40 transformation of mouse cell line 3T3. Virology. 1996;4:756–759. doi: 10.1016/0042-6822(66)90261-3. [DOI] [PubMed] [Google Scholar]
- Schreiber M, Kolbus A, Piu F, Szabowski A, Mohle-Steinlein U, Tian J, Karin M, Angel P, Wagner EF. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev. 1999;13:607–619. doi: 10.1101/gad.13.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbus A, Herr I, Schreiber M, Debatin KM, Wagner EF, Angel P. c-Jun-dependent CD95-L expression is a rate-limiting step in the induction of apoptosis by alkylating agents. Mol Cell Bio. 2000;20:575–582. doi: 10.1128/mcb.20.2.575-582.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenteau NL, Nolte CM, Bilbo P, Rosenberg K, Wilkins LM, Johnson EW, Watson S, Mason VS, Bell E. Epidermis generated in vitro: practical considerations and applications. J Cell Biochem. 1991;45:245–251. doi: 10.1002/jcb.240450304. [DOI] [PubMed] [Google Scholar]
- Contard P, Bartel RL, Jacobs L, Perlish JS, MacDonald ED, Handler L, Cone D, Fleischmajer R. Culturing keratinocytes and fibroblasts in a three-dimensional mesh results in epidermal differentiation and formation of a basal lamina-anchoring zone. J Invest Dermatol. 1993;100:35–39. doi: 10.1111/1523-1747.ep12349952. [DOI] [PubMed] [Google Scholar]
- Maas-Szabowski N, Stark HJ, Fusenig NE. Keratinocyte growth regulation in defined organotypic cultures through IL–1-induced KGF expression in resting fibroblasts. J Invest Dermatol. 2000;114:1075–1084. doi: 10.1046/j.1523-1747.2000.00987.x. [DOI] [PubMed] [Google Scholar]
- Stark HJ, Maas-Szabowski N, Smola H, Breitkreutz D, Mirancea N, Fusenig NE. Organotypic keratinocyte-fibroblast cocultures: in vitro skin equivalents to study the molecular mechanisms of cutaneous regeneration. In: Cultured human keratinocytes and tissue engeneered skin substitutes. Horch, Munster, Achauer (eds.); Thieme Verlag, Stuttgart 2001; pp 161-170.
- Turksen K, Choi Y, Fuchs E. Transforming growth factor alpha induces collagen degradation and cell migration in differentiating human epidermal raft cultures. Cell Regul. 1991;2:613–625. doi: 10.1091/mbc.2.8.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Carter WG. Integrin expression and differentiation in transformed human epidermal cells is regulated by fibroblasts. J Cell Sci. 1992;103:755–763. doi: 10.1242/jcs.103.3.755. [DOI] [PubMed] [Google Scholar]
- Choi Y, Fuchs E. TGF-β and retinoic acid: regulators of growth and modifiers of differentiation in human epidermal cells. Cell Regulation. 1994;4:791–809. doi: 10.1091/mbc.1.11.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabowski A, Maas-Szabowski N, Andrecht S, Kolbus A, Schorpp-Kistner M, Fusenig NE, Angel P. c-Jun and JunB antagonistically control cytokine-regulated mesenchymal-epidermal interaction in skin. Cell. 2000;103:745–755. doi: 10.1016/s0092-8674(00)00178-1. [DOI] [PubMed] [Google Scholar]
- Boukamp P, Popp S, Altmeyer S, Hulsen A, Fasching C, Cremer T, Fusenig NE. Sustained nontumorigenic phenotype correlates with a largely stable chromosome content during long-term culture of the human keratinocyte line HaCaT. Genes Chromosomes Cancer. 1997;19(4):201–214. doi: 10.1002/(SICI)1098-2264(199708)19:4<201::AID-GCC1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]


