Abstract
Central to the pathogenesis of atypical haemolytic uraemic syndrome (aHUS) is over-activation of the alternative pathway of complement. Inherited defects in complement genes and autoantibodies against complement regulatory proteins have been described. The use of plasma exchange to replace non-functioning complement regulators and hyper-functional complement components in addition to the removal of CFH-autoantibodies made this the ‘gold-standard’ for management of aHUS. In the last 4 years the introduction of the complement inhibitor Eculizumab has revolutionised the management of aHUS. In this review we shall discuss the available literature on treatment strategies to date.
Keywords: Eculizumab, Haemolytic Uraemic Syndrome, Treatment, Complement
1. Introduction
Atypical HUS (aHUS) is the prototypical disease of complement over activation (Kavanagh et al., 2008a). Thrombocytopenia, microangiopathic haemolytic anaemia and acute renal failure are the hallmarks of haemolytic uraemic syndrome (HUS). Atypical HUS is the term used to classify any HUS not due to Shiga toxin (Stx)-producing bacteria, typically Escherichia coli O157:H7 (Besbas et al., 2006).
The discovery of mutations in the complement system in aHUS by Warwicker et al. (1998) was to set in train the research which was to ultimately result in the successful use of the complement inhibitor Eculizumab in aHUS.
2. The complement system
Complement is an ancient pathway that sits at the nexus of the immune system (Ricklin et al., 2010): protecting against invading pathogens; bridging innate and adaptive immunity (Kemper and Atkinson, 2007); and disposing of immune complexes and injured tissues and cells (Richards et al., 2007b).
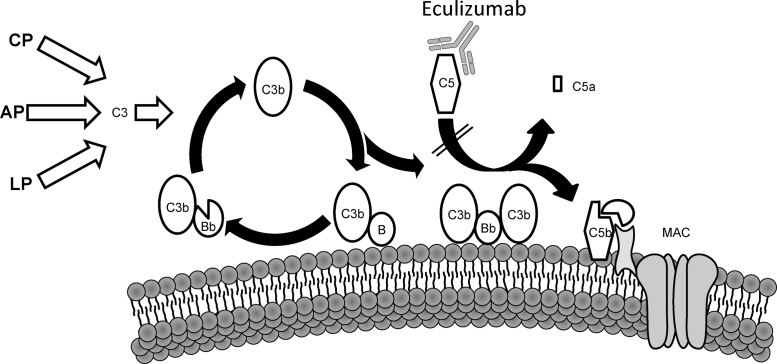
Complement activation is mediated via different initiating triggers. The classical pathway (CP) can be initiated via IgM and IgG as well as the pattern recognition molecule (PRM) C1q. In the lectin pathway (LP) the PRMs, mannose binding lectin (MBL) and ficolins bind carbohydrates to trigger complement activation. The alternative pathway (AP) constantly “ticks over” depositing C3b on surfaces which is inactivated on host cells and amplified on foreign cells. Properdin can also bind to foreign and apoptotic cells to propagate the AP. The AP is also recruited by C3 convertases formed by the CP and LP and as such, it serves as an amplification step accounting for ∼80% of all complement activation regardless of the initial trigger (Harboe and Mollnes, 2008). All pathways subsequently converge to produce the common terminal pathway effector molecules (Ricklin et al., 2010) (Fig. 1).
Fig. 1.
Complement activation and the mechanism of action of Eculizumab. The AP constantly undergoes ‘tick-over’ but can also be primed by the CP and LP pathways. The C3b that is formed interacts with factor B (B), which is then cleaved by factor D to form the AP C3 convertase (C3bBb). This enzyme complex is attached to the target covalently via C3b while Bb is the catalytic serine protease subunit. Because C3 is the substrate for this convertase, a powerful feedback loop is created. Unchecked, this will lead to activation of the terminal complement pathway with generation of the effector molecules; the anaphylatoxin C5a and the membrane attack complex (MAC). Eculizumab binds C5 and prevents its entry into the C5 convertase (C3bBbC3b), thus precluding cleavage into the effector molecules, C5a and C5b and ultimately the MAC.
The runaway complement activation of the AP has evolved to rapidly destroy invading microorganisms but to prevent collateral damage to host tissue, fluid phase (e.g. complement factor H (CFH) and complement factor I (CFI)) and membrane bound (e.g. membrane cofactor protein (MCP)) complement regulatory proteins are present. It is an imbalance between this activation and regulation on the glomerular vasculature which underlies the pathogenesis of aHUS.
3. The role of complement in aHUS
The last 15 years has seen the elucidation of the critical pathways involved in the pathogenesis of aHUS. Loss of function mutations in complement regulatory proteins and gain of function mutations in complement components have been described in aHUS. Similarly, autoantibodies to complement regulatory proteins have been described.
3.1. Complement factor H
CFH is the critical fluid-phase regulator of the AP acting via its N-terminal domains (CCPs 1–4) (Richards et al., 2007b). CFH can also protect host surfaces by binding to polyanions such as the glycosaminoglycans (GAG) of endothelial cells and exposed basement membranes (Meri and Pangburn, 1994, Schmidt et al., 2008). CFH has two GAG binding domains in CCPs 6–8 and CCPs 19–20 which have different sulphate specificities. CCPs 6–8 are predominantly responsible for binding in the eye while the C-terminal domains (CCPs 19–20) account for kidney binding (Clark et al., 2013). Additionally CFH also binds to the lipid peroxidation product malondialdehyde (Weismann et al., 2011), the acute phase proteins, C-reactive protein (Hakobyan et al., 2008, Laine et al., 2007, Sjoberg et al., 2007) and pentraxin 3 (Kopp et al., 2012) as well as necrotic cells (Sjoberg et al., 2007).
Mutations in CFH were first described in 1998 (Warwicker et al., 1998) and mutations in this gene are the most common genetic predisposition to aHUS, accounting for around 25% of all cases (Caprioli et al., 2001, Dragon-Durey et al., 2004, Fan et al., 2013, Geerdink et al., 2012, Maga et al., 2010, Neumann et al., 2003, Perez-Caballero et al., 2001, Richards et al., 2001, Warwicker et al., 1998). The majority of mutations in CFH are located in CCPs 19–20 and do not usually result in a quantitative deficiency. These C-terminal mutations fail to bind to cell surfaces and result in ineffective control of complement activation on the glomerular vasculature (Abarrategui-Garrido et al., 2008, Ferreira et al., 2009, Vaziri-Sani et al., 2006). C-terminal CFH mutants have also been demonstrated to have reduced binding to platelets resulting in increased complement activation with consequent platelet activation, aggregation and release of tissue factor-expressing micro-particles (Stahl et al., 2008). Although clustering in the C-terminal, mutations are reported throughout the molecule. N-terminal mutations in CFH (CCPs 1–4) result in ineffective control of the AP both in the fluid phase and on cell surface (Pechtl et al., 2011). The functional effects of normally secreted genetic variants in other regions of the protein remain to be determined (Kavanagh and Anderson, 2012, Tortajada et al., 2012).
CFH and the five factor H-related proteins, which arose from several large genomic duplications, reside in the RCA cluster. This homology predisposes to gene conversions and genomic rearrangements through non-allelic homologous recombination (NAHR) and microhomology-mediated end joining (MMEJ). The CFH mutations S1191L, V1197A, and combined S1191L/V1197A arose through gene conversion between CFHR1 and CFH (Heinen et al., 2006). A hybrid (fusion) gene comprising the 21 N-terminal exons of CFH and the 2 C-terminal exons of CFHR1 has been demonstrated to have arisen through NAHR resulting in aHUS (Venables et al., 2006). More recently a hybrid gene consisting of the 22 N-terminal exons of CFH and the 5 C-terminal domains of CFHR3 arising through MMEJ has been reported in aHUS (Francis et al., 2012). As with C-terminal point mutations in CFH, these hybrid genes result in loss of cell surface complement regulation.
3.2. Complement factor I
CFI is a serum serine protease, which functions as a critical mediator of complement regulation by cleaving C3b and C4b in the presence of its cofactors (CFH for C3b; C4BP for C4b; MCP and CR1 for both). It is predominantly synthesised by the liver. Mutations in CFI account for between 5 and 10% of aHUS (Caprioli et al., 2006, Fremeaux-Bacchi et al., 2005, Kavanagh et al., 2005, Kavanagh et al., 2008b, Maga et al., 2010, Nilsson et al., 2010, Nilsson et al., 2007, Sullivan et al., 2010, Westra et al., 2010). The CFI mutations described in aHUS are all heterozygous. These mutations cluster in the serine protease domain and the majority result in a non-secreted protein. Functional analysis has been undertaken for a number of mutants and demonstrates a loss of both AP and CP regulatory activity in the fluid phase and on cell surfaces (Kavanagh et al., 2008b, Nilsson et al., 2010).
3.3. Membrane cofactor protein
MCP is a surface bound complement regulatory protein which acts as a cofactor for the CFI mediated cleavage of C3b and C4b that are deposited on host cells (Richards et al., 2007a). Mutations in MCP are found in around 10% of patients with aHUS (Caprioli et al., 2006, Fremeaux-Bacchi et al., 2006, Maga et al., 2010, Richards et al., 2007a, Richards et al., 2003, Westra et al., 2010). Most mutations described in aHUS reside in the extracellular 4 CCP domains that are responsible for C3b and C4b binding. Most MCP mutations described to date result in a quantitative defect in MCP (∼75%) (Richards et al., 2007a). The remaining mutations have been demonstrated to result in a secreted, non-functional protein (Richards et al., 2007a).
3.4. Activating mutations
In addition to loss of function mutations in complement regulatory proteins, gain of function mutations have been described in the complement components C3 and factor B (CFB). C3 is cleaved to form the anaphylatoxin C3a and C3b, which is highly reactive, and can bind to cell surfaces via its reactive thioester. C3b can then interact with CFB in the presence of factor D to form the AP C3 convertase (C3bBb), which cleaves further C3, introducing a positive-amplification loop (Fig. 1).
Gain of function mutations in CFB are rare (Fan et al., 2013, Geerdink et al., 2012, Goicoechea de Jorge et al., 2007, Kavanagh et al., 2006, Noris et al., 2010, Roumenina et al., 2009, Tawadrous et al., 2010). The mutations have been demonstrated to either enhance formation of the C3bB proenzyme or form a C3 convertase more resistant to decay by the complement regulators decay accelerating factor (DAF; CD55) and CFH (Goicoechea de Jorge et al., 2007, Roumenina et al., 2009). Ultimately these mutations result in increased complement deposition on endothelial cells (Roumenina et al., 2009).
Mutations in C3 appear to be more common occurring in 2–10% of aHUS (Fan et al., 2013, Fremeaux-Bacchi et al., 2013, Fremeaux-Bacchi et al., 2008, Geerdink et al., 2012, Lhotta et al., 2009, Maga et al., 2010, Noris et al., 2010, Roumenina et al., 2012, Sartz et al., 2012). As with CFB mutations, the C3 mutants either have increased resistance to regulation or bind to CFB with higher affinity resulting in increased C3 convertase formation (Roumenina et al., 2012, Sartz et al., 2012). These mutations result in increased complement activation on platelets (Sartz et al., 2012) and glomerular endothelium (Roumenina et al., 2012)
3.5. Acquired complement abnormalities in aHUS
As well as the genetic abnormalities described in aHUS, autoantibodies to CFH have also been linked to disease in 4–14% of aHUS patients (Abarrategui-Garrido et al., 2009, Dragon-Durey et al., 2005, Foltyn Zadura et al., 2012, Jozsi et al., 2007, Maga et al., 2010, Moore et al., 2010, Noris et al., 2010). In cohorts of paediatric patients, this figure is as high at 25% (Hofer et al., 2012). Most of the reported studies suggest that the anti-CFH Abs bind predominantly to the C-terminus (Dragon-Durey et al., 2005, Jozsi et al., 2007, Moore et al., 2010) although in some cases there is a polyclonal response (Blanc et al., 2012). Cross reactivity of the anti-CFH Ab has also been seen to CFHR1 (Blanc et al., 2012, Moore et al., 2010, Strobel et al., 2011) and CFHR2 (Blanc et al., 2012). Several studies have demonstrated various functional consequences of anti-CFH Abs. The antibodies have been demonstrated to reduce binding to C3b (Blanc et al., 2012, Jozsi et al., 2007). They perturb CFH-mediated cell surface protection and in some individuals the autoantibodies also impair cofactor activity (Blanc et al., 2012) or decay accelerating activity (Dragon-Durey et al., 2004). These functional studies suggest a pathogenic role for CFH autoantibodies in aHUS.
Autoantibodies to CFI are much rarer than anti-CFH Abs (0–2%) (Foltyn Zadura et al., 2012, Kavanagh et al., 2012). Anti-CFI Abs were seen to form immune complexes in serum however functional analysis revealed only a minor effect on fluid phase co-factor activity (Kavanagh et al., 2012). The co-existence of functionally significant mutants in the majority of patients, added to the lack of correlation of anti-CFI Ab titre and disease activity raise the possibility that they are an epiphenomenon rather than a direct cause of disease.
4. Incomplete penetrance
Incomplete penetrance has been reported for all the genes associated with aHUS. Penetrance has been reported at around 50% for individuals carrying CFH, CFI, MCP, and CFB mutations (Caprioli et al., 2003, Kavanagh and Goodship, 2010, Sullivan et al., 2011) and slightly lower for C3 mutations, albeit with small numbers (Lhotta et al., 2009). This suggests that the penetrance is altered by other environmental and genetic modifiers.
It is increasingly recognised that patients may have mutations in more than one complement gene (Bienaime et al., 2010, Cruzado et al., 2009, Esparza-Gordillo et al., 2006, Maga et al., 2010, Sellier-Leclerc et al., 2007) or mutations in one complement gene in addition to autoantibodies to complement regulators (Kavanagh et al., 2012, Moore et al., 2010). In a study of 795 aHUS patients the European Working Party on Complement Genetics demonstrated that at least 3.4% of aHUS cases will have more than one mutation. 8–10% of patients with mutations in CFH, C3 or CFB had combined mutations whereas 25% of patients with mutations in CFI or MCP had combined mutations (Bresin et al., 2013). The penetrance increased as the number of mutations in a patient increased (Bresin et al., 2013).
In addition to mutations in complement genes a number of single nucleotide polymorphisms (SNPs) in CFH have been demonstrated to be associated with aHUS in several studies (Abarrategui-Garrido et al., 2009, Caprioli et al., 2003, Ermini et al., 2012, Esparza-Gordillo et al., 2005, Fremeaux-Bacchi et al., 2005, Pickering et al., 2007). A haplotype in CFH (CFH-H3; tgtgt) composed of these SNPs increases this risk of aHUS 2–4-fold (Fremeaux-Bacchi et al., 2013, Pickering et al., 2007). A haplotype block in MCP (MCPggaac) comprising 2 SNPs in the promoter region has been associated with a 2–3 fold increased risk of aHUS (Esparza-Gordillo et al., 2005, Fremeaux-Bacchi et al., 2013, Fremeaux-Bacchi et al., 2005). Some of these studies have suggested that this risk occurs exclusively in those patients already carrying complement mutations (Ermini et al., 2012, Esparza-Gordillo et al., 2005). A SNP in C4b binding protein (R240H) was associated with aHUS in cohorts from the UK and France but could not be replicated in a Spanish cohort (Blom et al., 2008, Martinez-Barricarte et al., 2009). In a study examining SNPs in 47 complement genes in 2 separate cohorts, SNPs in CFHR2 and CFHR4 were also associated with aHUS. In this study there were no reproducible associations between SNPs and aHUS outside the RCA cluster (Ermini et al., 2012).
Thus, haplotypes and SNPs act together with mutations and inhibitory autoantibodies to increase the penetrance of disease. However even when a patient has multiple genetic risk factors, disease may not present until middle age suggesting a triggering stimuli is required for disease to manifest. In individuals with mutations, these stimuli have been suggested to be upper respiratory tract infections, fevers, pregnancy, drugs and non Escherichia coli diarrhoeal illnesses as potential triggers (Bento et al., 2010, Caprioli et al., 2006, Edey et al., 2008, Fakhouri et al., 2010, Fremeaux-Bacchi et al., 2013, Goodship and Kavanagh, 2010, Noris et al., 2010). It is likely that these events trigger the AP which susceptible individuals are unable to adequately control, resulting in aHUS.
5. Diacylglycerol kinase ɛ and HUS
In addition to complement mediated aHUS (Lemaire et al., 2013) have recently demonstrated that homozygous or compound heterozygous mutations in diacylglycerol kinase ɛ (DGKɛ) cause disease. The clinical phenotype of these patients appears distinct from complement mediated aHUS. All individuals presented with aHUS before one year (mean 0.5 years, range 0.3–0.9 years). In those recovering from the acute episode of aHUS, microscopic haematuria and proteinuria persisted and progression to CKD was common. In keeping with this distinct phenotype, a recessively inherited MPGN like illness with proteinuria and renal failure has also been linked to DGKɛ (Ozaltin et al., 2013). In contrast to serum complement mediated aHUS, recurrence in renal transplants was not seen.
6. Diagnosing aHUS
Having diagnosed a thrombotic microangiopathy (TMA), the initial management involves differentiating between thrombotic thrombocytopenic purpura (TTP), Stx-HUS, and aHUS (reviewed Loirat and Fremeaux-Bacchi, 2011). Rapid exclusion by microbiological analysis for Stx-producing E. coli and analysis of ADAMTS13 activity can lead to a diagnosis of aHUS. Following exclusion of Stx-HUS and TTP, precipitating events and the underlying genetic defects predisposing to aHUS should be sought.
Prior to initiation of plasma exchange (PE) serum should be obtained for levels of C3, C4, CFH and CFI, and a complement antibody screen. FACS analysis of peripheral blood mononuclear cells for MCP expression should be performed. Genetic testing including a method to detect copy number variation should be undertaken.
7. Prognosis
Historically, the overall prognosis for patients with aHUS has been poor with up to 48% of children and 67% of adults dying or reaching end-stage renal disease (ESRD) within 5 years (Fremeaux-Bacchi et al., 2013, Noris et al., 2010). The outlook is predicted by the genotype with MCP mutations carrying the best prognosis. In several large cohorts, no patient with an MCP mutation died at first presentation and at 5 years only 35% had reached ESRD. Mutations in CFH, CFI or C3 all carry poor outcomes. At 3–5 years follow up, up to 77% of patients with CFH mutations had developed ESRD or had died. Only 30–40% of individuals with CFI and C3 mutations will be alive with native kidney function at 3–5 years (Fremeaux-Bacchi et al., 2013, Noris et al., 2010). The prognosis of aHUS with CFB mutations is also poor (Goicoechea de Jorge et al., 2007, Noris et al., 2010, Roumenina et al., 2009).
In addition to predicting the outcome in native kidneys, the outcome following renal transplantation is determined largely by the underlying genetic abnormality. Graft failure is predominantly due to aHUS recurrence which occurs in 60–70% of patients (Bresin et al., 2006, Le Quintrec et al., 2013). In individuals with mutations in CFH the recurrence rate is >80%. Similarly activating mutations in C3 and CFB also have a high risk of renal recurrence. Initial studies all suggested that mutations in CFI carried a poor prognosis although more recently one study failed to replicate this data (Le Quintrec et al., 2013). Unlike the serum complement proteins, the recurrence rate in individuals with mutations in MCP is very low (Loirat and Fremeaux-Bacchi, 2008). As MCP is a membrane regulator, a renal allograft will correct the complement defect and protect against aHUS.
8. Treatment
8.1. Plasma exchange
Until the introduction of Eculizumab, PE has been considered the ‘gold-standard’ for management of aHUS. The replacement of non-functioning complement regulators and hyper-functional complement components (e.g. gain of function mutations) in addition to the removal of CFH-autoantibodies made PE a logical choice (reviewed in European (Ariceta et al., 2009) and UK (Taylor et al., 2010) guidelines on aHUS treatment). The consensus based guidelines recommended that PE should be commenced as soon as possible on diagnosis of aHUS and performed daily with dose titration to control haemolysis. Once haemolysis has been controlled, PE can be slowly withdrawn, although individuals with genetic defects in the complement system are frequently plasma dependent and require long term plasma therapy (weekly/biweekly) to maintain remission. In adults, only once ADAMTS13 deficiency is excluded should Eculizumab be considered.
9. Eculizumab
9.1. Pharmacology
Eculizumab is a recombinant, monoclonal antibody directed against human complement component C5 (Rother et al., 2007). Molecular modelling has suggested that Eculizumab binds C5 and prevents its entry into the C5 convertase, thus precluding cleavage into the effector molecules, C5a and C5b (Zuber et al., 2012a) (Fig. 1).
Eculizumab has been humanized, replacing the murine heavy-chain constant region with a human hybrid IgG2/IgG4 constant region (Thomas et al., 1996). This hybrid region utilises the desirable properties both of IgG2, which fails to bind Fc receptors (Canfield and Morrison, 1991), and IgG4, which does not activate complement (Tao et al., 1993), to reduce the pro-inflammatory potential of the antibody.
Eculizumab is administered by intravenous infusion and has a half-life of ∼11 days (Rother et al., 2007). Complete blockade of the terminal pathway of complement occurs in vivo with serum concentrations above 35 μg/mL (Park et al., 2011). Human tissue cross-reactivity has not been seen in Eculizumab binding studies (Rother et al., 2007) and little transplacental transfer of Eculizumab has been reported in pregnant women with paroxysmal nocturnal haemoglobinuria (PNH) on Eculizumab (Kelly et al., 2010, Luzzatto et al., 2010).
9.2. Evidence in animal models for the use of Eculizumab in aHUS
Pickering et al. (2007) generated a transgenic mouse lacking the C-terminal domains of factor H (Cfh−/−Δ16–20). Without the GAG binding domains, endothelial cell complement regulation was lacking and the mice spontaneously developed aHUS. Goicochea de Jorge et al. (2008) subsequently crossed the Cfh−/−Δ16–20 with a C5-deficient mouse which did not develop aHUS, suggesting a critical role downstream of C3b generation in aHUS and thus providing a rationale for the use of Eculizumab in human disease.
9.3. The use of Eculizumab in aHUS
The use of Eculizumab in aHUS was first reported by Gruppo and Rother (2009) and Nurnberger et al. (2009) as two separate cases published in the New England Journal of Medicine in 2009. In this review, we describe the published experience of the use of Eculizumab in the treatment of aHUS. In total 44 cases have been summarised in Table 1, Table 2, Table 3. These are limited to individual case reports and series. Data from prospective clinical trials of Eculizumab in the treatment of aHUS awaits publication.
Table 1.
Summary of 18 patients receiving Eculizumab for the treatment of aHUS in the native kidney.
| Reference | Mutation | Age at onset of aHUS | Response to PE | Time from aHUS diagnosis to Ecu | Response to PE at time of Ecu | SCr (μmol/L) at time of Ecu | Achieved TMA remission | Ecu dosing | Evolution of aHUS last SCr (μmol/L) | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Gruppo and Rother (2009) | NI | <8d | PI sensitive 3 relapses over 11m |
19m | Resistant | 265 | Y | Ongoing | Remission 35 |
2y 4m |
| Fremont et al. (2009) | CFH | 4y | PE partially sensitive | Months | Partially sensitive | 80 | Y | Ongoing | Remission 26 |
10w |
| Mache et al. (2009) | NI | 17.8m | PE sensitive 3 relapses at PE taper |
3m | Resistant | Dialysis | Y | Single dose | Relapse 2 weeks further 3 doses ESRD |
Ecu discontinued |
| Kose et al. (2010) | NI | 18y | NK | Months | Resistant | ∼300 | Y | Single dose | Relapse at 2m ESRD |
NA |
| Lapeyraque et al. (2011) | CFH S1191L V1197A | 7m | PE/PI sensitive 11 relapses over 5 y |
Years | Resistantb | 108 | Y | Ongoing | Remission 44 |
1y 3m |
| Prescott et al. (2010) | CFI A258T | 47y | PE sensitive relapse 2w after PE cessation |
18d | Resistant | 610 | Y | Ongoing | Remission 230 |
7m |
| Ohanian et al., 2011a, Ohanian et al., 2011b | NI | 50y | NAa | 6d | No PEa | 600 | Y | Ongoing | Remission 125 |
6m |
| Tschumi et al. (2011) | CFH C611Y | 9y | 2 relapses during PE taper | 126d | Sensitivec | 220 | Ye | Ongoing | Remission ∼100 |
2y |
| Ariceta et al. (2012) | NI | 28d | Resistant to 4× PI Intolerant of PE |
11d | Resistantb | Dialysis | Y | Ongoing | Remission normal SCr |
14m |
| Carr and Cataland (2012) | CFH | 20y | Resistant | <2w | Resistant | Dialysis | Y | 9m | Relapse 6m later Remission again with Ecu |
NK |
| Cayci et al. (2012) |
CFI K434R |
10y | 10 sessions of PE | <2w | Resistant | Dialysis | Y | 3× doses | Remission ∼44 |
4m |
| Garjau et al. (2012) | MCP c.286 + 1G > C | 44y | PE for 90 days | 90d | Resistant | Dialysis | Y | 27w | ESRD at 27w | Ecu discontinued |
| Kim et al. (2012) | CFH Y1190H | 7m | 3 relapses despite PE | 4m | Resistant | Dialysis | Y. | Ongoing | Remission 75 |
18m |
| Dorresteijn et al. (2012) | NI | 6y | Initial response to PE, relapse on PI | 11w | Resistant | Dialysis | Y | Ongoing | Remission 80 |
9m |
| Giordano et al. (2012) | CFH c3514G > T | 1y | 21 PI | 3m | Resistant | Dialysis | Y | Ongoing | Remission 44 |
12m |
| Vilalta et al. (2012) | CFH D1119N | 1y | PE sensitive | 5m | No PEd | Dialysis | Y | Single dose | Relapse after 8w restarted on Ecu remission 26.5 |
2.5y |
| De et al. (2010) and Heinen et al. (2013) | CFH S1191L | 6m | PE dependent | 11y | Sensitive | Dialysis | Y | Ongoing | ESRD Ecu continued to maintain TMA remission |
NK |
| Povey et al. (2013) | NK | NK | PE resistant | Months | Resistant | Dialysis | Y | Ongoing | Remission near-normal SCr |
NK |
| Gilbert et al. (2013) |
CFB K323Q |
4m | NAa | 7 days | No PEa | 20 | Y | Ongoing | Remission 12 |
6m |
NI: not identified; NK: not known; NA: not applicable; SCr serum creatinine; Ecu: Eculizumab; PI plasma infusion; PE plasma exchange.
Commenced on Ecu first line.
Was receiving plasma infusion.
Stopped due to allergic reaction.
PE previously stopped due to intolerance.
Already in remission when Ecu commenced.
Table 2.
Summary of 15 patients receiving Eculizumab for the treatment of aHUS recurrence following renal transplantation.
| Reference | Mutation | Previous transplants | Age and post-Tx course | Time from recurrence to Ecu | SCr (μmol/L) at time of Ecu | Eculizumab dosing | TMA remission achieved | Recurrence if Ecu stopped | Outcome Ecu continued SCr (μmol/L) | Follow up |
|---|---|---|---|---|---|---|---|---|---|---|
| Nurnberger et al. (2009) | CFH Y475S | 1st Tx recurrence at 5w, PE resistant, graft loss | 37y 2nd Tx, recurrence at 6w. PE resistant |
5d | 132 | Single dose | Y | Likely (21m) graft loss | NA | NA |
| Chatelet et al. (2010) |
C3 R570Q |
1st Tx, recurrence at 5m Graft loss at 2y. |
43y 2nd Tx, recurrence at 3y, PE dependent |
15m | 320 | Ongoing | Y | NA | 2 recurrences of TMAb 230 |
2y 5m |
| Legault and Boelkins (2009) | ND | No | 34y 1st Tx recurrence at 1m and 5m. PE sensitive then resistant |
9m | 323 | Ongoing | Y | NA | Remission 238 |
6m |
| Davin et al. (2010) | CFH S1191L | 1st Tx, recurrence at 3d, graft loss. 2nd Tx under PE, recurrence at 10w graft loss |
17y 3rd Tx, prophylactic PE. Recurrence at 4m. rescue PE intolerant at 10m. |
10m | 131 | Ongoing | Ya | NA | Remission 130 |
1y 10m |
| Larrea et al. (2010) and Loirat and Fremeaux-Bacchi (2011) | NI | No | 22y 1st Tx recurrence at 12d PE resistant |
9d | 415 | Single dose | Y | Recurrence (11.5m) Ecu resumed |
Subsequent humoral rejection Ecu stopped and graft loss |
NA |
| Zuber et al. (2011) | CFH | 1st Tx, recurrence, graft loss | 24y 2nd Tx, prophylactic PI/PE recurrence 1d PE resistant |
4d | 500 | Ongoing | Y | NA | Remission 62 |
9m |
| Al-Akash et al. (2011) |
C3 R570W |
1st Tx, recurrence at 4y, graft loss 2nd Tx recurrence at 2m graft loss |
15y 3rd Tx, prophylactic PE, recurrence at 2m, PE partially sensitive |
∼20d | 202 | Ongoing | Y | NA | Remission 115 |
1y 5m |
| Duran et al. (2012) | CFH Q1172X | No | 32y 1st Tx,. recurrence at 1m PE sensitive further recurrence at 2m |
1m | Dialysis | Ongoing | Y | NA | Remission 228 |
10m |
| Alachkar et al. (2012) | NI | 1st Tx recurrence 2m, graft loss | 32y 2nd Tx recurrence at 10 weeks. PE resistant |
∼2w | Dialysis | 8m | Y | Recurrence 5m after Ecu stopped – pneumoniac | Ecu restarted but graft loss after ATN. Ecu discontinued |
NA |
| Ardissino et al. Zuber et al. (2012b) | CFH | No | 6y 1st Tx, recurrence 2m, PE resistant |
2d | 442 | Ongoing | Y | NA | Remission 48 |
25m |
| Zuber et al. (2012b) | CFH S1191L V1197A | 1st Tx recurrence, graft loss |
23 y 2nd Tx recurrence 3d. PE resistant |
3d | 627 | Ongoing | Y | NA | Remission 65 |
17m |
| Zuber et al. (2012b) | CFH/CFHR1 hybrid | 4 previous Tx – 2 due to recurrence, 2 due to thrombosis | 27y 5th Tx, recurrence 3d PE partially sensitive |
1m | 237 | Ongoing | Y | Fresh TMA lesions at 3 monthsd | Remission 204 |
12m |
| Zuber et al. (2012b) | Anti FH Ab ΔCFHR1/3 |
4 previous Tx, 3 due to recurrence | 41y 5th Tx, recurrence 5ye PE partially sensitive |
3m | 89 | Ongoing | Y | NA | Remission 80 |
9m |
| Guentin et al. Zuber et al. (2012b) |
CFI G101R |
1st Tx, recurrence graft loss |
42y 2nd Tx, 15m of prophylactic PE, 8m taper recurrence at 13m after stopping. PE resistant |
9w | 190 | Ongoing | Y | Relapse following delay prior to 5th infusion | Remission 156 |
4.5m |
| Heyne Zuber et al. (2012b) |
NI | 1st Tx recurrence graft loss |
43y 2nd Tx, recurrence 8d no PE |
1d | 176 | 8m | Y | Relapse 3 months after stopping influenza vaccine triggered |
Remission 123 |
14m |
NI: not identified; ND: not documented; Tx: renal transplant; Ecu: Eculizumab; SCr: serum creatinine.
In remission already.
aHUS recurrence with AKI when injection delayed by 6–8 days.
Following Ecu resumption, patient had endovascular procedure leading to severe ATN and subsequent graft loss.
Biopsy of transplant allograft in response to falling haptoglobin level.
Graft biopsy due to slight decrease in renal function disclosed fresh TMA lesions.
Table 3.
Summary of 10 patients who received Eculizumab as pre-emptive treatment for aHUS in renal transplantation.
| Reference | Mutation | Previous transplants | Age (y) at current Tx | Type of donor | Received PEa | When Eculizumab starteda | Outcome SCr (μmol/L) | Follow Up |
|---|---|---|---|---|---|---|---|---|
| Zimmerhackl et al. (2010) |
CFH W1183C |
No | 10 | DD | 9 PE day 0 to day 9 |
Day 10 | No recurrence 44 |
2y 1m |
| Weitz et al. (2011) |
CFH E1198X |
No | 7 | DD | No | Day-21b | No recurrence normal SCr |
7m |
| Nester et al. (2011) | CFH/CFHR1 hybrid | No | 12 | LNR | 2 PE day-7 and day-1 |
Day 7 and -1 | No recurrence 80 |
4m |
| Krid et al. (2012) | CFH/CFHR1 hybrid | No | 7.5 | DD | No | At time of transplant | No recurrence normal SCr |
16m |
| Rondeau et al. Zuber et al. (2012b) | Complex recombination between CFH and CFHR1 | No | 18 | DD | 6PE day 0 to day 5 |
Day 5 | No recurrence | 14m |
| Lahoche Zuber et al. (2012b) |
C3 R161W |
No | 6.4 | DD | No | At time of transplant | No recurrence 44 |
4.5m |
| Krid and Niaudet Zuber et al. (2012b) |
CFH Q1137X |
No | 9 | DD | No | At time of transplant | No recurrence 58 |
2m |
| Hourmant Zuber et al. (2012b) |
CFH S1191L |
1st Tx, recurrence. | 18 | DD | 1PE day 0 |
At time of transplantc | Graft lossd | NA |
| Zuber and Legendre Zuber et al. (2012b) |
CFH Y1177C |
1st and 2nd Tx recurrence | 41 | DD | No | At time of transplant | No recurrence 176 |
2m |
| Xie et al. (2012) |
CFH E625X |
No | 30 | LNR | 2PE day-7 and day-1 |
Day-7 and day-1 | No recurrence ∼77 |
1y |
NA: not applicable; DD: deceased donor; LNR: live non-related donor; SCr: serum creatinine; Ecu: Eculizumab.
Timings in days in relation to day of renal transplantation.
Received weekly doses until transplantation.
Discontinued after nephrectomy.
Early arterial thrombosis at day 1, nephrectomy day 3.
9.4. Eculizumab and the treatment of aHUS in native kidneys
There are currently 19 reported cases of the use of Eculizumab in aHUS in native kidneys (Table 1). Over half were in children and the oldest patient was 50 years. While the clinical course of disease leading to the commencement of Eculizumab was variable, response was overwhelmingly positive, albeit with the caveat of publication bias towards successful cases. Plasma exchange was attempted in 17/19 patients prior to the use of Eculizumab. Most (13/19) patients had severe renal failure or had already commenced renal replacement therapy. The time from diagnosis until use of Eculizumab was variable. There were 6 patients who received Eculizumab early in their clinical course (<2 weeks). Five were on dialysis and four patients had some plasma exchange. The fifth patient described by (Ohanian et al., 2011a, Ohanian et al., 2011b) had no plasma exchange – concerns regarding her neurological status prompted the first-line use of Eculizumab. In a further paediatric case Eculizumab was used as first line treatment successfully (Gilbert et al., 2013).
The general outcome was of an improvement in TMA and most patients had improvement in their renal function, including many of those already on dialysis. Povey et al. (2013) reported a case of a 21-year old unsuccessfully treated with PE who responded to Eculizumab and regained almost normal renal function despite over 3 months of dialysis requirement. Three patients did not recover renal function. Mache et al. (2009) describe resolution of the TMA and some renal recovery following a single dose of Eculizumab, given 3 months after diagnosis of aHUS. Following a relapse 2 weeks later, 3 more doses of Eculizumab were given. This again corrected the TMA but the patient had developed ESRD and Eculizumab was stopped. Kose et al. (2010) report initial control of TMA and renal improvement following a single dose of Eculizumab but a relapse after 2 months led to ESRD. Garjau et al. (2012) report failure to recover renal function following initial treatment with PE and commencing treatment with Eculizumab 90 days after diagnosis for a total of 27 weeks. The TMA, however, did improve.
Heinen et al. (2013) and Lapeyraque et al. (2011) report treatment following a long relapsing-remitting course highly dependent on PE. The period of plasma treatments had spanned 11 and 5 years respectively. In the former case, a switch to Eculizumab maintained TMA remission (though the patient already had established ESRD without improvement). In the latter case, PE resistant TMA had developed and Eculizumab successfully corrected TMA with improvement in renal function.
9.5. Eculizumab in recurrent aHUS in renal transplantation
There are currently 15 reported cases of the use of Eculizumab for the treatment of recurrence of aHUS in renal allografts (Table 2). Nine patients had documented mutations which are considered high risk for recurrence. The experience in early childhood is limited – with only one child (6 years). The others ranged from 15 years to 43 years. Eight patients received Eculizumab within one month of disease recurrence. The longest time from recurrence to commencement of Eculizumab was 15 months. All, but one patient, received PE as part the treatment for recurrence of aHUS (prior to receiving Eculizumab), including five patients who were on pre-emptive PE strategies. All patients responded to Eculizumab, irrespective of their clinical course leading up to the use of Eculizumab. Eight patients have remained on Eculizumab and have been in remission throughout.
Nurnberger et al. (2009) report giving a single dose of Eculizumab resulting in early remission although the patient subsequently lost their graft at 21 months – without biopsy proven TMA. In a separate case, a single dose initially controlled disease but aHUS recurred at 11 months necessitating reintroduction of Eculizumab (Larrea et al., 2010). Subsequent humoral rejection ultimately led to graft loss.
In two reported cases (Alachkar et al., 2012, Zuber et al., 2012b) Eculizumab initially controlled aHUS recurrence in the transplant and treatment was stopped at 8 months. One patient developed pneumonia 5 months after discontinuation of Eculizumab leading to a relapse of aHUS and worsening renal function. Eculizumab was recommenced resulting in improvement in TMA and renal function. Following an endovascular procedure, this patient developed severe acute tubular necrosis with poor recovery. Progressive renal failure followed leading to graft loss after 2 years. Eculizumab was only discontinued at this time-point – there was no evidence of TMA throughout this time. The other patient had a relapse of aHUS 3 months after discontinuation of Eculizumab, triggered by influenza vaccination. Eculizumab was restarted and remission has been maintained. In two further cases (Chatelet et al., 2010, Zuber et al., 2012b) minor relapses followed delays in dosing. Zuber and Legendre (Zuber et al., 2012b) report one patient who had a low haptoglobin level between 6 and 12 weeks of starting Eculizumab. Renal biopsy was therefore undertaken demonstrating fresh TMA lesions despite ongoing Eculizumab treatment. CH50 was below the level of detection and renal allograft function was stable.
9.6. Pre-emptive use of Eculizumab in renal transplantation
With the high aHUS recurrence rate in renal allografts in individuals with complement mutations, pre-emptive Eculizumab has been given in ten cases (Table 3). All patients had a complement mutation that is associated with a high risk of disease recurrence. Two patients had PE prior to renal transplantation and continued until days 5 and 10 respectively before receiving Eculizumab. Three patients had PE followed by Eculizumab pre-operatively and the remaining five patients received only Eculizumab (no PE) at the time of transplantation. Most (9/10) patients remain in disease remission (and still receiving Eculizumab) with excellent graft function with follow-up ranging from 2 months to over 2 years. Only 1 patient had graft loss and this was due to arterial thrombosis in a patient with S1191L mutation in CFH.
9.7. Prospective trials
Although the results of the prospective trials have as yet, only been reported in meeting abstracts (Greenbaum et al., 2011, Greenbaum, 2011, Legendre et al., 2010, Licht, 2011, Licht et al., 2011, Muus et al., 2010) from the information available, Eculizumab seems highly effective, with ∼85% of patients becoming disease free in both plasma-resistant and plasma-dependent aHUS (reviewed Zuber et al., 2011). It has been suggested that Eculizumab achieves better control of disease as witnessed by improvement in renal function following switching from PE and in rescuing plasma resistant individuals (Zuber et al., 2011). It should be noted however that a randomised trial of Eculizumab against PE was not and is unlikely to be performed.
9.8. Adverse effects of Eculizumab
There were few side effects documented in the review of the case reports. Adverse effects were reported in preliminary data from the prospective open-label clinical trials of Eculizumab in aHUS. Campistol et al. (2013) summarise this data and note 4 reports (out of 37 patients) of serious adverse effects (peritonitis, influenza infections, venous disorder and severe hypertension). The use of Eculizumab has been approved for use in PNH following a successful clinical trial. In this study there were only 4 serious adverse effects (compared to 9 in placebo). These were exacerbation of PNH, renal colic, lumbar- or sacral-disc prolapsed and α-haemolytic streptococcal bacteraemia (Hillmen et al., 2006). Vaccination against Streptococcus pneumonia and Haemophilus influenza B in children treated with Eculizumab has been recommended (Zuber et al., 2011).
9.9. Risk of meningococcal infection
It is well recognised that loss of ability to form membrane attack complex is associated with infection due to Neisseria meningitidis. Patients receiving Eculizumab should therefore receive the tetravalent vaccine (A,C,Y,W135). Because the vaccine does not cover the most prevalent serogroup in Europe – serogroup B (Bouts et al., 2011) we recommend ongoing prophylactic penicillin in Eculizumab treated patients.
9.10. When to start Eculizumab?
We believe that there is a clear role for the use of Eculizumab in the treatment of aHUS. Despite this PE should remain the initial treatment in adults until ADAMTS13 deficiency can be excluded. In paediatric cases where the risks and difficulties of administrating PE are high, it has been suggested that Eculizumab be used first line. The success of such a strategy depends on the differentiation of aHUS from TTP and the authors are aware of at least one case where congenital ADAMTS13 deficiency would have been inappropriately treated with Eculizumab (based on platelet count, creatinine and clinical presentation).
Given the apparent superior efficacy of Eculizumab over PE (Zuber et al., 2011), the authors believe that once ADAMTS13 deficiency has been excluded all patients should be managed with Eculizumab. While genetic testing should be performed in all patients with aHUS, it need not delay treatment with Eculizumab. Clearly, the prohibitive cost of Eculizumab will mean that in many parts of the World, PE remains the only available treatment.
In patients undergoing transplantation with high risk mutations (e.g. CFH, C3 and CFB), the unavoidable ischaemia–reperfusion injury induced complement activation make pre-emptive Eculizumab the treatment of choice in our opinion.
The penetrance rate in families with mutations is low and as such there will be many family members potentially at risk of disease when exposed to a triggering stimuli. Currently in family members with known mutations our strategy has been to advise monitoring at times of high risk (e.g. pregnancy, respiratory tract infections, etc.).
9.11. When to stop Eculizumab?
Ultimately, the aim is to balance the risk of Eculizumab (see side-effects) against effective treatment and prevention of recurrence of TMA and the organ-specific damage associated with it. Early cessation of Eculizumab has given rise to recurrence of disease. This is perhaps unsurprising with a terminal cascade blocking agent, as the C3 convertase will initially still be active on the glomerular vasculature. As such, a sustained course of treatment should be given to maintain inhibition of complement activity. The reported experiences suggest that even in individuals who have presented requiring dialysis, Eculizumab treatment can control disease eventually leading to recovery of renal function after several months. It is tempting to treat every patient with long-term Eculizumab, but the cost and unknown long-term effects of this treatment need to be considered. There are certain groups of individuals where withdrawal of Eculizumab is likely to be successful (e.g. MCP mutations). Such a strategy should be performed under the auspices of a clinical trial with careful monitoring.
Likewise in individuals treated with Eculizumab where genetic analysis subsequently reveals mutations in DGKɛ, treatment can be stopped. This pathway does not seem to involve the complement system and as such complement modulatory therapy is unlikely to be efficacious. Indeed, one patient with mutations in DGK ɛ developed aHUS while on treatment with Eculizumab (Lemaire et al., 2013).
In individuals where TMA has been controlled but renal function has not recovered Eculizumab has usually been discontinued. There are, however, rare reports of severe extra-renal manifestations such as cerebral artery stenoses (Kaplan et al., 2000, Loirat et al., 2010, Vergouwen et al., 2008) which have been attributed to ongoing complement activation. The experience is currently too limited to recommend routine ongoing treatment with Eculizumab in individuals on long term dialysis.
9.12. Single nucleotide polymorphisms in C5
It had been noted in a Japanese PNH population that certain individuals did not respond to Eculizumab despite adequate serum levels (Nishimura et al., 2012). In vitro assays of these non-responders’ serum demonstrated an inability of Eculizumab to prevent haemolysis. An antibody against a different epitope on C5 did, however, block haemolysis. Genetic analysis of these non-responders revealed a SNP in C5 (p.R885H) which predicted a poor response to Eculizumab (Nishimura et al., 2012). This SNP was present in around 2% of a Japanese population. In individuals with aHUS who fail to respond to Eculizumab, PE should be re-instituted and genetic analysis of C5 performed.
9.13. Long term treatment with Eculizumab
While the effect of Eculizumab in preventing ESRD in aHUS seems inescapable, long term follow up is still awaited. It is interesting to note that an aHUS patient who received the drug seems to have deposited the Eculizumab in glomeruli and tubular basement membranes (Herlitz et al., 2012). This has also been reported in individuals receiving treatment for C3 glomerulopathies and DDD where Eculizumab was deposited in a similar distribution and was said to resemble monoclonal immunoglobulin deposition disease (Herlitz et al., 2012). Similarly, as would be expected in a terminal pathway blocking therapy, these individuals continued to deposit C3 in the kidney (Herlitz et al., 2012). While the outcome of these effects remains to be established, the longer term evidence from PNH treatment would not suggest worsening of kidney function or development of proteinuria (Hillmen et al., 2010).
10. Summary
In the last 15 years the elucidation of the role of complement in pathogenesis of aHUS has seen a sea change in the management of disease. The complement inhibitor, Eculizumab, has now been demonstrated to be effective in controlling aHUS, however, its prohibitive cost will mean that in many parts of the World, PE will remain the only treatment option.
Added in press
The prospective trial of Eculizumab in aHUS demonstrating efficacy has now been published (Legendre et al., 2013).
Acknowledgements
EKSW is a Medical Research Council Clinical Research Training Fellow. DK is a Wellcome Trust Intermediate Clinical Fellow. THG and DK have received fees from Alexion Pharmaceuticals for invited lectures and attendance at Global aHUS Advisory panel meetings.
References
- Abarrategui-Garrido C., Martinez-Barricarte R., Lopez-Trascasa M., de Cordoba S.R., Sanchez-Corral P. Characterization of complement factor H-related (CFHR) proteins in plasma reveals novel genetic variations of CFHR1 associated with atypical hemolytic uremic syndrome. Blood. 2009;114:4261–4271. doi: 10.1182/blood-2009-05-223834. [DOI] [PubMed] [Google Scholar]
- Abarrategui-Garrido C., Melgosa M., Pena-Carrion A., de Jorge E.G., de Cordoba S.R., Lopez-Trascasa M., Sanchez-Corral P. Mutations in proteins of the alternative pathway of complement and the pathogenesis of atypical hemolytic uremic syndrome. American Journal of Kidney Diseases. 2008;52:171–180. doi: 10.1053/j.ajkd.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Al-Akash S.I., Almond P.S., Savell V.H., Jr., Gharaybeh S.I., Hogue C. Eculizumab induces long-term remission in recurrent post-transplant HUS associated with C3 gene mutation. Pediatric Nephrology. 2011;26:613–619. doi: 10.1007/s00467-010-1708-6. [DOI] [PubMed] [Google Scholar]
- Alachkar N., Bagnasco S.M., Montgomery R.A. Eculizumab for the treatment of two recurrences of atypical hemolytic uremic syndrome in a kidney allograft. Transplant International. 2012;25:e93–e95. doi: 10.1111/j.1432-2277.2012.01497.x. [DOI] [PubMed] [Google Scholar]
- Ariceta G., Arrizabalaga B., Aguirre M., Morteruel E., Lopez-Trascasa M. Eculizumab in the treatment of atypical hemolytic uremic syndrome in infants. American Journal of Kidney Diseases. 2012;59:707–710. doi: 10.1053/j.ajkd.2011.11.027. [DOI] [PubMed] [Google Scholar]
- Ariceta G., Besbas N., Johnson S., Karpman D., Landau D., Licht C., Loirat C., Pecoraro C., Taylor C.M., Van de Kar N., Vandewalle J., Zimmerhackl L.B. Guideline for the investigation and initial therapy of diarrhea-negative hemolytic uremic syndrome. Pediatric Nephrology. 2009;24:687–696. doi: 10.1007/s00467-008-0964-1. [DOI] [PubMed] [Google Scholar]
- Bento D., Mapril J., Rocha C., Marchbank K.J., Kavanagh D., Barge D., Strain L., Goodship T.H., Meneses-Oliveira C. Triggering of atypical hemolytic uremic syndrome by influenza A (H1N1) Renal Failure. 2010;32:753–756. doi: 10.3109/0886022X.2010.486491. [DOI] [PubMed] [Google Scholar]
- Besbas N., Karpman D., Landau D., Loirat C., Proesmans W., Remuzzi G., Rizzoni G., Taylor C.M., Van de Kar N., Zimmerhackl L.B. A classification of hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and related disorders. Kidney International. 2006;70:423–431. doi: 10.1038/sj.ki.5001581. [DOI] [PubMed] [Google Scholar]
- Bienaime F., Dragon-Durey M.A., Regnier C.H., Nilsson S.C., Kwan W.H., Blouin J., Jablonski M., Renault N., Rameix-Welti M.A., Loirat C., Sautes-Fridman C., Villoutreix B.O., Blom A.M., Fremeaux-Bacchi V. Mutations in components of complement influence the outcome of factor I-associated atypical hemolytic uremic syndrome. Kidney International. 2010;77:339–349. doi: 10.1038/ki.2009.472. [DOI] [PubMed] [Google Scholar]
- Blanc C., Roumenina L.T., Ashraf Y., Hyvarinen S., Sethi S.K., Ranchin B., Niaudet P., Loirat C., Gulati A., Bagga A., Fridman W.H., Sautes-Fridman C., Jokiranta T.S., Fremeaux-Bacchi V., Dragon-Durey M.A. Overall neutralization of complement factor H by autoantibodies in the acute phase of the autoimmune form of atypical hemolytic uremic syndrome. Journal of Immunology. 2012;189:3528–3537. doi: 10.4049/jimmunol.1200679. [DOI] [PubMed] [Google Scholar]
- Blom A.M., Bergstrom F., Edey M., Diaz-Torres M., Kavanagh D., Lampe A., Goodship J.A., Strain L., Moghal N., McHugh M., Inward C., Tomson C., Fremeaux-Bacchi V., Villoutreix B.O., Goodship T.H. A novel non-synonymous polymorphism (p.Arg240His) in C4b-binding protein is associated with atypical hemolytic uremic syndrome and leads to impaired alternative pathway cofactor activity. Journal of Immunology. 2008;180:6385–6391. doi: 10.4049/jimmunol.180.9.6385. [DOI] [PubMed] [Google Scholar]
- Bouts A., Monnens L., Davin J.C., Struijk G., Spanjaard L. Insufficient protection by Neisseria meningitidis vaccination alone during eculizumab therapy. Pediatric Nephrology. 2011;26:1919–1920. doi: 10.1007/s00467-011-1929-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresin E., Daina E., Noris M., Castelletti F., Stefanov R., Hill P., Goodship T.H., Remuzzi G. Outcome of renal transplantation in patients with non-Shiga toxin-associated hemolytic uremic syndrome: prognostic significance of genetic background. Clinical Journal of American Society of Nephrology. 2006;1:88–99. doi: 10.2215/CJN.00050505. [DOI] [PubMed] [Google Scholar]
- Bresin E., Rurali E., Caprioli J., Sanchez-Corral P., Fremeaux-Bacchi V., Rodriguez de Cordoba S., Pinto S., Goodship T.H., Alberti M., Ribes D., Valoti E., Remuzzi G., Noris M. Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. Journal of the American Society of Nephrology. 2013;24:475–486. doi: 10.1681/ASN.2012090884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campistol J.M., Arias M., Ariceta G., Blasco M., Espinosa M., Grinyo J.M., Praga M., Torra R., Vilalta R., Rodriguez de Cordoba S. An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrologia. 2013;33:27–45. doi: 10.3265/Nefrologia.pre2012.Nov.11781. [DOI] [PubMed] [Google Scholar]
- Canfield S.M., Morrison S.L. The binding affinity of human IgG for its high affinity Fc receptor is determined by multiple amino acids in the CH2 domain and is modulated by the hinge region. Journal of Experimental Medicine. 1991;173:1483–1491. doi: 10.1084/jem.173.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli J., Bettinaglio P., Zipfel P.F., Amadei B., Daina E., Gamba S., Skerka C., Marziliano N., Remuzzi G., Noris M. The molecular basis of familial hemolytic uremic syndrome: mutation analysis of factor H gene reveals a hot spot in short consensus repeat 20. Journal of the American Society of Nephrology. 2001;12:297–307. doi: 10.1681/ASN.V122297. [DOI] [PubMed] [Google Scholar]
- Caprioli J., Castelletti F., Bucchioni S., Bettinaglio P., Bresin E., Pianetti G., Gamba S., Brioschi S., Daina E., Remuzzi G., Noris M. Complement factor H mutations and gene polymorphisms in haemolytic uraemic syndrome: the C-257T, the A2089G and the G2881T polymorphisms are strongly associated with the disease. Human Molecular Genetics. 2003;12:3385–3395. doi: 10.1093/hmg/ddg363. [DOI] [PubMed] [Google Scholar]
- Caprioli J., Noris M., Brioschi S., Pianetti G., Castelletti F., Bettinaglio P., Mele C., Bresin E., Cassis L., Gamba S., Porrati F., Bucchioni S., Monteferrante G., Fang C.J., Liszewski M.K., Kavanagh D., Atkinson J.P., Remuzzi G. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267–1279. doi: 10.1182/blood-2005-10-007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr R., Cataland S.R. Relapse of aHUS after discontinuation of therapy with eculizumab in a patient with aHUS and factor H mutation. Annals of Hematology. 2012 doi: 10.1007/s00277-012-1622-z. [DOI] [PubMed] [Google Scholar]
- Cayci F.S., Cakar N., Hancer V.S., Uncu N., Acar B., Gur G. Eculizumab therapy in a child with hemolytic uremic syndrome and CFI mutation. Pediatric Nephrology. 2012;27:2327–2331. doi: 10.1007/s00467-012-2283-9. [DOI] [PubMed] [Google Scholar]
- Chatelet V., Lobbedez T., Fremeaux-Bacchi V., Ficheux M., Ryckelynck J.P., Hurault de Ligny B. Eculizumab: safety and efficacy after 17 months of treatment in a renal transplant patient with recurrent atypical hemolytic-uremic syndrome: case report. Transplantation Proceedings. 2010;42:4353–4355. doi: 10.1016/j.transproceed.2010.09.125. [DOI] [PubMed] [Google Scholar]
- Clark S.J., Ridge L.A., Herbert A.P., Hakobyan S., Mulloy B., Wurzner R., Morgan B.P., Uhrin D., P.N. B., Day A.J. Tissue-specific host recognition by complement factor H is mediated by differential activities of its glycoaminoglycans-binding regions. Journal of Immunology. 2013:190. doi: 10.4049/jimmunol.1201751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzado J.M., de Cordoba S.R., Melilli E., Bestard O., Rama I., Sanchez-Corral P., Lopez-Trascasa M., Navarro I., Torras J., Goma M., Grinyo J.M. Successful renal transplantation in a patient with atypical hemolytic uremic syndrome carrying mutations in both factor I and MCP. American Journal of Transplantation. 2009;9:1477–1483. doi: 10.1111/j.1600-6143.2009.02647.x. [DOI] [PubMed] [Google Scholar]
- Davin J.C., Gracchi V., Bouts A., Groothoff J., Strain L., Goodship T. Maintenance of kidney function following treatment with eculizumab and discontinuation of plasma exchange after a third kidney transplant for atypical hemolytic uremic syndrome associated with a CFH mutation. American Journal of Kidney Diseases. 2010;55:708–711. doi: 10.1053/j.ajkd.2009.08.011. [DOI] [PubMed] [Google Scholar]
- De S., Waters A.M., Segal A.O., Trautmann A., Harvey E.A., Licht C. Severe atypical HUS caused by CFH S1191L—case presentation and review of treatment options. Pediatric Nephrology. 2010;25:97–104. doi: 10.1007/s00467-009-1306-7. [DOI] [PubMed] [Google Scholar]
- Dorresteijn E.M., van de Kar N.C., Cransberg K. Eculizumab as rescue therapy for atypical hemolytic uremic syndrome with normal platelet count. Pediatric Nephrology. 2012;27:1193–1195. doi: 10.1007/s00467-012-2130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragon-Durey M.A., Fremeaux-Bacchi V., Loirat C., Blouin J., Niaudet P., Deschenes G., Coppo P., Herman Fridman W., Weiss L. Heterozygous and homozygous factor h deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. Journal of the American Society of Nephrology. 2004;15:787–795. doi: 10.1097/01.asn.0000115702.28859.a7. [DOI] [PubMed] [Google Scholar]
- Dragon-Durey M.A., Loirat C., Cloarec S., Macher M.A., Blouin J., Nivet H., Weiss L., Fridman W.H., Fremeaux-Bacchi V. Anti-factor H autoantibodies associated with atypical hemolytic uremic syndrome. Journal of the American Society of Nephrology. 2005;16:555–563. doi: 10.1681/ASN.2004050380. [DOI] [PubMed] [Google Scholar]
- Duran C., Blasco M., Maduell F., Campistol J.M. Rescue therapy with eculizumab in a transplant recipient with atypical haemolytic-uraemic syndrome. Clinical Kidney Journal. 2012;5:28–30. doi: 10.1093/ndtplus/sfr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edey M.M., Mead P.A., Saunders R.E., Strain L., Perkins S.J., Goodship T.H., Kanagasundaram N.S. Association of a factor H mutation with hemolytic uremic syndrome following a diarrheal illness. American Journal of Kidney Diseases. 2008;51:487–490. doi: 10.1053/j.ajkd.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Ermini L., Goodship T.H., Strain L., Weale M.E., Sacks S.H., Cordell H.J., Fremeaux-Bacchi V., Sheerin N.S. Common genetic variants in complement genes other than CFH, CD46 and the CFHRs are not associated with aHUS. Molecular Immunology. 2012;49:640–648. doi: 10.1016/j.molimm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza-Gordillo J., Goicoechea de Jorge E., Buil A., Carreras Berges L., Lopez-Trascasa M., Sanchez-Corral P., Rodriguez de Cordoba S. Predisposition to atypical hemolytic uremic syndrome involves the concurrence of different susceptibility alleles in the regulators of complement activation gene cluster in 1q32. Human Molecular Genetics. 2005;14:703–712. doi: 10.1093/hmg/ddi066. [DOI] [PubMed] [Google Scholar]
- Esparza-Gordillo J., Jorge E.G., Garrido C.A., Carreras L., Lopez-Trascasa M., Sanchez-Corral P., de Cordoba S.R. Insights into hemolytic uremic syndrome: segregation of three independent predisposition factors in a large, multiple affected pedigree. Molecular Immunology. 2006;43:1769–1775. doi: 10.1016/j.molimm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Fakhouri F., Roumenina L., Provot F., Sallee M., Caillard S., Couzi L., Essig M., Ribes D., Dragon-Durey M.A., Bridoux F., Rondeau E., Fremeaux-Bacchi V. Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. Journal of the American Society of Nephrology. 2010;21:859–867. doi: 10.1681/ASN.2009070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Yoshida Y., Honda S., Matsumoto M., Sawada Y., Hattori M., Hisanaga S., Hiwa R., Nakamura F., Tomomori M., Miyagawa S., Fujimaru R., Yamada H., Sawai T., Ikeda Y., Iwata N., Uemura O., Matsukuma E., Aizawa Y., Harada H., Wada H., Ishikawa E., Ashida A., Nangaku M., Miyata T., Fujimura Y. Analysis of genetic and predisposing factors in Japanese patients with atypical hemolytic uremic syndrome. Molecular Immunology. 2013;54:238–246. doi: 10.1016/j.molimm.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Ferreira V.P., Herbert A.P., Cortes C., McKee K.A., Blaum B.S., Esswein S.T., Uhrin D., Barlow P.N., Pangburn M.K., Kavanagh D. The binding of factor H to a complex of physiological polyanions and C3b on cells is impaired in atypical hemolytic uremic syndrome. Journal of Immunology. 2009;182:7009–7018. doi: 10.4049/jimmunol.0804031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltyn Zadura A., Zipfel P.F., Bokarewa M.I., Sturfelt G., Jonsen A., Nilsson S.C., Hillarp A., Saxne T., Trouw L.A., Blom A.M. Factor H autoantibodies and deletion of Complement Factor H-Related protein-1 in rheumatic diseases in comparison to atypical hemolytic uremic syndrome. Arthritis Research and Therapy. 2012;14:R185. doi: 10.1186/ar4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis N.J., McNicholas B., Awan A., Waldron M., Reddan D., Sadlier D., Kavanagh D., Strain L., Marchbank K.J., Harris C.L., Goodship T.H. A novel hybrid CFH/CFHR3 gene generated by a microhomology-mediated deletion in familial atypical hemolytic uremic syndrome. Blood. 2012;119:591–601. doi: 10.1182/blood-2011-03-339903. [DOI] [PubMed] [Google Scholar]
- Fremeaux-Bacchi V., Fakhouri F., Garnier A., Bienaime F., Dragon-Durey M.A., Ngo S., Moulin B., Servais A., Provot F., Rostaing L., Burtey S., Niaudet P., Deschenes G., Lebranchu Y., Zuber J., Loirat C. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide french series comparing children and adults. Clinical Journal of American Society of Nephrology. 2013 doi: 10.2215/CJN.04760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeaux-Bacchi V., Kemp E.J., Goodship J.A., Dragon-Durey M.A., Strain L., Loirat C., Deng H.W., Goodship T.H. The development of atypical haemolytic-uraemic syndrome is influenced by susceptibility factors in factor H and membrane cofactor protein: evidence from two independent cohorts. Journal of Medical Genetics. 2005;42:852–856. doi: 10.1136/jmg.2005.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeaux-Bacchi V., Miller E.C., Liszewski M.K., Strain L., Blouin J., Brown A.L., Moghal N., Kaplan B.S., Weiss R.A., Lhotta K., Kapur G., Mattoo T., Nivet H., Wong W., Gie S., Hurault de Ligny B., Fischbach M., Gupta R., Hauhart R., Meunier V., Loirat C., Dragon-Durey M.A., Fridman W.H., Janssen B.J., Goodship T.H., Atkinson J.P. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood. 2008;112:4948–4952. doi: 10.1182/blood-2008-01-133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeaux-Bacchi V., Moulton E.A., Kavanagh D., Dragon-Durey M.A., Blouin J., Caudy A., Arzouk N., Cleper R., Francois M., Guest G., Pourrat J., Seligman R., Fridman W.H., Loirat C., Atkinson J.P. Genetic and functional analyses of membrane cofactor protein (CD46) mutations in atypical hemolytic uremic syndrome. Journal of the American Society of Nephrology. 2006;17:2017–2025. doi: 10.1681/ASN.2005101051. [DOI] [PubMed] [Google Scholar]
- Fremont O., Gordon C., Hand M. Eculizumab treatment for aHUS in a child with positive family history. JASN. 2009;20 988A Abstr PUB715. [Google Scholar]
- Garjau M., Azancot M., Ramos R., Sanchez-Corral P., Montero M., Seron D. Early treatment with eculizumab in atypical haemolytic uraemic syndrome. Clinical Kidney Journal. 2012;5:31–33. doi: 10.1093/ndtplus/sfr157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerdink L.M., Westra D., van Wijk J.A., Dorresteijn E.M., Lilien M.R., Davin J.C., Komhoff M., Van Hoeck K., van der Vlugt A., van den Heuvel L.P., van de Kar N.C. Atypical hemolytic uremic syndrome in children: complement mutations and clinical characteristics. Pediatric Nephrology. 2012;27:1283–1291. doi: 10.1007/s00467-012-2131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R.D., Fowler D.J., Angus E., Hardy S.A., Stanley L., Goodship T.H. Eculizumab therapy for atypical haemolytic uraemic syndrome due to a gain-of-function mutation of complement factor B. Pediatric Nephrology. 2013 doi: 10.1007/s00467-013-2492-x. [DOI] [PubMed] [Google Scholar]
- Giordano M., Castellano G., Messina G., Divella C., Bellantuono R., Puteo F., Colella V., Depalo T., Gesualdo L. Preservation of renal function in atypical hemolytic uremic syndrome by Eculizumab: a case report. Pediatrics. 2012;130(5):e1385–e1388. doi: 10.1542/peds.2011-1685. Epub 2012 Oct 1. [DOI] [PubMed] [Google Scholar]
- Goicochea de Jorge E., Paixao-Cavalcante D., Rose K.L., Cook H.T., Botto M., Pickering M.C. C5 activation is required for the development of atypical haemolytic uraemic syndrome in CFH−/−FH Delta 16–20 mice. Molecular Immunology. 2008;45:4100–4101. [Google Scholar]
- Goicoechea de Jorge E., Harris C.L., Esparza-Gordillo J., Carreras L., Arranz E.A., Garrido C.A., Lopez-Trascasa M., Sanchez-Corral P., Morgan B.P., Rodriguez de Cordoba S. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:240–245. doi: 10.1073/pnas.0603420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodship T.H., Kavanagh D. Pulling the trigger in atypical hemolytic uremic syndrome: the role of pregnancy. Journal of the American Society of Nephrology. 2010;21:731–732. doi: 10.1681/ASN.2010030308. [DOI] [PubMed] [Google Scholar]
- Greenbaum L., Babu S., Furman R. Eculizumab is an effective long-term treatment in patients with atypical hemolytic uremic syndrome resistant to plasma exchange/infusion: results of an extension study. 53th Annual Meeting of the American Society of Hematology; San Diego, USA; 2011. (Abstract) [Google Scholar]
- Greenbaum L.A. Continued improvement in renal function with sustained eculizumab in patients with atypical HUS resistant to plasma exchange/infusion. 44th Annual Meeting of the American Society of Nephrology; Philadelphia, USA; 2011. (Abstract) [Google Scholar]
- Gruppo R.A., Rother R.P. Eculizumab for congenital atypical hemolytic-uremic syndrome. New England Journal of Medicine. 2009;360:544–546. doi: 10.1056/NEJMc0809959. [DOI] [PubMed] [Google Scholar]
- Hakobyan S., Harris C.L., van den Berg C.W., Fernandez-Alonso M.C., de Jorge E.G., de Cordoba S.R., Rivas G., Mangione P., Pepys M.B., Morgan B.P. Complement factor H binds to denatured rather than to native pentameric C-reactive protein. Journal of Biological Chemistry. 2008;283:30451–30460. doi: 10.1074/jbc.M803648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Mollnes T.E. The alternative complement pathway revisited. Journal of Cellular and Molecular Medicine. 2008;12:1074–1084. doi: 10.1111/j.1582-4934.2008.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen S., Pluthero F.G., van Eimeren V.F., Quaggin S.E., Licht C. Monitoring and modeling treatment of atypical hemolytic uremic syndrome. Molecular Immunology. 2013;54:84–88. doi: 10.1016/j.molimm.2012.10.044. [DOI] [PubMed] [Google Scholar]
- Heinen S., Sanchez-Corral P., Jackson M.S., Strain L., Goodship J.A., Kemp E.J., Skerka C., Jokiranta T.S., Meyers K., Wagner E., Robitaille P., Esparza-Gordillo J., Rodriguez de Cordoba S., Zipfel P.F., Goodship T.H. De novo gene conversion in the RCA gene cluster (1q32) causes mutations in complement factor H associated with atypical hemolytic uremic syndrome. Human Mutation. 2006;27:292–293. doi: 10.1002/humu.9408. [DOI] [PubMed] [Google Scholar]
- Herlitz L.C., Bomback A.S., Markowitz G.S., Stokes M.B., Smith R.N., Colvin R.B., Appel G.B., D‘Agati V.D. Pathology after Eculizumab in dense deposit disease and C3 GN. Journal of the American Society of Nephrology. 2012;23:1229–1237. doi: 10.1681/ASN.2011121186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmen P., Elebute M., Kelly R., Urbano-Ispizua A., Hill A., Rother R.P., Khursigara G., Fu C.L., Omine M., Browne P., Rosse W. Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. American Journal of Hematology. 2010;85:553–559. doi: 10.1002/ajh.21757. [DOI] [PubMed] [Google Scholar]
- Hillmen P., Young N.S., Schubert J., Brodsky R.A., Socie G., Muus P., Roeth A., Szer J., Elebute M.O., Nakamura R., Browne P., Risitano A.M., Hill A., Schrezenmeier H., Fu C.-L., Maciejewski J., Rollins S.A., Mojcik C.F., Rother R.P., Luzzatto L. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. New England Journal of Medicine. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- Hofer J., Janecke A.R., Zimmerhackl L.B., Riedl M., Rosales A., Giner T., Cortina G., Haindl C.J., Petzelberger B., Pawlik M., Jeller V., Vester U., Gadner B., van Husen M., Moritz M.L., Wurzner R., Jungraithmayr T. Complement factor H-related protein 1 deficiency and factor H antibodies in pediatric patients with atypical hemolytic uremic syndrome. Clinical Journal of American Society of Nephrology. 2012;8:407–415. doi: 10.2215/CJN.01260212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozsi M., Strobel S., Dahse H.M., Liu W.S., Hoyer P.F., Oppermann M., Skerka C., Zipfel P.F. Anti factor H autoantibodies block C-terminal recognition function of factor H in hemolytic uremic syndrome. Blood. 2007;110:1516–1518. doi: 10.1182/blood-2007-02-071472. [DOI] [PubMed] [Google Scholar]
- Kaplan B.S., Garcia C.D., Chesney R.W., Segar W.E., Giugno K., Chem R. Peripheral gangrene complicating idiopathic and recessive hemolytic uremic syndromes. Pediatric Nephrology. 2000;14:985–989. doi: 10.1007/s004670050058. [DOI] [PubMed] [Google Scholar]
- Kavanagh D., Anderson H.E. Interpretation of genetic variants of uncertain significance in atypical hemolytic uremic syndrome. Kidney International. 2012;81:11–13. doi: 10.1038/ki.2011.330. [DOI] [PubMed] [Google Scholar]
- Kavanagh D., Goodship T. Genetics and complement in atypical HUS. Pediatric Nephrology. 2010;25:2431–2442. doi: 10.1007/s00467-010-1555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh D., Kemp E.J., Mayland E., Winney R.J., Duffield J.S., Warwick G., Richards A., Ward R., Goodship J.A., Goodship T.H. Mutations in complement factor I predispose to development of atypical hemolytic uremic syndrome. Journal of the American Society of Nephrology. 2005;16:2150–2155. doi: 10.1681/ASN.2005010103. [DOI] [PubMed] [Google Scholar]
- Kavanagh D., Kemp E.J., Richards A., Burgess R.M., Mayland E., Goodship J.A., Goodship T.H. Does complement factor B have a role in the pathogenesis of atypical HUS? Molecular Immunology. 2006;43:856–859. doi: 10.1016/j.molimm.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Kavanagh D., Pappworth I.Y., Anderson H., Hayes C.M., Moore I., Hunze E.M., Bennaceur K., Roversi P., Lea S., Strain L., Ward R., Plant N., Nailescu C., Goodship T.H., Marchbank K.J. Factor I autoantibodies in patients with atypical hemolytic uremic syndrome: disease-associated or an epiphenomenon? Clinical Journal of American Society of Nephrology. 2012;7:417–426. doi: 10.2215/CJN.05750611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh D., Richards A., Atkinson J. Complement regulatory genes and hemolytic uremic syndromes. Annual Review of Medicine. 2008;59:293–309. doi: 10.1146/annurev.med.59.060106.185110. [DOI] [PubMed] [Google Scholar]
- Kavanagh D., Richards A., Noris M., Hauhart R., Liszewski M.K., Karpman D., Goodship J.A., Fremeaux-Bacchi V., Remuzzi G., Goodship T.H., Atkinson J.P. Characterization of mutations in complement factor I (CFI) associated with hemolytic uremic syndrome. Molecular Immunology. 2008;45:95–105. doi: 10.1016/j.molimm.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Kelly R., Arnold L., Richards S., Hill A., Bomken C., Hanley J., Loughney A., Beauchamp J., Khursigara G., Rother R.P., Chalmers E., Fyfe A., Fitzsimons E., Nakamura R., Gaya A., Risitano A.M., Schubert J., Norfolk D., Simpson N., Hillmen P. The management of pregnancy in paroxysmal nocturnal haemoglobinuria on long term eculizumab. British Journal of Haematology. 2010;149:446–450. doi: 10.1111/j.1365-2141.2010.08099.x. [DOI] [PubMed] [Google Scholar]
- Kemper C., Atkinson J.P. T-cell regulation: with complements from innate immunity. Nature Reviews Immunology. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Waller S.C., Reid C.J. Eculizumab in atypical haemolytic-uraemic syndrome allows cessation of plasma exchange and dialysis. Clinical Kidney Journal. 2012;5:34–36. doi: 10.1093/ndtplus/sfr174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp A., Strobel S., Tortajada A., Rodriguez de Cordoba S., Sanchez-Corral P., Prohaszka Z., Lopez-Trascasa M., Jozsi M. Atypical hemolytic uremic syndrome-associated variants and autoantibodies impair binding of factor h and factor h-related protein 1 to pentraxin 3. Journal of Immunology. 2012;189:1858–1867. doi: 10.4049/jimmunol.1200357. [DOI] [PubMed] [Google Scholar]
- Kose O., Zimmerhackl L.B., Jungraithmayr T., Mache C., Nurnberger J. New treatment options for atypical hemolytic uremic syndrome with the complement inhibitor eculizumab. Seminars in Thrombosis and Hemostasis. 2010;36:669–672. doi: 10.1055/s-0030-1262889. [DOI] [PubMed] [Google Scholar]
- Krid S., Roumenina L., Beury D., Charbit M., Boyer O., Fremeaux-Bacchi V., Niaudet P. Renal transplantation under prophylactic Eculizumab in atypical hemolytic uremic syndrome with CFH/CFHR1 hybrid protein. American Journal of Transplantation. 2012;12:1938–1944. doi: 10.1111/j.1600-6143.2012.04051.x. [DOI] [PubMed] [Google Scholar]
- Laine M., Jarva H., Seitsonen S., Haapasalo K., Lehtinen M.J., Lindeman N., Anderson D.H., Johnson P.T., Jarvela I., Jokiranta T.S., Hageman G.S., Immonen I., Meri S. Y402H polymorphism of complement factor H affects binding affinity to C-reactive protein. Journal of Immunology. 2007;178:3831–3836. doi: 10.4049/jimmunol.178.6.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapeyraque A.L., Fremeaux-Bacchi V., Robitaille P. Efficacy of eculizumab in a patient with factor-H-associated atypical hemolytic uremic syndrome. Pediatric Nephrology. 2011;26:621–624. doi: 10.1007/s00467-010-1719-3. [DOI] [PubMed] [Google Scholar]
- Larrea C.F., Cofan F., Oppenheimer F., Campistol J.M., Escolar G., Lozano M. Efficacy of eculizumab in the treatment of recurrent atypical hemolytic-uremic syndrome after renal transplantation. Transplantation. 2010;89:903–904. doi: 10.1097/TP.0b013e3181ccd80d. [DOI] [PubMed] [Google Scholar]
- Le Quintrec M., Zuber J., Moulin B., Kamar N., Jablonski M., Lionet A., Chatelet V., Mousson C., Mourad G., Bridoux F., Cassuto E., Loirat C., Rondeau E., Delahousse M., Fremeaux-Bacchi V. Complement genes strongly predict recurrence and graft outcome in adult renal transplant recipients with atypical hemolytic and uremic syndrome. American Journal of Transplantation. 2013;13:663–675. doi: 10.1111/ajt.12077. [DOI] [PubMed] [Google Scholar]
- Legault D., Boelkins M. Successful treatment of aHUS recurrence and arrest of plasma exchange resistant TMA post-renal transplantation with the terminal complement inhibitor Eculizumab. Blood. 2009;114 abstrac 2421. [Google Scholar]
- Legendre C., Furman R., Sheerin N. Safety and efficacy of eculizumab in aHUS resistant to plasma therapy: interim analysis from a phase II trial. 43rd Annual Meeting of the American Society of Nephrology; Denver, USA; 2010. [Google Scholar]
- Legendre C.M., Licht C., Muus P., Greenbaum L.A., Babu S., Bedrosian C., Bingham C., Cohen D.J., Delmas Y., Douglas K., Eitner F., Feldkamp T., Fouque D., Furman R.R., Gaber O., Herthelius M., Hourmant M., Karpman D., Lebranchu Y., Mariat C., Menne J., Moulin B., Nurnberger J., Ogawa M., Remuzzi G., Richard T., Sberro-Soussan R., Severino B., Sheerin N.S., Trivelli A., Zimmerhackl L.B., Goodship T., Loirat C. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. The New England Journal of Medicine. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- Lemaire M., Fremeaux-Bacchi V., Schaefer F., Choi M., Tang W.H., Le Quintrec M., Fakhouri F., Taque S., Nobili F., Martinez F., Ji W., Overton J.D., Mane S.M., Nurnberg G., Altmuller J., Thiele H., Morin D., Deschenes G., Baudouin V., Llanas B., Collard L., Majid M.A., Simkova E., Nurnberg P., Rioux-Leclerc N., Moeckel G.W., Gubler M.C., Hwa J., Loirat C., Lifton R.P. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nature Genetics. 2013;45:531–536. doi: 10.1038/ng.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhotta K., Janecke A.R., Scheiring J., Petzlberger B., Giner T., Fally V., Wurzner R., Zimmerhackl L.B., Mayer G., Fremeaux-Bacchi V. A large family with a gain-of-function mutation of complement C3 predisposing to atypical hemolytic uremic syndrome, microhematuria, hypertension and chronic renal failure. Clinical Journal of American Society of Nephrology. 2009;4:1356–1362. doi: 10.2215/CJN.06281208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht C. Phase II study of eculizumab in patients with atypical HUS receiving chronic plasma exchange/infusion. Presented at the 44th Annual Meeting of the American Society of Nephrology; Philadelphia, USA; 2011. [Google Scholar]
- Licht C., Muus P., Legendre C. Eculizumab is an effective long-term treatment in patients with atypical haemolytic uremic syndrome previously receiving chronic plasma exchange/infusion: extension study results. Presented at the 53th Annual Meeting of the American Society of Hematology; San Diego, USA; 2011. [Google Scholar]
- Loirat C., Fremeaux-Bacchi V. Hemolytic uremic syndrome recurrence after renal transplantation. Pediatric Transplantation. 2008;12:619–629. doi: 10.1111/j.1399-3046.2008.00910.x. [DOI] [PubMed] [Google Scholar]
- Loirat C., Fremeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet Journal of Rare Diseases. 2011;6:60. doi: 10.1186/1750-1172-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loirat C., Macher M.A., Elmaleh-Berges M., Kwon T., Deschenes G., Goodship T.H., Majoie C., Davin J.C., Blanc R., Savatovsky J., Moret J., Fremeaux-Bacchi V. Non-atheromatous arterial stenoses in atypical haemolytic uraemic syndrome associated with complement dysregulation. Nephrology, Dialysis, Transplantation. 2010;25:3421–3425. doi: 10.1093/ndt/gfq319. [DOI] [PubMed] [Google Scholar]
- Luzzatto L., Risitano A.M., Notaro R. Paroxysmal nocturnal hemoglobinuria and eculizumab. Haematologica. 2010;95:523–526. doi: 10.3324/haematol.2009.017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mache C.J., Acham-Roschitz B., Fremeaux-Bacchi V., Kirschfink M., Zipfel P.F., Roedl S., Vester U., Ring E. Complement inhibitor eculizumab in atypical hemolytic uremic syndrome. Clinical Journal of American Society of Nephrology. 2009;4:1312–1316. doi: 10.2215/CJN.01090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maga T.K., Nishimura C.J., Weaver A.E., Frees K.L., Smith R.J. Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Human Mutation. 2010;31:E1445–E1460. doi: 10.1002/humu.21256. [DOI] [PubMed] [Google Scholar]
- Martinez-Barricarte R., Goicoechea de Jorge E., Montes T., Layana A.G., Rodriguez de Cordoba S. Lack of association between polymorphisms in C4b-binding protein and atypical haemolytic uraemic syndrome in the Spanish population. Clinical and Experimental Immunology. 2009;155:59–64. doi: 10.1111/j.1365-2249.2008.03798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meri S., Pangburn M.K. Regulation of alternative pathway complement activation by glycosaminoglycans: specificity of the polyanion binding site on factor H. Biochemical and Biophysical Research Communications. 1994;198:52–59. doi: 10.1006/bbrc.1994.1008. [DOI] [PubMed] [Google Scholar]
- Moore I., Strain L., Pappworth I., Kavanagh D., Barlow P.N., Herbert A.P., Schmidt C.Q., Staniforth S.J., Holmes L.V., Ward R., Morgan L., Goodship T.H., Marchbank K.J. Association of factor H autoantibodies with deletions of CFHR1, CFHR3, CFHR4, and with mutations in CFH, CFI, CD46, and C3 in patients with atypical hemolytic uremic syndrome. Blood. 2010;115:379–387. doi: 10.1182/blood-2009-05-221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muus P., Douglas K., Hurmant M. Safety and efficacy of eculizumab in aHUS patients on chronic plasma therapy: Interim analysis of a phase II trial. Presented at the 43rd Annual Meeting of the American Society of Nephrology; Denver, USA; 2010. [Google Scholar]
- Nester C., Stewart Z., Myers D., Jetton J., Nair R., Reed A., Thomas C., Smith R., Brophy P. Pre-emptive eculizumab and plasmapheresis for renal transplant in atypical hemolytic uremic syndrome. Clinical Journal of American Society of Nephrology. 2011;6:1488–1494. doi: 10.2215/CJN.10181110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H.P., Salzmann M., Bohnert-Iwan B., Mannuelian T., Skerka C., Lenk D., Bender B.U., Cybulla M., Riegler P., Konigsrainer A., Neyer U., Bock A., Widmer U., Male D.A., Franke G., Zipfel P.F. Haemolytic uraemic syndrome and mutations of the factor H gene: a registry-based study of German speaking countries. Journal of Medical Genetics. 2003;40:676–681. doi: 10.1136/jmg.40.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S.C., Kalchishkova N., Trouw L.A., Fremeaux-Bacchi V., Villoutreix B.O., Blom A.M. Mutations in complement factor I as found in atypical hemolytic uremic syndrome lead to either altered secretion or altered function of factor I. European Journal of Immunology. 2010;40:172–185. doi: 10.1002/eji.200939280. [DOI] [PubMed] [Google Scholar]
- Nilsson S.C., Karpman D., Vaziri-Sani F., Kristoffersson A.C., Salomon R., Provot F., Fremeaux-Bacchi V., Trouw L.A., Blom A.M. A mutation in factor I that is associated with atypical hemolytic uremic syndrome does not affect the function of factor I in complement regulation. Molecular Immunology. 2007;44:1835–1844. doi: 10.1016/j.molimm.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Nishimura J., Yamamoto M., Hayashi S., Ohyashiki K., Ando K., Noji H., Kitamura K., Eto T., Ando T., Masuko M., Shibayama H., Hase M., Lan L., Tamburini P., Inazawa J., Kinoshita T., Kanakura Y. 2012. A Rare Genetic Polymorphism in C5 Confers Poor Response to the Anti-C5 Monoclonal Antibody Eculizumab by Nine Japanese Patients with PNH, Atlanta. [Google Scholar]
- Noris M., Caprioli J., Bresin E., Mossali C., Pianetti G., Gamba S., Daina E., Fenili C., Castelletti F., Sorosina A., Piras R., Donadelli R., Maranta R., van der Meer I., Conway E.M., Zipfel P.F., Goodship T.H., Remuzzi G. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clinical Journal of American Society of Nephrology. 2010;5:1844–1859. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger J., Witzke O., Saez A.O., Vester U., Baba H.A., Kribben A., Zimmerhackl L.B., Janecke A.R., Nagel M., Kirschfink M. Eculizumab for atypical hemolytic-uremic syndrome. New England Journal of Medicine. 2009;360:542–544. doi: 10.1056/NEJMc0808527. [DOI] [PubMed] [Google Scholar]
- Ohanian M., Cable C., Halka K. Eculizumab safely reverses neurologic impairment and eliminates need for dialysis in severe atypical hemolytic uremic syndrome. Clinical Pharmacology. 2011;3:5–12. doi: 10.2147/CPAA.S17904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanian M., Cable C., Halka K. Reduced dose maintenance eculizumab in atypical hemolytic uremic syndrome (aHUS): an update on a previous case report. Clinical Pharmacology. 2011;3:45–50. doi: 10.2147/CPAA.S23687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaltin F., Li B., Rauhauser A., An S.W., Soylemezoglu O., Gonul I.I., Taskiran E.Z., Ibsirlioglu T., Korkmaz E., Bilginer Y., Duzova A., Ozen S., Topaloglu R., Besbas N., Ashraf S., Du Y., Liang C., Chen P., Lu D., Vadnagara K., Arbuckle S., Lewis D., Wakeland B., Quigg R.J., Ransom R.F., Wakeland E.K., Topham M.K., Bazan N.G., Mohan C., Hildebrandt F., Bakkaloglu A., Huang C.L., Attanasio M. DGKE variants cause a glomerular microangiopathy that mimics membranoproliferative GN. Journal of the American Society of Nephrology. 2013;24:377–384. doi: 10.1681/ASN.2012090903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.J., Kim J.H., Shin J.I., Ha T.S. The significance of T helper 1 cell-derived cytokines in patients with systemic lupus erythematosus with acquired thrombotic thrombocytopenic purpura. American Journal of Clinical Pathology. 2011;136:659–660. doi: 10.1309/AJCPCWQ2PZ4NLWRB. [DOI] [PubMed] [Google Scholar]
- Pechtl I.C., Kavanagh D., McIntosh N., Harris C.L., Barlow P.N. Disease-associated N-terminal complement factor H mutations perturb cofactor and decay-accelerating activities. Journal of Biological Chemistry. 2011;286:11082–11090. doi: 10.1074/jbc.M110.211839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Caballero D., Gonzalez-Rubio C., Gallardo M.E., Vera M., Lopez-Trascasa M., Rodriguez de Cordoba S., Sanchez-Corral P. Clustering of missense mutations in the C-terminal region of factor H in atypical hemolytic uremic syndrome. American Journal of Human Genetics. 2001;68:478–484. doi: 10.1086/318201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering M.C., de Jorge E.G., Martinez-Barricarte R., Recalde S., Garcia-Layana A., Rose K.L., Moss J., Walport M.J., Cook H.T., de Cordoba S.R., Botto M. Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. Journal of Experimental Medicine. 2007;204:1249–1256. doi: 10.1084/jem.20070301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povey H., Vundru R., Junglee N., Jibani M. Renal recovery with eculizumab in atypical hemolytic uremic syndrome following prolonged dialysis. Clinical Nephrology. 2013 doi: 10.5414/CN107958. [DOI] [PubMed] [Google Scholar]
- Prescott H.C., Wu H.M., Cataland S.R., Baiocchi R.A. Eculizumab therapy in an adult with plasma exchange-refractory atypical hemolytic uremic syndrome. American Journal of Hematology. 2010;85:976–977. doi: 10.1002/ajh.21862. [DOI] [PubMed] [Google Scholar]
- Richards A., Buddles M.R., Donne R.L., Kaplan B.S., Kirk E., Venning M.C., Tielemans C.L., Goodship J.A., Goodship T.H. Factor H mutations in hemolytic uremic syndrome cluster in exons 18–20, a domain important for host cell recognition. American Journal of Human Genetics. 2001;68:485–490. doi: 10.1086/318203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A., Kathryn Liszewski M., Kavanagh D., Fang C.J., Moulton E., Fremeaux-Bacchi V., Remuzzi G., Noris M., Goodship T.H., Atkinson J.P. Implications of the initial mutations in membrane cofactor protein (MCP; CD46) leading to atypical hemolytic uremic syndrome. Molecular Immunology. 2007;44:111–122. doi: 10.1016/j.molimm.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Richards A., Kavanagh D., Atkinson J.P. Inherited complement regulatory protein deficiency predisposes to human disease in acute injury and chronic inflammatory statesthe examples of vascular damage in atypical hemolytic uremic syndrome and debris accumulation in age-related macular degeneration. Advances in Immunology. 2007;96:141–177. doi: 10.1016/S0065-2776(07)96004-6. [DOI] [PubMed] [Google Scholar]
- Richards A., Kemp E.J., Liszewski M.K., Goodship J.A., Lampe A.K., Decorte R., Muslumanoglu M.H., Kavukcu S., Filler G., Pirson Y., Wen L.S., Atkinson J.P., Goodship T.H. Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12966–12971. doi: 10.1073/pnas.2135497100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nature Immunology. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rother R.P., Rollins S.A., Mojcik C.F., Brodsky R.A., Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nature Biotechnology. 2007;25:1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- Roumenina L.T., Frimat M., Miller E.C., Provot F., Dragon-Durey M.A., Bordereau P., Bigot S., Hue C., Satchell S.C., Mathieson P.W., Mousson C., Noel C., Sautes-Fridman C., Halbwachs-Mecarelli L., Atkinson J.P., Lionet A., Fremeaux-Bacchi V. A prevalent C3 mutation in aHUS patients causes a direct C3 convertase gain of function. Blood. 2012;119:4182–4191. doi: 10.1182/blood-2011-10-383281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumenina L.T., Jablonski M., Hue C., Blouin J., Dimitrov J.D., Dragon-Durey M.A., Cayla M., Fridman W.H., Macher M.A., Ribes D., Moulonguet L., Rostaing L., Satchell S.C., Mathieson P.W., Sautes-Fridman C., Loirat C., Regnier C.H., Halbwachs-Mecarelli L., Fremeaux-Bacchi V. Hyperfunctional C3 convertase leads to complement deposition on endothelial cells and contributes to atypical hemolytic uremic syndrome. Blood. 2009;114:2837–2845. doi: 10.1182/blood-2009-01-197640. [DOI] [PubMed] [Google Scholar]
- Sartz L., Olin A.I., Kristoffersson A.C., Stahl A.L., Johansson M.E., Westman K., Fremeaux-Bacchi V., Nilsson-Ekdahl K., Karpman D. A novel C3 mutation causing increased formation of the C3 convertase in familial atypical hemolytic uremic syndrome. Journal of Immunology. 2012;188:2030–2037. doi: 10.4049/jimmunol.1100319. [DOI] [PubMed] [Google Scholar]
- Schmidt C.Q., Herbert A.P., Kavanagh D., Gandy C., Fenton C.J., Blaum B.S., Lyon M., Uhrin D., Barlow P.N. A new map of glycosaminoglycan and C3b binding sites on factor H. Journal of Immunology. 2008;181:2610–2619. doi: 10.4049/jimmunol.181.4.2610. [DOI] [PubMed] [Google Scholar]
- Sellier-Leclerc A.L., Fremeaux-Bacchi V., Dragon-Durey M.A., Macher M.A., Niaudet P., Guest G., Boudailliez B., Bouissou F., Deschenes G., Gie S., Tsimaratos M., Fischbach M., Morin D., Nivet H., Alberti C., Loirat C. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. Journal of the American Society of Nephrology. 2007;18:2392–2400. doi: 10.1681/ASN.2006080811. [DOI] [PubMed] [Google Scholar]
- Sjoberg A.P., Trouw L.A., Clark S.J., Sjolander J., Heinegard D., Sim R.B., Day A.J., Blom A.M. The factor H variant associated with age-related macular degeneration (His-384) and the non-disease-associated form bind differentially to C-reactive protein, fibromodulin, DNA, and necrotic cells. Journal of Biological Chemistry. 2007;282:10894–10900. doi: 10.1074/jbc.M610256200. [DOI] [PubMed] [Google Scholar]
- Stahl A.L., Vaziri-Sani F., Heinen S., Kristoffersson A.C., Gydell K.H., Raafat R., Gutierrez A., Beringer O., Zipfel P.F., Karpman D. Factor H dysfunction in patients with atypical hemolytic uremic syndrome contributes to complement deposition on platelets and their activation. Blood. 2008;111:5307–5315. doi: 10.1182/blood-2007-08-106153. [DOI] [PubMed] [Google Scholar]
- Strobel S., Abarrategui-Garrido C., Fariza-Requejo E., Seeberger H., Sanchez-Corral P., Jozsi M. Factor H-related protein 1 neutralizes anti-factor H autoantibodies in autoimmune hemolytic uremic syndrome. Kidney International. 2011;80:397–404. doi: 10.1038/ki.2011.152. [DOI] [PubMed] [Google Scholar]
- Sullivan M., Erlic Z., Hoffmann M.M., Arbeiter K., Patzer L., Budde K., Hoppe B., Zeier M., Lhotta K., Rybicki L.A., Bock A., Berisha G., Neumann H.P. Epidemiological approach to identifying genetic predispositions for atypical hemolytic uremic syndrome. Annals of Human Genetics. 2010;74:17–26. doi: 10.1111/j.1469-1809.2009.00554.x. [DOI] [PubMed] [Google Scholar]
- Sullivan M., Rybicki L.A., Winter A., Hoffmann M.M., Reiermann S., Linke H., Arbeiter K., Patzer L., Budde K., Hoppe B., Zeier M., Lhotta K., Bock A., Wiech T., Gaspert A., Fehr T., Woznowski M., Berisha G., Malinoc A., Goek O.N., Eng C., Neumann H.P. Age-related penetrance of hereditary atypical hemolytic uremic syndrome. Annals of Human Genetics. 2011;75:639–647. doi: 10.1111/j.1469-1809.2011.00671.x. [DOI] [PubMed] [Google Scholar]
- Tao M.H., Smith R.I., Morrison S.L. Structural features of human immunoglobulin G that determine isotype-specific differences in complement activation. Journal of Experimental Medicine. 1993;178:661–667. doi: 10.1084/jem.178.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawadrous H., Maga T., Sharma J., Kupferman J., Smith R.J., Schoeneman M. A novel mutation in the complement factor B gene (CFB) and atypical hemolytic uremic syndrome. Pediatric Nephrology. 2010;25:947–951. doi: 10.1007/s00467-009-1415-3. [DOI] [PubMed] [Google Scholar]
- Taylor C.M., Machin S., Wigmore S.J., Goodship T.H. Clinical practice guidelines for the management of atypical haemolytic uraemic syndrome in the United Kingdom. British Journal of Haematology. 2010;148:37–47. doi: 10.1111/j.1365-2141.2009.07916.x. [DOI] [PubMed] [Google Scholar]
- Thomas T.C., Rollins S.A., Rother R.P., Giannoni M.A., Hartman S.L., Elliott E.A., Nye S.H., Matis L.A., Squinto S.P., Evans M.J. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Molecular Immunology. 1996;33:1389–1401. doi: 10.1016/s0161-5890(96)00078-8. [DOI] [PubMed] [Google Scholar]
- Tortajada A., Pinto S., Martinez-Ara J., Lopez-Trascasa M., Sanchez-Corral P., de Cordoba S.R. Complement factor H variants I890 and L1007 while commonly associated with atypical hemolytic uremic syndrome are polymorphisms with no functional significance. Kidney International. 2012;81:56–63. doi: 10.1038/ki.2011.291. [DOI] [PubMed] [Google Scholar]
- Tschumi S., Gugger M., Bucher B.S., Riedl M., Simonetti G.D. Eculizumab in atypical hemolytic uremic syndrome: long-term clinical course and histological findings. Pediatric Nephrology. 2011;26:2085–2088. doi: 10.1007/s00467-011-1989-4. [DOI] [PubMed] [Google Scholar]
- Vaziri-Sani F., Holmberg L., Sjoholm A.G., Kristoffersson A.C., Manea M., Fremeaux-Bacchi V., Fehrman-Ekholm I., Raafat R., Karpman D. Phenotypic expression of factor H mutations in patients with atypical hemolytic uremic syndrome. Kidney International. 2006;69:981–988. doi: 10.1038/sj.ki.5000155. [DOI] [PubMed] [Google Scholar]
- Venables J.P., Strain L., Routledge D., Bourn D., Powell H.M., Warwicker P., Diaz-Torres M.L., Sampson A., Mead P., Webb M., Pirson Y., Jackson M.S., Hughes A., Wood K.M., Goodship J.A., Goodship T.H. Atypical haemolytic uraemic syndrome associated with a hybrid complement gene. PLoS Medicine. 2006;3:e431. doi: 10.1371/journal.pmed.0030431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergouwen M.D., Adriani K.S., Roos Y.B., Groothoff J.W., Majoie C.B. Proximal cerebral artery stenosis in a patient with hemolytic uremic syndrome. American Journal of Neuroradiology. 2008;29:e34. doi: 10.3174/ajnr.A0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilalta R., Lara E., Madrid A., Chocron S., Munoz M., Casquero A., Nieto J. Long-term eculizumab improves clinical outcomes in atypical hemolytic uremic syndrome. Pediatric Nephrology. 2012;27:2323–2326. doi: 10.1007/s00467-012-2276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwicker P., Goodship T.H., Donne R.L., Pirson Y., Nicholls A., Ward R.M., Turnpenny P., Goodship J.A. Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney International. 1998;53:836–844. doi: 10.1111/j.1523-1755.1998.00824.x. [DOI] [PubMed] [Google Scholar]
- Weismann D., Hartvigsen K., Lauer N., Bennett K.L., Scholl H.P., Charbel Issa P., Cano M., Brandstatter H., Tsimikas S., Skerka C., Superti-Furga G., Handa J.T., Zipfel P.F., Witztum J.L., Binder C.J. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478:76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz M., Amon O., Bassler D., Koenigsrainer A., Nadalin S. Prophylactic eculizumab prior to kidney transplantation for atypical hemolytic uremic syndrome. Pediatric Nephrology. 2011;26:1325–1329. doi: 10.1007/s00467-011-1879-9. [DOI] [PubMed] [Google Scholar]
- Westra D., Volokhina E., van der Heijden E., Vos A., Huigen M., Jansen J., van Kaauwen E., van der Velden T., van de Kar N., van den Heuvel L. Genetic disorders in complement (regulating) genes in patients with atypical haemolytic uraemic syndrome (aHUS) Nephrology, Dialysis, Transplantation. 2010;25:2195–2202. doi: 10.1093/ndt/gfq010. [DOI] [PubMed] [Google Scholar]
- Xie L., Nester C.M., Reed A.I., Zhang Y., Smith R.J., Thomas C.P. Tailored eculizumab therapy in the management of complement factor h-mediated atypical hemolytic uremic syndrome in an adult kidney transplant recipient: a case report. Transplantation Proceedings. 2012;44:3037–3040. doi: 10.1016/j.transproceed.2012.07.141. [DOI] [PubMed] [Google Scholar]
- Zimmerhackl L.B., Hofer J., Cortina G., Mark W., Wuerzner R., Jungraithmayr T.C., Khursigara G., Kliche K.O., Radauer W. Prophylactic Eculizumab after renal transplantationin atypical hemolytic-uremic syndrome. New England Journal of Medicine. 2010;362:1746–1748. doi: 10.1056/NEJMc1001060. [DOI] [PubMed] [Google Scholar]
- Zuber J., Fakhouri F., Roumenina L.T., Loirat C., Fremeaux-Bacchi V. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nature Reviews Nephrology. 2012;8:643–657. doi: 10.1038/nrneph.2012.214. [DOI] [PubMed] [Google Scholar]
- Zuber J., Le Quintrec M., Krid S., Bertoye C., Gueutin V., Lahoche A., Heyne N., Ardissino G., Chatelet V., Noel L.H., Hourmant M., Niaudet P., Fremeaux-Bacchi V., Rondeau E., Legendre C., Loirat C., French Study Grp Atypical H. U. S. Eculizumab for atypical hemolytic uremic syndrome recurrence in renal transplantation. American Journal of Transplantation. 2012;12:3337–3354. doi: 10.1111/j.1600-6143.2012.04252.x. [DOI] [PubMed] [Google Scholar]
- Zuber J., Le Quintrec M., Sberro-Soussan R., Loirat C., Fremeaux-Bacchi V., Legendre C. New insights into postrenal transplant hemolytic uremic syndrome. Nature Reviews Nephrology. 2011;7:23–35. doi: 10.1038/nrneph.2010.155. [DOI] [PubMed] [Google Scholar]