Abstract
Candida is the most common fungal pathogen of humans worldwide and has become a major clinical problem because of the growing number of immunocompromised patients, who are susceptible to infection. Moreover, the number of available antifungals is limited, and antifungal-resistant Candida strains are emerging. New and effective antifungals are therefore urgently needed. Here, we discovered a small molecule with activity against Candida spp. both in vitro and in vivo. We screened a library of 50,240 small molecules for inhibitors of yeast-to-hypha transition, a major virulence attribute of Candida albicans. This screening identified 20 active compounds. Further examination of the in vitro antifungal and anti-biofilm properties of these compounds, using a range of Candida spp., led to the discovery of SM21, a highly potent antifungal molecule (minimum inhibitory concentration (MIC) 0.2 – 1.6 µg/ml). In vitro, SM21 was toxic to fungi but not to various human cell lines or bacterial species and was active against Candida isolates that are resistant to existing antifungal agents. Moreover, SM21 was relatively more effective against biofilms of Candida spp. than the current antifungal agents. In vivo, SM21 prevented the death of mice in a systemic candidiasis model and was also more effective than the common antifungal nystatin at reducing the extent of tongue lesions in a mouse model of oral candidiasis. Propidium iodide uptake assay showed that SM21 affected the integrity of the cell membrane. Taken together, our results indicate that SM21 has the potential to be developed as a novel antifungal agent for clinical use.
Introduction
Candida is the most common fungal pathogen of humans and the fourth leading pathogen in nosocomial bloodstream infections [1]. It causes a wide range of infections, from invasive to superficial, at various anatomical sites [2]. Invasive candidiasis is associated with high mortality among immunocompromised populations, and superficial mucosal candidiases are highly prevalent and persistent, especially among immunocompromised patients. The most common superficial candidiases are oral candidiasis and Candida-associated denture stomatitis [3]–[5]. Candida-associated denture stomatitis could affect up to 70% of denture wearers [6].
The most prevalent candidiasis-causing species is Candida albicans. It has a versatile morphogenesis, existing in three forms (i.e., yeasts, pseudohyphae and hyphae), and has two lifestyles (planktonic and biofilm), permitting C. albicans to thrive under diverse environmental conditions. In particular, the yeast-to-hypha (Y-H) transition is a major virulence attribute that facilitates tissue invasion by C. albicans [7], [8]. In addition to Y-H transition, biofilm formation on host tissue or abiotic devices is also a significant basis for Candida infections [5], examples include Candida-associated denture stomatitis [9]. The formation of Candida biofilms, which are more resistant to antifungal agents than planktonic cells, is directly associated with treatment failure [5], [10]. Although Candida infections have always posed a heavy burden on public health, this situation has recently worsened. First, the worldwide incidence of candidiasis has been increasing over the past few decades [11], which may be attributable to increased numbers of immunocompromised patients and the use of broad-spectrum antibiotics [12]. Second, the increased incidence of invasive candidiasis caused by non-albicans Candida (NAC) species, such as Candida glabrata, Candida tropicalis, Candida krusei or Candida parapsilosis, has been a major concern [13], [14], because these infections are often associated with higher mortality and antifungal resistance than those caused by C. albicans [15], [16]. Lastly, the emergence of antifungal resistance and treatment side effects have further restricted treatment options because of the already limited arsenal of current antifungal agents [17], [18].
Only a few classes of antifungal drugs (polyenes, azoles, echinocandins, allylamines and DNA analogues) are available for candidiasis treatment [19], but these drugs are far from ideal. Polyenes, for example, have dose-related toxicity, particularly nephrotoxicity (even though the recent introduction of lipid formulations has now improved the risk-benefit ratio) [20]. In addition, rising drug resistance is an inevitable problem. This is pertinent for fluconazole, a drug of choice for treating AIDS patients with Candida infections. While C. glabrata and C. krusei are intrinsically resistant to fluconazole, the emergence of fluconazole-resistant C. albicans strains is also on the rise [21], [22]. Similarly, emerging resistance has also been reported for the more recently introduced echinocandins [23]–[26]. Therefore, the situation is likely to be ameliorated only by the discovery and introduction of safe and new antifungal agents.
Small molecules are an invaluable source of novel antifungal agents [27]. Previous screening of the same small-molecule library has resulted in the identification of novel compounds effective for severe acute respiratory syndrome-associated coronavirus [28]. Here, we sought small molecules with anti-Candida properties (with the overall strategy depicted in Figure 1). We first screened a collection of over 50,000 small molecules for inhibitors of the Y-H transition in C. albicans. The identified hits were further assessed for antifungal and anti-biofilm activity, which led us to discover a novel antifungal small molecule, which we designated SM21. We examined the in vitro antifungal activity of SM21, as well as its in vivo efficacy in mouse infection models of oral and systemic candidiases. Last, the potential mechanism of action of SM21 was also investigated.
Figure 1. Strategy for screening for novel antifungal small molecules.

We screened for Y-H inhibitors in a library containing 50,240 small molecules and found 20 active compounds. These 20 primary hits were further validated by assessing their activity in a dose-dependent manner, which led to the identification of eight potent Y-H inhibitors. The antifungal properties of the eight compounds were analysed in antifungal susceptibility tests, and the four most potent molecules were selected. Finally, the anti-biofilm activity of the four hits was evaluated, and SM21 was chosen for comprehensive in vitro and in vivo assays. HTS, high-throughput screening; AST, antifungal susceptibility test; ABT, anti-biofilm test.
Materials and Methods
High-throughput screening for Candida Y-H inhibitors
High-throughput screening was performed at the Chemical Genetics Unit, Department of Microbiology, Research Center of Infection and Immunology, Li Ka Shing Faculty of Medicine, University of Hong Kong, on a library with 50,240 small molecules (ChemBridge, San Diego, CA, USA) to identify inhibitors of Y-H transition in C. albicans SC5314, as was previously described [29]. C. albicans SC5314 were seeded at 5×103 cells per well in complete yeast peptone dextrose (YPD) (1% yeast extract, 2% peptone, 2% glucose/dextrose) supplemented with 20% heat-inactivated foetal bovine serum (Invitrogen, Carlsbad, CA, USA) in a total volume of 50 µl in 384-well microtitre plates. The small molecules were dissolved in dimethyl sulfoxide (DMSO) and were added to the wells at final concentration of 20 µg/ml, whereas the controls contained the same amount of DMSO but without small molecules. Assay plates were incubated at 37°C in 5% CO2 for 12 h. Morphologies of the Candida were scored using a Leica DMIL inverted microscope equipped with DC300F digital imaging system (Leica Microsystems, Heidelberg, Germany). Small molecules with lower scores than that of the control (yeast-to-hypha transition inhibitors) were selected as primary hits.
Further evaluation of Y-H inhibition of the primary hits
Dose-dependent Y-H inhibition by the primary hits was further examined using the reference strain C. albicans SC5314 and ten clinical isolates of C. albicans (strains CL1 – CL10, of oral origin from the archival collection of Oral Biosciences, Faculty of Dentistry, University of Hong Kong) under robust hypha-inducing conditions (Lee's medium [30] and 37°C incubation). Each sample contained 100 µl of Candida suspension (1×106 CFUs/ml) and 100 µl of the small molecules of different final concentrations (3.13 – 25 µg/ml). After incubation for 24 h, cell morphologies were observed by light microscopy (Olympus BH-2, Tokyo, Japan). The degree of Y-H inhibition was quantified by calculating the percentage of hyphal cells in one hundred cells in a single sample [29]. Counting was performed in quadruplicate for each sample. The minimum inhibitory concentration (MIC) of Y-H transition (MICY-H) was determined as the minimum concentration at which no hyphae were observed. The assay was performed on three different occasions in duplicate.
Antifungal susceptibility tests of the small molecules
The antifungal activity of small molecules was assessed by disk diffusion and broth dilution assays (see below) using four groups of Candida isolates. The first group consisted of ATCC strains: C. albicans ATCC 90028 (quality control strain), C. glabrata ATCC 90030, C. krusei ATCC 6258, C. parapsilosis ATCC 22019 and C. tropicalis ATCC 13803.
The other three groups were clinical isolates: 92 bloodstream isolates (Queen Mary Hospital and Queen Elizabeth Hospital, Hong Kong), 46 oral isolates from nasopharyngeal carcinoma patients (Queen Mary Hospital, Hong Kong) and 10 denture stomatitis clinical isolates (from the archival collection of Oral Biosciences, Faculty of Dentistry, University of Hong Kong). All of the isolates were subcultured on Sabouraud dextrose agar (SDA) and were incubated aerobically at 37°C overnight before the assay. All of the assays were performed on three different occasions in duplicate per isolate.
Disk diffusion assay
The standard disk diffusion assay (Clinical and Laboratory Standards Institute (CLSI) M44-A2) was used. In brief, a 1×106 CFUs/ml yeast suspension was prepared for each isolate in phosphate-buffered saline (PBS) and distributed evenly by a spiral plater on Mueller-Hinton (M-H) agar supplemented with 2% glucose and 0.5 µg/ml methylene blue. Antifungal tablets (10 µg amphotericin B, 5 µg caspofungin, 15 µg ketoconazole and 25 µg fluconazole) (Neo-Sensitabs, Rosco Diagnostica, Taastrup, Denmark) were used as positive controls. Paper coupons were placed on the agar, and either 2 µg of the small molecule or PBS (as a negative control) was added onto the coupons. The M-H agar plates were then incubated aerobically at 37°C for 24 h, and diameters of the resultant inhibition zones were measured.
Broth dilution assay
The standard protocol for broth microdilution assay (CLSI M27-A3) was followed with modifications [31]. Yeast suspensions (approximately 1×103 colony forming units per millilitre (CFUs/ml)) were prepared in RPMI 1640 medium (Life Technologies, New York, USA) and were added to a 96-well microtitre plate (Iwaki, Tokyo, Japan). Serial dilutions of the small molecules were prepared with the medium and added to the wells. Amphotericin B was used as a positive control. The plate was incubated at 37°C for 48 h, and then Candida growth on the well bottoms was visually observed to determine the minimum inhibitory concentration (MIC). Activity against Cryptococcus neoformans, Aspergillus fumigatus and Penicillium marneffei was similarly assessed after 72 h of incubation.
Anti-biofilm assay in microtitre plates
Candida biofilms were formed according to a previously described method [32]. In brief, yeast cells were subcultured on SDA and incubated at 37°C aerobically for 18 h. They were then transferred to yeast nitrogen base (YNB) liquid medium supplemented with 50 mM glucose, and incubated aerobically at 37°C, 75 rpm overnight. The harvested cells were washed twice with 10 ml PBS and were resuspended to a density of 1×107 CFUs/ml in YNB medium supplemented with 100 mM glucose. Each of the wells of the microtitre plate (Iwaki) was inoculated with 100 µl yeast suspension. The cells were allowed to adhere to the well bottoms by incubating at 37°C, 75 rpm for 1.5 h (adhesion phase). Then, the biofilm was washed with 100 µl PBS to remove the non-adherent cells, and 200 µl fresh medium was added to each well. The biofilm was then incubated at 37°C, 75 rpm for 24 h. For the screening assay, the small molecules (0.2 – 100 µg/ml) were added to the biofilm before or after the adhesion phase. The effect of SM21 on mature biofilms was further investigated by adding SM21 (0.2 – 100 µg/ml) to the biofilm at 24 h and 48 h.
Cell viability of the treated biofilms was measured by the XTT reduction assay [33]. The medium in each well was discarded, and 200 µl XTT solution was added. The XTT solution consisted of 40 µl XTT stock solution (1 mg/ml in PBS), 2 µl menadione (0.4 mM in acetone) and 158 µl PBS. The microtitre plate was incubated at 37°C in the dark for 3 h. Then, 160 µl XTT solution from each well was transferred to a new microtitre plate, and the absorbance was measured at 490 nm. The minimum inhibitory concentration for biofilm (MICbiofilm) was determined as the concentration that caused a 50% reduction in cell viability. All of the assays were performed on three different occasions in duplicate.
Anti-biofilm assay using an in vitro denture stomatitis model
Cold-cured polymethylmethacrylate (PMMA) discs were prepared according to the manufacturers' instructions (Vertex RS, Vertex-Dental BV, Zeist, The Netherlands) and as described previously with some modifications [34]. In brief, transparent self-polymerising acrylic powder was mixed with monomer liquid at a 1.5∶1 ratio (w/v). This mixture was then immediately spread onto and pressed tightly between two aluminium foil-covered glass slides that had been secured with two binder clips to ensure similar thickness and surface of the resultant acrylic strips. After curing for 15 min at 40°C, 2 – 6 bar, the strips were carefully retrieved and cut into 1-cm2 squares. The strips were immersed in distilled water for 7 days to remove excess monomers, and then disinfected in 70% alcohol for 1 min and washed thrice with sterile distilled water. To simulate conditions present in the mouth and to promote Candida adhesion, the strips were treated with saliva for 30 min at 37°C [35]. The saliva had been collected from five healthy volunteers and centrifuged at 12,000× g for 15 min at 4°C, and the resulting supernatant was stored at −70°C until use [36].
C. albicans isolate BF-1, which was identified as a stronger biofilm former in a previous study [36], was subcultured on SDA one day before the assay. The yeast inoculum was standardised to 1×107 CFUs/ml in YNB supplemented with 100 mM glucose.
The acrylic strips were placed in 24-well plates (Iwaki), and 1 ml yeast inoculum was added to each well, completely covering the acrylic strips. SM21 was added at MICbiofilm (1.56 µg/ml) before, after, or both before and after the adhesion phase. The adhesion phase was assessed 1.5 h after inoculation, and the plate was then placed in a shaking incubator at 37°C. The cell viability of the biofilm on the acrylic strips was quantified by the XTT reduction assay and was also observed by confocal laser scanning microscopy (Olympus Fluoview FV1000) after staining using a LIVE/DEAD BacLight Viability Kit (Invitrogen).
Analysis of HWP1 expression
Engineered C. albicans strain PHWP1-GFP, which expressed GFP under the control of either the HWP1 promoter, was gift from Dr. B. P. Krom [37]. The PHWP1-GFP strain was used to test the effect of SM21 on HWP1 expression. The strains were cultivated overnight at 37°C in Lee's medium containing SM21 at MIC (0.2 µg/ml) or 2×MIC (0.4 µg/ml). GFP expression was then observed using confocal laser scanning microscopy (Olympus Fluoview FV1000).
Fungal specificity assay
The disk diffusion assay (CLSI M44-A2) was also used to assess the potential activity of SM21 against six bacterial species (Escherichia coli, Lactobacillus acidophilus, Streptococcus mutans, Streptococcus mitis, Streptococcus sanguinis and Aggregatibacter actinomycetemcomitans). The bacteria were subcultured one day before the assay. Bacterial cell suspensions were prepared for each sample at a density of 1×106 CFUs/ml and were then distributed evenly on sheep blood agar plates with a spiral plater. Paper coupons inoculated with 10 µl of drugs or controls (0.2 µg/µl SM21, 1 µg/µl amphotericin B and 0.5 µg/µl caspofungin) were placed on the agar. Chlorhexidine gluconate (0.2%) was used as a positive (antibacterial) control, and PBS as a negative (harmless) control. The plates were incubated anaerobically (except for the E. coli plate) at 37°C for 24 h, and the diameters of inhibition zones were measured.
Effects on antifungal-resistant isolates
The efficacy of SM21 against 16 antifungal-resistant Candida strains was evaluated by antifungal susceptibility tests (broth microdilution and disk diffusion assays) as described above. The strains were clinical blood isolates belonging to various Candida spp., which had been obtained from Helsinki University Central Hospital (Helsinki, Finland) and that had been confirmed to have resistance to one or more antifungal agents by standard CLSI antifungal susceptibility tests in our previous study [25].
Cytotoxicity assays
Primary human oral keratinocytes (HOKs) (ScienCell Research Laboratories, Carlsbad, CA, USA) at passage three were seeded into a 96-well plate (Iwaki) in oral keratinocyte medium (ScienCell Research Laboratories). The serum-free medium contained 1% oral keratinocyte growth supplement, 1% penicillin and 1% streptomycin. Each well initially contained approximately 1×104 HOKs, which were incubated in the presence of 5% CO2 at 37°C (medium was changed every day) until the cells reached confluence. A dilution series of SM21 (0.05 – 54.8 µg/ml) was then supplied to the wells so that each well contained 200 µl of medium in total. The plate was incubated at 37°C for 24 h, and then the viability of the HOKs was determined by MTT assay (see below).
T-75 flasks were also used to assess cell viability in the presence of SM21. Besides HOKs, human gingival fibroblasts (HGFs) and monocyte cell lines (ScienCell Research Laboratories) were also used. Each cell line was seeded in T-75 flasks, and after confluence was reached, 2 µg SM21 in 10 ml of growth medium (0.2 µg/ml final concentration) was added to each flask. For comparison, amphotericin B was used at the same concentration. After 24 h-incubation at 37°C, MTT assay was performed to measure the cell viability.
MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) is a yellow tetrazolium dye that is converted into a purple compound by mitochondrial enzymes. MTT solution (5 mg/ml) was prepared in growth medium and added to the wells or flasks. After incubation at 37°C for 3 h in dark, the MTT solution was discarded. The converted dye was solubilised with an equal amount of DMSO. The absorbance of the converted dye was measured at 570 nm with background subtraction at 650 nm. The concentration at which the cell viability had dropped by 50% was recorded as the cytotoxic concentration (CC50) [38].
Co-culture of C. albicans and HOKs
HOK culture was performed as mentioned above. HOKs at passage three were seeded into an 8-well plate (ibidi, Martinsried, Germany), which was compatible for the observation with confocal laser scanning microscope. The cells were incubated at 37°C in the presence of 5% CO2 until confluence was reached, with the medium changed every day. Each well contained approximately 1×105 HOKs at confluence. The cells were washed once with PBS, and fresh medium without antibiotics (as antibiotics could inhibit Candida growth) was added. A suspension of C. albicans SC5314 (1×104 CFUs/ml obtained by subculturing on SDA plates and incubating at 37°C one day before inoculation) was prepared in the growth medium (without antibiotics), and 100 µl of this cell suspension was added to each well. SM21 was added at various final concentrations (0.5, 1 and 2 µg/ml), whereas the positive controls contained only the growth medium. The plate was then incubated at 37°C for 24 h in the presence of 5% CO2. The viability of HOKs and yeast cells was assessed by confocal laser scanning microscopy using fluorescent probes (LIVE/DEAD BacLight Viability Kit and LIVE/DEAD Viability/Cytotoxicity Kit for mammalian cells; Invitrogen).
Ethics Statement
The animal studies strictly followed the recommendations in “Guide for the Care and Use of Laboratory Animals” published by National Institutes of Health. The protocols were approved by the Committee on the Use of Live Animals in Teaching and Research (CULATR), University of Hong Kong (Permit Number: 2461-11).
Mouse model of systemic candidiasis
The mice used were 8-week-old male C57BL/6 mice that were obtained from the Laboratory Animal Unit, University of Hong Kong. Mouse model of systemic candidiasis was established according to a previously described method [39], with modifications. The mice were injected via the tail vein with 100 µl cell suspension of C. albicans SC5314 (1×106 CFUs/ml) 3 h before the start of antifungal treatment. Drugs were given to the mice twice a day for 5 days via intraperitoneal injection. The test group (n = 5) was treated with 0.01, 0.1, 1 and 10 mg/kg SM21 in 100 µl PBS, while the control group (n = 5) was treated with PBS. The mice were weighed every day. All of the mice were sacrificed after 5 days, and the kidneys were harvested (as most of the Candida cells in systemic candidiasis murine models are found in the kidneys [39]). The kidneys were divided into equal portions for fungal burden determination and histopathological evaluation. For fungal burden determination, the kidneys were weighed and then homogenised in PBS, and the homogenates were serially diluted before plating on SDA. The plates were incubated at 37°C, and fungal burden was expressed as the ratio of colony forming units (CFUs) to the organ weight. For histopathological analysis, organs were fixed in 4% paraformaldehyde, embedded with paraffin wax and stained with periodic acid-Schiff (PAS).
Mouse model of oral candidiasis
The murine model of oral candidiasis was established according to a previously described method [40], [41]. One day before infection, C57BL/6 mice were immunosuppressed by subcutaneous injection of two doses of prednisolone (100 mg/kg body weight), and the antibiotic tetracycline hydrochloride (0.83 mg/ml) was administered in the drinking water. Immediately before infection, the mice were anesthetised with 50 µl chlorpromazine chloride (2 mg/ml) via intramuscular injection on each femur. The oral cavities of the anesthetised mice were swabbed with a sterilised cotton swab that had been dipped in a cell suspension of C. albicans SC5314 (2.5×107 CFUs/ml; from cultures grown overnight at 37°C on SDA). Subsequently, 10 µl SM21 (200 µg/ml; n = 3), 10 µl nystatin (10 µg/ml; n = 3) or 10 µl PBS (as a control; n = 3) was administered to the mice by pipetting into their mouths; this was done five times, at 3, 6, 12, 24 and 36 h after inoculation. The mice were sacrificed 72 h after infection and were immediately observed macroscopically for tongue lesions. The tongue lesion degree was evaluated using a scoring system of 0 – 3 [40], with 0 denoting a healthy tongue surface and 3 the most severe stage. The entire oral cavities of the mice were swabbed with sterilised cotton swabs, which were then submerged in 500 µl PBS and vortexed vigorously. The resultant suspension was spiral plated on SDA, and the number of CFUs was counted after incubation at 37°C for 24 h. In addition, the invasion of tongue tissues by Candida was evaluated histologically by PAS staining, as described above.
Propidium iodide uptake assay
Propidium iodide, a membrane-impermeable fluorescent dye that binds to nucleic acids, is widely used to differentiate cells that have damaged plasma membranes from healthy ones [42], [43]. To evaluate the effect of SM21 on the fungal plasma membrane, C. albicans SC5314 cells (approximately 1×103 CFUs/ml) were obtained from logarithmic phase cultures and suspended in RPMI 1640 medium, as described above. The cells were then exposed to sub-MIC of SM21 (0.1 µg/ml) for 1 h at 30°C with gentle shaking. Subsequently, cells were harvested, incubated with propidium iodide using a LIVE/DEAD BacLight Viability Kit (Invitrogen) for 15 min, and then observed by confocal microscopy (Olympus FluoView FV1000).
Statistical analysis
One-way Analysis of Variance (ANOVA) was employed to evaluate the mean difference between the control and the test groups. Statistical significance of the data was analyzed using SPSS software package (SPSS version 20; SPSS Inc, Chicago, IL, USA). Data was considered significant if p-values are less than 0.05.
Results
Discovery of the lead compound
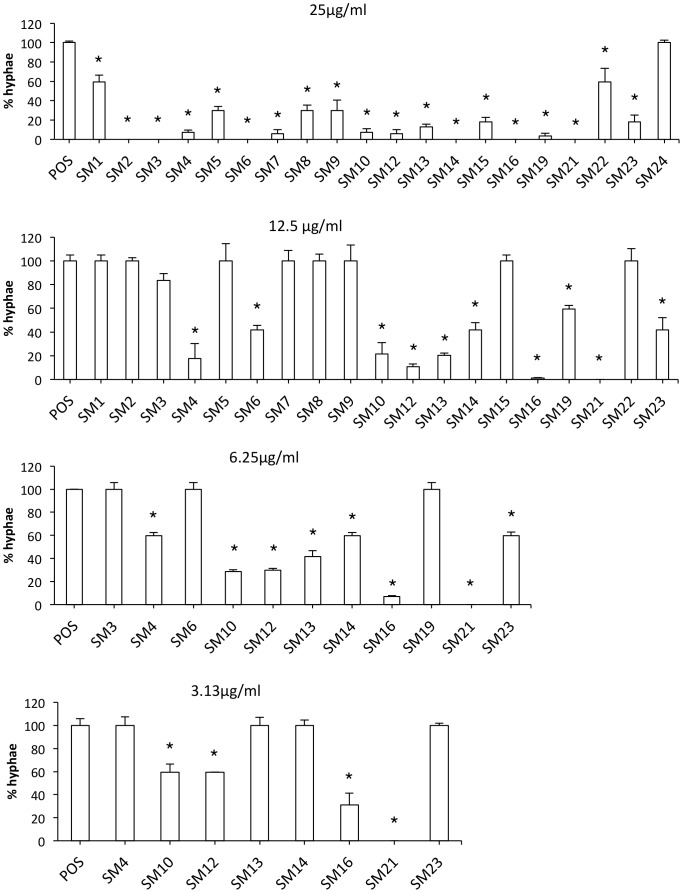
To obtain small molecule inhibitors of Candida spp., we first screened 50,240 small molecules for inhibitors of the Y-H transition in C. albicans, and 20 active compounds were identified. Of these, eight compounds displayed strong dose-dependent Y-H inhibition under hypha-inducing conditions for the reference strain C. albicans SC5314 and ten clinical isolates of C. albicans (Figure 2). Of these eight compounds, four (SM10, SM12, SM16 and SM21) formed inhibition zones in disk diffusion assays using the standard reference strain C. albicans ATCC 90028, indicating fungicidal activity (The result of SM21 is shown in Figure S1). In addition, SM21 had the most potent anti-biofilm activity out of the four compounds (Figure 3). Therefore, SM21 (molecular weight = 438 g/mol, ChemBridge ID# 6633321), which had not been previously identified as an antifungal agent, was chosen for further study (Figure 4).
Figure 2. Dose-dependent Y-H inhibition of C. albicans SC5314 by the 20 primary hits under hyphal-inducing conditions.
Cell morphology of C. albicans SC5314 was observed by light microscopy after incubation in the presence of different concentrations of the 20 primary hits under hyphal-inducing conditions (Lee's medium and incubation at 37°C). The degree of Y-H inhibition was quantified by calculating the percentage of hyphal cells in one hundred cells in a single sample. The average percentage of hyphae in the treated samples was normalised to the average percentage of hyphae in the positive control (taken as 100%). Starting with the highest concentration, those small molecules showing no Y-H inhibition were progressively eliminated in subsequent tests, which used lower concentrations. The assay identified eight potent Y-H inhibiting small molecules. The standard deviations are shown for each sample, and asteriks indicate p-value <0.05.
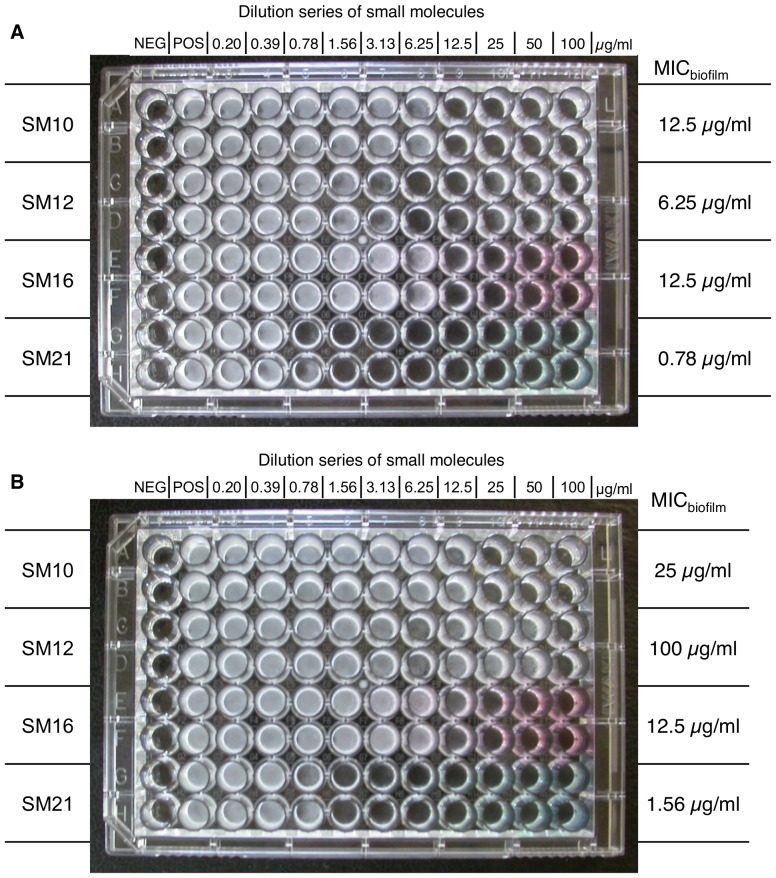
Figure 3. Anti-biofilm properties of SM10, SM12, SM16 and SM21.
The small molecules were added to the C. albicans SC5314 biofilm (A) before and after the adhesion phase; (B) after the adhesion phase; and incubated in 37°C for 24 h. The MICbiofilm was determined as the concentration where the viability of the biofilm was reduced by 50% as compared with the positive control. SM21 had the lowest MICbiofilm among the four hits in both cases, indicating its potent anti-biofilm property. NEG, negative control; POS, positive control.
Figure 4. Structure of SM21.
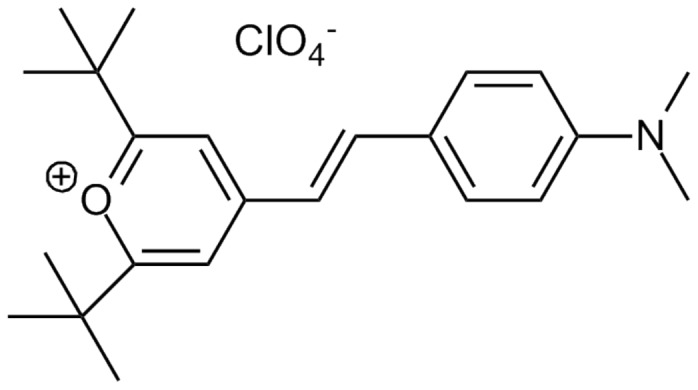
The molecular weight of SM21 (ChemBridge ID# 6633321) is 438 g/mol.
SM21 is a potent Y-H inhibitor
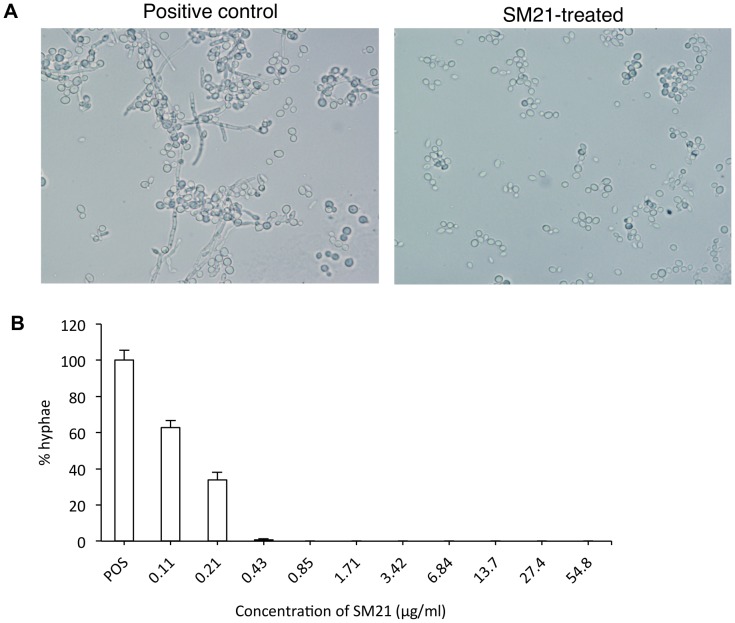
To further examine the Y-H inhibition of SM21, the phenotype of a panel of Candida isolates, including reference strain C. albicans SC5314 and ten C. albicans clinical isolates of oral origins was observed under robust hypha-inducing condition in the presence of SM21. In the control, where SM21 was not added, extensive hyphae could be observed after 24-h incubation. In contrast, only yeast cells, and not hyphae, could be observed in samples laced with SM21 of concentration 0.43 µg/ml or higher (Figure 5A). The MICY-H of SM21 was 0.43 µg/ml (equivalent to 0.98 µM) (for 1×106 CFUs/ml C. albicans SC5314; Figure 5B), which was similar to the average MICY-H for ten clinical isolates (0.5 µg/ml) (Table 1). Furthermore, the MICY-H was directly proportional to the cell density (Table 1).
Figure 5. Y-H inhibition by SM21.
C. albicans cell morphology was observed by light microscopy after incubation in the presence of SM21. (A) SM21-mediated Y-H inhibition against strain CL1, a C. albicans clinical isolate. Under hyphal inducing conditions, hyphae could be observed in the positive control (no SM21), whereas, no hyphae were observed in the SM21-treated samples. (B) Dose-dependent effect of SM21 on Y-H inhibition of C. albicans SC5314 (1×106 CFUs/ml). POS, positive control (no SM21). The degree of Y-H inhibition of each concentration was quantified by calculating the mean percentage of hyphal cells in one hundred cells in quadruplicate. The average percentage of hyphae in the treated samples was normalised to the average percentage of hyphae in the positive control (taken as 100%). MICY-H of SM21 was taken at 0.43 µg/ml, where only minimal amount of hyphae was observed. mean differences between the tests and control were all statistically significant (p-value <0.05).
Table 1. Relationship between C. albicans cell density and MICY-H of SM21.
| Cell density | MICY-H | |
| (CFUs/ml) | µg/ml | µM |
| 104 | 0.125 | 0.29 |
| 106 | 0.5 | 1.14 |
| 107 | 1 | 2.28 |
| 108 | 10 | 22.8 |
Results are the mean values of three independent experiments, performed in duplicate for each of the ten clinical C. albicans isolates CL1 – CL10.
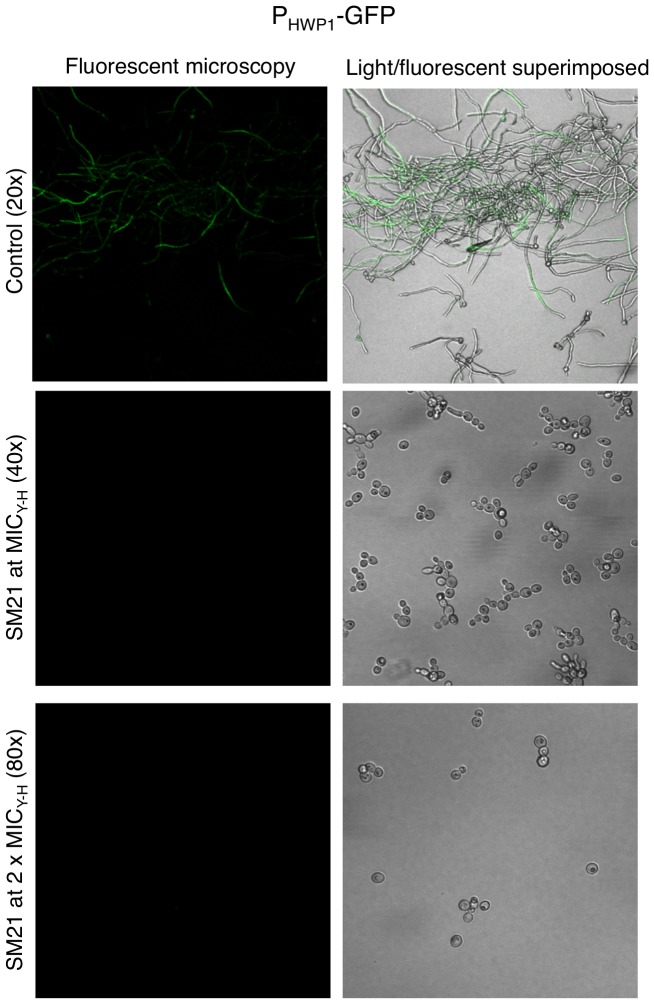
In addition, we used a C. albicans PHWP1-GFP strain to test whether SM21 affected HWP1 expression. GFP was expressed in candidal hyphae under untreated conditions (as observed by confocal fluorescence microscopy; Figure 6). By contrast, no trace of fluorescence could be detected from the SM21-treated cells at both MICY-H and 2×MICY-H, suggesting that SM21 influenced HWP1 expression.
Figure 6. Inhibition of HWP1 expression by SM21.
The inhibition of HWP1 expression of SM21 was examined by C. albicans strain PHWP1-GFP at MICY-H (0.43 µg/ml) and 2×MICY-H (0.86 µg/ml). Fluorescence, which indicated GFP expression under the control of the HWP1 promoter, was observed by confocal microscopy. In the control, multiple hyphae layers were observed under hypha-inducing conditions, however, only the fluorescence from the top layer could be captured by the confocal microscopy. After treatment with SM21 at MICY-H or 2×MICY-H, no trace of fluorescence was detected.
SM21 is active against fungi but not bacteria
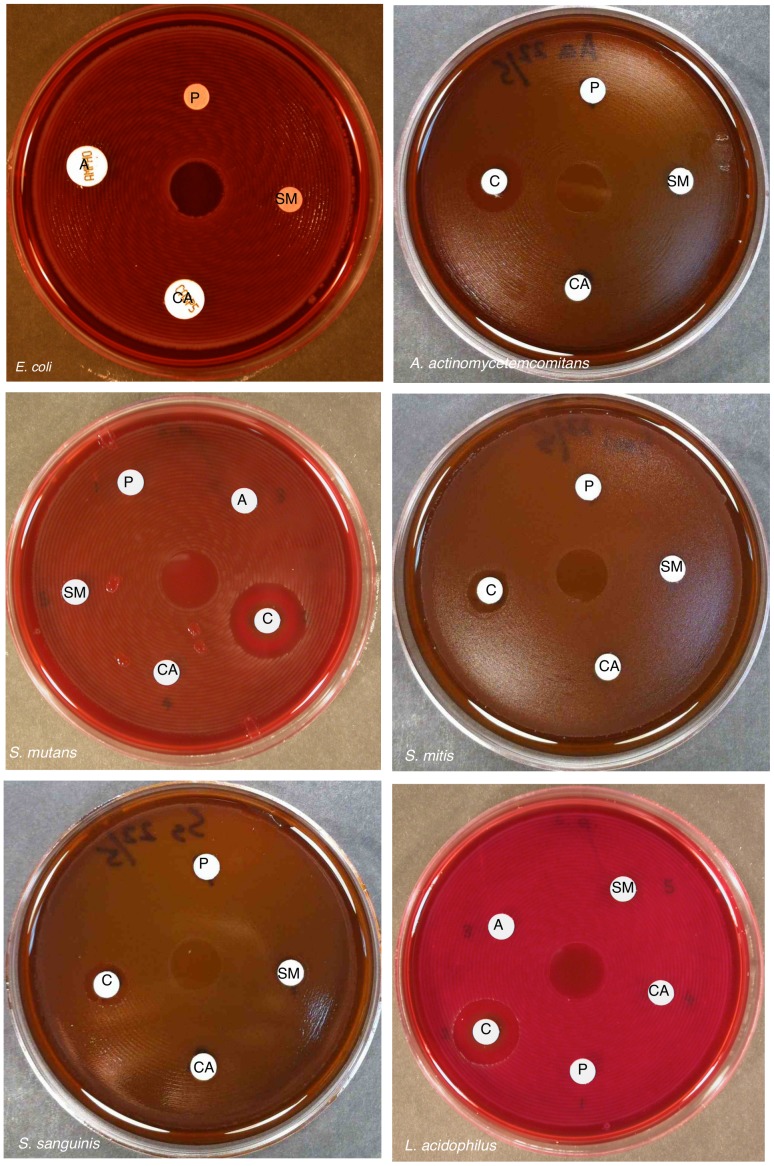
An ideal antifungal agent should be active against fungal species but not against bacteria. The antifungal activity of SM21 was exhibited by disk diffusion assay and broth microdilution assay. SM21 formed inhibition zones with C. albicans SC5314, C. albicans ATCC 90028 and all of the 148 clinical isolates tested. The average diameter of the inhibition zones of SM21 against these isolates was 27.7 mm (range = 25 – 31 mm) (The growth inhibition zones of SM21 against C. albicans SC5314 and C. albicans ATCC 90028 were shown in Figure S1.). For the broth microdilution assay, the MIC of SM21 against C. albicans ATCC 90028 was found to be 0.2 µg/ml, which was similar to that of the conventional antifungal amphotericin B, suggesting a comparable potency (Table 2). The MIC of SM21 for a wide range of Candida spp. (including clinical isolates) and other fungal species (except A. fumigatus) ranged from 0.2 to 1.6 µg/ml (Table 2). Lower susceptibility to SM21 was noted with the fungus A. fumigatus (MIC = 6.25 µg/ml), as was also observed for amphotericin B (MIC = 1.56 µg/ml). SM21 did not inhibit the growth of any of the six bacterial species evaluated in disk diffusion assay, implying the fungal specificity of the novel molecule (Figure 7).
Table 2. MICs of SM21 and amphotericin B against a range of Candida and other fungal species.
| MIC (µg/ml) | ||
| Fungal strain | SM21 | AMB |
| C. albicans ATCC90028 | 0.2 | 0.2 |
| C. albicans SC5314 | 0.2 | 0.2 |
| C. albicans CL1 | 0.2 | 0.2 |
| C. albicans CL2 | 0.2 | 0.4 |
| C. albicans CL3 | 1.6 | 0.4 |
| C. albicans CL3 | 1.6 | 0.4 |
| C. albicans CL4 | 0.8 | 0.2 |
| C. albicans CL5 | 1.6 | 0.2 |
| C. albicans CL6 | 1.6 | 0.2 |
| C. albicans CL7 | 0.8 | 0.2 |
| C. albicans CL8 | 0.8 | 0.4 |
| C. albicans CL9 | 1.6 | 0.4 |
| C. albicans CL10 | 1.6 | 0.2 |
| C. glabrata ATCC90030 | 1.6 | 0.4 |
| C. krusei ATCC6258 | 0.4 | 0.4 |
| C. tropicalis ATCC13803 | 0.8 | 0.8 |
| C. parapsilosis ATCC22019 | 1.6 | 0.8 |
| C. neoformans | 0.4 | 0.4 |
| A. fumigatus | 6.25 | 1.56 |
| P. marneffei | 0.2 | 0.05 |
AMB – amphotericin B. The MICs of SM21 against the Candida isolates tested ranged from 0.2 – 1.6 µg/ml, while the MICs of amphotericin B ranged from 0.2 – 0.8 µg/ml. The similar range of MICs suggested the comparable potency of SM21 and amphotericin B. For the other fungal species, lower susceptibility of SM21 against A. fumigatus was observed. Such trend was also observed for amphotericin B.
Figure 7. Effect of SM21 on several bacterial species.
Disk diffusion assays revealed that SM21 was harmless to E. coli, A. actinomycetemcomitans, S. mutans, S. mitis, S. sanguinis, and L. acidophilus. P – PBS, C – chlorhexidine (0.2%), CA – caspofungin (5 µg), A – amphotericin B (10 µg), SM – SM21 (2 µg).
SM21 inhibits biofilm formation
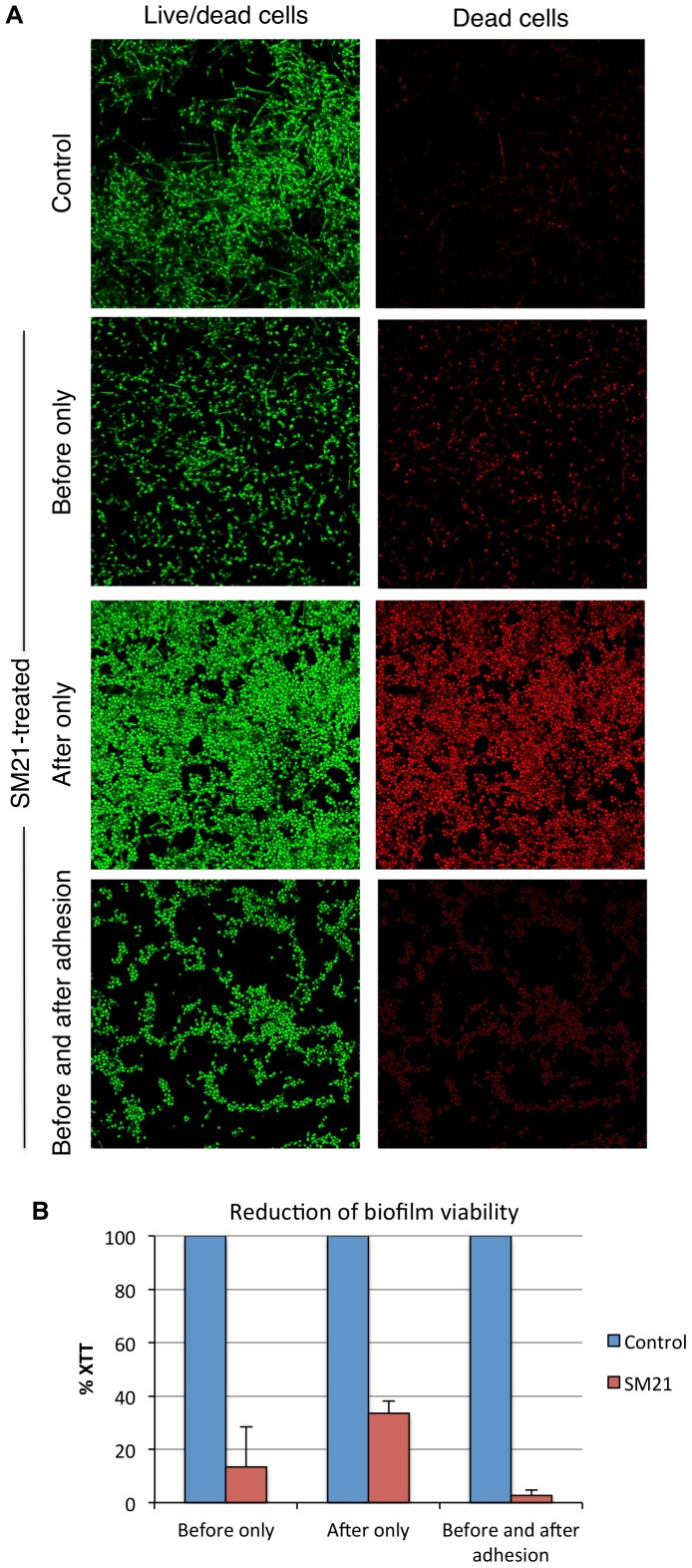
We then assessed the activity of SM21 against Candida biofilm formation. We first used a microtitre-plate biofilm model in which SM21 was added to the 24-h and 48-h biofilm of C. albicans, C. glabrata and C. krusei. SM21 demonstrated a lower MICbiofilm than amphotericin B and caspofungin for both 24-h and 48-h biofilm (Table 3). In an in vitro model of denture stomatitis, SM21 effectively prevented candidal adhesion and biofilm development on denture acrylic surfaces (Figure 8A). In this model, biofilm viability was reduced by 85%, 66% and 97%, when SM21 was added before, after, or both before and after the 1.5 h adhesion phase respectively (Figure 8B). Summarized from the two assays, SM21 was effective against mature Candida biofilm and inhibited biofilm formation on denture acrylic surfaces.
Table 3. MICbiofilm of SM21 against three different Candida species.
| MIC (µg/ml) | ||||||
| Candida species | 24-h biofilm | 48-h biofilm | ||||
| SM21 | AMB | CASP | SM21 | AMB | CASP | |
| C. albicans | 3.2 | 32 | 50 | 25 | 32 | 100 |
| C. glabrata | 3.2 | 16 | 50 | 25 | 16 | 100 |
| C. krusei | 3.2 | 32 | 50 | 25 | 32 | 100 |
Candida biofilms of 24-h or 48-h were incubated with serial dilution of SM21 for 24 h. Thereafter, the MICbiofilm of SM21 was determined as the concentration at which the cell viability of the biofilm was reduced by 50% as compared with the untreated control. (AMB – amphotericin B, CASP – caspofungin.)
Figure 8. Effect of SM21 on C. albicans biofilm formation on denture acrylic.
(A) Confocal images of C. albicans biofilm after treatment with SM21 and staining with fluorescent labels that distinguish between live and dead cells. All cells were labeled with the green fluorescence, while only the dead cells were labeled with red fluorescence. (B) Biofilm cell viability, quantified by XTT reduction assay, was reduced by 85% and 66%, respectively, when SM21 was added before or after the adhesion phase. The reduced biofilm viability was maximised (97%) when SM21 was added both before and after the adhesion phase. The standard deviations of each sample are shown in the graph, and all the mean differences between the control and test (SM21) were statistically significant (p-value<0.05).
SM21 is active against antifungal-resistant Candida spp.
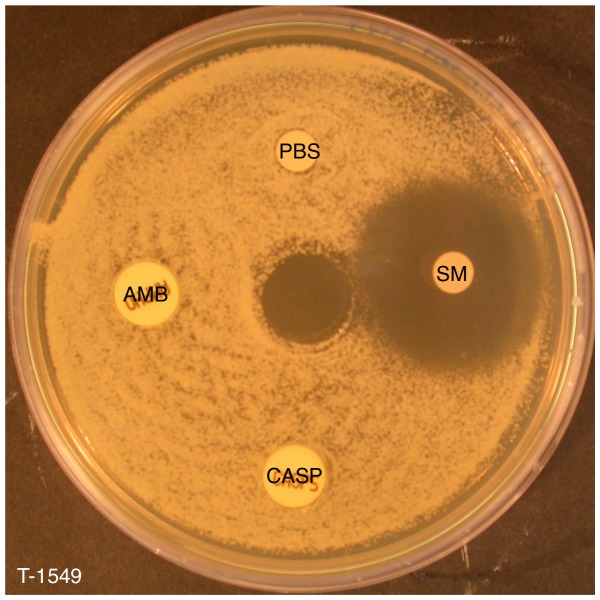
The emergence of antifungal-resistant Candida strains has led to the urgent need of new antifungal agents. All of the drug-resistant isolates tested were susceptible to SM21. For instance, a clear SM21 inhibition zone was observed for T-1549, a multidrug-resistant C. guilliermondii strain (Figure 9). The MIC of SM21 against drug-resistant Candida isolates in broth microdilution assays ranged from 0.5 to 1 µg/ml (Table 4), which was marginally higher than the MIC for C. albicans ATCC 90028.
Figure 9. Effect of SM21 on a multidrug-resistant isolate.
SM21 (SM) produced a clear inhibition zone in a disk diffusion assay against T-1549, a C. guilliermondii strain with multidrug resistance to amphotericin B (AMB), caspofungin (CASP) and fluconazole.
Table 4. MICs of SM21 for antifungal-resistant Candida strains.
| Drug-resistant isolates | Antifungal-resistance | MIC (µg/ml) |
| C. albicans T1675 | F,I | 0.5 |
| C. parapsilosis T1677 | C,F,I,K,V | 1 |
| C. parapsilosis T1565 | C,I | 1 |
| C. parapsilosis T1545 | C | 1 |
| C. parapsilosis T1688 | I | 0.5 |
| C. glabrata T1585 | F,I,K,V | 1 |
| C. glabrata T1672 | I,K | 0.5 |
| C. glabrata T1570 | I | 0.5 |
| C. tropicalis T1427 | I | 1 |
| C. tropicalis T789 | I | 1 |
| C. tropicalis T1148 | I | 0.5 |
| C. krusei T1472 | F,I,K | 1 |
| C. krusei T1266 | F | 0.5 |
| C. krusei T1562 | F | 1 |
| C. krusei T1266 | F | 1 |
| C. guilliermondii T1549 | A,C,F | 1 |
A – amphotericin B, C – caspofungin, F – fluconazole, I – itraconazole, K – ketoconazole, V – voriconazole.
SM21 displays low toxicity to human cells
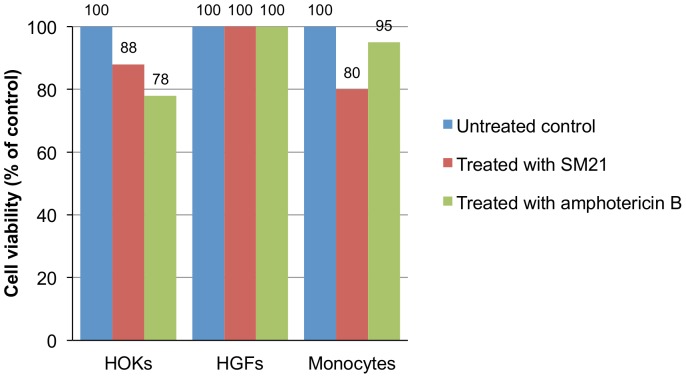
Cytotoxicity assays performed in a 96-well plate revealed the CC50 of SM21 to be 3.4 µg/ml (7.8 µM) for HOKs. In assays conducted in T-75 flasks, 0.2 µg/ml SM21 reduced the viability of HOKs by 12% compared with the untreated controls, which is lower than the 22% reduction observed for amphotericin B. Treatment with SM21 or amphotericin B caused, respectively, a 20% or 5% reduction in monocyte viability compared with the controls. Neither SM21 nor amphotericin B affected the viability of HGFs (Figure 10).
Figure 10. Cytotoxicity of SM21 against three primary cell lines.
HOK – human oral keratinocyte, HGF – human gingival fibroblast, AMB – amphotericin B. Both SM21 (0.2 µg/ml) and amphotericin B (0.2 µg/ml) reduced the viability of HOKs and monocytes. SM21 caused a reduction of 12% in cell viability compared with the untreated controls, which is lower than the 22% reduction observed for amphotericin B. Treatment with SM21 or amphotericin B caused, respectively, a 20% or 5% reduction in monocyte viability compared with the controls. Neither SM21 nor amphotericin B affected the viability of HGFs.
SM21 prevents candidal invasion of human cells in vitro
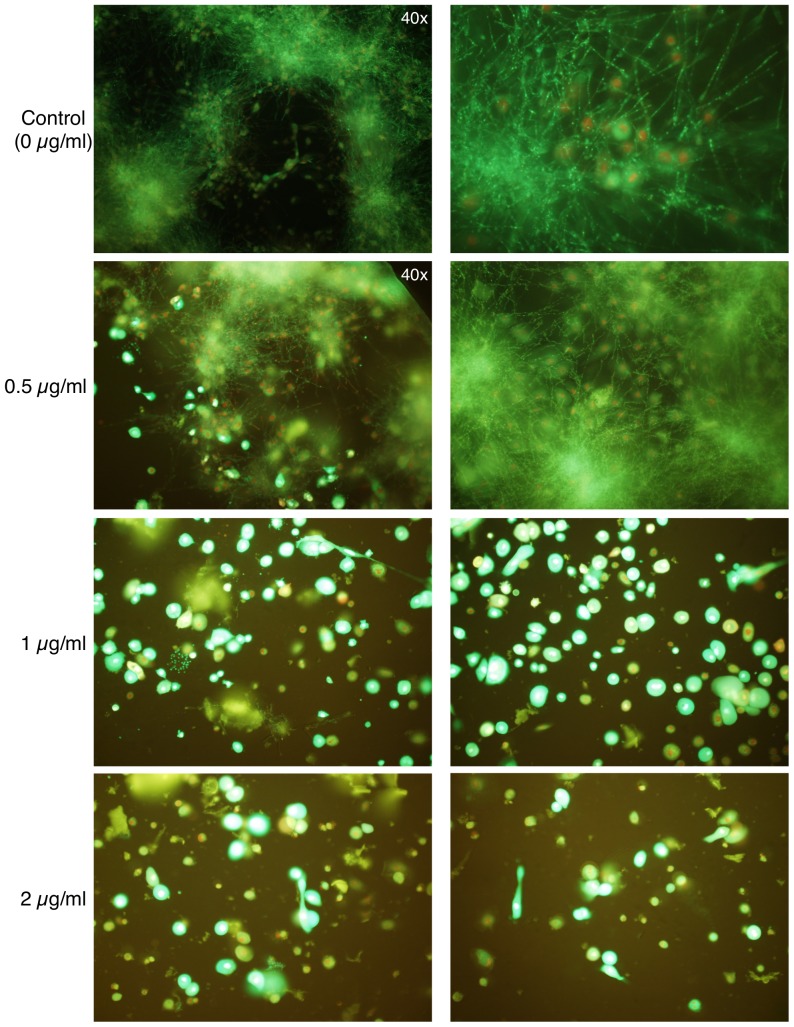
To test whether SM21 was able to prevent C. albicans invasion of human cells, a co-culture of HOKs and C. albicans SC5314 was exposed to various concentrations of SM21. In the co-culture control (in which no SM21 was present), many hyphae were observed. The hyphal invaded and caused the death of most of the HOKs (Figure 11). At 0.5 µg/ml SM21, Candida hyphae could still be observed, however, the proportion of live HOKs increased. At 2 µg/ml and 4 µg/ml SM21, most of the HOKs were alive, and no Candida were observed. Therefore, this SM21 concentration was safe to HOKs and was sufficient to prevent Candida from invading the human cells.
Figure 11. Effect of SM21 treatment in Candida–HOK co-culture model.
The viability of co-cultured HOKs and yeast cells in the presence of SM21 was assessed by confocal laser scanning microscopy using fluorescent probes. In the untreated control, many hyphae were observed, and most of the HOKs were dead (the nuclei of the dead cells were labeled with red fluorescence). Samples containing 0.5 µg/ml SM21 still included many hyphae, but they contained more live HOKs than the untreated control. Samples containing 1 µg/ml or 2 µg/ml SM21 included few yeast cells and no hyphae, and most of the HOKs were alive. Magnification = 20× (unless specified otherwise).
SM21 improves survival in a mouse model of systemic candidiasis
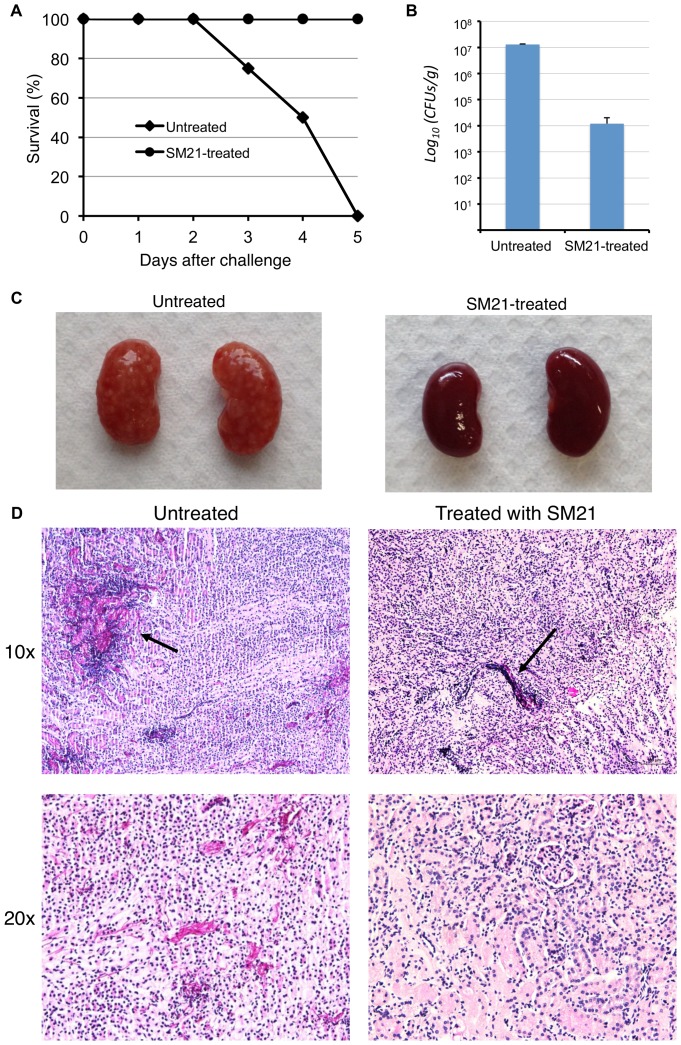
A mouse model of systemic candidiasis was used to assess the in vivo activity of SM21. Infection was established in mice by injecting 100 µl of 1×106 CFUs/ml C. albicans SC5314 via tail vein. The test group was treated with 0.01, 0.1, 1 and 10 mg/kg SM21, while control group was treated with PBS. At day 5 (the experimental end point) after infection with C. albicans, all of the untreated mice with systemic candidiasis had died, whereas all of the SM21-treated mice were alive (Figure 12A). The fungal burden in the kidneys was significantly lower in the SM21-treated mice than in the untreated ones (Figure 12B). Moreover, white Candida lesions were visible covering the surfaces of the kidneys of the untreated mice, while the kidneys of the SM21-treated mice appeared healthy (Figure 12C). Clusters of Candida hyphae were easily identified in PAS-stained kidneys of the untreated mice, whereas very few Candida cells were observed in the kidney parenchyma of the SM21-treated mice (Figure 12D). These results indicated that SM21 was effective in preventing systemic spread and invasion of Candida in the mouse model.
Figure 12. Effect of SM21 treatment on a mouse model of systemic candidiasis.
(A) Survival rate of the mice. After the experimental period (day 5), the survival rates for untreated and SM21-treated mice were 0% and 100%, respectively. (B) Fungal burden in the kidneys of the mice (Error bars indicate standard deviation). SM21 significantly reduced the renal fungal burden in the mice by an order of magnitude of 3 (p-value of mean difference <0.05). (C) Surfaces of the kidneys of untreated mice were covered with Candida lesions, while the kidneys of the SM21-treated mice appeared healthy. (D) PAS staining of the kidneys. Candida hyphae (indicated by black arrows) were easily spotted in the kidneys of untreated mice, whereas few were detected in the kidneys of SM21-treated mice.
SM21 reduces tongue lesions in a mouse model of oral candidiasis
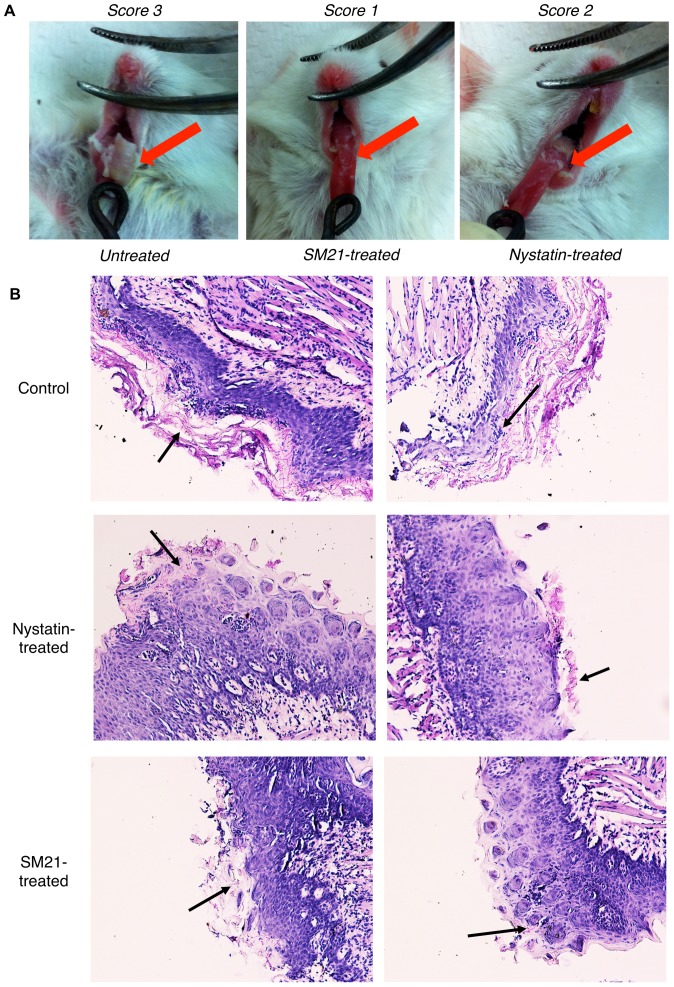
We then assessed the effects of SM21 and nystatin treatments in a mouse model of oral candidiasis established by inoculating C. albicans SC5314 (2.5×107 CFUs/ml) in the oral cavities of the mice. Treatment was given to the mice at five time intervals. Immediately after sacrifice (72 h after inoculation), scores were assigned for tongue lesions from 0 (healthy tongue surface) to 3 (most severe stage). Thick oral thrush was observed in the untreated mice (score = 3; Figure 13A). Tongue lesions in the nystatin-treated mice were less severe than in the untreated controls, although considerable oral thrush was noted at the back of the tongue (score = 2). Compared with the other two groups, SM21-treated mice displayed the least severe tongue lesions (score = 1). Moreover, Candida cell counts from tongue swabs of the SM21-treated mice (4.5×102 CFUs/ml) were consistently lower than those of the control mice (4.8×103 CFUs/ml) and the nystatin-treated animals (6.0×102 CFUs/ml). Numerous candidal hyphae were noted in PAS-stained tongue sections of the untreated mice, whereas less profuse candidal growth was observed in samples from the mice treated with SM21 or nystatin (Figure 13B).
Figure 13. Effect of SM21 treatment on a mouse model of oral candidiasis.
(A) The degree of tongue lesions in the oral candidiasis mouse model was evaluated by a scoring system of 0 – 3 (0 denotes healthy tongue surface and 3 denotes the most severe stage). A thick oral thrush (score = 3) was observed in untreated mice. Nystatin-treated mice displayed less oral thrush on the tongue surface than the untreated controls, but a considerable amount of oral thrush was observed at the back of the tongue (score = 2). SM21-treated mice displayed the least severe tongue lesions (score = 1). (B) PAS staining of tongue sections. Abundant hyphae (indicated by black arrows) covered most of the tongue surface in the untreated mice, whereas comparatively fewer hyphae were observed in nystatin- and SM21-treated mice.
SM21 affects permeability of the fungal cell membrane
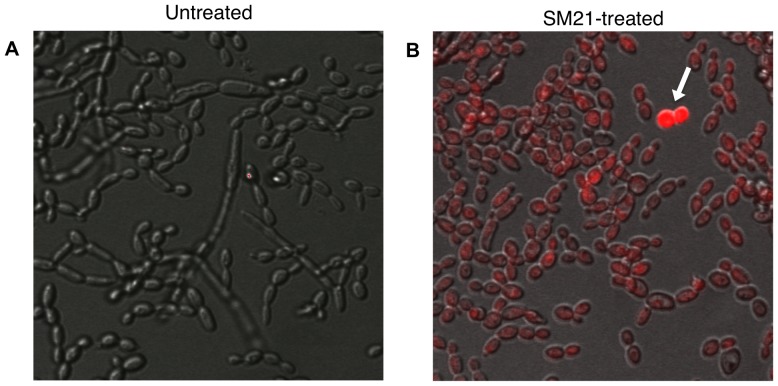
To shed some light on the mechanism of action of SM21, we performed propidium-iodide uptake assays. Candida exposed to sub-MIC of SM21 (0.1 µg/ml) displayed abundant propidium iodide uptake, indicating cell membrane damage (Figure 14). Therefore, the molecular target of SM21 might be a component of the cell membrane.
Figure 14. Confocal microscopic analysis of propidium-iodide uptake assays.
(A) Untreated control samples showed hyphal elements of live C. albicans cells. (B) C. albicans treated with sub-MIC of SM21 (0.1 µg/ml) showed propidium iodide uptake (labeled by red fluorescence), indicating cell membrane damage. Some of the cells (denoted by white arrow) were non-viable.
Discussion
A handful of small molecules have been reported to possess activity against C. albicans [29], [44]–[47]. However, the therapeutic potential of these small molecules is unclear, because they have been evaluated mainly for the ability to inhibit C. albicans virulence properties such as Y-H transition or biofilm formation in vitro. It has been hypothesised that arresting Candida in the unicellular yeast form would result in reduced virulence. This hypothesis is supported by the finding that non-filamentous C. albicans mutants are not virulent in a mouse infection model [48], however, this is only relevant to C. albicans. Some NAC species (which do not form true hyphae) are also virulent. Moreover, the strategy of inhibiting virulence factors could facilitate the emergence of resistant strains because it only inhibits the growth of virulent strains but does not kill them. Complete elimination of pathogen is clearly a safer option; therefore, fungicidal drugs are preferred.
For all of the above reasons, we set out to identify new antifungal compounds with both fungicidal and anti-virulence properties. To this end, we first screened for Y-H inhibitors a library of 50,240 small molecules. It should be noted that the Y-H inhibitor screening assay selects for molecules that block the change from the yeast form of C. albicans to the hyphal form, as assessed by morphology. Therefore, this assay does not discriminate between molecules that inhibit the growth of C. albicans, which would also prevent the Y-H transition, and true inhibitors of the Y-H transition. Moreover, conventional antifungal agents such as fluconazole, amphotericin B and 5-fluorocytosine, whose mechanisms are not directly related to Y-H inhibition, also inhibit the formation of hyphae [49]. Nevertheless, this ambiguity of the screening assay was favourable for increasing the hit rate in our initial screening.
After several in vitro analyses of the antifungal properties of 20 small molecules identified by our initial screening, we found that one of them, SM21, is a potent Y-H inhibitor that is effective at low concentrations. Among the Y-H inhibitors identified in the study by Midkiff et al, the lowest MICY-H is 20 µM for 1×104 cells [46], which is much higher than the SM21 MICY-H (0.98 µM for 1×106 CFUs/ml). The number of Candida decreased after SM21 treatment in vitro, suggesting that this compound is fungicidal. Fungicidal drugs inhibit Y-H transition at sub-MIC, whereas fungistatic drugs inhibit Y-H transition only at much higher concentrations than their MIC [50]. It has been suggested that fungicidal drugs inhibit hyphal development and the budding process, whereas fungistatic drugs inhibit only the budding process [50].
The in vitro antifungal activity of SM21 is comparable to that of commonly used antifungal drugs such as amphotericin B (a polyene). In disk diffusion assays, SM21 forms clear inhibition zones that resemble those produced by polyenes and echinocandins, and unlike the partial inhibition zones formed by azoles, for which trailing endpoint is observed [51]. The MIC of SM21 in broth microdilution assays for a wide range of C. albicans strains (0.2 – 1.6 µg/ml) is similar to the MIC of amphotericin B, which has been considered to be a gold standard of antifungal agents [52]. Moreover, the activity of SM21 against C. albicans biofilms grown on microtitre plates is similar or more potent than that of amphotericin B or caspofungin (Table 3). The MICbiofilm of SM21 increases as the biofilm matures, a common phenomenon among antifungal agents [53].
Attachment to mucosal tissue and biofilm formation are the basis of mucosal candidiases such as oral candidiasis and Candida-associated denture stomatitis [4], [9]. SM21 is effective at reducing candidal attachment to denture acrylic surfaces and inhibiting biofilm development in an in vitro model of denture stomatitis. These promising data indicate that SM21 might have potential as a denture disinfectant or as the active ingredient in an oral drug delivery system.
We also analysed the in vivo effects of SM21 treatment on mouse models of oral and systemic candidiasis. We showed that oral rinses containing 200 µg/ml SM21 significantly reduced tongue lesions in the oral candidiasis mouse model. Moreover, the efficacy of SM21 was better than that of nystatin, which is the most commonly used antifungal for oral candidiasis [4]. In particular, oral thrush was observed at the back of the tongues in nystatin-treated mice (indicating oropharyngeal candidiasis) but not in SM21-treated animals. However, very little is known about the pharmacokinetics and pharmacodynamics of nystatin in murine models of oral candidiasis, and therefore the concentration used in our experiments (10 µg/ml) might not be high enough to produce an optimal outcome. In addition, the duration of an oral rinse cannot be controlled in a mouse model.
We evaluated SM21 toxicity against human cell lines in vitro. In contrast to bacteria, the cells of fungi and humans are eukaryotic and, therefore, very similar. This similarity is a major hurdle in antifungal development, which requires drug targets to be fungus-specific to avoid undesirable toxicity [54]. We found that the CC50 of SM21 for HOKs is 7.8 µM (3.4 µg/ml) in a 96-well plate assay. Although this value is lower than the minimum CC50 (16 µM) reported for 14 antifungal compounds that were identified by LaFleur et al (tested against human fibroblasts in 96-well plates; [45]), the CC50 of SM21 is still higher than its MIC (0.2 µg/ml), which indicates that there is a range of concentrations at which SM21 could be used as an antifungal agent without causing significant toxicity to human cells. Indeed, no detrimental effects were observed in mice that had been injected with SM21 doses as high as 10 mg/kg twice daily. In addition, SM21 does not seem to have antibacterial properties, indicating that its target is present in fungi but not in bacteria.
We demonstrated that intraperitoneal administration of SM21 is an effective treatment for systemic candidiasis in a mouse model. An SM21 dose of 0.1 mg/kg or higher twice daily beginning 3 h post-infection significantly reduced fungal burden in non-neutropenic mice. The in vivo efficacy of SM21 is similar to that of conventional antifungal agents in comparable non-neutropenic mouse models of systemic C. albicans candidiasis. For example, the intraperitoneal ED50 (50% effective dose) of fluconazole is 4.56 mg/kg when administered to the mice as a single dose 5 h after infection; the animals were sacrificed 24 h later [55]. Intraperitoneal amphotericin B (1 mg/kg dose daily) decreases fungal burden in mouse kidneys from day 1 to day 3 after infection (with treatment beginning 24 h after infection) [56]. In addition, intravenous micafungin treatment (0.125 mg/kg or higher daily dose, starting 1 h after infection for 4 days) prolongs survival of infected mice [57]. However, it is difficult to compare the effective doses from different studies because there is no standard protocol, and differences in the Candida strain, inoculum size, mouse strain, mouse immune status and the start and interval of antifungal therapy could interfere with the outcome. In addition, there are differences in the in vivo drug efficacy among normal and neutropenic murine models, which may be due to the possible interactions between the drug and host defence mechanisms [56]. Detailed pharmacodynamics-pharmacokinetics studies would need to be performed in the future to determine the time/concentration relationship of the drug at the infection sites and, thus, to generate the dosing regimens that produce the best treatment outcome in vivo [58].
SM21 is effective against antifungal-resistant clinical isolates, indicating that its mechanism of action is distinct from those of conventional antifungals. Our preliminary results described here indicated that SM21 could be targeting a component of the fungal cell membrane. Nevertheless, extensive assays are needed to elucidate the mechanism of action of this new drug.
In conclusion, in the present study we have characterised novel antifungal small molecule, SM21 using a comprehensive set of in vitro and in vivo assays. Our findings support SM21 as a promising compound for development of novel antifungal agent for treating local and systemic candidiasis, which could bring enormous benefit to the patients suffering from this recalcitrant fungal pathogen.
Supporting Information
Antifungal susceptibility test (disk diffusion assay) of SM21 against C. albicans SC5314 and C. albicans ATCC 90028. 21 – SM21, A – amphotericin B, F – fluconazole, K – ketoconazole. Growth inhibition zones were observed for SM21 against the two C. albicans strains tested. The growth inhibition zones produced by SM21 were clear, similar to those produced by amphotericin B, but dissimilar to those produced by fluconazole. This phenomenon suggested that SM21 is, like amphotericin B, fungicidal in nature.
(TIF)
Acknowledgments
We acknowledge the editorial assistance by Nature Publishing Group (NPG) accredited MSC editorial service.
Funding Statement
This study was funded by Health and Medical Research Fund, HKU Research Output Prize and HKU Small project funding to CJS and Tah-Wah Endowed Professorship fund to LPS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, et al. (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 39: 309–317. [DOI] [PubMed] [Google Scholar]
- 2.Odds FC (1988) Candida and candidosis: a review and bibliography. Bailliere Tindall.
- 3. Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW (1998) Candida-associated denture stomatitis. Aetiology and management: a review. Part 2. Oral diseases caused by Candida species. Australian dental journal 43: 160–166. [DOI] [PubMed] [Google Scholar]
- 4. Akpan A, Morgan R (2002) Oral candidiasis. Postgraduate medical journal 78: 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Douglas LJ (2003) Candida biofilms and their role in infection. Trends in microbiology 11: 30–36. [DOI] [PubMed] [Google Scholar]
- 6. Gendreau L, Loewy ZG (2011) Epidemiology and etiology of denture stomatitis. Journal of Prosthodontics 20: 251–260. [DOI] [PubMed] [Google Scholar]
- 7. Calderone RA, Fonzi WA (2001) Virulence factors of Candida albicans . Trends in microbiology 9: 327–335. [DOI] [PubMed] [Google Scholar]
- 8. Sudbery PE (2011) Growth of Candida albicans hyphae. Nature reviews Microbiology 9: 737–748. [DOI] [PubMed] [Google Scholar]
- 9. Ramage G, Tomsett K, Wickes BL, Lopez-Ribot JL, Redding SW (2004) Denture stomatitis: a role for Candida biofilms. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics 98: 53–59. [DOI] [PubMed] [Google Scholar]
- 10. Seneviratne CJ, Jin L, Samaranayake LP (2008) Biofilm lifestyle of Candida: a mini review. Oral diseases 14: 582–590. [DOI] [PubMed] [Google Scholar]
- 11. Eggimann P, Garbino J, Pittet D (2003) Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. The Lancet infectious diseases 3: 685–702. [DOI] [PubMed] [Google Scholar]
- 12. Ortega M, Marco F, Soriano A, Almela M, Martínez JA, et al. (2011) Candida species bloodstream infection: epidemiology and outcome in a single institution from 1991 to 2008. Journal of Hospital Infection 77: 157–161. [DOI] [PubMed] [Google Scholar]
- 13. Pfaller MA, Diekema DJ (2007) Epidemiology of invasive candidiasis: a persistent public health problem. Clinical microbiology reviews 20: 133–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sardi JC, Scorzoni L, Bernardi T, Fusco-Almeida AM, Mendes Giannini MJ (2013) Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. Journal of medical microbiology 62: 10–24. [DOI] [PubMed] [Google Scholar]
- 15. Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M (2011) Candida bloodstream infections: comparison of species distributions and antifungal resistance patterns in community-onset and nosocomial isolates in the SENTRY Antimicrobial Surveillance Program, 2008-2009. Antimicrobial agents and chemotherapy 55: 561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silva S, Negri M, Henriques M, Oliveira R, Williams DW, et al. (2012) Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS microbiology reviews 36: 288–305. [DOI] [PubMed] [Google Scholar]
- 17. Ostrosky-Zeichner L, Casadevall A, Galgiani JN, Odds FC, Rex JH (2010) An insight into the antifungal pipeline: selected new molecules and beyond. Nature reviews Drug discovery 9: 719–727. [DOI] [PubMed] [Google Scholar]
- 18. Pfaller MA (2012) Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. The American journal of medicine 125: S3–13. [DOI] [PubMed] [Google Scholar]
- 19. Sanglard D, Coste A, Ferrari S (2009) Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS yeast research 9: 1029–1050. [DOI] [PubMed] [Google Scholar]
- 20. Chandrasekar P (2011) Management of invasive fungal infections: a role for polyenes. Journal of Antimicrobial Chemotherapy 66: 457–465. [DOI] [PubMed] [Google Scholar]
- 21. Siikala E, Rautemaa R, Richardson M, Saxen H, Bowyer P, et al. (2010) Persistent Candida albicans colonization and molecular mechanisms of azole resistance in autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy (APECED) patients. Journal of Antimicrobial Chemotherapy 65: 2505–2513. [DOI] [PubMed] [Google Scholar]
- 22. Kanafani ZA, Perfect JR (2008) Antimicrobial resistance: resistance to antifungal agents: mechanisms and clinical impact. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 46: 120–128. [DOI] [PubMed] [Google Scholar]
- 23. Ben-Ami R, Garcia-Effron G, Lewis RE, Gamarra S, Leventakos K, et al. (2011) Fitness and virulence costs of Candida albicans FKS1 hot spot mutations associated with echinocandin resistance. Journal of Infectious Diseases 204: 626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clancy CJ, Nguyen MH (2011) At what cost echinocandin resistance? Journal of Infectious Diseases 204: 499–501. [DOI] [PubMed] [Google Scholar]
- 25. Seneviratne CJ, Wong SS, Yuen KY, Meurman JH, Parnanen P, et al. (2011) Antifungal susceptibility and virulence attributes of bloodstream isolates of Candida from Hong Kong and Finland. Mycopathologia 172: 389–395. [DOI] [PubMed] [Google Scholar]
- 26. Hakki M, Staab JF, Marr KA (2006) Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrobial agents and chemotherapy 50: 2522–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Persidis A (1999) Antibacterial and antifungal drug discovery. Nature biotechnology 17: 1141–1142. [DOI] [PubMed] [Google Scholar]
- 28. Kao RY, Tsui WHW, Lee TSW, Tanner JA, Watt RM, et al. (2004) Identification of novel small-molecule inhibitors of severe acute respiratory syndrome-associated coronavirus by chemical genetics. Chemistry & biology 11: 1293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toenjes KA, Munsee SM, Ibrahim AS, Jeffrey R, Edwards JE Jr, et al. (2005) Small-molecule inhibitors of the budded-to-hyphal-form transition in the pathogenic yeast Candida albicans . Antimicrobial agents and chemotherapy 49: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee KL, Buckley HR, Campbell CC (1975) An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans . Sabouraudia 13: 148–153. [DOI] [PubMed] [Google Scholar]
- 31.John H. Rex MAG (2008) Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved standard—third edition. Clinical and Laboratory Standards Institute document M27-A3 28.
- 32. Jin Y, Yip HK, Samaranayake YH, Yau JY, Samaranayake LP (2003) Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. Journal of clinical microbiology 41: 2961–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. da Silva WJ, Seneviratne J, Parahitiyawa N, Rosa EA, Samaranayake LP, et al. (2008) Improvement of XTT assay performance for studies involving Candida albicans biofilms. Brazilian dental journal 19: 364–369. [DOI] [PubMed] [Google Scholar]
- 34. Ellepola AN, Samaranayake LP (1998) Adhesion of oral Candida albicans isolates to denture acrylic following limited exposure to antifungal agents. Arch Oral Biol 43: 999–1007. [DOI] [PubMed] [Google Scholar]
- 35. Chandra J, Mukherjee PK, Leidich SD, Faddoul FF, Hoyer LL, et al. (2001) Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J Dent Res 80: 903–908. [DOI] [PubMed] [Google Scholar]
- 36. Jin Y, Samaranayake LP, Samaranayake Y, Yip HK (2004) Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch Oral Biol 49: 789–798. [DOI] [PubMed] [Google Scholar]
- 37. Jarosz LM, Krom BP (2011) Rapid screening method for compounds that affect the growth and germination of Candida albicans, using a real-time PCR thermocycler. Applied and environmental microbiology 77: 8193–8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miyazaki M, Horii T, Hata K, Watanabe NA, Nakamoto K, et al. (2011) In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrobial agents and chemotherapy 55: 4652–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lionakis MS, Lim JK, Lee CC, Murphy PM (2011) Organ-specific innate immune responses in a mouse model of invasive candidiasis. Journal of innate immunity 3: 180–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takakura N, Sato Y, Ishibashi H, Oshima H, Uchida K, et al. (2003) A novel murine model of oral candidiasis with local symptoms characteristic of oral thrush. Microbiology and immunology 47: 321–326. [DOI] [PubMed] [Google Scholar]
- 41. Kamagata-Kiyoura Y, Abe S, Yamaguchi H, Nitta T (2004) Protective effects of human saliva on experimental murine oral candidiasis. Journal of infection and chemotherapy: official journal of the Japan Society of Chemotherapy 10: 253–255. [DOI] [PubMed] [Google Scholar]
- 42. Ramani R, Ramani A, Wong SJ (1997) Rapid flow cytometric susceptibility testing of Candida albicans . Journal of clinical microbiology 35: 2320–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ali I, Khan FG, Suri KA, Gupta BD, Satti NK, et al. (2010) In vitro antifungal activity of hydroxychavicol isolated from Piper betle L. Annals of clinical microbiology and antimicrobials 9: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Toenjes KA, Stark BC, Brooks KM, Johnson DI (2009) Inhibitors of cellular signalling are cytotoxic or block the budded-to-hyphal transition in the pathogenic yeast Candida albicans . Journal of medical microbiology 58: 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. LaFleur MD, Lucumi E, Napper AD, Diamond SL, Lewis K (2011) Novel high-throughput screen against Candida albicans identifies antifungal potentiators and agents effective against biofilms. J Antimicrob Chemother 66: 820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Midkiff J, Borochoff-Porte N, White D, Johnson DI (2011) Small molecule inhibitors of the Candida albicans budded-to-hyphal transition act through multiple signaling pathways. PloS one 6: e25395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grald A, Yargosz P, Case S, Shea K, Johnson DI (2012) Small-molecule inhibitors of biofilm formation in laboratory and clinical isolates of Candida albicans . Journal of medical microbiology 61: 109–114. [DOI] [PubMed] [Google Scholar]
- 48. Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, et al. (1997) Nonfilamentous C. albicans mutants are avirulent. Cell 90: 939–949. [DOI] [PubMed] [Google Scholar]
- 49. Odds FC, Cockayne A, Hayward J, Abbott AB (1985) Effects of imidazole- and triazole-derivative antifungal compounds on the growth and morphological development of Candida albicans hyphae. J Gen Microbiol 131: 2581–2589. [DOI] [PubMed] [Google Scholar]
- 50. Hawser S, Islam K (1999) Comparisons of the effects of fungicidal and fungistatic antifungal agents on the morphogenetic transformation of Candida albicans . J Antimicrob Chemother 43: 411–413. [DOI] [PubMed] [Google Scholar]
- 51. Rex JH, Pfaller MA, Rinaldi MG, Polak A, Galgiani JN (1993) Antifungal susceptibility testing. Clinical microbiology reviews 6: 367–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ostrosky-Zeichner L, Marr KA, Rex JH, Cohen SH (2003) Amphotericin B: time for a new “gold standard”. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 37: 415–425. [DOI] [PubMed] [Google Scholar]
- 53. Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, et al. (2001) Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. Journal of bacteriology 183: 5385–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Krcmery V, Kalavsky E (2007) Antifungal drug discovery, six new molecules patented after 10 years of feast: why do we need new patented drugs apart from new strategies? Recent patents on anti-infective drug discovery 2: 182–187. [DOI] [PubMed] [Google Scholar]
- 55. Louie A, Drusano GL, Banerjee P, Liu QF, Liu W, et al. (1998) Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrobial agents and chemotherapy 42: 1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van't Wout JW, Mattie H, van Furth R (1989) Comparison of the efficacies of amphotericin B, fluconazole, and itraconazole against a systemic Candida albicans infection in normal and neutropenic mice. Antimicrobial agents and chemotherapy 33: 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ikeda F, Wakai Y, Matsumoto S, Maki K, Watabe E, et al. (2000) Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrobial agents and chemotherapy 44: 614–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Andes D, Craig WA (2002) Animal model pharmacokinetics and pharmacodynamics: a critical review. International journal of antimicrobial agents 19: 261–268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antifungal susceptibility test (disk diffusion assay) of SM21 against C. albicans SC5314 and C. albicans ATCC 90028. 21 – SM21, A – amphotericin B, F – fluconazole, K – ketoconazole. Growth inhibition zones were observed for SM21 against the two C. albicans strains tested. The growth inhibition zones produced by SM21 were clear, similar to those produced by amphotericin B, but dissimilar to those produced by fluconazole. This phenomenon suggested that SM21 is, like amphotericin B, fungicidal in nature.
(TIF)