Abstract
The prevention of cyto- and genotoxicity of nanocarriers is an important task in nanomedicine. In the present investigation, we, at the first time using similar experimental conditions, compared genotoxicity of nanocarriers with different composition, architecture, size, molecular weight and charge. Poly(ethylene glycol) polymers, neutral and cationic liposomes, micelles, poly(amindo amine) and poly(propyleneimine) dendrimers, quantum dots, mesoporous silica, and supermagnetic iron oxide (SPIO) nanoparticles were studied. All nanoparticles were used in non-cytotoxic concentrations. However, even in these concentrations, positively charged cationic liposomes, dendrimers, and SPIO nanoparticles induced genotoxicity leading to the significant formation of micronuclei in cells. Negatively charged and neutral nanocarriers were not genotoxic. A strong positive correlation was found between the number of formed micronuclei and the positive charge of nanocarriers. We proposed modifications of both types of dendrimers and SPIO nanoparticles that substantially decreased their genotoxicity and allowed for an efficient intracellular delivery of nucleic acids.
Keywords: Nanotoxicology, nanogenotoxicology, formation of micronuclei, nanoparticles, siRNA delivery
Background
Different nanocarriers are currently being used for the delivery of various drugs and nucleic acids. The advantages of nanocarriers as drug vehicles include but are not limited to improving solubility and stability of the delivered agents as well as enhancing their uptake by targeted cells. A rapid expansion of nanotechnology raises several environmental, health, and safety issues that should be understood, investigated, and regulated [1, 2]. The study of possible threats of nanocarriers led to the emergence of two novel branches of bionanoscience: nanotoxicology and most recently nanogenotoxicology[3–6]. Nanotoxicology studies mechanisms of cytotoxicity of nanomaterials while nanogenotoxicology is focused on analyzing the potential of engineered nanomaterials on damaging DNA. While cytotoxicity of nanocarriers is relatively widely analyzed for different nanomaterials, genotoxicity of nanocarriers were investigated only in limited number of recent publications [3–5, 7–15]. Meanwhile, genotoxicity of drug carriers should be avoided especially for prolonged chronic treatment. If nanocarriers are used for the delivery of cytotoxic drugs (e.g. for chemotherapy of cancer) and are delivered specifically for the targeted site of action (e.g. tumor), then their own cyto- and genotoxicity is less important when compared with other factors (delivery efficiency, profile of drug release, cost, etc.). However, when the carriers are used for the delivery of non-cytotoxic agents and may be accumulated in healthy tissues, then it is critically important to prevent cytotoxicity of nanocarriers and their adverse genetic effects.
In the present study, we selected one representative nanocarrier from the most widely used types of drugs and nucleic acid delivery vehicles and analyzed nanoparticles with different composition, size, molecular weight, and electrical charge: (1) supermagnetic iron oxide (SPIO) nanoparticles; (2) – 2, 10 and 20 kDa poly(ethylene glycol) (PEG) polymer; (3) – quantum dots (QD); (4) – poly(propyleneimine) (PPI) dendrimers and poly(amido amine) (PAMAM) dendrimers; (5) – polymeric micelles; (6) – “neutral” liposomes (120 nm and 600 nm); (7) – cationic liposomes; and (8) – mesoporous silica (MS) nanoparticles. The size of studied nanocarriers varied from 10 to 600 nm while surface charge values covered a region from −10 to +90 mV. Here we tried to answer two questions. First, which type of nanoparticles can cause genetic aberrations under non-cytotoxic concentrations by inducing the formation of micronuclei during cell division? Second, how one can limit genotoxicity of nanocarriers by their modifications suitable for the delivery of siRNA? Consequently, the present study was aimed at evaluating and comparing the genotoxicity of different nanocarriers and developing possible modifications of genotoxic nanoparticles in order to limit their adverse effects on DNA and to use for intracellular delivery of siRNA.
Methods
Chinese Hamster Ovary (CHO-K1) cells were employed in all in vitro experiments. The cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA 20108) and cultured in F-12K medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and penicillin-streptomycin (100 UI/ml-100ug/ml, Sigma, St. Louis, Mo). Poly(ethylene glycol) (PEG) polymer, neutral and cationic liposomes, polymeric micelles, poly(amido amine) (PAMAM) and poly(propyleneimine) (PPI) dendrimers, quantum dots (QD), mesoporous silica (MS) and supermagnetic iron oxide (SPIO) nanoparticles were investigated. PEG polymers (2, 10 and 20 kDa) were purchased from Rapp Polymere GmbH (Tubingen, Germany). Liposomes were prepared as previously described [8, 16]. Briefly, neutral PEGylated liposomes were formulated from egg phosphatidylcholin: cholesterol:1,2,-distearoyl-sn-glycero-3-phosphoethanolamine-N-aminopolyethelenglycol (DSPE-PEG) in mole ratio 51:44:5, respectively, using the ethanol injection method with final concentration 10 mM. Cationic liposomes were prepared from positively charged dioleoyl-2-trimethylammonium propane (DOTAP, Avanti Polar Lipids, Alabaster, AL) in concentration 10 mM. DSPE-PEG 2000 micelles were prepared as previously described [17]. Briefly, DSPE-PEG powder was dissolved in tert-butanol, lyophilized overnight followed by rehydration in 0.9% NaCl to a final concentration above the lipopolymer critical micelle concentration (10 mM). PAMAM generation 4.0 (ethylenediamine core) and PPI tetrahexacontaamine generation 5 dendrimers were obtained from Sigma-Aldrich (Milwaukee, WI). Amine terminated quantum dots were prepared as previously described [18]. Mobile Crystaline Material-41 (MCM-41) type MS nanoparticles were synthesized using a surfactant-templated, base-catalyzed condensation method as previously reported [19]. SPIO nanoparticles were synthesized as previously described [20]. Briefly, iron oxide nanocrystals of 5 nm in diameter were synthesized in organic solvents at high temperature. For solubilization of iron oxide nanoparticles in water, micelles were formed with amphiphilic polymers by transferring iron oxide nanocrystals from organic solvents into water. Carboxyl terminated Quantum Dots (QD-COOH) with an emission peak at 490 nm were purchased from eBioscience, Inc. (San Diego, CA). The structure of these functionalized eFluornanocrystals consists of a core particle that is composed of cadmium selenide (CdSe) surrounded by a zinc sulfide (ZnS) shell. Quantum dots have a lipid based coating containing PEG molecules attached that enables water solubility, and carboxyl groups available for conjugation. These quantum dots were previously evaluated and used in our lab as imaging agents and drug carriers [18]. The samples of nanocarriers were imaged with a tapping mode atomic force microscope (Nanoscope III A, Veeco Digital Instruments, Chadds Ford, PA) as previously described [21]. The height differences on the surface are indicated by the color code: lighter regions indicate higher heights. Complexes of siRNA with cationic liposomes, mesoporous silica nanoparticles, PAMAM and PPI dendrimers and SPIO nanoparticles were performed in our laboratory as previously described [16, 19–24].
Cytotoxicity of all synthesized carriers was analyzed using a modified 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) (MTT) assay as previously described [25]. Particle size was measured by dynamic light scattering using 90 Plus Particle Sizer Analyzer (Brookhaven Instruments Corp., New York, NY). To characterize a surface charge of nanoparticles, zeta potential was measured on PALS Zeta Potential Analyzer (Brookhaven Instruments Corp., Holtsville, NY).
Genotoxicity of the studied carriers were evaluated using the in vitro micronucleus assay on CHO-K1 cells as previously described [26]. Briefly, about 300,000 cells were cultured with the media in 25 cm2 flasks and held 24 hours before treatment. They were then incubated with tested nanocarriers for 24 h. Negative control cells were incubated with fresh media, while positive control cells were treated with ethyl methanesulfonate(400 μg/ml). After incubation, the cells were fixed in a cold solution of 100 % methanol. The methanol was removed and the cells were washed with phosphate buffer and the cells’ nuclei were then stained with 600 nM of 4, 6 diamidino-2-phenylindole (DAPI) for 8 minutes. This solution was removed and all the flasks were washed with PBS containing 0.05 % Tween 20 (Sigma Aldrich, St Louis, MO). After staining, the formation of micronuclei was detected by a fluorescent microscope (Olympus, New York, NY) and documented by counting the number of micronuclei per 1000 cells.
Cellular internalization of 6-FAM labeled siRNA-nanoparticle complexes was analyzed by laser scanning spectral confocal (Leica Microsystems Inc., Bannockburn, IL) microscopes. Prior the visualization cells were plated (20,000 cells/well) in 6-well tissue culture plates and treated with analyzed drug carrier-siRNA complexes for 24 h. The concentration of siRNA was 0.25 μM. After 24 h of treatment, cells were washed three times with DPBS, 1 mL of fresh medium was added to each well and photographed by a confocal microscope.
Results
Cytotoxicity of all studied nanocarriers was studied. It was found that QD, PEG polymers (2, 10 and 20 kDa) and “neutral” liposomes (120 and 600 nm) were not toxic at all available concentrations. PPI dendrimers at highest available concentrations demonstrated low cytotoxicity (cellular viability was 30% lower when compared with the control level). In contrast, MS nanoparticles, PAMAM dendrimers and SPIO nanoparticles showed substantial cytotoxicity when their concentrations exceeded 0.1 mg/mL, 0.8 mg/mL and 80 μg/mL, respectively. Based on these measurements, maximal non-toxic concentrations of each nanocarrier were selected for the further studies. These working concentrations were equal to: 0.078 mg/mL (PEG Polymers), 2.50 mM (“neutral” liposomes), 0.117 mM (cationic liposomes), 0.0195 mM (polymeric micelles), 0.391 μg/mL (PAMAM dendrimer), 0.0978 μM (PPI dendrimer), 7.03 nM (QD), 3.91 μg/mL (MS nanoparticles), and 1.526 μg/mL (SPIO nanoparticles).
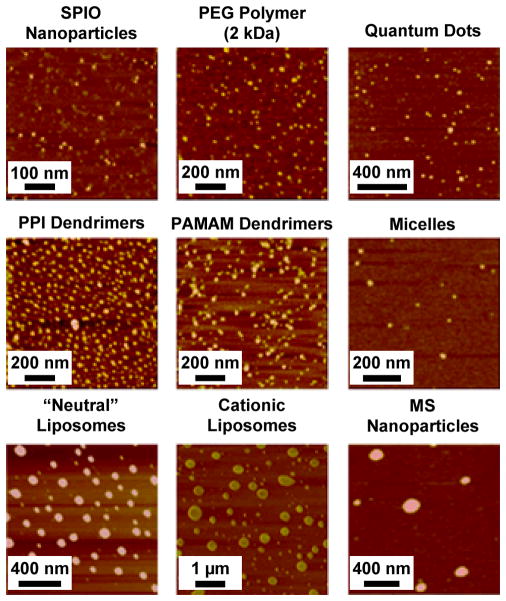
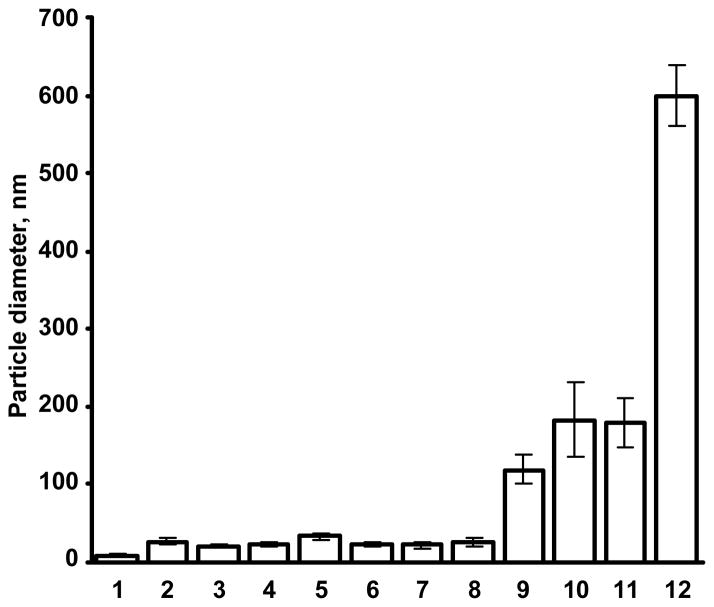
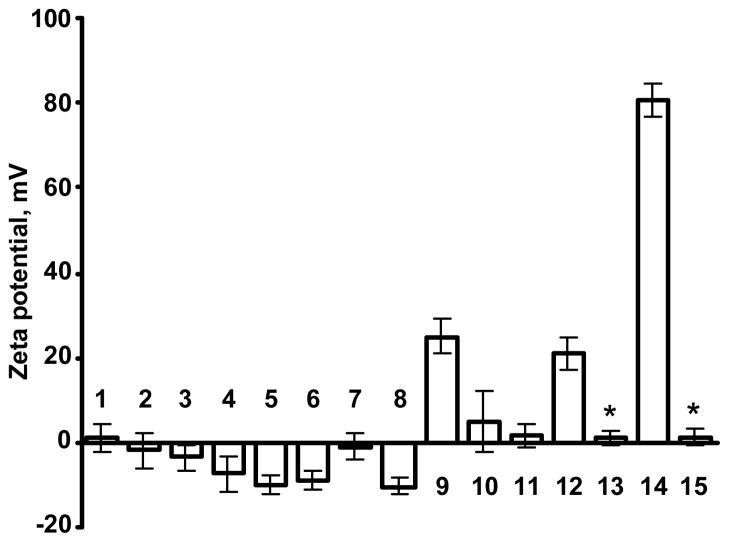
All used carriers were characterized by atomic force microscope imaging and measurements of their size and surface charge. Atomic force microscope analysis showed that all studied vehicles formed well-defined nanoparticle-like structures (Fig. 1). These results allowed us to designate all studied carriers as true compact “particles.” The measurements of particle size showed that the size of the particles varied from 10 to 600 nm (Fig. 2). Therefore, carriers employed covered almost the entire range of carrier sizes designated for nanocarriers. Consequently, carriers used can be referred as “nanoparticles”. Zeta potential of all nanocarriers is presented in Fig. 3. It ranged from approximately −10 mV for micelles and “neutral” liposomes to more than + 90 mV for SPIO nanoparticles. Therefore, these parameters of the selected nanocarriers covered almost entire region of nanocarrier charges that are currently used in nanomedicine for intracellular delivery of drugs, siRNA and other bioactive materials or are employed for bioimaging.
Figure 1.
Representative atomic force microscope (AFM) images of different nanocarriers: supermagnetic iron oxide (SPIO) nanoparticles; poly(ethylene glycol) (PEG); quantum dots (QD); poly(propyleneimine) (PPI) dendrimers; poly(amido amine) (PAMAM) dendrimers; polymeric micelles; “neutral” liposomes; cationic liposomes and mesoporous silica (MS) nanoparticles.
Figure 2.
Average size of different nanocarriers: 1 – supermagnetic iron oxide (SPIO) nanoparticles; 2 – 2 kDa poly(ethylene glycol) (PEG) polymer; 3 – 10 kDa PEG polymer; 4 – 20 kDa PEG polymer; 5 – quantum dots (QD); 6 – poly(propyleneimine) (PPI) dendrimers; 7 – poly(amido amine) (PAMAM) dendrimers; 8 – polymeric micelles; 9 – “neutral” liposomes (120 nm); 10 – cationic liposomes; 11 – mesoporous silica (MS) nanoparticles; and 12–“neutral” liposomes (600 nm). Means ± SD are shown.
Figure 3.
Zeta potential of different nanocarriers: 1 – 2 kDa poly(ethylene glycol) (PEG) polymer; 2 – 10 kDa PEG polymer; 3 – 20 kDa PEG polymer; 4 – quantum dots (QD); 5 – “neutral” liposomes (120 nm); 6 – “neutral” liposomes (600 nm);7 – mesoporous silica (MS) nanoparticles; 8 – polymeric micelles; 9 – cationic liposomes; 10 – poly(amido amine) (PAMAM) dendrimers; 11 – modified PAMAM dendrimers; 12 – poly(propyleneimine) (PPI) dendrimers; 13 – modified PPI dendrimers; 14 – supermagnetic iron oxide (SPIO) nanoparticles; and 15 – modified SPIO nanoparticles. Means ± SD are shown. *P< 0.05 when compared with corresponding non-modified nanocarrier.
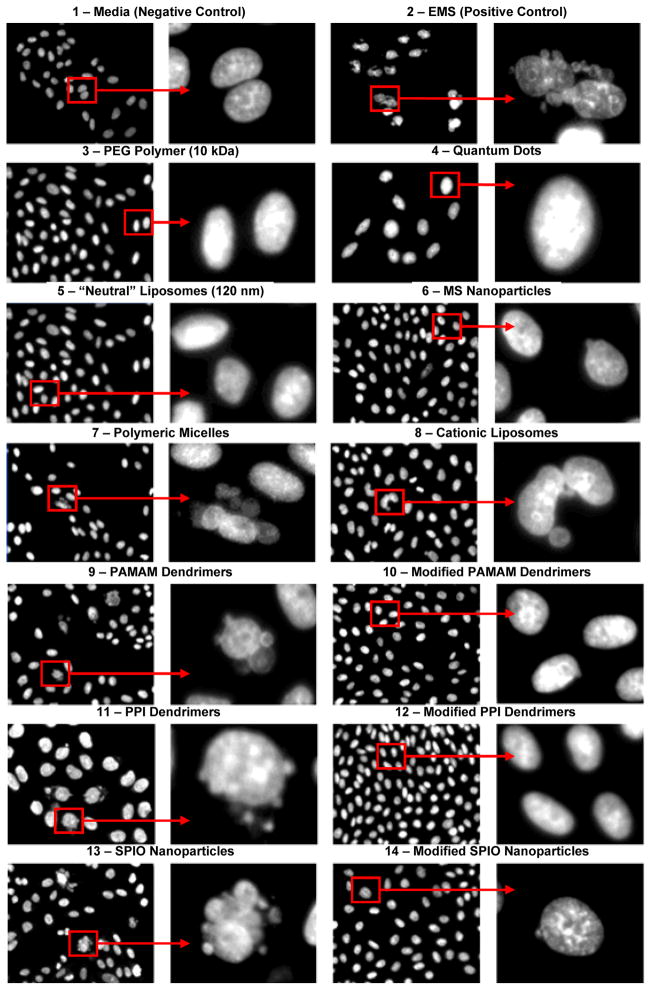
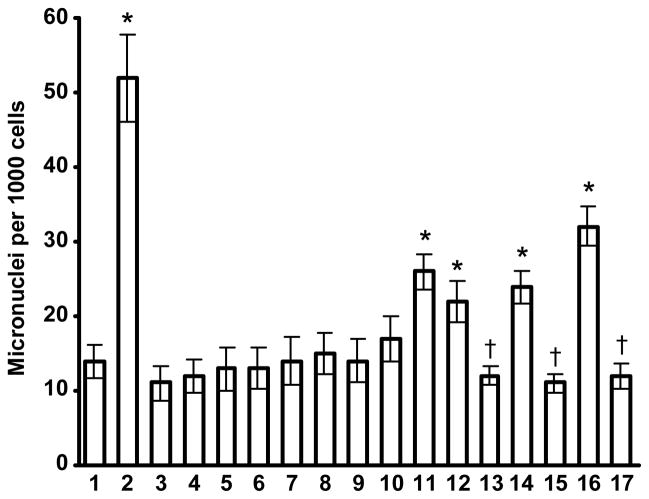
Representative images of stained cells incubated with different nanocarriers and corresponding negative and positive controls are shown in Fig. 4. Quantitative analysis of genotoxicity is presented in Fig. 5. It was found that PEG polymer, QD, “neutral” liposomes, MS nanoparticles, and micelles in non-cytotoxic concentrations did not induce measurable genotoxic effects in terms of formation of micronuclei (please see Fig. 4, images 3–7 and Fig. 5, bars 3–10). In contrast, cationic liposomes, PAMAM and PPI dendrimers, and SPIO nanoparticles significantly increased the formation of micronuclei in tested cells (please see Fig. 4, images 8, 9, 11, 13 and Fig. 5, bars 11, 12, 14, 16).
Figure 4.
Genotoxicity (formation of micronuclei) of different nanocarriers and corresponding controls. Representative fluorescence microscopy images of CHO-K1 cells incubated within 24 hours with different substances: 1 – media (negative control); 2 – ethyl methanesulfonate (EMS, positive control); 3 – 10 kDa poly(ethylene glycol) (PEG) polymer; 4 – quantum dots (QD); 5 – neutral liposomes (120 nm); 6 – mesoporous silica (MS) nanoparticles; 7 – polymeric micelles; 8 – cationic liposomes; 9 – poly(amido amine) (PAMAM) dendrimers; 10 – modified PAMAM dendrimers; 11 – poly(propyleneimine) (PPI) dendrimers; 12 – Modified PPI dendrimers; 13 – supermagnetic iron oxide (SPIO) nanoparticles; and 14 – modified SPIO nanoparticle. The cells were stained with DAPI nuclear dye. For each substance, images on the right panel show magnified cells marked by the square on the left panel.
Figure 5.
Genotoxicity of different nanocarriers: 1 – media (negative control); 2 – ethyl methanesulfonate (EMS, positive control); 3 – 2 kDa poly(ethylene glycol) (PEG) polymer; 4 – 10 kDa PEG polymer; 5 – 20 kDa PEG polymer; 6 – quantum dots (QD); 7 – “neutral” liposomes (120 nm); 8 – “neutral” liposomes (600 nm);9 – mesoporous silica (MS) nanoparticles; 10 – polymeric micelles; 11 – cationic liposomes; 12 – poly(amido amine) (PAMAM) dendrimers; 13 – modified PAMAM dendrimers; 14 – poly(propyleneimine) (PPI) dendrimers; 15 – modified PPI dendrimers; 16 – supermagnetic iron oxide (SPIO) nanoparticles; and 17 – modified SPIO nanoparticles. Means ± SD are shown. *P< 0.05 when compared with media (negative control). †P< 0.05 when compared with a corresponding non-modified carrier.
In order to decrease genotoxicity of nanocarriers, we performed the following modifications. PAMAM dendrimers were internally quaternized and surface acetylated as previously described [22]. The modified dendrimers also did not demonstrate any signs of cytotoxicity for all available concentrations. Analysis of genotoxicity showed that the modification of PAMAM dendrimers prevented not only cytotoxic effects but also limited the formation of micronuclei (please compare images 9 and 10 in Fig. 4 and bars 12 and 13 in Fig. 5). For the purpose of decreasing genotoxicity of PPI dendrimers and use them for the delivery of nucleic acids, PPI dendrimers were condensed with siRNA and the formed siRNA nanoparticles were caged with a dithiol containing cross-linker molecules followed by coating them with PEG polymer as described [32]. As can be seen from the present experimental data, the modification substantially limited genotoxicity of the carriers. In fact, such modification almost completely prevented the formation of micronuclei during the incubation with cells (please compare images 11 and 12 in Fig. 4 and bars 14 and 15 in Fig. 5). To decrease the toxicity of SPIO nanoparticles and make them suitable for siRNA delivery, the following approach was used. Complexes were formed by cooperative condensation of siRNA with 5 nm SPIO nanoparticles and PPI generation 5 dendrimers, then the resulted complexes were coated with PEG polymer as previously described [20]. Experimental data obtained show that the modification also significantly limited genotoxicity of nanoparticles preventing the formation of micronuclei in the cells (please compare images 13 and 14 in Fig. 4 and bars 16 and 17 in Fig. 5).
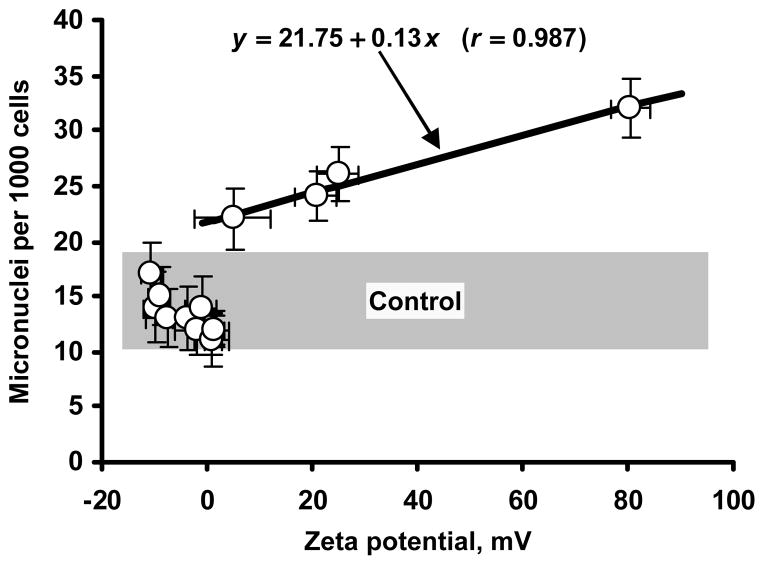
To reveal an influence of surface charge on genotoxicity of nanoparticles, we analyzed the correlation between zeta potential of nanocarriers and their genotoxicity (Fig. 6). It was found that negatively charged and neutral nanocarriers did not induce the formation of additional micronuclei when compared with spontaneous micronucleus formation in control (cells incubated with media). A strong positive linear correlation (r = 0.987) was found between the value of zeta potential and genotoxicity of positively charged nanocarriers.
Figure 6.
Correlation between the zeta potential of nanocarriers and their genotoxicity. Shaded area represents the control range of micronuclei formation. Means ± SD are shown.
To analyze the relationship between the cellular internalization of siRNA and genotoxicity of nanocarriers, we studied the internalization of fluorescently labeled siRNA delivered by nanocarriers with different genotoxicity: mesoporous silica nanoparticles, cationic liposomes, modified poly(amido amine) and poly(propyleneimine) dendrimers (Fig. 7). It was found that all studied nanocarrier-siRNA complexes successfully penetrated the cells and siRNA was efficiently released from the complexes into the cellular cytoplasm. We did not found substantial differences between all studied nanocarriers with different genotoxicity in the efficiency of siRNA delivery inside the cells.
Figure 7.
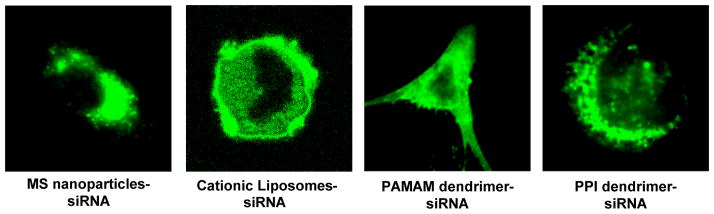
Cellular internalization of siRNA complexes with different nanocarriers. Representative confocal microscope images of cancer cells incubated within 24 h with fluorescently labeled siRNA delivered by mesoporous silica (MS) nanoparticles, cationic liposomes, modified poly(amido amine) (PAMAM) dendrimers and modified poly(propyleneimine) (PPI) dendrimers.
Discussion
Our ultimate goal was to select a single representative of each of the most frequently used classes of nanocarriers in order to cover a wide range of sizes, molecular weights, compositions, and electrical charges. Such a selection is based upon the following considerations. First, we tried to select a typical representative from each of nanocarriers that have been developed, well characterized, and widely used in various nanomedical applications. Secondly, we intended to cover a wide range of carrier composition, architecture, size, and electrical charge. Consequently, the following nanocarriers have been selected for the analysis: supermagnetic iron oxide nanoparticles, poly(ethylene glycol) polymers with different molecular weight, quantum dots, poly(propyleneimine) and poly(amido amine) dendrimers, polymeric micelles, “neutral” and cationic liposomes, and mesoporous silica nanoparticles. Such a selection allowed us to analyze the influence of architecture, molecular weight, size, and surface charge on genotoxicity of nanocarriers. It is understandable that in most cases nanocarriers are used in concentrations that do not induce significant cytotoxic effects in vitro or in doses that do not exceed the maximal tolerated doses in vivo. It is generally assumed that such concentrations are safe and will not induce severe side effects, including undesirable genotoxic effects. We also used all nanocarriers in concentrations that did not induce substantial cell death in vitro. The viability of cells incubated with working concentrations of nanoparticles employed in the present study was 90% or higher when compared with untreated cells incubated with fresh media. However, some nanocarriers demonstrated marked genotoxicity even under non-toxic concentrations. We found that PEG polymer, QD, “neutral” liposomes, MS nanoparticles, and micelles did not demonstrate signs of genotoxicity in non-cytotoxic concentrations. In contrast, cationic liposomes, PAMAM and PPI dendrimers, and SPIO nanoparticles being applied in non-cytotoxic concentrations did induce micronuclei formation in tested cells. These results are in good agreement with the literature data reporting that different dendrimers can induce not only cell death but also moderate genotoxic effects [7]. Genotoxicity of iron oxide nanoparticles has also been recently reported [13]. However, it should be stressed that in the available literature we did not find a comparison of different nanocarriers in terms of their genotoxicity analyzed in the similar conditions in one experimental study. In this sense, the present work fills the gap and allows us to select genotoxic carriers for further modifications.
PAPAM dendrimers were modified in order to eliminate surface charges and protect siRNA conjugated with dendrimers from the harsh action of the environment during its voyage in the blood stream towards the targets [21]. This modification included acetylation of surface branches and internal quaternization. The modified dendrimers have neutral surfaces and cationic charges inside the dendrimers (not on the outer surface). Our previous data showed that these modified dendrimers formed well-condensed, spherical particles (polyplexes) with siRNA, protected nucleic acids from degradation, and provided their effective cellular internalization [21]. In summary, the proposed modification of PAMAM dendrimers decreased their surface charges creating internal sites with positive charges for conjugation with negatively charged siRNA molecules. Consequently, one or several dendrimers can cover a siRNA molecule forming well-defined spherical nanocomplexes and protecting the payload from the degradation during the journey in the systemic circulation to the site of the action. We found [21] that such complexes efficiently penetrate inside the cells and release siRNA in the cytoplasm probably as a result of pH decreasing and braking the electrostatic bonds between the dendrimers and nucleic acids. In contrast, the conjugation of siRNA with externally charged dendrimers led to the formation of nanotubes that were unable to efficiently deliver siRNA inside the cells. Present experimental data also show that such a modification substantially limits the genotoxicity of these type of dendrimers.
PPI dendrimers were condensed with siRNA and the formed siRNA nanoparticles were caged with a dithiol containing cross-linker molecule followed by coating them with PEG polymer as described [24]. Previously, we found that, this layer-by-layer modification of dendrimers increased the siRNA stability in plasma and intracellular bioavailability, provided for their efficient cellular uptake, accumulation of siRNA inside the cells, and efficient gene silencing [24]. Present experimental data support this finding. In addition, it was found in the present experimental work, that this modification decreased genotoxicity of formed dendrimer-siRNA complexes.
The modification of SPIO nanoparticles included two steps. First, complexes were formed by cooperative condensation of siRNA with SPIO nanoparticles and PPI dendrimers. Secondly, the resulted complexes were coated with PEG polymer as previously described [20]. Previously, we found that such a modification decreased the cytotoxicity of the nanoparticles, protected the payload in the blood stream, enhanced the efficiency of cellular internalization of siRNA and increased the efficiency of targeted gene suppression [20]. In addition, as was shown in the present study, these modifications of SPIO nanoparticles decreased their genotoxicity. In summary, the proposed modifications of nanoparticles eliminated their positive surface charge, allowed for stable complexes with siRNA, provided for an efficient intracellular delivery of nucleic acids, and limited cytotoxicity and genotoxicity of nanocarriers.
The exact mechanisms of genotoxic effects of nanoparticles are still unknown. One can suggest that the following factors can potentially affect genotoxicity of nanocarriers: composition, size, molecular weight, particle geometry, and surface charge. In the present study, all of these parameters varied substantially in the investigated nanoparticles. Recently, it was suggested based on the results of the comparative analysis that particle composition probably played a primary role in the cytotoxic effects of different nanoparticles while the potential genotoxicity might be mostly attributed to particle shape [11]. However, based on the results obtained in the present study, it is most probable that the surface charge of nanocarriers plays a central role in genotoxic effects. Three major sets of data support this assumption. First, the degree of genotoxicity of nanocarriers does not correlate with their size or molecular weight. In fact, comparable genotoxicity was found in nanocarriers with huge differences in size (from ~20 nm in PEG polymers to ~600 nm in large “neutral” liposomes). At the same time, nanocarriers with comparable size demonstrated dramatically different genotoxicity. For instance, PEG polymers (~20 nm), QD (~40 nm), polymeric micelles (~30 nm) did not induce genotoxicity, but SPIO nanoparticles (~10 nm), PAMAM and PPI dendrimers (~20 nm) were genotoxic; larger carriers like “neutral” liposomes (~120 nm) and MS nanoparticles (~180 nm) did not lead to the formation of micronuclei, but cationic liposomes (~190 nm) were genotoxic (Fig. 2–5). The increase in molecular weight of PEG-based nanocarrier from 2 to 20 kDa also did not influence its genotoxicity. Secondly, some nanocarriers of similar size, molecular weight, and shape possessed substantially different genotoxicity. Examples from the present study include “neutral” (non-genotoxic) and cationic (highly genotoxic) liposomes. It seems that the presence of positive charge substantially increases the genotoxicity of nanoparticles. Thirdly, modifications of nanocarriers that eliminate their surface charge (e.g. PAMAM, PPI dendrimers and SPIO nanoparticles) reduced their genotoxicity. A strong positive correlation between micronuclei formation and surface charge of nanoparticles revealed in the present study support this conclusion. The results also agreed with the literature data showing that toxic effects of different nanocarriers correlates with positive charge of their surface [27–30].
Theoretically, differences in the internalization of siRNA inside the cells could potentially influence genotoxicity of complexes of siRNA with nanocarriers. To examine this probability, we studied cellular internalization and release of siRNA delivered by nanocarriers with substantially different genotoxicity. We did not found substantial differences between all studied nanocarriers with different genotoxicity in the efficiency of siRNA delivery inside the cells. Therefore, it is unlikely that genotoxicity of nanocarriers depend on efficiency of cellular internalization of the siRNA.
Additional investigations are planned in our laboratory in order to elucidate possible mechanisms of genotoxicity of nanocarriers. It should be stressed again that nanocarriers were used in the present study for the genotoxic study in non-cytotoxic concentrations that did not decrease cellular viability. However, some carriers did demonstrate substantial genotoxicity, which confirms the importance of testing genotoxicity together with cytotoxicity in order to assess the safety of nanocarriers.
Conclusions
In summary, the present experimental data clearly showed the importance of genotoxicity testing during the characterization of nanocarriers. In fact, even in non-cytotoxic concentrations, nanocarriers with positive surface charges induced formation of micronuclei after incubation with cells. Genotoxicity of nanocarriers correlated well with their surface charges. We also proposed several modifications of genotoxic dendrimers and SPIO nanoparticles in order to limit their genotoxicity. These modifications prevented the formation of micronuclei in cells incubated with nanocarriers, limited their cytotoxicity, and increased the stability and efficiency of cellular internalization of complexated siRNA, making such modified nanocarriers attractive for the delivery of nucleic acid for clinical applications.
Acknowledgments
This research was supported in part by grants from the National Cancer Institute (R01 CA100098, R01 CA138533, and R01 CA111766) and National Science Foundation (CBET-0933966).
References
- 1.Vega-Villa KR, Takemoto JK, Yanez JA, Remsberg CM, Forrest ML, Davies NM. Clinical toxicities of nanocarrier systems. Adv Drug Deliv Rev. 2008;60:929–38. doi: 10.1016/j.addr.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Warheit DB, Donner EM. Rationale of genotoxicity testing of nanomaterials: regulatory requirements and appropriateness of available OECD test guidelines. Nanotoxicology. 2010;4:409–13. doi: 10.3109/17435390.2010.485704. [DOI] [PubMed] [Google Scholar]
- 3.Ng CT, Li JJ, Bay BH, Yung LY. Current studies into the genotoxic effects of nanomaterials. J Nucleic Acids. 2010;2010:1–12. doi: 10.4061/2010/947859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norppa H, Catalan J, Falck G, Hannukainen K, Siivola K, Savolainen K. Nano-specific genotoxic effects. J Biomed Nanotechnol. 2011;7:19. doi: 10.1166/jbn.2011.1179. [DOI] [PubMed] [Google Scholar]
- 5.Singh N, Manshian B, Jenkins GJ, Griffiths SM, Williams PM, Maffeis TG, Wright CJ, Doak SH. NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials. 2009;30:3891–914. doi: 10.1016/j.biomaterials.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Stone V, Johnston H, Schins RP. Development of in vitro systems for nanotoxicology: methodological considerations. Crit Rev Toxicol. 2009;39:613–26. doi: 10.1080/10408440903120975. [DOI] [PubMed] [Google Scholar]
- 7.Choi YJ, Kang SJ, Kim YJ, Lim YB, Chung HW. Comparative studies on the genotoxicity and cytotoxicity of polymeric gene carriers polyethylenimine (PEI) and polyamidoamine (PAMAM) dendrimer in Jurkat T-cells. Drug Chem Toxicol. 2010;33:357–66. doi: 10.3109/01480540903493507. [DOI] [PubMed] [Google Scholar]
- 8.Garbuzenko OB, Saad M, Betigeri S, Zhang M, Vetcher AA, Soldatenkov VA, Reimer DC, Pozharov VP, Minko T. Intratracheal versus intravenous liposomal delivery of siRNA, antisense oligonucleotides and anticancer drug. Pharm Res. 2009;26:382–94. doi: 10.1007/s11095-008-9755-4. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Gao H, Gou M, Ye H, Liu Y, Gao Y, Peng F, Qian Z, Cen X, Zhao Y. Acute toxicity and genotoxicity studies on poly(varepsilon-caprolactone)-poly(ethylene glycol)-poly(varepsilon-caprolactone) nanomaterials. Mutat Res. 2010;696:101–6. doi: 10.1016/j.mrgentox.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Moreira S, Silva NB, Almeida-Lima J, Rocha HA, Medeiros SR, Alves C, Jr, Gama FM. BC nanofibres: in vitro study of genotoxicity and cell proliferation. Toxicol Lett. 2009;189:235–41. doi: 10.1016/j.toxlet.2009.06.849. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Liu C, Yang D, Zhang H, Xi Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol. 2009;29:69–78. doi: 10.1002/jat.1385. [DOI] [PubMed] [Google Scholar]
- 12.Yin L, Zhao X, Cui L, Ding J, He M, Tang C, Yin C. Cytotoxicity and genotoxicity of superporous hydrogel containing interpenetrating polymer networks. Food Chem Toxicol. 2009;47:1139–45. doi: 10.1016/j.fct.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 13.Zuzana M, Alessandra R, Lise F, Maria D. Safety assessment of nanoparticles cytotoxicity and genotoxicity of metal nanoparticles in vitro. J Biomed Nanotechnol. 2011;7:20–1. doi: 10.1166/jbn.2011.1180. [DOI] [PubMed] [Google Scholar]
- 14.Muller RH, Shegokar R, Keck CM. 20 years of lipid nanoparticles (SLN and NLC): present state of development and industrial applications. Current drug discovery technologies. 2011;8:207–27. doi: 10.2174/157016311796799062. [DOI] [PubMed] [Google Scholar]
- 15.Simoni J, Simoni G, Wesson DE, Feola M. ATP-Adenosine-Glutathione Cross-Linked Hemoglobin as Clinically Useful Oxygen Carrier. Current drug discovery technologies. 2011 doi: 10.2174/157016312802650797. [DOI] [PubMed] [Google Scholar]
- 16.Betigeri S, Zhang M, Garbuzenko O, Minko T. Non-viral systemic delivery of siRNA or antisense oligonucleotides targeted to Jun N-terminal kinase 1 prevents cellular hypoxic damage. Drug Deliv Transl Res. 2011;1:13–24. doi: 10.1007/s13346-010-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garbuzenko O, Zalipsky S, Qazen M, Barenholz Y. Electrostatics of PEGylated micelles and liposomes containing charged and neutral lipopolymers. Langmuir. 2005;21:2560–8. doi: 10.1021/la0479105. [DOI] [PubMed] [Google Scholar]
- 18.Savla R, Taratula O, Garbuzenko O, Minko T. Tumor targeted quantum dot-mucin 1 aptamer-doxorubicin conjugate for imaging and treatment of cancer. J Control Release. 2011;153:16–22. doi: 10.1016/j.jconrel.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Chen AM, Zhang M, Wei D, Stueber D, Taratula O, Minko T, He H. Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small. 2009;5:2673–7. doi: 10.1002/smll.200900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taratula O, Garbuzenko O, Savla R, Wang YA, He H, Minko T. Multifunctional nanomedicine platform for cancer specific delivery of siRNA by superparamagnetic iron oxide nanoparticles-dendrimer complexes. Curr Drug Deliv. 2010;8:59–69. doi: 10.2174/156720111793663642. [DOI] [PubMed] [Google Scholar]
- 21.Patil ML, Zhang M, Betigeri S, Taratula O, He H, Minko T. Surface-modified and internally cationic polyamidoamine dendrimers for efficient siRNA delivery. Bioconjug Chem. 2008;19:1396–403. doi: 10.1021/bc8000722. [DOI] [PubMed] [Google Scholar]
- 22.Minko T, Patil ML, Zhang M, Khandare JJ, Saad M, Chandna P, Taratula O. LHRH-targeted nanoparticles for cancer therapeutics. Methods in molecular biology. 2010;624:281–94. doi: 10.1007/978-1-60761-609-2_19. [DOI] [PubMed] [Google Scholar]
- 23.Taratula O, Garbuzenko OB, Chen AM, Minko T. Innovative strategy for treatment of lung cancer: targeted nanotechnology-based inhalation co-delivery of anticancer drugs and siRNA. Journal of drug targeting. 2011;19:900–14. doi: 10.3109/1061186X.2011.622404. [DOI] [PubMed] [Google Scholar]
- 24.Taratula O, Garbuzenko OB, Kirkpatrick P, Pandya I, Savla R, Pozharov VP, He H, Minko T. Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. Journal of controlled release : official journal of the Controlled Release Society. 2009;140:284–93. doi: 10.1016/j.jconrel.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pakunlu RI, Wang Y, Tsao W, Pozharov V, Cook TJ, Minko T. Enhancement of the efficacy of chemotherapy for lung cancer by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense: novel multicomponent delivery system. Cancer Res. 2004;64:6214–24. doi: 10.1158/0008-5472.CAN-04-0001. [DOI] [PubMed] [Google Scholar]
- 26.Dayan N, Shah V, Minko T. Genotoxic potential evaluation of a cosmetic insoluble substance by the micronuclei assay. J Cosmet Sci. 2011;62:29–39. [PubMed] [Google Scholar]
- 27.Arvizo RR, Miranda OR, Thompson MA, Pabelick CM, Bhattacharya R, Robertson JD, Rotello VM, Prakash YS, Mukherjee P. Effect of nanoparticle surface charge at the plasma membrane and beyond. Nano letters. 2010;10:2543–8. doi: 10.1021/nl101140t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asati A, Santra S, Kaittanis C, Perez JM. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS nano. 2010;4:5321–31. doi: 10.1021/nn100816s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharjee S, de Haan LH, Evers NM, Jiang X, Marcelis AT, Zuilhof H, Rietjens IM, Alink GM. Role of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Particle and fibre toxicology. 2010;7:25. doi: 10.1186/1743-8977-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nabeshi H, Yoshikawa T, Arimori A, Yoshida T, Tochigi S, Hirai T, Akase T, Nagano K, Abe Y, Kamada H, Tsunoda S, Itoh N, Yoshioka Y, Tsutsumi Y. Effect of surface properties of silica nanoparticles on their cytotoxicity and cellular distribution in murine macrophages. Nanoscale research letters. 2011;6:93. doi: 10.1186/1556-276X-6-93. [DOI] [PMC free article] [PubMed] [Google Scholar]