Abstract
Background
Gastric injections of botulinum toxin A (BTA) may induce changes in gastric emptying and body weight, but results vary. BTA dose and depth of injection may affect efficacy. This study assessed changes in gastric emptying, satiation, symptoms, and body weight after endoscopic ultrasound (EUS)-guided injection of 100 or 300 U BTA into gastric antral muscularis propria of obese subjects.
Methods
Open label study of ten healthy, obese adults (age=29–49 years, body mass index=31–54 kg/m2) who received 100 U (n=4) or 300 U (n=6) BTA and were followed for 16 weeks. Measures included gastric emptying of solids (by scintigraphy), satiation (by maximum tolerated volume [MTV] during nutrient drink test), gastrointestinal symptoms (by the Gastrointestinal Symptom Rating Scale), caloric intake (by food frequency questionnaire), and body weight.
Results
For the entire cohort, MTV decreased from 1,380 cc (range: 474–2,014) at baseline to 620 cc (range: 256–1,180) 2 weeks after BTA injection; decreases were statistically significant in the subjects receiving 300 U BTA (p=0.03). Average body weight loss was 4.9 (±6.3) kg after 16 weeks. Gastric emptying T1/2 was prolonged in the 300 U BTA group, but not significantly different from baseline (p=0.17). BTA injections were well tolerated without significant adverse effects.
Conclusion
EUS-guided injection of BTA into gastric muscularis propria can be performed safely with minimal adverse effects. A dose of 300 U BTA significantly enhances satiation, is associated with weight loss, and may slow gastric emptying. Further study of higher dose BTA in obese subjects is warranted.
Keywords: Obesity, Endoscopic ultrasound, Botulinum toxin, Gastric emptying, Satiation
Introduction
Obesity is an important public health concern in the USA. Although most pharmacologic therapies have targeted the central nervous system, surgical treatments induce satiation by decreasing the size of the gastric reservoir and inducing maldigestion or malabsorption [1]. Surgery is effective but invasive and expensive, and some obese persons are not good surgical candidates. Promising pharmacologic and endoscopic approaches to obesity include treatments that affect gastric volume, gastric emptying, gastric compliance, and satiation [2].
In rats, botulinum toxin A (BTA) injected subserosally into the antrum resulted in significant decreases in caloric intake and body weight when compared to control and sham-injected rats [3, 4]. Subsequent open-label human studies and three small randomized trials have reported conflicting results, with some studies finding little or no body weight loss after gastric BTA injection [5–10], and one randomized controlled trial showing statistically significant decreases in gastric emptying and body weight [11]. These trials differed in BTA dose (100 to 300 U in Botox formulation equivalents) and location of injections (antrum alone vs. antrum, body, and cardia). Depth of injection in the gastric wall was largely unknown in these studies, as endoscopic ultrasound (EUS) was not used to guide injection into the muscularis propria or subserosal layers.
We hypothesized that BTA would have dose-related effects on gastric emptying, satiation, and body weight loss and that EUS guidance would help to assure injection of BTA in the gastric muscularis propria and subserosal layers of the gastric wall. In this pilot study, BTA was injected into gastric antral muscularis propria under EUS guidance, and changes in gastric emptying, satiation, gastrointestinal symptoms, and body weight were assessed during a 16-week follow-up period. We studied doses of 100 U (reportedly efficacious in the first human case report) and 300 U (the maximum dose of BTA “Botox” formulation administered to patients at one time in previous human studies) [12].
Materials and Methods
Subjects
Twelve overweight, healthy subjects were recruited; ten of whom underwent BTA injection. Persons with known gastroparesis, diabetes, peptic ulcer disease, active upper gastrointestinal ulceration, prior gastric or small bowel surgery, ASA Class 3 (ASA, American Society of Anesthesiologists) or higher, or patients with more than mild, infrequent symptoms of upper abdominal pain or nausea were excluded. Women of childbearing potential underwent urinary pregnancy tests before scintigraphic and endoscopic procedures. One of the 12 enrolled subjects was excluded because of the presence of delayed gastric emptying on baseline scintigraphy, and a second was excluded because of the presence of active esophageal ulceration identified during endoscopy.
Measures
Gastric emptying of solids in a mixed meal was measured using a scintigraphic method that has been previously validated [13]. Briefly, one mCi 99mTc-sulfur colloid was added to two raw eggs during the scrambling, cooking process. The eggs were served on one slice of bran bread along with a 240-ml glass of skim milk (total calories: 296 kcal, 32% protein, 35% fat, 33% carbohydrate). Anterior and posterior gamma camera images were obtained immediately after the meal ingestion, every 15 min for the first 2 h and, then, every 30 min for the next 2 h (for a total of 4 h after the radiolabeled meal) to assess gastric emptying.
Satiation was assessed with the nutrient drink test, following the method of Tack et al. [14] as previously reported [7]. Subjects ingested 120 ml of a nutrient drink (Ensure®) per 4 min. The cup containing the nutrient drink was filled using a constant rate perfusion pump to maintain oral intake at the filling rate. Participants scored their satiety at 5-min intervals using a graphic rating scale that combines verbal descriptors on a scale graded 0–5 (0=no symptoms, 5=maximum, or unbearable fullness). Participants stopped meal intake when a score of 5 was reached, and the maximum tolerated volume (MTV) of nutrient drink was recorded. Normal values for MTV in adults in our laboratory are ≥850 cc [7]. Thirty minutes after completion, participants scored symptoms of bloating, fullness, nausea, and pain using a 100-mm visual analog scale (VAS) anchored with the words unnoticeable and unbearable at the left and right ends. The aggregate symptom score was defined as the sum of the VAS scores for each symptom (i.e., maximum 400).
Gastrointestinal symptoms were assessed with the Gastrointestinal Symptom Rating Scale (GSRS) [15], a validated questionnaire comprised of 15 items rating gastrointestinal symptoms using 7-point Likert scales with values ranging from 1 to 7. In addition to an overall score ranging from 15 to 105, the GSRS may be scored for five-symptom subscales (reflux, diarrhea, constipation, abdominal pain, indigestion) [15] with recall referring to the past week. It is responsive to change [16, 17].
Caloric intake was measured using the Three-Day Nutrition/Cholesterol Control Reporter (CCR), a validated dietary questionnaire [18]. Subjects identified foods eaten over a 72-h time period. Barcoded study data sheets were used to standardize data entry, and standardized software permitted calculation of carbohydrate, fat, and protein intake as well as total caloric intake.
Procedures
During a 2-week baseline period, subjects underwent a gastric emptying test, a nutrient drink test, were weighed weekly, and completed both the GSRS and the CCR questionnaires weekly. They then underwent esophagogastroduodenoscopy (EGD) under conscious sedation. If no ulceration or retained food was found during EGD, EUS and BTA injection (Botox®, Allergan Corporation, Irvine, CA, USA) were performed under the same sedation.
EUS examinations were performed with either GF-UC140P Olympus/Aloka or XGIF-UCT140-AT8 Olympus/ATL linear array echoendoscopes (Olympus America, Center Valley, PA, USA). BTA injections were made via a 25-gauge EUS needle (Cook Endoscopy, Winston-Salem, NC, USA; Fig. 1a–c). A ring of five injections was made into the gastric antral muscularis propria, 2 to 3 cm proximal to the pylorus. The first four subjects received 20 U at each injection site (total dose 100 U), and the subsequent six subjects received 60 U at each injection site (total dose 300 U). Subjects were assessed for complications after recovery from conscious sedation and, again, by telephone the next day.
Fig. 1.
EUS-guided injections of BTA into gastric antral muscularis propria. Arrows indicate antral muscularis propria, arrowheads indicate the injection needle. a Before BTA injection. b During BTA injection. c Immediately after BTA injection
During a 16-week follow-up period after BTA injections, subjects were weighed and completed the GSRS weekly. They underwent repeat studies: a gastric emptying test 2 weeks after BTA injection, nutrient drink tests 4 and 16 weeks after BTA injection, and completed the CCR questionnaire during weeks 4, 8, 12, and 16. No behavioral or dietary interventions were offered to study subjects.
Data Analysis
Changes in the mean MTV achieved during nutrient drink tests, and changes in gastric emptying T1/2, were compared between baseline and follow-up studies with the Wilcoxon signed rank test. The median change in caloric intake and body weight from baseline to each 4 weeks of follow-up was also compared using the Wilcoxon signed rank test. GSRS scores were compared over time using a longitudinal regression model.
Ethical Approval
The research protocol was approved by the Mayo Clinic Institutional Review Board. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Results
Gastric Emptying
As shown in Fig. 2, median gastric emptying T1/2 was not delayed after antral injections of 100 U BTA; however, there was delay in gastric emptying T1/2 in the subjects receiving 300 U BTA, although this did not reach statistical significance (p=0.17). Three of the six subjects who received 300 U BTA demonstrated both a ≥15-min increase in time to 50% gastric emptying (T1/2) as well as an abnormally low 1-h gastric emptying (<10% emptying in our lab), as shown in Fig. 3.
Fig. 2.
Change in gastric emptying T1/2 2 weeks after BTA injection. Box plots show median and interquartile ranges, error bars show SD. p values are for comparison between baseline and 2-week postinjection values for each dosage tested
Fig. 3.
Gastric emptying curves at baseline (squares) and 2 weeks after BTA injection (diamonds) in a subject receiving 300 U BTA who developed delayed 1-h emptying (<10%) as well as delayed GE T1/2 after BTA injection
Satiation
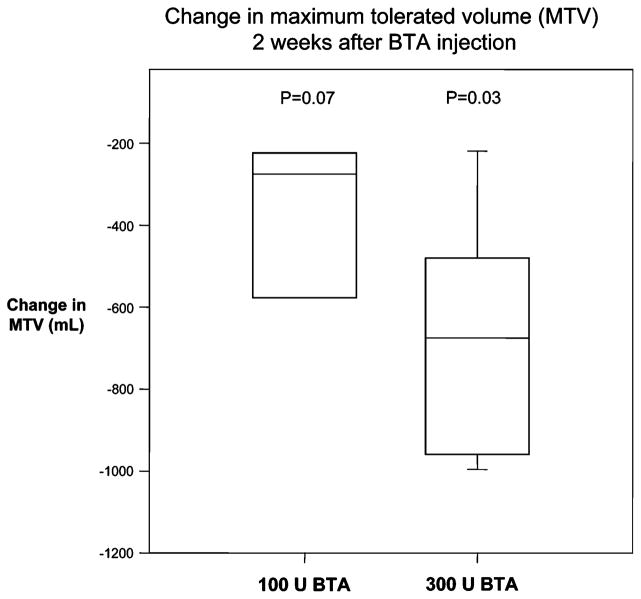
For the entire cohort, median MTVs during nutrient drink tests decreased from a baseline value of 1,380 cc (range: 474–2,014) to 620 cc (range: 256=1,180) 2 weeks after BTA injection, with all but one subject having early satiation (MTV<850 cc). Statistically significant decreases in MTV were observed in the subjects receiving 300 U BTA (n=6, p=0.03), with a trend for those 100 U BTA (n= 4, p=0.07) as shown in Fig. 4. For the entire cohort, the median MTV increased at 16 weeks, but still was quantitatively lower than baseline (median=840 cc, range: 480–1750, p=0.09). At each time point, subjects reported similar symptom scores 30 min after completing meal intake (data not shown).
Fig. 4.
Change in MTVs during nutrient drink tests 2 weeks after BTA injection. Box plots show median and interquartile ranges, error bars show SD. p values are for comparison between baseline and 2-week postinjection values for each dosage tested
Body Weight
Table 1 lists initial BMI, initial body weight, and week-16 body weight for all subjects. Eight of the ten subjects lost body weight, and mean weight reduction was 4.9 (±6.3) kg at 16 weeks. Mean body weight reduction was similar in subjects receiving 100 or 300 U BTA (5.0 vs. 4.8 kg). There was a nonsignificant trend toward loss of more body weight in the three subjects who developed decreased gastric emptying after receiving 300 U BTA. Mean body weight continued to decrease until the end of the 16-week study period.
Table 1.
Subject BMI and body weight at baseline and week 16
| Subject no. | BTA dose (U) | Initial BMI | Initial body weight (kg) | Week 16 body weight (kg) |
|---|---|---|---|---|
| 1 | 100 | 36.8 | 112.8 | 104.4 |
| 2 | 100 | 41.5 | 105.8 | 96.5 |
| 3 | 100 | 30.2 | 76.3 | 76.5 |
| 4 | 100 | 32.3 | 76.0 | 73.5 |
| 5 | 300 | 38.0 | 106.1 | 105.8 |
| 6 | 300 | 38.0 | 104.1 | 99.9 |
| 7 | 300 | 37.6 | 101.0 | 81.1 |
| 8 | 300 | 37.8 | 128.0 | 124.4 |
| 9 | 300 | 40.8 | 110.4 | 111.0 |
| 10 | 300 | 37.2 | 91.2 | 90.2 |
Caloric Intake
No significant change in caloric intake was identified between baseline and follow-up, as determined by the CCR diet questionnaire. However, large intra-subject variability in results of the two baseline CCR questionnaires precluded meaningful analysis of the effect of treatment on caloric intake.
Symptoms
Mean GSRS scores increased an average of 0.18 per week (p=0.006), a clinically insignificant change (Fig. 5). Mild elevations in mean global GSRS scores at weeks 12 to 16 were associated with reports of loose stools and diarrhea in two subjects, one of whom also reported fecal urgency. These symptoms resolved or improved by week 16.
Fig. 5.

Mean weekly global GSRS scores at baseline (weeks 1 and 2) and for 16 weeks after antral BTA injections for subjects with (diamonds) and without (open squares) delayed gastric emptying,
Adverse Effects
All studies were completed without complications. One subject (who received 100 U BTA) experienced nausea and back pain the day after BTA injections; laboratory evaluation and abdominal CT scan were normal and her symptoms resolved spontaneously within 48 h. Self-limited diarrhea occurred in two subjects as described above. No other adverse events occurred.
Discussion
This study shows that antral injections of 300 U BTA can be accomplished safely and that EUS can be used to guide injection into the gastric antral muscularis propria. A trend toward delayed gastric emptying was seen with the 300 U BTA dose, as well as a statistically significant increase in satiation. These changes in gastric function were associated with an average weight loss of ~5 kg over 16 weeks of follow up.
Prior studies demonstrated the importance of gastric antral contractility to the normal emptying of solids [19]. Reduced antral motility is associated with retardation of gastric emptying with prolongation of the lag time and gastric emptying half-time (T1/2), and a slower post-lag gastric emptying phase [20]. BTA inhibits cholinergic transmission, a pivotal mechanism in the excitation of gastrointestinal contractility [3]. BTA injection is used therapeutically to treat human gastrointestinal disorders, including achalasia and anal fissure, at doses ranging from 100 to 300 U of the Botox formulation [21]. Doses of 200 U of Botox injected into the pylorus have been reported to improve symptoms of gastroparesis, without adverse effects [5, 22]. Doses of up to 500 U of the lower-potency Dysport formulation of BTA have been injected in the stomach [6], but 500 U of Dysport has an estimated potency equivalent to 125 U of the Botox formulation [23].
Conflicting data are available regarding the physiologic effects of gastric BTA injections in obese subjects. Most open-label studies and two randomized trials exploring doses of 100 to 300 U showed little effect of gastric BTA injections [5, 6, 8, 9, 24], although a randomized, placebo-controlled trial of 200 U BTA demonstrated increased satiety in response to a standard meal, delayed gastric emptying, decreased maximal gastric capacity, and increased weight loss when compared to saline control [11]. Potential explanations for these discordant results include not only BTA dose but also depth of injection into the gastric wall. No human study has ensured that injections were performed into the gastric muscularis propria or subserosa (the space between serosa and muscularis propria), the locations where BTA was injected in animal studies [3, 4]. In one study, EUS was performed after completion of all injections in a small subset of subjects [5]. In our experience, injection into the antral muscularis propria or subserosa is moderately difficult even under direct EUS guidance. The injection needle tends to stop in the submucosa or pass through the muscular layer and serosa into the peritoneal cavity. Some of the variability in the literature may thus reflect differences in delivery of BTA to the muscularis propria or subserosa.
In this pilot study, antral muscularis propria BTA injections performed under EUS guidance resulted in dose-dependent increased satiation (significant with the 300 U BTA dose), and there was a trend toward delayed gastric emptying T1/2 in subjects receiving the higher tested dose (300 U). Potential mechanisms for increased satiation include delayed gastric emptying, reduced antral capacity leading to increased sensation of intragastric contents, BTA-induced inflammation of the antrum, alteration of gastric hormone secretion (such as ghrelin, gastrin, or somatostatin), or a placebo effect. Four of ten subjects had early satiation at baseline, a finding that appears to be uncommon in obese persons (based on previous studies conducted at our center) [25] and could in part be because of palatability of the nutrient drink or psychological influences. However, even these four subjects tolerated lower volumes of the nutrient drink after BTA injection. The finding of a dose-dependent change in satiation suggests that changes in satiation were not because of a placebo effect alone, and future studies should explore further the potential effects of BTA on antral and whole gastric capacity, as well as levels of gastric hormones or peptides associated with satiation.
The trend toward delayed gastric emptying T1/2 in subjects receiving 300 U BTA is in accord with the findings of a previous small study with this dosage [5]. In the present study, three of six subjects receiving 300 U developed abnormally low 1-h emptying and delays of at least 15 min in gastric emptying T1/2, without a slower post-lag gastric emptying phase. It is notable that delays in gastric emptying were not accompanied by adverse symptoms. On the other hand, delayed emptying at 1 h may conceivably contribute to the satiation reported using the challenge with a caloric nutrient drink. Higher doses of BTA may be required to more effectively retard gastric emptying, potentially optimizing the clinical benefit of BTA injections in obese persons, but it is unclear whether higher doses would be well tolerated.
The average body weight loss after BTA injection in this study compares favorably to that obtained with pharmacologic interventions for obesity [26, 27], but was less than the 11-kg body weight loss reported in one recent double-blind randomized trial of gastric BTA injections [11]. We noted that gradual body weight loss continued throughout the 16 week study period, and satiation was increased at 16 weeks compared to baseline. This apparently continued efficacy of antral muscularis BTA is beyond the presumed duration of action of BTA in skeletal muscle [28, 29], although similar prolonged responses to BTA have been reported in the treatment of achalasia. The mechanism underlying these continued benefits at 16 weeks is unclear; they may be because of a biological effect of BTA on gastrointestinal smooth muscle that is prolonged relative to its effects on skeletal muscle, inflammation (e.g., myenteric ganglionitis) induced by BTA injection, a behavioral benefit of BTA injection persisting after the pharmacological action has resolved, or a placebo effect. Clearly, this requires further study.
In summary, the beneficial effects of 300 U BTA on satiation and weight loss suggest that further study of gastric antral BTA injections is merited, including studies of higher doses. Injection of BTA under EUS guidance clarifies the location at which BTA is deposited and may optimize its efficacy. The therapeutic value of antral BTA injections for obesity should be further assessed in additional blinded trials comparing an optimal dose of BTA to placebo.
Acknowledgments
The authors thank Mr. Duane Burton, Ms. Judy Peterson, and Ms. Julie Schreiber for their assistance in the conduct of this study. Dr. Camilleri is support by DK67071 and DK54681 from the National Institutes of Health.
References
- 1.Steinbrook R. Surgery for severe obesity. N Engl J Med. 2004;350:1075–9. doi: 10.1056/NEJMp048029. [DOI] [PubMed] [Google Scholar]
- 2.Padwal R, Majumdar S. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007;369:71–7. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- 3.James A, Ryan J, Parkman H. Inhibitory effects of botulinum toxin on pyloric and antral smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003;285:G291–7. doi: 10.1152/ajpgi.00296.2002. [DOI] [PubMed] [Google Scholar]
- 4.Coskun H, Duran Y, Dilege E, Mihmanli M, Seymen H, Demirkol M. Effect on gastric emptying and weight reduction of botulinum toxin-A injection into the gastric antral layer: an experimental study in the obese rat model. Obes Surg. 2005;15:1137–43. doi: 10.1381/0960892055002275. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso Junior A, Savassi-Rocha P, Vaz Coelho L, de Mello Sposito M, Albuquerque W, Costa Diniz M, et al. Botulinum A toxin injected into the gastric wall for the treatment of class III obesity: a pilot study. Obes Surg. 2006;16:335–43. doi: 10.1381/096089206776116408. [DOI] [PubMed] [Google Scholar]
- 6.Albani G, Petroni M, Mauro A, Liuzzi A, Lezzi G, Verti B, et al. Safety and efficacy of therapy with botulinum toxin in obesity: a pilot study. J Gastroenterol. 2005;40:833–5. doi: 10.1007/s00535-005-1669-x. [DOI] [PubMed] [Google Scholar]
- 7.Delgado-Aros S, Cremonini F, Castillo J, Chial H, Burton D, Ferber I, et al. Independent influences of body mass and gastric volumes on satiation in humans. Gastroenterology. 2004;126:432–40. doi: 10.1053/j.gastro.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Gui D, Mingrone G, Valenza V, Spada P, Mutignani M, Runfola M, et al. Effect of botulinum toxin antral injection on gastric emptying and weight reduction in obese patients: a pilot study. Aliment Pharmacol Ther. 2006;23:675–80. doi: 10.1111/j.1365-2036.2006.02773.x. [DOI] [PubMed] [Google Scholar]
- 9.Mittermair R, Keller C, Geibel J. Intragastric injection of botulinum toxin A for the treatment of obesity. Obes Surg. 2007;17:732–6. doi: 10.1007/s11695-007-9135-x. [DOI] [PubMed] [Google Scholar]
- 10.Rollnik JD, Manns MP, Goke M. Antral injections of botulinum A toxin for the treatment of obesity. Ann Intern Med. 2003;138:359–60. doi: 10.7326/0003-4819-138-4-200302180-00026. [DOI] [PubMed] [Google Scholar]
- 11.Foschi D, Corsi F, Lazzaroni M, Sangaletti O, Riva P, La Tartara G, et al. Treatment of morbid obesity by intraparietogastric administration of botulinum toxin: a randomized, double-blind, controlled study. Int J Obes. 2007;31:707–12. doi: 10.1038/sj.ijo.0803451. [DOI] [PubMed] [Google Scholar]
- 12.Schurch B, Stohrer M, Kramer G, Schmid D, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol. 2000;164:692–7. doi: 10.1097/00005392-200009010-00018. [DOI] [PubMed] [Google Scholar]
- 13.Cremonini F, Mullan B, Camilleri M, Burton D, Rank M. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther. 2002;16:1781–90. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 14.Tack J, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–52. doi: 10.1016/s0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 15.Dimenas E, Glise H, Hallerback B, Hernqvist H, Svedlund J, Wiklund I. Well-being and gastrointestinal symptoms among patients referred to endoscopy owing to suspected duodenal ulcer. Scand J Gastro. 1995;30:1046–52. doi: 10.3109/00365529509101605. [DOI] [PubMed] [Google Scholar]
- 16.Dimenas E, Glise H, Hallerback B, Hernqvist H, Svedlund J, Wiklund I. Quality of life in patients with upper gastrointestinal symptoms. An improved evaluation of treatment regimens? Scand J Gastroenterol. 1993;28:681–7. doi: 10.3109/00365529309098272. [DOI] [PubMed] [Google Scholar]
- 17.Talley N, Fullerton S, Junghard O, Wiklund I. Quality of life in patients with endoscopy-negative heartburn: reliability and sensitivity of disease-specific instruments. Am J Gastroenterol. 2001;96:1998–2004. doi: 10.1111/j.1572-0241.2001.03932.x. [DOI] [PubMed] [Google Scholar]
- 18.Hodis H, Mack W, Lobo R, Shoupe D, Sevanian A, Mahrer P, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–53. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 19.Camilleri M, Malagelada J, Brown M, Becker G, Zinsmeister AR. Relation between antral motility and gastric emptying of solids and liquids in humans. Am J Physiol. 1985;249:G580–5. doi: 10.1152/ajpgi.1985.249.5.G580. [DOI] [PubMed] [Google Scholar]
- 20.Camilleri M, Brown M, Malagelada J. Relationship between imparied gastric emptying and abnormal gastrointestinal motility. Gastroenterology. 1986;91:94–9. doi: 10.1016/0016-5085(86)90444-0. [DOI] [PubMed] [Google Scholar]
- 21.Qureshi W. Gastrointestinal uses of botulinum toxin. J Clin Gastroenterol. 2002;34:126–8. doi: 10.1097/00004836-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Lacy B, Crowell M, Schettler-Duncan A, Mathis C, Pasricha P. The treatment of diabetic gastroparesis with botulinum toxin injection of the pylorus. Diabetes Care. 2004;27:2341–7. doi: 10.2337/diacare.27.10.2341. [DOI] [PubMed] [Google Scholar]
- 23.Sampaio C, Ferreira J, Simoes F, Rosas M, Magalhaes M, Correia A, et al. DYSBOT: a single-blind, randomized parallel study to determine whether any differences can be detected in the efficacy and tolerability of two formulations of botulinum toxin type A-Dysport and Botox-assuming a ratio of 4:1. Mov Disord. 1997;12:1013–8. doi: 10.1002/mds.870120627. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Compean D, Mendoza-Fuerte E, Martinez J, Villarreal I, Maldonado H. Endoscopic injection of botulinum toxin in the gastric antrum for the treatment of obesity. Results of a pilot study. Gastroenterol Clin Biol. 2005;29:789–91. doi: 10.1016/s0399-8320(05)86349-3. [DOI] [PubMed] [Google Scholar]
- 25.Delgado-Aros S, Camilleri M, Castillo E, Cremonini F, Stephens D, Ferber I, et al. Effect of gastric volume or emptying on meal-related symptoms after liquid nutrients in obesity: a pharmacologic study. Clin Gastroenterol Hepatol. 2005;3:997–1006. doi: 10.1016/s1542-3565(05)00285-5. [DOI] [PubMed] [Google Scholar]
- 26.Wadden T, Berkowitz R, Womble L, Sarwer D, Phelan S, Cato R, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111–20. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 27.Berkowitz R, Fujioka K, Daniels S, Hoppin A, Owen S, Perry A, et al. Effects of sibutramine treatment in obese adolescents: a randomized trial. Ann Intern Med. 2006;145:81–90. doi: 10.7326/0003-4819-145-2-200607180-00005. [DOI] [PubMed] [Google Scholar]
- 28.Abbruzzese G, Berardelli A. Neurophysiological effects of botulinum toxin type A. Neurotox Res. 2006;9:109–14. doi: 10.1007/BF03033927. [DOI] [PubMed] [Google Scholar]
- 29.Botulinum toxin type A: drug information. UpToDate Online version 14.3 2006.