Summary
Background
Studies using allergen challenge models have suggested Th2 cytokines promote airway inflammation in asthma. We assessed mediators of airway inflammation during the chronic asymptomatic phase of asthma.
Methods
Nine non-atopic asthma (NAA) patients, 19 atopic asthma (AA) patients, 20 atopic controls (AC), and 38 normal controls (NC) underwent sputum induction while asymptomatic. Sputum total cell counts and differentials were determined; levels of cytokines IL-4, IL-5, IL-13, GM-CSF, and IFN-γ, and chemokines eotaxin (CCL11) and RANTES (CCL5) were measured by ELISA; and levels of eosinophil-derived neurotoxin (EDN) were measured by radioimmunoassay.
Results
NAA patients showed higher % eosinophils and total eosinophils compared to AA. NAA and AA patients showed higher IFN-γ and EDN levels compared to AC and NC, with no differences in IL-4, IL-5, or IL-13 levels among the four groups. GM-CSF levels were higher in AA patients compared to AC or NC. In NAA, AA, and AC patients, % eosinophils and EDN levels correlated positively with IFN-γ, GM-CSF, eotaxin, and RANTES, but not with IL-5 levels.
Conclusions
Baseline airway inflammation of intrinsic and extrinsic asthma is characterized by eosinophilic inflammation and the Th1 cytokine, IFN-γ. GM-CSF, instead of IL-5, and chemokines may coordinate airway eosinophilia during the chronic asymptomatic phase of asthma.
Keywords: Eosinophil, Cytokine, Intrinsic asthma
Introduction
It has been known for over 100 years that the eosinophil is associated with bronchial asthma.1 Over the past two decades, the underlying airway inflammation of asthma, characterized by marked infiltration of eosinophils, has received increasing attention. Numerous human airway studies have provided consistent evidence for the strong association between the inflammatory nature of asthma and airway eosinophils.2,3 Eosinophil granular proteins, such as eosinophil major basic protein and eosinophil peroxidase, have functions relevant to the features of human asthma, including histopathologic changes, reversible airway narrowing, and bronchial hyperreactivity.4,5 Thus, evidence supports a central role for eosinophils in the pathophysiology of bronchial asthma. However, the precise mechanism of airway eosinophil involvement in asthma remains enigmatic.
Over the last decade, interest has been focused on the Th1/Th2 paradigm in the pathogenesis of asthma. Th2 cells mediate eosinophil influx by releasing Interleukin (IL)-4 and IL-56; IL-5 is the major eosinophil specific growth factor. Indeed, an increase in Th2 cytokine production in the airway has been found in asthma.7 However, the role of eosinophils as effector cells in asthma pathogenesis has been questioned. Leckie et al.8 showed anti-IL-5 Ab treatment of patients with asthma decreased blood and sputum eosinophils but did not affect the late asthmatic responses and airway hyperresponsiveness. These results from the allergen challenge model are difficult to interpret, since they do not necessarily reflect events in the natural course of asthma. This was clearly demonstrated in a study by Lommatzsch et al.9 who found eosinophil and lymphocyte infiltration into the airways occurred at significantly different times when comparing human and murine asthma. More recent studies by Haldar et al.10 and Nair et al.11 demonstrated reductions in eosinophil numbers and clinical improvement after administration with anti-IL-5 Ab treatment of patients with refractory eosinophilic asthma.
Intrinsic (non-atopic) asthma is considered a variant of asthma, since unlike extrinsic (atopic) asthma, patients with the disease are skin test-negative to common aeroallergens, lack circulating specific IgE, and have later clinical onset.12 Analyses of airway specimens in patients with extrinsic asthma have revealed the activation of typical Th2 lymphocytes producing IL-4 and IL-5, but no IL-2 or interferon (IFN)-γ.13,14 In contrast, intrinsic asthma appears to have a more distinct T-cell activation pattern, producing IL-5, IL-2 and IFN-γ, but not IL-4, which is incompatible with a typical Th2 pattern.15,16 However, recent studies indicate more similarities than differences in regard to cytokine gene expression between intrinsic and extrinsic asthma.17
The aim of the present study was to compare intrinsic and extrinsic asthma in terms of their patterns of eosinophilic inflammation and cytokine profiles (Th1 or Th2) in the airway. To perform the study, we examined eosinophils, eosinophil-associated cytokines (IL-4, IL-5, IL-13, IFN-γ, and GM-CSF) and chemokines eotaxin (CCL11) and RANTES (CCL5) in sputum from intrinsic and extrinsic asthmatic subjects during a stable period of natural asthma. These results were compared with those of atopic and normal control subjects.
Methods
Study subjects
Induced sputum was obtained from nine non-atopic asthmatic (NAA), 19 atopic asthmatic (AA), 20 nonasthmatic atopic control (AC), and 38 nonasthmatic non-atopic normal control (NC) subjects. At the time of study, asthmatic patients had stable bronchial asthma, with no acute asthma symptoms nor evidence of chest infection. Fifteen out of 20 AC subjects had a history of allergic rhinitis. All subjects underwent sputum induction during the winter time. All subjects were nonsmokers and had not taken oral glucocorticoids for at least 8 weeks prior to the study. Four out of 9 NAA subjects and 9 out of 19 AA subjects were receiving regular inhaled corticosteroid therapy. Subjects were recruited from a pool of patients or healthy individuals seen at the Mayo Clinic, Rochester, MN. The Mayo Clinic Institutional Review Board reviewed and approved this study.
Asthmatic subjects had a clear history of asthma. They had a greater than 15% increase in FEV1 after 180 µg inhaled albuterol and/or a positive methacholine challenge as defined by a 20% reduction from baseline in FEV1 following inhaled methacholine.
Atopy was defined as the presence of at least one positive skin prick reaction (>3 mm wheal diameter) to a battery of 15 common aeroallergens. Nonatopic subjects had negative skin test responses to the battery of 15 common aeroallergens, and negative RAST test responses (Pharmacia Diagnostics, Uppsala, Sweden) to the same common aeroallergens.
Sputum induction
All subjects were pre-medicated with 2 puffs of albuterol, total dose 180 µg, via spacer tube. Sputum induction was performed with aerosolized 3% hypertonic saline solution generated by a DeVilbiss Ultra-Neb 99 ultrasonic nebulizer (the DeVilbiss Co, Somerset, PA), as described previously.18
Sputum processing
The collected sputum was processed, as described by Pizzichini et al.19 with modifications. Sputum was processed immediately within a time limit of 2 h. Equal volumes of sputum were placed into two 15-ml polypropylene tubes. One aliquot was treated with an equal volume of saline solution containing 0.1% dithiothreitol (DTT) (1:10 diluted DTT) (Sputolysin 1%; Calbiochem Corp., San Diego, CA) (“with DTT” aliquot). The other aliquot was mixed with two times the volume of saline solution without DTT (“without DTT” aliquot). Both mixtures were aspirated up and down with a Pasteur pipette, then vortexed for 15 s, and placed on a bench rocker for 20 min “with DTT” aliquots were filtered through 40-µm strainers (Beckton Dickinson, Franklin Lakes, NJ) and then both aliquots were centrifuged at 833 g for 10 min at 4 °C. The supernatants from each sample were collected and then frozen at −70 °C for later assays. Cell pellets from the original aliquot “with DTT” were resuspended in saline solution, stained with trypan blue and a total nonsquamous cell count was performed with a hemocytometer. A nonsquamous cell viability was then calculated. Cytospin preparations were adjusted to equal approximately 1.0 × 106/ml, and then stained with Diff-Quik stain (Dade Behring Inc., Newark, DE). Four hundred differential nonsquamous cells were counted. The specimen was considered adequate if morphologic identification of total and differential cell counts could be obtained.20 Individual cell number/ml was determined by multiplying the percentage specific cell type by the total cell number/ml. Supernatant “with DTT” aliquot and cell pellet “without DTT” were not used in this study.
Fluid-phase markers measurements
The levels of IL-4, IL-5, IL-13, IFN-γ (Endogen, Woburn, MA), GM-CSF, eotaxin and RANTES (R&D Systems, Minneaplois, MN) were measured in the thawed supernatant using sandwich ELISAs according to the respective manufacturers’ protocols, and the results were expressed in pg/ml. The sensitivities for IL-4 and IL-5 were less than 2.0 pg/ml and those for IL-13, IFN-γ, GM-CSF, eotaxin and RANTES were less than 10 pg/ml, 1 pg/ml, 0.36 pg/ml, 5.0 pg/ml, and 2.5 pg/ml, respectively. The eosinophil-derived neurotoxin (EDN) value was determined using a double antibody competitive RIA and the results were expressed in ng/ml. The sensitivity for EDN was less than 1 ng/ml. The result was adjusted for the dilution. When cytokine and chemokine levels were below sensitivity, 0 level was used for comparisons of the levels and 1/2 level of the sensitivity was used for correlations.
Statistical analysis
Cellularity and fluid-phase marker levels were presented as medians with interquartile ranges [25th–75th]. Fluid-phase marker levels were analyzed only in the “without DTT” aliquot. Because most of the data were not normally distributed, differences between the four subject groups were screened with the nonparametric Kruskal–Wallis test. When this test indicated a significant difference, the difference between two groups was tested for significance with a Mann–Whitney U test. Correlations were analyzed by the nonparametric Spearman correlation test. A P value of <0.05 was considered statistically significant.
Results
Subject characteristics
Subject characteristics are as follows: 1) age [median(range)]: NA, 44 (23–71 yr); AA, 28 (22–44); AC, 35 (22–70); NC, 36.5 (22–75); 2) sex (M:F): NA, 1:8 (P < 0.05 compared to AA); AA, 11:8; AC, 6:14; NC, 12:26; 3) FEV1 (% predicted) [median (range)]: NA, 92 (61–116); AA, 96 (68–114); AC, 101 (83–118); NC, 103 (90–122); 4) FEV1FVC (%) [median(range)]: NA, 83 (54–101); AA, 90 (64–104); AC, 87 (60–111); NC, 91 (80–104); 5) patients on inhaled corticosteroids: NA, 4/9; AA, 9/19; AC, 0/20;NC, 0/38.All subjects tolerated sputum induction with no adverse effects. Three subjects were excluded: two because of poor sputum induction (one AA and one NC) and one because of a low FEV1% predicted (NC). There were significantly more women among NAA (P<0.05) subjects than among AA subjects. There were no statistical differences between the four groups in terms of age, FEV1% predicted, or FEV1/FVC ratio.
Cell and mediator profiles
Cell viability, the number of total cells per ml, and the percentages for each cell type in induced sputum are shown in Table 1. No differences were found in cell viability. Numbers of total cells were significantly higher in NAA subjects compared with AA (P < 0.05), AC (P < 0.05), and NC (P < 0.01) subjects. With regard to cell types, there were significant differences in the percentages of macrophages, eosinophils, and epithelial cells among the 4 groups. Eosinophil percentages were significantly higher in NAA subjects than AA (P < 0.01) and AC/NC (P < 0.001) subjects. In AA subjects, eosinophil percentages were higher than in AC/NC subjects. Epithelial cells percentages were significantly higher in AA subjects than in NAA (P < 0.01), AC (P < 0.05), and NC (P < 0.01) subjects. Percentage of macrophages was lower in NAA (P < 0.01) subjects than in NC subjects, in conjunction with a marked increase in the percentage of eosinophils. Percentages of neutrophils and lymphocytes showed no significant differences among the 4 groups.
Table 1.
Sputum cell profiles of study subjects.a
| Non-atopic asthma (n = 9) |
Atopic asthma (n = 19) |
Atopic control (n = 20) |
Normal control (n = 38) |
|
|---|---|---|---|---|
| Viability, % | 77.0 [76.0–85.3] | 77.4 [69.8–85.0] | 85.2 [72.5–88.5] | 80.0 [71.8–84.3] |
| Total cells, 105/ml | 11.0†‡§ [7.7–13.0] | 7.0 [5.3–8.1] | 7.0 [4.3–9.6] | 6.3 [3.7–7.6] |
| Macrophages, % | 35.0§ [21.5–41.5] | 55.0 [30.0–66.5] | 57.3 [30.3–68.0] | 58.8 [46.3–68.5] |
| Neutrophils, % | 22.0 [12.8–47.5] | 18.0 [9.0–34.0] | 29.8 [18.5–58.8] | 28.0 [17.0–48.6] |
| Lymphocytes, % | 3.0 [1.3–7.5] | 3.0 [1.5–4.5] | 3.0 [1.1–4.8] | 2.5 [1.0–6.0] |
| Eosinophils, % | 24.0¶#** [14.0–48.3] | 8.0#** [2.5–21.0] | 1.3§ [0–2.8] | 0 [0–1.0] |
| Epithelial cells, % | 1.5¶ [1.0–2.8] | 8.5‡§ [2.0–21.5] | 4.5 [1.3–8.0] | 3.5 [0.9–6.0] |
P < 0.05 compared with Atopic asthma.
P < 0.05 compared with Atopic control.
P < 0.01 compared with Normal control.
P < 0.01 compared with Atopic asthma.
P < 0.001 compared with Atopic control.
P < 0.001 compared with Normal control.
Data are expressed as median and interquartile range [25th–75th].
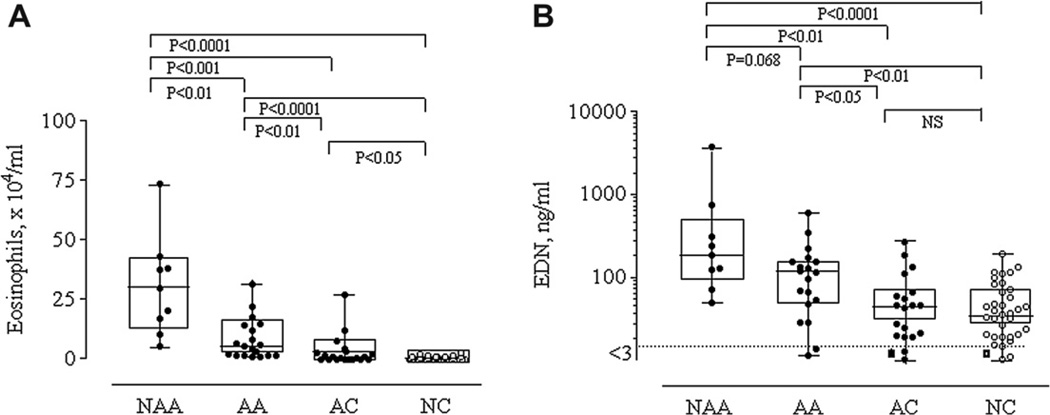
Absolute numbers of eosinophils and levels of EDN from the four groups are shown in Fig. 1-A and Fig. 1-B. A significant increase in the absolute eosinophil number was evident in asthmatic subjects (NAA and AA) compared with control subjects (AC and NC; P < 0.001, P < 0.0001, P < 0.01, P < 0.0001, respectively). Between the two asthmatic groups, NAA subjects showed significantly higher eosinophil absolute numbers compared to AA subjects (P < 0.01). Similarly, between the two control groups, AC subjects showed significantly higher eosinophil absolute numbers compared to NC subjects (P < 0.05). Absolute numbers of macrophages, neutrophils, lymphocytes and epithelial cells showed no significant differences among the 4 groups.
Figure 1.
Absolute numbers of sputum eosinophils (Fig. 1-A) and sputum EDN (Fig. 1-B) levels from the four groups. Levels are expressed as box and whisker plots with range and values displayed. Dot lines represent the sensitivity levels of the EDN assay.
A significant increase in EDN levels was also evident in asthmatic subjects (NAA and AA) compared with control subjects (AC and NC). Between the two asthmatic groups, NAA subjects showed higher EDN levels than AA subjects, although the difference did not reach statistical significance (P = 0.068). No differences in EDN levels were detected between AC and NC subjects.
Cytokine profiles
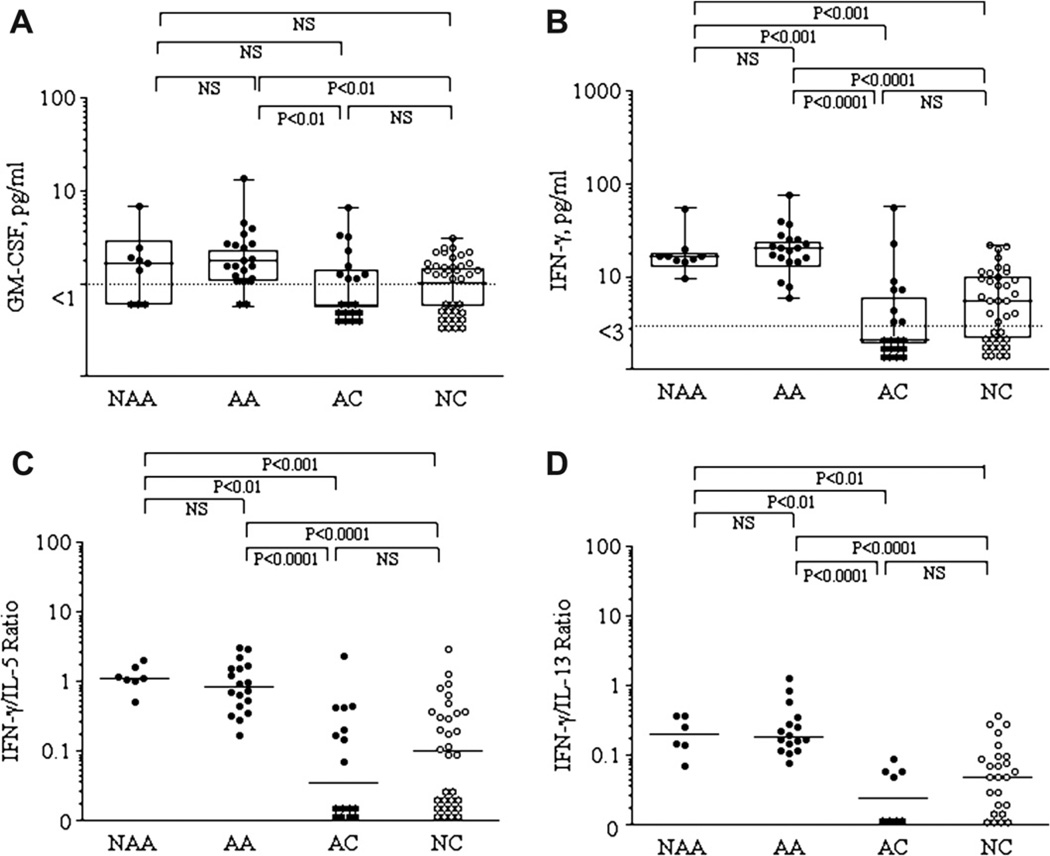
IL-4, IL-5, IL-13, GM-CSF and IFN-γ levels in the 4 groups were compared. No differences were observed in sputum levels of IL-4, IL-5, or IL-13 among the four groups (data not shown). GM-CSF levels (Fig. 2-A) were higher in NAA (1.7[0.0–2.2] pg/ml) and AA (1.8[1.2–2.7] pg/ml) patients compared to AC (0.0[0.0–1.5] pg/ml) and NC (1.3[0.0–1.7] pg/ml) subjects, although statistical significance was only reached in AA (P<0.01) patients. IFN-γ levels (Fig. 2-B) were higher in NAA (16.5[14.9–18.5] pg/ml) and AA (20.9[14.5–25.8] pg/ml) patients compared to AC (0.0[0.0–6.6] pg/ml, P < 0.001 and P < 0.0001) and NC (5.5[0.0–10.5] pg/ml, P < 0.001 and P < 0.0001) subjects. The ratios of IFN-γ/IL-4 (not shown), IFN-γ/IL-5 (Fig. 2-C) and IFN-γ/IL-13 (Fig. 2-D) were similarly higher in NAA and AA patients compared to AC and NC subjects. No differences were found between the two asthma groups or between the two control groups.
Figure 2.
Comparisons of sputum cytokines GM-CSF (2-A) and IFN-γ (Fig. 2-B) levels in the 4 groups, including IFN-γ/IL-5 (Fig. 2-C) and IFN-γ/IL-13 (Fig. 2-D) ratios. Levels are expressed as box and whisker plots with range and values displayed. Dot lines represent the sensitivity levels of the assay.
Chemokine profiles
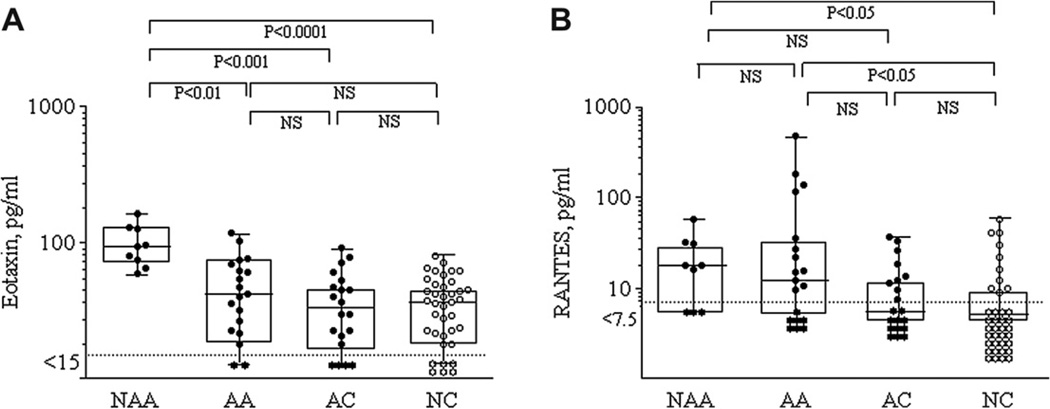
Comparisons of eotaxin and RANTES levels in the 4 groups are shown in Fig. 3A and B. Only NAA (94.4[70.7–129.1] pg/ml) patients showed higher eotaxin levels compared to AA (42.4 [22.8–70.8] pg/ml, P < 0.01), AC (33.4[18.6–51.7] pg/ml, P < 0.001), and NC (37.5[21.4–50.4] pg/ml, P < 0.0001). NAA (17.8[0–32.3] pg/ml) and AA (12.2[0–35.7] pg/ml) patients showed higher RANTES levels compared to NC (0[0–9.3] pg/ml) (P < 0.05). No differences were found between the two asthma groups or between the two control groups.
Figure 3.
Comparisons of sputum eotaxin (Fig. 3-A) and RANTES (Fig. 3-B) levels in the 4 groups. Levels are expressed as box and whisker plots with range and values displayed. Dot lines represent sensitivity levels of the assay.
Correlations
Correlations of cytokines IL-4, IL-5, IL-13, IFN-γ and GM-CSF and the chemokines eotaxin and RANTES with sputum eosinophils and EDN in NAA, AA, and AC subjects are shown in Table 2. Percentage of sputum eosinophils and EDN levels correlated positively with the levels of IFN-γ (r = 0.606 and p < 0.0001, r = 0.432 and p < 0.01), GM-CSF (r = 0.541 and p < 0.0001, r = 0.453 and p < 0.01), eotaxin (r = 0.640 and p < 0.0001, r = 0.514 and p < 0.001), and RANTES (r = 0.506 and p < 0.001, r = 0.480 and p < 0.001), but not with IL-4, IL-5, and IL-13 levels.
Table 2.
Correlations of sputum cytokines and chemokines with sputum eosinophils and EDN in NAA, AA, and AC patients.
| IL-4 | IL-5 | IL-13 | IFN-γ | GM-CSF | Eotaxin | RANTES | |
|---|---|---|---|---|---|---|---|
| Eosinophils, % | R = −0.160, P═NS | R = 0.099, P═NS | R = −0.049, P═NS | R = 0.606, P < 0.0001 | R = 0.541, P < 0.0001 | R = 0.640, P < 0.0001 | R = 0.506, P < 0.001 |
| Eosinophils, total | R = −0.132, P═NS | R = −0.130, P═NS | R = −0.121, P═NS | R = 0.524, P < 0.0001 | R = 0.273, P═NS | R = 0.675, P < 0.0001 | R = 0.541, P < 0.0001 |
| EDN | R = −0.201, P═NS | R = −0.058, P═NS | R = −0.227, P═NS | R = 0.432, P < 0.01 | R = 0.453, P < 0.01 | R = 0.514, P < 0.001 | R = 0.480, P < 0.001 |
Discussion
Measurement of mediators in an allergen challenge model or during acute exacerbation of asthma has been used widely to elucidate the roles of inflammatory mediators and cytokines in asthma patients. However, these models may not reflect the mechanism of chronic disease because of the acute exposure to extremely high concentrations of allergen. Therefore, we assessed airway inflammation in sputum from intrinsic and extrinsic asthmatics during their stable period of chronic asthma. In this study, we observed that NAA and AA patients had higher sputum eosinophils (% and total) and EDN levels compared to AC and NC. No differences were observed in IL-4, IL-5, and IL-13 levels among the four groups. In contrast, IFN-γ levels were higher in NAA and AA patients compared to AC and NC. GM-CSF levels were higher in AA patients compared to AC and NC. NAA and AA patients showed higher RANTES levels compared to NC; NAA patients showed higher eotaxin levels compared to AA, AC, and NC. The percentage of sputum eosinophils and EDN levels correlated positively with the levels of IFN-γ, GM-CSF, RANTES and eotaxin, but not with IL-5 levels. Thus, these sputum findings demonstrate that the baseline airway inflammation of asthma, irrespective of atopic or non-atopic diathesis, is characterized by eosinophilic inflammation and a Th1 cytokine, IFN-γ. GM-CSF, instead of IL-5, and chemokines may coordinate airway eosinophilia during the asymptomatic period of chronic asthma. Between non-atopic asthma and atopic asthma, the only differences were in sputum eosinophils and eotaxin. There was no qualitative difference between the 2 groups in terms of mediators.
A potential pitfall of our study was the difficulty in optimal measurement of the fluid-phase markers. There is currently no consensus among researchers as to whether DTT or protease inhibitors should be used in sputum processing. Most authors process sputum with DTT as a mucolytic19 that reduces disulfide bonds.21 However, evidence suggests that DTT has the potential to interfere with the detection of inflammatory mediators in the sputum fluid phase, either by affecting the three dimensional structure of proteins, or by interfering directly with the immunoassay.22 Furthermore, we observed that levels of IL-5 and eotaxin, but not EDN, were significantly lower in “with DTT” aliquots compared to “without DTT” aliquots (data not shown). Hence, we analyzed the fluid-phase marker levels only in the “without DTT” aliquot in the present study. Sputum proteases may also be responsible for degrading the mediators or interfering with the antibodies in the assay in some cases. Proteases may attain high levels in sputum, proportional to the clinical severity of asthma.23 Kelly et al.24 observed increased detection of IL-5 in sputum by addition of protease inhibitors. However, we believe this is not problematic in this study because most fluid-phase markers including IL-5 were recovered in our assay.
The “Th2 hypothesis for asthma”25 is controversial and more recent studies suggest it is far too simplistic.26 Animal studies have shown that Th1 cells do not counterbalance Th2-mediated effects but may worsen airway inflammation,27 while human asthma subjects have exhibited significant increases in IFN-γ, with no significant differences in IL-4 production in the supernatants of cultured bronchoalveolar cells.28 More recently, Magnan et al.29 demonstrated increased IFN-γ-producing CD8+ T cells in asthmatic airways, and the levels of IFN-γ correlated with asthma severity, bronchial hyperresponsiveness, and blood eosinophilia. Human eosinophils are known to express and be activated through the IFN-γ receptor.30 These observations question the concept that Th1 cytokine levels are decreased in patients with asthma.
Epidemiological studies suggest that allergy and asthma are not identical.31 Illi et al.32 demonstrated that only about a third of individuals with allergic rhinitis (caused by Th2 responses in the upper respiratory tract) develop asthma (caused by Th2 responses in the lower airways). Our results indicate the significant increase in a Th1 cytokine, IFN-γ, in both atopic and non-atopic asthma. There were no significant increases in Th2 cytokines, IL-4, IL-5, or IL-13 in the two asthma groups, whereas a typical Th2 pattern was found in AC subjects based on decreased IFN-γ/IL-4, IFN-γ/IL-5 and IFN-γ/IL-13 ratios. These results are consistent with the previous observation that IFN-γ production prominently appears in chronic phase lesions of atopic dermatitis.33 In our study, IFN-γ levels were higher in non-atopic and atopic asthmatics when compared to controls, and correlated with eosinophil-active cytokine GM-CSF and chemokines eotaxin and RANTES. The increased IFN-γ levels may be due to its release by airway eosinophils, leading to increased eosinophilic infiltration and greater IFN-γ release through autocrine stimulation.30,34 IFN-γ also induces/enhances the expression of intercellular adhesion molecule-1 (ICAM-1),35 which is important in eosinophil migration because it plays a critical role in eosinophil adhesion to endothelial cells in vivo36 and in vitro.37 This seems plausible as both non-atopic and atopic asthmatics had significantly greater sputum eosinophils (% and total) when compared to controls. In a murine model, activated eosinophils strongly induced IFN-γ expression in lungs, while Th2 inflammatory and regulatory responses, such as IL-4, IL-6, IL-9, IL-10, IL-13, and TGF-β, were minor or insignificant.34 Yamaguchi et al.38 found IFN-γ significantly enhanced GM-CSF-induced eosinophil degranulation. Other studies have shown that IFN-γ upregulated RANTES mRNA and protein expression,39 and enhanced eotaxin expression in airway epithelial cells.40 Although further confirmation is required, our sputum findings suggest that both intrinsic and extrinsic asthma may not follow a typical Th2 pattern, at least in asymptomatic chronic asthma.
The role of IL-5 in human asthma is not as strong as previously suspected. There are several reasons for low IL-5 or lack of difference in IL-5 levels between asthmatic and nonasthmatic patients other than protease activity, inhaled steroid use or asymptomatic phase of asthma. First, Flood-Page et al.41 observed that anti-IL-5 only reduced airway tissue eosinophils by 50%. Furthermore, the level of eosinophilic degranulation (MBP) within the bronchial mucosa remained unchanged by anti IL-5 treatment. Second, Liu et al.42 suggested that when eosinophils are recruited to the airway, regulation of their function becomes IL-5 independent. There was a striking reduction in membrane IL-5Rα on BAL eosinophils compared to blood eosinophils; in contrast, membrane GM-CSFRα was expressed on nearly 100% of BAL eosinophils. Furthermore, Hamilos et al.43 observed that GM-CSF mRNA, but not IL-5, was increased in chronic sinusitis with nasal polyposis and the degree of tissue eosinophilia most strongly associated with GM-CSF, not IL-5. GM-CSF activates mature eosinophils, increasing their degranulation, cytotoxicity, and response to chemoattractants. It is particularly important because mature, activated eosinophils lose their expression of IL-5Rs, while upregulating GM-CSF receptors; consequently, it is essential to prolonging eosinophil survival and function.44 These data raise the possibility that airway eosinophils are less dependent on IL-5 for recruitment and degranulation, and that GM-CSF may play a more prominent role by prolonging the survival and function of eosinophils in the airways. In partial contrast, a recently published study by Fanat et al.45 found anti-IL-5 and GM-CSF therapy reduced the eosinophilopoietic potential of airway smooth muscle cells, suggesting they may promote in situ eosinophilopoiesis in asthmatic lungs. As well, Haldar et al.10 and Nair et al.11 demonstrated the ability of anti-IL-5 therapy to reduce eosinophil numbers and asthma control in refractory eosinophilic asthma. It should be noted, however, in our study the lack of IL-5 in sputum during chronic asymptomatic asthma does not rule out its contribution to airway eosinophilia, as it may prime circulating eosinophils prior to their egress to the airway.
In this study, GM-CSF, IFN-γ, eotaxin and RANTES – but not IL-5 – correlated strongly with the percentage of sputum eosinophils. Therefore, we suggest that GM-CSF, instead of IL-5, and chemokines may coordinate airway eosinophilia during the chronic asymptomatic phase of asthma.
Acknowledgments
Funding
Supported in part by a grant from the National Institutes of Health (AI50494) and the Korea Science and Engineering Foundation (KOSEF 02-12-30-1).
Abbreviations
- IL
Interleukin
- NAA
Nonatopic asthmatic
- AA
Atopic asthmatic
- AC
Atopic control
- NC
Normal control
- EDN
Eosinophil-derived neurotoxin
- FEV1
Forced expiratory volume in 1 s
- DTT
Dithiothreitol
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population? Thorax. 1994;49:171–174. doi: 10.1136/thx.49.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 3.Wardlaw AJ, Dunnette S, Gleich GJ, Collins JV, Kay AB. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am Rev Respir Dis. 1988;137:62–69. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]
- 4.Gleich GJ, Adolphson CR, Kita H. The eosinophil and asthma. In: Busse WW, Holgate ST, editors. Asthma and rhinitis. 2nd ed. vol. 1. Oxford, England: Blackwell Science Ltd; 2000. pp. 429–479. [Google Scholar]
- 5.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651–663. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- 6.Garlisi CG, Falcone A, Kung TT, Stelts D, Pennline KJ, Beavis AJ, et al. T cells are necessary for Th2 cytokine production and eosinophil accumulation in airways of antigen-challenged allergic mice. Clin Immunol Immunopathol. 1995;75:75–83. doi: 10.1006/clin.1995.1055. [DOI] [PubMed] [Google Scholar]
- 7.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar Tlymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 8.Leckie MJ, ten Brinke A, Khan J, Diamant Z, O’Connor BJ, Walls CM, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 9.Lommatzsch M, Julius P, Kuepper M, Garn H, Bratke K, Irmscher A, et al. The course of allergen-induced leukocyte infiltration in human and experimental asthma. J Allergy Clin Immunol. 2006;118:91–97. doi: 10.1016/j.jaci.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 12.Barnes PJ. Intrinsic asthma: not so different from allergic asthma but driven by superantigens? Clin Exp Allergy. 2009;39:1145–1151. doi: 10.1111/j.1365-2222.2009.03298.x. [DOI] [PubMed] [Google Scholar]
- 13.Virchow JC, Jr, Walker C, Hafner D, Kortsik C, Werner P, Matthys H, et al. T cells and cytokines in bronchoalveolar lavage fluid after segmental allergen provocation in atopic asthma. Am J Respir Crit Care Med. 1995;151:960–968. doi: 10.1164/ajrccm/151.4.960. [DOI] [PubMed] [Google Scholar]
- 14.Humbert M, Durham SR, Ying S, Kimmitt P, Barkans J, Assoufi B, et al. IL-4 and IL-5 mRNA and protein in bronchial biopsies from patients with atopic and nonatopic asthma: evidence against “intrinsic” asthma being a distinct immunopathologic entity. Am J Respir Crit Care Med. 1996;154:1497–1504. doi: 10.1164/ajrccm.154.5.8912771. [DOI] [PubMed] [Google Scholar]
- 15.Walker C, Bauer W, Braun RK, Menz G, Braun P, Schwarz F, et al. Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia. Am J Respir Crit Care Med. 1994;150:1038–1048. doi: 10.1164/ajrccm.150.4.7921434. [DOI] [PubMed] [Google Scholar]
- 16.Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow JC, Jr, et al. Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992;146:109–115. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- 17.Humbert M, Menz G, Ying S, Corrigan CJ, Robinson DS, Durhan SR, et al. The immunopathology of extrinsic (atopic) and intrinsic (non-atopic) asthma: more similarities than differences. Immunol Today. 1999;20:528–533. doi: 10.1016/s0167-5699(99)01535-2. [DOI] [PubMed] [Google Scholar]
- 18.Kim CK, Hagan JB. Sputum tests in the diagnosis and monitoring of asthma. Ann Allergy Asthma Immunol. 2004;93:112–123. doi: 10.1016/S1081-1206(10)61462-7. [DOI] [PubMed] [Google Scholar]
- 19.Pizzichini E, Pizzichini MM, Efthimiadis A, Evans S, Morris MM, Squillace D, et al. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996;154:308–317. doi: 10.1164/ajrccm.154.2.8756799. [DOI] [PubMed] [Google Scholar]
- 20.Belda J, Leigh R, Parameswaran K, O’Byrne PM, Sears MR, Hargreave FE. Induced sputum cell counts in healthy adults. Am J Respir Crit Care Med. 2000;161:475–478. doi: 10.1164/ajrccm.161.2.9903097. [DOI] [PubMed] [Google Scholar]
- 21.Cleland WW. Dithiothreitol, a new protective reagent for SH groups. Biochemistry. 1964;35:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- 22.Grebski E, Peterson C, Medici TC. Effect of physical and chemical methods of homogenization on inflammatory mediators in sputum of asthma patients. Chest. 2001;119:1521–1525. doi: 10.1378/chest.119.5.1521. [DOI] [PubMed] [Google Scholar]
- 23.Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol. 1995;95:843–852. doi: 10.1016/s0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 24.Kelly MM, Leigh R, Carruthers S, Horsewood P, Gleich GJ, Hargreave FE, et al. Increased detection of interleukin-5 in sputum by addition of protease inhibitors. Eur Respir J. 2001;18:685–691. doi: 10.1183/09031936.01.00098501. [DOI] [PubMed] [Google Scholar]
- 25.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 26.Boushey HA, Fahy JV. Targeting cytokines in asthma therapy: round one. Lancet. 2000;356:2114–2116. doi: 10.1016/S0140-6736(00)03486-3. [DOI] [PubMed] [Google Scholar]
- 27.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest. 1999;103:175–183. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krug N, Madden J, Redington AE, Lackie P, Djukanovic R, Schauer U, et al. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Respir Cell Mol Biol. 1996;14:319–326. doi: 10.1165/ajrcmb.14.4.8600935. [DOI] [PubMed] [Google Scholar]
- 29.Magnan AO, Mely LG, Camilla CA, Badier MM, Montero-Julian FA, Guillot CM, et al. Assessment of the Th1/Th2 paradigm in whole blood in atopy and asthma. Increased IFN-gamma-producing CD8 (+) T cells in asthma. Am J Respir Crit Care Med. 2000;161:1790–1796. doi: 10.1164/ajrccm.161.6.9906130. [DOI] [PubMed] [Google Scholar]
- 30.Ishihara C, Ochiai K, Kagami M, Takashahi H, Matsuyama G, Yoshida S, et al. Human peripheral eosinophils express functional interferon-gamma receptors (IFN-gammaR) Clin Exp Immunol. 1997;110:524–529. doi: 10.1046/j.1365-2249.1997.4511469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearce N, Douwes J, Beasley R. Is allergen exposure the major primary cause of asthma? Thorax. 2000;55:424–431. doi: 10.1136/thorax.55.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Illi S, von Mutius E, Lau S, Nickel R, Niggemann B, Sommerfeld C, et al. Multicenter allergy study group: the pattern of atopic sensitization is associated with the development of asthma in childhood. J Allergy Clin Immunol. 2001;108:709–714. doi: 10.1067/mai.2001.118786. [DOI] [PubMed] [Google Scholar]
- 33.Werfel T, Morita A, Grewe M, Renz H, Wahn U, Krutmann J, et al. Allergen specificity of skin-infiltrating T cells is not restricted to a type-2 cytokine pattern in chronic skin lesions of atopic dermatitis. J Invest Dermatol. 1996;107:871–876. doi: 10.1111/1523-1747.ep12331164. [DOI] [PubMed] [Google Scholar]
- 34.Kanda A, Driss V, Hornez N, Abdallah M, Roumier T, Abboud G, et al. Eosinophil-derived IFN-γ induces airway hyper-responsiveness and lung inflammation in the absence of lymphocytes. J Allergy Clin Immunol. 2009;124:573–582. doi: 10.1016/j.jaci.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 35.Czech W, Krutmann J, Budnik A, Schopf E, Kapp A. Induction of intercellular adhesion molecule 1 (ICAM-1) expression in normal human eosinophils by inflammatory cytokines. J Invest Dermatol. 1993;100:417–423. doi: 10.1111/1523-1747.ep12472082. [DOI] [PubMed] [Google Scholar]
- 36.Zhenying N, Nelson CS, Jacoby DB, Fryer AD. Expression and regulation of intercellular adhesion molecule-1 on airway parasympathetic nerves. J Allergy Clin Immunol. 2007;119:1415–1422. doi: 10.1016/j.jaci.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Stark JM, Godding V, Sedgwick JB, Busse WW. Respiratory syncytial virus infection enhances neutrophil and eosinophil adhesion to cultured respiratory epithelial cells. J Immunol. 1996;156:4774–4782. [PubMed] [Google Scholar]
- 38.Yamaguchi T, Kimura H, Kurabayashi M, Kozawa K, Kato M. Interferon-gamma enhances human eosinophil effector functions induced by granulocyte-macrophage colony-stimulating factor or interleukin-5. Immunol Lett. 2008;118:88–95. doi: 10.1016/j.imlet.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Lacy P, Mahmudi-Azer S, Bablitz B, Hagen SC, Velazquez JR, Man SF, et al. Rapid mobilization of intracellularly stored RANTES in response to interferon-gamma in human eosinophils. Blood. 1999;94:23–32. [PubMed] [Google Scholar]
- 40.Matsukura S, Kokubu F, Kuga H, Kawaguchi M, Ieki K, Odaka M, et al. Differential regulation of eotaxin expression by IFN-gamma in airway epithelial cells. J Allergy Clin Immunol. 2003;111:1337–1344. doi: 10.1067/mai.2003.1513. [DOI] [PubMed] [Google Scholar]
- 41.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 42.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, et al. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: I. Loss of membrane IL-5 receptor alpha on airway eosinophils and increased soluble IL-5 receptor alpha in the airway after allergen challenge. J Immunol. 2002;169:6452–6458. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 43.Hamilos DL, Leung DY, Wood R, Cunningham L, Bean DK, Yasruel Z, et al. Evidence for distinct cytokine expression in allergic versus nonallergic chronic sinusitis. J Allergy Clin Immunol. 1995;96:537–544. doi: 10.1016/s0091-6749(95)70298-9. [DOI] [PubMed] [Google Scholar]
- 44.Simon HU. Regulation of cell survival. In: Adkinson NF Jr, Bochner BS, Busse WW, Holgate ST, Lemanske RF Jr, Simons FER, editors. Adkinson: middleton’s allergy: principles and practice. 7th ed. St. Louis: Mosby; 2008. pp. 413–422. [Google Scholar]
- 45.Fanat AI, Thomson JV, Radford K, Nair P, Sehmi R. Human airway smooth muscle promotes eosinophil differentiation. Clin Exp Allergy. 2009;39:1009–1117. doi: 10.1111/j.1365-2222.2009.03246.x. [DOI] [PubMed] [Google Scholar]