Abstract
Robust, facile high throughput assays based on non-peptidic probes are available to detect the enzyme activity of protein tyrosine phosphatases. However, these assays cannot replace the use of peptide-based probes in many applications; for example when a closer mimic of the physiological target is desired or in substrate profiling expeditions. Phosphotyrosine peptides are often used in these assays, but their use is complicated by either poor sensitivity or the need for indirect detection methods, among other pitfalls. Novel peptide-based probes for protein tyrosine phosphatases are needed to replace phosphotyrosine peptides and accelerate the field of tyrosine phosphatase substrate profiling. Here we review a type of peptidic probe for tyrosine phosphatases, which is based on the incorporation of the phosphotyrosine-mimic phosphocoumaryl amino propionic acid (pCAP) into peptides. The resulting fluorogenic pCAP peptides are dephosphorylated by tyrosine phosphatases with similar efficiency as the homologous phosphotyrosine peptides. pCAP peptides outperform phosphotyrosine peptides, providing an assay that is as robust, sensitive and facile as the non-peptidic fluorogenic probes on the market. Finally the use of pCAP can expand the range of phosphatase assays, facilitating the investigation of multiphosphorylated peptides and providing an in-gel assay for phosphatase activity.
Keywords: fluorogenic enzyme substrates, peptide synthesis, high-throughput screening, enzyme activity gel, multiply phosphorylated peptides
1 Introduction
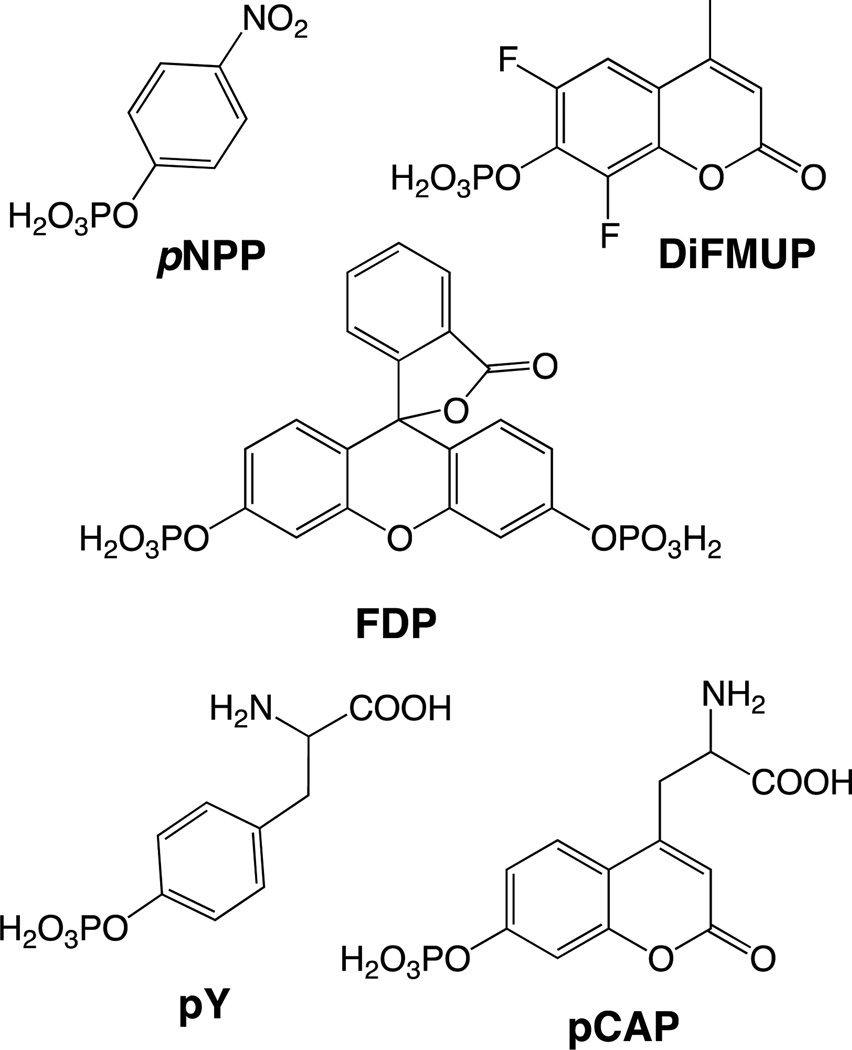
Ready availability of a convenient assay is a prerequisite for understanding the activity of any family of enzymes. Several assays have been developed for monitoring the activity of the protein tyrosine phosphatases (PTPs), key enzymes responsible for controlling numerous aspects of cellular signaling [1]. For example, the activity of a PTP on a phosphotyrosine (pY) containing peptide can be quantified by monitoring the increase in absorbance or fluorescence upon tyrosine dephosphorylation [2, 3] or by monitoring the release of inorganic phosphate [4, 5]. These methods work well, but measuring the absorbance or fluorescence of pY dephosphorylation offers only modest sensitivity and monitoring phosphate release requires quenching the reaction and adding additional reagents, resulting in a discontinuous assay. Alternatively, phosphotyrosine mimics have been used as substrates in PTP activity assays, including para-nitrophenylphosphate (pNPP) as a colorimetric reagent and difluoromethylumbelliferone phosphate (DiFMUP) [6] and fluorescein diphosphate (FDP) [7] as fluorogenic substrates. These substrates (depicted in Figure 1) provide excellent sensitivity, but cannot be used to address certain questions, such as the influence of peptide sequence on PTP-mediated dephosphorylation. The shortcomings of these commonly used substrates underscore the importance of expanding our toolkit of PTP probes.
Figure 1.
Chemical structures of the PTP substrates para-nitrophenylphosphate (pNPP), difluoromethylumbelliferyl phosphate (DiFMUP), fluorescein diphosphate (FDP), phosphotyrosine (pY) and phosphocoumaryl aminopropionic acid (pCAP).
It has become clear that the development of novel peptide-based assays for PTP activity would facilitate the study of these important enzymes. Several groups have made important contributions to this end [8, 9], designing substrates that provide a fluorescent signal after dephosphorylation and treatment with a protease. However, although these substrates can be good mimics of native PTP substrates, the assays are discontinuous and require the addition of a proteolytic enzyme to generate the fluorescent signal. We hypothesized that a sensitive, continuous, peptide-based assay would prove invaluable in probing the substrate specificity of the PTPs, provide an excellent substrate for use in high-throughput screening for novel PTP inhibitors, and perhaps lead to other applications in the study of PTP activity. To this end, we designed the phosphocoumaryl amino acid pCAP (Figure 1) [10]. By incorporating a phosphotyrosine mimetic phosphocoumarin moiety into peptides, we were able to obtain highly sensitive, fluorogenic, peptide-based PTP substrates. These pCAP peptides provide a facile assay for PTP activity in many different applications, representing a significant improvement over existing peptide-based assays. In addition, they can easily replace non-peptidic probes in well-established PTP assays, where they bring the advantage of a more physiological substrate without compromising sensitivity or robustness. In this review, we provide a standardized synthetic route to pCAP and pCAP peptides and highlight several general applications for these novel PTP substrates.
2. Materials and Methods
Chemicals, Fmoc-protected amino acids and peptide synthesis reagents required for the studies reported here were obtained from commercial sources and used as received, unless otherwise noted. The enzymatically active catalytic domains of several phosphatases are commercially available, including CD45, YopH, PTP1B, PTPH1 and MKP-5. Other phosphatase catalytic domains were expressed and purified as reported previously, including LYP [11], PTP-PEST [12], HePTP [13] and VHR [14]. The standard assay buffer used in PTP activity assays contained 50 mM Tris pH 6.5, 100 mM NaCl, 1 mM EDTA, 0.01% Brij 35, 0.1 mM DTT and between 1–10 nM of enzyme.
2.1. Synthesis of pCAP
The optimized synthesis of phosphocoumaryl amino propionic acid (pCAP) outlined in Scheme 1 is based on our previously reported work [10, 15]. The phosphotyrosine mimetic amino acid is synthesized from aspartate and resorcinol in high yield. Compound 1 is appropriately protected and fully compatible with Fmoc-based solid phase peptide synthesis. Note that variations can also be synthesized, for example, using glutamate or a fluorinated resorcinol [15]. While, in principle, a fluorinated phosphocoumarin could provide a more sensitive fluorogenic assay for PTP activity [6], and the glutamate-derived phosphocoumaryl amino acid could be a slightly better substrate than the aspartate-derived pCAP for some PTPs [15], the ease of pCAP synthesis coupled with its excellent utility as a substrate for many PTPs render it the fluorogenic phosphotyrosine mimic of choice in most applications.
Scheme 1. Synthesis of protected pCAP (1).
(i) Trichloroethanol, DCC, DMAP, 0 °C, 16 h, 99%. (ii) neet TFA, rt, 30 min, >99%. (iiia) DCC, Meldrum’s acid, DMAP, CH2Cl2, 0 °C- 2 h, rt-3 h. (iiib) CH3OH, Benzene (4:1), reflux, 12 h, 99%. (iv) CH3SO3H, 0 °C-6 h, 4 °C-24 h, 77%. (v) (EtO)2POCl, diisopropyl-ethylamine, CHCl3, rt, 14 h. 82%. (vi) Zn dust (6 equiv) 50% acetic acid in THF, rt, 12 h, 99%.
2.2. Synthesis of pCAP peptides
Peptides containing pCAP or pCAP derivatives can be synthesized using standard solid phase peptide synthesis (SPPS) protocols as reported previously [10, 15, 16]. The peptides synthesized for this work are summarized in Table 1. It may be desirable to perform a double coupling on the amino acid immediately N-terminal to the pCAP residue, however this is not always necessary. The one critical deviation from standard SPPS occurs after the peptide is fully synthesized at the resin cleavage and side chain deprotection step. After the final amino acid is incorporated and amine-deprotected or acylated (etc.) as desired, the phosphate ethyl ester protecting groups must be removed. To do this, the resin-bound peptide is treated with a 1 M solution of trimethylsilyliodide in dichloromethane for 1.5 h. The resin-bound peptide is then washed with methanol and dichloromethane and dried under vacuum prior to cleavage of the peptide from the resin. The successful synthesis of pCAP peptides has been carried out manually, on an automated peptide synthesizer, and by the University of Utah peptide synthesis facility (peptide 2). It should be noted that the yield for these peptides is a bit low (yields can be found in Table 1) – these phosphorylated peptides are often difficult peptide sequences to synthesize. However, in each case, we were able to obtain enough purified peptide to carry out kinetic studies.
Table 1.
Peptide sequences synthesized.
| Cmpd # |
Peptide sequence | Yield | MS (calc’d) |
MS (found) | reference |
|---|---|---|---|---|---|
| 2 | Ac-ARLIEDNE-pCAP-TAREG-NH2 | NA | 1826 | 1827 | [12] |
| 2a | Ac-ARLIEDNE-pY-TAREG-NH2 | NA | 1758 | 1759 | |
| 3 | Ac-DIDE-pY-LAA-NH2 | 13% | 1098 | 1120 (+Na) | [16] |
| 4 | Ac-FnGA-pCAP-QLEE-NH2 | 13% | 1258 | 1280 (+Na) | [16] |
| 5 | Ac-VFDQ-pCAP-HESP-NH2 | 25% | 1310 | 1310 | [16] |
| 6 | Ac-DPHR-pCAP-VWKR-NH2 | 35% | 1445 | 1445 | [16] |
| 7 | Ac-QEGL-pCAP-NELQKDKMAEA-pY-SEIG-NH2 | 20% | 2686 | 2687 | |
| 8 | Ac-QEGL-pY-NELQKDKMAEA-pCAP-SEIG-NH2 | 30% | 2686 | 2687 | |
| 9 | Ac-QEGL-pCAP-NELQKDKMAEA-Y-SEIG-NH2 | 27% | 2606 | 2607 | |
| 10 | Ac-QEGL-Y-NELQKDKMAEA-pCAP-SEIG-NH2 | 16% | 2606 | 2607 | |
| 11 | Ac-HDGL-pCAP-QGLSTATKDT-pY-DALH-NH2 | 10% | 2475 | 2476 | |
| 12 | Ac-HDGL-pY-QGLSTATKDT-pCAP-DALH-NH2 | 12% | 2475 | 2476 | |
| 13 | Ac-HDGL-pCAP-QGLSTATKDT-Y-DALH-NH2 | 12% | 2395 | 2396 | |
| 14 | Ac-HDGL-Y-QGLSTATKDT-pCAP-DALH-NH2 | 14% | 2395 | 2396 | |
| 15 | Ac-HTGFL-pT-E-pCAP-VATRW-NH2 | 7% | 1849 | 1851 | |
| 16 | Ac-HTGFL-T-E-pCAP-VATRW-NH2 | 21% | 1770 | 1770 | |
| 17 | Acr-Ahx-DADE-pCAP-LIPQQG-NH2 | 3% | 1564 | 1566 | |
| 18 | Acr-Ahx-DADE-Et2pCAP-LIPQQG-NH2 | 3% | 1620 | 1642 (+Na) | |
| 19 | Ac-EDNE-pCAP-TARE-NH2 | NA | 1314 | 1336 (+ Na) | |
| 20 | C14-βAla-R7-EDNE-pCAP-TARE-NH2 | NA | 2646 | 2630 (−H2O) | [17] |
| 21 | Cyclo[(pCAP)FΦRRRQ] (Φ=L-2-naphthylalanine) | NA | 1538 | 1538 | [18] |
2.3. Assaying PTP activity using pCAP peptides
The PTP enzyme activity assays can be carried out at room temperature or a fixed temperature (30°C or 37°C, for example) in an appropriate PTP activity buffer. For an excellent review of standard conditions commonly employed to assay PTP activity, see reference [1]. Enzyme concentrations in the low nanomolar range (5–500 nM, for example) are often sufficient to measure kinetic parameters using pCAP peptides. To obtain Michaelis-Menten parameters, initial velocities can be measured at several different substrate concentrations in the range between approximately 0.5 and 250 µM. The increase in fluorescence at 460 nm when the substrate is exposed to a PTP can be measured in a fluorimeter with 340 nm excitation. Generally, reactions can be monitored continuously for 30 min. No appreciable fluorescence is observed in the absence of enzyme, consistent with the stability of pCAP against spontaneous hydrolysis.
In order to compare the utility of the pCAP peptide assay with that of other commonly used PTP activity assays, we measured LYP-mediated hydrolysis of peptides 2 and 2a, methylumbelliferyl phosphate (MUP) and difluoromethylumbelliferyl phosphate (DiFMUP). The hydrolysis of peptide 2a was measured using both the absorbance-based Malachite Green assay [4, 5] and also the tyrosine fluorescence assay [2, 3]. In these experiments, 5 nM of LYP was activated by pre-incubation for one hour in a buffer containing 50 mM Tris, pH 6.5, 100 mM NaCl, 1 mM dithiothreitol (DTT), 2 mM EDTA and 0.01 % Brij 35. After this preincubation period, the substrate was added and the reaction monitored as appropriate for the individual assay [1].
2.4. Synthesis and utility of a peptide substrate library
In addition to the synthesis of individual peptide substrates, libraries of pCAP peptides can also be synthesized and used to assess enzyme substrate selectivity and to obtain peptide sequences targeted to an individual PTP. For example, two positionally scanned combinatorial libraries were synthesized, one scanning the four positions N-terminal to a central pCAP residue in the sequence XXXX-pCAP-LAA and the other scanning the four positions C-terminal to the pCAP residue in the sequence DIDE-pCAP-XXXX [16]. In addition to this positional scanning approach where one position is spatially anchored and the other three positions are fully randomized, it is also possible to use pCAP peptides in an inverse alanine scanning approach [19, 20] or a one-bead, one-compound combinatorial peptide library approach [21, 22].
2.5. Using pCAP peptides to assess enzyme selectivity on multiply phosphorylated peptides
The pCAP residue is an excellent tool for probing PTP substrate preferences within multiply phosphorylated peptides. Peptides containing pCAP and pY/pT/pS can readily be synthesized using the standard peptide synthesis methodologies discussed in section 2.2. above. By substituting pCAP for a phosphotyrosine and taking advantage of the fluorescent readout provided by pCAP peptides, one can isolate the dephosphorylation of a single phosphotyrosine residue in the presence of other phosphorylated residues in the same substrate.
2.5.1. Dephosphorylation of substrates with tandem phosphotyrosine residues
To investigate the ability of PTPs to discriminate among substrates containing tandem phosphotyrosine residues, we analyzed the dephosphorylation of peptides designed from the immunoreceptor tyrosine-based activation motifs (ITAMs) of the ζ-chain of the T cell receptor (TCR). The TCR ζ-chain contains three sequential ITAMs (termed A, B and C), each containing the consensus sequence YXX(L/I)X6–8YXX(L/I) with two potential tyrosine phosphorylation sites [23, 24]. Substituting pCAP for either phosphotyrosine, we examined the efficiency of dephosphorylation of each phosphosite in ITAM B and ITAM C by two PTPs expressed in T cells and proposed as regulators of TCR ζ-chain ITAM phosphorylation; Protein Tyrosine Phosphatase H1 (PTPH1) and Lymphoid Tyrosine Phosphatase (LYP) [25, 26]. Importantly, we were able to monitor whether the efficiency of dephosphorylation of each phosphosite was affected by the phosphorylation status of the additional tyrosine residue in the peptide. We synthesized pCAP-based peptides derived from the ITAM B and ITAM C consensus sequences (see peptides 7–10 and 11–14, respectively, in Table 1). To obtain kinetic parameters, increasing concentrations of each peptide were incubated with 50 nM recombinant PTPH1 (MBL International) or 50 nM recombinant catalytic domain of LYP (amino acids 2–309, isolated in our laboratories [27]) in a buffer containing 50 mM Bis-Tris pH 6.0 and 1 mM DTT at 37°C. The resulting dephosphorylation of the pCAP residue was measured by monitoring the increase in fluorescence at λex=340 nm with λem=460 nm.
2.5.2. Dephosphorylation of substrates containing phosphotyrosine and phosphoserine/threonine by Dual-Specificity Phosphatases
As an example of substrates containing tandem phosphotyrosine and phosphoserine/threonine residues, we analyzed the dephosphorylation of peptides designed from the activation motif of the Extracellular signal-Related Kinases 1 and 2 (ERK1 and ERK2). ERK1, ERK2 and other Mitogen-Activated Protein Kinases (MAPKs) contain a TXY motif in the activation loop of the kinase that can be phosphorylated on both the threonine and the tyrosine residues [28, 29]. Using pCAP as a substitute for phosphotyrosine, we examined the efficiency of dephosphorylation of the pCAP residue in the presence of either phosphothreonine or threonine by two Dual-Specificity Phosphatases (DSPs) proposed to regulate MAPK phosphorylation, Vaccinia H1-Related Phosphatase (VHR) [30] and Map Kinase Phosphatase 5 (MKP-5) [31]. We synthesized pCAP-based peptides derived from the ERK1/2 activation loop (see peptides 15 and 16 in Table 1). To obtain kinetic parameters, increasing concentrations of each peptide were incubated with 250 nM recombinant VHR (isolated in our laboratory) or 50 nM recombinant MKP-5 (Enzo Life Sciences) in a buffer containing 50 mM Bis-Tris pH 6.0 and 1 mM DTT at 37°C. The resulting dephosphorylation of the pCAP residue was measured by monitoring the increase in fluorescence at λex=340 nm with λem=460 nm.
2.6. pCAP peptides in inhibitor screens
Based on the ease of detecting PTP activity using pCAP peptides, it is not surprising that these peptides should be excellent substrates for high throughput screening. We have carried out low [12] and medium [32] throughput assays in 96-well plate format using a buffer containing 50 mM Tris pH 6.5, 100 mM NaCl, 1 mM EDTA, 0.01% Brij 35, 0.1 mM DTT, 5 nM of LYP and 10 µM [32] or 30 µM [12] of peptide 2 (Table 1). The assay can also be miniaturized into 1536-well plate format for high throughput screening. In collaboration with the Conrad Prebys Center for Chemical Genomics (CPCCG) at the Sanford|Burnham Medical Research Institute (S|B), we carried out a screen of the NIH Molecular Libraries Small Molecules Repository (MLSMR) to identify novel inhibitors of LYP (PubChem AID:1779). The assay buffer contained 50 mM Bis-Tris pH 6.0, 1 mM DTT, 0.1% BSA and 0.005% Tween 20. The pCAP peptide 2 (Table 1) was used as the substrate at a final concentration of 25 µM. Approximately 290,000 compounds were tested at a final concentration of 11.5 µM each using this approach. The Z’ value for the assay was 0.88, indicating excellent performance in high throughput screening [33]. The signal to background ratio of ∼55, signal to noise ratio of ∼730, and signal to window of 27, also supported a very robust 1536-well assay. The HTS campaigns yielded a relatively low hit rate of 0.05% and ultimately a compound (PubChem CID9595032) was obtained with a verified 3 to 5-fold greater potency when assayed using the pCAP substrate (0.53 µM IC50) versus the OMFP substrate (1.5 µM IC50).
2.7. Phosphogram gels
The ability to visualize enzyme activity in a gel is often desirable. To this end, pCAP peptides were incorporated into polyacrylamide gels and the resulting “phosphogram” gels were useful in monitoring PTP activity directly in the gel, analogous to the zymogram gels that facilitate direct detection of protease activity.
2.7.1. Synthesis of acryloyl pCAP peptides
Acryloylated peptides 18 and 19, suitable for polymerization into polyacrylamide gels, were synthesized as described in section 2.2 above. N-terminal acryloylation of the peptides was carried out using a slight modification of a previously published procedure [34]. After synthesis of the base peptide sequence, 6-aminohexanoic acid (Ahx) was coupled using standard HOBT/DICI techniques. The N-terminus was deprotected with 1 mL 2% DBU and the resin washed with amine-free DMF and dichloromethane. The resin-bound peptide was then transferred into a round bottom flask under nitrogen, swelled with 1.5 mL anhydrous DMF for 0.5 h and cooled on ice. To this, 20 equiv of DIPEA in 2 mL anhydrous DMF and 10 equiv of acryloyl chloride in 1 mL DMF were added sequentially and the reaction mixture stirred under a nitrogen atmosphere at 0 °C for 1 h and at room temperature for another h. The resin was transferred back into a peptide synthesis column and rinsed with DMF and dichloromethane. The diethylphosphate protecting group of the pCAP residue in peptide 18 was removed with a 1 M solution of trimethylsilyliodide (TMSI) in DCM for 1 h (this was not done for peptide 19). The side chain protecting groups were then removed simultaneously with peptide cleavage from the resin by treatment with a cleavage cocktail containing 88% TFA, 5% water, 2% triisopropylsilane (TIS) and 5% phenol for at least 4 h. Peptides were purified on a Varian ProStar 210 semi-preparative scale HPLC and purified peptides characterized by MALDI-TOF on a Micromass MALDI Micro MX spectrometer using α-cyano-4-hydroxycinnamic acid as matrix. The yield for these peptides is low but unoptimized.
2.7.2. Copolymerization of acryloyl pCAP peptides into gel
A 1:87.5 or 1:175 molar ratio of peptide to acrylamide was used in the 10% acrylamide gels, corresponding to 4 mg and 2 mg of peptide to 0.35 g of acrylamide, respectively. The resolving gel was prepared by adding the peptide to 3.5 mL of a mixture consisting of 1.7 mL Milli-Q water, 0.875 mL 1.5 M Tris pH 8.8, 0.875 mL of 40% acrylamide solution, 35 µL of 10% SDS, 35 µL of 10% ammonium persulfate and the mixture degassed for 5 min. To this, 1.4 µL of N,N,N’,N’-tetramethylethylenediamine (TMEDA) was added to initiate polymerization. The stacking gel consisted of 1.48 mL Milli-Q water, 0.250 mL 1.5 M Tris pH 6.8, 0.250 mL of 40% acrylamide solution, 20 µL of 10 % SDS, 20 µL of 10% ammonium persulfate and 2 µL TMEDA.
2.7.3. SDS-PAGE and Gel Treatments
Varying amounts of purified PTPs (0.25 µg to 1 µg) were treated with 20, 40 or 50 mM iodoacetic acid (IAA) or left untreated prior to SDS-PAGE. IAA treated samples were prepared as follows: the PTP was first treated with 0.5 mM tris(2-carboxyethyl)phosphine (TCEP) for 0.5 h at room temperature, followed by treatment with IAA (20, 40 or 50 mM, depending on the PTP) for a further 0.5 h at room temperature. Following IAA treatments samples were treated with NuPAGE LDS sample buffer 4x, heated for 4 min at 95 °C and subjected to SDS-PAGE.
Aliquots of IAA treated or untreated PTPs were run on gels copolymerized with the peptide, under standard conditions using a Bio-Rad Mini Protean gel electrophoresis system with a running buffer containing 25 mM Tris, 250 mM glycine and 0.1% SDS at 25 mA. Following electrophoresis, gels were subjected to the following treatments modified from a previously published procedure [35]. Gels were first treated with 50 mM Tris•HCl, pH 7.2 containing 20% isopropanol for 1.5 h, washed twice with 50 mM Tris•HCl, pH 7.2 and 0.3% β-mercaptoethanol for 0.5 h each and incubated in the above buffer with 6 M guanidine hydrochloride and 1 mM EDTA for 1.5 h. Gels were then incubated in renaturing buffer consisting of 50 mM Tris•HCl, pH 7.2, 0.3% β-mercaptoethanol, 0.04% Tween-20, 1 mM EDTA twice for 1 h each. Final renaturation of PTPs was achieved by incubation with the above renaturing buffer containing 4 mM DTT overnight. PTPs were then imaged on a quantity one GelDoc System from BioRad Laboratories and stained using GelCode Blue.
2.8. Visualizing intracellular PTP activity using pCAP peptides
In order to investigate whether pCAP peptides could be useful for visualizing PTP activity in cells, we investigated a series of complementary approaches for introducing pCAP peptides into cells. Peptide 19 could be introduced into cells via microinjection (sea urchin oocytes [17]) or electroporation (Jurkat T cells [17]). The addition of a basic and hydrophobic tag, either in a linear peptide (peptide 20) or a cyclic peptide (peptide 21), facilitated spontaneous uptake primarily to the cytosol in a variety of cell types, including Jurkat T cells, RAW 264.7 macrophages and MCF-7 cells [17, 18]. In all cases, the internalized pCAP peptide was rapidly dephosphorylated by intracellular phosphatases to produce fluorescent cells. Importantly, the pCAP peptides can be hydrolyzed only by PTP activity. Serine/threonine phosphatases are not able to dephosphorylate the pCAP peptides, and kinases are unable to appreciably rephosphorylate the resulting CAP peptides [17, 18].
3. Results and Discussion
3.1. pCAP: A Fluorogenic Phosphotyrosine Mimic
Early on, we and others recognized that having a peptide based, fluorogenic assay for PTP activity would be critical for studying this family of enzymes. We hypothesized that an amino acid derivative of DiFMUP would be a very useful tool for monitoring PTP activity, identifying substrate sequence preferences and performing high-throughput screens for novel PTP inhibitors. To this end, we devised the synthesis of pCAP and derivatives.
Enantiomerically pure L-aspartic acid was chosen as the starting point in this synthesis, as outlined in Scheme 1. The α-carboxylic acid of aspartic acid was first protected as a trichloroethyl (TCE) ester. The β-carboxylic acid was then deprotected and acylated using isopropenyl chloroformate and Meldrum’s acid to obtain the β-ketoester in an overall yield of 90% starting from aspartic acid. Methanesulfonic acid had been reported to catalyze the condensation between an amino acid derived β-ketoester and resorcinol at room temperature, preserving the chirality of the stereocenter [36]. In our laboratory, the reaction yield of the N and C-protected coumaryl amino acid was higher when carried out at 0–4°C. Subsequent phosphorylation with diethylchlorophosphate and diisopropylethylamine followed by removal of the TCE group using 50% acetic acid in tetrahydrofuran in the presence of activated zinc dust generated the phosphorylated coumaryl amino acid (pCAP) (1). As illustrated in Scheme 1, this approach generates an enantiomerically pure, appropriately protected phosphocoumaryl-amino-propionic acid (1, pCAP) in high yield via six facile steps from readily available starting materials. Full synthetic details can be found in reference [15]. This synthesis is superior to other reported syntheses of coumaryl amino acids in its simplicity, yield, and versatility.
3.2. Synthesis of pCAP peptides
In order to demonstrate the utility of pCAP in in vitro PTP assays, we synthesized a series of peptide substrates with the general formula Ac-ARLIEDNE-X-TAREG-NH2, where X = pCAP (peptide 2, Table 1) or phosphotyrosine (peptide 2a, Table 1). The coumarin-based amino acids are compatible with standard, Fmoc-based SPPS methodologies [37]. Deprotection of the phosphate moiety was accomplished by the addition of trimethylsilyliodide to the peptide prior to cleavage from the resin. The peptides were then fully deprotected and cleaved from the resin, purified by reverse phase HPLC, and characterized by mass spectrometry. The peptide sequence Ac-ARLIEDNE-X-TAREG-NH2 is expected to be a reasonable, though not optimal, substrate for many PTPs.
3.3. pCAP peptides as substrates for assaying PTP activity
Because the pCAP moiety is slightly larger than phosphotyrosine, we set out to establish the efficiency of enzymatic turnover of 2. As shown in Table 2, peptide 2 is readily dephosphorylated by the lymphoid tyrosine phosphatase, LYP. Peptide 2 (kcat/Km = 27,000,000 M−1s−1) is hydrolyzed about as efficiently as the phosphotyrosine containing peptide 2a (kcat/Km = 52,000,000 M−1s−1). The kinetic data are summarized in Table 2. LYP readily accepts the pCAP moiety as a phosphotyrosine mimic. Indeed, we have not yet found a PTP that is unable to hydrolyze pCAP peptides.
Table 2.
Kinetics of YOP-mediated phosphopeptide dephosphorylation.
| Peptide # |
Peptide sequence | kcat(s−1) | Km (µM) | kcat/Km (M−1s−1) |
|---|---|---|---|---|
| 2 | Ac-ARLIEDNE-pCAP-TAREG-NH2 | 5400 ± 1000 | 200 ± 60 | 27,000,000 |
| 2a | Ac-ARLIEDNE-pY-TAREG-NH2 | 6700 ± 400 | 130 ± 20 | 52,000,000 |
We also carried out a direct comparison of our pCAP peptide-based assay for LYP activity with some of the most commonly used existing assays. In these experiments, we attempted to standardize the assays for comparison, optimizing conditions to achieve a signal to background ratio of approximately 10 using 5 nM of LYP in a standard activity buffer. As highlighted in Table 3, the assays with phosphotyrosine peptide and MUP required an exorbitant amount of substrate (500 µM) to achieve S/B ratios of 2–6. While the malachite green assay provided greater S/B than the fluorescence assay with peptide 2a, neither is optimal and both require large amounts of peptide. In contrast, the DiFMUP and pCAP peptide assays achieve S/B ratios of 10 or more at a much lower substrate concentration. When saturating substrate concentrations are desirable (for example, under some inhibitor screening conditions [27]), a commercially available phosphotyrosine mimic such as DiFMUP (or pNPP, not compared here) would be preferable. But in experiments where a non-saturating substrate concentration or a better mimic of the biological substrate is desired, pCAP peptides are the best option.
Table 3.
A comparison of commonly used PTP assays using 5 nM LYP.
| Substrate | Method | Readout | [substrate] | S/B |
|---|---|---|---|---|
| Peptide 2 | Fluorescence | λex= 360 nm, λem = 455 nm |
10 µM | 10 |
| Peptide 2a | Fluorescence | λex = 280 nm, λem = 305 nm |
500 µM | 2.0 |
| Peptide 2a | Malachite green | Absorbance at 620 nm |
500 µM | 6.0 |
| MUP | Fluorescence | λex = 360 nm, λem = 455 nm |
500 µM | 6.5 |
| DiFMUP | Fluorescence | λex = 360 nm, λem = 455 nm |
0.50 µM | 25 |
3.4. Using pCAP peptides to profile substrate selectivity
In addition to serving as substrates for monitoring PTP activity, libraries of pCAP peptides can be used to profile PTP substrate selectivity. In principle, many combinatorial peptide library formats could be used, including inverse alanine scanning libraries [19], one bead one compound libraries [21] and positionally scanned peptide libraries [38, 39]. As an example, we developed a positionally scanned synthetic combinatorial library of pCAP peptides, scanning four amino acids on either side of a central pCAP residue [16]. Using this library, we profiled the substrate selectivity of TCPTP. As shown in Figure 2, TCPTP exhibited slight preferences for Phe and Leu at the −4 position and Ala at the −1 position, a marked preference for hydrophobic residues at −3 and little selectivity at the −2 position. Interestingly, acidic amino acids were not preferred residues at any of the N-terminal sites. This result, though in contrast to conventional wisdom about PTP substrate recognition and other published work [40], validates other recent data on the substrate selectivity of TCPTP [21]. On the C-terminal side, TCPTP displays a marked bias against Pro at the +1 position, a preference for Glu at the +3 position and little selectivity at the +2 and +4 positions.
Figure 2. The N- and C-terminal substrate selectivity profiles of TCPTP.
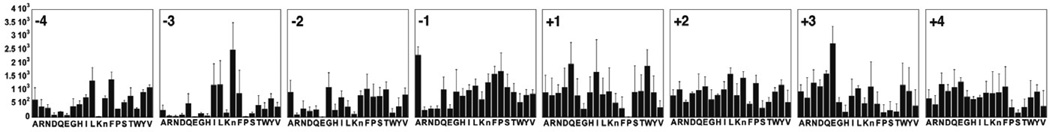
The y-axis shows the increase in relative fluorescence intensity produced upon substrate hydrolysis. The amino acids at each position are spatially addressed along the x-axis. The numbering scheme represents the relative position with respect to the pCAP residue, as defined in the sequence (−4)–(−3)–(−2)–(−1)–pCAP–(+1)–(+2)–(+3)–(+4). Adapted from reference [14].
In order to verify the library profile of TCPTP, we synthesized individual peptide substrates based on the most and least preferred amino acids at each position. When the “best” amino acids at each position are combined, the resulting peptide, Ac-FnGA-pCAP-QLEE-NH2 (peptide 4), is hydrolyzed readily by the enzyme with significantly higher turnover than the library base sequence Ac-DIDE-pCAP-LAA-NH2 (peptide 3) (see Table 4). On the other hand, when some of the “worst” amino acids at each position are combined, no hydrolysis of the resulting peptide, Ac-VFDQ-pCAP-HESP-NH2 (peptide 5), was observed. A peptide based on mediocre amino acids at each position, Ac-DPHR-pCAP-VWKR-NH2 (peptide 6), was hydrolyzed over ten times less efficiently than the “best” substrate. The results obtained with purified peptide substrates validate the library profiles for TCPTP. The peptide sequence optimized for TCPTP can be converted into a TCPTP-selective inhibitor by replacing the pCAP residue with a nonhydrolyzable phosphotyrosine mimetic such as phosphonomethylphenylalanine (Pmp) as reported previously [16].
Table 4.
Kinetic parameters for TCPTP-mediated hydrolysis of several peptide substrates
| Peptide # | Peptide sequence | kcat (s−1) | Km (mM) | kcat/Km (M−1s−1) |
|---|---|---|---|---|
| 3 | Ac-DIDE-pCAP-LAA-NH2 | 92 ± 5 | 0.32 ± 0.02 | 290,000 |
| 4 | Ac-FnGA-pCAP-QLEE-NH2 | 160 ± 30 | 0.27 ± 0.05 | 600,000 |
| 5 | Ac-VFDQ-pCAP-HESP-NH2 | 11.5 ± 0.5 | 0.28 ± 0.03 | 41,000 |
| 6 | Ac-DPHR-pCAP-VWKR-NH2 | NR | NR | NR |
3.5. Using pCAP peptides to assess selectivity in multiply phosphorylated peptides
Another critical question regarding the substrate selectivity of the PTPs is how these enzymes act on multiply phosphorylated substrates. While the use of singly-phosphorylated phosphotyrosine peptides would allow kinetic examination of the dephosphorylation of each phosphotyrosine residue, this approach would not allow isolation of the effect of the phosphorylation on a second tyrosine residue in the substrate.
The ζ-chain of the TCR is a prototypical signaling molecule with multiple tandem tyrosine phosphorylated residues. The kinetics and order of phosphorylation and dephosphorylation of tandem Tyr residues in the ζ-chain affects the biological response to engagement of the TCR [41]. The generation of combined pCAP/pTyr ζ-chain-derived pCAP peptides extended the range of approaches available for the biochemical study of tandem site dephosphorylation. While PTPH1 and LYP have been proposed as candidate PTPs regulating the phosphorylation status of the TCR ζ-chain [25, 26], dissection of the efficiency of these enzymes at dephosphorylation of each phosphorylation site has yet to be conducted. Results from these studies are summarized in Table 5.
Table 5.
Kinetic parameters for PTPH1 and LYP-mediated hydrolysis of peptides based on TCR ζ-chain ITAMs
| Peptide | KM (µM) [95% CI] | kcat (s−1) [95% CI] | kcat/Km (s−1mM−1) | # of Experiments |
|
|---|---|---|---|---|---|
| PTPH1 | |||||
| 7; ITAM B pCAP/pY | 4.44 [3.88–5.08] | 0.77 [0.74–0.81] | 173.4 | 5 | |
| 8; ITAM B pY/pCAP | 4.26 [3.76–4.81] | 1.05 [1.01–1.09] | 246.5 | 5 | |
| 9; ITAM B pCAP/Y | 9.49 [8.68–10.34] | 2.16 [2.09–2.22] | 227.3 | 6 | |
| 10; ITAM B Y/pCAP | 8.64 [7.92–9.41] | 2.02 [1.96–2.07] | 233.1 | 6 | |
| 11; ITAM C pCAP/pY | 14.41 [10.90–17.91] | 0.33 [0.31–0.35] | 22.8 | 3 | |
| 12; ITAM C pY/pCAP | 19.09 [14.98–23.20] | 0.69 [0.65–0.72] | 36.0 | 3 | |
| 13; ITAM C pCAP/Y | 13.70 [10.36–17.04] | 1.12 [1.06–1.18] | 81.7 | 3 | |
| 14; ITAM C Y/pCAP | 25.13 [20.88–29.37] | 1.45 [1.38–1.52] | 57.7 | 3 | |
| LYP | |||||
| 7; ITAM B pCAP/pY | 1.77 [1.38–2.24] | 0.22 [0.20–0.24] | 125.4 | 4 | |
| 8; ITAM B pY/pCAP | 1.87 [1.36–2.49] | 0.36 [0.32–0.39] | 191.4 | 4 | |
| 9; ITAM B pCAP/Y | 6.81 [6.20–7.49] | 0.78 [0.76–0.81] | 114.9 | 3 | |
| 10; ITAM B Y/pCAP | 6.09 [5.26–7.02] | 0.76 [0.72–0.79] | 124.2 | 3 | |
PTPH1 showed generally lower efficiency of dephosphorylation of the ITAM C-based peptide versus the ITAM-B based peptide. LYP on the other hand, dephosphorylated the ITAM B peptide, but showed nearly undetectable hydrolysis of the ITAM C peptide, suggesting that PTPH1 is a more diffuse regulator of the TCR ζ-chain than LYP. On the singly phosphorylated ITAM B peptides, PTPH1 showed near equal efficiency whether the pCAP was in the first or second position. Phosphorylation of the additional residue increased the affinity of PTPH1 for pCAP at either position, as shown by the decreased KM values on the double-phosphorylated peptides. The increased affinity is likely due to a cooperative mechanism to recruit the enzyme to the multi-phosphorylated substrate. The turnover rate, however, also decreased for pCAP in either position with the addition of the phosphate at the other site, as the two sites were thus in competition for binding to the PTP active site. The cumulative effect of the double phosphorylation was to increase efficiency of PTPH1 dephosphorylation of the peptide when pCAP was in the second position, but decrease efficiency when pCAP was in the first position. These results suggest that when ITAM B is in full phosphorylation status, the second phosphotyrosine is likely to be dephosphorylated first by PTPH1. LYP showed a nearly identical trend of dephosphorylation of the ITAM B peptides as PTPH1.
3.5.2. Dephosphorylation of substrates containing phosphotyrosine and phosphoserine/threonine by DSPs
We also analyzed the effect of threonine phosphorylation on the dephosphorylation of phosphotyrosine in ERK1/2 based peptides by the DSPs VHR and MKP-5. This is an example of a biologically critical double pThr/pTyr phosphorylated site that is regulated by several phosphatases, including the dual specificity enzymes VHR and MKP-5 [42]. The combined pThr/pCAP peptide enabled an easy and direct approach to the biochemical study of the dephosphorylation of doubly phosphorylated peptide by enzymes that can in principle dephosphorylate both pThr and pTyr. Surprisingly, dephosphorylation of the pCAP residue by VHR was unaffected by the phosphorylation status of the threonine residue (see Table 6). Both the KM and kcat values were nearly identical between the single and double phosphorylated peptides. These results confirm previous findings that within the activation loop of ERK1 or ERK2, the phosphotyrosine is the predominant substrate of VHR [42]. MKP-5, on the other hand, showed a slight preference for the single phosphorylated pCAP peptide. Addition of phosphate to the threonine reduced the affinity of MKP-5 for the pCAP peptide, yet also intriguingly increased the rate of hydrolysis of the pCAP.
Table 6.
Kinetic parameters for VHR and MKP-5-mediated hydrolysis of peptides based on ERK1/2 activation loop
| Peptide | KM (µM) [95% CI] | kcat (s−1) [95% CI] | kcat/Km (s−1mM−1) | # of Experiments |
|
|---|---|---|---|---|---|
| VHR | |||||
| 15; ERK1/2 pT/pCAP | 48.9 [36.6–61.2] | 0.26 [0.23–0.28] | 5.22 | 3 | |
| 16; ERK1/2 T/pCAP | 47.6 [33.1–62.0] | 0.28 [0.25–0.31] | 5.88 | 3 | |
| MKP-5 | |||||
| 15; ERK1/2 pT/pCAP | 25.8 [20.3–31.2] | 0.24 [0.22–0.25] | 9.19 | 3 | |
| 16; ERK1/2 T/pCAP | 15.5 [13.7–17.4] | 0.16 [0.16–0.17] | 10.63 | 3 | |
3.6. pCAP peptides as substrates in inhibitor screens
It had been proposed that the choice of substrate in an enzyme inhibitor screen is important in guiding the outcome of the screen [43], and we hypothesized that peptide substrates could be superior to phosphotyrosine mimetic substrates such as DiFMUP and pNPP in identifying hits with physiologically relevant activity and selectivity for the enzyme of interest [12, 27]. To test this hypothesis, we optimized our pCAP peptide assay for high-throughput screening in both 96- and 1536-well plate format. We decided to utilize peptide 2, a 14-amino acid peptide based on a known biological substrate of LYP [44]. This peptide performed well in a small-scale pilot screen [12], and provided excellent results both in our screen of the 2000 member Spectrum collection from Microsource Discovery Systems, Inc. [32] and also in our screen of 290,000 compounds from the NIH MLSMR in collaboration with the CPCCG at the Sanford|Burnham Institute (unpublished work). After carrying out parallel screens of the 2000 member Spectrum collection from Microsource Discovery Systems, Inc. using DiFMUP and peptide 2 as the substrates, we found that there were significant differences in hit rate and spectrum of hits depending on which substrate was used [32]. For example, suramin, a known PTP inhibitor [45, 46] would not have been identified as a hit in a DiFMUP-based screen of this library, inhibiting LYP activity only ∼40%. In the peptide-based screen, suramin inhibited nearly all LYP activity and arose as one of the top hits [32].
3.7. In-gel activity assay: phosphograms
Among the various in-gel techniques used to probe enzymatic function, perhaps the most familiar method is zymography [47, 48]. This commercially available gel-based protocol is routinely used to study the activity of proteases including collagenases, serine proteases and matrix metalloproteinases. For example, zymography has been used to determine the metastatic potential of tumors by identifying the type and amount of metalloproteinases expressed by cancer cells [49]. This electrophoretic technique entails running a protease on a polyacrylamide “native” gel containing a protease substrate under “non-denaturing” conditions. Once proteins are resolved by PAGE, proteolytic activity is identified by UV-imaging or conventional staining of the resulting “zymogram”. A similar tool for the protein tyrosine phosphatases would facilitate the detection and characterization of this important family of signaling enzymes. Several different approaches to visualizing phosphatase activity in SDS-PAGE gels have been described in the literature. One approach involves soaking a pre-cast acrylamide gel containing the resolved phosphatase(s) with a solution of fluorogenic substrate after reactivation. Such an approach has been used to visualize the activity of aminopeptidases, glucosidases and esterases with 7-amino-4-methylcoumarin-leucine as the substrate [50], and more recently to visualize alkaline phosphatase and serine/threonine phosphatase activity in gels using 4-methylumbelliferone phosphate and 6,8-difluoro-4-methylumbelliferone phosphate [51]. However, we found that this method does not have the sensitivity required to visualize PTP activity in a gel. Another approach involves co-polymerization of a 32P-labeled poly(Glu4pTyr) substrate into a polyacrylamide gel, followed by resolution of phosphatases, reactivation and visualization of activity using X-ray film [35, 52, 53] This approach provides a sensitive signal in the presence of PTPs, but has the drawback of requiring a radioactive substrate. Our laboratory recently developed a fluorogenic peptide-based assay for PTP activity using the phosphocoumaryl amino acid, pCAP (Figure 1) [10]. Peptides containing the pCAP residue are excellent substrates for PTP activity, and we hypothesized that immobilization of a pCAP peptide in a polyacrylamide gel might provide the sensitivity required for in-gel visualization of PTP activity without the need for a radiolabeled substrate.
To test this hypothesis, we first incorporated the pCAP moiety into the amino acid sequence, DADE- pCAP-LIPQQG-NH2, a peptide that is based on the residues surrounding the Y992 of the EGF receptor and is a pan-specific PTP substrate [54]. In order to copolymerize the peptide into the acrylamide gel matrix, the peptide was acryloylated (Acr) by the addition of acryloyl chloride to a 6-aminohexanoic acid (Ahx) spacer attached at the N-terminus of the peptide [34]. Following this, an excess of acrylamide was copolymerized with the acryloyl-appended peptide and cast into a 10% SDS gel using standard procedures. The catalytic domain of the human T cell derived PTP CD45 provides an illustrative example of the utility of the phosphogram technology. The gel was loaded with varying concentrations of CD45, either left untreated or pre-treated with a large excess of iodoacetic acid (IAA), a covalent inactivator of tyrosine phosphatase activity. As shown in Figure 3, strong fluorescence is observed in lanes containing CD45 following electrophoresis, enzyme renaturation and activation, while the addition of IAA effectively abrogates the activity of CD45 and the fluorescence of the bands.
Figure 3.
A 4 mg portion of the peptide Acrylamide-DADE-pCAP-LIPQQG-NH2 was polymerized with 0.35 g of acrylamide using standard gel-casting methods. Aliquots of 0.25, 0.5 and 1 µg CD45 were either treated with 20, 40 or 50 mM iodoacetic acid (IAA) or left untreated (0 mM), were boiled for 4 min at 95°C and run on “Phosphogram gels”. Following electrophoresis, samples were incubated in an activating buffer and imaged to identify PTP activity (Top panel) or stained with GelCode Blue (Bottom panel).
In order to demonstrate the broader applicability of this phosphogram approach, we loaded a series of human PTPs on a phosphogram gel alongside YopH, a bacterial PTP with high catalytic activity. As before, the phosphatases were either untreated or pretreated with 50 mM IAA, loaded on the gel, subjected to electrophoresis followed by incubation of the gel in an activation buffer, and finally, imaged. As shown in Figure 4, a strong fluorescent band is present in almost all of the lanes containing untreated PTP, while significantly less or no fluorescence is seen in the IAA treated lanes. The failure of the covalently inactivated PTPs to hydrolyze the in-gel substrate and provide a phosphogram signal suggests that active phosphatases are required in order to produce a fluorescent signal. This facile, in-gel assay for PTP activity should have utility in monitoring selective covalent inhibition of PTPs in a cell as well as reversible oxidation of cellular PTPs [53].
Figure 4.
A series of human tyrosine phosphatases and the bacterial phosphatase YopH show activity on a phosphogram gel. 2 mg of the peptide Acr-Ahx-DADE-pCAP-LIPQQG-NH2 was polymerized with 0.35 g of acrylamide using standard gel-casting methods. 0.5 ug of YopH, CD45, HePTP, PTP1B, PTP-PEST and 1 ug LYP samples were either treated with 50 mM iodoacetic acid (IAA) or left untreated (0 mM), boiled for 4 min at 95°C and run on the phosphogram gel. Following electrophoresis, samples were incubated in an activating buffer and imaged to identify PTP activity. peptide will not result in fluorescence unless intracellular PTPs dephosphorylate the pCAP peptide. Finally, selective pCAP peptides can be used to monitor intracellular inhibition of PTP activity. If a cell is incubated both with a cell-penetrating pCAP peptide and a PTP inhibitor, limited fluorescence will develop, indicative of PTP inhibition.
3.8. Monitoring intracellular PTP activity using pCAP peptides
In addition to their considerable utility for in vitro applications, pCAP peptides can be used for cellular imaging applications as well. While simple pCAP peptides such as peptide 19 can be directly introduced into cells using microinjection or electroporation, these techniques are not suitable for all cell types. The introduction of a cell penetrating tag expands the scope of cellular applications for pCAP peptides. We have discovered that a combination of a hydrophobic and a basic cell penetrating tag provides optimal cell uptake. Linear peptides with a myrisitoylated polyarginine tag, such as peptide 20, facilitate spontaneous cellular update [17]. In addition, cyclic peptides containing a naphthylalanine and four arginine residues are excellent vehicles for peptide internalization [18]. Once inside a cell, these pCAP peptides can serve as selective reporters of PTP activity, as they are not dephosphorylated by serine/threonine phosphatases nor rephosphorylated by intracellular kinases. Furthermore, by tuning the sequence of the peptide, the probes can be rendered selective for one member of the PTP family (peptide 20 is preferentially dephosphorylated by CD45, for example [17]). By using a PTP-selective pCAP peptide, inhibition of the intracellular activity of a given PTP can be monitored directly and high-content screens for novel PTP inhibitors can be carried out [17]. The utility of pCAP peptides for monitoring intracellular PTP activity is highlighted in Scheme 2. First, cellular uptake of a fluorescent CAP-containing peptide will result in fluorescent staining of the cell. Second, internalization of a pCAP
Scheme 2.
Visualizing intracellular PTP activity and inhibition using pCAP peptides.
4. Conclusions
The fluorogenic phosphotyrosine mimic pCAP provides a facile assay for PTP activity in several different applications, as highlighted in this review. In addition to providing a convenient kinetic assay for PTP activity, pCAP peptides are excellent substrates for high-throughput screens aimed at discovering new PTP inhibitors. Peptides containing the pCAP moiety facilitate a thorough investigation of the substrate preferences of the PTPs, with the added advantage of identifying selectivity in multiply phosphorylated peptides. Incorporation of pCAP peptides into a polyacrylamide gel provides a simple approach to monitoring in-gel PTP activity, analogous to the zymogram gels that are commonly used to monitor in-gel protease activity. Based on the ease with which PTP activity can be assayed using pCAP peptides and the broad applicability of these assays, we anticipate that pCAP peptides will find additional uses in the study of PTP chemistry and biology. As an example, we have recently demonstrated that pCAP peptides can be used to monitor intracellular PTP activity [55]
Highlights.
A fluorogenic, phosphotyrosine mimetic amino acid (pCAP) has been developed.
pCAP peptides provide a facile, fluorogenic assay for monitoring PTP activity.
pCAP peptides have utility in vitro, in gels and in living cells.
Acknowledgements
We thank all those who have worked on this project in the Barrios and Bottini labs since its inception. This work was partially supported by a grant from the Zumberge Research fund at the University of Southern California to AMB and NB, NIH grants R21 GM079386 to AMB, R01 DK080165 to NB and AMB, and R21 NS056945-01 to NB and was accepted via the “Fast-Track” mechanism into the National Institutes of Health’ Roadmap Molecular Libraries Program (MLP) network of Probe Production Centers Network (MLPCN) with assistance of Drs. Chung and Sergienko to assignment to the Sanford|Burnham MLPCN Center. The HTS portion of this work was supported by National Institutes of Health Roadmap Program Award U54HG005033 to Sanford|Burnham Medical Research Institute (SBMRI) and performed by the Conrad Prebys Center for Chemical Genomics (CPCCG) at SBMRI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Montalibet J, Skorey KI, Kennedy BP. "Protein tyrosine phosphatases: Enzymatic assays". Methods. 2005;2005;35:2–8. doi: 10.1016/j.ymeth.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z-Y, Maclean D, Thieme-Sefler A, Roeske R, Dixon J. "A continuous spectrometric and fluorimetric assay for protein tyrosine phosphatase using phosphotyrosine-containing peptides". Anal Biochem. 1993;1993;211(1):7–15. doi: 10.1006/abio.1993.1224. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Z, Zander NF, Malencik DA, Anderson SR, Fischer EH. "Continuous spectrophotometric assay of protein tyrosine phosphatase using phosphotyrosine". Anal Biochem. 1992;1992;202:361–366. doi: 10.1016/0003-2697(92)90119-r. [DOI] [PubMed] [Google Scholar]
- 4.Lanzetta P, Alvarez L, Reinach P, Candia O. "An improved assay for nanomole amounts of inorganic phosphate". Anal Biochem. 1979;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- 5.Geladopoulos T, Sotiroudis T, Evangelopoulos A. "A malachite green colorimetric assay for protein phosphatase activity". Anal Biochem. 1991;192(1):112–116. doi: 10.1016/0003-2697(91)90194-x. [DOI] [PubMed] [Google Scholar]
- 6.Gee KR, Sun W-C, Bhalgat MK, Upson R, Klaubert DH, Latham KA, Haugland RP. "Fluorogenic substrates based on fluorinated umbelliferones for continuous assays of phosphatases and β-galactosidases". Anal Biochem. 1999;1999;273:41–48. doi: 10.1006/abio.1999.4202. [DOI] [PubMed] [Google Scholar]
- 7.Huang Z, Wang Q, Ly H, Gorvindarajan A, Scheigetz J, Zamboni R, Desmarais S, Ramachandran C. "3,6-fluorescein diphosphate: A sensitive fluorogenic and chromogenic substrate for protein tyrosine phosphatases". J Biomol Screen. 1999;1999;4(6):327–334. doi: 10.1177/108705719900400608. [DOI] [PubMed] [Google Scholar]
- 8.Kupcho K, Hsiao K, Bulleit B, Goueli S. "A homogeneous, nonradioactive high-throughput fluorogenic protein phosphatase assay". J Biomol Screen. 2004;2004;9:223–231. doi: 10.1177/1087057103262840. [DOI] [PubMed] [Google Scholar]
- 9.Nishikata M, Suzuki K, Yoshimura Y, Deyama Y, Matsumoto A. "A phosphotyrosine-containing quenched fluorogenic peptide as a novel substrate for protein tyrosine phosphatases". Biochem J. 1999;1999;343:385–391. [PMC free article] [PubMed] [Google Scholar]
- 10.Mitra S, Barrios AM. "Highly sensitive peptide-based probes for protein tyrosine phosphatase activity utilizing a fluorogenic mimic of phosphotyrosine". Bioorg & Med Chem Lett. 2005;15 doi: 10.1016/j.bmcl.2005.08.054. 5142–5145. [DOI] [PubMed] [Google Scholar]
- 11.Tsai S, Sen U, Zhao L, Greenleaf W, Dasgupta J, Fiorillo E, Orru V, Bottini N, Chen X. "Crystal structure of the human lymphoid tyrosine phosphatase catalytic domain: Insights into redox regulation". Biochem. 2009;2009;48:4838–4845. doi: 10.1021/bi900166y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karver MR, Krishnamurthy D, Kulkarni RA, Bottini N, Barrios AM. "Identifying potent, selective protein tyrosine phosphatase inhibitors from a library of Au(I) complexes". J Med Chem. 2009;2009;52:6912–6918. doi: 10.1021/jm901220m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mustelin T, Tautz L, Page R. "Structure of the hematopoietic tyrosine phosphatase (HePTP) catalytic domain: Structure of a kim phosphatase with phosphate bound at the active site". J Mol Biol. 2005;354(1):150–163. doi: 10.1016/j.jmb.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 14.Wu S, Vossius S, Rahmouni S, Miletic AV, Vang T, Vazquez-Rodriguez J, Cerignoli F, Arimura Y, Williams S, Hayes T, Moutschen M, Vasile S, Pellecchia M, Mustelin T, Tautz L. "Multidentate small-molecule inhibitors of vaccinia h1-related (VHR) phosphatase decrease proliferation of cervix cancer cells". J Med Chem. 2009;2009;52(21):6716–6723. doi: 10.1021/jm901016k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitra S, Barrios AM. "A series of peptide-based, fluorogenic probes for protein tyrosine phosphatase activity". Anal Biochem. 2007;2007;370:249–251. doi: 10.1016/j.ab.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Mitra S, Barrios A. "A rapid and general method for profiling phosphatase substrate specificity generates PTP-selective substrates and inhibitors". ChemBioChem. 2008;2008;9:1216–1219. doi: 10.1002/cbic.200800046. [DOI] [PubMed] [Google Scholar]
- 17.Stanford SM, Panchal R, Walker L, Wu D, Falk M, Mitra S, Damle S, Ruble D, Kaltcheva T, Zhang S, Zhang Z-Y, Bavari S, Barrios AM, Bottini N. "High-throughput screen using a single-cell tyrosine phosphatase assay reveals biologically active inhibitors of tyrosine phosphatase CD45". Proc Natl Acad Sci, USA. 2012;109(35):13972–13977. doi: 10.1073/pnas.1205028109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian Z, Liu T, Liu Y-Y, Briesewitz R, Barrios A, Jhiang S, Pei D. "Efficient delivery of cyclic peptides into mammalian cells with short sequence motifs". ACS Chem Biol. 2012;2012;8:423–431. doi: 10.1021/cb3005275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vetter SW, Keng Y-F, Lawrence DS, Zhang Z-Y. "Assessment of protein-tyrosine phosphatase 1b substrate specificity using “inverse alanine scanning”". J Biol Chem. 2000;2000;275(4):2265–2268. doi: 10.1074/jbc.275.4.2265. [DOI] [PubMed] [Google Scholar]
- 20.Yu X, Chen M, Zhang S, Yu Z-H, Sun J-P, Wang L, Liu S, Imasaki T, Takagi Y, Zhang Z-Y. "Substrate specificity of lymphoid-specific tyrosine phosphatase (LYP) and indentification of src kinase-associated protein of 55 kda homolog (SKAP-HOM) as a LYP substrate". J Biol Chem. 2011;286(35):30526–30534. doi: 10.1074/jbc.M111.254722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garaud M, Pei D. "Substrate profiling of protein tyrosine phosphatase PTP1B by screening a combinatorial library". J Am Chem Soc. 2007;129(17):5366–5367. doi: 10.1021/ja071275i. [DOI] [PubMed] [Google Scholar]
- 22.Ren L, Chen X, Leuchapanichkul R, Selner N, Meyer, Wavreille A-S, Chan R, Iorio C, Zhou X, Neel B, Pei D. "Substrate specificity of protein tyrosine phosphatases 1b, RPTPα, SHP-1 and SHP-2". Biochem. 2011;50(12):2339–2356. doi: 10.1021/bi1014453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kersh EN, Shaw AS, Allen PM. "Fidelity of T cell activation through multistep T cell receptor zeta phosphorylation". Science. 1998;1998;281(5376):572–575. doi: 10.1126/science.281.5376.572. [DOI] [PubMed] [Google Scholar]
- 24.Pitcher LA, van Oers NS. "T-cell receptor signal transmission: Who gives an itam?". Trends in immunology. 2003;2003;24(10):554–560. doi: 10.1016/j.it.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Sozio MS, Mathis MA, Young JA, Walchli S, Pitcher LA, Wrage PC, Bartok B, Campbell A, Watts JD, Aebersold R, Hooft van Huijsduijnen R, van Oers NS. "PTPH1 is a predominant protein-tyrosine phosphatase capable of interacting with and dephosphorylating the t cell receptor zeta subunit". J Biol Chem. 2004;2004;279(9):7760–7769. doi: 10.1074/jbc.M309994200. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Katrekar A, Honigberg LA, Smith AM, Conn MT, Tang J, Jeffery D, Mortara K, Sampang J, Williams SR, Buggy J, Clark JM. "Identification of substrates of human protein-tyrosine phosphatase PTPN22". J Biol Chem. 2006;2006;281(16):11002–11010. doi: 10.1074/jbc.M600498200. [DOI] [PubMed] [Google Scholar]
- 27.Stanford SM, Krishnamurthy D, Falk M, Messina R, Debnath B, Li S, Liu T, Kazemi R, Dahl R, He Y, Yu X, Chan A, Zhang Z-Y, Barrios AM, Woods V, Neamati N, Bottini N. "Discovery of a novel series of inhibitors of lymphoid tyrosine phosphatase with activity in human T cells". J Med Chem. 2011;2011;54:1640–16454. doi: 10.1021/jm101202j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang R, Hammer M, Mages J. DUSP meet immunology: Dual specificity mapk phosphatases in control of the inflammatory response". J Immunol. 2006;177(11):7497–7504. doi: 10.4049/jimmunol.177.11.7497. [DOI] [PubMed] [Google Scholar]
- 29.Owens DM, Keyse SM. "Differential regulation of map kinase signalling by dual-specificity protein phosphatases". Oncogene. 2007;2007;26(22):3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 30.Rahmouni S, Cerignoli F, Alonso A, Tsutji T, Henkens R, Zhu C, Louis-dit-Sully C, Moutschen M, Jiang W, Mustelin T. "Loss of the VHR dual-specific phosphatase causes cell-cycle arrest and senescence". Nature Cell Biol. 2006;2006;8(5):524–531. doi: 10.1038/ncb1398. [DOI] [PubMed] [Google Scholar]
- 31.Theodosiou A, Smith A, Gillieron C, Arkinstall S, Ashworth A. "MKP5, a new member of the map kinase phosphatase family, which selectively dephosphorylates stress-activated kinases". Oncogene. 1999;18(50):6981–6988. doi: 10.1038/sj.onc.1203185. [DOI] [PubMed] [Google Scholar]
- 32.Kulkarni RA, Vellore N, Bliss M, Stanford S, Falk M, Bottini N, Baron R, Barrios AM. "Substrate selection influences molecular recognition in a screen for lymphoid tyrosine phosphatase inhibitors". ChemBioChem. 2013 doi: 10.1002/cbic.201300273. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang JH, Chung TD, Oldenburg KR. "A simple statistical parameter for use in evaluation and validation of high throughput screening assays". J Biomol Screen. 1999;1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien-Simpson N, Ede N, Brown L, Swan J, Jackson D. "Polymerization of unprotected synthetic peptides: A view toward synthetic peptide vaccines". J Am Chem Soc. 1997;119:1183–1188. [Google Scholar]
- 35.Burridge K, Nelson A. "An in-gel assay for protein tyrosine phosphatase activity: Detection of widespread distribution in cells and tissues". Anal Biochem. 1995;1995;232:56–64. doi: 10.1006/abio.1995.9961. [DOI] [PubMed] [Google Scholar]
- 36.Huyer G, Kelly J, Moffat J, Zamboni R, Jia Z, Gresser MJ, Ramachandran C. "Affinity selection from peptide libraries to determine substrate specificity of protein tyrosine phosphatases". Anal Biochem. 1998;1998;258:19–30. doi: 10.1006/abio.1997.2541. [DOI] [PubMed] [Google Scholar]
- 37.Cammish LE, Kates SA. In: Fmoc solid phase peptide synthesis: A practical approach. Chan WC, White PD, editors. Oxford: Oxford University Press; 2000. [Google Scholar]
- 38.Pinella C, Appel J, Blanc P, Houghten R. "Rapid identification of high affinity peptide ligands using positionally scanned synthetic peptide combinatorial libraries". Biotechniques. 1992;1992;13(6):901–905. [PubMed] [Google Scholar]
- 39.Pinilla C, Appel J, Blondelle S, Dooley C, Dorner B, Eichler J, Ostresh J, Houghten RA. "A review of the utility of soluble peptide combinatorial libraries". Biopolymers. 1995;37(3):221–240. doi: 10.1002/bip.360370306. [DOI] [PubMed] [Google Scholar]
- 40.Ruzzene M, Donella-Deana A, Marin O, Perich JW, Ruzza P, Borin G, Calderan A, Pinna LA. "Specificity of T-cell protein tyrosine phosphatase toward phosphorylated synthetic peptides". Eur J Biochem. 1993;1993;211:289–295. doi: 10.1111/j.1432-1033.1993.tb19897.x. [DOI] [PubMed] [Google Scholar]
- 41.Housden HR, Skipp PJ, Crump MP, Broadbridge RJ, Crabbe T, Perry MJ, Gore MG. "Investigation of the kinetics and order of tyrosine phosphorylation in the T-cell receptor zeta chain by the protein tyrosine kinase Lck". Eur J Biochem / FEBS. 2003;2003;270(11):2369–2376. doi: 10.1046/j.1432-1033.2003.03604.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhou B, Wang ZX, Zhao Y, Brautigan DL, Zhang ZY. "The specificity of extracellular signal-regulated kinase 2 dephosphorylation by protein phosphatases". J Biol Chem. 2002;2002;277(35):31818–31825. doi: 10.1074/jbc.M203969200. [DOI] [PubMed] [Google Scholar]
- 43.Holmes C, Macher N, Grove J, Lang L, Irvine J. "Designing better coumarin-based fluorogenic substrates for PTP1B". Bioorg Med Chem Lett. 2008;18(11):3382–3385. doi: 10.1016/j.bmcl.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 44.Wu J, Katrekar A, Honigberg LA, Smith AM, Conn MT, Tang J, Jeffery D, Mortara K, Sampang J, Williams SR, Buggy J, Clark JM. "Identification of substrates of human protein-tyrosine phosphatase PTPN22". J Biol Chem. 2006;2006;281(16):11002–11010. doi: 10.1074/jbc.M600498200. [DOI] [PubMed] [Google Scholar]
- 45.Heneberg P. "Use of protein tyrosine phosphatase inhibitors as promising targeted therapeutic drugs". Curr Med Chem. 2009;16(6):706–733. doi: 10.2174/092986709787458407. [DOI] [PubMed] [Google Scholar]
- 46.McCain D, Wu L, Nickel P, Kassack M, Kreimeyer A, Gagliardi A, Collins D, Zhang Z-Y. "Suramin derivatives as inhibitors and activators of protein tyrosine phosphatases". J Biol Chem. 2004;2004;279(15):14713–14725. doi: 10.1074/jbc.M312488200. [DOI] [PubMed] [Google Scholar]
- 47.Hunter R, Markert G. "Histochemical demonstration of enzymes separated by zone electrophoresis in starch gels". Science. 1957;125(3261):1294–1295. doi: 10.1126/science.125.3261.1294-a. [DOI] [PubMed] [Google Scholar]
- 48.Lantz M, Ciborowski P. "Zymographic techniques for detection and characterization of microbial proteases". Methods Enzymol. 1994;1994;235:563–594. doi: 10.1016/0076-6879(94)35171-6. [DOI] [PubMed] [Google Scholar]
- 49.Law B, Hsiao JK, Bugge TH, Weissleder R, Tung CH. "Optical zymography for specific detection of urokinase plasminogen activator activity in biological samples". Anal Biochem. 2005;2005;338(1):151–158. doi: 10.1016/j.ab.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 50.Konopka A, Zakharova T. "Evaluation of methods to solubilize and analyze cell-associated ectoenzymes". J Microbiol Methods. 2002;51:273–282. doi: 10.1016/s0167-7012(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 51.Kameshita I, Baba H, Umeda Y, Sueyoshi N. "In-gel protein phosphatase assay using fluorogenic substrates". Anal Biochem. 2010;2010;400:118–122. doi: 10.1016/j.ab.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 52.Meng T, Hsu S, Tonks N. "Development of a modified in-gel assay to identify protein tyrosine phosphatases that are oxidized and inactivated in vivo". Methods. 2005;35 doi: 10.1016/j.ymeth.2004.07.005. 28–36. [DOI] [PubMed] [Google Scholar]
- 53.Markova B, Gulati P, Herrlich P, Böhmer F. "Investigation of protein-tyrosine phosphatases by in-gel assays". Methods. 2005;2005;35:22–27. doi: 10.1016/j.ymeth.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Z-Y, Thieme-Sefler AM, Maclean D, McNamara DJ, Dobrusin EM, Sawyer TK, Dixon JE. "Substrate specificity of the protein tyrosine phosphatases". Proc Natl Acad Sci, USA. 1993;1993;90:4446–4450. doi: 10.1073/pnas.90.10.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stanford SM, Panchal R, Walker L, Wu D, Falk M, Mitra S, Damle S, Ruble D, Kaltcheva T, Zhang S, Zhang Z-Y, Bavari S, Barrios AM, Bottini N. "High-throughput screen using a single-cell tyrosine phosphatase assay reveals biologically active inhibitors". Proc Nat Aca Sci. 2012;109(35):13972–13977. doi: 10.1073/pnas.1205028109. [DOI] [PMC free article] [PubMed] [Google Scholar]