Abstract
The spatial organization of the genome inside the nucleus affects many nuclear processes, such as DNA replication, DNA repair, and gene transcription. In metazoans, the nuclear periphery harbors mainly repressed genes that associate with the nuclear lamina. This review discusses how peripheral positioning is connected to transcriptional regulation in yeasts. Tethering of reporter genes to the nuclear envelope was found to result in transcriptional silencing. Similarly, repression of the silent mating type loci and subtelomeric genes is influenced by their position close to the nuclear envelope. In contrast, active genes are bound by nucleoporins and inducible genes associate with the nuclear pore complex upon activation. Taken together, these results portray the nuclear envelope as a platform for transcriptional regulation, both through activation at nuclear pores and silencing at the nuclear envelope.
Keywords: nuclear envelope, transcriptional regulation, telomeres, heterochromatin, nuclear pore complex
Introduction
The most obvious function of the nuclear envelope is the separation of the cell into two compartments: the nucleus and the cytoplasm. Exchange between the two occurs through the nuclear pores, which perforate the nuclear envelope. Far from being passive openings, the large multi-protein nuclear pore complexes tightly control what comes in and what goes out of the nucleus. The nuclear envelope itself is a double membrane system, with the outer nuclear membrane (ONM), which is continuous with the endoplasmic reticulum (ER), and the inner nuclear membrane (INM), facing toward the inside of the nucleus which is continuous with the ONM at the pores. In metazoans, the nuclear envelope is lined by the nuclear lamina, a mesh-like protein layer that provides structural stability to the nucleus. Numerous transmembrane proteins are embedded into the INM and interact with the lamina. Importantly, the chromatin is not distributed randomly inside the nucleus. Instead, there are several levels of nuclear organization, affecting both the chromosomes and the protein complexes involved in their replication, maintenance, and transcription.
In metazoan cells, chromosomes tend not to intermingle, but rather occupy distinct chromosome territories (see Cremer and Cremer1 for review). Recent studies have also observed this organization in yeast cells.2-4 The relative positioning of the chromosomes within the nucleus also appears to be non-random: chromosomes with lower gene density are preferentially located toward the nuclear periphery, chromosomes with higher gene density are positioned in the interior.5 Below the level of entire chromosomes, the chromatin is organized in domains that share similar chromatin properties. One example are lamina associated domains (LADs), which contain repressive histone modifications and low levels of transcription.6 LADs are bordered by binding sites for CTCF, a zinc-finger protein involved in maintaining contacts between different parts of the genome (see Phillips and Corces7 for review).
Apart from chromatin itself, the different biological processes that occur in the nucleus also have a spatial component. Transcription occurs in factory-like structures, where high concentrations of transcription factors and polymerases allow efficient transcription of multiple genes (see Sutherland and Bickmore8 for review). DNA replication and DNA repair are organized in similar foci (see Chagin et al.9 and Giglia-Mari et al.10 for review, respectively).
Through various studies, both the nuclear pore complex (NPC) and INM proteins have emerged as key players in organizing a variety of nuclear processes. This review discusses how the protein components of the nuclear envelope are involved in the regulation of gene expression. The main focus will be on the basic mechanisms established most thoroughly in the budding yeast Saccharomyces cerevisiae (S. cerevisiae) model system, with additional comparative studies on the fission yeast Schizosaccharomyces pombe (S. pombe) and metazoan cells.
Tethering Experiments
Early electron micrographs of the nucleus revealed two distinct regions: one in the nuclear interior and one adjacent to the nuclear envelope. These peripheral areas were later identified as enriched in heterochromatin that harbors mainly silent genes, and which differs in structure and composition from highly transcribed euchromatin. Consistent with these studies, labeling of nascent RNA in the nuclei of living cells showed that the bulk of transcription happens in the nuclear interior rather than at the nuclear periphery.11
Since then, the impact of the nuclear periphery on gene expression has become apparent in tethering experiments. This approach makes use of the GAL4/UAS system12 to bind a chromosome locus to a membrane protein at the nuclear periphery. The first study13 integrated GAL4 binding UAS sites into a crippled silencer DNA element of the HMR locus. The GAL4-DNA binding domain (DBD) was then fused to Yip1, a Golgi membrane protein. Overexpression of this construct leads to accumulation of the protein in the ER, the ONM, and eventually also the INM, where interactions with chromatin are possible. Expression of a reporter gene integrated at the HMR locus was silenced only when the fusion construct was expressed. However, the repression depended on the presence of at least one intact silencing element, suggesting that silencing also requires the interaction between DNA and silencing factors (e.g., SIR proteins).
A second study14 used the same experimental system to find other factors involved in transcriptional silencing at the nuclear periphery. Repression of the reporter gene at the HMR locus was alleviated in a strain lacking Mlp1 and Mlp2. These coiled-coil proteins are homologs of the human Tpr and form part of the basket-like protrusion of the NPC into the nucleoplasm.15 Deletion of MLP1 and MLP2 was also shown to alleviate silencing at an ectopic locus with the same silencer elements, suggesting a more general role of the NPC in transcriptional regulation. In human HeLa cells, Tpr is involved in excluding heterochromatin from the vicinity of the nuclear pores.16 It is possible that the nuclear basket is required for separation of silent and active chromatin environments at the nuclear periphery.
Since these initial studies in S. cerevisiae, tethering experiments have been performed in metazoan cells as well.17-20 While the extent of transcriptional repression varied depending on the construct and cell line, those studies confirmed that it is possible to silence transcription by relocating a transcriptionally active gene locus to the nuclear periphery (reviewed in Ruault et al.21 and Towbin et al.22). This conclusion is consistent with genome-wide association studies showing that the nuclear lamina interacts mainly with transcriptionally repressed genes.6,23 Although neither S. cerevisiae nor S. pombe has a nuclear lamina, we have shown previously that the INM proteins Man1 and Ima1 in S. pombe interact preferentially with lowly expressed genes.24 This suggests that peripheral localization of repressed regions might be a common feature of all eukaryotes, irrespective of the presence of a nuclear lamina.
Regulation of Inducible Genes
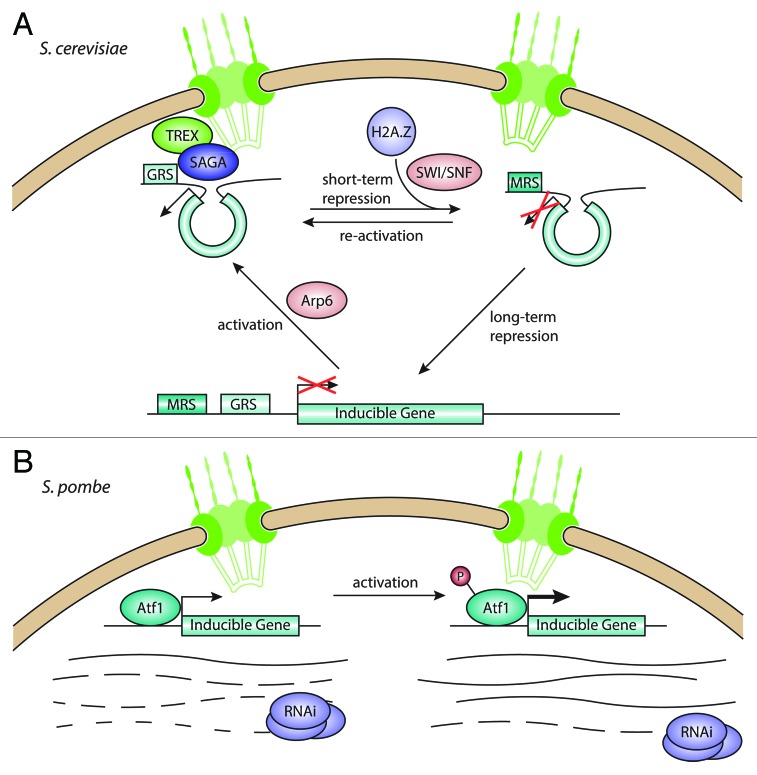
A study by Casolari et al. in 2004 challenged the paradigm of the nuclear periphery as a silencing environment. Interactions between chromatin and various components of the NPC in S. cerevisiae showed a strong preference for highly transcribed genes to be associated with the nuclear pores.25 Among those genes were the GAL genes, a group of inducible genes that are expressed when S. cerevisiae is grown in galactose but not in glucose. When repressed by the presence of glucose, these genes are localized in the nuclear interior. However, when induced, they move to the nuclear periphery and associate with nucleoporins.25 Another inducible gene, INO1, not only localizes to the nuclear periphery upon activation, but its activation requires peripheral positioning26 (Fig. 1A).
Figure 1. Interactions between the nuclear pore complex and inducible genes. (A) In S. cerevisiae, inducible genes move from the nuclear interior to the NPC upon activation, mediated by the SAGA and TREX complexes. Genes can stay at the NPC during short-term repression, allowing for fast activation, but move toward the interior under long-term repression. (B) In S. pombe, inducible genes are bound by the transcriptional regulator Atf1 and associate with the NPC. Under repressive conditions, the RNAi machinery degrades the transcripts coming from these genes, keeping mRNA levels low. When induced, Atf1 gets phosphorylated and leads to increased transcription, overcoming the degradation of transcripts through RNAi.
A microscopic study of GAL gene positioning in the nucleus used a GFP-fused Tet-Repressor bound to recognition sequences inserted close to the gene loci.27 Under repressive conditions, the GAL loci move randomly in the nuclear interior. When activated, this movement becomes restricted to a back-and-forth movement close to the nuclear envelope.
Previous studies of the GAL genes found that their activation is mediated by the histone acetyltransferase complex SAGA.28-30 Interestingly, deletion of the lysine acetyltransferase Gcn5 itself had no effect on peripheral positioning,27 suggesting that acetylation might occur after recruitment to the NPC. However, mutations in Sus1 and Ada2, both non-enzymatic SAGA components, impaired recruitment to the NPC. Both proteins are involved in linking the SAGA complex to the mRNA export machinery,31 which in turn interacts with the NPC through Nup1.32 This model is further strengthened by the observation that Sac3, a component of the TREX mRNA export complex, is also required for GAL gene relocation to the NPC. Taken together, these results demonstrate that inducible genes localize to the NPC when activated and require the SAGA and TREX complexes, but not the lysine acetylation activity of Gcn5, for anchoring to the NPC.
While translocation of inducible genes to the NPC appears to be an important factor in their activation,26,33 it was unclear what marks a gene for recruitment to the periphery. By sequential deletion of different regions in the upstream sequence of the INO1 gene, Ahmed et al. were able to identify two different DNA sequence elements required for peripheral targeting.34 These gene recruitment sequences (GRS) are relatively short (8–20 bp) and function like zip codes, both on the INO1 gene and at an ectopic locus with a reporter gene. In both cases, the GRS was necessary and sufficient for peripheral positioning and induction of expression. The GRS sequence elements are over-represented in stress-induced genes. Interestingly, a reporter locus with a S. cerevisiae GRS in S. pombe was also localized preferentially at the nuclear periphery, indicating that this recruitment mechanism might be conserved, at least in yeasts. However, it is unclear whether GRS-mediated recruitment to the NPC would also alter gene expression in S. pombe. GRS signals with the same sequence were shown to cluster at the NPC with the help of the transcription factor Put3,35 pointing to a role for DNA zip codes in organizing intra-chromosomal interactions.
In 2007, several studies reported the discovery of a “transcriptional memory” for the GAL genes.33,36,37 In S. cerevisiae cells cultured in galactose, expression of the GAL genes is induced to enable cells to metabolize galactose instead of glucose. If the cells are then shifted into glucose-containing medium for a short time, GAL gene expression is rapidly repressed. However, upon re-activation by a return to galactose, the increase in transcripts from the GAL genes occurs much more rapidly than in the initial activation. This process has been described as “transcriptional memory,” since it allows the cell to “remember” recently activated loci upon recurring changes in nutrient conditions.
Different studies have since been published that try to elucidate the mechanism behind transcriptional memory. A study from the Brickner laboratory showed that GAL1 is moved to the nuclear periphery when activated and stays there after repression.33 This peripheral localization depends on the NPC component Nup2. Also, deletion of the histone variant H2A.Z reduced mRNA levels during re-activation. In human and S. cerevisiae, H2A.Z is required for efficient transcription of inducible genes by recruiting of Pol II.38,39 Data from the Peterson group also demonstrated the transcriptional memory behavior of GAL1 and showed that faster expression after re-activation depends on the chromatin remodeling complex SWI/SNF.36 Taken together, these results point to a chromatin-based transcriptional memory at the NPC, with H2A.Z marking genes that were recently expressed for quick induction upon re-activation. However, the question remains in which order chromatin modification, NPC anchoring and activation occur.
More recently it has emerged that the transcriptional memory observed for the GAL genes could primarily be regulated by signal transduction and only to a lesser extent governed by epigenetic factors. Using a heterokaryon assay, Zacharioudakis et al. showed that cytoplasmic factors are involved in the transcriptional memory of GAL1.37 In this assay, two haploid cells of opposite mating type are fused together to generate a diploid, but a mutation in a nuclear fusion factor prevents the two nuclei from fusing. In this way, the authors were able to fuse one cell that had experienced glucose starvation and one that had been growing in glucose. In the resulting diploid, expression of a GFP-Gal1 fusion was upregulated only in the nucleus from the non-induced cell, suggesting that exposure to the cytoplasm of the induced cell is sufficient to trigger induction. The Gal1 protein itself appears to work in a positive feedback loop to enhance GAL gene expression during re-activation. Indeed, deletion of GAL1 abolished transcriptional memory for GAL10.40 In the case of the GAL genes, a memory appears to be achieved first through feedback loops of the proteins themselves, and only second through changes in chromatin. It remains to be seen if other inducible genes use similar mechanisms.
A different perspective on transcriptional memory comes from chromatin confirmation capture (3C) data, which showed that some inducible genes are arranged in chromatin loops the ends of which are anchored at the NPC.41 The HXK1 gene, which encodes a Hexokinase isoenzyme 1 induced by glucose starvation, forms a loop structure between its 3′ and 5′ ends when activated. This loop is preserved during repression and is required for fast Pol II recruitment during re-activation, possibly by maintaining a platform for transcription factors. Loop formation depends on the transcription factor TFIIIB and the nuclear pore basket component Mlp1.41 These results suggest that transcriptional memory is not only a matter of protein binding, but also of the 3D arrangement of chromatin. Maintaining the loop structure during short-term repression may allow for faster expression upon re-activation, since the loop does not need to be formed de novo.
While GRS are involved in attachment of activated genes, a different DNA zip code ensures that they remain attached after repression.42 The memory recruitment sequence (MRS) was identified by sequential deletions in the upstream sequence of the INO1 gene and screening for release of the recently repressed locus from the periphery. Without the MRS, but with the GRS intact, the INO1 gene moves to the NPC upon induction, but does not remain attached there after repression.
It is important to point out that the INO1 gene differs from GAL1 and HXK1 in its expression behavior. When re-activated after induction and repression, it does not increase in transcription as rapidly as in the initial activation.33,41 Therefore, it has been debated whether INO1 does in fact display a transcriptional memory or not. While INO1 forms a loop structure like HXK1 upon activation, this is not maintained after switching to repressive conditions.41 However, if not attached to the NPC like GAL1, the mRNA levels increase even slower,33 suggesting that the attachment to the NPC does affect the transcription to a certain extent. The incorporation of H2A.Z at inducible genes through the remodeler Swr1 appears to influence transcription levels and nuclear positioning as well,33,42 indicating that there is a chromatin-based mechanism for the transcriptional behavior of INO1.
Other factors have also been implicated in transcriptional activation of genes at nuclear pores. The Swr1 chromatin remodeling complex subunit Arp6 mediates the movement of a locus to the nuclear periphery, even in the absence of Swr1.43 This suggests that activation of genes at the NPC is possible without incorporation of H2A.Z. The peripheral localization of the GAL2 locus and its induction were disrupted in yeast strains carrying mutations in cohesin components.44
In contrast to the majority of eukaryotes, S. cerevisiae lacks an RNA interference (RNAi) system. S. pombe makes use of its RNAi machinery in various ways: to maintain centromeric heterochromatin,45 to prevent read-through transcription at convergent gene pairs46 and to suppress antisense transcription.47 Recently, the regulation of stress-response genes was added to that list (Fig. 1B). Woolcock et al. used DamID experiments to map the interactions between RNAi components and chromatin.48 They found that Dicer (Dcr1) and the RNA-dependent RNA polymerase (Rdp1) exhibit a strong preference for the promoters of genes also bound by the Atf1 transcriptional regulator that binds its targets constitutively and increases their transcription in response to stress. Deletion of the RNAi components, but not deletion of the HP1 homolog Swi6, increases expression of Atf1 target genes, indicating that they are not heterochromatic. Argonaute (Ago1) bound small RNAs were enriched for RNA derived from these target genes at well. The authors propose that the RNAi machinery prevents expression of the inducible genes in the absence of stress, while induction by phosphorylation of Atf1 leads to increased expression that overcomes the repression by Dcr1/Rdp1. Interestingly, Atf1 targets were also bound by the nucleoporin Nup85. In contrast to S. cerevisiae, these inducible genes appear to associate with NPCs constitutively, suggesting that there might be multiple ways of regulating activation at the nuclear periphery.
Silencing at the Mating Type Loci
The silent mating type loci of S. cerevisiae and S. pombe represent a well-studied model for heterochromatin formation and boundary function (see Haber49 for review). For both yeasts, there are two mating type loci that are silenced and one that is active. The content of the active locus can be switched through recombination between the active locus and the silent locus with the opposite mating type information. In S. cerevisiae, the active mating type cassette is excised by the HO endonuclease enzyme and the gap is filled by homologous recombination from one of the two silent loci (see Haber49 for review). In S. pombe, mating type switching occurs through a replication-coupled mechanism (see Klar50 for review).
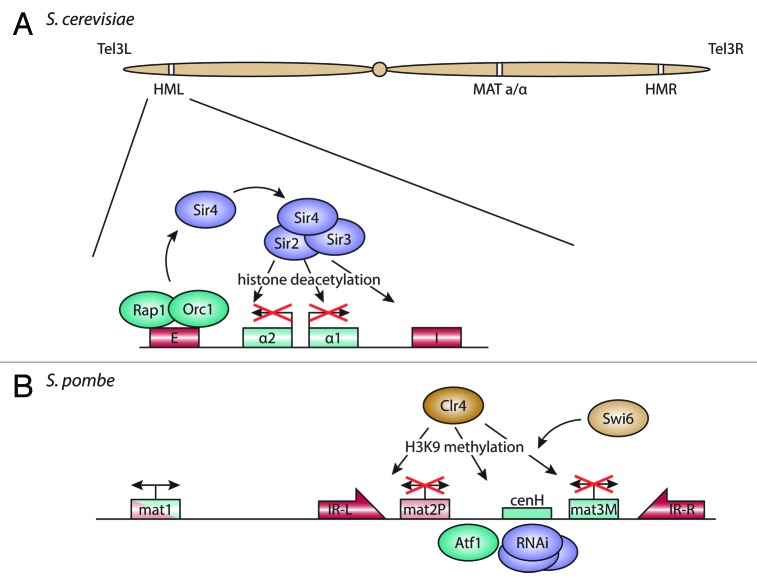
In the S. cerevisiae genome, the two silent mating type loci are spatially separated, with the HML and HMR loci on opposite ends of chromosome 3 (Fig. 2A). Both loci contain silencer DNA elements that recruit Rap1 and Orc1, which in turn recruit Sir4. This serves as a binding site for the other Sir-proteins, including the histone deacetylase (HDAC) Sir2.51,52 Sir2 removes the acetylation mark on lysine 16 of histone 4 (H4K16), so that Sir3 can bind.53 The deacetylated histone tails provide a platform for further recruitment of Sir proteins, which establish heterochromatin over the entire locus and repress transcription between the silencing elements (see Fox and McConnell54 for review).
Figure 2. Organization and repression of the silent mating type loci. (A) In S. cerevisiae, the E and I silencer elements at the silent mating type loci HML and HMR are bound by Rap1 and Orc1. These factors recruit the SIR protein complex, containing the histone deacetylase Sir2, which leads to silencing of the mating type alleles between the silencers. (B) In S. pombe, the two mating type cassettes mat2-P and mat3-M are kept silent through recruitment of Atf1 and the RNAi machinery to sequence elements between the two genes. This in turn brings in Clr4, a histone methyltransferase, which methylates histone H3 on lysine 9. Together with the HP1 homolog Swi6, this establishes heterochromatin on the locus, with the inverted repeats IR-L and IR-R maintaining the border to adjacent euchromatin.
The location of HMR/HML in the nucleus appears to have some impact on silencing. Positioning of both HMR and HML close to the telomeres could serve to restrict their position to the proximity of the nuclear envelope, since the telomeres themselves are anchored at the nuclear membrane. The ability of the silencer elements HMR-E and HMR-I to repress transcription of nearby genes is reduced if they are inserted farther away from the telomeres.55-58 Indeed, the silent mating type loci localize mainly close to the nuclear envelope, while the active locus resides mainly in the nuclear interior.59 Using 3C, Miele et al. have shown that HMR and HML loci cluster when silenced, both in the nucleoplasm and at the nuclear envelope.60 Surprisingly, tethering the HMR locus with a defective silencer to the NPC restores silencing, in contrast to the NPC interaction observed for active genes.61
But is peripheral positioning cause or consequence of silencing at the mating type loci? Gartenberg et al. used a recombinase to excise the silent HMR locus and form an extrachromosomal circle, which stayed at the nuclear envelope.62 This anchoring depends on the INM protein Esc1 and the Ku protein yKu70, which is involved in double strand break (DSB) repair and telomere tethering to the nuclear envelope. In the absence of both proteins the HMR ring detached from the nuclear envelope and moved into the interior.62 Silencing of the locus persisted even in the nucleoplasm, suggesting that at least maintenance of silencing is independent of peripheral positioning.
Boundaries around the mating type loci are necessary to prevent spreading of heterochromatin onto neighboring genes. Ishii and colleagues have shown that tethering of NPC components and exportins is sufficient for boundary function.63 Even if periphery interaction is not required for maintaining silencing, it might instead be important for limiting the spreading of heterochromatin and preventing the silencing of adjacent genes. However, loss of nuclear pore components was not sufficient to inhibit boundary function of a tDNA insulator at the HMR locus.61
In S. pombe, the silent mating type loci are located close together, in a 20 kb region of chromosome 2 flanked by two inverted repeats (Fig. 2B). The cenH DNA element between the two mating type cassettes shows homology to centromeric outer repeats and is at least partially required for silencing of the mat2-mat3 region.64,65 The silencing mechanism itself appears to rely on two pathways. While RNAi components are not required for maintenance of mating type silencing, they may be necessary for de novo establishment of heterochromatin in the silent region.66 In addition, a cis-acting DNA element between cenH and mat2-P may initiate heterochromatin formation through an Atf1-dependent pathway.67
In FISH experiments, the mating type loci localize close to the nuclear envelope.68,69 At the nuclear envelope, the mat2-mat3 region is positioned close to the spindle pole body (SPB).70 This distance increases if the inverted repeat border elements are missing, but the region stays attached to the nuclear envelope. In cells lacking Clr4, the histone methyltransferase required for heterochromatin formation in S. pombe, or Swi6, the HP1 homolog, the mating type region moves away from the nuclear periphery and the SPB.70 It has been hypothesized that the nuclear envelope is involved in anchoring and loop formation at the mating type locus,69 but this needs to be tested experimentally.
Telomere Attachment and Subtelomeric Chromatin Domains
Linear chromosomes present a challenge for the eukaryotic cell: the free ends need to be protected from degradation by exonucleases. Also, the DNA repair machinery needs to be prevented from recognizing the chromosome ends as DSBs, the repair of which would result in concatenated chromosomes and disrupt chromosome segregation. These problems are circumvented by the telomeres and their associated proteins. In yeasts, the telomeres consist of a single-stranded DNA sequence, maintained by telomerase, followed by a double-stranded section of telomeric sequence repeats of varying length. The neighboring subtelomeres are influenced by the telomeres and possess a unique chromatin structure.
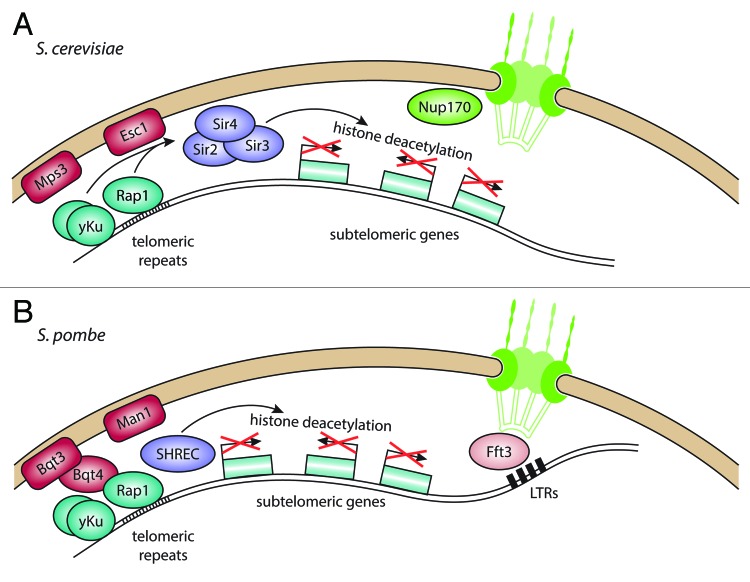
In S. cerevisiae, telomeric repeats are recognized and bound by Rap1, which recruits the SIR proteins to the telomeres71 (Fig. 3A). Additionally, the Ku proteins yKu70/80 bind the chromosome ends and also serve as binding sites for Sir4p.72,73 Similar to the situation at mating type loci, the SIR proteins establish silencing in the adjacent chromatin, in this case the subtelomeres. This gives rise to telomere position effect (TPE), in which genes inserted within a certain distance of the telomeres are silenced through spreading of heterochromatin from the telomeres (see Huang74 for review).
Figure 3. Telomere anchoring at the nuclear envelope and transcriptional regulation of subtelomeric genes. (A) In S. cerevisiae, telomeres are bound by Rap1 and yKu, which interact with the inner nuclear membrane (INM) proteins Esc1 and Mps3, respectively. This recruits the SIR protein complex, which deacetylates histones in the adjacent subtelomeres, leading to repression of genes in this region. (B) In S. pombe, yKu and Rap1 also bind to telomeres, but bind to the INM protein Bqt3 through Bqt4. The histone deacetylase complex SHREC is recruited through Rap1, leading to deacetylation of histones in the subtelomeres. The subtelomeres are also found close to the nuclear envelope and associate with the INM protein Man1. The border of the subtelomeres is maintained by the chromatin remodeler Fft3, which binds to long-terminal repeat (LTR) elements in proximity to the NPC.
Telomeres are anchored at the nuclear envelope through two redundant pathways. The SIR complex interacts directly with the INM protein Esc1, allowing tethering of both the telomeres and subtelomeres as well as the silent mating type loci.62,75 Independent of silencing, the SUN-domain protein Mps3 interacts with the Ku heterodimer and telomerase subunits.76,77 Bühler and Gasser have proposed a model of sequential silencing and recruitment of telomeres,78 in which Sir4 is first recruited to DNA elements via DNA binding proteins such as Rap1 and Orc1. It is then anchored to the nuclear envelope, where higher concentrations of SIR proteins lead to silencing. The clustering of SIR proteins at the telomeres appears to build silencing sub-compartments at the nuclear periphery, through which expression of subtelomeric loci is regulated. Loss of telomere attachment at the nuclear envelope breaks up these foci and enables SIR proteins to diffuse freely in the nucleoplasm.79 This leads to silencing of genes away from the telomeres, suggesting that compartmentalization of silencing factors helps prevent promiscuous repression.
It has also been suggested that the nuclear basket components Mlp1 and Mlp2 are involved in telomere tethering through their interaction with yKu.80 A recent study showed that a deletion of the nucleoporin Nup170 results in upregulation of subtelomeric genes.81 Nup170 binds to subtelomeric genes and Sir4 disperses into the nucleoplasm in nup170∆ cells, resulting in telomere detachment from the nuclear envelope. The authors therefore propose that Nup170 is involved in recruiting Sir4 to telomeres and subtelomeric silencing.
In S. pombe, the telomeres are also attached to the nuclear envelope,82 although this appears to occur through a slightly different mechanism (Fig. 3B). Studies from the Hiraoka lab have shown that the Bqt proteins are required for telomere tethering.83,84 The telomeres are bound by the DNA binding protein Taz1, which in turn recruits Rap1. Bqt3 is an INM transmembrane protein that forms a complex with Bqt4, which binds to Rap1. This interaction ensures telomere attachment during vegetative growth and allows dissociation during mitosis by phosphorylation of Rap1.85 The INM membrane proteins Lem2 and Man1 are also required for peripheral positioning of telomeres.86 Both proteins contain a helix-extension-helix (HeH) domain, which shows limited sequence homology but a DNA-binding fold similar to that of the LEM domain found in many mammalian INM proteins.87 As in S. cerevisiae, the Ku heterodimer also binds to S. pombe telomeres.88 While deletion of Ku70/80 results in dissociation of the telomeres from the nuclear envelope, it does not alleviate silencing close to the telomeres.
The subtelomeres of chromosomes 1 and 2 in S. pombe show an unusual chromatin structure and contain mainly lowly expressed genes.89,90 Chromosome 3 has rDNA repeats in the subtelomeric region. While the repressive chromatin mark H3K9me2 is restricted to a 10–20 kb telomere-proximal region on chromosomes 1 and 2,91 the active marks H3K4me2 and H2A.Z stay at relatively low levels in an extensive region reaching about 80–100 kb away from the telomere. Using DamID experiments, we have shown that these 80–100 kb subtelomeric regions are enriched for interactions with Man1, a HeH domain INM protein.24 This chromatin state depends on the JmjC-domain protein Msc1 and the Fun30 chromatin remodeler Fft3. Deletion of either results in increased H2A.Z incorporation in the subtelomeres and upregulation of subtelomeric genes.89,90 Interestingly, Fft3 does not bind to the subtelomeres themselves, but rather to retrotransposon-derived long-terminal repeats (LTRs) at their borders. Recently we discovered that peripheral localization of subtelomeres and repression of subtelomeric genes depend on catalytic activity of Fft3 at these border elements (Strålfors et al., unpublished). The border LTRs are normally associated with the NPC but lose this interaction in the absence of Fft3.91 Tethering of insulators to nucleoporins has been observed previously,63 making it plausible that chromatin remodeling by Fft3 is required for nuclear pore attachment and insulator function of subtelomeric borders.
How the repressed chromatin state of the subtelomeres is achieved is largely unknown. The HDAC complex SHREC is recruited to telomeres through its Ccq1 subunit,92 which also has other functions at telomeres such as recruitment of telomerase.93 The HDAC in the SHREC complex, Clr3, is required for peripheral localization and repression of a subtelomeric gene cluster involved in nitrogen starvation response.94 Interestingly, expression of subtelomeric genes is also affected by cohesin mutants,95 suggesting that local chromosome organization by cohesin is important for proper gene regulation in these regions.
Links to Genome Stability, Recombination, and DNA Repair
Many of the proteins and complexes involved in gene regulation at the nuclear periphery have recently been shown to be involved in other nuclear processes, such as maintenance of genome stability and DNA repair.
One such example is the organization and regulation of the rDNA. In S. cerevisiae, the rRNA genes are organized in repeats of DNA encoding the 35S rRNA precursor and a 5S rRNA, separated by intergenic spacers (IGS). The number of these repeats can vary between strains. Because of the repeat structure, this region is a prime target for the homologous recombination pathway for double strand break repair. Since uneven numbers of rDNA repeats between sister chromosomes can lead to segregation problems, the rDNA repeats need to be protected from recombination to prevent genomic instability. Furthermore, recombination can produce extrachromosomal rDNA circles which accumulate in the nucleus of the mother cell during cell division and trigger senescence.96
Various factors are involved in silencing of rDNA repeats and thereby preventing homologous recombination and genomic instability. In S. cerevisiae, these are the RENT complex (“regulator of nucleolar silencing and telophase exit”) and the cohibin complex. Cohibin components were shown to physically interact with two INM proteins, Nur1 and Heh1.97 Deletion of the two membrane proteins increases the recombination frequency between rDNA repeats, but does not affect silencing of Pol II transcription at the IGS. Using fusion-proteins, Mekhail et al. were able to show that Heh1 is involved in tethering the rDNA repeats to the nuclear envelope at the nucleolar side of the nucleus. This interaction appears to be bridged by Lsm4, since fusion of Sir2 and Heh1 can rescue the repeat instability and detachment in an lsm4∆ mutant. The authors conclude that peripheral tethering of rDNA repeats is crucial for stability of the repeats independent of repeat silencing.97
A follow-up study showed that cooperation between cohibin and INM proteins is not restricted to the nucleolus, but also involves telomere anchoring and silencing of subtelomeres.98 The authors show that the cohibin complex and its membrane anchor proteins Heh1 and Nur1 bind to the telomeres through interaction between the SIR complex and cohibin. Cells lacking cohibin show alleviation of telomeric silencing and increased instability of telomeric repeats. Taken together with its function at rDNA repeats, cohibin appears to be of key importance in the maintenance of genome stability. This adds another functionality for the cohibin complex, which has now been shown to organize chromatin in various contexts, such as DNA replication and chromosome segregation (see Poon and Mekhail99 for review).
Peripheral positioning also appears to affect mating type switching. In S. cerevisiae cells of mating type a, the HMLα locus is bound by yKu70, a factor involved in double strand break (DSB) repair.59 In wild type cells, the HMLα locus can move away from the periphery to a certain extent, while HMRa stays close to the nuclear envelope. In cells lacking yKu70, HMLα loses this movement toward the interior and instead moves only along the nuclear membrane.59 In this way, yKu70 and membrane positioning of the silent locus that carries the current mating type allele might direct recombination between the active mating type locus and the alternate silent locus.
There are also indications that the nuclear periphery is involved in organizing DNA repair. S. cerevisiae strains lacking the nuclear basket component Mlp2 are sensitive to Bleomycin, suggesting an impairment in DSB repair.80 The Nup84 complex, part of the NPC, is required for efficient repair of a DSB specifically in subtelomeric regions,100 possibly through interaction with a SUMO-protease required for sumoylation of DNA repair factors such as Yku70.101 Recruitment of damaged DNA to the NPC was shown using live-cell imaging and chromatin immunoprecipitation.102 This targeting appears to be mediated by a SUMO-dependent E3-ligase, which interacts with the NPC.
In an alternative pathway, DSBs can also be bound by telomerase, possibly to recruit unrepaired or slowly repairing breaks to a silent compartment at the nuclear periphery.103
Conclusions
There is mounting evidence for a role of peripheral positioning in gene transcription from observations in organisms from yeast to human, pointing to a common mechanism that is conserved throughout evolution. However, many questions are still open regarding how peripheral positioning and transcriptional silencing are achieved and in which order these events occur.
Recently, two studies were able to shed some light on what regulates peripheral positioning and transcriptional silencing.104,105 Using mouse embryonic fibroblasts and Caenorhabditis elegans embryos, respectively, the authors identified histone methyltransferase enzymes which are required for stepwise methylation of lysine 9 on histone 3 (H3K9). Mono- and di-methylation of H3K9 are required for targeting of a locus to the nuclear periphery, while trimethylation is needed for stable attachment and silencing. Together with the action of histone demethylase enzymes, this allows for plasticity in interactions between the nuclear lamina and chromatin, which have been observed through DamID experiments in different stages of development.106
Positioning of genes at the nuclear periphery has been linked both to transcriptional activation and repression. While interactions with the nuclear lamina and INM proteins mainly occurs at repressed genes,6,23,24,107 NPC components were shown to interact with active loci.25,26 To explain this dichotomy, the existence of micro-environments at the nuclear envelope, which contain silent and active loci, has been proposed,. It is however unclear what would shield these compartments from each other. Furthermore, it has been debated whether interactions between active genes and nucleoporins happen at the NPC. Data from DamID experiments in Drosophila show that NPC-tethered and free forms of the same nucleoporin have different targets in the genome, with only the freely diffusing nucleoporin binding to active genes.108 This suggests that the interaction happens at least in part in the nucleoplasm, with nucleoporins shuttling between the NPC and the nuclear interior.
The rapid recruitment of inducible genes to the nuclear periphery in S. cerevisiae poses the question how this motion is achieved. The movement of a single locus is normally constrained by the surrounding chromatin, but can be enhanced through chromatin remodeling.109 Interestingly, actin and actin-related proteins are involved in transcriptional regulation, e.g., as components of many chromatin remodeling complexes in yeasts and humans (see Oma and Harata110 for review). In human cells, it has been shown that movement of an active locus depends on actin,111 suggesting that actin might be involved in directed chromatin movements. This could occur in cooperation with nuclear myosin, which has also been implicated in transcription in human cells.112 It is conceivable that chromatin remodeling at an active locus opens up the chromatin structure in its vicinity, allowing it to be relocated to the nuclear periphery.
We have come a long way from electron micrographs showing condensed heterochromatin at the nuclear periphery to a biochemical description of histone modifications associated with heterochromatin and a mechanistic understanding of how the location of a gene in the nucleus influences is transcriptional status. The general model that is emerging from studies in yeast and mammalian cells is that the nuclear periphery is not a uniform environment: genes targeted to the inner nuclear membrane are silenced whereas inducible genes are activated when they associate with the nuclear pore complex. We also know that specific chromosomal regions, such as telomeres, and DNA-based processes such as DNA repair are enriched at the nuclear periphery. Understanding the complex landscape of the nuclear periphery will provide further insight into the regulation of transcription and the non-random organization of chromatin within the nucleus.
Acknowledgments
This work was supported by grants from the Swedish Cancer Society and the Swedish Research council (to Ekwall K). This material is based in part on work supported by the National Science Foundation under Grant number 0744945 (to Sazer S). Any opinions, findings and conclusions or recommendations expressed in this article are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/nucleus/article/26394
References
- 1.Cremer T, Cremer M. Chromosome territories. Cold Spring Harb Perspect Biol. 2010;2:a003889. doi: 10.1101/cshperspect.a003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molnar M, Kleckner N. Examination of interchromosomal interactions in vegetatively growing diploid Schizosaccharomyces pombe cells by Cre/loxP site-specific recombination. Genetics. 2008;178:99–112. doi: 10.1534/genetics.107.082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duan Z, Andronescu M, Schutz K, McIlwain S, Kim YJ, Lee C, Shendure J, Fields S, Blau CA, Noble WS. A three-dimensional model of the yeast genome. Nature. 2010;465:363–7. doi: 10.1038/nature08973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanizawa H, Iwasaki O, Tanaka A, Capizzi JR, Wickramasinghe P, Lee M, Fu Z, Noma K-II. Mapping of long-range associations throughout the fission yeast genome reveals global genome organization linked to transcriptional regulation. Nucleic Acids Res. 2010;38:8164–77. doi: 10.1093/nar/gkq955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle S, Gilchrist S, Bridger JM, Mahy NL, Ellis JA, Bickmore WA. The spatial organization of human chromosomes within the nuclei of normal and emerin-mutant cells. Hum Mol Genet. 2001;10:211–9. doi: 10.1093/hmg/10.3.211. [DOI] [PubMed] [Google Scholar]
- 6.Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–51. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- 7.Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell. 2009;137:1194–211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutherland H, Bickmore WA. Transcription factories: gene expression in unions? Nat Rev Genet. 2009;10:457–66. doi: 10.1038/nrg2592. [DOI] [PubMed] [Google Scholar]
- 9.Chagin VO, Stear JH, Cardoso MC. Organization of DNA replication. Cold Spring Harb Perspect Biol. 2010;2:a000737–000737. doi: 10.1101/cshperspect.a000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giglia-Mari G, Zotter A, Vermeulen W. DNA damage response. Cold Spring Harb Perspect Biol. 2011;3:a000745. doi: 10.1101/cshperspect.a000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wansink DG, Schul W, van der Kraan I, van Steensel B, van Driel R, de Jong L. Fluorescent labeling of nascent RNA reveals transcription by RNA polymerase II in domains scattered throughout the nucleus. J Cell Biol. 1993;122:283–93. doi: 10.1083/jcb.122.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 13.Andrulis ED, Neiman AM, Zappulla DC, Sternglanz R. Perinuclear localization of chromatin facilitates transcriptional silencing. Nature. 1998;394:592–5. doi: 10.1038/29100. [DOI] [PubMed] [Google Scholar]
- 14.Feuerbach F, Galy V, Trelles-Sticken E, Fromont-Racine M, Jacquier A, Gilson E, Olivo-Marin J-C, Scherthan H, Nehrbass U. Nuclear architecture and spatial positioning help establish transcriptional states of telomeres in yeast. Nat Cell Biol. 2002;4:214–21. doi: 10.1038/ncb756. [DOI] [PubMed] [Google Scholar]
- 15.Strambio-de-Castillia C, Blobel G, Rout MP. Proteins connecting the nuclear pore complex with the nuclear interior. J Cell Biol. 1999;144:839–55. doi: 10.1083/jcb.144.5.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krull S, Dörries J, Boysen B, Reidenbach S, Magnius L, Norder H, Thyberg J, Cordes VC. Protein Tpr is required for establishing nuclear pore-associated zones of heterochromatin exclusion. EMBO J. 2010;29:1659–73. doi: 10.1038/emboj.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumaran RI, Spector DL. A genetic locus targeted to the nuclear periphery in living cells maintains its transcriptional competence. J Cell Biol. 2008;180:51–65. doi: 10.1083/jcb.200706060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452:243–7. doi: 10.1038/nature06727. [DOI] [PubMed] [Google Scholar]
- 19.Finlan LE, Sproul D, Thomson I, Boyle S, Kerr E, Perry P, Ylstra B, Chubb JR, Bickmore WA. Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genet. 2008;4:e1000039. doi: 10.1371/journal.pgen.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dialynas G, Speese S, Budnik V, Geyer PK, Wallrath LL. The role of Drosophila Lamin C in muscle function and gene expression. Development. 2010;137:3067–77. doi: 10.1242/dev.048231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruault M, Dubarry M, Taddei A. Re-positioning genes to the nuclear envelope in mammalian cells: impact on transcription. Trends Genet. 2008;24:574–81. doi: 10.1016/j.tig.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Towbin BD, Meister P, Gasser SM. The nuclear envelope--a scaffold for silencing? Curr Opin Genet Dev. 2009;19:180–6. doi: 10.1016/j.gde.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Pickersgill H, Kalverda B, de Wit E, Talhout W, Fornerod M, van Steensel B. Characterization of the Drosophila melanogaster genome at the nuclear lamina. Nat Genet. 2006;38:1005–14. doi: 10.1038/ng1852. [DOI] [PubMed] [Google Scholar]
- 24.Steglich B, Filion GJ, van Steensel B, Ekwall K. The inner nuclear membrane proteins Man1 and Ima1 link to two different types of chromatin at the nuclear periphery in S. pombe. Nucleus. 2012;3:77–87. doi: 10.4161/nucl.18825. [DOI] [PubMed] [Google Scholar]
- 25.Casolari JM, Brown CR, Komili S, West J, Hieronymus H, Silver PA. Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell. 2004;117:427–39. doi: 10.1016/S0092-8674(04)00448-9. [DOI] [PubMed] [Google Scholar]
- 26.Brickner JH, Walter P. Gene recruitment of the activated INO1 locus to the nuclear membrane. PLoS Biol. 2004;2:e342. doi: 10.1371/journal.pbio.0020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabal GG, Genovesio A, Rodriguez-Navarro S, Zimmer C, Gadal O, Lesne A, Buc H, Feuerbach-Fournier F, Olivo-Marin J-C, Hurt EC, et al. SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature. 2006;441:770–3. doi: 10.1038/nature04752. [DOI] [PubMed] [Google Scholar]
- 28.Larschan E, Winston F. The S. cerevisiae SAGA complex functions in vivo as a coactivator for transcriptional activation by Gal4. Genes Dev. 2001;15:1946–56. doi: 10.1101/gad.911501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhaumik SR, Green MR. SAGA is an essential in vivo target of the yeast acidic activator Gal4p. Genes Dev. 2001;15:1935–45. doi: 10.1101/gad.911401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huisinga KL, Pugh BF. A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in Saccharomyces cerevisiae. Mol Cell. 2004;13:573–85. doi: 10.1016/S1097-2765(04)00087-5. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez-Navarro S, Fischer T, Luo M-J, Antúnez O, Brettschneider S, Lechner J, Pérez-Ortín JE, Reed R, Hurt E. Sus1, a functional component of the SAGA histone acetylase complex and the nuclear pore-associated mRNA export machinery. Cell. 2004;116:75–86. doi: 10.1016/S0092-8674(03)01025-0. [DOI] [PubMed] [Google Scholar]
- 32.Fischer T, Strässer K, Rácz A, Rodriguez-Navarro S, Oppizzi M, Ihrig P, Lechner J, Hurt E. The mRNA export machinery requires the novel Sac3p-Thp1p complex to dock at the nucleoplasmic entrance of the nuclear pores. EMBO J. 2002;21:5843–52. doi: 10.1093/emboj/cdf590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brickner DG, Cajigas I, Fondufe-Mittendorf Y, Ahmed S, Lee P-C, Widom J, Brickner JH. H2A.Z-mediated localization of genes at the nuclear periphery confers epigenetic memory of previous transcriptional state. PLoS Biol. 2007;5:e81. doi: 10.1371/journal.pbio.0050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed S, Brickner DG, Light WH, Cajigas I, McDonough M, Froyshteter AB, Volpe T, Brickner JH. DNA zip codes control an ancient mechanism for gene targeting to the nuclear periphery. Nat Cell Biol. 2010;12:111–8. doi: 10.1038/ncb2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brickner DG, Ahmed S, Meldi L, Thompson A, Light W, Young M, Hickman TL, Chu F, Fabre E, Brickner JH. Transcription factor binding to a DNA zip code controls interchromosomal clustering at the nuclear periphery. Dev Cell. 2012;22:1234–46. doi: 10.1016/j.devcel.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kundu S, Horn PJ, Peterson CL. SWI/SNF is required for transcriptional memory at the yeast GAL gene cluster. Genes Dev. 2007;21:997–1004. doi: 10.1101/gad.1506607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zacharioudakis I, Gligoris T, Tzamarias D. A yeast catabolic enzyme controls transcriptional memory. Curr Biol. 2007;17:2041–6. doi: 10.1016/j.cub.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 38.Adam M, Robert F, Larochelle M, Gaudreau L. H2A.Z is required for global chromatin integrity and for recruitment of RNA polymerase II under specific conditions. Mol Cell Biol. 2001;21:6270–9. doi: 10.1128/MCB.21.18.6270-6279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardy S, Jacques P-E, Gévry N, Forest A, Fortin M-E, Laflamme L, Gaudreau L, Robert F. The euchromatic and heterochromatic landscapes are shaped by antagonizing effects of transcription on H2A.Z deposition. PLoS Genet. 2009;5:e1000687. doi: 10.1371/journal.pgen.1000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kundu S, Peterson CL. Dominant role for signal transduction in the transcriptional memory of yeast GAL genes. Mol Cell Biol. 2010;30:2330–40. doi: 10.1128/MCB.01675-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan-Wong SM, Wijayatilake HD, Proudfoot NJ. Gene loops function to maintain transcriptional memory through interaction with the nuclear pore complex. Genes Dev. 2009;23:2610–24. doi: 10.1101/gad.1823209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Light WH, Brickner DG, Brand VR, Brickner JH. Interaction of a DNA zip code with the nuclear pore complex promotes H2A.Z incorporation and INO1 transcriptional memory. Mol Cell. 2010;40:112–25. doi: 10.1016/j.molcel.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshida T, Shimada K, Oma Y, Kalck V, Akimura K, Taddei A, Iwahashi H, Kugou K, Ohta K, Gasser SM, et al. Actin-related protein Arp6 influences H2A.Z-dependent and -independent gene expression and links ribosomal protein genes to nuclear pores. PLoS Genet. 2010;6:e1000910. doi: 10.1371/journal.pgen.1000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gard S, Light W, Xiong B, Bose T, McNairn AJ, Harris B, Fleharty B, Seidel C, Brickner JH, Gerton JL. Cohesinopathy mutations disrupt the subnuclear organization of chromatin. J Cell Biol. 2009;187:455–62. doi: 10.1083/jcb.200906075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volpe TA, Kidner C, Hall IM, Teng G, Grewal SIS, Martienssen RA. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science. 2002;297:1833–7. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- 46.Gullerova M, Moazed D, Proudfoot NJ. Autoregulation of convergent RNAi genes in fission yeast. Genes Dev. 2011;25:556–68. doi: 10.1101/gad.618611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang K, Fischer T, Porter RL, Dhakshnamoorthy J, Zofall M, Zhou M, Veenstra T, Grewal SIS. Clr4/Suv39 and RNA quality control factors cooperate to trigger RNAi and suppress antisense RNA. Science. 2011;331:1624–7. doi: 10.1126/science.1198712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woolcock KJ, Stunnenberg R, Gaidatzis D, Hotz H-R, Emmerth S, Barraud P, Bühler M. RNAi keeps Atf1-bound stress response genes in check at nuclear pores. Genes Dev. 2012;26:683–92. doi: 10.1101/gad.186866.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haber JE. Mating-type genes and MAT switching in Saccharomyces cerevisiae. Genetics. 2012;191:33–64. doi: 10.1534/genetics.111.134577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klar AJS. Lessons learned from studies of fission yeast mating-type switching and silencing. Annu Rev Genet. 2007;41:213–36. doi: 10.1146/annurev.genet.39.073103.094316. [DOI] [PubMed] [Google Scholar]
- 51.Tanny JC, Dowd GJ, Huang J, Hilz H, Moazed D. An enzymatic activity in the yeast Sir2 protein that is essential for gene silencing. Cell. 1999;99:735–45. doi: 10.1016/S0092-8674(00)81671-2. [DOI] [PubMed] [Google Scholar]
- 52.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 53.Suka N, Luo K, Grunstein M. Sir2p and Sas2p opposingly regulate acetylation of yeast histone H4 lysine16 and spreading of heterochromatin. Nat Genet. 2002;32:378–83. doi: 10.1038/ng1017. [DOI] [PubMed] [Google Scholar]
- 54.Fox CA, McConnell KH. Toward biochemical understanding of a transcriptionally silenced chromosomal domain in Saccharomyces cerevisiae. J Biol Chem. 2005;280:8629–32. doi: 10.1074/jbc.R400033200. [DOI] [PubMed] [Google Scholar]
- 55.Thompson-Stewart D, Karpen GH, Spradling AC. A transposable element can drive the concerted evolution of tandemly repetitious DNA. Proc Natl Acad Sci U S A. 1994;91:9042–6. doi: 10.1073/pnas.91.19.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shei GJ, Broach JR. Yeast silencers can act as orientation-dependent gene inactivation centers that respond to environmental signals. Mol Cell Biol. 1995;15:3496–506. doi: 10.1128/mcb.15.7.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maillet L, Boscheron C, Gotta M, Marcand S, Gilson E, Gasser SM. Evidence for silencing compartments within the yeast nucleus: a role for telomere proximity and Sir protein concentration in silencer-mediated repression. Genes Dev. 1996;10:1796–811. doi: 10.1101/gad.10.14.1796. [DOI] [PubMed] [Google Scholar]
- 58.Marcand S, Buck SW, Moretti P, Gilson E, Shore D. Silencing of genes at nontelomeric sites in yeast is controlled by sequestration of silencing factors at telomeres by Rap 1 protein. Genes Dev. 1996;10:1297–309. doi: 10.1101/gad.10.11.1297. [DOI] [PubMed] [Google Scholar]
- 59.Bystricky K, Van Attikum H, Montiel M-D, Dion V, Gehlen L, Gasser SM. Regulation of nuclear positioning and dynamics of the silent mating type loci by the yeast Ku70/Ku80 complex. Mol Cell Biol. 2009;29:835–48. doi: 10.1128/MCB.01009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miele A, Bystricky K, Dekker J. Yeast silent mating type loci form heterochromatic clusters through silencer protein-dependent long-range interactions. PLoS Genet. 2009;5:e1000478. doi: 10.1371/journal.pgen.1000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruben GJ, Kirkland JG, MacDonough T, Chen M, Dubey RN, Gartenberg MR, Kamakaka RT. Nucleoporin mediated nuclear positioning and silencing of HMR. PLoS One. 2011;6:e21923. doi: 10.1371/journal.pone.0021923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gartenberg MR, Neumann FR, Laroche T, Blaszczyk M, Gasser SM. Sir-mediated repression can occur independently of chromosomal and subnuclear contexts. Cell. 2004;119:955–67. doi: 10.1016/j.cell.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 63.Ishii K, Arib G, Lin C, Van Houwe G, Laemmli UK. Chromatin boundaries in budding yeast: the nuclear pore connection. Cell. 2002;109:551–62. doi: 10.1016/S0092-8674(02)00756-0. [DOI] [PubMed] [Google Scholar]
- 64.Grewal SIS, Klar AJ. A recombinationally repressed region between mat2 and mat3 loci shares homology to centromeric repeats and regulates directionality of mating-type switching in fission yeast. Genetics. 1997;146:1221–38. doi: 10.1093/genetics/146.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thon G, Friis T. Epigenetic inheritance of transcriptional silencing and switching competence in fission yeast. Genetics. 1997;145:685–96. doi: 10.1093/genetics/145.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hall IM, Shankaranarayana GD, Noma K-II, Ayoub N, Cohen A, Grewal SIS. Establishment and maintenance of a heterochromatin domain. Science. 2002;297:2232–7. doi: 10.1126/science.1076466. [DOI] [PubMed] [Google Scholar]
- 67.Martienssen RA, Zaratiegui M, Goto DB. RNA interference and heterochromatin in the fission yeast Schizosaccharomyces pombe. Trends Genet. 2005;21:450–6. doi: 10.1016/j.tig.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Ekwall K, Javerzat JP, Lorentz A, Schmidt H, Cranston G, Allshire RC. The chromodomain protein Swi6: a key component at fission yeast centromeres. Science. 1995;269:1429–31. doi: 10.1126/science.7660126. [DOI] [PubMed] [Google Scholar]
- 69.Noma K, Allis CD, Grewal SIS. Transitions in distinct histone H3 methylation patterns at the heterochromatin domain boundaries. Science. 2001;293:1150–5. doi: 10.1126/science.1064150. [DOI] [PubMed] [Google Scholar]
- 70.Alfredsson-Timmins J, Henningson F, Bjerling P. The Clr4 methyltransferase determines the subnuclear localization of the mating-type region in fission yeast. J Cell Sci. 2007;120:1935–43. doi: 10.1242/jcs.03457. [DOI] [PubMed] [Google Scholar]
- 71.Luo K, Vega-Palas MA, Grunstein M. Rap1-Sir4 binding independent of other Sir, yKu, or histone interactions initiates the assembly of telomeric heterochromatin in yeast. Genes Dev. 2002;16:1528–39. doi: 10.1101/gad.988802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gravel S, Larrivée M, Labrecque P, Wellinger RJ. Yeast Ku as a regulator of chromosomal DNA end structure. Science. 1998;280:741–4. doi: 10.1126/science.280.5364.741. [DOI] [PubMed] [Google Scholar]
- 73.Martin SG, Laroche T, Suka N, Grunstein M, Gasser SM. Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell. 1999;97:621–33. doi: 10.1016/S0092-8674(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 74.Huang Y. Transcriptional silencing in Saccharomyces cerevisiae and Schizosaccharomyces pombe. Nucleic Acids Res. 2002;30:1465–82. doi: 10.1093/nar/30.7.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taddei A, Hediger F, Neumann FR, Bauer C, Gasser SM. Separation of silencing from perinuclear anchoring functions in yeast Ku80, Sir4 and Esc1 proteins. EMBO J. 2004;23:1301–12. doi: 10.1038/sj.emboj.7600144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bupp JM, Martin AE, Stensrud ES, Jaspersen SL. Telomere anchoring at the nuclear periphery requires the budding yeast Sad1-UNC-84 domain protein Mps3. J Cell Biol. 2007;179:845–54. doi: 10.1083/jcb.200706040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schober H, Ferreira H, Kalck V, Gehlen LR, Gasser SM. Yeast telomerase and the SUN domain protein Mps3 anchor telomeres and repress subtelomeric recombination. Genes Dev. 2009;23:928–38. doi: 10.1101/gad.1787509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bühler M, Gasser SM. Silent chromatin at the middle and ends: lessons from yeasts. EMBO J. 2009;28:2149–61. doi: 10.1038/emboj.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taddei A, Van Houwe G, Nagai S, Erb I, van Nimwegen E, Gasser SM. The functional importance of telomere clustering: global changes in gene expression result from SIR factor dispersion. Genome Res. 2009;19:611–25. doi: 10.1101/gr.083881.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galy V, Olivo-Marin JC, Scherthan H, Doye V, Rascalou N, Nehrbass U. Nuclear pore complexes in the organization of silent telomeric chromatin. Nature. 2000;403:108–12. doi: 10.1038/47528. [DOI] [PubMed] [Google Scholar]
- 81.Van de Vosse DW, Wan Y, Lapetina DL, Chen W-M, Chiang J-H, Aitchison JD, Wozniak RW. A role for the nucleoporin Nup170p in chromatin structure and gene silencing. Cell. 2013;152:969–83. doi: 10.1016/j.cell.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Funabiki H, Hagan IM, Uzawa S, Yanagida M. Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J Cell Biol. 1993;121:961–76. doi: 10.1083/jcb.121.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chikashige Y, Tsutsumi C, Yamane M, Okamasa K, Haraguchi T, Hiraoka Y. Meiotic proteins bqt1 and bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell. 2006;125:59–69. doi: 10.1016/j.cell.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 84.Chikashige Y, Yamane M, Okamasa K, Tsutsumi C, Kojidani T, Sato M, Haraguchi T, Hiraoka Y. Membrane proteins Bqt3 and -4 anchor telomeres to the nuclear envelope to ensure chromosomal bouquet formation. J Cell Biol. 2009;187:413–27. doi: 10.1083/jcb.200902122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujita I, Nishihara Y, Tanaka M, Tsujii H, Chikashige Y, Watanabe Y, Saito M, Ishikawa F, Hiraoka Y, Kanoh J. Telomere-nuclear envelope dissociation promoted by Rap1 phosphorylation ensures faithful chromosome segregation. Curr Biol. 2012;22:1932–7. doi: 10.1016/j.cub.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 86.Gonzalez Y, Saito A, Sazer S. Fission yeast Lem2 and Man1 perform fundamental functions of the animal cell nuclear lamina. Nucleus. 2012;3:60–76. doi: 10.4161/nucl.18824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brachner A, Foisner R. Evolvement of LEM proteins as chromatin tethers at the nuclear periphery. Biochem Soc Trans. 2011;39:1735–41. doi: 10.1042/BST20110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tanaka A, Tanizawa H, Sriswasdi S, Iwasaki O, Chatterjee AG, Speicher DW, Levin HL, Noguchi E, Noma K-II. Epigenetic regulation of condensin-mediated genome organization during the cell cycle and upon DNA damage through histone H3 lysine 56 acetylation. Mol Cell. 2012;48:532–46. doi: 10.1016/j.molcel.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buchanan L, Durand-Dubief M, Roguev A, Sakalar C, Wilhelm B, Strålfors A, Shevchenko A, Aasland R, Shevchenko A, Ekwall K, et al. The Schizosaccharomyces pombe JmjC-protein, Msc1, prevents H2A.Z localization in centromeric and subtelomeric chromatin domains. PLoS Genet. 2009;5:e1000726. doi: 10.1371/journal.pgen.1000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Strålfors A, Walfridsson J, Bhuiyan H, Ekwall K. The FUN30 chromatin remodeler, Fft3, protects centromeric and subtelomeric domains from euchromatin formation. PLoS Genet. 2011;7:e1001334. doi: 10.1371/journal.pgen.1001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cam HP, Sugiyama T, Chen ES, Chen X, FitzGerald PC, Grewal SIS. Comprehensive analysis of heterochromatin- and RNAi-mediated epigenetic control of the fission yeast genome. Nat Genet. 2005;37:809–19. doi: 10.1038/ng1602. [DOI] [PubMed] [Google Scholar]
- 92.Sugiyama T, Cam HP, Sugiyama R, Noma K-II, Zofall M, Kobayashi R, Grewal SIS. SHREC, an effector complex for heterochromatic transcriptional silencing. Cell. 2007;128:491–504. doi: 10.1016/j.cell.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 93.Tomita K, Cooper JP. Fission yeast Ccq1 is telomerase recruiter and local checkpoint controller. Genes Dev. 2008;22:3461–74. doi: 10.1101/gad.498608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alfredsson-Timmins J, Kristell C, Henningson F, Lyckman S, Bjerling P. Reorganization of chromatin is an early response to nitrogen starvation in Schizosaccharomyces pombe. Chromosoma. 2009;118:99–112. doi: 10.1007/s00412-008-0180-6. [DOI] [PubMed] [Google Scholar]
- 95.Dheur S, Saupe SJ, Genier S, Vazquez S, Javerzat J-P. Role for cohesin in the formation of a heterochromatic domain at fission yeast subtelomeres. Mol Cell Biol. 2011;31:1088–97. doi: 10.1128/MCB.01290-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sinclair DA, Guarente L. Extrachromosomal rDNA circles--a cause of aging in yeast. Cell. 1997;91:1033–42. doi: 10.1016/S0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 97.Mekhail K, Seebacher J, Gygi SP, Moazed D. Role for perinuclear chromosome tethering in maintenance of genome stability. Nature. 2008;456:667–70. doi: 10.1038/nature07460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chan JNY, Poon BPK, Salvi J, Olsen JB, Emili A, Mekhail K. Perinuclear cohibin complexes maintain replicative life span via roles at distinct silent chromatin domains. Dev Cell. 2011;20:867–79. doi: 10.1016/j.devcel.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 99.Poon BPK, Mekhail K. Cohesin and related coiled-coil domain-containing complexes physically and functionally connect the dots across the genome. Cell Cycle. 2011;10:2669–82. doi: 10.4161/cc.10.16.17113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Therizols P, Fairhead C, Cabal GG, Genovesio A, Olivo-Marin J-C, Dujon B, Fabre E. Telomere tethering at the nuclear periphery is essential for efficient DNA double strand break repair in subtelomeric region. J Cell Biol. 2006;172:189–99. doi: 10.1083/jcb.200505159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Palancade B, Liu X, Garcia-Rubio M, Aguilera A, Zhao X, Doye V. Nucleoporins prevent DNA damage accumulation by modulating Ulp1-dependent sumoylation processes. Mol Biol Cell. 2007;18:2912–23. doi: 10.1091/mbc.E07-02-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nagai S, Dubrana K, Tsai-Pflugfelder M, Davidson MB, Roberts TM, Brown GW, Varela E, Hediger F, Gasser SM, Krogan NJ. Functional targeting of DNA damage to a nuclear pore-associated SUMO-dependent ubiquitin ligase. Science. 2008;322:597–602. doi: 10.1126/science.1162790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oza P, Jaspersen SL, Miele A, Dekker J, Peterson CL. Mechanisms that regulate localization of a DNA double-strand break to the nuclear periphery. Genes Dev. 2009;23:912–27. doi: 10.1101/gad.1782209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pinheiro I, Margueron R, Shukeir N, Eisold M, Fritzsch C, Richter FM, Mittler G, Genoud C, Goyama S, Kurokawa M, et al. Prdm3 and Prdm16 are H3K9me1 methyltransferases required for mammalian heterochromatin integrity. Cell. 2012;150:948–60. doi: 10.1016/j.cell.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 105.Towbin BD, González-Aguilera C, Sack R, Gaidatzis D, Kalck V, Meister P, Askjaer P, Gasser SM. Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell. 2012;150:934–47. doi: 10.1016/j.cell.2012.06.051. [DOI] [PubMed] [Google Scholar]
- 106.Peric-Hupkes D, Meuleman W, Pagie L, Bruggeman SWM, Solovei I, Brugman W, Gräf S, Flicek P, Kerkhoven RM, van Lohuizen M, et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell. 2010;38:603–13. doi: 10.1016/j.molcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ikegami K, Egelhofer TA, Strome S, Lieb JD. Caenorhabditis elegans chromosome arms are anchored to the nuclear membrane via discontinuous association with LEM-2. Genome Biol. 2010;11:R120. doi: 10.1186/gb-2010-11-12-r120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kalverda B, Pickersgill H, Shloma VV, Fornerod M. Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell. 2010;140:360–71. doi: 10.1016/j.cell.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 109.Neumann FR, Dion V, Gehlen LR, Tsai-Pflugfelder M, Schmid R, Taddei A, Gasser SM. Targeted INO80 enhances subnuclear chromatin movement and ectopic homologous recombination. Genes Dev. 2012;26:369–83. doi: 10.1101/gad.176156.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oma Y, Harata M. Actin-related proteins localized in the nucleus: from discovery to novel roles in nuclear organization. Nucleus. 2011;2:38–46. doi: 10.4161/nucl.2.1.14510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dundr M, Ospina JK, Sung MH, John S, Upender M, Ried T, Hager GL, Matera AG. Actin-dependent intranuclear repositioning of an active gene locus in vivo. J Cell Biol. 2007;179:1095–103. doi: 10.1083/jcb.200710058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sarshad A, Sadeghifar F, Louvet E, Mori R, Böhm S, Al-Muzzaini B, Vintermist A, Fomproix N, Östlund A-K, Percipalle P. Nuclear myosin 1c facilitates the chromatin modifications required to activate rRNA gene transcription and cell cycle progression. PLoS Genet. 2013;9:e1003397. doi: 10.1371/journal.pgen.1003397. [DOI] [PMC free article] [PubMed] [Google Scholar]