Abstract
Objective
To evaluate the secular trend in incidence of and mortality from Type 1 diabetes mellitus (T1DM) in Taiwan, 1999–2010.
Methods
All 7,225 incident cases of T1DM were retrospectively retrieved from Taiwan's National Health Insurance Research Database from 1999 to 2010. Trend of bi-annual age- and sex-specific incidence rates of T1DM was calculated and tested with Poisson regression model. Standardized mortality ratios (SMRs) were calculated, using age-, sex-, and calendar years-specific mortality rates of the general population as the reference, to estimate the relative mortality risk of T1DM.
Results
The number of male and female T1DM was 3,471 (48%) and 3,754 (52%), respectively. The annual number of incident T1DM increased from 543 in 1999 to 737 in 2010. The overall bi-annual incidence rate rose from 1999–00 to 2003–04 and mildly declined thereafter rose to 2009–10, with an insignificant trend (P = 0.489) over the study period. Regardless of gender, the higher age-specific incidence rate was noted in the younger groups (<30 years) and highest at <15 years. The incidence rates in younger groups were constantly higher in female population than in male one. The SMR from all causes was significantly increased at 3.00 (95% Confidence Interval (CI) 2.83–3.16) in patients with T1DM. The sex-specific SMR was 2.66 (95% CI 2.46–2.85) and 3.58 (95% CI 3.28–3.87) for male and female patients, respectively. For both sexes, the age-specific SMR peaked at 15–29 years.
Conclusions
Among T1DM patients in Taiwan, there were significant increasing trends in males and female aged <15 years. We also noted a significantly increased overall and sex-specific SMR from all causes in patients with TIDM which suggests a need for improvements in treatment and care of patients with T1DM.
Introduction
Type 1 diabetes mellitus (T1DM) has been reported to increase the risk of death [1], [2], mainly resulting from hyperglycemia, which is linked to a number of acute (e.g., diabetic ketoacidosis) and chronic (e.g., diabetic nephropathy and cardiovascular disease) complications [3]. Although treatment for T1DM improved evidently during the 1980s and 1990s with the advent of blood glucose self-monitoring, hemoglobin A1c testing, and use of angiotensin-converting-enzyme inhibitors/angiotensin II receptor blocker [4]–[6], T1DM complications still frequently lead to premature mortality [7]. Recent reports from Western Europe have shown a 3 to 4-fold higher long-term mortality rate (≧15 years follow-up) in T1DM, as compared to the general population [1], [8]. In the U.S., for example, the mortality rate of T1DM ranges from 5 to 7 times that of the general population [2], [7], and such increased risk of mortality was particularly notable for women and African Americans with T1DM. Risk of mortality from T1DM in Asian societies has rarely been reported in the literature.
A steady increase in the incidence of T1DM has been reported worldwide [9]. The incidence of this disease varies considerably among countries-e.g., it is lowest in China and Venezuela and highest in Finland and Sardinia [10]. However, long-term population-based data on T1DM incidence in the ethnic Chinese population is very limited [11], [12]. One national survey on whole diabetes found that T1DM was present in less than 1% of the diabetic population and the standardized incidence of T1DM remained constant over the recent 10 years in Taiwan [13]. No nationwide research focusing on T1DM mortality in Taiwan has been published. Using a large population-based T1DM cohort in Taiwan diagnosed between 1999 and 2010, we now investigated the long-term trends of incidence rate of T1DM in all sex and age stratifications after diagnosis and explored the overall as well as the age and sex-specific risk of mortality in patients with T1DM. Data analyzed in this study were derived from Taiwan's National Health Insurance Research Database (NHIRD) that provides a valid and nation-wide registration system for T1DM.
Patients and Methods
Data Sources
The National Health Insurance (NHI) service was launched in Taiwan on 1st March 1995. The coverage rate of the NHI from 2000 to 2007 had increased steadily from 96.1% to 98.6% [14]. The NHIRD, a large-scale computerized database, derived from this system by the Bureau of NHI and maintained by the National Health Research Institutes (NHRI), is provided to scientists in Taiwan for research purposes. Data in NHIRD that could be used to identify patients or care providers, including medical institutions and physicians, are scrambled before being sent to the National Health Research Institutes for database construction. Data are further scrambled before being released to each researcher. Therefore, individuals cannot be identified from the database.
In Taiwan, the certification of various catastrophic illnesses is subject to evaluation and review by the Bureau of NHI. Patients with catastrophic illness certificates (CICs) are eligible for exemption from insurance premiums and co-payments. Therefore, the CICs data are highly accurate and reliable [14]. The CICs will be terminated once the patients died. Access of this study to the NHIRD has been approved by the Review Committee of the NHRI, and conduct of this study was approved by the Institutional Review Board of National Cheng Kung University Hospital.
Identification of newly-diagnosed type 1 diabetes mellitus
We searched the Taiwan NHIRD for the T1DM population from 1 January 1999 to 31 December 2010. (as per the International Classification of Diseases, 9th Revision, Clinical Modification, ICD-9-CM code 250). We considered the first ever registered CICs for T1DM as the newly-diagnosed T1DM patients, and excluded all CICs registered before 1999. T1DM is recognized as a catastrophic illness by the Taiwan NHI. Due to the fact that no co-payment is required for admission, emergency, and outpatient services, this certification is only applied when detailed and specific clinical data are met, e.g., regular insulin use with a history of diabetic ketoacidosis, a positive glucagon test, or the presence of glutamic-acid-decarboxylase (GAD) antibodies [13]. This CICs registration is a valid and reliable source of data for the T1DM retrieval. Besides, cases of newly-diagnosed T1DM retrieved from the CICs are considered complete [15].
Validation
We validated the ICD-9-CM code for the identification of T1DM by analyzing the chart records of 60 patients who were randomly selected using the study method from the inpatient claims database in National Cheng Kung University Hospital, a 1143-bed tertiary medical center in southern Taiwan, between 2002 and 2010. The contents of this database were used for reimbursements and were similar to those of the NHIRD. T1DM was ascertained by the patients who had 3 or more outpatient diabetes diagnoses with insulin prescriptions, and a history of diabetic ketoacidosis, a positive glucagon test, or the presence of glutamic-acid-decarboxylase (GAD) antibodies [16], [17].
Among the randomly selected 60 patients coded with T1DM, 59 were confirmed by chart review, yielding a positive predictive value of 98.3%. Additionally, T1DM was listed in the principal diagnosis in 100.0% of the patients (data not shown in the table).
Statistical Analysis
We first calculated the age- and sex-specific proportion of T1DM in Taiwan during the study period with the age stratifications of <15, 15–29, 30–44, 45–59, and 60 years or older. To obtain the reliable estimates of incidence rates, we calculated the age- and sex-specific biannual incidence rates of T1DM over the study period. The bi-annual incident rate of T1DM was calculated by dividing the number of incident T1DM cases by the averaged mid-year population of every two years. To examine the secular trend of T1DM incidence rate across the study period, we treated the calendar year as a continuous variable and testing the statistical significance of regression coefficient derived from the Poisson regression model that simultaneously included age, sex, and calendar year in the multivariable regression model. We also employed the 2000 WHO standard population as the reference to calculate the age- and sex-standardized incidence rate of overall T1DM bi-annually.
We selected age- and sex-matched control subjects by identifying from the national database with the age distribution of standard population and yearly mortality counting (i.e., population of Taiwan since 1999 to 2010) collected from Department of Statistics, Ministry of the Interior, Executive Yuan, Taiwan. Compared to the all-causes mortality rates of the general population of the same age and sex, we also calculated the age, sex, and calendar year standardized mortality ratio (SMR) of patients with T1DM. The 95% confidence intervals (CIs) of SMR were estimated using the Poisson distribution. The analyses were performed using SAS software (SAS Institute Inc., Cary, NC, USA). A P-value below 0.05 was considered statistically significant.
Results
Proportion and incidence of type 1 diabetes mellitus
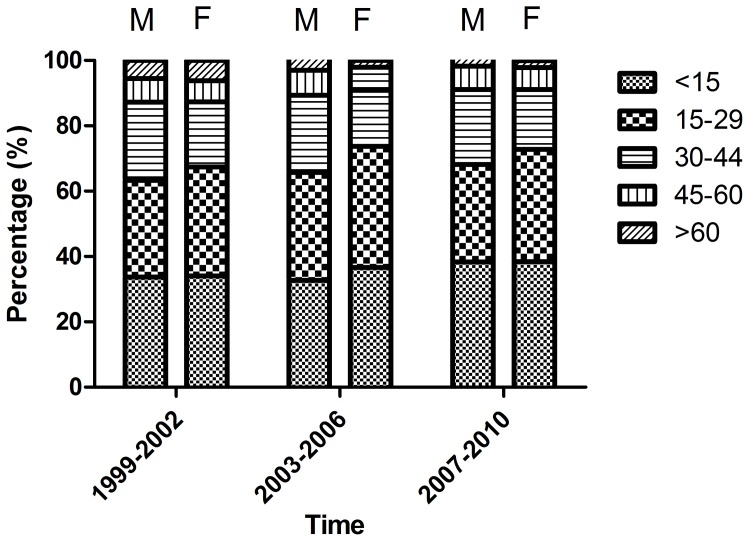
Figure 1 shows the age and sex specific proportions of newly-diagnosed T1DM in Taiwan from 1999 to 2010. A total of 7,225 T1DM patients were identified from the CICs between 1999 and 2010 including 3,471 males and 3,754 females. The number of newly-diagnosed T1DM was increased from 1,133 in 1999–00 to 1,276 in 2009–10, and the number of female patients was generally greater than that of male patients. The majority of T1DM were diagnosed at ages of 30 years or less in both males and females. The age and sex-specific proportions of T1DM were generally constant over the study period. In male patients, the younger group (i.e., <30 years) accounted for about 60% of the patients, while the corresponding figure for female patients was even higher at 70%.
Figure 1. Age and sex specific proportions of newly-diagnosed type 1 diabetes in Taiwan from 1999 to 2010.
(M: male, F: female).
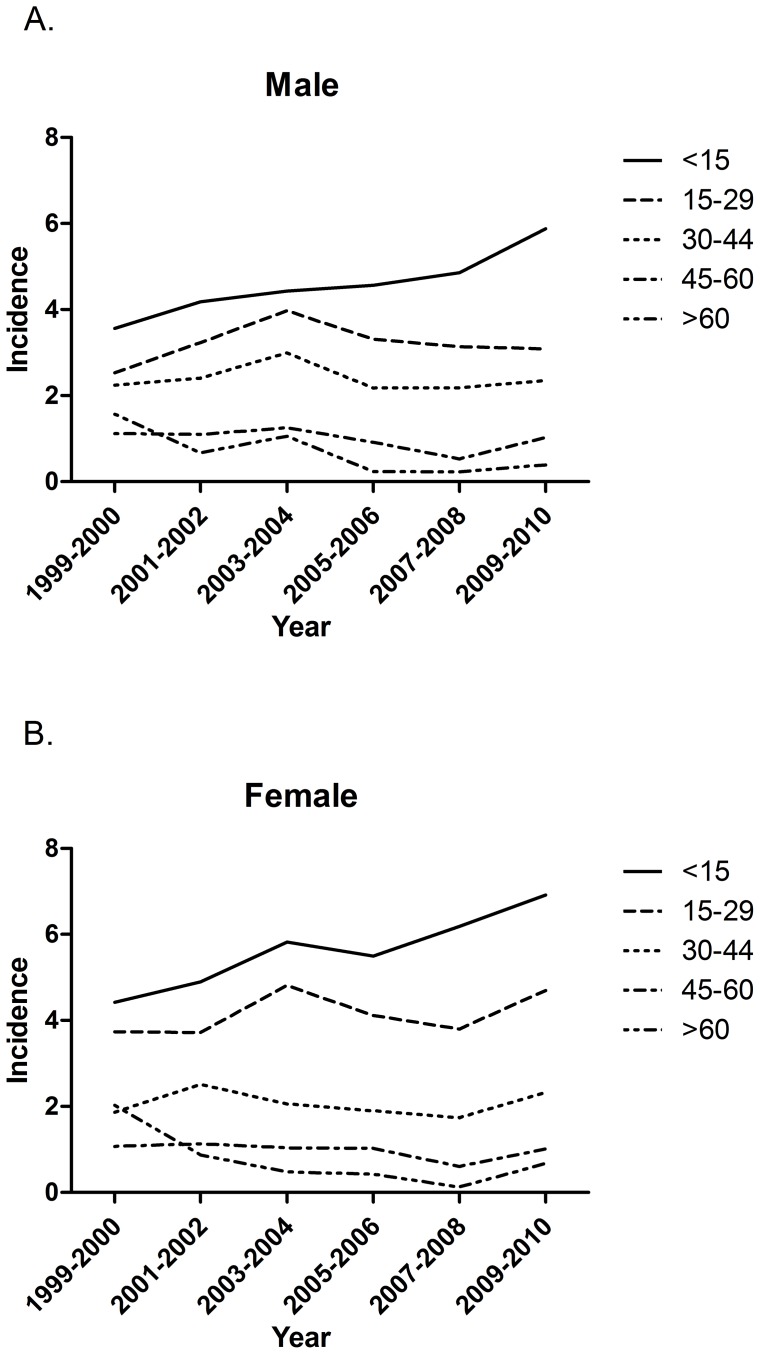
Table 1 shows the bi-annual overall as well as age- and sex-specific incidence rates of T1DM throughout 1999 to 2010. The standardized bi-annual overall incidence rate rose from 1999–00 (2.63 per 100,000) to 2003–04 (3.22 per 100,000) and then mildly declined thereafter rose to 2009–10 (3.34 per 100,000). Over the study period, the β value was −0.002 with an insignificant secular trend (P = 0.489). Regardless of gender, the highest age-specific incidence rate was noted in the younger group (<30 years) and especially highest at <15 years. Additionally, the incidence rate was constantly higher in females than in males in subjects aged <30 years during 1999 to 2010 (Figure 2). For both subjects, the mostly notable increase in incidence rate was noted for ages <15 yr in male (β = 0.043) and female (β = 0.041), respectively. Those aged 45–60 yr (β = −0.036) and ≧60 yr (β = −0.176) in male subjects and those aged ≧60 yr (β = −0.169) in female subjects showed the greater decreases.
Table 1. Bi-annual sex- and age-specific incidence rate (per 100,000 inhabitants) of type 1 diabetes in Taiwan, 1999–2010.
| 1999–00 | 2001–02 | 2003–04 | 2005–06 | 2007–08 | 2009–10 | β | P for trend | |
| Male | ||||||||
| <15 yr | 3.56 | 4.19 | 4.43 | 4.56 | 4.86 | 5.88 | 0.043 | <0.001* |
| 15–29 yr | 2.53 | 3.23 | 3.97 | 3.31 | 3.14 | 3.08 | 0.009 | 0.322 |
| 30–44 yr | 2.24 | 2.40 | 2.99 | 2.18 | 2.18 | 2.35 | −0.006 | 0.590 |
| 45–60 yr | 1.12 | 1.10 | 1.25 | 0.92 | 0.53 | 1.02 | −0.036 | 0.049* |
 60 yr
60 yr |
1.57 | 0.67 | 1.06 | 0.23 | 0.23 | 0.39 | −0.176 | <0.001* |
| Female | ||||||||
| <15 yr | 4.42 | 4.89 | 5.82 | 5.50 | 6.19 | 6.92 | 0.041 | <0.001* |
| 15–29 yr | 3.74 | 3.72 | 4.81 | 4.12 | 3.80 | 4.70 | 0.015 | 0.063 |
| 30–44 yr | 1.87 | 2.51 | 2.06 | 1.89 | 1.74 | 2.32 | −0.001 | 0.916 |
| 45–60 yr | 1.07 | 1.13 | 1.03 | 1.02 | 0.60 | 1.01 | −0.027 | 0.142 |
 60 yr 60 yr |
2.03 | 0.87 | 0.48 | 0.42 | 0.12 | 0.67 | −0.169 | <0.001* |
| Total | 2.63 | 2.83 | 3.22 | 2.85 | 2.81 | 3.34 | −0.002 | 0.489 |
P for linear trend test <0.05.
Figure 2. Trend of bi-annual age- and sex-specific incidence rate (per 100,000 inhabitants) of type 1 diabetes in Taiwan, 1999–2010.
A. In male patients B. In female patients.
Mortality of type 1 diabetes mellitus
The all causes SMR in patients with T1DM was significantly increased at 3.00 (95% CI, 2.83–3.16). The sex-specific SMR for men and women was also significantly increased at 2.66 (95% CI 2.46–2.85) and 3.58 (95% CI 3.28–3.87) (Table 2). Among the age stratifications, the SMR peaked when age at onset was 15–29 years for both male (5.97, 95% CI 4.99–6.95) and female (12.91, 95% CI 10.96–14.86) subjects. The SMR was significantly increased at all age stratifications in females, but age at onset <60 years in male subjects.
Table 2. Standardized mortality ratio of type 1 diabetes in Taiwan according to sex and age at onset, 1999–2010.
| Observedno. of deaths | Expected no. of deaths | SMR | 95% CI | |
| All patients | 333 | 111.08 | 3.00 | 2.83–3.16 |
| Male | 186 | 69.99 | 2.66 | 2.46–2.85 |
| Female | 147 | 41.08 | 3.58 | 3.28–3.87 |
| Age at onset, male | ||||
| <15 yr | 11 | 2.39 | 4.60 | 3.21–5.98 |
| 15–29 yr | 37 | 6.20 | 5.97 | 4.99–6.95 |
| 30–44 yr | 75 | 14.15 | 5.30 | 4.69–5.91 |
| 45–60 yr | 30 | 13.37 | 2.24 | 1.83–2.65 |
 60 yr 60 yr |
33 | 35.33 | 0.93 | 0.77–1.10 |
| Age at onset, female | ||||
| <15 yr | 10 | 2.30 | 4.34 | 2.97–5.71 |
| 15–29 yr | 44 | 3.41 | 12.91 | 10.96–14.86 |
| 30–44 yr | 34 | 4.29 | 7.92 | 6.56–9.27 |
| 45–60 yr | 25 | 5.49 | 4.55 | 3.64–5.46 |
 60 yr 60 yr |
34 | 26.56 | 1.28 | 1.06–1.50 |
Abbreviations: CI, confidence interval; SMR, standardized mortality rate.
Table 3 shows the SMR of T1DM according to the duration since diagnosis of T1DM. Among the age stratifications after T1DM onset, the SMR were significantly increased at all following time for both gender after T1DM diagnosis. The SMR for male was increased from 1.78 (95% CI 1.35–4.63) in the first year to 2.84 (95% CI 2.57–3.52) for the period of <5 yr then mildly declined to 2.66 (95% CI 2.46–2.85) for the period of <12 yr. The SMR for female was highest from 5.00 (95% CI 4.02–9.09) then declined to 3.58 (95% CI 3.28–3.87) for the period of <12 yr.
Table 3. Standardized mortality ratio of type 1 diabetes in Taiwan according to duration of disease by gender, 1999–2010.
| Observedno. of deaths | Expected no. of deaths | SMR | 95% CI | |
| Duration (yrs), male | ||||
| <1 | 17 | 9.53 | 1.78 | 1.35–4.63 |
| <3 | 69 | 26.02 | 2.65 | 2.33–3.69 |
| <5 | 113 | 39.76 | 2.84 | 2.57–3.52 |
| <12 | 186 | 69.99 | 2.66 | 2.46–2.85 |
| Duration (yrs), female | ||||
| <1 | 26 | 5.20 | 5.00 | 4.02–9.09 |
| <3 | 56 | 14.08 | 3.98 | 3.45–5.49 |
| <5 | 89 | 21.87 | 4.07 | 3.64–5.04 |
| <12 | 147 | 41.08 | 3.58 | 3.28–3.87 |
Abbreviations: CI, confidence interval; SMR, standardized mortality rate.
To assess the duration specific SMR in children and adults separately, we further presented age stratified results (Table 4). Among the group of <15 yr, the SMR was 3.65 (95% CI 1.83–5.48) in the first year and slightly went down to 2.35 (95% CI 1.46–3.24) for the period of <3 yr, then rose to 4.47 (95% CI 3.50–5.45) for the period of <12 yr. Among the group of ≧15 yr, the SMR rose from 2.86 (95% CI 2.40–3.32) in the first year to 3.31 (95% CI 3.07–3.55) for the period of <5 yr then declined to 2.87 (95% CI 2.71–3.03) for the period of <12 yr.
Table 4. Standardized mortality ratio of type 1 diabetes in Taiwan according to duration of disease by <15 yr and ≧15 yr, 1999–2010.
| Observedno. of deaths | Expected no. of deaths | SMR | 95% CI | |
| Duration (yrs), <15 yr | ||||
| <1 | 4 | 1.10 | 3.65 | 1.83–5.48 |
| <3 | 7 | 2.98 | 2.35 | 1.46–3.24 |
| <5 | 13 | 4.47 | 2.91 | 2.10–3.72 |
| <12 | 21 | 4.70 | 4.47 | 3.50–5.45 |
Duration (yrs),  15 yr 15 yr
|
||||
| <1 | 39 | 13.64 | 2.86 | 2.40–3.32 |
| <3 | 118 | 37.12 | 3.18 | 2.89–3.47 |
| <5 | 189 | 57.16 | 3.31 | 3.07–3.55 |
| <12 | 312 | 108.81 | 2.87 | 2.71–3.03 |
Abbreviations: CI, confidence interval; SMR, standardized mortality rate.
Discussion
This is the largest population-based T1DM cohort study with at least 12 years of follow-up in Taiwan. We noted that the incidence rate of T1DM was higher in women, especially in the younger group (age at onset <30 years) and was highest when age at onset <15 years both in both sexes. Additionally, the all causes SMR was significantly increased in female patients of all age stratifications. The age-specific SMR was peaking when age at onset was 15–29 years for both sexes. Moreover, the significantly increased SMR was also noted for both gender in the variable following time especially highest for female after disease diagnosis less than one year. From our study we also found that the trend of SMR is different if the diagnosis of T1DM is in childhood (<15 yr) or in adulthood (≧15 yr).
It has been well-documented that the incidence of T1DM is higher among Caucasoid populations than among Mongoloids and Blacks. The distribution of ethnic diversity shows the importance of the differential genetic susceptibility among populations. Within ethnic groups, however, there are also geographical differences in incidence largely explained by the admixture between racial groups and possible environmental exposures [18]. The incidence of T1DM possibly has been underestimated in earlier studies because of incomplete case ascertainment and death from undiagnosed diabetes. Compared with the Chinese population study in Shanghai [19], [20], our group may provide more reliable and updated estimates of incidence and mortality rates in patients with ethnic Chinese T1DM patients due to certain methodological strengths including a much larger and representative study cohort. A steady increase in the incidence of T1DM has been reported worldwide [9]. From our study, we found an upward trend in the incidence of T1DM in younger age-groups (<15 yr), which is similar to the findings of other countries, but a downward trend in older age-groups (≧60 yr). There are several possible reasons that may explain the downward trend among old ages in our study. Free health check-ups have been available for adults aged 40 years or older since 1995 when Taiwan's National Health Insurance program was lunched. A diagnostic protocol for diabetes was also proposed using fasting plasma glucose and hemoglobin A1c levels as criteria. The American Diabetes Association also lowered the fasting plasma glucose level (i.e.,126 mg/dL) in determining diabetes in 1997. Since we enrolled the study patients registered in 1999 and thereafter, therefore, the elderly T1D registered in earlier years might include some patients who had delayed diagnosis.
Studies of patients with T1DM have shown relative mortality peaking in young adults. For example, both the Pittsburgh and Denmark studies showed that relative mortality was highest in the 30–39 ages [21], [22]. The New Zealand and UK studies showed that the highest relative mortality occurred in the 0–29 age group [23], [24]. Our study also revealed a higher relative risk of mortality noted in the younger (age at onset <45 years) group and the highest when age at onset was 15–29 years, which was compatible with findings of the previous studies [21]–[24]. The higher relative risk of death for T1DM patients diagnosed during adolescence and young adults may reflect that these patients are essentially out of their parents' control at the time of diagnosis, increasing the risk of diabetic ketoacidosis (DKA) or poor overall diabetes management [25]. Besides, the severity of T1DM itself would be different across age groups; that is, patients who have severe conditions or rapidly progressive T1DM could be detected in younger age and subsequently have a higher mortality risk.
Many studies have shown that there are no gender differences in mortality associated with T1DM, either in terms of death rates or relative risk of mortality [26]–[28]. In contrast, the risk of mortality relative to the New Zealand general population was higher at all ages in female T1DM than in males except in the 40–49 age group at onset, and the largest difference was noted in the 0–29 age group at onset [23]. Results from the British Diabetic Association Cohort Study also showed a higher mortality in diabetic females than in males at all ages at onset compared to the general population [24]. Finland was the country with the world's highest incidence of T1DM and had many related researches [10], [29]. In a population based nationwide cohort study conducted in Japan and Finland, women had a higher relative mortality in both country but men had a higher absolute mortality in the Finnish cohort. These previous findings suggested a greater effect of T1DM on mortality in female population than in males [10], [29].These previous findings are generally further supported by our study findings, showing a significantly higher SMR among female patients. One possible explanation for a higher SMR in female T1DM patients may be related to our previous findings that although the overall incidence of DKA linearly decreased between 1997 and 2005 in Taiwan, significantly sharp rising trends were still observed in female diabetic patients aged <35 years [30]. It has been reported that, to have a better body image, girls with diabetes often omit insulin injections to avoid weight gain [31]. Rodin et al also reported that young female patients with T1DM tend to have a relatively high prevalence of eating disorders, which might contribute to impaired metabolic control, and consequently result in hyperglycemia and DKA [32]. More aggressive diabetic care programs aimed at young female patients should be considered to reduce this emergency and possibly fatal diabetic complications. Since women usually have lower absolute rates of mortality than men at these ages in Taiwan, clinicians must be acutely aware of this differential and be aggressive in diabetes management.
The Pittsburgh Epidemiology of Diabetes Complications (EDC) data confirmed the importance of renal disease, including persistent microalbuminuria, as a marker of mortality risk and suggested that T1DM patients without renal disease achieve long-term survival comparable to the general population [33]. Taiwan was a country with a very high prevalence of end stage renal disease (ESRD) and diabetes was the leading cause [34]. Whether or to what extent the ESRD may have explained the increased risk of mortality from Taiwanese T1DM population warrants further investigations.
For males, the SMR gradually increased with the increasing duration of T1DM. But for females, the highest SMR was noted during the first year, and decreased after 5 years. We also found that the SMR went down after the first year, and then increased with increasing duration of follow-up in children aged <15 yr. Patients with T1DM have been found to experience various acute and chronic complications. DKA has been reported to be the leading cause of early mortality in T1DM, which may occur long before the peak incidence of chronic complications of diabetes [8], [35]–[37]. The EURODIAB mortality study reported that as many as 35% of the deaths in children diagnosed after 1989 were due to acute complications with DKA [35]. Although the aetiology is not fully clear, it is found that the incidence of T1DM is relative low, but the frequency of DKA in T1DM is very high (65%) in Taiwan [38], [39]. A previous Taiwanese study [30] reported a significant rising trend of DKA incidence in female diabetic patients aged <15 years between 1997 and 2005, but such rising trend was not observed in male patients of the same age, which may largely contribute to our finding that a high SMR in the first years of T1DM diagnosis in females, especially in the first year. It is therefore suggested that more aggressive diabetic cares aiming at young female patients are warranted. Physicians should also be more alert of the increased risk of death, especially from DKA, in children or younger adults diagnosed with T1DM.
The strengths of this work included its nationwide population-based cohort design, relative long duration of follow-up (up to 12 years), and the large number of incident cases of T1DM, which allowed us to calculate the incidence and mortality according to various sex and age stratifications. In addition, the study used the registration system of catastrophic diseases in Taiwan, which provides a good validation of disease diagnosis. Besides, based on the universal coverage system, all T1DM patients were enrolled in the study and a nationally representative sample can be assumed in our study. Findings from our study may provide the updated information on T1DM incidence and mortality in Asian populations. Despite the above strengths, certain limitations involved in our study should be noticed. First, the claim data do not cover laboratory data and clinical details specific to T1DM, which made it impossible to determine the severity of T1DM. Second, there is no information on the underlying cause of death in the NHI claims, thus, we were unable to estimate the cause-specific risk of mortality in T1DM.
Conclusions
In summary, the incidence rate of T1DM was highest in the young-aged group (age at onset <15 years) in both women and men in Taiwan, and the difference in incidence rate between sexes was also greatest in this young group. We also noted an excess mortality in Taiwan's T1DM patient population of both sexes, and of many age stratifications. Targeted strategies aimed at reducing mortality among individuals with T1DM must be considered from both clinical and health policy perspectives.
Acknowledgments
Data from the National Health Insurance Research Database was provided by the Taiwan Bureau of National Health Insurance, Department of Health. The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health or National Health Research Institutes.
Funding Statement
This study was supported by a grant from the National Scientific Council (grant number: NSC101-2314-B-006-076 -MY3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Asao K, Sarti C, Forsen T, Hyttinen V, Nishimura R, et al. (2003) Long-term mortality in nationwide cohorts of childhood-onset type 1 diabetes in Japan and Finland. Diabetes Care 26: 2037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishimura R, LaPorte RE, Dorman JS, Tajima N, Becker D, et al. (2001) Mortality trends in type 1 diabetes. The Allegheny County (Pennsylvania) Registry 1965–1999. Diabetes Care 24: 823–7. [DOI] [PubMed] [Google Scholar]
- 3. Shankar A, Klein R, Klein BE, Moss SE (2007) Association between glycosylated hemoglobin level and cardiovascular and all-cause mortality in type 1 diabetes. Am J Epidemiol 166(4): 393–402. [DOI] [PubMed] [Google Scholar]
- 4. Goldstein DE, Walker B, Rawlings SS, Hess RL, England JD, et al. (1980) Hemoglobin A1c levels in children and adolescents with diabetes mellitus. Diabetes Care 3: 503–7. [DOI] [PubMed] [Google Scholar]
- 5. Ziegler R, Heidtmann B, Hilgard D, Hofer S, Rosenbauer J, et al. (2011) Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes 12: 11–7. [DOI] [PubMed] [Google Scholar]
- 6. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD (1993) The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 329: 1456–62. [DOI] [PubMed] [Google Scholar]
- 7. Secrest AM, Becker DJ, Kelsey SF, LaPorte RE, Orchard TJ (2010) All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes: the Allegheny County type 1 diabetes registry. Diabetes Care 33: 2573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, et al. (2006) Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 49: 298–305. [DOI] [PubMed] [Google Scholar]
- 9. Onkamo P, Vaananen S, Karvonen M, Tuomilehto J (1999) Worldwide increase in incidence of Type I diabetes – the analysis of the data on published incidence trends. Diabetologia 42: 1395–403. [DOI] [PubMed] [Google Scholar]
- 10. The DIAMOND Project Group (2006) Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabetic Medicine 23: 857–66. [DOI] [PubMed] [Google Scholar]
- 11. Wei JN, Sung FC, Lin CC, Lin RS, Chiang CC, et al. (2003) National surveillance for type 2 diabetes mellitus in Taiwanese children. JAMA 290: 1345–50. [DOI] [PubMed] [Google Scholar]
- 12. Wei JN, Li HY, Chang CH, Sung FC, Li CY, et al. (2006) Birth weight and type 1 diabetes among schoolchildren in Taiwan – A population-based case-controlled study. Diabetes Res Clin Pract 74: 309–15. [DOI] [PubMed] [Google Scholar]
- 13. Jiang YD, Chang CH, Tai TY, Chen JF, Chuang LM (2012) Incidence and prevalence rates of diabetes mellitus in Taiwan: analysis of the 2000–2009 Nationwide Health Insurance database. J Formos Med Assoc 111: 599–604. [DOI] [PubMed] [Google Scholar]
- 14. Chiu YM, Lai CH (2010) Nationwide population-based epidemiologic study of systemic lupus erythematosus in Taiwan. Lupus 19: 1250–5. [DOI] [PubMed] [Google Scholar]
- 15. Chen HF, Chen P, Li CY (2011) Risk of malignant neoplasm of the pancreas in relation to diabetes: a population-based study in Taiwan. Diabetes Care 34: 1177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Diabetes Association (2004) Diagnosis and classification of diabetes mellitus. Diabetes Care 27 Suppl 1S5–S10. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence JM, Black MH, Zhang JL, Slezak JM, Takhar HS, et al. (2014) Validation of Pediatric Diabetes Case Identification Approaches for Diagnosed Cases by Using Information in the Electronic Health Records of a Large Integrated Managed Health Care Organization. Am J Epidemiol 179: : 27–38. [DOI] [PubMed] [Google Scholar]
- 18. Karvonen M, Tuomilehto J, Libman I, LaPorte R (1993) A review of the recent epidemiological data on the worldwide incidence of type 1 (insulin-dependent) diabetes mellitus. World Health Organization DIAMOND Project Group. Diabetologia 36: 883–92. [DOI] [PubMed] [Google Scholar]
- 19. Fu H, Shen SX, Chen ZW, Wang JJ, Ye TT, et al. (1994) Shanghai, China, has the lowest confirmed incidence of childhood diabetes in the world. Diabetes Care 17: 1206–8. [DOI] [PubMed] [Google Scholar]
- 20. Shen SX, Wang HB, Chen ZW, Shen YE, Fu H, et al. (1996) The incidence of insulin-dependent diabetes mellitus in urban districts of Shanghai (1989–1993). J Pediatr Endocrinol Metab 9: 469–73. [DOI] [PubMed] [Google Scholar]
- 21. Chen F, Florkowski CM, Dever M, Beaven DW (2004) Death Certification and New Zealand Health Information Service (NZHIS) statistics for diabetes mellitus: an under-recognised health problem. Diabetes Res Clin Pract 63: 113–8. [DOI] [PubMed] [Google Scholar]
- 22. DERI Mortality Study Group (1991) Sex differences in the mortality associated with insulin-dependent diabetes mellitus in four countries. The Diabetes Epidemiology Research International (DERI) Study. Am J Epidemiol 133: 577–84. [PubMed] [Google Scholar]
- 23. Dawson SI, Willis J, Florkowski CM, Scott RS (2008) All-cause mortality in insulin-treated diabetic patients: a 20-year follow-up. Diabetes Res Clin Pract 80: e6–9. [DOI] [PubMed] [Google Scholar]
- 24. Laing SP, Swerdlow AJ, Slater SD, Botha JL, Burden AC, et al. (1999) The British Diabetic Association Cohort Study, I: all-cause mortality in patients with insulin-treated diabetes mellitus. Diabetic Medicine 16: 459–65. [DOI] [PubMed] [Google Scholar]
- 25. Burnet DL, Cooper AJ, Drum ML, Lipton RB (2007) Risk factors for mortality in a diverse cohort of patients with childhood-onset diabetes in Chicago. Diabetes Care 30: 2559–63. [DOI] [PubMed] [Google Scholar]
- 26. Deckert T, Poulsen JE, Larsen M (1978) Prognosis of diabetics with diabetes onset before the age of thirty-one. I. Survival, causes of death, and complications. Diabetologia 14: 363–70. [DOI] [PubMed] [Google Scholar]
- 27. Riley MD, McCarty DJ, Couper DJ, Humphrey AR, Dwyer T, et al. (1995) The 1984 Tasmanian insulin treated diabetes mellitus prevalence cohort: an eight and a half year mortality follow-up investigation. Diabetes Res Clin Pract 29: 27–35. [DOI] [PubMed] [Google Scholar]
- 28. Borch-Johnsen K, Kreiner S, Deckert T (1986) Mortality of type 1 (insulin-dependent) diabetes mellitus in Denmark: a study of relative mortality in 2930 Danish type 1 diabetic patients diagnosed from 1933 to 1972. Diabetologia 29: 767–72. [DOI] [PubMed] [Google Scholar]
- 29. Harjutsalo V, Sjoberg L, Tuomilehto J (2008) Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 371: 1777–82. [DOI] [PubMed] [Google Scholar]
- 30. Liu CC, Chen KR, Chen HF, Huang HL, Ko MC, et al. (2010) Trends in hospitalization for diabetic ketoacidosis in diabetic patients in Taiwan: analysis of national claims data, 1997–2005. J Formos Med Assoc 109: 725–34. [DOI] [PubMed] [Google Scholar]
- 31. Meltzer LJ, Johnson SB, Prine JM, Banks RA, Desrosiers PM, et al. (2001) Disordered eating, body mass, and glycemic control in adolescents with type 1 diabetes. Diabetes Care 24: 678–82. [DOI] [PubMed] [Google Scholar]
- 32. Rodin GM, Daneman D (1992) Eating disorders and IDDM. A problematic association. Diabetes Care 15: 1402–12. [DOI] [PubMed] [Google Scholar]
- 33. Orchard TJ, Secrest AM, Miller RG, Costacou T (2010) In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 53: 2312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Renal Data System (2012) Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.
- 35. Patterson CC, Dahlquist G, Harjutsalo V, Joner G, Feltbower RG, et al. (2007) Early mortality in EURODIAB population-based cohorts of type 1 diabetes diagnosed in childhood since 1989. Diabetologia 50: 2439–42. [DOI] [PubMed] [Google Scholar]
- 36. Laing SP, Jones ME, Swerdlow AJ, Burden AC, Gatling W (2005) Psychosocial and socioeconomic risk factors for premature death in young people with type 1 diabetes. Diabetes Care 28: 1618–23. [DOI] [PubMed] [Google Scholar]
- 37. Laing SP, Swerdlow AJ, Slater SD, Botha JL, Burden AC, et al. (1999) The British Diabetic Association Cohort Study, II: cause-specific mortality in patients with insulin-treated diabetes mellitus. Diabetic Medicine 16: 466–71. [DOI] [PubMed] [Google Scholar]
- 38. Usher-Smith JA, Thompson M, Ercole A, Walter FM (2012) Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia 55: 2878–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ting WH, Huang CY, Lo FS, Hung CM, Chan CJ, et al. (2007) Clinical and laboratory characteristics of type 1 diabetes in children and adolescents: experience from a medical center. Acta paediatrica Taiwanica 48: 119–24. [PubMed] [Google Scholar]