Abstract
Targeting early infection in mucosal sites is one of the primary goals for mucosal vaccines so as to prevent pathogen mucosal transmission and infection. The TLR5 agonist flagellin was deemed to be a mucosal adjuvant candidate for clinical usage. However, the high antigenicity of flagellin and the possible inflammatory injury induced by flagellin might restrict its clinical usage. Here HIV-1 p24 protein was selected as an antigen model and we replaced the main antigenicity region domains D2 and D3 of non-pathogenic E.coli-derived flagellin (KF). The derived soluble protein KFD-p24 3D was then compared with KF-p24, which fused p24 directly to the C-terminal of KF. In vitro and ex vivo experiments showed that KFD-p24 3D has lower TLR5 agonist efficacy and less immunocyte-activating efficacy. Interestingly, the production of KF- specific antibody was highly reduced, and KFD-p24 3D induced IgA-biased antibody responses in mucosal sites. Moreover, KFD-p24 3D induced far fewer systemic inflammatory responses and abrogated detectable inflammatory side effects on mice, even at the high dose. The properties of enhanced IgA generation and attenuated inflammatory responses broaden the safe-dose range of KFD-p24 3D flagellin, creating a potentially promising mucosal adjuvant.
Keywords: flagellin, mucosal, adjuvant, IgA, inflammatory response
Introduction
Most microbial pathogens gain access to their host through mucosal surfaces and therefore mucosal immune responses function as a first line of defense.1,2 Hence, the best strategy to prevent pathogen mucosal infection is to target early infection in mucosal sites.3,4 As a key mediator of mucosal immune defenses–in addition to preventing attachment, invasion and replication of mucosal pathogens–mucosal IgA antibodies are unique in their capacity to bind to newly synthesized viral antigens inside cells so as to reduce the viral replication of HIV-1, for example.5-7 The SHIV vaginal challenge model in nonhuman primates showed protection of HIV-1-specific antibodies in mucosal sites.7
For development of mucosal vaccines against virus infection through mucosal surfaces, intranasal (i.n.) immunization has been widely used, because both systemic and mucosal immune responses could be induced, including those in the genital tract and rectum.8-10 However, the weak immunogenicity of most protein antigens when administered via mucosa presents a grave challenge for the development of safe and effective mucosal vaccines.11
Flagellin, the major structural protein of bacterial flagella, can induce proinflammatory responses and dendric cells (DCs) maturation through the TLR5 pathway.12 The flagellin molecule is composed of highly conserved N/C regions (domains D0/D1) crucial for TLR5 agonist activity, and the middle hyper-variable region (domains D2/D3) has vast diversity in size and composition in various bacterial strains.13-15 During the past decade, a number of studies have described the mucosal adjuvant property of flagellin in the context of a broad range of recombinant vaccines against pathogens like Yersinia pestis and West Nile virus, largely due to its TLR5-activating potential.16-18 The most attractive advantage of flagellin as adjuvant is its plasticity for generation of antigen-flagellin fusion proteins.19 A number of vaccine candidates with this strategy have reached early stage clinical studies, representing one promising new direction in vaccine development.20-24 Two formulations of flagellin and targeted antigen fusion proteins are currently used: fusion to the C-terminus of flagellin or replacing the D3 domain to preserve greater TLR5 agonist efficacy.22,25 Our previous study showed that a virulent fusion protein of S. mutans attached to the C-terminus of the nonpathogenic E. coli K12 strain-derived flagellin (KF) is a highly potent mucosal vaccine against carries.26
However, the very potent antigenicity of flagellin itself led to a concern that immunity to flagellin might affect the potency of this molecule and induce possible side effects when used as a mucosal adjuvant,27 although there were reports that prior immunity to flagellin does not impair its adjuvant activity and does not lead to serious systemic effects.18 In addition, the inflammatory reactions caused by flagellin created more concern. To human being, less than 10 μg of recombinant antigen-flagellin fusion protein are believed to constitute a safe dose.23,28,29
This paper is therefore focused on the effects of replacement of most of the hyper-variable region of flagellin with HIV-1 p24 on the mucosal p24-specific IgA generations, the immunogenicity of flagellin, and the systemic inflammatory response induced by the fusion protein. Based on the flagellin derived from E. coli (KF), we successfully obtained a soluble KFD-p24 3D recombinant protein in which the main antigenicity region (i.e., domains D2 and D3) was replaced by HIV-1 p24. Surprisingly, KFD-p24 3D has been shown to induce high IgA-biased antibody responses at different mucosal sites and far fewer flagellin-induced systemic inflammatory responses.
Results
Replacement of hypervariable region domains D2 and D3 retained the TLR5 agonist function of flagellin
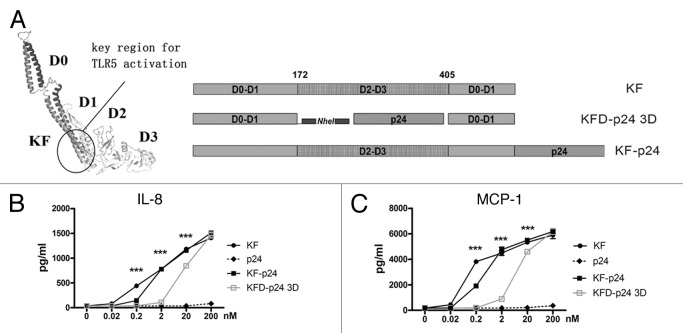
The mucosal adjuvanticity of flagellin was mostly due to its TLR5 agonist capability, and the hypervariable region was functionally dissociated from the TLR5-activating domains.27 In Salmonella-derived flagellin, high immunogenicity was induced by the hypervariable region but not by the conserved region critical for TLR5 activation.27,30 To reduce the antigenicity and immunogenicity of flagellin, we attempted to replace the entire hyper-variable region domains D2 and D3 by HIV-p24; and compared this with KF-p24, in which the p24 was directly fused to the C-terminal of KF (Fig. 1A and S1A). KFD-p24 3D was generated by replacing domains D2 and D3 and linking p24 with two repeats of 11 amino acids in the human IgG3 hinge region to enhance molecule flexibility (Fig. 1A and Table 1). The epithelial cell line Caco-2 (which expresses TLR5 constitutively), was then adopted to test TLR5 agonist efficacy of flagellin derivants.30 KFD-p24 3D induced IL-8 and MCP-1 in a dose-dependent manner similar to that for KF and KF-p24, although with less activity in the concentrations lower than 200 nM (Fig. 1B and C). The EC50s of KF, KF-p24 and KFD-p24 3D for inducing MCP-1 were 0.17 nM, 0.85 nM, and 11 nM, respectively (Fig. 1C). Therefore, replacement of the hyper-variable domain D2 and D3 retained the TLR5 agonist activity to stimulate epithelial cells, which is essential for modification and optimization of flagellin as a mucosal adjuvant (Fig. S1B and C).
Figure 1. Flagellin KFD-p24 3D (with D2 and D3 domains replaced) manifested partially preserved TLR5 agonist efficacy. (A) Schematic diagram showing domain structures and variants. Left: The 3D structures of KF predicted by ESyPred3D Web Server 1.052; KFD-p24 3D: D2 and D3 domains of KF were replaced by HIV-1 protein p24, KF-p24: fusion protein of KF and p24. (B) IL-8 and (C) MCP-1 in cell culture supernatants of Caco-2 cells 6 h post stimulation were tested by ELISA to reflect TLR5 agonist efficacy. Data are presented as the means ± SEM from triplicates of one experiment that was repeated at least three times.
Table 1. Oligonucleotide primers for generation of KF-p24 and KFD-p24 3D.
| Plasmid | Restricted site | Sequence (5′-3′) |
|---|---|---|
| pKF-p24 | NcoI | GCG CCA TGGCAC AAG TCA TTA ATA CC |
| EcoRI | GCG AAT TCA CCC TGC AGC AGA GAC AGA AC | |
| EcoRI | GCC GAA TTC CCT ATA GTG CAG AAC CTC CAG | |
| XhoI | GGA CTC GAG CAA AAC TCT TGC TTT ATG GCC | |
| pKFN3 | NcoI | GGC GTC CAT GGC ACA AGT CAT TAA TAC |
| BamHI | TAC TAG GAT CCG TCG TGC TAG CTC CAG ATG TGT GAG TTG TGT CAC CAA GTG GAG TAT CAA GGC CAA GAG TTT TAG |
|
| pKFD-p24 3D | NheI | ATC GAG CTA GCA CAC CTC TTG GTG ATA CTA CAC ACA CAT CAG GAC CTA TAG TGC AGA ACC TCC A |
| BamHI | TAT GCG GAT CCG TCG TCA AAA CTC TTG CTT TAT GGC |
KFD-p24 3D subtly activated antigen-presenting cells and improved cytokine production
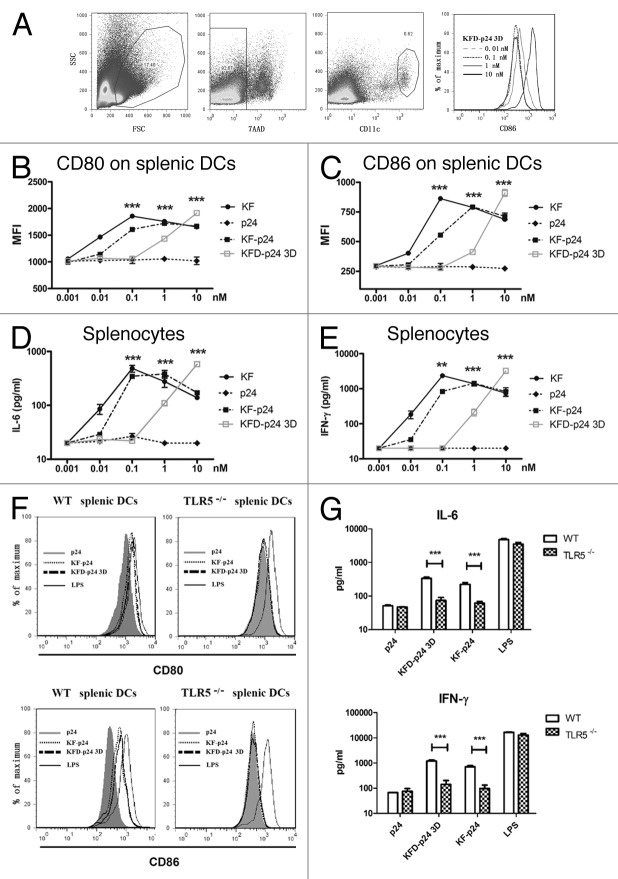
Most TLR agonists function as adjuvants by stimulating the production of cytokines and the maturation of dendritic cells (DCs), thereby linking innate and adaptive immunity.18 To test the adjuvant potential, splenocytes, which can be activated through the TLR5- signaling pathway in vivo, were selected initially as an ex vivo immunocyte model.17,31 We tested expression levels in BALB/c mice derived splenic DCs, gated by 7AAD- CD11chigh (Fig. 2A), of the co-stimulatory molecules CD80 and CD86, and cytokine secretion in cell culture supernatants. Although the activity of KFD-p24 3D was much lower, dose-dependent stimulation occurred similarly to that for KF-p24 and KF (Fig. 2B and C). The dose-dependent induction of cytokines IL-6 and IFN-γ also showed that the activating immunocyte efficacy of KFD-p24 3D was about 10 times less than that of KF-p24 (Fig. 2D and E). These activations on splenocytes mainly depended upon the TLR5 pathway as all of these actions could only be observed on splenocytes separated from wild-type mice but not from Tlr5-knockout mice (on a C57BL/6 background; Fig. 2F and G). The activating potential of KFD-p24 3D could also be observed on DCs separated from neck-associated lymph nodes and mesenteric lymph nodes (data not shown).
Figure 2. The stimulatory effects of recombinant flagellin on splenocytes ex vivo. Cells and supernatants were separately harvested for analysis 20 h after stimulation of splenocytes separated from naïve BALB/c mice in A–E or from C57BL/6 mice background in F and G. (A) CD86 expression level changes for gated 7AAD- CD11chigh splenic DCs after 0.01–10 nM KFD-p24 stimulation. (B and C) Dose-dependent CD80 and CD86 expression levels for CD11chigh splenic DCs were tested by FACS. (D and E) Dose-dependent IL-6 and IFN-γ secreted into the culture supernatant from splenocytes were tested by ELISA. (F and G) CD80, CD86 expression level changes on CD11chigh splenic DCs and IL-6, IFN-γ secreted by splenocytes after 10 nM p24, KF-p24, KFD-p24 3D or 1 μg/ml LPS stimulation. Data are presented as the means ± SEM from triplicates of one experiment that was repeated at least three times.
KFD-p24 3D reduced the immunogenicity of flagellin and induced IgA-biased antibody responses
To determine the immunogenicity of KFD-p24 3D, BALB/c mice were immunized intranasally with p24, p24 plus KF, KF-p24 or KFD-p24 3D. As the main antigenicity region of flagellin KF was replaced, KFD-p24 3D showed about a 2-log reduction in the generation of KF-specific serum IgG (Fig. 3A), serum IgA (Fig. 3B) and mucosal IgAs (data not shown), compared with both full-length KF plus p24-mixed protein and full-length KF-p24 fusion protein. This demonstrated that the replacement of the hyper-variable domains D2 and D3 reduced by more than 95% the immunogenicity against full-length flagellin KF protein. Compared with KF plus p24, KFD-p24 3D induced significantly higher p24-specific serum IgG, serum IgA and mucosal IgAs (Fig. 3C-E). Compared with KF-p24, KFD-p24 3D induced slightly less p24-specific serum IgG, but slightly higher p24-specific serum IgA and mucosal IgAs (although the differences were not always significant except in saliva IgA; Fig. 3C-E). Nevertheless, it is important to note that the ratios of p24-specific mucosal IgA to serum IgG induced by KFD-p24 3D were all significantly higher than those for KF-p24 and the KF-plus-p24 group (Fig. 3F). This indicated that KFD-p24 3D induced IgA-biased responses in mucosal sites, while KFD-p24 3D induced Th2-biased immune responses; and these were identical to responses to KF and KF-p24, according to the serum IgG2a/IgG1 ratio (data not shown). All of the other KFD-p24s shown in Figure S1A induced IgA and IgG responses similar to those for KFD-p24 3D upon intranasal immunization (data not shown).
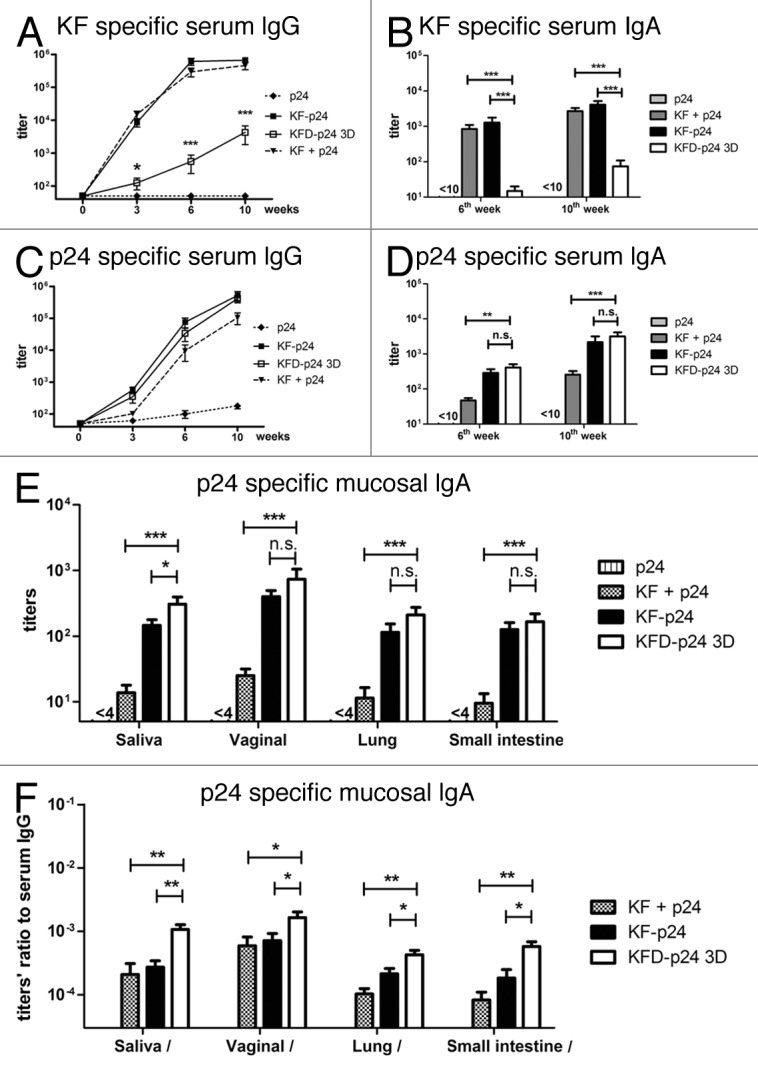
Figure 3. p24- and KF-specific antibody responses after intranasal immunization. BALB/c mice were immunized intranasally with 5 μg p24, 5 μg p24 plus 5 μg KF, 10 μg KF-p24 or 10 μg KFD-p24 3D at 0, 4th, and 8th weeks. (A and C) Time-course of KF-specific or p24-specific IgG titers in serum. (B and D) KF-specific or p24-specific IgA titers in serum at 6th and 10th weeks. (E) p24-specific IgA titers in mucosal sites including saliva, vaginal lavage fluid, and lung and small intestine at 10th week. (F) Ratios of p24-specific IgA titers in mucosal sites to serum IgG titers at 10th week. Data are presented as the means ± SEM from one experiment that was repeated three times (n = 6 per group).
KFD-p24 3D induced much less of a systemic inflammatory response than KF-p24
To evaluate nasal immunization of KF-derived recombinant flagellins for observing potential flagellin-induced inflammatory responses and possible side-effects, proinflammatory cytokines, body-weight of treated mice, and serum transaminases were evaluated. From the cytokine chip assay, we observed that G-CSF, GM-CSF, IL-6, KC, MIP-1α, MIP-1γ and TNF-α were significantly upregulated in bronchoalveolar lavage fluids (BALFs) (data not shown). Among them, IL-6, a marker of the synthetic proinflammatory cytokines32; KC, a neutrophil chemoattractant chemokine33; and TNF-α were then quantified by ELISA. IL-6, KC, and TNF-α were detectable in BALF until 24 h after intranasal administration of flagellin; and the responses peaked at 4 h, at which time KFD-p24 3D induced lower responses in all three cytokines than did KF-p24 (Fig. 4A-C). KFD-p24 3D also lowered IL-6 and KC responses compared with those of KF-p24 in serum 4 h after intranasal administration (Fig. 4D), but induced similar IL-6 and KC responses in nose lavage fluid (NLF) (Fig. 4E). Consistent with relatively lower TLR5 agonist efficacy, intranasal administration of KFD-p24 3D attenuated the loss in body mass compared with 10 μg of KF-p24 (Fig. 4F). Even when the dosage was increased to 50 μg, no significant loss in body mass was observed after intranasal administration of KFD-p24 3D (data not shown).
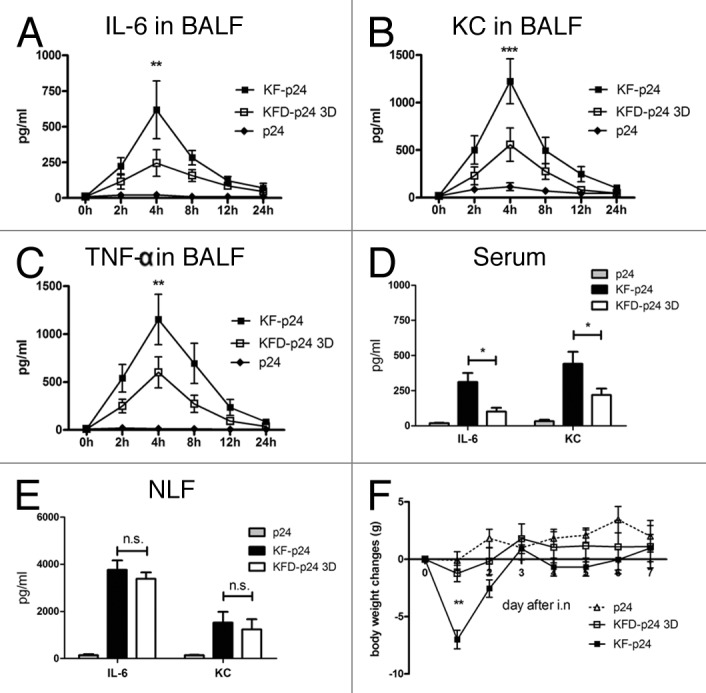
Figure 4. KFD-p24 3D induced a lowered systemic inflammatory response after intra-nasal administration. Control protein p24 (10μg) or recombinant flagellin with PBS (10 μl) was administrated intranasally to BALB/c mice. Time- course of (A) IL-6, (B) KC and (C) TNF-α in BALFs after administration. IL-6 and KC in serum (D) and nose lavage fluid (E) 4 h after administration. (F) Body mass changes after administration. Data are presented as the means ± SEM from one experiment that was repeated three times (n = 6 per group).
It was reported in mice that high-dose flagellin could induce severe systemic inflammation, which might in turn result in transient liver injury.34 We therefore further evaluated the safety of the KF-derived recombinant flagellins by quantifying serum IL-6, KC, ALT and AST using an ultra-high dose in the exquisitely sensitive C57BL/6 mouse. We administered up to 250 μg of p24, KF-p24 or KFD-p24 3D intranasally, and samples were collected and evaluated as described in Materials and Methods. Both KF-p24 and KFD-p24 3D upregulated IL-6, IL-12 p40, exotaxin, CXCL16, RANTES, KC, fractalkine, MCP-1, BLC, TIMP-1 and GCSF using the RayBio mouse cytokine antibody array III (Fig. 5A and B). However, compared with KF-p24, KFD-p24 3D induced significantly less of these proinflammatory cytokines and chemokines by semi-quantitative analysis of the RayBio mouse cytokine antibody array III assay (Fig. 5C). These lowered cytokine and chemokine responses were further confirmed by ELISA tests of IL-6 (Fig. 5D) and KC (Fig. 5E) at different time-points after intranasal administration. Unexpectedly, intranasal administration of 250 μg KFD-p24 3D induced no change in levels of serum transaminase ALT or AST, while 250 μg of KF-p24 produced a 5-fold increase in ALT and a 3-fold increase in AST; this indicated that no overt liver injury was induced by KFD-p24 3D (Fig. 5F). After intra peritoneal (i.p.) administration of KFD-p24 3D, we observed similar effects on IL-6 and KC in serum (Fig. S2A-D), on body mass loss and liver-injury indicators (Fig. S2E and F).
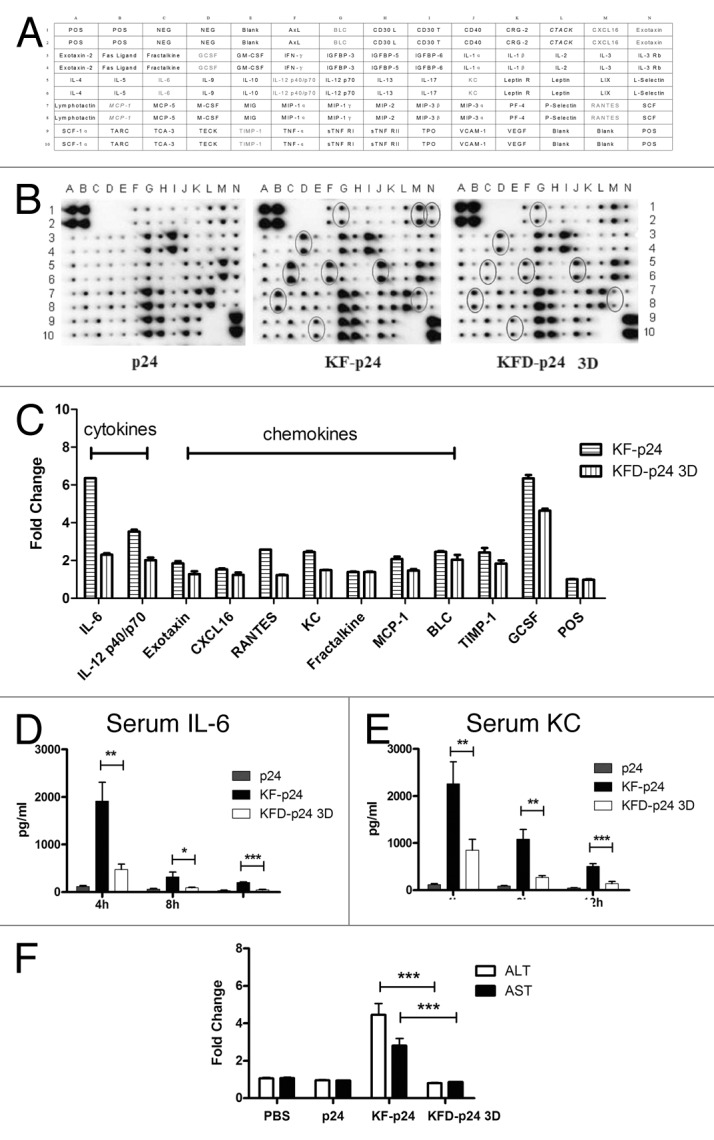
Figure 5. At the high dose, KFD-p24 3D induced much less of a systemic inflammatory response after intra-nasal administration than did KF-p24. C57BL/6 mice were treated intranasally with 250 μg of p24, KFD-p24 3D or KF-p24 in 25 μl PBS. (A) Template showing the location of cytokine antibodies spotted in duplicate onto the RayBio mouse cytokine antibody array III.51 (B) Representative photographs of cytokine arrays using pooled serum samples. Circles designating differentially expressed cytokines are labeled gray in panel B. (C) Fold-changes in levels of proteins were significantly increased compared with the p24 control treatment. Data are represented as mean ± SEM for duplicate spots. (D) IL-6 and (E) KC in serum after administration. (F) Fold-changes in levels of serum transaminase were evaluated 24 h post-administration and compared with the control group (250 μg p24). Data are presented as the means ± SEM from one experiment that was repeated three times (n = 6 per group).
Collectively, replacement of the hyper-variable domains D2 and D3 in flagellin with HIV-1 p24 attenuated flagellin-induced inflammatory responses and abrogated potential liver injury even at high dose. KFD-p24 3D might thereby possess a high safety range for use as a mucosal adjuvant.
Discussion
The present study was part of our ongoing efforts to seek an optimized adjuvant for mucosal vaccines to combat infectious diseases such as HIV and measles. We have previouely shown that deletion of the D3 region could only partially reduce the immunogenicity of flagellin derived from Salmonella.30 In order to fully explore the structural locations of the immunogenicity of flagellin, a series of KF-derived recombinant proteins were designed and expressed (Fig. S3A and B). Results showed that about 10%, 30% and 90% antigenicities were located on domains D0/D1, D3 and D2/D3, respectively (Fig. S3C). Our previous study also showed that the partial deletion of the hypervariable region of flagellin changed mucosal IgA responses.30 Some others reported a flagellin with truncated variable region incoperated into HIV VLPs as adjuvant was more effective in inducing HIV-specific mucosal IgA responses.35 We therefore further replaced the main antigenicity region domains D2 and D3 with HIV-1 p24 protein and successfully obtained a soluble KFD-p24 3D recombinant protein. As we expected, KFD-p24 3D reduced by more than 95% the immunogenicity against full-length flagellin KF protein (Fig. 3A and B). The most notable finding in our study was that KFD-p24 3D could induce IgA-biased antibody responses at different mucosal sites and resulted in far fewer flagellin-induced systemic inflammatory responses. The propensity to produce IgA-biased responses in mucosal sites is important and promising for mucosal vaccines, especially for HIV-1 vaccines.
Flagellin is comprised of a middle hypervariable region harboring flagellin’s major antigenic epitopes, a N-terminal conserved domain and a C-terminal conserved domain, where the two conserved domains form a dimer for binding to TLR5 as well as inflammasome NLRC4, mediating the adjuvant effects of flagellin. Some previous studies in the literature support a functional dichotomy between conserved domain for adjuvanticity and hypervariable domain for antigenicity by showing that complete or partial deletions of the hypervariable domain decreased the flagellin’s antigenicity with no apparent effects on the production of IL-8 and CCL20 and the flagellin’s adjuvanticity.17,27 However, some other groups reported that complete or partial deletions of the hypervariable domain caused reduced productions of IL-8 and TNF-α.27 Through the present study, we attempted to gain more insights into the effects of structures especially the hypervariable region on the antigenicity and adjuvanticity of recombinant flagellins by generating new fusion recombinant proteins. Our results that the replacement of the domains D2 and D3 by target antigen abrogated high immunogenicity of flagellin itself are in support of the notion that the hypervariable domain is for antigenicity. But surprisingly, it is the first time we showed that the replacement of the domains D2 and D3 by target antigen induced IgA-biased antibody responses at different mucosal sites and lowered flagellin-induced systemic inflammatory responses. Our study also further supported the notion that the hypervariable domain was involved in the cytokine production by flagellin and more importantly implied that the changed cytokine profile might influence the mucosal adjuvanticity and possible side effect of flagellin detectable on high dose. More tests should be performed to replace the domains D2 and D3 with some other antigens to show the universality, though we got similar results by replacement with a Streptococcus mutans surface protein PAc (unpublished data). Furthermore, pathogen-related protective antigen is worth testing for neutralizing antibody generation and protective effect on mice as well as in guinea pigs, rabbits or even non-human primates.
The reason why KFD-p24 3D has propensity to produce IgA-biased responses in mucosal sites is intriguing and worth further exploration. The most successful replacement case so far is replacing the D3 domain of flagellin with influenza viral haemagglutinin globular head domain, which showed highly effective at eliciting protective HAI titers and protecting mice from disease and death, and thus made the influenza vaccine candidate VAX125 go into clinical trial.22,25 However, the vaccination of this study was administered subcutaneously, which did not show any data on IgA responses. While the mechanisms by which flagellin acts as an adjuvant are not fully understood at present, it is plausible to speculate the underlying mechanisms by which KFD-p24 3D induced the IgA biased responses. First, KFD-p24 3D may promote more B cell isotype switching to IgA and facilitate IgA plasma cell maturation in mucosal sites as that of cholera toxin acts as mucosal adjuvant.36 But the detail mechanism should be elucidated further. Second, as the mucosal IgA generation involves various cells, including epithelial cells, IECs, stromal cells, various DCs, macrophages, Treg and T follicular helper (Tfh) cells, and many cytokines, chemokines, or even cell surface molecules, such as transforming growth factor-β (TGF-β) and co-stimulatory signals triggered by CD40L; or the tumor-necrosis factor (TNF) family members BAFF, a proliferation inducing ligand APRIL, IL-6 and IL-10,37-39 the underlying mechanisms may be very complex. However, our data that KFD-p24 3D induced significantly less of the proinflammatory cytokines and chemokines (Fig. 5A-C) than KF-p24 give us hint that the decreases in the proinflammatory cytokines and chemokines may influence the local IgA production. Consider the role of IgA and IgA Fc receptor as anti-inflammatory agents,40,41 we cannot tell which is the initial inducer. Third, the Naip5/NLRC4 pathway, which could be activated by cytosolic flagellin in macrophages,42,43 might also involve in the antibody responses by intranasal immunization.
As for the systemic inflammatory response, it was reported in mice that high-dose flagellin could induce severe systemic inflammation, which might in turn result in transient liver injury.34 It was reported in the clinical trial (NCT00921947) that one serious adverse event, hydronephrosis, occurred occasionally when 1μg flagellin fusion protein was given intramuscularly, but other symptoms including arm pain, muscle aches, headache, fatigue and swelling were also induced.29 Flagellin as adjuvant, the maximal responses could be obtained with doses in about 1 µg by systemic usage, but about 10-folds flagellin was needed in mice model to elicit stable and efficient responses by mucosal immunization in preventing pathogen mucosal infection (data not shown). To get more insight into the systemic proinflammatory effect, we tested cytokine responses with antibody array and serum transaminases at high dose of flagellin by intranasal administration in mice. As similar as previous studies, in which high amounts of pro-inflammatory cytokines in plasma, indicators of oxidative stress, liver damage and neutrophil infiltration into the lungs were observed when high doses of flagellin administrated,34,44 KF-p24 also induced certain high level of proinflammatory cytokines and chemokines (Fig. 5B-E), and serum transaminases (Fig. 5F). However, KFD-p24 3D significantly reduced all these flagellin-induced systemic inflammatory responses (Fig. 4), even at an ultra-high dose (Fig. 5; Fig. S2). The underlying mechanism for this reduction remains unknown, though we may speculate its relationship with its relatively lower TLR5 agonist efficacy and the changed cytokine profile (Figs. 4 and 5).
In conclusion, compared with KF-p24 (where p24 was directly linked to the C-terminal of KF), the replacement of D2 and D3 domains of flagellin KF-derived recombinant protein KFD-p24 3D nearly abrogated the generation of flagellin-specific antibody but enhanced p24 specific IgA generation and, more importantly, to a great extent reduced systemic inflammation and possible unwanted side effect. The properties of enhanced IgA generation and attenuated inflammatory responses broaden the safe-dose range of KFD-p24 3D flagellin, creating a potentially promising mucosal adjuvant.
Material and Methods
Animals
Female C57BL/6 and BALB/c mice, aged 6–8 weeks (18–20 g per mouse) were obtained from Beijing Laboratory Animal Research Center and housed in the Animal Center of Wuhan Institute of Virology (WIV), Chinese Academy of Sciences (CAS) under specific pathogen-free (SPF) conditions. C57BL/6 background TLR5−/− mice in Jackson laboratory origin were bred and housed in the Animal Center of Wuhan Institute of Virology (WIV), Chinese Academy of Sciences (CAS) under specific pathogen-free (SPF) conditions. Animal studies were performed according to Regulations for the Administration of Affairs Concerning Experimental Animals in China (1988), and the Guideline for Animal Care and Use, WIV, CAS (permit number WIVA09201105). All animal studies and methods conformed to the ARRIVE guidelines. All animals were randomly assigned to groups before immunization.
Cloning and expression of recombinant proteins
Recombinant Escherichia coli K-12 MG1655 flagellin (KF)- (GenBank Accession number 949101),26 and plasmid pNL4–3- derived HIV-1 p24 clones were cloned into restriction enzyme sites (NdeI and XhoI) of the pET-30a-c (+) vector (Novagen). KF-p24 was generated by insertion of NcoI- and EcoRI-restricted KF-derived fragments and EcoRI- and XhoI-restricted p24-derived fragments into a pET-28a-c (+) vector (Novagen). The hypervariable region-deleted clones KFD3 were generated by linking (NcoI and BamHI restricted) the N-terminal of the KF-derived fragment (KFN3) and (BamHI and XhoI enzyme digested) C-terminal of the KF-derived fragment (KFC) into a pET-28a-c (+) vector. KFD-p24 3D was generated by inserting (NheI and BamHI restricted) p24-derived hypervariable fragments into the middle of (NheI and BamHI enzyme digested) the plasmid KFD3 (Table 1).
The transformed E. coli BL21 DE3 containing the expression constructs was grown overnight at 37°C in Luria–Bertani broth with 50 μg/ml kanamycin. Log-phase bacteria were induced with isopropyl b-D-thiogalactoside (IPTG) plus lactose and harvested by centrifugation. To improve the yield of soluble protein KFD-p24 3D, 1% ethanol was added 1 h before induction and the culture temperature was reduced to 22°C.
Purification and verification of recombinant protein
Lysis was performed in binding buffer by freeze thawing and sonication. The recombinant proteins were purified by affinity chromatography on a Ni–NTA column (Qiagen) and dialyzed with PBS at 4°C. Contaminating lipopolysaccharide (LPS) was removed by Triton X-114 phase separation, which retains the physical integrity and the biological activity of the protein and is harmless to experimental animals.45,46 The purified protein was treated with Acrodisc syringe filters (Pall, Port Washington, NY) and preserved at -80°C. Quantification of the purified proteins was performed using the Bradford assay. The residual LPS content was determined using the Limulus assay (Associates of Cape Cod) and was determined to be less than 0.002 EU / μg protein. Purified proteins were tested by SDS-PAGE and verified by western blotting with an anti-His-tag monoclonal antibody (Qiagen) and a secondary alkaline phosphatase (AP)-conjugated goat anti-mouse IgG antibody (Southern Biotech). Visualization of the antibody–antigen complex formation was accomplished using NTB and BCIP (Beyotime, China). RAW 264.7 cells, which are sensitive to LPS and bacterial DNA but not to flagellin,47,48 were used to further ensure that residual bacterial DNA and LPS contamination were undetectable.
The response of Caco-2 cell to flagellins
The human intestinal epithelial Caco-2 cells were grown in DMEM supplemented with 10% FBS (GIBCO) and 100 U/ml of penicillin/streptomycin at 37°C in 5% CO2, and used as previously described.30 For the cytokine release assays, the cells were seeded at a density of 200,000 per well into 24-well polystyrene plates (Costar) and used 5 d later. After an overnight culture in medium without FBS, the cells were stimulated with flagellins for 6 h at the indicated concentrations. The supernatants were then collected and quantified for IL-8 and MCP-1.
The response of splenocytes to flagellin
The spleens obtained from 6–8 weeks old female BALB/c mice, C57BL/6 mice and C57BL/6 background TLR5−/− mice were smashed using syringe pistons in PBS and filtered through strainers (BD Biosciences). After washing with PBS and RPMI 1640 medium containing 10% FBS and 100 U/ml of penicillin/streptomycin, single cells were seeded into 48-well plates (0.4 × 107 cells / well, 0.2 ml) in medium. The cells and supernatants were collected separately to assess the levels of co-stimulatory molecules and cytokines after stimulation. The cells were blocked with anti-Fc receptor (BD Biosciences) before staining. For the detection of co-stimulatory molecules, the cells were stained with APC-labeled anti-CD11c, FITC-labeled anti-CD86, PE-labeled anti-CD80 and 7-amino-actinomycin D (7-AAD) (BD Biosciences). The samples were analyzed using a FACSCalibur (Becton Dickinson, Heidelberg, Germany). The IL-6 and IFN-γ in supernatants were quantified by ELISA.
Mice immunization, sample preparation and antibody measurement
Female BALB/c mice were anesthetized and immunized intranasally with recombinant flagellin and/or p24 in 10 μl PBS three times at four-week intervals. Immunized sera and mucosal secretions were collected after immunization for analysis of p24- or KF-specific antibody responses as previously described.30,49 Briefly, blood samples were collected from the retro-orbital plexus. Saliva was procured after carbamylcholine (Sigma) intraperitoneal injection. Vaginal lavage fluid was obtained by washing the genital tract with 40 μl of sterile PBS three times. Thoracolaparotomy was performed after sacrifice to harvest the lungs and small intestines, which were then ground in 800 μL of grinding buffer (PBS with 0.2% saponin, 0.2% NaN3 and 50 μM PMSF) or 1.6 ml, respectively. The ground samples were then oscillated for 4 h at 4°C with centrifugation. All samples were stored at -80°C until they were assayed by ELISA. One hundred μl of sera or mucosal samples was subsequently applied as a 4-fold dilution series for ELISA.30 Alkaline phosphatase-labeled goat anti-mouse IgG, IgA (Southern Biotechnology), IgG1 or IgG2a (BETHYL) polyclonal antibodies and substrate (p-nitrophenyl phosphate; Sigma) were used for detection.
Collection of bronchoalveolar lavage (BALF) and nose lavage fluids (NLFs)
Female BALB/c mice and C57BL/6 mice in 6–8 weeks old were anesthetized and immunized intranasally with 10 μg stimulus in 10 μl PBS or with a 250 μg stimulus in 25 μl PBS. The animals were sacrificed by cervical dislocation at the indicated time points and the bronchoalveolar lavage (BALFs) and nasal lavage fluids (NLFs) were collected as described previously.50 Briefly, 1 ml sterile PBS was inserted into the bronchoalveoli of each mouse twice. PBS (0.3 ml) was inserted into the trachea, followed by two washes.
Cytokine antibody assay
RayBio mouse cytokine antibody array III (Ray-Biotech, Inc., Norcross, GA) was used for cytokine antibody assay as described previously.51 Signal intensities were quantified directly with a chemiluminescence imaging system (FluorChem HD2; Alpha Innotech) and analyzed with its corresponding software. Spots were digitized into pixel densities (PDs), and these were exported into an Excel program for analysis. For the control group (p24 treatment), the digitized pixel densities (PDs) of each protein were determined as the mean of duplicate spots, and the fold-change for each protein was calculated as PD KF-p24 / PD control or PD KFD-p24 3D / PD control.
Cytokines were quantified by using ELISA kits (BD Biosciences for Human IL-8, MCP-1 and Mouse TNF-α, IL-6, IFN-γ; Antigenix America for mouse KC) according to the manufacturer’s instructions.
Serum aminotransferase and cytokine assay
Twenty-four hours after intranasal administration of a 250 μg stimulus in 25 μl PBS or after intraperitoneal injection of a 250 μg stimulus in 250 μl PBS on 6–8 weeks old C57BL/6 mice, blood was obtained by cardiac puncture after pentobarbital anesthetization, and allowed to clot at room temperature. Serum was then separated by centrifugation (3,000 rpm, 10 min) and stored at -80°C. Concentrations of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured in serum samples at Wuhan University’s Zhongnan Hospital.
Statistical analysis
All of the results are presented as the means ± standard error of the mean (SEM) from one experiment that was repeated at least three times. Statistical analyses were performed with Instat GraphPad software, version 5.0. Time course and dose dependent effects were analyzed by two-way analysis of variance (ANOVA); all other data analyses were performed with one-way ANOVA. When the P value was significant at the 5% level, further pairwise comparisons were made using Bonferroni posttests and the differences between the KFD-p24 3D group and KF-p24 group were labeled if not specially indicated. Statistical significance is indicated by * (p < 0.05), ** (p < 0.01), and *** (p < 0.001). A P value less than 0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgments
We sincerely thank Xuefang An, Wei Li and Yang Xiao for valuable assistance in the animal studies.
This work was supported by grants from the Ministry of Science and Technology of the People’s Republic of China (973 Program, No. 2012CB518904; the National S&T Major Project on Major Infectious Diseases, Nos. 2012ZX10001–008 and 2008ZX10001–010; the National Key R&D Program, No. 2007BAI28B04) and the National Natural Science Foundation of China (Nos. 31270207 and 81202381).
Glossary
Abbreviations:
- KF
E.coli K12 strain-derived flagellin
- DC
dendritic cell
- BALF
bronchoalveolar lavage fluids
- NLF
nose lavage fluids
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/23809
References
- 1.Mestecky J, Michalek SM, Moldoveanu Z, Russell MW. Routes of immunization and antigen delivery systems for optimal mucosal immune responses in humans. Behring Inst Mitt. 1997:33–43. [PubMed] [Google Scholar]
- 2.Jakobsen H, Jonsdottir I. Mucosal vaccination against encapsulated respiratory bacteria--new potentials for conjugate vaccines? Scand J Immunol. 2003;58:119–28. doi: 10.1046/j.1365-3083.2003.01292.x. [DOI] [PubMed] [Google Scholar]
- 3.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–23. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 4.Virgin HW, Walker BD. Immunology and the elusive AIDS vaccine. Nature. 2010;464:224–31. doi: 10.1038/nature08898. [DOI] [PubMed] [Google Scholar]
- 5.Lamm ME. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997;51:311–40. doi: 10.1146/annurev.micro.51.1.311. [DOI] [PubMed] [Google Scholar]
- 6.Mazanec MB, Nedrud JG, Kaetzel CS, Lamm ME. A three-tiered view of the role of IgA in mucosal defense. Immunol Today. 1993;14:430–5. doi: 10.1016/0167-5699(93)90245-G. [DOI] [PubMed] [Google Scholar]
- 7.Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–80. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Bonenfant C, Dimier-Poisson I, Velge-Roussel F, Buzoni-Gatel D, Del Giudice G, Rappuoli R, et al. Intranasal immunization with SAG1 and nontoxic mutant heat-labile enterotoxins protects mice against Toxoplasma gondii. Infect Immun. 2001;69:1605–12. doi: 10.1128/IAI.69.3.1605-1612.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, Cu-Uvin S, et al. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J Immunol. 2002;169:566–74. doi: 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]
- 10.Mestecky J, Fultz PN. Mucosal immune system of the human genital tract. J Infect Dis. 1999;179(Suppl 3):S470–4. doi: 10.1086/314806. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Patel GB, Yan H, Zhang J. Recent advances in the development of novel mucosal adjuvants and antigen delivery systems. Hum Vaccin. 2010;6:6. doi: 10.4161/hv.6.9.11561. [DOI] [PubMed] [Google Scholar]
- 12.Ramos HC, Rumbo M, Sirard JC. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 2004;12:509–17. doi: 10.1016/j.tim.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Homma M, Fujita H, Yamaguchi S, Iino T. Regions of Salmonella typhimurium flagellin essential for its polymerization and excretion. J Bacteriol. 1987;169:291–6. doi: 10.1128/jb.169.1.291-296.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eaves-Pyles TD, Wong HR, Odoms K, Pyles RB. Salmonella flagellin-dependent proinflammatory responses are localized to the conserved amino and carboxyl regions of the protein. J Immunol. 2001;167:7009–16. doi: 10.4049/jimmunol.167.12.7009. [DOI] [PubMed] [Google Scholar]
- 15.Murthy KG, Deb A, Goonesekera S, Szabó C, Salzman AL. Identification of conserved domains in Salmonella muenchen flagellin that are essential for its ability to activate TLR5 and to induce an inflammatory response in vitro. J Biol Chem. 2004;279:5667–75. doi: 10.1074/jbc.M307759200. [DOI] [PubMed] [Google Scholar]
- 16.McDonald WF, Huleatt JW, Foellmer HG, Hewitt D, Tang J, Desai P, et al. A West Nile virus recombinant protein vaccine that coactivates innate and adaptive immunity. J Infect Dis. 2007;195:1607–17. doi: 10.1086/517613. [DOI] [PubMed] [Google Scholar]
- 17.Mizel SB, Bates JT. Flagellin as an adjuvant: cellular mechanisms and potential. J Immunol. 2010;185:5677–82. doi: 10.4049/jimmunol.1002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honko AN, Sriranganathan N, Lees CJ, Mizel SB. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect Immun. 2006;74:1113–20. doi: 10.1128/IAI.74.2.1113-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huleatt JW, Jacobs AR, Tang J, Desai P, Kopp EB, Huang Y, et al. Vaccination with recombinant fusion proteins incorporating Toll-like receptor ligands induces rapid cellular and humoral immunity. Vaccine. 2007;25:763–75. doi: 10.1016/j.vaccine.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Adar Y, Singer Y, Levi R, Tzehoval E, Perk S, Banet-Noach C, et al. A universal epitope-based influenza vaccine and its efficacy against H5N1. Vaccine. 2009;27:2099–107. doi: 10.1016/j.vaccine.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Bargieri DY, Leite JA, Lopes SC, Sbrogio-Almeida ME, Braga CJ, Ferreira LC, et al. Immunogenic properties of a recombinant fusion protein containing the C-terminal 19 kDa of Plasmodium falciparum merozoite surface protein-1 and the innate immunity agonist FliC flagellin of Salmonella typhimurium. Vaccine. 2010;28:2818–26. doi: 10.1016/j.vaccine.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Song L, Zhang Y, Yun NE, Poussard AL, Smith JN, Smith JK, et al. Superior efficacy of a recombinant flagellin:H5N1 HA globular head vaccine is determined by the placement of the globular head within flagellin. Vaccine. 2009;27:5875–84. doi: 10.1016/j.vaccine.2009.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Treanor JJ, Taylor DN, Tussey L, Hay C, Nolan C, Fitzgerald T, et al. Safety and immunogenicity of a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125) in healthy young adults. Vaccine. 2010;28:8268–74. doi: 10.1016/j.vaccine.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Taylor DN, Treanor JJ, Strout C, Johnson C, Fitzgerald T, Kavita U, et al. Induction of a potent immune response in the elderly using the TLR-5 agonist, flagellin, with a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125, STF2.HA1 SI) Vaccine. 2011;29:4897–902. doi: 10.1016/j.vaccine.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Bargieri DY, Soares IS, Costa FT, Braga CJ, Ferreira LC, Rodrigues MM. Malaria Vaccine Development: Are Bacterial Flagellin Fusion Proteins the Bridge between Mouse and Humans? J Parasitol Res 2011; 2011:965369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y, Shi W, Yang JY, Zhou DH, Chen YQ, Zhang Y, et al. Flagellin-PAc fusion protein is a high-efficacy anti-caries mucosal vaccine. J Dent Res. 2012;91:941–7. doi: 10.1177/0022034512457684. [DOI] [PubMed] [Google Scholar]
- 27.Nempont C, Cayet D, Rumbo M, Bompard C, Villeret V, Sirard JC. Deletion of flagellin’s hypervariable region abrogates antibody-mediated neutralization and systemic activation of TLR5-dependent immunity. J Immunol. 2008;181:2036–43. doi: 10.4049/jimmunol.181.3.2036. [DOI] [PubMed] [Google Scholar]
- 28.Taylor DN, Treanor JJ, Sheldon EA, Johnson C, Umlauf S, Song L, et al. Development of VAX128, a recombinant hemagglutinin (HA) influenza-flagellin fusion vaccine with improved safety and immune response. Vaccine. 2012;30:5761–9. doi: 10.1016/j.vaccine.2012.06.086. [DOI] [PubMed] [Google Scholar]
- 29.Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, et al. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine. 2011;29:5145–52. doi: 10.1016/j.vaccine.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 30.Liu F, Yang J, Zhang Y, Zhou D, Chen Y, Gai W, et al. Recombinant flagellins with partial deletions of the hypervariable domain lose antigenicity but not mucosal adjuvancy. Biochem Biophys Res Commun. 2010;392:582–7. doi: 10.1016/j.bbrc.2010.01.077. [DOI] [PubMed] [Google Scholar]
- 31.Sanders CJ, Franchi L, Yarovinsky F, Uematsu S, Akira S, Núñez G, et al. Induction of adaptive immunity by flagellin does not require robust activation of innate immunity. Eur J Immunol. 2009;39:359–71. doi: 10.1002/eji.200838804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barton BE. IL-6: insights into novel biological activities. Clin Immunol Immunopathol. 1997;85:16–20. doi: 10.1006/clin.1997.4420. [DOI] [PubMed] [Google Scholar]
- 33.Schumacher C, Clark-Lewis I, Baggiolini M, Moser B. High- and low-affinity binding of GRO alpha and neutrophil-activating peptide 2 to interleukin 8 receptors on human neutrophils. Proc Natl Acad Sci U S A. 1992;89:10542–6. doi: 10.1073/pnas.89.21.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liaudet L, Murthy KG, Mabley JG, Pacher P, Soriano FG, Salzman AL, et al. Comparison of inflammation, organ damage, and oxidant stress induced by Salmonella enterica serovar Muenchen flagellin and serovar Enteritidis lipopolysaccharide. Infect Immun. 2002;70:192–8. doi: 10.1128/IAI.70.1.192-198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vassilieva EV, Wang BZ, Vzorov AN, Wang L, Wang YC, Bozja J, et al. Enhanced mucosal immune responses to HIV virus-like particles containing a membrane-anchored adjuvant. MBio. 2011;2:e00328–10. doi: 10.1128/mBio.00328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lycke NY. Cholera toxin promotes B cell isotype switching by two different mechanisms. cAMP induction augments germ-line Ig H-chain RNA transcripts whereas membrane ganglioside GM1-receptor binding enhances later events in differentiation. J Immunol. 1993;150:4810–21. [PubMed] [Google Scholar]
- 37.Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol. 2009;70:505–15. doi: 10.1111/j.1365-3083.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 38.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–84. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Chorny A, Puga I, Cerutti A. Innate signaling networks in mucosal IgA class switching. Adv Immunol. 2010;107:31–69. doi: 10.1016/B978-0-12-381300-8.00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monteiro RC. Role of IgA and IgA fc receptors in inflammation. J Clin Immunol. 2010;30:1–9. doi: 10.1007/s10875-009-9338-0. [DOI] [PubMed] [Google Scholar]
- 41.Ben Mkaddem S, Rossato E, Heming N, Monteiro RC. Anti-inflammatory role of the IgA Fc receptor (CD89): From autoimmunity to therapeutic perspectives. Autoimmun Rev. 2012 doi: 10.1016/j.autrev.2012.10.011. In Press. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Y, Yang J, Shi J, Gong Y-N, Lu Q, Xu H, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 43.Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol. 2007;29:275–88. doi: 10.1007/s00281-007-0078-z. [DOI] [PubMed] [Google Scholar]
- 44.Honko AN, Mizel SB. Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect Immun. 2004;72:6676–9. doi: 10.1128/IAI.72.11.6676-6679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132:191–5. doi: 10.1016/0022-1759(90)90029-U. [DOI] [PubMed] [Google Scholar]
- 46.Liu S, Tobias R, McClure S, Styba G, Shi Q, Jackowski G. Removal of endotoxin from recombinant protein preparations. Clin Biochem. 1997;30:455–63. doi: 10.1016/S0009-9120(97)00049-0. [DOI] [PubMed] [Google Scholar]
- 47.McCoy SL, Kurtz SE, Hausman FA, Trune DR, Bennett RM, Hefeneider SH. Activation of RAW264.7 macrophages by bacterial DNA and lipopolysaccharide increases cell surface DNA binding and internalization. J Biol Chem. 2004;279:17217–23. doi: 10.1074/jbc.M303837200. [DOI] [PubMed] [Google Scholar]
- 48.West AP, Dancho BA, Mizel SB. Gangliosides inhibit flagellin signaling in the absence of an effect on flagellin binding to toll-like receptor 5. J Biol Chem. 2005;280:9482–8. doi: 10.1074/jbc.M411875200. [DOI] [PubMed] [Google Scholar]
- 49.Gai W, Zou W, Lei L, Luo J, Tu H, Zhang Y, et al. Effects of different immunization protocols and adjuvant on antibody responses to inactivated SARS-CoV vaccine. Viral Immunol. 2008;21:27–37. doi: 10.1089/vim.2007.0079. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Yang J, Bao R, Chen Y, Zhou D, He B, et al. Unpolarized release of vaccinia virus and HIV antigen by colchicine treatment enhances intranasal HIV antigen expression and mucosal humoral responses. PLoS One. 2011;6:e24296. doi: 10.1371/journal.pone.0024296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong M, He B, Yang J, Bao R, Zhang Y, Zhou D, et al. L-selectin and P-selectin are novel biomarkers of cervicovaginal inflammation for preclinical mucosal safety assessment of anti-HIV-1 microbicide. Antimicrob Agents Chemother. 2012;56:3121–32. doi: 10.1128/AAC.05950-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lambert C, Léonard N, De Bolle X, Depiereux E. ESyPred3D: Prediction of proteins 3D structures. Bioinformatics. 2002;18:1250–6. doi: 10.1093/bioinformatics/18.9.1250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.