Abstract
Italy was one of the first countries in the world to introduce a routine vaccination program against HBV for newborns and 12-y-old children. From a clinical point of view, such strategy was clearly successful. The objective of our study was to verify whether, at 20 y from its implementation, hepatitis B universal vaccination had positive effects also from an economic point of view. An a posteriori analysis evaluated the impact that the hepatitis B immunization program had up to the present day. The implementation of vaccination brought an extensive reduction of the burden of hepatitis B-related diseases in the Italian population. As a consequence, the past and future savings due to clinical costs avoided are particularly high. We obtained a return on investment nearly equal to 1 from the National Health Service perspective, and a benefit-to-cost ratio slightly less than 1 for the Societal perspective, considering only the first 20 y from the start of the program. In the longer-time horizon, ROI and BCR values were positive (2.78 and 2.46, respectively). The break-even point was already achieved few years ago for the NHS and for the Society, and since then more and more money is progressively saved. The implementation of universal hepatitis B vaccination was very favorable during the first 20 y of adoption, and further benefits will be increasingly evident in the future. The hepatitis B vaccination program in Italy is a clear example of the great impact that universal immunization is able to provide in the medium-long-term when health care authorities are so wise as to invest in prevention.
Keywords: infection, HBV, hepatitis B, chronic hepatitis, cirrhosis, hepatocellular carcinoma, cost-effectiveness, vaccine, universal immunization, Italy
Introduction
Hepatitis B virus (HBV) infection is still a relevant worldwide public health problem: as a matter of fact, HBV infection may lead to many serious acute and chronic liver diseases, including cirrhosis and hepatocellular carcinoma (HCC). Despite improvements in hygienic conditions and behaviors had a crucial importance in the last decades, vaccination remains the most effective measure to control the transmission of HBV and to prevent hepatitis B diseases. In accordance with WHO recommendations, endorsed by the World Health Assembly in 1992, 93% of countries in the world have integrated vaccination against HBV in their national routine immunization schedule.1 The adoption of such vaccination programs led to a substantial decrease in disease burden, HBsAg carrier rates and hepatitis B-related morbidity and mortality in the world.2,3
In 1991, Italy was the first industrialized country to introduce a program of routine vaccination against HBV with immunization of all newborns in the first years of life and all 12 y old children during the first 12 y of the program.4 The double cohort policy of mandatory immunization aimed at reducing, and, in the long-term, at eliminating the transmission of the infection.
In Italy, during the 1980s, about 11,000 symptomatic cases of acute viral hepatitis per year (19/100,000 inhabitants) were notified. At that time, 64,000 people were affected by chronic viral hepatitis or cirrhosis (prevalence rate 112/100,000) and 3,400 by hepatocellular carcinoma (prevalence rate 5.9/100,000).5 In 1991, hepatitis B incidence had declined to 5 cases / 100,000 subjects (1, 12 and 4 cases per 100,000 in subjects 0–14, 15–24 and ≥ 25 y-old, respectively), due to several changes in social behavior and improvement of health procedures. In 2010, after 20 y of universal vaccination, the rate of notified cases of hepatitis B had decreased to 0.9 cases/100,000 (0.0, 0.5 and 1.2 cases per 100,000 in subjects 0–14, 15–24 and ≥ 25 y old, respectively.6
In 1999, Da Villa et al. outlined a first preliminary cost-effectiveness evaluation of this preventive intervention during the first six years of implementation, with particular focus on the occurrence of new acute hepatitis B cases. The results of that study highlighted that averted costs following the decline of acute hepatitis B cases amounted to 2/3 of the expenditure for vaccination during the first 6 y of immunization.5
Since the impact of hepatitis B immunization is increasingly evident in the middle-long-term, we could perform a more complete clinical and economic evaluation 20 y after the introduction of the immunization program, starting from the first cost-effectiveness study of Da Villa et al.5 The calculation of all costs incurred and avoided in the first 20 y of implementation seemed a particularly interesting approach, taking into account that many a priori models of preventive interventions forecast future clinical and economic benefits, but very few models are subsequently developed to show the actual impact with real data. For this reason, we performed an a posteriori economic analysis of universal hepatitis B vaccination in Italy, but also projected the further future clinical/economic benefits due to the immunization of our population in the first 20 y of the program. Particularly, the main objective of this study was to determine the economic impact of the adoption of universal vaccination against hepatitis B in Italy from 1991 to 2010, comparing the past and future medical care and social costs avoided by reducing all diseases related to infection with hepatitis B virus vs. the overall cost of the immunization program in the first 20 y of implementation.
Results
Table 1 shows the total number of clinical cases related to HBV infection (HBV infections, acute hepatitis B, chronic hepatitis without evolution toward cirrhosis and carcinoma, compensated and decompensated cirrhosis, hepatocellular carcinoma, DC and HCC patients requiring liver transplantation), as calculated by the simulation model, with and without the adoption of the vaccination strategy. The implementation of the immunization program reduces largely the overall burden of hepatitis B disease. Particularly, the number of HBV infections was decreased by 75% .
Table 1. Total number of clinical cases related to HBV infection in Italy.
| CLINICAL CASES | No-vaccination | Vaccination | Avoided cases | % reduction |
|---|---|---|---|---|
| HBV infection | 168,930 | 42,038 | 126,892 | 75 |
| AHB | 43,140 | 28,520 | 14,621 | 34 |
| CHB | 5,465 | 1,360 | 4,105 | 75 |
| CC | 129 | 59 | 70 | 54 |
| DC | 9 | 4 | 5 | 54 |
| HCC | 86 | 22 | 64 | 74 |
| LT | 24 | 7 | 17 | 72 |
Clinical costs during the period 1991–2010, 2011–2059 and 1991–2059 according to the NHS and to the Societal perspective in the no-vaccination and vaccination scenario are presented in Table 2. The implementation of vaccination against HBV infection compared with the no-vaccination scenario implies a reduction in clinical costs of 53% during the past period (1991–2010), 77% during the future period (2011–2059) and 66% in the overall analyzed period. The predominant clinical costs avoided are due to treatment of prevented chronic hepatitis B cases and acute hepatitis B related costs.
Table 2. Clinical costs during the period 1991–2010, 2011–2059 and 1991–2059 according to NHS and Societal perspective in the no-vaccination and vaccination scenario.
| PAST COSTS (1991–2010) | ||||
|---|---|---|---|---|
| NHS perspective | ||||
| No-vaccination | Vaccination | Avoided costs | % reduction | |
| AHB | 572,051,723 | 362,160,953 | 209,890,771 | 37 |
| CHB | 649,157,949 | 210,059,569 | 439,098,380 | 68 |
| CC | 18,485,689 | 8,914,521 | 9,571,168 | 52 |
| DC | 1,193,807 | 575,700 | 618,107 | 52 |
| HCC | 8,330,359 | 2,830,361 | 5,499,999 | 66 |
| LT | 3,135,545 | 1,117,773 | 2,017,771 | 64 |
| Total | 1,252,355,072 | 585,658,877 | 666,696,195 | 53 |
| Societal perspective | ||||
|---|---|---|---|---|
| No-vaccination | Vaccination | Avoided costs | % reduction | |
| AHB | 689,926,159 | 436,786,229 | 253,139,930 | 37 |
| CHB | 763,780,696 | 247,150,087 | 516,630,609 | 68 |
| CC | 21,749,733 | 10,488,571 | 11,261,162 | 52 |
| DC | 1,404,599 | 677,353 | 727,247 | 52 |
| HCC | 9,801,263 | 3,330,121 | 6,471,141 | 66 |
| LT | 3,681,249 | 1,311,056 | 2,370,193 | 64 |
| Total | 1,490,343,698 | 699,743,417 | 790,600,281 | 53 |
| FUTURE COSTS (2011–2059) | ||||
|---|---|---|---|---|
| NHS perspective | ||||
| No-vaccination | Vaccination | Avoided costs | % reduction | |
| AHB | 0 | 0 | 0 | 0 |
| CHB | 1,407,135,406 | 312,317,653 | 1,094,817,753 | 78 |
| CC | 19,147,161 | 8,519,755 | 10,627,406 | 56 |
| DC | 795,200 | 342,980 | 452,219 | 57 |
| HCC | 61,994,027 | 16,534,566 | 45,459,461 | 73 |
| LT | 5,855,495 | 1,471,071 | 4,384,425 | 75 |
| Total | 1,494,927,289 | 339,186,025 | 1,155,741,264 | 77 |
| Societal perspective | ||||
|---|---|---|---|---|
| No-vaccination | Vaccination | Avoided costs | % reduction | |
| AHB | 0 | 0 | 0 | 0 |
| CHB | 1,655,595,315 | 367,464,028 | 1,288,131,288 | 78 |
| CC | 22,528,002 | 10,024,100 | 12,503,902 | 56 |
| DC | 935,609 | 403,541 | 532,069 | 57 |
| HCC | 73,523,198 | 19,623,408 | 53,899,790 | 73 |
| LT | 6,546,747 | 1,625,042 | 4,921,705 | 75 |
| Total | 1,759,128,872 | 399,140,118 | 1,359,988,754 | 77 |
| TOTAL COSTS (1991–2059) | ||||
|---|---|---|---|---|
| NHS perspective | ||||
| No-vaccination | Vaccination | Avoided costs | % reduction | |
| AHB | 572,051,723 | 362,160,953 | 209,890,771 | 37 |
| CHB | 2,056,293,355 | 522,377,222 | 1,533,916,133 | 75 |
| CC | 37,632,849 | 17,434,276 | 20,198,573 | 54 |
| DC | 1,989,007 | 918,681 | 1,070,326 | 54 |
| HCC | 70,324,386 | 19,364,927 | 50,959,459 | 72 |
| LT | 8,991,040 | 2,588,844 | 6,402,196 | 71 |
| Total | 2,747,282,361 | 924,844,902 | 1,822,437,459 | 66 |
| Societal perspective | ||||
|---|---|---|---|---|
| No-vaccination | Vaccination | Avoided costs | % reduction | |
| AHB | 689,926,159 | 436,786,229 | 253,139,930 | 37 |
| CHB | 2,419,376,011 | 614,614,115 | 1,804,761,896 | 75 |
| CC | 44,277,735 | 20,512,671 | 23,765,064 | 54 |
| DC | 2,340,208 | 1,080,893 | 1,259,315 | 54 |
| HCC | 83,324,461 | 22,953,529 | 60,370,932 | 72 |
| LT | 10,227,996 | 2,936,098 | 7,291,898 | 71 |
| Total | 3,249,472,570 | 1,098,883,535 | 2,150,589,035 | 66 |
Note: costs assessed during 2011–59 are limited to those that acquired HBV during the 1991–2010 period.
Particularly, when analyzing the reduction rates of past and future costs, the main clinical savings are due to avoided treatment of acute hepatitis B cases and of chronic/cirrhosis cases in patients who subsequently evolved to sequelae in the 20 y vaccination period. Instead, most future savings are related to untreated patients with secondary diseases due to chronic HBV infection (Fig. 1).
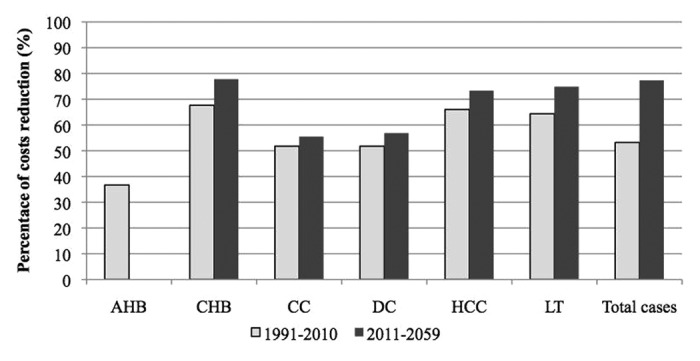
Figure 1. Percentage of clinical costs reduction due to implementation of the vaccination program in past and future years.
According to the mathematical model, the immunization of 20 infant cohorts and 12 adolescent cohorts during the period 1991–2010 cost 655,675,042 Euro in the NHS perspective and 872,002,316 in the Societal perspective. Comparing vaccination costs with clinical savings, the immunization program results in savings for the NHS perspective but not for the Societal perspective during the period 1991–2010. Instead, the preventive intervention becomes cost saving in both perspectives during the overall analyzed period. Particularly, a ROI value of 1.02 and a BCR value of 0.91 was calculated for the past period, but an overall ROI of 2.78 and BCR of 2.47 in the long-time horizon (Table 3).
Table 3. Savings during the first 20 y of the vaccination program (1991–2010) and overall savings (1991–2059).
| Savings during the first 20 y of the vaccination program (1991–2010) | ||
|---|---|---|
| NHS perspective | Societal perspective | |
| Clinical savings | 666,696,195 | 790,600,281 |
| Vaccination cost | 655,675,042 | 872,002,316 |
| Net costs | -11,021,153 | 81,402,035 |
| ROI/BCR | 1.02 | 0.91 |
| Overall savings (1991–2059) | ||
|---|---|---|
| NHS perspective | Societal perspective | |
| Clinical savings | 1,822,437,459 | 2,150,589,035 |
| Vaccination cost | 655,675,042 | 872,002,316 |
| Net costs | -1,166,762,417 | -1,278,586,719 |
| ROI/BCR | 2.78 | 2.47 |
Analyzing the cumulative costs during the period 1991–2010, clinical savings exceeded vaccination costs in 2010 in the NHS perspective, while the break-even occurred in 2012 in the Societal perspective (Figs. 2 and 3).
Figure 2. Cumulative clinical savings and vaccination costs in the NHS perspective during the period 1991–2010.
Figure 3. Cumulative clinical savings and vaccination costs in the Societal perspective during the period 1991–2010.
The sensitivity analysis shows that the increasing rate of symptomatic subjects represents the most sensitive parameter input in the mathematical model. However, the adoption of universal vaccination against HBV infection in Italy results again favorable in the long-time horizon even if the values of the analyzed factors are changed (ROI and BCR > 1) (Table 4).
Table 4. Sensitivity analysis: NHS and Societal perspective.
| NHS perspective | N. HBV infection avoided | Net costs (1991–2010) |
Net costs (1991–2059) |
ROI (1991–2010) |
ROI (1991–2059) |
|---|---|---|---|---|---|
| Baseline results | 126,892 | -11,021,153 | -1,166,762,417 | 1.02 | 2.78 |
| Adolescent vaccination coverage (95%) | 126,892 | 5,268,429 | -1,150,472,835 | 0.99 | 2.71 |
| > % symptomatic subjects (+10%) | 88,351 | 128,629,092 | -676,047,683 | 0.80 | 2.03 |
| Median age of chronic evolution (36 y) | 126,892 | -11,021,153 | -1,080,801,052 | 1.02 | 2.65 |
| Societal perspective |
N. HBV infection avoided | Net costs (1991–2010) |
Net costs (1991–2059) |
BCR (1991–2010) |
BCR (1991–2059) |
|---|---|---|---|---|---|
| Baseline results | 126,892 | 81,402,035 | -1,278,586,719 | 0.91 | 2.47 |
| Adolescent vaccination coverage (95%) | 126,892 | 109,211,314 | -1,250,777,440 | 0.88 | 2.39 |
| > % symptomatic subjects (+10%) | 88,351 | 245,709,317 | -701,172,609 | 0.72 | 1.80 |
| Median age of chronic evolution (36 y) | 126,892 | 81,402,035 | -1,177,470,063 | 0.91 | 2.35 |
Discussion
Italy was one of the first industrialized countries to introduce a program of universal vaccination against HBV infection in 1991, after many heated debates among national experts and careful evaluations of both epidemiological and economic data.7,8
At present, 20 y after the introduction of this broad preventive intervention, it is possible to evaluate all related outcomes based on real data. Particularly, from a clinical point of view, the universal immunization of newborns and adolescents in Italy resulted in favorable evident effects: an extensive decline of hepatitis B incidence in all age-groups, especially in immunized cohorts (100%, 97%, 70% and 82% in 0–14, 15–24, > 24 y old subjects and total population, respectively, from 1990 to 2010), with the simultaneous achievement of high vaccination coverage (95.8% for completed 3-doses schedule in 24 mo-old infants in 2009).6,9 The objective of this study was to verify whether the implementation of hepatitis B vaccination was favorable also from an economic point of view.
The economic evaluation is usually one of the useful tools required from decision makers before approving the implementation of a new health intervention. In this paper, however, we performed an a posteriori analysis, examining the past impact of the adoption of the hepatitis B immunization program and highlighting further benefits. Therefore, the aim of this study was to confirm the importance of the Italian authorities’ decision to implement the immunization program in Italy after 20 y. Particularly, we compared the real incidence of acute hepatitis B notified in Italy during the vaccination period and the following related diseases with the hepatitis B burden in a hypothetic no-vaccination scenario, with a different approach from usual pharmaco-economic studies (real no-vaccination scenario vs. hypothetic vaccination scenario). In addition, since the real impact of the vaccination of specific cohorts (infants and adolescent) on the hepatitis B burden in the overall population was available, we did not perform a classic cohort model, but valued the effects of universal vaccination not only on immunized groups, but on the whole Italian population. In our study, we started the analysis from the same costs data collected and used by the Technical-Scientific and Health Planning Committee of the Ministry of Health that actively contributed to the decision of the Italian authority to implement universal vaccination against HBV infection for all newborns and adolescents. After 6 y of routine immunization, Da Villa et al. performed an encouraging cost-effectiveness study analyzing only the first short-term outcome of HBV infections (acute hepatitis B cases). However, the same authors concluded the paper stating that the more relevant effects of vaccination would be obtained only after 15 y of immunization, when savings due to avoided costs for treatments of cirrhosis and hepatocellular carcinoma cases would start to become evident.5 Therefore, we tried to continue and complete the previous study, as hoped by the Authors, and to confirm the favorable outcomes in a broader time horizon.
In our simulation, the implementation of universal vaccination of infants and adolescent in Italy has determined an extensive reduction of the burden of hepatitis B disease in the overall population. Particularly, the number of HBV infections has decreased by 75% after the implementation of the vaccination program. Consequently, the past and future savings due to clinical costs avoided by the adoption of the immunization program is particularly high both for the NHS and the Societal perspective. Comparing clinical savings with vaccination costs, in the first 20 y of vaccination we obtained a return on investment nearly equal to 1 from the NHS perspective and a BCR slightly less than 1 from the Societal perspective. This result (a better impact of vaccination from the NHS than from the Societal perspective) differs from the usual outcomes of pharmaco-economic analyses, and can be explained by the fact that, in the first times, the indirect costs of vaccination were higher than indirect savings. Indeed, considering also the long-term effects of vaccination on hepatitis B diseases, the ROI and the BCR resulted largely positive (2.78 and 2.46, respectively). Such result is due to the fact that vaccination costs are paid immediately by the NHS, whereas the main impact of immunization is obtained after many years (15–20 y, when, in the absence of the vaccination program, some chronic HBsAg carriers would evolve to cirrhosis and hepatocellular carcinoma). Significantly, the break-even point (the time when savings overcome costs) was already achieved for the NHS (2010) and for the Society (2012): since then, we got clinical savings following the initial investment for vaccination of 32 birth cohorts during 1991–2010.
The study has several limitations. First of all, we did not take into account the shift from monovalent hepatitis B vaccine to combined vaccines, which might have changed the cost of the hepatitis B component (although probably not in a substantial way). Second, we included the medical assistance costs but not the costs of antiviral drug therapy in the analysis. This is particularly relevant vis-à-vis the progressively higher costs incurred in modern therapies, which make chronic viral hepatitis treatment extremely heavy from an economic viewpoint. For these reasons our analysis represents an underestimation of the real costs for chronic hepatitis management. In addition, we did not calculate the indirect costs due to liver transplantation because of lack of Italian available data on this issue. Third, no effects of co-infections from HCV, HDV, HIV, or from alcohol and illegal drug consumption were taken into account in the model. Furthermore, the life expectancy for chronic hepatitis B patients (not for cirrhosis and hepatocellular carcinoma cases) was considered equal to healthy people. Overall, our model is probably highly conservative regarding the economic impact of vaccination (i.e., we probably largely underestimated economic benefits of the vaccination program).
Our study did not consider the simultaneous implementation of the HBV vaccination program for risk groups in Italy: this additional immunization activity could only increase the favorable impact of vaccination against HBV infection on our national territory and improve the results of this study.
In conclusion, the introduction of universal hepatitis B vaccination in Italy was clinically and economically favorable during the first 20 y of adoption. Further clinical and economic benefits for this first period of vaccination will be increasingly evident in the future, when we will observe the reduction of chronic hepatitis B, cirrhosis, hepatocellular carcinoma cases and, therefore, of costs for treatments, due to HBV infections avoided by the vaccination program. Both in the payer and in the Societal perspective, we already reached the break-even point, and we are now progressively saving an ever-increasing amount of resources. The universal hepatitis B vaccination continues to be supported from both healthcare providers and the public, making Italy a candidate country for the elimination of HBV-related diseases in few decades.
Materials and Methods
Mathematical simulation model
In order to determine the clinical/economic impact of the universal infant and adolescent hepatitis B vaccination program in Italy, a mathematical simulation model was developed using the Microsoft Excel 2010 software (Microsoft Corporation, Redmond, Washington, USA). The mathematical model includes two modules: (1) a clinical model that estimates the number of clinical cases related to hepatitis B diseases and the related costs, with and without the adoption of the vaccination program; (2) a vaccination module that computes the number of vaccine doses administered between 1991 and 2010, and the total costs of vaccination.
The impact of the vaccination program was evaluated over a broad period: the past immunization period 1991–2010 (that is the first 20 y of vaccination in Italy) and the future period 2011–2059, when potential cases of hepatitis B infection, that would have occurred during the vaccination period 1991–2010, would subsequently evolve to chronic hepatitis B, cirrhosis and hepatocellular carcinoma, and should be treated. Lastly, in order to better understand the real impact of the immunization strategy, the current analysis was performed according to both the perspective of the National Health Service (NHS) and that of the Society, including indirect costs.
Specifically, the real clinical and economic outcomes due to the introduction of hepatitis B vaccination were compared with an hypothetical no-vaccination scenario, assuming a slowly decreasing hepatitis B incidence. In the latter case, the incidence of acute hepatitis B in Italy would be supposed to maintain a downward trend, as evidenced in previous years, due to the implementation of other general preventive activities (improvement in diagnostic tests, reduction of vertical transmission, use of safer blood products, increasing attention to universal precautions, etc.), and therefore, assuming a gradual decreasing incidence of acute hepatitis B cases, but obviously less pronounced than in the vaccination period. Particularly, the costs of diseases related to HBV infection in this situation were compared with the respective costs calculated for the real cases notified in the 20 y following the introduction of universal vaccination, and the differences of those costs were evaluated taking into account the cost of the immunization program. The annual hepatitis B incidence in the no-vaccination scenario during the period 1991–2010 was calculated as shown in Table 5. In the absence of vaccination, the decline of acute hepatitis B cases would have been 75%, 50%, and 25% of the decline observed during the vaccination period for ages 0–14, 15–24, and > 24 y, respectively.
Table 5. Methodology for the calculation of the annual number of acute hepatitis B cases, by age group, in the no-vaccination scenario.
| No-vaccination scenario: slow decrease of acute hepatitis B incidence |
|---|
| 0–14 y (reduction of incidence mainly due to adoption of preventive interventions other than vaccination) |
| 75% of calculated cases = same incidence of vaccination period |
| 25% of calculated cases = incidence of 1991 |
| 15–24 y |
|---|
| 50% of calculated cases = same incidence of vaccination period |
| 50% of calculated cases = incidence of 1991 (intermediate importance of sexual transmission) |
| ≥ 25 y |
|---|
| 25% of calculated cases = same incidence of vaccination period |
| 75% of calculated cases = incidence of 1991 (maximal importance of sexual transmission) |
In the simulation model, the following possible cost-generating health statuses related to HBV infection were considered: symptomatic acute HBV infection (AHB), chronic hepatitis B (CHB), compensated cirrhosis (CC), decompensated cirrhosis (DC), and hepatocellular carcinoma (HCC). In addition, the number of liver transplantations (LT) required in decompensated cirrhosis and hepatocellular carcinoma cases were calculated. Particularly, in our study, CHB cases mean subjects with chronic hepatitis B without spontaneous resolution or compensated cirrhosis/hepatocellular carcinoma evolution. CCs are subjects with compensated cirrhosis, that is cirrhosis without decompensation or carcinoma development. DCs are people with decompensated cirrhosis that did not require liver transplantation. HCCs are chronic hepatitis B or compensated cirrhosis cases evolved in hepatocellular carcinoma that did not require liver transplantation. Lastly, LTs are subjects with decompensated cirrhosis or hepatocellular carcinoma that required liver transplantation.
In order to estimate the number of cases in each of the above statuses in the analysis period, and the related-costs, the flowchart on the natural history of HBV infection was built based on data from the national and international literature (Fig. 4).

Figure 4. Natural history of HBV infection (Note: 0–14 y, 15–24 y, and > 24 y refer to age groups).
The outcomes of the mathematical model include the annual number of clinical cases of the above health statuses with and without the adoption of the vaccination program, and, consequently, the number of avoided cases and the rate of incidence reduction. Furthermore, the model calculates the annual clinical costs, divided into medical assistance costs and social costs. The amount of net costs was calculated comparing immunization costs with the difference in clinical costs between the no-vaccination and the vaccination scenario, in the NHS and in the Societal perspective, respectively. However, the main economic outcome obtained by the model is the Benefit-to-Cost Ratio (BCR), defined as the net savings due to vaccination (in terms of disease treatment costs avoided plus indirect cost savings when the Societal perspective is adopted) divided by the vaccination costs: a BCR of ≥ 1 means that for each Euro invested in the vaccination program, one Euro or more would be saved through avoided costs attributable to prevented hepatitis B disease cases. Similarly, the ratio of benefit to costs is defined as Return On Investment (ROI) in the perspective of the National Health Service.
Epidemiological/clinical data
Incidence of acute hepatitis B, chronic hepatitis B, cirrhosis and hepatocellular carcinoma
The study was performed on the annual resident population in Italy as of January 1st from 1991 to 2010, grouped by year and age, as reported by the National Institute of Statistics (ISTAT).10
The annual incidence of acute hepatitis B for the years 1991–2010 was obtained from the Integrated Epidemiological System Of Acute Viral Hepatitis (Sistema Epidemiologico Integrato dell'Epatite Virale Acuta, SEIEVA).6 Particularly, the incidence rates were specified for the following three age groups: 0–14, 15–24 and ≥ 25 y of age. A discount rate for underreporting was not applied because SEIEVA is a sentinel surveillance system.
In the mathematical model, it was assumed that the number of acute hepatitis B cases, calculated applying the age-specific SEIEVA incidence rate to the Italian resident population, corresponded to the symptomatic cases. Symptomatic cases represented 10% of HBV infections in children between 0 and 14 y, 20% for subjects between 15 and 24 y, and 30% in adults > 24 y of age.11-13 Six percent of all HBV infected cases (asymptomatic and symptomatic) progress to chronic infection (Fig. 4).11-19 Ninety percent of those with chronic infection undergo spontaneous anti-HBe seroconversion. Twenty percent of those who seroconverted revert to HBeAg positive. The remaining HBeAg negative subjects (80%) continue to have chronic hepatitis in 20% of cases while the residual 80% of cases become chronic inactive carriers. Lastly, 20% of chronic inactive carriers reactivate.20 In Italy, of all subjects with chronic hepatitis B, 85% turn out to be HBeAg negative.18,21
Chronic hepatitis B cases can subsequently progress to spontaneous resolution (6.9% of HBeAg positive subjects and 1.6% of HBeAg negative subjects), compensated cirrhosis (3.0% of HBeAg positive subjects and 4.6% of HBeAg negative subjects) and hepatocellular carcinoma (1.5%). Some of the compensated cirrhosis cases evolve to decompensated cirrhosis (7.3%) or hepatocellular carcinoma (3.4%). Finally, 21% of decompensated cirrhosis cases and 25% of hepatocellular carcinoma cases need a liver transplantation.22,23
It was assumed that the time interval between the onset of chronic hepatitis B and compensated cirrhosis was 15 y, between compensated cirrhosis and decompensated cirrhosis was 3 y, and between the onset of compensated cirrhosis and the development of hepatocellular carcinoma was 44 mo.11
Median age of diagnosis and survival rates
In the mathematical model, the median age of initial presentation with HBeAg positive chronic hepatitis B was assumed to be 31 y.11 The median age of patients at diagnosis of compensated cirrhosis results equal to 46 y (and 3 y later for decompensated cirrhosis); meanwhile, for the development of hepatocellular carcinoma is 50 y.11 Hepatocellular carcinoma and decompensated cirrhosis patients requiring liver transplants wait on average 3 mo for surgery.24
The survival rate for chronic hepatitis B cases was assumed equal to 100%, according to life expectancy for the general population.10 The survival rate for cirrhosis diagnosis was assumed to be 99.1% at 5 y from onset, 76.8% at 10 y, 49.4% at 15 y25 and was estimated to be 25% at 20 y, and, lastly, 0% at 25 y. Instead, decompensated cirrhosis patients have a survival rate of 14% after 5 y from the onset.15,20,26 The survival rate of hepatocellular carcinoma cases was obtained by Santi et al., and corresponds to 78.7%, 50.4% and 28.9%, at 1, 3 and 5 y, respectively.27 Five percent of patients requiring liver transplantation encounters transplant-related operative death, while the others have a 72% post-transplant survival rate 5 y after surgery.24,28
Vaccine data
The total number of subjects in Italy targeted by vaccination against HBV (infant and 12-y-old adolescent) inside the immunization program from 1991 to 2010, was obtained from the number of age-specific residents in Italy, as reported by ISTAT.10
In the simulation model, hepatitis B vaccination coverage of newborns increased from 94% in 2000 to 96% in 2009, as reported by the Ministry of Health.9 For lack of official data, the immunization coverage for the previous period 1991–1999 and for the year 2010 was assumed equal to 94% and 96%, respectively. However, the vaccination coverage for adolescents (vaccinated in the 12th year of life or rather at 11 y of age) was estimated at 90% throughout the period 1991–2003 in which the immunization program was applied. By the end of 2003, the first infant cohort vaccinated in 1991 reached 12 y of age, and the vaccination of adolescents was discontinued.
According to the vaccination schedule, 3 doses of pediatric HBV vaccine were administrated to infants (born to HBsAg-negative mothers) and 3 adult doses to adolescents. However, 4 doses of pediatric vaccine against HBV were administrated to children born to HBsAg positive mothers starting immediately after birth (first dose together with specific immune globulins).
For the calculation of the number of children born to HBsAg-positive mothers, a prevalence of 2.4% for 1991,29-31 1.7% for 200132 and 0.86% for 200833 was assumed. The values of prevalence for the other years were estimated starting from the above values. The prevalence for 2009 and 2010, in the absence of published data to date, has been kept constant, equal to the 2008 prevalence.
In this evaluation, for the calculation of costs and vaccine-related data, the administration of monovalent HBV vaccine was assumed to have remained steady during all 20 y of the vaccination period, although an hexavalent vaccine became available in Italy since 2001 and was progressively used thereafter.
Economic data
Table 6 shows the annual values of medical care and social costs (in Euro) for each case of acute viral hepatitis B, chronic hepatitis B, cirrhosis, and hepatocellular carcinoma, referred to 1990, as reported by Da Villa et al. and calculated by the Technical-Scientific and Health Planning Committee of the Ministry of Health that worked on the evaluation of costs related to HBV diseases.5 The respective costs for the years 1991–2010 were calculated by applying the annual inflation rate (reported by ISTAT) to the 1990-costs. Since 2011, a discount rate (3%) was applied to calculate the future annual assistance and social costs. This analysis does not include expenditure for antiviral drug therapy.
Table 6. Annual clinical costs of HBV diseases: medical care and social costs per case (values referred to 1990)5.
| Annual costs of HBV diseases (1990) | |
|---|---|
| Annual assistance costs/case | |
| Acute viral hepatitis (Euro) | 18,000,000 lire (9,296 Euro) |
| Chronic viral hepatitis viral and cirrhosis (hospital stay and home assistance) (Euro) | 14,000,000 lire (7,230 Euro) |
| Hepatocellular carcinoma (Euro) | 54,000,000 lire (27,889 Euro) |
| Annual social costs /case | |
|---|---|
| Acute viral hepatitis (Euro) | 3,709,000 lire (1,916 Euro) |
| Chronic viral hepatitis viral and cirrhosis (Euro) | 2,472,000 lire (1,277 Euro) |
| Hepatocellular carcinoma (Euro) | 22,565,000 lire (11,654 Euro) |
In addition, to the above costs, we assumed an annual direct cost for liver transplantation and, subsequently, for post-transplantation follow-up amounting to 82,867.40 Euro and 6,358.04 Euro (year 2009 values),23 respectively, in accordance with other studies.34
The direct and indirect costs of HBV vaccination, referred to 1991, are shown in Table 7, as reported by Da Villa et al.5 The respective annual costs for the years 1992–2010 have been calculated applying the annual inflation rate (reported by ISTAT) to the 1991-costs.
Table 7, Hepatitis B vaccination costs (values referred to 1991)5.
| HBV vaccination costs (1991) | |
|---|---|
| Direct costs | |
| Vaccine | 12,000 lire (6.20 Euro)/dose (pediatric dose) 18,000 lire (9.30 Euro)/dose (adult dose) |
| Vaccine preservation | 172 lire (0.09 Euro)/dose |
| Vaccine administration | 12,500 lire (6.46 Euro)/person |
| Treatment of adverse reactions (1% of all vaccine doses administered) | 9,545 lire (4.93 Euro)/case |
| Passive immunization of babies born to HBsAg+ mothers | 29,000 lire (14.98 Euro)/newborn |
| Indirect costs | |
|---|---|
| Lost working days for vaccination (only for adolescents) | 15,455 lire (7.98 Euro)/dose |
| Lost working days for treatment of adverse reactions (1% of administered doses) | 41,000 lire (21.17 Euro)/dose |
Costs presented in Table 6 and 7 referred, specifically, to 1990 and 1991, as reported by Da Villa et al.5 However, as underlined above, costs for the past 20 y were updated in the mathematical model according to the yearly inflation rate. On the other hand, a common baseline year (2010), was the starting point to which a 3% yearly inflation rate was applied for the future cost (period 2011–2059), according to the standard methods. In addition, 1990 was the last year without any implementation of hepatitis B routine vaccination in Italy; the reported clinical costs were calculated by the Technical-Scientific and Health Planning Committee of the Ministry of Health in order to evaluate the possible implementation of universal HBV vaccination program. The vaccination costs, instead, referred to 1991 that was the first year of immunization. This is the reason of the apparent discrepancy in the baseline years for the calculation of the costs. It seemed to us reasonable to keep the original real costs referred to diseases costs in 1990 and to immunization costs in 1991, updating them according to the yearly inflation rate.
Treatments and their duration
In the mathematical model, we assumed that the symptomatic acute hepatitis B cases were treated only during the year of diagnosis, in order to calculate treatment costs. The treatment for chronic hepatitis B cases is applied to all cases for the remaining years of life, according to life expectancy of the general population, starting from the median age of diagnosis. Compensated cirrhosis cases are treated during the first 15 y as chronic hepatitis B, successively as cirrhosis according to the 5-yearly survival rates for the following 25 y. Decompensated cirrhosis cases are treated during the first 15 y as chronic hepatitis B, for the following 3 y as compensated cirrhosis, and, successively, as decompensated cirrhosis according to the 5-yearly survival rate, and, last, as compensated cirrhosis cases according to the remaining life expectancy. Hepatocellular carcinoma cases are treated as chronic hepatitis B during the first 20 y or as chronic hepatitis B during the first 15 y, and as compensated cirrhosis in the following 5 y; successively, patients are treated for 1 y as hepatocellular carcinoma, then for the following 4 y as chronic hepatitis B/cirrhosis according to the survival rate and, in the end, as chronic hepatitis B/cirrhosis according to life expectancy. HCC and decompensated cirrhosis patients requiring liver transplantation, after the onset of disease are treated 1 y for this specific pathology and simultaneously for LT, with a successive post-transplantation follow-up for the remaining years, according to the combined calculation of post-transplants survival rates and population life expectancy.
Sensitivity analysis
In order to verify the impact of uncertainty of input data in the mathematical model, one-way sensitivity analysis was performed. Differently from other model-based analyses, we started from real-life epidemiological data and evaluated the additional costs that would have been needed if the same number of disease cases had been prevented under a higher coverage for adolescents (95% vs. 90%). We also evaluated the scenario where the real-life incidence of notified acute hepatitis B corresponded to 20%, 30% and 40% of total infections instead of 10%, 20% and 30% in the age groups 0–14, 15–24 and > 24 y, respectively. Furthermore, we increased the median age of chronic evolution from 31 y to 36 y (and, consequently, the median age of hepatocellular carcinoma from 50 to 55 y), respectively.
Acknowledgments
The authors are very grateful to Professor Alessandro Zanetti, University of Milan, for providing suggestions and critical input to their work.
Glossary
Abbreviations:
- NHS
National Health Service
- HBV
hepatitis B virus
- AHB
symptomatic acute HBV infection
- CHB
chronic hepatitis B
- CC
compensated cirrhosis
- DC
decompensated cirrhosis
- HCC
hepatocellular carcinoma
- LT
liver transplantation
- BCR
benefit-to-cost ratio
- ROI
return on investment
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/23827
References
- 1.World Health Organization (WHO). Immunization surveillance, assessment and monitoring. Hepatitis B. WHO website. Available to: http://www.who.int/immunization_monitoring/diseases/hepatitis/en/index.html
- 2.Romano’ L, Paladini S, Van Damme P, Zanetti AR. The worldwide impact of vaccination on the control and protection of viral hepatitis B. Dig Liver Dis. 2011;43(Suppl 1):S2–7. doi: 10.1016/S1590-8658(10)60685-8. [DOI] [PubMed] [Google Scholar]
- 3.Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine. 2008;26:6266–73. doi: 10.1016/j.vaccine.2008.09.056. [Review] [DOI] [PubMed] [Google Scholar]
- 4.Law 27 May 1991, n. 165. Obbligatorietà della vaccinazione contro l'epatite virale B. Gazz. Uff. 1° giugno 1991, n. 127.
- 5.Da Villa G, Sepe A. Immunization programme against hepatitis B virus infection in Italy: cost-effectiveness. Vaccine. 1999;17:1734–8. doi: 10.1016/S0264-410X(98)00414-9. [DOI] [PubMed] [Google Scholar]
- 6.Istituto Superiore di Sanità. Integrated Epidemiological System Of Acute Viral Hepatitis (Sistema Epidemiologico Integrato dell'Epatite Virale Acuta, SEIEVA). SEIEVA website. Available at: http://www.iss.it/seieva
- 7.Crovari P, Coppola RC, Icardi GC, Bonanni P. Efficacia e sicurezza dei vaccini contro l’epatite B. In: Fondazione SmithKline, editor. La vaccinazione contro l’epatite B: una scelta prioritaria di politica sanitaria ed economica. Milano, Franco Angeli Editore; 1990. p. 59–84. [Google Scholar]
- 8.Dellamano R. Vaccinare contro l’epatite B: un costo senza ritorno o un buon investimento? In: Ghetti V, editor. La Vaccinazione di Massa contro l’Epatite B in Italia. Basi Fisio-patologiche, Epidemiologiche e Socio-economiche. Milano, Franco Angeli Editore; 1991. p. 31–76. [Google Scholar]
- 9.Italian Ministry of Health. Hepatitis B vaccination coverage. Italian Ministry of Health website. Available to: http://www.salute.gov.it
- 10.Istituto Nazionale di Statistica (ISTAT)/National Institute of Statistics. Demografia in cifre. Population resident in Italy at the 1st January from 1991 to 2010, grouped by years and age range, divided by year and age. Demo.istat website. Available at: http://demo.istat.it
- 11.Fattovich G. Natural history of hepatitis B. J Hepatol. 2003;39(Suppl 1):S50–8. doi: 10.1016/S0168-8278(03)00139-9. [DOI] [PubMed] [Google Scholar]
- 12.Hyams KC. Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis. 1995;20:992–1000. doi: 10.1093/clinids/20.4.992. [DOI] [PubMed] [Google Scholar]
- 13.McMahon BJ, Alward WL, Hall DB, Heyward WL, Bender TR, Francis DP, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151:599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 14.Lavarini C, Farci P, Chiaberge E, Veglio V, Giacobbi D, Bedarida G, et al. IgM antibody against hepatitis B core antigen (IgM anti-HBc): diagnostic and prognostic significance in acute HBsAg positive hepatitis. Br Med J (Clin Res Ed) 1983;287:1254–6. doi: 10.1136/bmj.287.6401.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lok AS, McMahon BJ, Practice Guidelines Committee, American Association for the Study of Liver Diseases Chronic hepatitis B. Hepatology. 2001;34:1225–41. doi: 10.1053/jhep.2001.29401. [DOI] [PubMed] [Google Scholar]
- 16.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–39. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 17.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–52. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Stroffolini T. The changing pattern of hepatitis B virus infection over the past three decades in Italy. Dig Liver Dis. 2005;37:622–7. doi: 10.1016/j.dld.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006;43(Suppl 1):S173–81. doi: 10.1002/hep.20956. [DOI] [PubMed] [Google Scholar]
- 20.McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49(Suppl):S45–55. doi: 10.1002/hep.22898. [DOI] [PubMed] [Google Scholar]
- 21.Stroffolini T, Almasio PL, Sagnelli E, Mele A, Gaeta GB, Italian Hospitals’ Collaborating Group Evolving clinical landscape of chronic hepatitis B: A multicenter Italian study. J Med Virol. 2009;81:1999–2006. doi: 10.1002/jmv.21643. [DOI] [PubMed] [Google Scholar]
- 22.Idris BI, Brosa M, Richardus JH, Esteban R, Schalm SW, Buti M. Estimating the future health burden of chronic hepatitis B and the impact of therapy in Spain. Eur J Gastroenterol Hepatol. 2008;20:320–6. doi: 10.1097/MEG.0b013e3282f340c8. [Review] [DOI] [PubMed] [Google Scholar]
- 23.Colombo GL, Gaeta GB, Viganò M, Di Matteo S. A cost-effectiveness analysis of different therapies in patients with chronic hepatitis B in Italy. Clinicoecon Outcomes Res. 2011;3:37–46. doi: 10.2147/CEOR.S16655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cucchetti A, Cescon M, Trevisani F, Morelli MC, Ercolani G, Pellegrini S, et al. What is the probability of being too old for salvage transplantation after hepatocellular carcinoma resection? Dig Liver Dis. 2012;44:523–9. doi: 10.1016/j.dld.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Gentilini P, Laffi G, La Villa G, Romanelli RG, Buzzelli G, Casini-Raggi V, et al. Long course and prognostic factors of virus-induced cirrhosis of the liver. Am J Gastroenterol. 1997;92:66–72. [PubMed] [Google Scholar]
- 26.de Jongh FE, Janssen HL, de Man RA, Hop WC, Schalm SW, van Blankenstein M. Survival and prognostic indicators in hepatitis B surface antigen-positive cirrhosis of the liver. Gastroenterology. 1992;103:1630–5. doi: 10.1016/0016-5085(92)91188-a. [DOI] [PubMed] [Google Scholar]
- 27.Santi V, Buccione D, Di Micoli A, Fatti G, Frigerio M, Farinati F, et al. The changing scenario of hepatocellular carcinoma over the last two decades in Italy. J Hepatol. 2012;56:397–405. doi: 10.1016/j.jhep.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Vitale A, Volk ML, Pastorelli D, Lonardi S, Farinati F, Burra P, et al. Use of sorafenib in patients with hepatocellular carcinoma before liver transplantation: a cost-benefit analysis while awaiting data on sorafenib safety. Hepatology. 2010;51:165–73. doi: 10.1002/hep.23260. [DOI] [PubMed] [Google Scholar]
- 29.Stroffolini T, Pasquini P, Mele A. HBsAg carriers among pregnant women in Italy: results from the screening during a vaccination campaign against hepatitis B. Public Health. 1988;102:329–33. doi: 10.1016/S0033-3506(88)80102-1. [DOI] [PubMed] [Google Scholar]
- 30.Stroffolini T, Pasquini P, Mele A. A nationwide vaccination programme in Italy against hepatitis B virus infection in infants of hepatitis B surface antigen-carrier mothers. Vaccine. 1989;7:152–4. doi: 10.1016/0264-410X(89)90056-X. [DOI] [PubMed] [Google Scholar]
- 31.Stroffolini T, Pasquini P. Five years of vaccination campaign against hepatitis B in Italy in infants of hepatitis B surface antigen carrier mothers. Ital J Gastroenterol. 1990;22:195–7. [PubMed] [Google Scholar]
- 32.Stroffolini T, Bianco E, Szklo A, Bernacchia R, Bove C, Colucci M, et al. Factors affecting the compliance of the antenatal hepatitis B screening programme in Italy. Vaccine. 2003;21:1246–9. doi: 10.1016/S0264-410X(02)00439-5. [DOI] [PubMed] [Google Scholar]
- 33.Spada E, Tosti ME, Zuccaro O, Stroffolini T, Mele A, Collaborating Study Group Evaluation of the compliance with the protocol for preventing perinatal hepatitis B infection in Italy. J Infect. 2011;62:165–71. doi: 10.1016/j.jinf.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Filipponi F, Pisati R, Cavicchini G, Ulivieri MI, Ferrara R, Mosca F. Cost and outcome analysis and cost determinants of liver transplantation in a European National Health Service hospital. Transplantation. 2003;75:1731–6. doi: 10.1097/01.TP.0000063828.20960.35. [DOI] [PubMed] [Google Scholar]




