Abstract
Objective: Recently, a vaccine with the capacity to protect against serogroup B meningococcal (MenB) disease received a positive opinion of the European Medicines Agency. Previously, such a vaccine was estimated to be cost-effective. However, since then, the MenB disease incidence has declined drastically in the Netherlands. Therefore, we re-assessed the potential incremental cost-effectiveness ratio (ICER) of vaccinating infants in the Netherlands with a MenB vaccine.
Methods: A cohort of 185,000 Dutch newborns was followed in a Markov model to compare routine vaccination against MenB disease with no vaccination. The ICER was estimated for different disease incidences. The study was performed from a societal perspective.
Results: Routine infant vaccination (2, 3, 4+11 mo) could prevent 39 cases of MenB disease in a single birth cohort, corresponding to a total gain of 133 quality-adjusted life years (QALYs). However, this strategy is unlikely to be cost-effective if the vaccine costs €40 per dose (€243,778 per QALY). At a disease incidence of 5.7 per 100,000 person-years or a vaccine price of €10 per dose including administration costs, the ICER becomes more acceptable and remains below a threshold of €50,000 per QALY.
Conclusions: At the current low level of disease incidence, introduction of routine infant vaccination, following a 2, 3, 4+11 mo schedule, against MenB disease is unlikely cost-effective in the Netherlands. If the MenB disease incidence increases or the vaccine price is substantially lower than €40, routine infant vaccination has the potential to be cost-effective.
Keywords: meningococcal B, cost-effectiveness, vaccine, infants, disease incidence
Introduction
Meningococcal disease is an important cause of morbidity and mortality worldwide.1-6 Since the 1980s, the majority of meningococcal disease in the Netherlands has been caused by serogroup B and C meningococci.7 A meningococcal C conjugate vaccine was implemented in the Netherlands after a steep increase in the serogroup C meningococcal (MenC) disease incidence between 2000 and 2001, hereafter the incidence of MenC disease decreased substantially.
Various serogroup B meningococcal (MenB) vaccines have been used to control regional epidemics. However, these vaccines are all strain-specific.8 Therefore, vaccines against MenB disease with broader strain coverage are needed.
The European Medicines Agency recently gave a positive opinion about a broad-coverage vaccine with the capacity to protect against MenB disease (Bexsero, Novartis).9 Additionally, two other vaccines with the capacity to protect against this disease are currently under development [LP2086, Pfizer; Nonamen, RIVM (National Institute of Public Health and the Environment, Bilthoven, the Netherlands)]. Therefore, decision makers will soon have to decide whether and how a MenB vaccine should be implemented.
Previously, the incremental cost-effectiveness ratio (ICER) of the implementation of a MenB outer-membrane vesicle (OMV) vaccine (Hexamen, RIVM) in the Dutch National Immunization Program was estimated at €15,721 per quality-adjusted life year (QALY).10 Since then, the epidemiological situation has changed in the Netherlands. Although no vaccination against MenB disease was implemented in the Netherlands, the annual MenB disease incidence declined from 2–4 cases per 100,000 persons in 1993–199710 to 1 case per 100,000 persons in 2005–2009.7 The incidence of MenB disease naturally fluctuates.11,12 Thus, while the Netherlands is currently experiencing the lowest MenB incidence in the last three decades, the incidence may increase in the future. We therefore evaluated the cost-effectiveness of vaccine implementation at both the current low incidence and higher incidences.
Results
Cost-effectiveness 2, 3, 4+11 mo schedule
The model predicted 276 cases of MenB disease for a birth cohort followed for 99 y. In this timeframe, 39 cases would be prevented by routine infant vaccination, corresponding to a total gain of 95 life-years or 133 QALYs (discounted). Vaccination would cost approximately €33 million, but would prevent approximately €0.65 million of direct costs and €22,000 of indirect costs. The ICER was estimated at €243,778 per QALY or €340,384 per life-year gained. The vaccine price per dose including administration costs would need to be as low as €4.70 to remain below the threshold of €20,000 per QALY. For a threshold of €50,000 per QALY this would be €10.35. Introducing an additional booster vaccination at 12 y of age would prevent 12 additional cases of MenB disease, resulting in an ICER of €247,139 per QALY.
Cost-effectiveness 12 +14 mo schedule
In the Netherlands, a vaccination against serogroup C disease is given at 14 mo, because cost-effectiveness analyses showed that that strategy was much more cost-effective than strategies with multiple doses in the first year of life.13 Therefore we assessed the cost-effectiveness of vaccination against MenB disease at 12 + 14 mo of age. The ICER was lower (€221,132 per QALY) for this strategy than for the 2, 3, 4+11 mo strategy, however only 28 cases of MenB disease could be prevented with this strategy. Adding a booster vaccination at 12 y of age would prevent 12 additional disease cases (40 cases in total), resulting in an ICER of €234,548 per QALY.
Cost-effectiveness with increased incidence
We performed a scenario analysis with the average incidence seen during 1990–1993 (3.46 per 100,000 person-years), which is comparable to, for example, the current incidence in Ireland.14 This resulted in an ICER of €85,931 per QALY for the 2, 3, 4 +11 mo schedule and €70,898 per QALY for the 12 + 14 mo schedule. At this high incidence, adding an additional booster at the age of 12 y to the 2, 3, 4+11 mo schedule was more cost-effective (€77,392 per QALY) than a schedule without that booster (€85,931 per QALY). For a strategy with vaccinations at 12 + 14 mo and 12 y of age an ICER of €66,811 per QALY was obtained (Table 1).
Table 1. Influence of increasing the incidence of meningococcal B disease on the cost-effectiveness of routine infant vaccination with different vaccination strategies.
| Vaccination schedule | Base-case incidence*(€/QALY) | Incidence ’90-’93* (€/QALY) | Incidence per 100,000 person-years needed to stay below €20,000/QALYa | Incidence per 100,000 person-years needed to stay below €50,000/QALYa |
|---|---|---|---|---|
| 2, 3, 4, 11 mo | 243,778 | 85,931 | 12.58 | 5.72 |
| 2, 3, 4, 11 mo + 12 y | 247,139 | 77,392 | 11.49 | 5.19 |
| 12 + 14 mo | 221,132 | 70,898 | 10.60 | 4.79 |
| 12 + 14 mo + 12 y | 234,548 | 66,811 | 10.08 | 4.53 |
*Base-case incidence was 1.07 per 100,000 person-years; Incidence of ’90-’93 was 3.46 per 100,000 person-years. aUsing the meningococcal B disease age-distribution of ’90-’93.
We also estimated the incidence levels required for the ICER to remain below €20,000 and €50,000 per QALY, respectively, assuming a vaccine cost per dose of €40 (Table 1). For a threshold of €50,000 per QALY, the model predicts the 2, 3, 4+11 mo vaccination schedule to be cost-effective at an annual incidence of 5.7 per 100.000 persons. Because the incidence was one of the most important cost-effectiveness drivers, the maximum vaccine costs per dose increased approximately linearly with the incidence (Table 2).
Table 2. Maximum vaccine price per dose for routine infant vaccination (2, 3, 4+11 mo schedule) against MenB disease, while remaining cost-effective for different MenB disease incidences and strategies.
| MenB disease incidence per 100,000 person-years | Maximum vaccine price per dose for a 2, 3, 4+11 mo vaccination strategy and a threshold of €20,000 per QALY (€) | Maximum vaccine price per dose for a 2, 3, 4+11 mo vaccination strategy and a threshold of €50,000 per QALY (€) |
|---|---|---|
| 1 | - | 2.82 |
| 2 | 1.96 | 12.46 |
| 3 | 6.35 | 22.10 |
| 4 | 10.73 | 31.73 |
| 5 | 15.12 | 41.36 |
A vaccine price of €0 per dose means that only administration costs of €6.81 per dose are paid
Sensitivity analyses
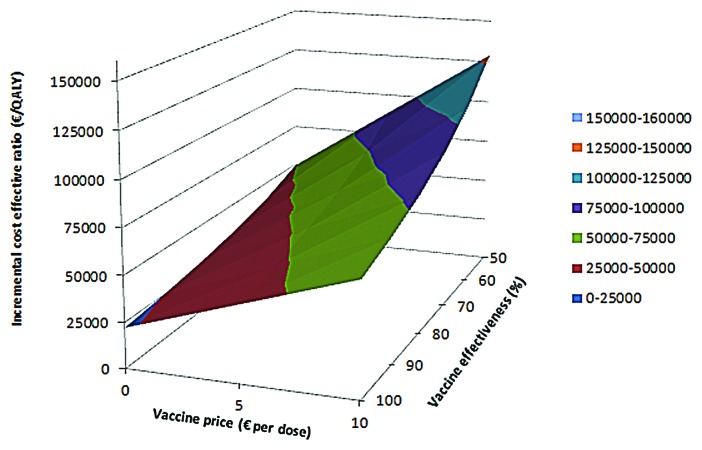
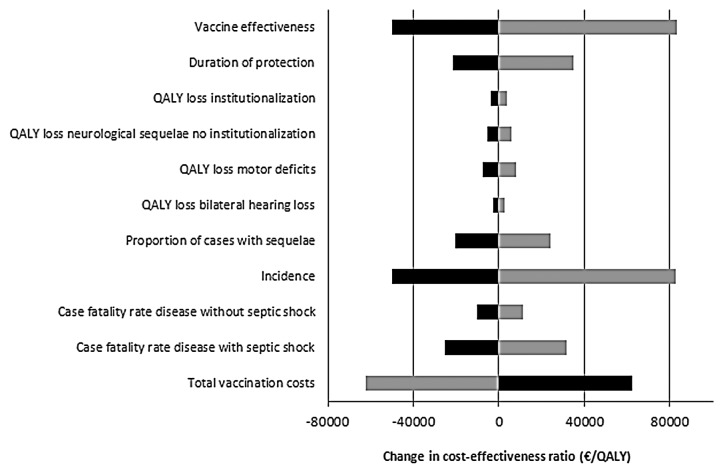
We estimated the impact of the vaccine effectiveness and the vaccine price on the cost-effectiveness for the base case (Fig. 1). Using base case assumptions, vaccination could be considered cost-effective for a few scenarios with a low vaccine price. Univariate sensitivity analysis showed that the vaccine effectiveness, the total vaccination costs, the case-fatality rate (CFR), the proportion of cases with sequelae, the incidence of MenB disease and the duration of protection provided by the vaccine were parameters with the highest impact on the ICER in our model (Fig. 2).
Figure 1. Incremental cost-effectiveness ratio for the base-case with vaccinations at 2,3,4+11 mo of age. The ICER is presented for different vaccine prices and vaccine effectiveness. A vaccine price of €0 per dose means that only administration costs of €6.81 per dose are paid.
Figure 2. Sensitivity analysis on the base-case ICER (2,3,4 +11 mo schedule). The parameters were varied by 25%. Light bars show the change in the ICER after a 25% decrease in the parameter, while dark bars represented the change in ICER after a 25% increase in the parameter. A change of €0 is representative for the ICER of the base-case (€243,778 per QALY). QALY, quality-adjusted life year.
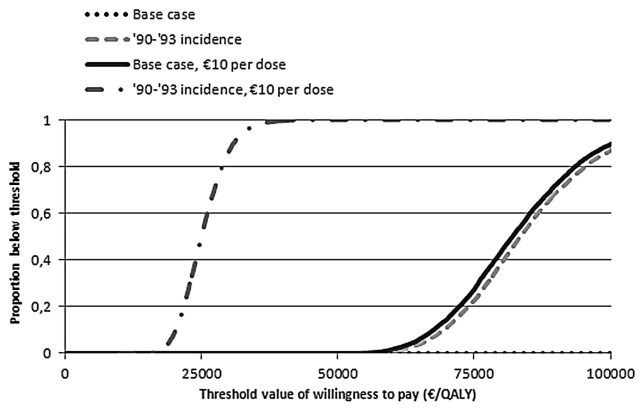
We used four scenarios to compare cost-effectiveness acceptability curves (Fig. 3). For the current MenB disease incidence, it is unlikely that vaccination could be considered cost-effective, at a vaccine price of €40 per dose. Even for the relatively high incidence seen during 1990–1993, the vaccine price per dose would need to be lower than €10 to remain below the threshold of €20,000 per QALY.
Figure 3. Cost-effectiveness acceptability curves for base-case scenario and for alternative scenarios for meningococcal B vaccination. Results are presented for a 2,3,4 + 11 mo schedule.
Discussion
Our analyses indicate that routine infant vaccination (2, 3, 4+11 mo schedule) against MenB disease in the Netherlands would be cost-effective only with a vaccine priced well below €10 per dose, given the current low MenB disease incidence. For higher MenB disease incidences or a vaccine price that is substantially lower than €40, vaccination is more likely cost-effective.
A strategy with vaccinations at 12 + 14 mo of age slightly decreased the ICER compared with a 2,3,4+11 mo schedule, although the vaccine price should be lower than €40 to let this strategy become cost-effective with the current epidemiology. Despite this strategy being slightly more cost-effective, fewer cases are prevented compared with a 2, 3, 4+11 mo schedule, since the MenB disease incidence is particularly high in the first year of life. A strategy with vaccinations at 12 + 14 mo and 12 y of age slightly decreased the ICER compared with a 2, 3, 4+11 schedule, while one additional case would be prevented. Especially when the MenB disease incidence increases, other vaccination strategies than the 2, 3, 4+11 schedule resulted in more favorable ICERs.
This study is timely because the European Medicines Agency recently gave a positive opinion about Bexsero (Novartis)9 and other vaccines are currently under development. In addition, ICERs for the current low incidence as well as possible higher incidences are presented, enabling policy makers to make informed decisions. In addition, we evaluated the effect of introducing a booster vaccination at 12 y of age, which likely reduces the disease burden.
For the base-case we assumed that infants would be vaccinated at 2, 3, 4 and 11 mo of age, because the incidence of MenB disease is particularly high during the first months of life and there is no evidence for a herd protection effect.15 However, vaccination against MenB disease would be a third injection at these ages, while a maximum of two injections per consult is currently accepted in the Netherlands.16 Possible solutions could be the acceptance of three injections at one vaccine consult or additional consultations. This second solution would negatively influence the ICER. Compared with simultaneous injections with already implemented routine vaccinations the vaccine price would need to be lowered with €17.77 to obtain equal ICERS when extra consults are implemented, due to extra productivity losses, travel costs and higher administration costs.
We did not model the cost-effectiveness for a specific MenB vaccine, because none of the vaccines was licensed for use in humans at the time of the analyses. However, of the potential vaccines against MenB disease, Bexsero will most likely be licensed by the European Medicines Agency, given the recent positive opinion about this vaccine.9 LP2086 (Pfizer) induces a robust SBA response against MenB strains in adolescents.17 However, this has not yet been shown for infants.
We based the safety of the MenB vaccine in our study on experience with a MenB OMV vaccine that was used in New Zealand.18 This resulted for our base case in the prediction of 479 adverse event related GP visits and 1 hospitalisation.
Recently, the safety of solely Bexsero or in combination with routine vaccines was assessed.19 Although the overall safety profile of Bexsero was similar to the other routine infant vaccines, the rates of fever were higher. Approximately 0.4% of children were hospitalized for fever following Bexsero vaccination, while no fever-related hospitalizations occurred with routine vaccinations alone.19 If we would have applied this to our base-case model, this would have resulted in 703 hospitalizations, while only 39 cases of MenB disease would be prevented. Therefore we may have underestimated the risk of adverse events associated with vaccination, although the estimate of the safety of the MenB OMV vaccine is based on a much larger population. Nevertheless, that recent study19 indicates that the risk for a transient vaccine-induced fever should be carefully weighed against the (country-specific) risk of a meningococcal infection, potentially leading to life-long disability or death.
There is no evidence that previous MenB vaccines or the new protein-based vaccines can induce herd protection.15 However, herd protection has been observed with MenC vaccination.20,21 Ignoring herd protection when present can substantially underestimate the impact of vaccination.22 However, implementing infant vaccination without boosters or a catch-up campaign would not likely result in herd protection.23 Herd protection is more likely to occur if a booster in adolescents is introduced, because carriage is particularly high in teenagers.24 If the new vaccines are subsequently shown to affect carriage our analyses with booster vaccinations at the age of 12 y, may underestimate the cost-effectiveness of the intervention. To assess the effect of herd protection on the cost-effectiveness, a transmission dynamic model should be used.25 On the other hand, we assumed that one vaccine dose at the age of 12 y would be sufficient to induce protective immunity in children that previously received multiple vaccine doses in the routine infant immunization program. If two doses are needed to induce a robust immune response at this age, we may have overestimated the cost-effectiveness of this booster dose at 12 y of age.
Because our model was sensitive to the vaccination costs, the vaccine effectiveness, future disease incidences, the duration of protection provided by the vaccine, and these parameters are uncertain, we varied these parameters over a range of plausible values.
Our model was also sensitive to the proportion of cases with sequelae, principally due to QALY losses. There are limited robust data about QALY losses associated with sequelae in children, but efforts are being made to get more reliable data.26 We excluded QALY losses associated with acute forms of disease or minor vaccine-related adverse events, because of the limited duration of these events and accurate estimates of QALY losses associated with such acute events in children are lacking.
In our study, introducing vaccination against MenB disease in the Netherlands was less cost-effective than previously estimated.10,27 This difference was mainly caused by a lower MenB disease incidence in our study. Additionally, in the study from 2001, Bos et al. calculated with a lower vaccine price per dose (€10 instead of €40).10 Using a vaccine price of €10 and the average incidence seen during ’90-’93 we estimated the ICER to be less favorable than in the study of Bos et al. (€27,631 vs. €15,721 per QALY),10 mainly because of a lower CFR used in our study. We estimated the average CFR at 5.4%, while Bos et al. estimated the average CFR at 9.5%.10,27 Our estimate is more similar to the CFR of 5.6% obtained when dividing the number of clinical hospitalizations for meningococcal disease by the number of deaths during these hospitalizations, using figures from 1981–2009 from Statistics Netherlands. A weighted average CFR of 6% was obtained from 7 studies in Europe using data after 1990.4,28-33
Our results show that routine infant vaccination against MenB-related disease is far more expensive per QALY gained than routine vaccination against MenC-related disease13 or human papilloma virus, which was recently implemented in the Dutch national immunization program.34 Cost-effectiveness analyses in England also indicate that vaccinating against MenB disease is cost-effective only when the vaccine is inexpensive (£7,-) using a threshold of £30,000 per QALY.35,36 If we would calculate with same annual disease incidence, the vaccine could cost £13,- using the same threshold of the willingness to pay. The maximum vaccine price is slightly higher in the Netherlands, mainly due to differences in discount rates between both countries.
Methods
Model
A Markov model was built using Excel 2010 (Microsoft) and @Risk (Palisade) with time cycles of 1 mo for children less than 2 y of age and annually thereafter (Fig. S1). A cohort of 185,000 newborns, representing the Dutch birth cohort, was followed once with, and once without, vaccination implemented. The time horizon was set at 99 y, taking lifetime effects and costs into account. The ICERs were calculated by dividing the incremental costs by either life-years or QALYs gained. The study was conducted from a societal perspective. Cost and health effects were discounted at 4% and 1.5% respectively.37
Epidemiology
The incidence of MenB disease was obtained from the Netherlands Reference Laboratory for Bacterial Meningitis (NRLBM, Academic Medical Center, Amsterdam, the Netherlands) (Fig. S2). For the base-case, the average incidence from 2005–2009 was used (1.07 cases per 100,000 person-years), and for scenario analyses we used the average incidence from 1990–1993 (3.46 cases per 100,000 person-years). We adjusted for underreporting using an estimate from the NRLBM for the base-case (15%) and a different estimate for a scenario analysis (38%).7,38 The proportion of MenB infections leading to septic shock and corresponding CFR figures were obtained from a study by De Greeff et al.28
Health care resource use
The average age-specific duration of meningococcal disease associated hospitalisations was obtained from the independent, nationwide hospital registry KiwaPrismant (Utrecht, the Netherlands).39 These averages were transformed to MenB disease with and without septic shock.10 MenB disease could result in complete recovery, death or sequelae, i.e., hearing loss, motor deficit, neurological sequelae, scars or amputations.2,4,5 The percentage of survivors with hearing loss that require cochlear hearing devices was estimated at 51%.1
We assumed that 33% of survivors with scars, and all survivors with amputations, needed to be hospitalized again within a few weeks for treatment of scars or amputations.40
Patients with cognitive difficulties, seizure disorders, visual disturbances, severe hearing loss and major clinical impairments might be eligible for special education or intensive institutional care. Approximately 3.6% of patients met these criteria,2 of which 25% would require intensive institutional care and 50% special education.10,41
Costs
Direct and indirect costs were considered at 2009 price levels (Table 3). Costs not available at 2009 price levels were inflated using the Dutch consumer price index. Special education costs were based on special education participation (Statistics Netherlands) and costs.42 Indirect costs for meningococcal disease and vaccinations were calculated using the friction cost method.37 For children less than 15 y of age, we assumed that one of the parents took time off during half the period that the child is in the hospital.43 For patients aged 15 y and over we conservatively assumed that the patient would miss the total duration of hospitalization plus one day.13 Although the indirect costs of death could be estimated, they were not used in the numerator for calculating ICERs to prevent possible double counting.25 Additional indirect costs (and traveling and higher administration costs) for vaccinations outside the current national immunization schedule were also taken into account. A vaccine price of €40 per dose was assumed in the base-case, based on vaccines previously introduced in developed countries.27
Table 3. Parameters used for the base-case scenario.
| Parameter | Mean or range | Distribution | References |
|---|---|---|---|
| Annual MenB related disease incidence (per 100,000 persons) | 1.07 | Scenario based variation (age dependent) | NRLBMa |
| Meningococcal infection leading to septic shock | 29.8% | Beta (161; 380) | 28 |
| Case-fatality rate meningococcal infections with septic shock | 13.1% | Beta (21; 140) | 28 |
| Case-fatality rate meningococcal infections without septic shock | 2.1% | Beta (8; 372) | 28 |
| Duration standard hospitalization care meningococcal infections without septic shock (days) | 10.1 | Gamma (age dependent) | 10, KPb |
| Duration standard hospitalization care meningococcal infections with septic shock (days) | 8.8 | Gamma (age dependent) | 10, KPb |
| Duration intensive care treatment meningococcal infections with septic shock (days) | 5.0 | Gamma (age dependent) | 10, KPb |
| Survivors requiring cochlear implantation | 1.9% | Beta (4.9; 254) | 1 , 2 |
| Survivors with other hearing loss | 1.8% | Beta (4.7; 254) | 1 , 2 |
| Survivors with motor deficit | 1.9% | Beta (5; 254) | 2 |
| Survivors with neurological sequelae | 3.9% | Beta (10; 249) | 2 |
| Survivors with scars | 3.6% | Beta (12.5; 333) | 4 , 5 |
| Survivors with amputations | 0.6% | Beta (2.5; 388) | 4 , 5 |
| Direct costs (€) | |||
| Standard care hospitalization day | 457 | Fixed | 37 |
| Hospitalization day at intensive care unit | 2183 | Fixed | 37 |
| GP visit | 28 | Fixed | 37 |
| Diagnostics | 261 | Triangular (196; 261; 326) | 10 |
| Full course of antibiotics | 287 | Triangular (215; 287; 359) | 10 |
| Extra medical assistance with shock | 1814 | Triangular (1361; 1814; 2268) | 10 |
| Pediatrician follow-up after recovery | 118 | Triangular (89; 118; 148) | 10 |
| Public health response to a case | 60 | Triangular (45; 60; 75) | 13 |
| Special education (annual costs) | 4500–23188 | Triangular (age dependent) | 42, CBSb |
| Institutional care (annual costs) | 40058 | Triangular (30044; 40058; 50073) | 43 |
| Cochlear implantation | 45562 | Fixed | 44 |
| Treatment of scars | 489 | Triangular (367; 489; 611) | 10 |
| Hospitalization associated with treatment of scars | 914 | Triangular (686; 914; 1143) | 10 , 37 |
| Treatment of amputations | 1573 | Triangular (1180; 1573; 1966) | 10 , 37 |
| Hospitalization associated with treatment of amputations | 3656 | Triangular (2742; 3656; 4570) | 10 , 37 |
| Traveling costs to hospital | 4.30 | Fixed | 37 |
| Traveling costs to general practitioner | 0.97 | Fixed | 37 |
| Traveling costs to municipal health service | 9.08 | Triangular (6.81; 9.08; 11.34) | 37 |
| Indirect costs (€) | |||
| Work loss per hour for parents children below 15 y old | 22.57 | Triangular (16.94; 22.57; 28.21) | CBSc |
| Work loss per hour for patient aged 15 y or older | 19.75 | Triangular (age dependent) | CBSc |
| Total drop in quality of life (QALYs) | |||
| Hearing loss requiring cochlear implantation (first year) | 0.45 | Beta (8.75; 10.70) | 43 , 45 |
| Hearing loss cochlear device (subsequent years) | 0.18 | Beta (1.18; 5.36) | 43 , 45 |
| All other hearing loss | 0.09 | Beta (1.06; 10.74) | 43 , 46 |
| Motor deficits | 0.17 | Beta (5.48; 28.51) | 47 |
| Neurological sequelae not requiring institutional care | 0.25 | Beta (10.47; 31.40) | 47 |
| Neurological sequelae requiring institutional care | 0.53 | Beta (1.58; 1.40) | 43 , 46 |
| Other parameters | |||
| Average QALY general population | 0.89 | Beta (age dependent) | 48 |
| Discount rate health effects | 1.5% | NA | 37 |
| Discount rate costs | 4% | NA | 37 |
NA, not applicable. aBased on data from the Netherlands Reference Laboratory for Bacterial Meningitis (NRLBM), Amsterdam, The Netherlands. bBased on data from KiwaPrismant, Utrecht, The Netherlands (KP). cBased on data from Statistics Netherlands (CBS).
Quality-adjusted life-years
We drew QALY losses due to sequelae from a number of Dutch studies using the most conservative QALY in each case (Table 3).43,45-47 Specific QALY losses were assigned to each health state in our model on a scale ranging from 0 (immediate death) to 1 (a state of perfect health). QALY losses due to hearing loss requiring choclear implantation were derived from Krabbe et al.45 who reported the quality of life of adults who received a cochlear implantation using the HUI-2 index. The QALY losses due to all other hearing loss and neurological sequelae requiring institutional care were derived from Oostenbrink et al. who used the EQ-5D index.46 For motor deficits and neurological sequelae not requiring institutional care, QALY losses were derived from Stouthard et al. who also used the EQ-5D index to estimate the quality of life of different health states.47
Vaccine characteristics
We assumed that the vaccine-type coverage multiplied by the vaccine efficacy would be 75% one month after the second vaccine dose.27 Vaccination was assumed to occur concurrently with vaccinations against pneumococcal disease, resulting in similar vaccination coverage.10 A dosing schedule with vaccinations at 2, 3, 4 and 11 mo of age was used in the base-case. The duration of protection after doses at 2, 3, 4 mo of age was assumed to be 1.5 y, which is comparable to that after immunization against MenC disease.49 The duration of protection was assumed to increase to 3 y following the booster at 11 mo of age. Because protection waned quite rapidly after MenC vaccination,50 we assumed that the vaccine effectiveness would decline in time following Equation: VE(t) = VE(0) * exp(-t/D) (Equation 1), where VE(0) is the vaccine effectiveness one month after the second dose, t is the time since protective immunity started, and D is the mean duration of protection.
For a scenario analysis we evaluated the cost-effectiveness of a dosing schedule with vaccinations at 12 and 14 mo of age, assuming an average duration of protection of 4 y starting half a month after the first vaccination. This duration of protection is comparable to that after immunization against MenC disease at an age of 14 mo.51 We based the frequency of vaccine related adverse events on data obtained with an OMV vaccine against MenB disease used in New Zealand.18 Table 4 lists all the vaccine parameters used in the model.
Table 4. Base-case vaccine parameters.
| Parameter | Mean or range | References |
|---|---|---|
| Vaccine effectiveness one month after second dose | 75% | Assumed |
| Vaccine effectiveness boosters | 75% | Assumed |
| Duration of protection after third dose | 1.5 y | 49 |
| Duration of protection after fourth dose | 3 y | Assumed |
| Duration of protection after boosters at 12 y of age | 8 y | Assumed |
| Vaccination coverage | 95% | 10 , 52 |
| Vaccine price per dose | €40 | 27 |
| Administration fee - routine vaccination (per dose) | €6.81 | 27 |
| Administration fee - vaccination if no other vaccine is already given at that moment (per dose) | €14.68 | 27 , 37 |
| Number of adverse event related general practitioner visits per 10,000 vaccine doses | 6.80 | 18 |
| Number of adverse event related hospitalizations per 10,000 vaccine doses | 0.01 | 18 |
Analyses with booster vaccinations
Data from OMV vaccines against MenB disease have shown that antibody persistence is shorter in infants than in older children, and can be prolonged by booster vaccinations.53,54 Therefore, we estimated the ICER for dosing schedules including an additional booster at 12 y of age. A booster uptake rate similar to the primary doses was used, because a similar uptake was observed among children and adolescents aged 14 mo to 19 y during the catch-up campaign for MenC vaccination.55 We assumed that the average duration of protection would increase to 8 y following the booster vaccinations at 12 y of age. Assuming waning occurs according to Equation 1, an average duration of protection of 8 y would, even for an initial vaccine effectiveness of 100%, result in approximately 50% of children being protected after 5 y; which is conservative compared with the percentage of children (more than 80%) found to have protective levels of antibody 5 y after MenC vaccination.51
Sensitivity analyses
Although there is no formal threshold for cost-effectiveness in the Netherlands, €20,000 per QALY is often mentioned for new vaccination programs to indicate highly favorable cost-effectiveness;16 beyond €50,000 per QALY is generally not considered to be cost-effective.43,56 We evaluated the level of MenB disease incidence required for vaccination to become cost-effective, given a vaccine price of €40 per dose and a willingness to pay of €20,000 or €50,000 per QALY. Additionally, we analyzed what the maximum vaccine price per dose could be for different MenB disease incidences. In univariate sensitivity analysis, relevant parameters were varied by 25% to determine which parameters have a large impact on the ICER. Additionally, we performed a two way sensitivity analyses to assess the impact of the vaccine effectiveness and the vaccine price on the cost-effectiveness.
To assess the uncertainty of the ICER, a probabilistic sensitivity analysis was performed using Monte Carlo simulation. Parameters were generated using random sampling within the specified range of the corresponding distribution of the parameters. Outcome values were generated by running the model 5,000 times. The probability that vaccination would be cost-effective was presented using a cost-effectiveness acceptability curve.
Conclusions
With the current incidence of MenB disease, routine infant vaccination against MenB is unlikely to be cost-effective at a vaccine price of €40 per dose. However, if the vaccine price would be reduced or the incidence of MenB disease increases and/or fewer vaccine doses are used, vaccination could be deemed cost-effective.
If the new vaccines are shown to influence carriage, a dynamic model for the Netherlands should be developed. Together with more robust data about QALY losses associated with sequelae and more information about the new vaccines, this could further enhance the validity of cost-effectiveness analyses of vaccination against MenB disease.
Supplementary Material
Acknowledgments
We would like to thank Patricia Kaaijk, PhD, for her helpful comments on drafts of this paper. This specific study was supported by a grant from the former Netherlands Vaccine Institute (since 2012 integrated into the National Institute of Public Health and the Environment). The sponsor had no role in the study design, the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Glossary
Abbreviations:
- CFR
case-fatality rate
- GP
general practitioner
- ICER
incremental cost-effectiveness ratio
- MenB
serogroup B meningococcal
- MenC
serogroup C meningococcal
- NRLBM
Netherlands Reference Laboratory for Bacterial Meningitis
- OMV
outer-membrane vesicle
- QALY
quality-adjusted life year
- VE
vaccine effectiveness
Submitted
12/12/12
Revised
01/24/13
Accepted
02/05/13
Disclosure of Potential Conflicts of Interest
Prof Postma received grants, honoraria and travel stipends from various pharmaceutical industries and institutes, inclusive those interested in the subject matter of this paper. Dr van der Ende reports receiving grant support from Pfizer for research on pneumococcal infections and pneumococcal vaccine studies and consulting fees from Pfizer and GlaxoSmithKline. Dr van den Dobbelsteen was employed at the National Institute for Public Health and the Environment (RIVM), Unit Vaccinology and is currently employed at Bacterial Vaccines, Crucell, Leiden, the Netherlands. The other authors have no conflicts of interest that are relevant to this article to disclose.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/23888
References
- 1.Baraff LJ, Lee SI, Schriger DL. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr Infect Dis J. 1993;12:389–94. doi: 10.1097/00006454-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:317–28. doi: 10.1016/S1473-3099(10)70048-7. [DOI] [PubMed] [Google Scholar]
- 3.Gottfredsson M, Reynisson IK, Ingvarsson RF, Kristjansdottir H, Nardini MV, Sigurdsson JF, et al. Comparative long-term adverse effects elicited by invasive group B and C meningococcal infections. Clin Infect Dis. 2011;53:e117–24. doi: 10.1093/cid/cir500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Healy CM, Butler KM, Smith EO, Hensey OP, Bate T, Moloney AC, et al. Influence of serogroup on the presentation, course, and outcome of invasive meningococcal disease in children in the Republic of Ireland, 1995-2000. Clin Infect Dis. 2002;34:1323–30. doi: 10.1086/340050. [DOI] [PubMed] [Google Scholar]
- 5.Scholten RJ, Bijlmer HA, Valkenburg HA, Dankert J. Patient and strain characteristics in relation to the outcome of meningococcal disease: a multivariate analysis. Epidemiol Infect. 1994;112:115–24. doi: 10.1017/S0950268800057472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spanjaard L, Bol P, de Marie S, Zanen HC. Association of meningococcal serogroups with the course of disease in the Netherlands, 1959-83. Bull World Health Organ. 1987;65:861–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Netherlands Reference Laboratory for Bacterial Meningitis. Amsterdam: University of Amsterdam. Bacterial meningitis in the Netherlands: Annual reports 2006-2009.
- 8.Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine. 2012;30(Suppl 2):B87–97. doi: 10.1016/j.vaccine.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novartis. Media release. Novartis receives positive CHMP opinion for Bexsero®, a groundbreaking vaccine to help prevent devastating MenB infections, the leading cause of meningitis in Europe. Available at: http://www.novartis.com/newsroom/media-releases/en/2012/1658747.shtml
- 10.Bos JM, Rümke HC, Welte R, Postma MJ, Jager JC. Health economics of a hexavalent meningococcal outer-membrane vesicle vaccine in children: potential impact of introduction in the Dutch vaccination program. Vaccine. 2001;20:202–7. doi: 10.1016/S0264-410X(01)00254-7. [DOI] [PubMed] [Google Scholar]
- 11.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl 2):B51–63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 12.Poolman JT, Lind I, Jónsdóttir K, Frøholm LO, Jones DM, Zanen HC. Meningococcal serotypes and serogroup B disease in north-west Europe. Lancet. 1986;2:555–8. doi: 10.1016/S0140-6736(86)90123-6. [DOI] [PubMed] [Google Scholar]
- 13.Welte R, van den Dobbelsteen G, Bos JM, de Melker H, van Alphen L, Spanjaard L, et al. Economic evaluation of meningococcal serogroup C conjugate vaccination programmes in The Netherlands and its impact on decision-making. Vaccine. 2004;23:470–9. doi: 10.1016/j.vaccine.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 14.European Centre for Disease Prevention and Control. Annual epidemiological report 2011. Reporting on 2009 surveillance data and 2010 epidemic intelligence data. Stockholm: ECDC; 2011. [Google Scholar]
- 15.Dellicour S, Greenwood B. Systematic review: Impact of meningococcal vaccination on pharyngeal carriage of meningococci. Trop Med Int Health. 2007;12:1409–21. doi: 10.1111/j.1365-3156.2007.01929.x. [DOI] [PubMed] [Google Scholar]
- 16.Health Council. (GezondheidsRaad; GR). The future of the national immunization program: Towards a program for all ages. The Hague: GR, 2007. Available at: http://www.gezondheidsraad.nl/sites/default/files/200702E_0.pdf
- 17.Richmond PC, Marshall HS, Nissen MD, Jiang Q, Jansen KU, Garcés-Sánchez M, et al. 2001 Study Investigators Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012;12:597–607. doi: 10.1016/S1473-3099(12)70087-7. [DOI] [PubMed] [Google Scholar]
- 18.McNicholas A, Galloway Y, Stehr-Green P, Reid S, Radke S, Sexton K, et al. Post-marketing safety monitoring of a new group B meningococcal vaccine in New Zealand, 2004-2006. Hum Vaccin. 2007;3:196–204. doi: 10.4161/hv.3.5.4458. [DOI] [PubMed] [Google Scholar]
- 19.Gossger N, Snape MD, Yu LM, Finn A, Bona G, Esposito S, et al. European MenB Vaccine Study Group Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA. 2012;307:573–82. doi: 10.1001/jama.2012.85. [DOI] [PubMed] [Google Scholar]
- 20.Maiden MC, Ibarz-Pavón AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197:737–43. doi: 10.1086/527401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trotter CL, Maiden MC. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev Vaccines. 2009;8:851–61. doi: 10.1586/erv.09.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brisson M, Edmunds WJ. Economic evaluation of vaccination programs: the impact of herd-immunity. Med Decis Making. 2003;23:76–82. doi: 10.1177/0272989X02239651. [DOI] [PubMed] [Google Scholar]
- 23.Larrauri A, Cano R, García M, Mateo Sd. Impact and effectiveness of meningococcal C conjugate vaccine following its introduction in Spain. Vaccine. 2005;23:4097–100. doi: 10.1016/j.vaccine.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 24.Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:853–61. doi: 10.1016/S1473-3099(10)70251-6. [DOI] [PubMed] [Google Scholar]
- 25.Kauf TL. Methodological concerns with economic evaluations of meningococcal vaccines. Pharmacoeconomics. 2010;28:449–61. doi: 10.2165/11535280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Christie D, Viner RM, Knox K, Coen PG, Wang H, El Bashir H, et al. Long-term outcomes of pneumococcal meningitis in childhood and adolescence. Eur J Pediatr. 2011;170:997–1006. doi: 10.1007/s00431-010-1390-5. [DOI] [PubMed] [Google Scholar]
- 27.Bos JM, Rümke HC, Welte R, Spanjaard L, van Alphen L, Postma MJ. Combination vaccine against invasive meningococcal B and pneumococcal infections: potential epidemiological and economic impact in the Netherlands. Pharmacoeconomics. 2006;24:141–53. doi: 10.2165/00019053-200624020-00004. [DOI] [PubMed] [Google Scholar]
- 28.de Greeff SC, de Melker HE, Schouls LM, Spanjaard L, van Deuren M. Pre-admission clinical course of meningococcal disease and opportunities for the earlier start of appropriate intervention: a prospective epidemiological study on 752 patients in the Netherlands, 2003-2005. Eur J Clin Microbiol Infect Dis. 2008;27:985–92. doi: 10.1007/s10096-008-0535-1. [DOI] [PubMed] [Google Scholar]
- 29.Domínguez A, Cardeñosa N, Pañella H, Orcau A, Companys M, Alseda M, et al. Working Group on the Study of Meningococcal Disease in Catalonia, 1990-1997 The case-fatality rate of meningococcal disease in Catalonia, 1990-1997. Scand J Infect Dis. 2004;36:274–9. doi: 10.1080/00365540410020163. [DOI] [PubMed] [Google Scholar]
- 30.Shigematsu M, Davison KL, Charlett A, Crowcroft NS. National enhanced surveillance of meningococcal disease in England, Wales and Northern Ireland, January 1999-June 2001. Epidemiol Infect. 2002;129:459–70. doi: 10.1017/S0950268802007549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobsson S, Olcén P, Löfdahl M, Fredlund H, Mölling P. Characteristics of Neisseria meningitidis isolates causing fatal disease. Scand J Infect Dis. 2008;40:734–44. doi: 10.1080/00365540802029565. [DOI] [PubMed] [Google Scholar]
- 32.Tsolia MN, Theodoridou M, Tzanakaki G, Vlachou V, Mostrou G, Stripeli F, et al. Invasive meningococcal disease in children in Greece: comparison of serogroup A disease with disease caused by other serogroups. Eur J Clin Microbiol Infect Dis. 2006;25:449–56. doi: 10.1007/s10096-006-0155-6. [DOI] [PubMed] [Google Scholar]
- 33.Biebl A, Hartmann G, Bernhard C, Bechter E, Luckner-Hornischer A, Frühwirth M, et al. Vaccine strategies of meningococcal disease: results of a 10-year population-based study. Eur J Pediatr. 2005;164:735–40. doi: 10.1007/s00431-005-1719-7. [DOI] [PubMed] [Google Scholar]
- 34.Rogoza RM, Westra TA, Ferko N, Tamminga JJ, Drummond MF, Daemen T, et al. Cost-effectiveness of prophylactic vaccination against human papillomavirus 16/18 for the prevention of cervical cancer: adaptation of an existing cohort model to the situation in the Netherlands. Vaccine. 2009;27:4776–83. doi: 10.1016/j.vaccine.2009.05.085. [DOI] [PubMed] [Google Scholar]
- 35.Christensen H, Trotter CL, Hickman M, Edmunds WJ. Modelling the potential impact of MenB vaccines. Presented at: 8th Meningitis Research Foundation International Conference 2011, London, United Kingdom, 8-9 November 2011. [Google Scholar]
- 36.Christensen H, Trotter CL, Hickman M, Edmunds WJ. Modelling the cost-effectiveness of new meningococcal vaccines in England. Presented at: 17th International Pathogenic Neisseria Conference, Banff, Alberta, Canada, 11-16 September 2010. [Google Scholar]
- 37.Hakkaart-van Roijen L, Tan SS, Bouwmans CAM. Guidelines for costing research, methods and standarized prices for economic evaluations in health care. Dutch Health Care Insurance Board, 2010. [Google Scholar]
- 38.de Greeff SC, Spanjaard L, Dankert J, Hoebe CJ, Nagelkerke N, de Melker HE. Underreporting of meningococcal disease incidence in the Netherlands: results from a capture-recapture analysis based on three registration sources with correction for false positive diagnoses. Eur J Epidemiol. 2006;21:315–21. doi: 10.1007/s10654-006-0020-z. [DOI] [PubMed] [Google Scholar]
- 39.Kiwa Prismant. Independent nation-wide hospital registry [in Dutch]. Available at: http://cognosserver.prismant.nl/cognos7/cgi-bin/ppdscgi.cgi?DC=Q&E=/Prisma-Landelijke-LMR/Landelijke+LMR-informatie+-+Diagnosen
- 40.Buysse CM, Oranje AP, Zuidema E, Hazelzet JA, Hop WC, Diepstraten AF, et al. Long-term skin scarring and orthopaedic sequelae in survivors of meningococcal septic shock. Arch Dis Child. 2009;94:381–6. doi: 10.1136/adc.2007.131862. [DOI] [PubMed] [Google Scholar]
- 41.Gruteke P, Postma MJ, Grosheide PM. Prevention of congenital syphilis: Economic evaluation using surveillance of the public-health laboratories [in Dutch]. Bilthoven, the Netherlands: National Institute of Public Health & the Environment (RIVM); 1995. [Google Scholar]
- 42.Minne B, Webbink D, van der Wiel H. Special care for children with learning and/or behavioural difficulties. Netherlands Bureau for Economic Policy Analysis. The Hague, the Netherlands. 192, 2006. [Google Scholar]
- 43.Rozenbaum MH, Sanders EA, van Hoek AJ, Jansen AG, van der Ende A, van den Dobbelsteen G, et al. Cost effectiveness of pneumococcal vaccination among Dutch infants: economic analysis of the seven valent pneumococcal conjugated vaccine and forecast for the 10 valent and 13 valent vaccines. BMJ. 2010;340:c2509. doi: 10.1136/bmj.c2509. [DOI] [PubMed] [Google Scholar]
- 44.Schippers EI. Available at: http://www.rijksoverheid.nl/bestanden/documenten-en-publicaties/kamerstukken/2011/04/21/antwoorden-kamervragen-niet-vergoeden-tweede-cochleaire-implantaat-bij-jonge-kinderen/antwoorden-kamervragen-niet-vergoeden-tweede-cochleaire-implantaat-bij-jonge-kinderen.pdf
- 45.Krabbe PF, Hinderink JB, van den Broek P. The effect of cochlear implant use in postlingually deaf adults. Int J Technol Assess Health Care. 2000;16:864–73. doi: 10.1017/S0266462300102132. [DOI] [PubMed] [Google Scholar]
- 46.Oostenbrink R, A Moll HA, Essink-Bot ML. The EQ-5D and the Health Utilities Index for permanent sequelae after meningitis: a head-to-head comparison. J Clin Epidemiol. 2002;55:791–9. doi: 10.1016/S0895-4356(02)00448-1. [DOI] [PubMed] [Google Scholar]
- 47.Stouthard MEA, Essink-Bot ML, Bonsel GJ, Barendregt JJ, Kramers PGN, van de Water HPA, et al. Disability weights for disease in The Netherlands. Amsterdam Medical Centre, Institute for Social Heatlh Care, Amsterdam. 1997. [Google Scholar]
- 48.Kind P, Hardman G, Macran S. UK population norms for EQ-5D. Discussion paper 172. The University of York Centre for Health Economics. 1999. Available at: http://www.york.ac.uk/inst/che/pdf/DP172.pdf
- 49.Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364:365–7. doi: 10.1016/S0140-6736(04)16725-1. [DOI] [PubMed] [Google Scholar]
- 50.De Wals P, Deceuninck G, Lefebvre B, Boulianne N, De Serres G. Effectiveness of serogroup C meningococcal conjugate vaccine: a 7-year follow-up in Quebec, Canada. Pediatr Infect Dis J. 2011;30:566–9. doi: 10.1097/INF.0b013e31820e8638. [DOI] [PubMed] [Google Scholar]
- 51.de Voer RM, Mollema L, Schepp RM, de Greeff SC, van Gageldonk PG, de Melker HE, et al. Immunity against Neisseria meningitidis serogroup C in the Dutch population before and after introduction of the meningococcal c conjugate vaccine. PLoS One. 2010;5:e12144. doi: 10.1371/journal.pone.0012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Lier EA, Oomen PJ, Giesbers H, Drijfhout IH, de Hoogh PAAM, de Melker HE. Immunization coverage National Immunization Programme in the Netherlands [in Dutch]. Bilthoven, the Netherlands: National Institute of Public Health & the Environment (RIVM); 2012. [Google Scholar]
- 53.Holst J, Martin D, Arnold R, Huergo CC, Oster P, O’Hallahan J, et al. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine. 2009;27(Suppl 2):B3–12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 54.Jackson C, Lennon D, Wong S, Yan J, Stewart J, Reid S, et al. Antibody persistence following MeNZB vaccination of adults and children and response to a fourth dose in toddlers. Arch Dis Child. 2011;96:744–51. doi: 10.1136/adc.2009.180596. [DOI] [PubMed] [Google Scholar]
- 55.Neppelenbroek SE, de Vries M, de Greeff SC, Timen A. 'da's goed gedaan?' Woordverslag van de landelijke vaccinatiecampagne meningokokken C, 2002 [in Dutch]. GGD Nederland; ISBN 90-72279-38-X. 2003. [Google Scholar]
- 56.Rozenbaum MH, Hak E, van der Werf TS, Postma MJ. Results of a cohort model analysis of the cost-effectiveness of routine immunization with 13-valent pneumococcal conjugate vaccine of those aged > or =65 years in the Netherlands. Clin Ther. 2010;32:1517–32. doi: 10.1016/j.clinthera.2010.06.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.