At cellular level, aging is often referred to as senescence and characterized by irreversible cell cycle arrest, morphological transformation, induction of senescence-associated β-galactosidase and heterochromatic foci, and secretory phenotype. A number of cellular events can induce senescence, including genetic lesions (e.g., DNA damage, genomic instability, and telomere attrition), oxidative stress, and epigenetic alterations.1 Telomere shortening, caused by replicative or pathological stress, is an essential cause of cellular aging and represents a well-studied mechanism of senescence.1 In multicellular organisms, aging is characterized by functional deteriorations across organs and tissues of the body in coordinated manners. Although various events such as oxidative stress and endocrine disorders have been extensively demonstrated to contribute to certain aspects of systemic aging,2,3 it is still a topic of much debate whether a program exists that guides aging development and lifespan control at the organism level. However, this fundamental question has recently been importantly addressed by an investigation that targeted the hypothalamus, a brain region that is known to regulate many basic life functions, such as growth, development, reproduction, and metabolism. Through hypothalamic manipulations in mouse models, findings in this study have led to the conclusion that the hypothalamus has a predominant role in coordinating whole-body aging.4
The context of this study was related to the recent understandings developed by the same group, showing that the mediobasal hypothalamus (MBH) is sensitive to mild but chronic environmental disturbances such as caloric excess. Indeed, chronic excess of calories can lead to molecular inflammatory changes in the hypothalamus, also briefly referred to as “hypothalamic inflammation.” Such onset of molecular inflammation in the MBH has been shown to impair the central regulation of energy homeostasis and therefore mediate the progression of body weight imbalance, obesity, and related metabolic disorders.5 Further, obesity-related hypothalamic inflammation works as a point to couple obesity with hypertension.6 More recently, it was revealed that hypothalamic inflammation acts to cause a neurodegenerative mechanism of obesity and type-2 diabetes, mechanistically medicated through inflammatory disruption on the hypothalamic neural stem cells and their neurogenesis.7 In this background, the authors recently obtained evidence showing that, in addition to overnutrition, age increase during early aging can sensitively induce molecular inflammation in the MBH.4 With detailed investigation, it was unveiled in this work that hypothalamic microglia can employ an IKKβ/NFκB-dependent innate immune reaction to crosstalk to MBH neurons during aging development.4 Subsequently, through using interventional strategies, this study demonstrated that inhibition of IKKβ/NFκB in either microglia or neurons of the MBH are both useful for aging retardation and lifespan extension. Of note, different studies in recent literature showed that microglia undergo phenotypic and functional changes with aging development, and these changes were suggested to be particularly important for development of neurodegenerative diseases.8 Altogether, these independent studies lend a support to the concept that the brain immune system has a crucial role in the development of systemic aging.4
A relevant subsequent question is, how does hypothalamic inflammation induce systemic impacts that promote systemic aging? In the same study, it was reported that activation of hypothalamic IKKβ/NFκB inhibits the production of gonadotropin-releasing hormone (GnRH) in the hypothalamus. GnRH is a decapeptide hormone that is produced mostly by hypothalamic GnRH neurons and acts on the anterior pituitary to further control the production and release of follicle-stimulating hormone and luteinizing hormone. In this study,4 the authors found that aging is associated with attenuation of hypothalamic GnRH gene expression, and, furthermore, IKKβ/NFκB activation can strongly inhibit GnRH gene transcription, thus providing an explanation for the phenomenon of aging-related GnRH decline. Therapeutically, GnRH treatment significantly although partially reversed aging-impaired neurogenesis in the hypothalamus, hippocampus and other brain regions. GnRH therapy also led to amelioration of various aging effects, including skin atrophy, muscle weakness, and bone loss. Therefore, in addition to the reproductive role, GnRH works in the brain and the periphery to regulate systemic aging. These findings further emphasize a central role of the hypothalamus in aging development, which is mediated via an interaction between innate immunity system and neuropeptide pathway. While many details are still unclear, one biological scenario could be that the inflammatory IKKβ/NFκB pathway in the hypothalamus reacts to aging-related environmental changes, leading to altered hypothalamic neuroendocrine outputs, which affect various aspects of systemic physiology to promote systemic aging. With these findings, based on GnRH-related aging mechanism, the authors further discussed that certain other hypothalamic factors could be involved as well in the central mechanism of aging, which calls for future exploration. Taken together, aging is an outcome of multiple physiological changes at advanced ages, and the hypothalamus can work as an underlying mediator and represent a target for interventional strategies in counteracting aging and related diseases. (Fig. 1)
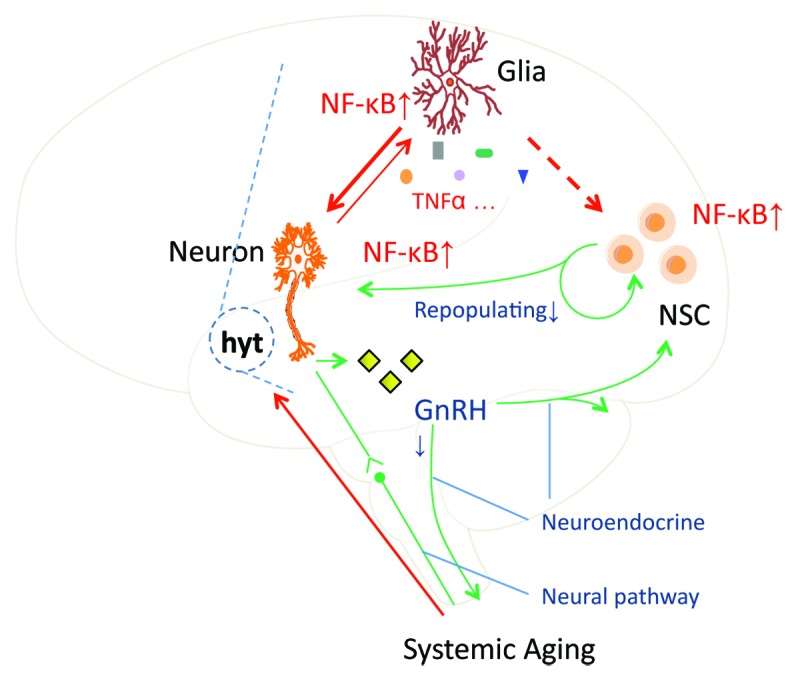
Figure 1. Hypothalamic control of systemic aging. Age increase-associated environmental insults activate proinflammatory IKKβ/NFκB in hypothalamic microglia, and this inflammatory event further employs cytokines-directed autocrine and paracrine crosstalks among different types of neural cells to subsequently induce neuronal IKKβ/NFκB activation and inflammatory changes. Such hypothalamic inflammation eventually causes various defects in regulatory functions and neurogenesis of the hypothalamus, and impairment of GnRH production represents a significant basis. Ultimately, hypothalamic control of whole-body physiology is collectively compromised, contributing profoundly to the development of systemic aging.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/26054
References
- 1.López-Otín C, et al. Cell. 2013;153:1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkel T, et al. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 3.Masoro EJ. Mech Ageing Dev. 2005;126:913–22. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Zhang G, et al. Nature. 2013;497:211–6. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, et al. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purkayastha S, et al. Nat Med. 2011;17:883–7. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, et al. Nat Cell Biol. 2012;14:999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saijo K, et al. Nat Rev Immunol. 2011;11:775–87. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]


