Abstract
The health-related hazards resulting from long-term exposure to radiation remain unknown. Thus, an appropriate molecular marker is needed to clarify these effects. Cyclin D1 regulates the cell cycle transition from the G1 phase to the S phase. Cyclin D1 is degraded as a G1/S checkpoint after 10 Gy of single acute radiation exposure, whereas conversely, cyclin D1 is stabilized when human tumor cells are exposed to fractionated radiation (FR) with 0.5 Gy of x-rays for 31 d. In this article, we review new findings regarding cyclin D1 overexpression in response to long-term exposure to FR. Cyclin D1 overexpression is associated with induction of genomic instability in irradiated cells. Therefore, repression of cyclin D1 expression is likely to cancel the harmful effects of long-term exposure to FR. Thus cyclin D1 may be a marker of long-term exposure to radiation and is a putative molecular radioprotection target for radiation safety.
Keywords: Cyclin D1, long-term fractionated radiation, AKT, double-strand breaks, radioprotection
Introduction
Ionizing radiation (IR) can induce various types of DNA damage, such as DNA base damage, DNA single-strand breaks (SSBs), and DNA double-strand breaks (DSBs). Radiation transfers energy to DNA and disrupts DNA chemical bonds, which results in direct induction of DNA damage. Radiation also affects water and causes the generation of reactive oxygen species (ROS) that react with DNA. In this case, radiation indirectly induces DNA damage via ROS production. Gamma irradiation or X-rays (1 Gy) can damage 500 DNA bases and create 1000 SSBs and 40 DDBs per cell.1,2 The most important biological consequence of IR is believed to be DSBs, which can trigger genomic instability in cells. For example, chromosomal aberrations, such as deletions, insertions, or translocations, can occur even after DSB repair.
Mammalian cells harbor a series of DNA damage responses (DDRs) that are induced after radiation in order to maintain genomic stability. The molecular mechanisms involved with DDRs have been thoroughly investigated using single radiation (SR) exposure regimes. DNA damage sensor kinases, ataxia telangiectasia mutated protein (ATM), and DNA-dependent protein kinase (DNA-PK) recognize DNA lesions and transfer these DNA damage signals to transducers, such as p53, checkpoint kinase1 (Chk1), and checkpoint kinase2 (Chk2). The G1/S checkpoint, which regulates the entry into the S phase in the presence of DSBs, functions by inactivating cyclin-dependent kinases (Cdks) via the degradation of positive regulators of Cdks, such as cyclin D1 and Cdc25A, and by activating a negative regulator such as p21.3-6
Cyclin D1 degradation occurs rapidly after irradiation and results in the release of p21 in a p53-independent manner.3 Thus, cell cycle progression ceases at the G1/S boundary by suppressing Cdks activity. Cell cycle checkpoints block cell cycle progression in the presence of DSBs to achieve adequate DNA damage repair after irradiation. After repairing DNA damage, the cells resume cell cycling by activating Cdks. On the other hand, cell death is induced in order to exclude abnormal cells that have been exposed to high doses of radiation. Induction of apoptosis after exposure to high doses of radiation is associated with mitochondrial transmembrane potential collapse, caspase activation, and DNA degradation.7,8
In order to analyze the biological effect of long-term exposure to radiation, human cells were exposed to fractionated radiation (FR) with 0.5-Gy x-ray fraction at dose rate of 1 Gy per minutes twice per day, 6 d a week for 31 d (Fig. 1). ATM and DNA-PK were constitutive activated by FR for 31 d.9 The cells treated with these exposure regimes were referred to as 31FR cells. Atotal dose of 27 Gy was delivered to cells for 31 d (Fig. 1). We further established 31FR-31NR cells by 31-d FR exposure followed by 31-d non-FR. We recently identified a unique DDR involving cyclin D1 in 31FR and 31FR-31NR cells.9,10 Cells acquired radioresistance because of cyclin D1 overexpression by downregulating the cyclin D1 degradation pathway after exposure to 31 d FR. Glycogen synthase kinase 3β (GSK3β) is a protein kinase that phosphorylates cyclin D1 on threonine 286 (Thr286) to facilitate its degradation.11 AKT-mediated phosphorylation of GSK3β on serine 9 decreases its kinase activity on cyclin D1 Thr286, which inhibits the nuclear export and cytoplasmic proteasomal degradation of cyclin D1.12,13 Constitutive activation of the AKT pro-survival pathway in 31FR cells resulted in downregulation of cyclin D1 proteolysis. Consequently, 31-d FR conferred radioresistance to tumor cells by cyclin D1 overexpression, which was mediated by the AKT/GSK3β pathway.9,10 Thus, cyclin D1 could be used as a marker of long-term exposure to radiation but not of SR exposure.
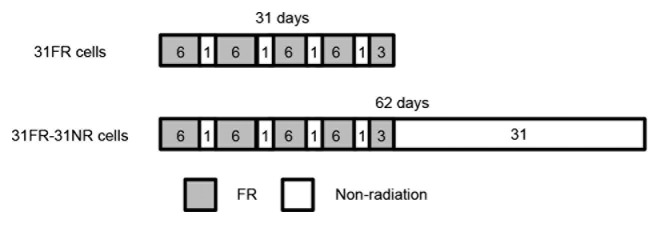
Figure 1. A fraction schedule to establish 31FR and 31FR-31NR cells. Number described in the box indicates the day. Cells were exposed to FR 6 d a week for 31 d to establish 31FR cells. Cells were further cultured without FR for 31 d to make 31FR-31NR cells.
This paper reviews a novel attractive DDR involving cyclin D1 in 31FR cells. Deregulation of cyclin D1 expression generates DSBs during DNA replication and is associated with induction of genomic instability in cells. Therefore, cyclin D1 may serve not only as a marker of long-term exposure to radiation, but may also be a molecular target to reduce the effects of radiation exposure for radioprotection.
Cyclin D1 Expression During Cell Cycling
Cyclin D1 is a positive cell cycle regulator during the G1/S transition. Cyclin D1 expression is regulated both at the transcriptional and post-translational levels. Cyclin D1 expression is upregulated by mitogenic signaling through the Ras-signaling pathway involving Ras/Raf/mitogen-activated protein (extracellular signal-regulated kinase [ERK] kinase) (MEK)/ERK.14 Cyclin D1 levels are also post-translationally regulated by its degradation through the following ubiquitin-proteasome pathway.
Phosphorylation of the cyclin D1 Thr286 residue facilitates the nuclear exclusion of cyclin D1 by the nuclear exporter chromosome maintenance region 1 (CRM1), and then it is degraded. When stimulated by insulin or a growth factor, phosphatidylinositol-3-OH kinase (PI3K) generates phosphatidylinositol-3,4,5-triphosphate, a lipid messenger that is essential for the translocation of AKT (protein kinase B) to the plasma membrane, where it is phosphorylated and activated by 3-phosphoinositide-dependent kinase 1.15 Activated AKT phosphorylates the GSK3β serine 9 residue to inactivate its kinase activity on the cyclin D1 Thr286 residue. This blocks cyclin D1 nuclear export, and cytoplasmic cyclin D1 subsequently undergoes proteasomal degradation.16 Thus, cyclin D1 accumulates in the nucleus as a result of PI3K- and AKT-mediated GSK3β inactivation.
Cyclin D1 mediates the G1/S transition by binding to Cdk4. Cyclin D1/Cdk4 phosphorylates Rb, and E2F is then released to transactivate genes required for the G1/S transition.17 In addition, cyclin D1 plays a role in the G1/S transition by sequestering a Cdk inhibitor of p21 and p27.18,19
Cyclin D1 levels vary throughout the cell cycle; cyclin D1 levels increase in the nucleus during the G1 phase and decline because of cyclin D1 nuclear exclusion when cells enter the S phase concomitant with an increase in GSK3β levels.20 Because cyclin D1 degradation during the S phase is essential for cell cycling, deregulating cyclin D1 expression during the S phase perturbs DNA replication.21,22
Cyclin D1 Regulation After Irradiation
After exposure to 10 Gy or 20 Gy of SR, cyclin D1 undergoes ubiquitin-proteasome degradation for a G1/S checkpoint and prevents entry of irradiated cells into the S phase.3,9 ATM signaling has been shown to play a critical role in regulating cyclin D1 degradation in response to DNA damage via F-box protein 31 (FBXO31), best known for their role as the substrate recognition components of the SCF (SKP/Cullin/F-box protein) class of E3 ubiquitin ligases. ATM directly phosphorylates FBXO31 to facilitate cyclin D1 degradation.23 CCND1 (Cyclin D1 gene) expression is also downregulated following irradiation by inhibiting CREB binding protein (CBP)/p300 histone acetyltransferase (HAT) activities via binding of an RNA binding protein, translocated in liposarcoma (TLS), that contains non-coding RNAs.24 Cdks inactivation by downregulating cyclin D1 expression results in Rb dephosphorylation, which then sequesters E2F to prevent its transactivating activity and to arrest cells at the G1/S boundary.
Conversely, cyclin D1 is stabilized in HepG2 and HeLa cells after exposure to 0.5 Gy of FR for 31 d, which results in cyclin D1 overexpression.9 The CCND1 mRNA levels were not dramatically different before and after 31-d FR.9 Therefore, 31 d FR-induced cyclin D1 overexpression was not due to some genetic change, such as gene amplification, but was due to the decreased protein degradation mediated by the AKT signaling pathway. AKT, a positive regulator of cyclin D1, is constitutively activated when cells are exposed to FR for >14 d (total dose is 12 Gy).9 In contrast, transient AKT activation has been reported in HepG2, HeLa, and human umbilical vein endothelial cells after 2 or 3 Gy of SR.9,25
Collectively, these results suggest that AKT pro-survival signals accumulate under the situation of constitutive activation of DNA-PK and ATM due to repeated radiation exposures. There is a threshold for the changes in the AKT radioresponse from a transient activation pattern to a constitutive activation pattern approximately 14 d of FR (Fig. 2). AKT activation and GSK3β inactivation precede cyclin D1 overexpression, because cyclin D1 overexpression is evident 31 d after FR. In addition, pro-survival signaling via the AKT/ERK pathway is activated at lower DSB levels (<2 Gy) but not at higher DSB levels (>2 Gy).26 Thus, the AKT pro-survival signaling pathway varies according to the magnitude of the irradiated dose and the duration of radiation exposure.
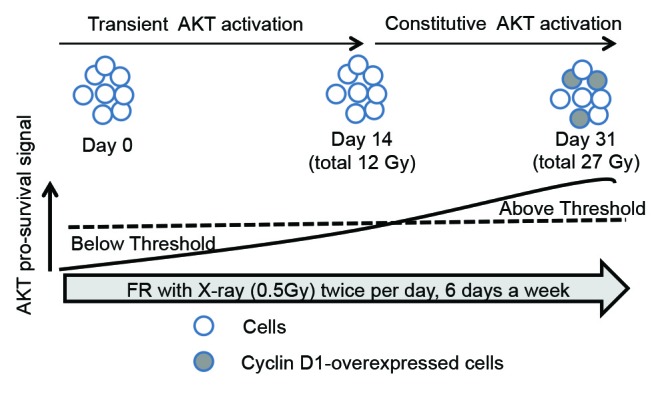
Figure 2. AKT radioresponse after 31-d FR. AKT pro-survival signals accumulate during exposure to FR. When these signals cross a threshold, the AKT response is changed from transient activation to constitutive activation after irradiation. Cyclin D1 is overexpressed in 31FR cells in which AKT is constitutively activated because of cumulative AKT pro-survival signals.
DNA-PK activates AKT in response to various genotoxic stresses, including low doses of radiation,27 and is the upstream target of the AKT pathway in 31FR cells.9 This epigenetic change in the DNA damage signaling pathway with DNA-PK/AKT/GSK3β-mediated cyclin D1 overexpression is irreversible, even after discontinuing FR for >1 mo. Cyclin D1-T286A that is mutated at the phosphorylation site on Thr286 resists radiation-induced cyclin D1 degradation by the ATM-FBXO31 pathway.23 This demonstrated that AKT-mediated cyclin D1 dephosphorylation on Thr286 invalidated ATM/FBXO31-mediated cyclin D1 degradation after 31 d FR.
Establishment of a Positive Feedback Loop Through the DNA-PK/AKT/GSK3β/Cyclin D1 Pathway by Replication-Associated DSBs Triggered by Cyclin D1 Overexpression
We previously reported that downregulation of cyclin D1 degradation resulted in persistent cyclin D1 expression during the S phase of 31FR cells.9 Deregulation of cyclin D1 expression perturbed DNA replication by inhibiting replication fork progression.22 Cyclin D1 has been shown to bind with the replication factor PCNA, a clamp loader of DNA polymerase.28-30 PCNA may recruit cyclin D1 to replication forks, and cyclin D1 binding to PCNA may inhibit replication fork movement in 31FR cells (Fig. 3). In response to aberrant replication forks induced by treatment with low-dose aphidicolin, an inhibitor of DNA polymerase α, DSBs were made by BLM helicase in cooperation with Mus81 nuclease for recovery.31 We also found that Mus81 controlled DSB formation in 31FR cells (Fig. 3).22 Using siRNAs, cyclin D1 or Mus81 knockdown decreased the amounts of DSBs in cyclin D1-overexpressing cells, whereas Cdk4 inactivation by siRNA or a CDK4 inhibitor of Cdk4-I had no effect.9,22 These results demonstrated that DSBs were mediated by cyclin D1 itself and did not require the activity of cyclin D1/Cdk4.
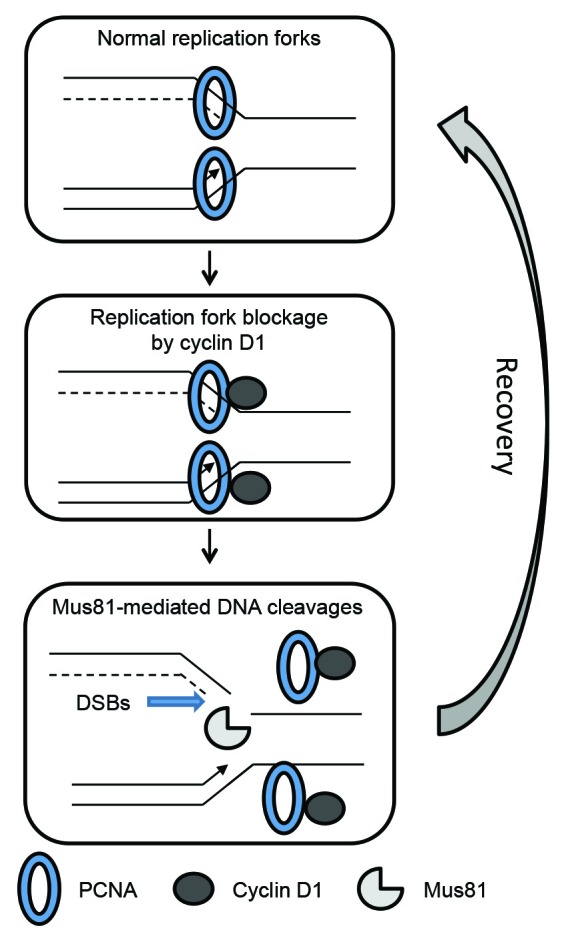
Figure 3. Induction of DSBs during the recovery of cyclin D1-mediated aberrant replication forks. PCNA, a clamp loader, is involved in the formation of the replication fork complex and regulates replication fork progression. Cyclin D1 binding to PCNA in a replication fork complex inhibits replication fork progression. Mus81 creates DSBs at a replication site to remove aberrant replication forks for the recovery from replication stress mediated by cyclin D1 overexpression. Replication forks are then reconstructed to resume DNA replication.
In contrast, overexpressing cyclin D1 using a cyclin D1 expression vector triggered DNA re-replication and induced DSBs in a Cdk4-dependent manner.21 Therefore, FR-induced DSBs were not induced by DNA re-replication, but were induced by suppressing replication fork movement. In addition to cyclin D1, cyclin A overexpression induces DSBs in human and mouse fibroblasts.32 Cyclin D1-mediated DSBs are also associated with induction of genomic instability in normal cells and increases cancer risk.
Figure 4 shows a schematic representation of DDR involving cyclin D1 in response to SR or 31 d FR. After SR with high doses, cyclin D1 is degraded by the ATM/FBXO31 pathway to inactivate Cdks. Cells are arrested at the G1/S boundary because of activation of a G1/S checkpoint. In contrast, cyclin D1 is stabilized after FR for 31 d by DNA-PK/AKT-mediated downregulation of its proteolysis. Persistent cyclin D1 expression during the S phase perturbs DNA replication and induces DSBs during the processing of abnormal DNA replication forks (Fig. 3). Cyclin D1-induced DSBs again activate the DNA-PK/AKT pathway, thus establishing a positive feedback loop of cyclin D1 overexpression cycle. Cyclin D1-induced DSBs are also associated with genomic instability and tumorigenesis in 31FR cells.
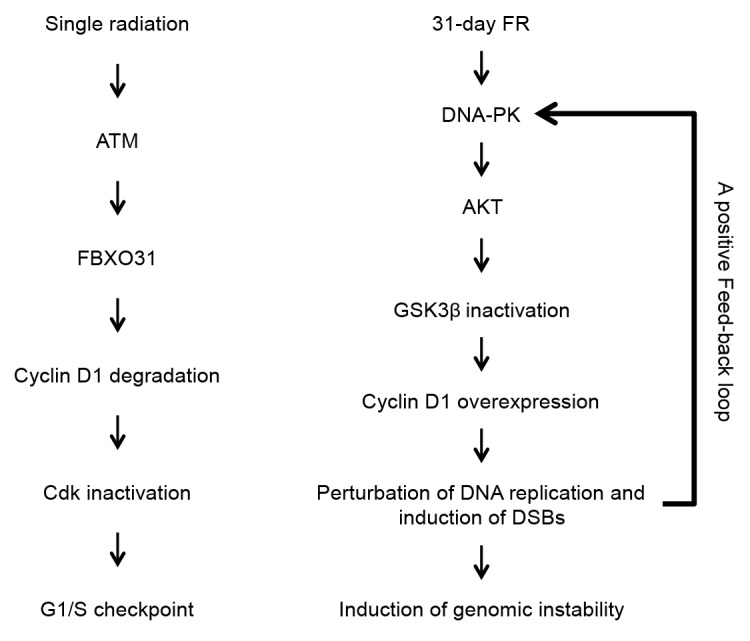
Figure 4. Cyclin D1 overexpression cycling in response to 31-d FR. After SR, cyclin D1 degradation causes Cdks inactivation as a G1/S checkpoint. In contrast, DNA-PK/AKT/GSK3β-mediated cyclin D1 overexpression results in perturbation of DNA replication and induction of DSBs. Cyclin D1-dependent DSBs establish a positive feedback loop for cyclin D1 overexpression cycling and the subsequent induction of genomic instability in 31FR cells.
Cyclin D1 as a Marker of Long-Term Exposure to Radiation
A biomarker is an indicator of a biological state that is defined as any measurement reflecting an interaction between a biological system and a genotoxic stress and is used to evaluate biological effects.33 Chromosomal aberrations are commonly used as IR biomarkers for radiation dose estimations, although the detection limit with this assay is <100 mGy. Radiation-induced DSBs are assessed by phosphorylation of H2AX (γ-H2AX), which can be detected with <20 mGy.34 However, it should be noted that DSBs are not unique to IR and can be induced by aging, oxidative stress, smoking, and certain chemicals. In addition, γ-H2AX has a disadvantage as a biomarker of long-term exposure to radiation, because it disappears when DSBs are repaired. Thus, another stable biomarker is needed to evaluate the effects of long-term exposure to radiation.
We are investigating suitable IR biomarkers from among molecules that are associated with DDRs of FR. Cyclin D1 is one candidate marker of FR, because cyclin D1 overexpression occurs after 31 d FR but not after SR and is long lasting even after FR is stopped. Further evaluation is needed to determine if cyclin D1 can be used as a biomarker of FR, especially by using normal human cells after long-term exposure to low doses of radiation.
A Role of Cyclin D1 Overexpression
Genetic aberrations in cell cycle regulators are frequently noted in human cancer and are linked to cancer development.35,36 Cyclin D1 overexpression is one of the most commonly observed alterations involving chromosomal translocations, gene amplification, and polymorphisms in various types of cancer cells.37 Cyclin D1 plays a role in controlling the transcription of several genes that regulate cell differentiation and proliferation by modulating the activities of transcription factors, co-activators, and co-repressors.38,39 Cyclin D1 is recruited to chromatin along with chromatin remodeling factors and alters acetylation and methylation of histones, which modulates the accessibility of transcription factors to chromatin. Cyclin D1 overexpression induces chromosomal instabilities, by interfering with the transcription of genes that govern the mitotic phase, and results in tumorigenesis.40,41
Overexpression of wild-type cyclin D1 has been shown to be insufficient for inducing cell transformation, as nuclear export and subsequent cytoplasmic proteolysis reduce the oncogenic potential of this protein.16,42 In contrast, overexpression of non-degradable cyclin D1-T286A results in tumorigenesis in transgenic mice.16,43-45 Thus, nuclear accumulation of cyclin D1 is associated with tumor-initiation event. Oncogene activation results in constitutive activation of the ATM-regulated DDR.46 ATM and DNA-PK activation due to cyclin D1 overexpression was also observed in 31FR and 31FR-31NR cells.9,22 Oncogene activation perturbs DNA replication and causes DSB accumulation because of replication stress and genomic instability in non-malignant cells.47-49 Therefore, cyclin D1 nuclear accumulation after exposure to 31 d FR possibly leads to the progression of malignancy in normal cells caused by the induction of genomic instability triggered by cyclin D1-dependent DSBs. On the other hand, cyclin D1 overexpression is considered as a marker of cellular senescence. It was reported that senescent cells express high levels of cyclin D1 in normal human fibroblasts.50-53
Targeting Cyclin D1 for Radioprotection
Accumulating evidence suggests that the PI3K/AKT signaling pathway regulates cell proliferation and survival processes that contribute to tumor progression.12,13,15 This pathway is upregulated after irradiation and is strongly correlated with radiosensitivity of irradiated cells. AKT can block apoptotic pathways by regulating various target molecules, including pro-apoptotic and anti-apoptotic proteins.15,54,55 Activated AKT, a common mediator of cell survival signals induced by radiation through multiple intracellular signaling pathways, modulates apoptosis and increases the apoptotic threshold.56,57 Thus, constitutive activation of the AKT/GSK3β/cyclin D1 pathway due to the accumulation of AKT survival signals results in radioresistance of 31FR cells.9
Cyclin D1 is considered a potential therapeutic target for cancer treatment.58,59 Aberrant cyclin D1 expression is often detected in premalignant and malignant tissues. Cyclin D1 overexpression is strongly correlated with a poor prognosis in oral carcinoma and head and neck squamous cell carcinoma after radiotherapy or chemoradiotherapy.60,61 Cyclin D1 levels are believed to be worth monitoring during the course of treatment to assess clinical responses. In addition to being a molecular target for cancer treatment, cyclin D1 is a selective molecular marker of long-term exposure to radiation in irradiated cells, where genomic instability is induced by cyclin D1 overexpression. API-2 was originally identified as a highly selective AKT inhibitor during screening by the National Cancer Institute and is considered an anticancer drug.62 Phase I and II clinical trials for API-2 have been conducted for advanced tumors.63,64 We also confirmed the efficacy of API-2 for suppressing the radioresistance of tumors derived from HepG2 and HeLa cells in animal experiments.65 We previously showed that inactivation of AKT/GSK3β-mediated cyclin D1 overexpression using an AKT inhibitor of API-2 abrogated cyclin D1 overexpression and rendered 31FR cells susceptible to radiation with increased apoptosis.9,65 Because API-2 facilitates GSK3β-mediated cyclin D1 proteolysis in 31FR and 31FR-31NR cells,9 it also can suppress tumoregenesis that is induced by cyclin D1 overexpression in normal cells. Therefore, targeting cyclin D1 is likely to cancel the effects of long-term exposure to FR that are caused by cyclin D1 overexpression.
Conclusions
We have described a unique DDR involving cyclin D1 after 31 d FR and its biological effects. Cyclin D1 overexpression generates DSBs during DNA replication and is associated with tumor initiation by inducing genomic instability in cells. Determination of cyclin D1 expression is proved to be a marker for monitoring long-term exposure of radiation. Furthermore, cyclin D1 is a molecular target for reducing the cancer risk posed by 31 d FR for radioprotection of humans.
Acknowledgments
We thank Prof Satish Kumar Adiga for his critical reading of the paper. The authors were supported by research grants from the Japanese Ministry of Education and Science Kiban C (24510063). This work was conducted at the Joint Usage/Research Center (RIRBM), Hiroshima University.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Sources of Support
Research grants from the Japanese Ministry of Education and Science Kiban C (24510063). This work was performed at the Joint Usage/ Research Center (RIRBM), Hiroshima University.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/25746
References
- 1.Elkind MM. Repair processes in radiation biology. Radiat Res. 1984;100:425–49. doi: 10.2307/3576409. [DOI] [PubMed] [Google Scholar]
- 2.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agami R, Bernards R. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell. 2000;102:55–66. doi: 10.1016/S0092-8674(00)00010-6. [DOI] [PubMed] [Google Scholar]
- 4.Cann KL, Hicks GG. Regulation of the cellular DNA double-strand break response. Biochem Cell Biol. 2007;85:663–74. doi: 10.1139/O07-135. [DOI] [PubMed] [Google Scholar]
- 5.Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell. 2007;28:739–45. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Lakin ND, Jackson SP. Regulation of p53 in response to DNA damage. Oncogene. 1999;18:7644–55. doi: 10.1038/sj.onc.1203015. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L, Yuan R, Serggio L. Molecular mechanisms of irradiation-induced apoptosis. Front Biosci. 2003;8:d9–19. doi: 10.2741/927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimura T, Kakuda S, Ochiai Y, Nakagawa H, Kuwahara Y, Takai Y, Kobayashi J, Komatsu K, Fukumoto M. Acquired radioresistance of human tumor cells by DNA-PK/AKT/GSK3beta-mediated cyclin D1 overexpression. Oncogene. 2010;29:4826–37. doi: 10.1038/onc.2010.238. [DOI] [PubMed] [Google Scholar]
- 10.Shimura T. Acquired radioresistance of cancer and the AKT/GSK3β/cyclin D1 overexpression cycle. J Radiat Res. 2011;52:539–44. doi: 10.1269/jrr.11098. [DOI] [PubMed] [Google Scholar]
- 11.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 13.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filmus J, Robles AI, Shi W, Wong MJ, Colombo LL, Conti CJ. Induction of cyclin D1 overexpression by activated ras. Oncogene. 1994;9:3627–33. [PubMed] [Google Scholar]
- 15.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–95. doi: 10.1016/S0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 16.Alt JR, Cleveland JL, Hannink M, Diehl JA. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 2000;14:3102–14. doi: 10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massagué J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 18.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 19.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 20.Yang K, Guo Y, Stacey WC, Harwalkar J, Fretthold J, Hitomi M, Stacey DW. Glycogen synthase kinase 3 has a limited role in cell cycle regulation of cyclin D1 levels. BMC Cell Biol. 2006;7:33. doi: 10.1186/1471-2121-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aggarwal P, Lessie MD, Lin DI, Pontano L, Gladden AB, Nuskey B, Goradia A, Wasik MA, Klein-Szanto AJ, Rustgi AK, et al. Nuclear accumulation of cyclin D1 during S phase inhibits Cul4-dependent Cdt1 proteolysis and triggers p53-dependent DNA rereplication. Genes Dev. 2007;21:2908–22. doi: 10.1101/gad.1586007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimura T, Ochiai Y, Noma N, Oikawa T, Sano Y, Fukumoto M. Cyclin D1 overexpression perturbs DNA replication and induces replication-associated DNA double-strand breaks in acquired radioresistant cells. Cell Cycle. 2013;12:773–82. doi: 10.4161/cc.23719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santra MK, Wajapeyee N, Green MR. F-box protein FBXO31 mediates cyclin D1 degradation to induce G1 arrest after DNA damage. Nature. 2009;459:722–5. doi: 10.1038/nature08011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Arai S, Song X, Reichart D, Du K, Pascual G, Tempst P, Rosenfeld MG, Glass CK, Kurokawa R. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454:126–30. doi: 10.1038/nature06992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan J, Geng L, Yazlovitskaya EM, Hallahan DE. Protein kinase B/Akt-dependent phosphorylation of glycogen synthase kinase-3beta in irradiated vascular endothelium. Cancer Res. 2006;66:2320–7. doi: 10.1158/0008-5472.CAN-05-2700. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins AJ, Golding SE, Khalil A, Valerie K. DNA double-strand break - induced pro-survival signaling. Radiother Oncol. 2011;101:13–7. doi: 10.1016/j.radonc.2011.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell. 2008;30:203–13. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Fukami-Kobayashi J, Mitsui Y. Cyclin D1 inhibits cell proliferation through binding to PCNA and cdk2. Exp Cell Res. 1999;246:338–47. doi: 10.1006/excr.1998.4306. [DOI] [PubMed] [Google Scholar]
- 29.Prosperi E, Scovassi AI, Stivala LA, Bianchi L. Proliferating cell nuclear antigen bound to DNA synthesis sites: phosphorylation and association with cyclin D1 and cyclin A. Exp Cell Res. 1994;215:257–62. doi: 10.1006/excr.1994.1341. [DOI] [PubMed] [Google Scholar]
- 30.Xiong Y, Zhang H, Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992;71:505–14. doi: 10.1016/0092-8674(92)90518-H. [DOI] [PubMed] [Google Scholar]
- 31.Shimura T, Torres MJ, Martin MM, Rao VA, Pommier Y, Katsura M, Miyagawa K, Aladjem MI. Bloom’s syndrome helicase and Mus81 are required to induce transient double-strand DNA breaks in response to DNA replication stress. J Mol Biol. 2008;375:1152–64. doi: 10.1016/j.jmb.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tane S, Chibazakura T. Cyclin A overexpression induces chromosomal double-strand breaks in mammalian cells. Cell Cycle. 2009;8:3900–3. doi: 10.4161/cc.8.23.10071. [DOI] [PubMed] [Google Scholar]
- 33.Pernot E, Hall J, Baatout S, Benotmane MA, Blanchardon E, Bouffler S, El Saghire H, Gomolka M, Guertler A, Harms-Ringdahl M, et al. Ionizing radiation biomarkers for potential use in epidemiological studies. Mutat Res. 2012;751:258–86. doi: 10.1016/j.mrrev.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Lassmann M, Hänscheid H, Gassen D, Biko J, Meineke V, Reiners C, Scherthan H. In vivo formation of gamma-H2AX and 53BP1 DNA repair foci in blood cells after radioiodine therapy of differentiated thyroid cancer. J Nucl Med. 2010;51:1318–25. doi: 10.2967/jnumed.109.071357. [DOI] [PubMed] [Google Scholar]
- 35.Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, Barnes D, Peters G. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 1994;54:1812–7. [PubMed] [Google Scholar]
- 36.Russell A, Thompson MA, Hendley J, Trute L, Armes J, Germain D. Cyclin D1 and D3 associate with the SCF complex and are coordinately elevated in breast cancer. Oncogene. 1999;18:1983–91. doi: 10.1038/sj.onc.1202511. [DOI] [PubMed] [Google Scholar]
- 37.Diehl JA. Cycling to cancer with cyclin D1. Cancer Biol Ther. 2002;1:226–31. doi: 10.4161/cbt.72. [DOI] [PubMed] [Google Scholar]
- 38.Fu M, Rao M, Bouras T, Wang C, Wu K, Zhang X, Li Z, Yao TP, Pestell RG. Cyclin D1 inhibits peroxisome proliferator-activated receptor gamma-mediated adipogenesis through histone deacetylase recruitment. J Biol Chem. 2005;280:16934–41. doi: 10.1074/jbc.M500403200. [DOI] [PubMed] [Google Scholar]
- 39.Hulit J, Wang C, Li Z, Albanese C, Rao M, Di Vizio D, Shah S, Byers SW, Mahmood R, Augenlicht LH, et al. Cyclin D1 genetic heterozygosity regulates colonic epithelial cell differentiation and tumor number in ApcMin mice. Mol Cell Biol. 2004;24:7598–611. doi: 10.1128/MCB.24.17.7598-7611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casimiro MC, Pestell RG. Cyclin d1 induces chromosomal instability. Oncotarget. 2012;3:224–5. doi: 10.18632/oncotarget.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lung JC, Chu JS, Yu JC, Yue CT, Lo YL, Shen CY, Wu CW. Aberrant expression of cell-cycle regulator cyclin D1 in breast cancer is related to chromosomal genomic instability. Genes Chromosomes Cancer. 2002;34:276–84. doi: 10.1002/gcc.10072. [DOI] [PubMed] [Google Scholar]
- 42.Quelle DE, Ashmun RA, Shurtleff SA, Kato JY, Bar-Sagi D, Roussel MF, Sherr CJ. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–71. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 43.Benzeno S, Lu F, Guo M, Barbash O, Zhang F, Herman JG, Klein PS, Rustgi A, Diehl JA. Identification of mutations that disrupt phosphorylation-dependent nuclear export of cyclin D1. Oncogene. 2006;25:6291–303. doi: 10.1038/sj.onc.1209644. [DOI] [PubMed] [Google Scholar]
- 44.Gladden AB, Diehl JA. Location, location, location: the role of cyclin D1 nuclear localization in cancer. J Cell Biochem. 2005;96:906–13. doi: 10.1002/jcb.20613. [DOI] [PubMed] [Google Scholar]
- 45.Lin DI, Lessie MD, Gladden AB, Bassing CH, Wagner KU, Diehl JA. Disruption of cyclin D1 nuclear export and proteolysis accelerates mammary carcinogenesis. Oncogene. 2008;27:1231–42. doi: 10.1038/sj.onc.1210738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mallette FA, Gaumont-Leclerc MF, Ferbeyre G. The DNA damage signaling pathway is a critical mediator of oncogene-induced senescence. Genes Dev. 2007;21:43–8. doi: 10.1101/gad.1487307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–7. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 48.Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre’ M, Nuciforo PG, Bensimon A, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–42. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 49.Murfuni I, Nicolai S, Baldari S, Crescenzi M, Bignami M, Franchitto A, et al. The WRN and MUS81 proteins limit cell death and genome instability following oncogene activation. Oncogene. 2013;32:610–20. doi: 10.1038/onc.2012.80. [DOI] [PubMed] [Google Scholar]
- 50.Dulić V, Drullinger LF, Lees E, Reed SI, Stein GH. Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-Cdk2 and cyclin D1-Cdk2 complexes. Proc Natl Acad Sci U S A. 1993;90:11034–8. doi: 10.1073/pnas.90.23.11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lucibello FC, Sewing A, Brüsselbach S, Bürger C, Müller R. Deregulation of cyclins D1 and E and suppression of cdk2 and cdk4 in senescent human fibroblasts. J Cell Sci. 1993;105:123–33. doi: 10.1242/jcs.105.1.123. [DOI] [PubMed] [Google Scholar]
- 52.Atadja P, Wong H, Veillete C, Riabowol K. Overexpression of cyclin D1 blocks proliferation of normal diploid fibroblasts. Exp Cell Res. 1995;217:205–16. doi: 10.1006/excr.1995.1080. [DOI] [PubMed] [Google Scholar]
- 53.Leontieva OV, Lenzo F, Demidenko ZN, Blagosklonny MV. Hyper-mitogenic drive coexists with mitotic incompetence in senescent cells. Cell Cycle. 2012;11:4642–9. doi: 10.4161/cc.22937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duronio V. The life of a cell: apoptosis regulation by the PI3K/PKB pathway. Biochem J. 2008;415:333–44. doi: 10.1042/BJ20081056. [DOI] [PubMed] [Google Scholar]
- 55.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, Hagan MP, Grant S, Schmidt-Ullrich R. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:SARIAO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt-Ullrich RK, Contessa JN, Dent P, Mikkelsen RB, Valerie K, Reardon DB, Bowers G, Lin PS. Molecular mechanisms of radiation-induced accelerated repopulation. Radiat Oncol Investig. 1999;7:321–30. doi: 10.1002/(SICI)1520-6823(1999)7:6<321::AID-ROI2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 58.Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–72. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 60.Jayasurya R, Francis G, Kannan S, Lekshminarayanan K, Nalinakumari KR, Abraham T, Abraham EK, Nair MK. p53, p16 and cyclin D1: molecular determinants of radiotherapy treatment response in oral carcinoma. Int J Cancer. 2004;109:710–6. doi: 10.1002/ijc.20042. [DOI] [PubMed] [Google Scholar]
- 61.Higuchi E, Oridate N, Homma A, Suzuki F, Atago Y, Nagahashi T, Furuta Y, Fukuda S. Prognostic significance of cyclin D1 and p16 in patients with intermediate-risk head and neck squamous cell carcinoma treated with docetaxel and concurrent radiotherapy. Head Neck. 2007;29:940–7. doi: 10.1002/hed.20632. [DOI] [PubMed] [Google Scholar]
- 62.Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, Hamilton AD, Polokoff M, Nicosia SV, Herlyn M, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–9. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- 63.Feun LG, Blessing JA, Barrett RJ, Hanjani P. A phase II trial of tricyclic nucleoside phosphate in patients with advanced squamous cell carcinoma of the cervix. A Gynecologic Oncology Group Study. Am J Clin Oncol. 1993;16:506–8. doi: 10.1097/00000421-199312000-00010. [DOI] [PubMed] [Google Scholar]
- 64.Feun LG, Savaraj N, Bodey GP, Lu K, Yap BS, Ajani JA, Burgess MA, Benjamin RS, McKelvey E, Krakoff I. Phase I study of tricyclic nucleoside phosphate using a five-day continuous infusion schedule. Cancer Res. 1984;44:3608–12. [PubMed] [Google Scholar]
- 65.Shimura T, Kakuda S, Ochiai Y, Kuwahara Y, Takai Y, Fukumoto M. Targeting the AKT/GSK3β/cyclin D1/Cdk4 survival signaling pathway for eradication of tumor radioresistance acquired by fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2011;80:540–8. doi: 10.1016/j.ijrobp.2010.12.065. [DOI] [PubMed] [Google Scholar]


