Abstract
The XPA1 human pancreatic cancer cell line is dimorphic, with spindle stem-like cells and round non-stem cells. We report here the in vitro IC50 values of stem-like and non-stem XPA1 human pancreatic cells cells for: (1) 5-fluorouracil (5-FU), (2) cisplatinum (CDDP), (3) gemcitabine (GEM), and (4) tumor-targeting Salmonella typhimurium A1-R (A1-R). IC50 values of stem-like XPA1 cells were significantly higher than those of non-stem XPA1 cells for 5-FU (P = 0.007) and CDDP (P = 0.012). In contrast, there was no difference between the efficacy of A1-R on stem-like and non-stem XPA1 cells. In vivo, 5-FU and A1-R significantly reduced the tumor weight of non-stem XPA1 cells (5-FU; P = 0.028; A1-R; P = 0.011). In contrast, only A1-R significantly reduced tumor weight of stem-like XPA1 cells (P = 0.012). The combination A1-R with 5-FU improved the antitumor efficacy compared with 5-FU monotherapy on the stem-like cells (P = 0.004). The results of the present report indicate A1-R is a promising therapy for chemo-resistant pancreatic cancer stem-like cells.
Keywords: Salmonella typhimurium, amino acid auxotrophy, selective tumor targeting, pancreatic cancer, stem cell, chemoresistance, RFP, GFP, fluorescence imaging, confocal microscopy
Introduction
Previously, we reported that XPA1 human pancreatic cancer cells are dimorphic, round, or spindle-shaped. The spindle-shaped cancer cells had the greater capability for distant metastasis and ascites formation, suggesting they are stem-like cells, which can be readily targeted for therapy.1
A tumor-targeting strain of S. typhimurium, A1-R, has been developed by our laboratory. S. typhimurium A1-R is auxotrophic for Leu-Arg, which prevents it from mounting a continuous infection in normal tissues. A1-R has no other attenuating mutations and, therefore, has very high tumor-targeting capability. A1-R was able to eradicate primary and metastatic tumors in monotherapy in nude mouse models of prostate, breast, and pancreatic cancer, as well as sarcoma and glioma.2-9
In the present study, we compared the efficacy of chemotherapy and S. typhimurium A1-R on the stem-like spindle and round non-stem cells of XPA1 pancreatic cancer cells.
Results and Discussion
Stem-like and non-stem XPA1 cells have a different drug-sensitivity profile
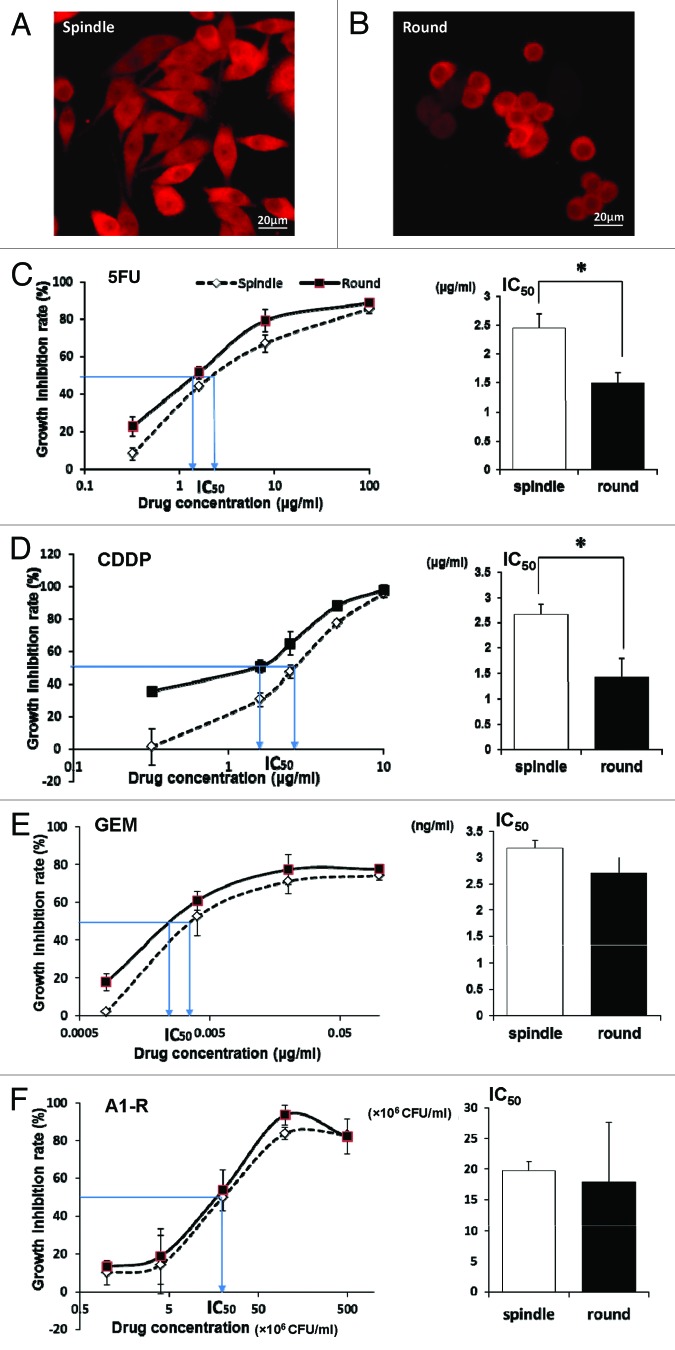
Stem-like spindle XPA1 cells spread throughout the surface of the culture flask, while non-stem round XPA1 cells tended to grow in a more clumped pattern (Fig. 1A and B).
Figure 1. XPA1 human pancreatic cancer cells are dimorphic. Stem-like XPA1 cells are spindle-shaped and spread throughout the surface of the culture flask (A), while non-stem XPA1 cells are round and tended to grow in a more clumped pattern (B). Dose response curves and the IC50 for different chemotherapeutic drugs for each morphological type of XPA1 pancreatic carcinoma cells (C–F). Stem-like XPA1 cells were resistant to 5-FU and CDDP compared with non-stem XPA1 cells (C and D). In contrast, there was no difference between the efficacy of A1-R on stem-like and non-stem XPA1 cells (F). Scale bars, 20 μm. *P < 0.05.
To determine the differences in the chemo-sensitivity behavior of the stem-like and non-stem XPA1 subtypes, we determined the IC50 for: (1) 5-FU, (2) cisplatinum (CDDP), (3) gemcitabine (GEM), and (4) A1-R. IC50 values of stem-like and non-stem XPA1 cells were 2.44 ± 0.25 μg/ml and 1.48 ± 0.19 μg/ml, respectively, for 5-FU (P = 0.007); 2.65 ± 0.22 μg/ml and 1.43 ± 0.36 μg/ml, respectively, for CDDP (P = 0.012); 3.17 ± 0.15 ng/ml and 2.70 ± 0.29 ng/ml, respectively, for GEM (P = 0.133); and (19.7 ± 1.46) × 106 colony forming units (CFU)/ml and (17.8 ± 9.78) × 106 CFU/ml for A1-R, respectively, (P = 0.771) (Fig. 1C–F). Stem-like XPA1 cells had significantly greater resistance to 5-FU and CDDP compared with non-stem XPA1 cells. In contrast, there was no difference between the efficacy of A1-R for stem-like and non-stem XPA1 cells (Table 1).
Table 1. Different drug-sensitivity profiles between stem-like and non-stem XPA1 cells in vitro.
| Treatment | IC50 of stem-like cells | IC50 of non-stem cells | P value |
|---|---|---|---|
| 5-FU | 2.44 ± 0.25 μg/ml | 1.48 ± 0.19 μg/ml | P = 0.007 |
| CDDP | 2.65 ± 0.22 μg/ml | 1.43 ± 0.36 μg/ml | P = 0.012 |
| GEM | 3.17 ± 0.15 ng/ml | 2.70 ± 0.29 ng/ml | N.S |
| A1-R | (19.7 ± 1.46) × 106 CFU/ml | (17.8 ± 9.78) × 106 CFU/ml | N.S |
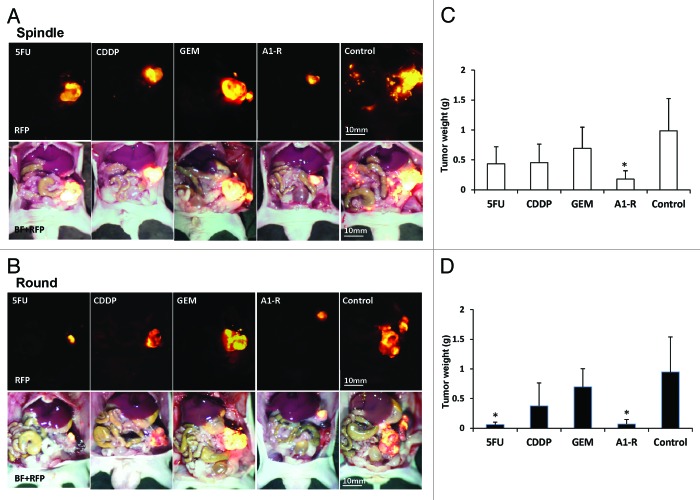
Next, we investigated the efficacy of the chemotherapeutic drugs and A1-R for stem-like and non-stem XPA1 cells in vivo. The tumor weight of non-stem XPA1 cells was 0.060 ± 0.043 g after 5-FU treatment; 0.376 ± 0.386 g after CDDP treatment; 0.696 ± 0.309 g after GEM treatment; 0.070 ± 0.075 g after A1-R treatment; and 0.948 ± 0.591 g for untreated control. 5-FU and A1-R significantly reduced tumor weight of XPA1 non-stem cells compared with untreated control (5-FU; P = 0.028; A1-R; P = 0.011) (Fig. 2B and D and Table 2). In contrast, the tumor weight of stem-like XPA1 cells was 0.436 ± 0.283 g after 5-FU treatment; 0.454 ± 0.310 g after CDDP treatment; 0.692 ± 0.354 g after GEM treatment; 0.178 ± 0.140 g after A1-R treatment; and 0.986 ± 0.539 g for untreated control. Only A1-R significantly reduced the tumor weight of stem-like XPA1 cells (P = 0.012) (Fig. 2A and C and Table 2).
Figure 2. Chemotherapy of both morphological types of XPA1 cells in vivo. (A and B) Images of tumors at termination. (C and D) Bar graphs of tumor weight. 5-FU and A1-R significantly reduced the tumor weight of round non-stem XPA1 cells (5-FU; P = 0.028, A1-R; P = 0.011) (B and D), whereas only A1-R significantly reduced the tumor weight of spindle-shaped stem-like XPA1 cells compared with control (P = 0.012) (A and C). *P < 0.05 (vs. control group). BF, bright field.
Table 2. Efficacy of chemotherapeutic drugs and A1-R on stem-like and non-stem XPA1 tumors.
| Treatment | Tumor weight of stem-like cells (g) | P value* | Tumor weight of non-stem cells (g) | P value* |
|---|---|---|---|---|
| 5-FU | 0.436 ± 0.283 | N.S | 0.060 ± 0.043 | P = 0.028 |
| CDDP | 0.454 ± 0.310 | N.S | 0.376 ± 0.386 | N.S |
| GEM | 0.692 ± 0.354 | N.S | 0.696 ± 0.309 | N.S |
| A1-R | 0.178 ± 0.140 | P = 0.012 | 0.070 ± 0.075 | P = 0.011 |
| Control | 0.986 ± 0.539 | ― | 0.948 ± 0.591 | ― |
*Compared with control.
Confocal imaging of cancer cells infected with S. typhimurium A1-R in vitro and in vivo
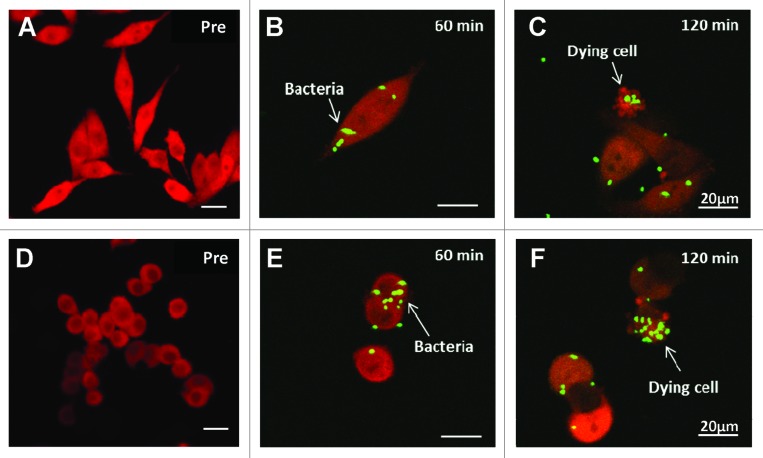
The interaction between A1-R expressing green fluorescent protein (GFP) and XPA1 pancreatic cancer cells labeled with red fluorescent protein (RFP) in the cytoplasm was observed with the Fluoview FV1000 confocal microscope (Olympus Corp) (Fig. 3). GFP-expressing A1-R invaded the stem-like and non-stem XPA1 pancreatic cancer cells expressing RFP as early as 60 min after infection (Fig. 3B and E). The stem-like and non-stem XPA1 cells appeared apoptotic within 120 min after bacterial infection (Fig. 3C and F). This result demonstrated virulence of A1-R for both stem-like and non-stem XPA1 pancreatic cancer cells.
Figure 3. Confocal imaging of stem-like (A–C) and non-stem (D–F) XPA1 pancreatic cancer cells infected with A1-R, expressing GFP, in vitro. XPA1 pancreatic cancer cell death was induced by A1-R targeting in vitro. A1-R infection was detected in both stem-like and non-stem XPA1 cells after 60 min (B and E, respectively). A1-R induced apoptosis in both stem-like and non-stem XPA1 cells (C and F, respectively). Scale bars, 20 μm. Pre, pre-infection.
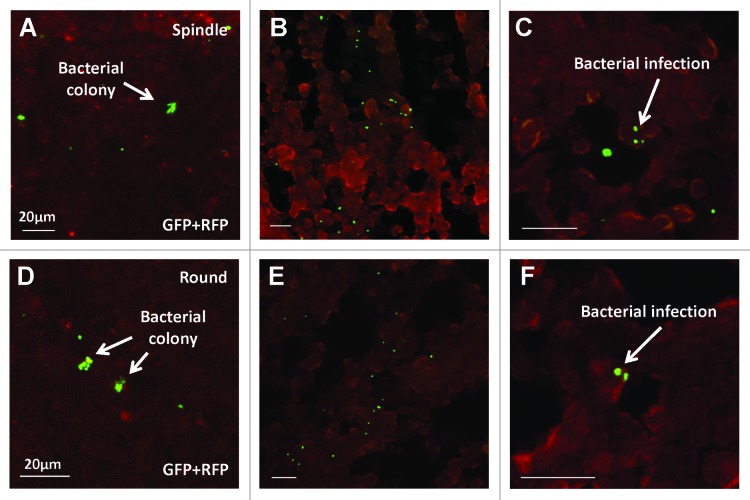
Bacterial colonies were detected in both stem-like (Fig. 4A) and non-stem (Fig. 4D) tumors in vivo after i.p. injection of A1-R. Frozen section microscopy showed A1-R infiltrated into both stem-like (Fig. 4B and C) and non-stem XPA1 cells (Fig. 4E and F). These results suggested that A1-R injected i.p. could survive in the nude mice and infiltrate into the stem-like and non-stem pancreatic cancer cells, resulting in cancer cell death.
Figure 4. Confocal imaging of cancer cells infected with A1-R, expressing GFP, in vivo. Bacterial colonies were detected in both stem-like (A) and non-stem (D) tumors. Frozen section microscopy showed infiltrating A1-R in both spindle-shaped stem-like (B and C) and round non-stem cells (E and F). (C and F) are high magnification images of (B and E), respectively. Scale bars, 20 μm.
Efficacy of 5-FU and 5-FU+A1-R combination on chemo-resistant stem-like XPA1 tumors
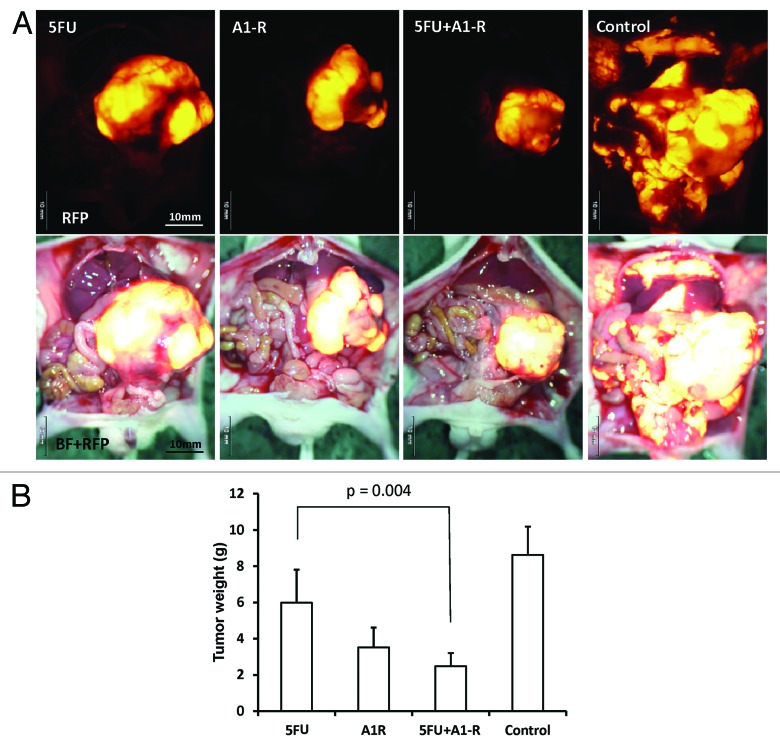
To examine the efficacy and the toxicity of A1-R in combination with other chemotherapeutic drugs, nude mice were orthotopically implanted with stem-like XPA1 cells and treated with the following drugs: (1) 5-FU (10 mg/kg, ip); (2) A1-R (1.5 × 108 CFU/body, ip); (3) 5-FU (6.5 mg/kg, ip) + A1-R (1.0 × 108 CFU/body, ip); and (4) saline (vehicle/control, ip). 5-FU and bacteria were injected weekly from day 21 after tumor implantation for 7 wk. Each treatment arm involved 8 tumor-bearing mice. No significant effects on body weight, morbidity, or severe toxicities were observed in any treatment arm. Animals were sacrificed at 10 wk, and tumors were weighed. Tumor weight was 5.982 ± 1.829 g for 5-FU; 3.518 ± 1.096 g for A1-R; 2.482 ± 0.722 g for 5-FU+A1-R; and 8.628 ± 1.560 g for saline. Every treatment significantly reduced the tumor weight compared with control (5-FU; P = 0.039; A1-R; P < 0.001; 5-FU+A1-R; P < 0.001); (Fig. 5A and B). The combination therapy (5-FU+A1-R) significantly reduced tumor weight compared with 5-FU (P = 0.004) (Table 3), even though the dosage of each drug was reduced compared with monotherapy (Fig. 5B).
Figure 5. Combination chemotherapy efficacy for stem-like XPA1 cells in vivo. (A) Images of tumors at termination. (B) Bar graphs of tumor weight. Every group significantly reduced tumor weight compared with control (5-FU, P = 0.039; A1-R, P < 0.001; 5-FU + A1-R, P < 0.001). Combination therapy (5-FU + A1-R) significantly reduced tumor weight compared with 5-FU (P = 0.004), even though the dosage of each drug was reduced compared with monotherapy (B) BF, brightfield.
Table 3. Efficacy of 5-FU and 5-FU+A1-R combination on chemo-resistant stem-like XPA1 tumors.
| Treatment | Tumor weight (g) | P value |
|---|---|---|
| 5-FU | 5.982 ± 1.829 | P = 0.039* |
| A1-R | 3.518 ± 1.096 | P < 0.001* |
| 5-FU+A1-R | 2.482 ± 0.722 | P < 0.001*, P = 0.004** |
| Control | 8.628 ± 1.560 | ― |
Compared with control, **compared with 5-FU.
Jia et al.10 demonstrated that the combination of Salmonella typhimurium and cyclophosphamide (CTX) significantly improved the efficacy of CTX and increased the number of bacteria within tumors.
In the present study, the combination A1-R with 5-FU had no adverse effects and improved the antitumor efficacy compared with monotherapy, even though the dosage of each agent was reduced (Fig. 5B). These findings suggest that the combination A1-R with chemotherapeutic agents may increase the efficacy and decrease the toxicity of conventional chemotherapy.
In conclusion, the stem-like XPA1 cells were resistant to chemotherapy but sensitive to A1-R. Thus, A1-R has promise for therapy of pancreatic cancer stem-like cells which are resistant to conventional chemotherapy. The combination of A1-R with chemotherapeutic agents has clinical potential for the treatment of pancreatic cancer stem cells.
Materials and Methods
Cell line and culture conditions
The XPA1 human pancreatic cancer cell line was a kind gift from Dr Anirban Maitra at Johns Hopkins University. Cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 2 mM glutamine from Gibco-BRL (Life Technologies Inc, Grand). All media were supplemented with penicillin and streptomycin (Gibco-BRL). Cells were cultured at 37 °C with 5% CO2.11
Production of RFP retroviral vector
The RFP (DsRed-2) gene (Clontech Laboratories) was inserted in the retroviral-based mammalian expression vector pLNCX (Clontech) to form the pLNCX DsRed-2 vector. Production of retrovirus resulted from transfection of pLNCX DsRed-2 in PT67 packaging cells, which produced retroviral supernatants containing the DSRed-2 gene. Briefly, PT67 cells were grown as monolayers in DMEM supplemented with 10% FCS (Gemini Bio-Products). Exponentially-growing cells (in 10 cm dishes) were transfected with 10 μg expression vector using Lipofectamine Plus (Life Technologies). Transfected cells were replated 48 h after transfection and 100 μg/ml G418 were added 7 h after transfection. Two days later, the amount of G418 was increased to 200 μg/ml. During the drug-selection period, surviving colonies were visualized under fluorescence microscopy (IX71 Inverted Microscope, Olympus), and RFP-positive colonies were isolated.12,13
RFP gene transduction of XPA1 cells
For RFP gene transduction, 20% confluent XPA1 cells were incubated with a 1:1 precipitated mixture of retroviral-containing supernatants of PT67 cells and RPMI 1640 or other culture media (Life Technologies) containing 10% FBS (Gemini Bio-Products) for 72 h. Fresh medium was replenished at this time. Cancer cells were harvested with trypsin/EDTA and subcultured at a ratio of 1:15 into selective medium, which contained 50 μg/ml G418. To select brightly fluorescent cells, the level of G418 was increased to 800 μg/ml in a stepwise manner. Clones of cancer cells expressing RFP were isolated with cloning cylinders (Bel-Art Products) by trypsin/EDTA and amplified and transferred by conventional culture methods in the absence of a selective agent.12,13
Preparation of bacteria
The S. typhimurium A1-R bacteria expressing GFP were grown overnight on LB medium and then diluted 1:10 in LB medium. Bacteria were harvested at late-log phase, washed with PBS, and then diluted in PBS. Bacteria were then used for in vitro and in vivo experiments.2
IC50 of chemotherapeutic agents
XPA1 human pancreatic cancer cells are dimorphic. Serial dilution was performed to obtain clones of each cellular morphology for further analysis. Each morphological type of XPA1 cells, in the exponential growth phase, was trypsinized to yield a cell suspension of 5 × 104 cells/ml and seeded onto 96-well plates (0.5–1 × 104 cells/well), in triplicate. Chemotherapeutic drugs were added after the cells were allowed to adhere for 24 h. The final concentrations of the chemotherapeutic drugs and S. typhimurium A1-R were as follows: 5-FU (Kyowa Hakko Kogyo), 0.32, 1.6, 8, 40, and 200 μg/ml; CDDP (Nippon Kayaku Co), 0.064, 0.32, 1.6, 8, and 40 μg/ml; GEM (Eli Lilly), 0.00016, 0.0008, 0.004, 0.02, and 0.1 μg/ml and S. typhimurium A1-R, 1, 4, 20, 100, and 500 × 106 colony forming units (CFU)/ml. After the plates were incubated at 37 °C in 5% CO2 for 48 h, MTS assays were performed at various time points using the Cell Titer 96 Assay (Promega) according to the manufacturer’s instructions.
The growth inhibition rates of the different drugs and concentrations were calculated by the following formula:
Inhibition rate = (A1 value of negative control – A value of experimental)/(A1 value of negative control – A0 value of blank) × 100%.
The IC50 value of each drug was calculated by the Karber method.14
Confocal imaging of cancer cells infected with S. typhimurium A1-R in vitro and in vivo
XPA1 pancreatic cancer cell death was induced by S. typhimurium A1-R targeting in vitro. Each morphological type of XPA1 pancreatic cancer cells was grown in 24-well tissue culture plates to a density of approximately 104 cells/well. A1-R was grown to late log in LB broth, diluted in cell culture medium, and added to the cancer cells (1 × 105 CFU/ml) and incubated at 37 °C. After 40 min, the cells were rinsed and cultured in medium containing gentamycin sulfate (20 μg/ml) to kill external, but not internal bacteria. The interaction of A1-R, expressing GFP, with cancer cells, expressing RFP, in vitro was observed with confocal microscopy (Fluoview FV1000, Olympus). Excitation sources were semiconductor lasers at 473 nm for GFP and 559 nm for RFP excitation. Fluorescence images were obtained using the 20×/1.0 XLUMPLFLN objective.15 In vivo, the resected specimens were embedded by optimal cutting temperature (OCT) compound (Tissue-Tek; Sakura Finetek Europe BV) and preserved in liquid nitrogen. Frozen sections of 7–10 μm thickness were prepared with a CM1850 cryostat (Leica). The frozen sections were directly observed with the FV1000.
Animal care
Nude (nu/nu) mice (AntiCancer Inc) were bred and maintained in a HEPA-filtered environment with cages, chow, and bedding sterilized by autoclaving. The animal diets were obtained from Harlan Teklad. Ampicillin (5.0%, w/v; Sigma) was added to the autoclaved drinking water. All surgical procedures and imaging were performed with the animal anesthetized by intramuscular injection of 0.02 ml of a solution of 50% ketamine, 38% xylazine, and 12% acepromazine maleate. All animal studies were conducted in accordance with the principles of and procedures outlined in the NIH guide for the care and use of laboratory animals under assurance number A3873-1.
Subcutaneous cancer cell implantation
Human XPA1-RFP pancreatic cancer cells were harvested by trypsinization and washed twice with serum-free medium. Cells (2 × 106 in 100 ml serum-free media) were injected subcutaneously, within 30 min of harvesting, over the right and left flanks in male nu/nu mice between 4 and 6 wk of age. Subcutaneous tumors were allowed to grow for 2–4 wk until large enough to supply adequate tumor to harvest for subsequent orthotopic implantation.
Orthotopic tumor implantation
Orthotopic human pancreatic cancer xenografts were established in nude mice, anesthetized as described above, by surgical orthotopic implantation (SOI) of fluorescent XPA1-RFP tumor fragments into the pancreas. A small 6 to 10 mm transverse incision was made on the left flank of the mouse through the skin and peritoneum. The tail of the pancreas was exposed through this incision and a single 1 mm3 tumor fragment from a XPA1-RFP subcutaneous tumor was sutured to the tail of the pancreas using 8–0 nylon surgical sutures (Ethilon; Ethicon Inc). On completion, the tail of the pancreas was returned to the abdomen, and the incision was closed in one layer using 6–0 nylon surgical sutures (Ethilon).16-21
Chemotherapy
Nude mice were orthotopically implanted with each morphological type of XPA1 as described above. The mice were treated in the following groups: (1) 5-FU (10 mg/kg, ip); (2) CDDP (5 mg/kg, ip); (3) GEM (150 mg/kg, ip); (4) A1-R (1.5 × 108 CFU/body, ip); and (5) saline (vehicle/control, ip). Chemotherapeutic drugs were injected weekly from day 21 after tumor implantation for 4 and 7 wk. Each treatment arm involved 8 tumor-bearing mice. No significant effects on body weight, morbidity, or severe toxicities were observed in any treatment arm. Animals were sacrificed at 7 wk, and tumors were weighed and harvested for analysis. GFP and RFP fluorescence was imaged using the OV100 variable magnification Small Animal Imaging System (Olympus)22 and the FV1000 confocal microscope (Olympus).15
Acknowledgment
This study was supported in part by National Cancer Institute grant CA126023 and Grants-in-Aid from the Japanese Ministry of Education, Culture, Sports, Science, and Technology for Fundamental Research (#23592018 to IE and # 24592009 to KT). This paper is dedicated to the memory of AR Moossa, MD.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/25872
References
- 1.Hassanein MK, Suetsugu A, Saji S, Moriwaki H, Bouvet M, Moossa AR, Hoffman RM. Stem-like and non-stem human pancreatic cancer cells distinguished by morphology and metastatic behavior. J Cell Biochem. 2011;112:3549–54. doi: 10.1002/jcb.23282. [DOI] [PubMed] [Google Scholar]
- 2.Zhao M, Yang M, Ma H, Li X, Tan X, Li S, Yang Z, Hoffman RM. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006;66:7647–52. doi: 10.1158/0008-5472.CAN-06-0716. [DOI] [PubMed] [Google Scholar]
- 3.Zhao M, Suetsugu A, Ma H, Zhang L, Liu F, Zhang Y, Tran B, Hoffman RM. Efficacy against lung metastasis with a tumor-targeting mutant of Salmonella typhimurium in immunocompetent mice. Cell Cycle. 2012;11:187–93. doi: 10.4161/cc.11.1.18667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci U S A. 2007;104:10170–4. doi: 10.1073/pnas.0703867104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yam C, Zhao M, Hayashi K, Ma H, Kishimoto H, McElroy M, Bouvet M, Hoffman RM. Monotherapy with a tumor-targeting mutant of S. typhimurium inhibits liver metastasis in a mouse model of pancreatic cancer. J Surg Res. 2010;164:248–55. doi: 10.1016/j.jss.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagakura C, Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Bouvet M, Hoffman RM. Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res. 2009;29:1873–8. [PubMed] [Google Scholar]
- 7.Momiyama M, Zhao M, Kimura H, Tran B, Chishima T, Bouvet M, Endo I, Hoffman RM. Inhibition and eradication of human glioma with tumor-targeting Salmonella typhimurium in an orthotopic nude-mouse model. Cell Cycle. 2012;11:628–32. doi: 10.4161/cc.11.3.19116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura H, Zhang L, Zhao M, Hayashi K, Tsuchiya H, Tomita K, Bouvet M, Wessels J, Hoffman RM. Targeted therapy of spinal cord glioma with a genetically modified Salmonella typhimurium. Cell Prolif. 2010;43:41–8. doi: 10.1111/j.1365-2184.2009.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman RM. Bugging tumors. Cancer Discov. 2012;2:588–90. doi: 10.1158/2159-8290.CD-12-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia LJ, Wei DP, Sun QM, Jin GH, Li SF, Huang Y, Hua ZC. Tumor-targeting Salmonella typhimurium improves cyclophosphamide chemotherapy at maximum tolerated dose and low-dose metronomic regimens in a murine melanoma model. Int J Cancer. 2007;121:666–74. doi: 10.1002/ijc.22688. [DOI] [PubMed] [Google Scholar]
- 11.McElroy M, Kaushal S, Bouvet M, Hoffman RM. Color-coded imaging of splenocyte-pancreatic cancer cell interactions in the tumor microenvironment. Cell Cycle. 2008;7:2916–21. doi: 10.4161/cc.7.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouvet M, Tsuji K, Yang M, Jiang P, Moossa AR, Hoffman RM. In vivo color-coded imaging of the interaction of colon cancer cells and splenocytes in the formation of liver metastases. Cancer Res. 2006;66:11293–7. doi: 10.1158/0008-5472.CAN-06-2662. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman RM, Yang M. Subcellular imaging in the live mouse. Nat Protoc. 2006;1:775–82. doi: 10.1038/nprot.2006.109. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton MA, Russo RC, Thurston RV. Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol. 1977;11:714–19. doi: 10.1021/es60130a004. [DOI] [Google Scholar]
- 15.Uchugonova A, Hoffman RM, Weinigel M, Koenig K. Watching stem cells in the skin of living mice noninvasively. Cell Cycle. 2011;10:2017–20. doi: 10.4161/cc.10.12.15895. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa T, Kubota T, Watanabe M, Kitajima M, Hoffman RM. A novel “patient-like” treatment model of human pancreatic cancer constructed using orthotopic transplantation of histologically intact human tumor tissue in nude mice. Cancer Res. 1993;53:3070–2. [PubMed] [Google Scholar]
- 17.Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci U S A. 1992;89:5645–9. doi: 10.1073/pnas.89.12.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs. 1999;17:343–59. doi: 10.1023/A:1006326203858. [DOI] [PubMed] [Google Scholar]
- 19.Bouvet M, Wang J-W, Nardin SR, Nassirpour R, Yang M, Baranov E, Jiang P, Moossa AR, Hoffman RM. Real-time optical imaging of primary tumor growth and multiple metastatic events in a pancreatic cancer orthotopic model. Cancer Res. 2002;62:1534–40. [PubMed] [Google Scholar]
- 20.Bouvet M, Yang M, Nardin S, Wang X, Jiang P, Baranov E, Moossa AR, Hoffman RM. Chronologically-specific metastatic targeting of human pancreatic tumors in orthotopic models. Clin Exp Metastasis. 2000;18:213–8. doi: 10.1023/A:1006767405609. [DOI] [PubMed] [Google Scholar]
- 21.Katz MH, Takimoto S, Spivack D, Moossa AR, Hoffman RM, Bouvet M. A novel red fluorescent protein orthotopic pancreatic cancer model for the preclinical evaluation of chemotherapeutics. J Surg Res. 2003;113:151–60. doi: 10.1016/S0022-4804(03)00234-8. [DOI] [PubMed] [Google Scholar]
- 22.Yamauchi K, Yang M, Jiang P, Xu M, Yamamoto N, Tsuchiya H, Tomita K, Moossa AR, Bouvet M, Hoffman RM. Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Res. 2006;66:4208–14. doi: 10.1158/0008-5472.CAN-05-3927. [DOI] [PubMed] [Google Scholar]