Abstract
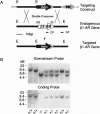
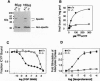
At least three distinct beta-adrenergic receptor (beta-AR) subtypes exist in mammals. These receptors modulate a wide variety of processes, from development and behavior, to cardiac function, metabolism, and smooth muscle tone. To understand the roles that individual beta-AR subtypes play in these processes, we have used the technique of gene targeting to create homozygous beta 1-AR null mutants (beta 1-AR -/-) in mice. The majority of beta 1-AR -/- mice die prenatally, and the penetrance of lethality shows strain dependence. Beta l-AR -/- mice that do survive to adulthood appear normal, but lack the chronotropic and inotropic responses seen in wild-type mice when beta-AR agonists such as isoproterenol are administered. Moreover, this lack of responsiveness is accompanied by markedly reduced stimulation of adenylate cyclase in cardiac membranes from beta 1-AR -/- mice. These findings occur despite persistent cardiac beta 2-AR expression, demonstrating the importance of beta 1-ARs for proper mouse development and cardiac function, while highlighting functional differences between beta-AR subtypes.
Full text
PDF

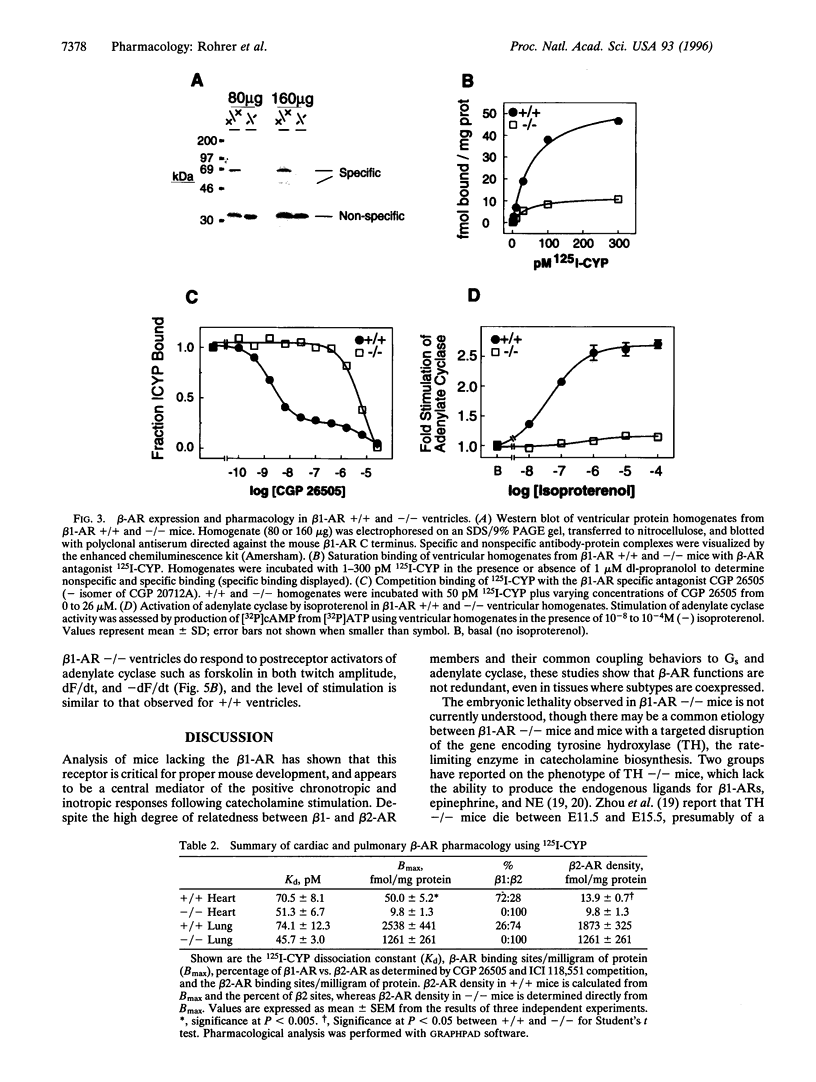

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bristow M. R., Hershberger R. E., Port J. D., Minobe W., Rasmussen R. Beta 1- and beta 2-adrenergic receptor-mediated adenylate cyclase stimulation in nonfailing and failing human ventricular myocardium. Mol Pharmacol. 1989 Mar;35(3):295–303. [PubMed] [Google Scholar]
- Broadley K. J., Williams R. G., Hawthorn M. H., Grassby P. F. Mechanisms of cardiac supersensitivity to sympathomimetic amines. Methods Find Exp Clin Pharmacol. 1984 Apr;6(4):179–186. [PubMed] [Google Scholar]
- Brodde O. E. Beta 1- and beta 2-adrenoceptors in the human heart: properties, function, and alterations in chronic heart failure. Pharmacol Rev. 1991 Jun;43(2):203–242. [PubMed] [Google Scholar]
- Böhm M., Lohse M. J. Quantification of beta-adrenoceptors and beta-adrenoceptor kinase on protein and mRNA levels in heart failure. Eur Heart J. 1994 Dec;15 (Suppl 500):30–34. doi: 10.1093/eurheartj/15.suppl_d.30. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Emorine L. J., Marullo S., Briend-Sutren M. M., Patey G., Tate K., Delavier-Klutchko C., Strosberg A. D. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989 Sep 8;245(4922):1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- Frielle T., Collins S., Daniel K. W., Caron M. G., Lefkowitz R. J., Kobilka B. K. Cloning of the cDNA for the human beta 1-adrenergic receptor. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7920–7924. doi: 10.1073/pnas.84.22.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. A., Holt B. D., Liggett S. B. Beta 1- and beta 2-adrenergic receptors display subtype-selective coupling to Gs. Mol Pharmacol. 1992 May;41(5):889–893. [PubMed] [Google Scholar]
- Hall J. A., Petch M. C., Brown M. J. Intracoronary injections of salbutamol demonstrate the presence of functional beta 2-adrenoceptors in the human heart. Circ Res. 1989 Sep;65(3):546–553. doi: 10.1161/01.res.65.3.546. [DOI] [PubMed] [Google Scholar]
- Hawthorn M. H., Broadley K. J. beta-Adrenoceptor ligand binding and supersensitivity to isoprenaline of ventricular muscle after chronic reserpine pretreatment. Naunyn Schmiedebergs Arch Pharmacol. 1982 Sep;320(3):240–245. doi: 10.1007/BF00510135. [DOI] [PubMed] [Google Scholar]
- Jasper J. R., Link R. E., Chruscinski A. J., Kobilka B. K., Bernstein D. Primary structure of the mouse beta 1-adrenergic receptor gene. Biochim Biophys Acta. 1993 Sep 13;1178(3):307–309. doi: 10.1016/0167-4889(93)90209-8. [DOI] [PubMed] [Google Scholar]
- Juberg E. N., Minneman K. P., Abel P. W. Beta 1- and beta 2-adrenoceptor binding and functional response in right and left atria of rat heart. Naunyn Schmiedebergs Arch Pharmacol. 1985 Sep;330(3):193–202. doi: 10.1007/BF00572434. [DOI] [PubMed] [Google Scholar]
- Kaumann A. J., Lemoine H. Beta 2-adrenoceptor-mediated positive inotropic effect of adrenaline in human ventricular myocardium. Quantitative discrepancies with binding and adenylate cyclase stimulation. Naunyn Schmiedebergs Arch Pharmacol. 1987 Apr;335(4):403–411. doi: 10.1007/BF00165555. [DOI] [PubMed] [Google Scholar]
- Kaumann A. J. The beta 1-adrenoceptor antagonist CGP 20712 A unmasks beta 2-adrenoceptors activated by (-)-adrenaline in rat sinoatrial node. Naunyn Schmiedebergs Arch Pharmacol. 1986 Apr;332(4):406–409. doi: 10.1007/BF00500096. [DOI] [PubMed] [Google Scholar]
- Klug S., Thiel R., Schwabe R., Merker H. J., Neubert D. Toxicity of beta-blockers in a rat whole embryo culture: concentration-response relationships and tissue concentrations. Arch Toxicol. 1994;68(6):375–384. doi: 10.1007/s002040050085. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Morita S., Sawada H., Mizuguchi T., Yamada K., Nagatsu I., Hata T., Watanabe Y., Fujita K., Nagatsu T. Targeted disruption of the tyrosine hydroxylase locus results in severe catecholamine depletion and perinatal lethality in mice. J Biol Chem. 1995 Nov 10;270(45):27235–27243. doi: 10.1074/jbc.270.45.27235. [DOI] [PubMed] [Google Scholar]
- Levy F. O., Zhu X., Kaumann A. J., Birnbaumer L. Efficacy of beta 1-adrenergic receptors is lower than that of beta 2-adrenergic receptors. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10798–10802. doi: 10.1073/pnas.90.22.10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano C. A., Allen L. F., Rockman H. A., Dolber P. C., McMinn T. R., Chien K. R., Johnson T. D., Bond R. A., Lefkowitz R. J. Enhanced myocardial function in transgenic mice overexpressing the beta 2-adrenergic receptor. Science. 1994 Apr 22;264(5158):582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- Muntz K. H., Zhao M., Miller J. C. Downregulation of myocardial beta-adrenergic receptors. Receptor subtype selectivity. Circ Res. 1994 Mar;74(3):369–375. doi: 10.1161/01.res.74.3.369. [DOI] [PubMed] [Google Scholar]
- Murphree S. S., Saffitz J. E. Delineation of the distribution of beta-adrenergic receptor subtypes in canine myocardium. Circ Res. 1988 Jul;63(1):117–125. doi: 10.1161/01.res.63.1.117. [DOI] [PubMed] [Google Scholar]
- Nagy A., Rossant J., Nagy R., Abramow-Newerly W., Roder J. C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piercy V. Method for assessing the activity of drugs at beta 1- and beta 2-adrenoceptors in the same animal. J Pharmacol Methods. 1988 Sep;20(2):125–133. doi: 10.1016/0160-5402(88)90072-1. [DOI] [PubMed] [Google Scholar]
- Rand M. J., Tung L. H., Louis W. J., Story D. F. Cardiac alpha-adrenoceptors: postjunctional and prejunctional. J Mol Cell Cardiol. 1986 Nov;18 (Suppl 5):17–32. doi: 10.1016/s0022-2828(86)80458-8. [DOI] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Fong T. M., Graziano M. P., Tota M. R. The family of G-protein-coupled receptors. FASEB J. 1995 Jun;9(9):745–754. [PubMed] [Google Scholar]
- Takei M., Furukawa Y., Narita M., Murakami M., Ren L. M., Karasawa Y., Chiba S. Sympathetic nerve stimulation activates both beta 1- and beta 2-adrenoceptors of SA and AV nodes in anesthetized dog hearts. Jpn J Pharmacol. 1992 May;59(1):23–30. doi: 10.1254/jjp.59.23. [DOI] [PubMed] [Google Scholar]
- Xiao R. P., Ji X., Lakatta E. G. Functional coupling of the beta 2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol Pharmacol. 1995 Feb;47(2):322–329. [PubMed] [Google Scholar]
- Zhou Q. Y., Quaife C. J., Palmiter R. D. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature. 1995 Apr 13;374(6523):640–643. doi: 10.1038/374640a0. [DOI] [PubMed] [Google Scholar]
- del Monte F., Kaumann A. J., Poole-Wilson P. A., Wynne D. G., Pepper J., Harding S. E. Coexistence of functioning beta 1- and beta 2-adrenoceptors in single myocytes from human ventricle. Circulation. 1993 Sep;88(3):854–863. doi: 10.1161/01.cir.88.3.854. [DOI] [PubMed] [Google Scholar]