Abstract
Alder (Alnus glutinosa) and more than 200 angiosperms that encompass 24 genera are collectively called actinorhizal plants. These plants form a symbiotic relationship with the nitrogen-fixing actinomycete Frankia strain HFPArI3. The plants provide the bacteria with carbon sources in exchange for fixed nitrogen, but this metabolite exchange in actinorhizal nodules has not been well defined. We isolated an alder cDNA from a nodule cDNA library by differential screening with nodule versus root cDNA and found that it encoded a transporter of the PTR (peptide transporter) family, AgDCAT1. AgDCAT1 mRNA was detected only in the nodules and not in other plant organs. Immunolocalization analysis showed that AgDCAT1 protein is localized at the symbiotic interface. The AgDCAT1 substrate was determined by its heterologous expression in two systems. Xenopus laevis oocytes injected with AgDCAT1 cRNA showed an outward current when perfused with malate or succinate, and AgDCAT1 was able to complement a dicarboxylate uptake-deficient Escherichia coli mutant. Using the E. coli system, AgDCAT1 was shown to be a dicarboxylate transporter with a Km of 70 μm for malate. It also transported succinate, fumarate, and oxaloacetate. To our knowledge, AgDCAT1 is the first dicarboxylate transporter to be isolated from the nodules of symbiotic plants, and we suggest that it may supply the intracellular bacteria with dicarboxylates as carbon sources.
Some plants and microorganisms engage in reciprocal symbiosis for the purpose of exchanging nutrients. For example, in nitrogen-fixing nodules, the intracellular bacteria supply the host plant with combined nitrogen and are in turn provided with carbon sources (Mylona et al., 1995). There are two types of nodule symbioses between nitrogen-fixing soil bacteria and higher plants, namely, the symbiosis between legumes and rhizobia, and the actinorhizal symbiosis between actinomycetes of the genus Frankia and a diverse group of angiosperms collectively called actinorhizal plants. In both cases, nutrient exchange between the host and its microsymbiont is controlled by the plant plasma membrane-derived interface enclosing the microsymbiont. In most legume symbioses, where the bacteria are taken up into the plant cells in a complete endocytotic process (Verma, 1992), this interface is the peribacteroid membrane (PBM). In primitive legume symbioses (de Faria et al., 1987) and the actinorhizal symbioses (Mylona et al., 1995), the interface is reported to be the invaginated and incompletely enclosed plasma membrane of the infected cell.
The nutrient exchange between the symbiotic partners requires transporters of the carbon sources and trace elements that flow from the plant to the microsymbiont along with the transporters of the products of bacterial nitrogen fixation that flow from the microsymbiont to the plant (Pawlowski and Bisseling, 1996). Soybean (Glycine max) nodules, which represent legume symbioses, show evidence of the physiological or biochemical activities of transporters of ammonium, which is the product of bacterial nitrogen fixation (Tyerman et al., 1995), and of transporters of dicarboxylates, the form of the carbon source that is supplied to the nitrogen-fixing microorganisms (Streeter, 1995). However, the genetic identities of these transporters remain unclear. It was initially suggested that nodulin-26 in the PBM of soybean nodules mediates the transport of dicarboxylates (Ou Yang et al., 1991), but it was later revealed that this molecule is an aquaporin that probably participates in osmoregulation (Rivers et al., 1997; Dean et al., 1999).
Dicarboxylates also appear to be the carbon source that actinorhizal plants provide to their Frankia sp. microsymbionts as suggested by studies on the enzymatic activities of Frankia sp. isolated from alder (Alnus glutinosa) nodules (Huss-Danell, 1997). Here, we identify a dicarboxylate transporter that is localized in the nitrogen-fixing nodules of alder. The nodule-specific expression and substrate specificity of this transporter support the notion that it participates in the flow of carbon sources from the plant cytoplasm to the symbiotic bacteria.
RESULTS
Isolation and Characterization of cDNAs Encoding a Nodule-Specific Transporter from Alder
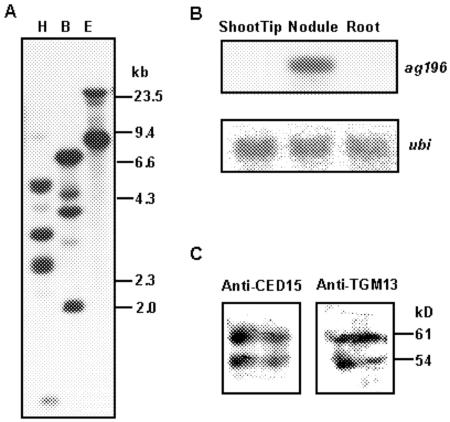
An alder nodule cDNA library was screened differentially using nodule versus root cDNA. Seven clones representing nodule-specific genes were identified and characterized. By sequencing the ends of the inserts, three were found to contain full-size cDNAs of the same gene, which was later termed AgDCAT1 (Alnus glutinosa dicarboxylate transporter 1) as the data we will describe in this paper indicate that it transports dicarboxylates. The insert of the three clones was sequenced completely (EMBL accession no. AGL488290). When we searched the Gen-Bank database, we found that the protein encoded by the cloned cDNAs is a novel member of the PTR (peptide transporter) family (Fig. 1) because it contains 12 putative transmembrane-spanning domains with a large hydrophilic loop between transmembrane domains VI and VII and the signature motif for the PTR family, F-Y-x-x-I-N-x-G-S-L, within transmembrane domain V. Furthermore, the central loop of AgDCAT1 contains the protein kinase C recognition motif (T-x-R/K) that is also conserved in the PTR family transporters (Steiner et al., 1995). When compared with the members of the PTR family that have been characterized already, it had the highest homology to CHL1, the nitrate transporter of Arabidopsis (Tsay et al., 1993; Frommer et al., 1994). DNA gel-blot analysis indicated that the corresponding gene is encoded by a small gene family (Fig. 2A). RNA gel-blot analysis showed expression of this gene only in nodules and not in roots, shoot tips, flowers, or developing fruits (Fig. 2B; data not shown for flowers and developing fruits).
Figure 1.
AgDCAT1 sequence analysis. A, Comparison of the amino acid sequences of AgDCAT1, the Arabidopsis nitrate transporter CHL1 (Tsay et al., 1993; AtCHL1A), and the Arabidopsis peptide transporter PTR2-B (Frommer et al., 1994; AtPTR2B). Gaps to optimize the alignment were introduced by using the Program ClustalW (EMBL), and the editor GeneDoc was used to present the alignment (Nicholas et al., 1997). Identical amino acids at conserved positions are labeled by inverse print, whereas chemically similar amino acids are shaded in gray. A signature motif of the PTR family within transmembrane domain V, F-Y-x-x-I-N-x-G-S-L, is shown in bold letters (amino acids 194–203). The protein kinase C recognition motif (T-x-R) in the central loop is shown in italics (amino acids 239–241). B, Hydropathy profile of AgDCAT1. Hydrophilicity was determined by the method of Kyte and Doolittle using a window of 19 amino acid residues. The numbers I to XII refer to the putative membrane-spanning segments.
Figure 2.
DNA, RNA, and protein gel-blot hybridization analysis. A, DNA gel blot containing the total DNA of alder digested with EcoRI (E), BamHI (B), and HindIII (H), respectively, was hybridized with the 32P-labeled insert of pAgDCAT1. The AgDCAT1 cDNA contains one BamHI site and two HindIII sites (223 bp apart). The numbers indicate the sizes of the marker fragments in kb. B, RNA gel blot containing the total RNA of shoot tips, nodules, and roots of alder was hybridized with the 32P-labeled insert of pAgDCAT1. Hybridization with a heterologous ubiquitin (ubi) probe (Kouchi and Hata, 1993) was used to quantify the amount of mRNA on the filter. C, Western blots of plasma membrane proteins from alder nodules. Plasma membrane proteins extracted from alder nodules were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and probed using the antisera specified. The numbers indicate protein molecular mass standards. The molecular masses of the two bands are approximately 54 and 61 kD. No signal was detected using plasma membrane proteins from alder leaves (data not shown).
AgDCAT1-Specific Antisera Recognize Two Bands in a Western Blot
Antisera were raised against two different peptides of AgDCAT1 (TGM13 and CED15; see “Materials and Methods”) and used for hybridization with blots containing plasma membrane proteins from alder nodules. In a dilution of 1:500, both the anti-TGM13 and anti-CED15 antisera hybridized with the same two bands that correspond to polypeptides with an apparent molecular masses of 54 and 61 kD (Fig. 2C). Peptide competition experiments were performed to test antibody specificity. The pre-incubation of anti-TGM13 with the immunizing peptide used to raise it prevented the appearance of both hybridizing bands in western blots (data not shown). The calculated molecular mass of AgDCAT1 is 64 kD. Hence, the 54-kD band might result from proteolysis of AgDCAT1. However, it should be noted that the estimation of the molecular mass of hydrophobic polypeptides on SDS-PAGE gels can be imprecise because they tend to run faster than typical standard proteins of similar molecular mass (Takagi, 1991). At any rate, both bands could correspond to AgDCAT1 and probably result from posttranslational modification of the original AgDCAT1 protein or its processed form. Glycosylation, for example, can change the apparent molecular mass of a transporter by up to 30 kD (Arima et al., 2002). Phosphorylation can also change the apparent molecular mass of a protein by several kilodaltons (Husain et al., 1996).
Immunolocalization of AgDCAT1 in Infected Alder Nodule Cells
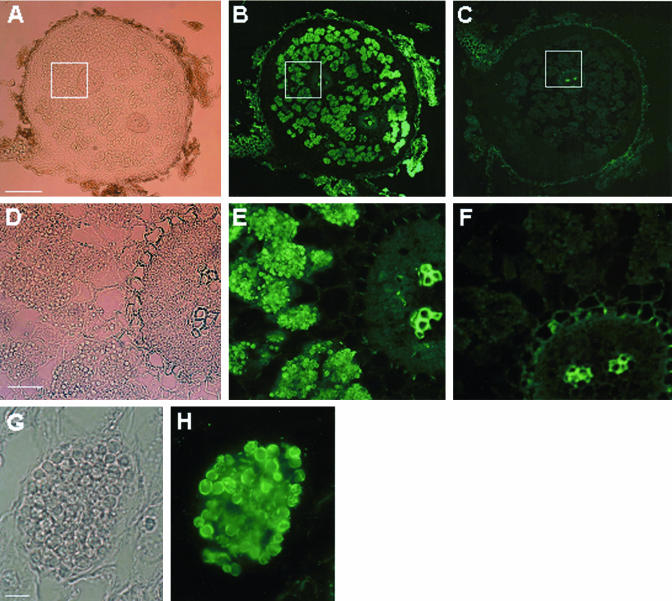
When Frankia sp.-infected alder root nodule sections were incubated with anti-TGM13 antiserum, the immunoreactant was found to be localized at the infected cells (Fig. 3, A, B, D, and E) and, more specifically, at the interface between the plant cell and the bacteria (Fig. 3, G and H). Together with our western-blot experiment that showed the presence of the protein in the plasma membrane-enriched fraction of the nodules (Fig. 2C), these images strongly support the notion that AgDCAT1 is localized at the plant plasma membrane-derived interface that encloses the microsymbiont. In contrast, hardly any fluorescence (except for the autofluorescence from the xylem cell wall) was visible from a negative control section from which the primary antiserum was omitted at the first incubation step (Fig. 3, C and F).
Figure 3.
Immunolocalization of AgDCAT1 in Frankia sp.-infected alder nodules using anti-TGM13 antiserum. A, Brightfield image of a section of a whole alder nodule. B, Fluorescence image corresponding to A. C, Negative control nodule section from which the primary antiserum was omitted at the first incubation step. D, Enlarged image of a part of A enclosed in a white frame. E, Enlarged image of a part of B enclosed in a white frame. F, Enlarged image of a part of C enclosed in a white frame. G, Bright-field image of an infected cell filled with symbiotic vesicles. H, Fluorescence image corresponding to G. Bars in A to C=500 μm, in D to F = 50 μm, and in G and H = 20 μm.
Functional Expression of AgDCAT1 in Xenopus laevis Oocytes
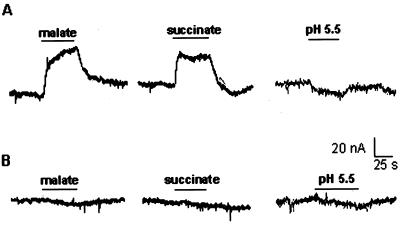
Members of the PTR family transport diverse substrates, namely, nitrate, amino acids or oligopeptides and their substrate specificities have been well characterized by their functional expression in X. laevis oocytes (Williams and Miller, 2001). Therefore, we used the X. laevis oocyte system to identify the substrate of AgDCAT1. Oocytes injected with AgDCAT1 cRNA did not respond to allantoin, citrulline, ammonia, dipeptides, or Glu (n = 13–18). However, when the injected oocytes were perfused with 10 mm malate (pH 5.5), an outward current was generated (Fig. 4A, left; n = 23), as would be expected because of the influx of a negatively charged substrate. Succinate mimicked malate (Fig. 4A, middle; n = 10), but Cl- or SO4- failed to generate similar outward currents (n = 10; data not shown). Noninjected oocytes or water-injected oocytes did not respond to either malate or succinate (Fig. 4B, left and middle; n = 18). The responses of the injected oocytes to NO3- were small and inconsistent (n = 20; data not shown). Upon perfusion with the buffer alone and changing the pH from 7.4 to 5.5 (Fig. 4A, right), the injected oocyte developed a small inward current. Although this could result from an AgDCAT1-associated change in leak conductance as has been found for a variety of other transporters (e.g. Sonders et al., 1997), further study is required to clarify this phenomenon. No distinct response was observed from the noninjected oocyte (Fig. 4B, right). These results suggest that AgDCAT1 is a dicarboxylate-specific transporter, with a much higher preference for malate and succinate than for the dicarboxylic amino acid Glu or for dipeptides.
Figure 4.
Representative current traces from AgDCAT1-injected or noninjected oocytes perfused with a malate- or succinate-containing solution. A, Outward currents were generated from an AgDCAT1-injected oocyte upon treatment with 10 mm malate (pH 5.5) or 10 mm succinate (pH 5.5). When only the pH of the perfusion solution was changed from 7.4 to 5.5 (right), the injected oocyte developed a small inward current. B, No distinct response was observed from a noninjected oocyte. The oocytes were first perfused with 230 mm mannitol, 0.3 mm CaCl2, and 10 mm MES/Tris (pH 7.4), and then either with the same solution adjusted to pH 5.5 (right) or with 10 mm malate or succinate plus 220 mm mannitol, 0.3 mm CaCl2, and 10 mm MES/Tris (pH 5.5; left and middle).
To test if the oocytes indeed took up malate via AgDCAT1, we measured [14C]malate accumulation in AgDCAT1-expressing oocytes. Thus, water- and AgDCAT1-injected oocytes were incubated in a bath containing 1 mm malate at pH 5.5 for 30 min. Although the malate accumulation of three separate batches of AgDCAT1-expressing oocytes was 205% ± 96% (se) of that of water-injected oocytes, the difference between the two groups was not statistically significant (P > 0.05, n = 18). Voltage clamping of the oocytes at -60, -30, or 0 mV during the uptake period did not sufficiently reduce the variability among the oocytes; therefore, we were not able to detect the significant accumulation of malate in AgDCAT1-expressing oocytes under these experimental conditions either (data not shown). This may be because of variability between the different oocyte batches and/or an additional modulatory interaction of the transporter with its own substrate malate, as has been shown before for other transporters (Lin et al., 1996; Sonders and Amara, 1996; Sonders et al., 1997).
AgDCAT1 Mediates Malate Transport in Budding Yeast (Saccharomyces cerevisiae) and an Escherichia coli dct Mutant
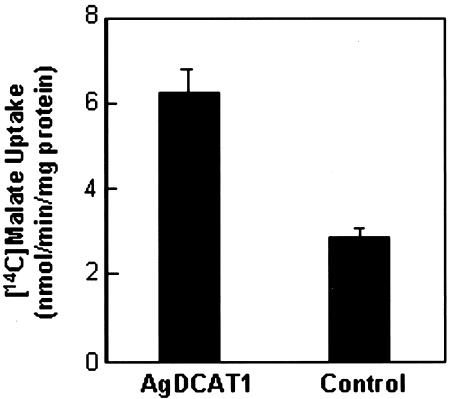
For further functional analysis of AgDCAT1, the gene was introduced into a wild-type strain of budding yeast that lacks an active dicarboxylate transporter (Camarasa et al., 2001) and into CBT315, a dicarboxylate transport mutant (dctA) strain of E. coli (Lo et al., 1972). Both systems showed enhanced malate uptake when AgDCAT1 was introduced. Yeast cells transformed with pFL61-AgDCAT1 took up 2-fold more malate than those transformed with empty vector (pFL61) when [14C]malate uptake was measured for 2 min with 1 mm malate (Fig. 5). Because E. coli showed more reproducible and higher rates of malate uptake in comparable conditions (see below), E. coli was chosen for further analysis. E. coli has been used successfully for the functional characterization of other plant transporters (Kim et al., 1998; Uozumi, 2001).
Figure 5.
Enhanced malate uptake by AgDCAT1-transformed yeast. DTY165 cells were transformed with pFL61-AgDCAT1 (AgDCAT1) or pFL61 (control), and [14C]malate uptake was measured for 2 min with 1 mm malate. Average values of two independent tests in triplicates are shown. Error bars = se; n = 6.
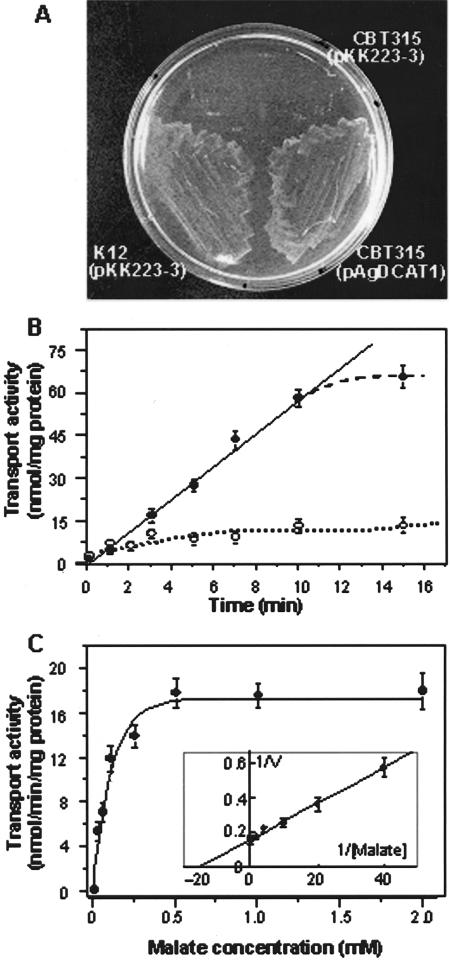
To test the ability of AgDCAT1 to complement CBT315, AgDCAT1 was cloned into the E. coli expression vector pKK223-3 under the control of the tac promoter (Brosius and Holy, 1984), yielding pAgDCAT1. The wild type that had been transformed with the empty vector, K-12(pKK223-3), was used as a positive control, and the empty vector transformant of CBT315, CBT315(pKK223-3), was used as a negative control. Because the tac promoter in pKK223-3 confers a low level of expression even in the absence of its inducer, isopropylthio-β-D-thiogalactoside, the experiments were performed in the absence of isopropylthio-β-D-thiogalactoside. The growth of the three transformants on M9 medium with 10 mm malate as the sole carbon source was then compared. K12(pKK223-3) and CBT315(pAgDCAT1) were able to grow under these conditions, whereas CBT315(pKK223-3) was not (Fig. 6A). Thus, AgDCAT1 complements the dicarboxylate uptake deficiency of CBT315, even when it is expressed at a very low level.
Figure 6.
Expression and characterization of AgDCAT1 by using an E. coli dct mutant. A, Complementation test on M9 media with l-malic acid as a sole carbon source. The E. coli wild-type K-12(pKK223-3) and its dct mutant strain CBT315 that harbored pAgDCAT1 grew on this medium, but CBT315(pKK223-3) did not. B, Time dependence of malate uptake at 1 mm malate (pH 5.9). Black circles, AgDCAT1-expressing CBT315(pAgDCAT1) cells; white circles, CBT315(pKK223-3) negative control. C, Rate of malate uptake over 10 min. Lineweaver-Burk plot analysis of the concentration-dependent uptake data revealed the Km and Vmax values to be 70 μm and 6.3 nmol min-1 mg-1 protein, respectively (inset). Black circles, AgDCAT1-expressing CBT315(pAgDCAT1) cells; white circles, CBT315(pKK223-3) negative control. Assays were performed in triplicate and repeated four times. Error bars = se; n = 12.
The function of AgDCAT1 in E. coli was analyzed further by comparing the malate uptake of CBT315(pKK223-3) and CBT315(pAgDCAT1). To obtain the initial rate of malate transport, time-dependent uptake was measured over 15 min in the presence of 1 mm malate (Fig. 6B). In CBT315(pAgDCAT1), malate uptake increased linearly during the first 10 min in the presence of 1 mm malate. During this period, CBT315(pKK223-3) cells accumulated only a background level of malate. Based on this, all of the subsequent uptake assays lasted 10 min (Fig. 6B). The concentration-dependent malate uptake by CBT315(pAgDCAT1) showed saturation kinetics (Fig. 6C) with an apparent Km of 70 μm according to the Lineweaver-Burk analysis (Fig. 6C, inset). This result indicates that AgDCAT1 mediates intermediate affinity malate transport, although it should be noted that the apparent Km value may not reflect the true Km within living plants.
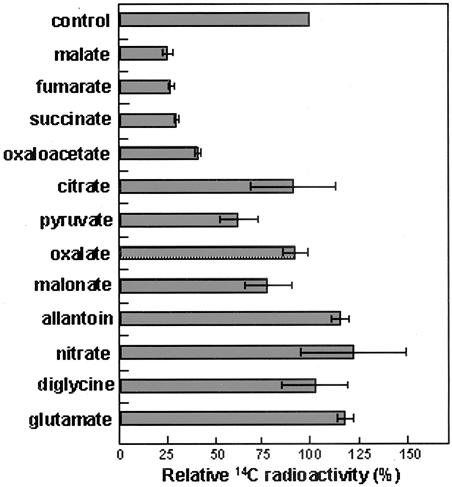
To determine the substrate specificity of AgDCAT1, competition assays were performed by adding potential competing substrates in a 3-fold molar excess to a reaction mixture containing 0.5 mm malate. As shown in Figure 7, the addition of 1.5 mm unlabeled malate to the reaction mixture decreased the uptake of radiolabeled malate to about 25%. The inhibitory effects of fumarate and succinate were at the same level as that of non-radiolabeled malate (Student's t test, P = 0.07 for succinate and 0.42 for fumarate). Oxaloacetate and pyruvate also competed with malate, reducing the uptake of radiolabeled malate to approximately 44% and 63%, respectively. Based on these results, and on the assumption of classical competitive inhibition and the Km and Vmax determined from the kinetics of malate transport (Fig. 6), the apparent binding constant (Ki) of the competing substrate was estimated in several trials so as to produce the fraction of residual malate transport as shown in Figure 7. Thus, the Ki value obtained was 145 and 315 μm for oxaloacetate and pyruvate, respectively. Other organic acids competed with malate only weakly. For example, malonate, an analog of l-malate, left 78% of the malate uptake intact, and citrate and oxalate inhibited only 8% (as a consequence, their calculated Ki values were 0.66 mm for malonate and 2 mm for citrate and oxalate). Substrates of other members of the PTR family, namely, nitrate, and di-Gly, did not compete, nor did nitrogenous compounds such as Glu or allantoin. It is interesting that Glu, a dicarboxylate, did not compete with malate. We speculate that the transport of this large dicarboxylic acid may be abrogated by its charged amino group. Thus, our results can be summarized as follows: In addition to malate, AgDCAT1 transports (or at least binds competitively at the transport-relevant site) several other C4-dicarboxylates, namely, succinate and fumarate with the same affinity, oxaloacetate with a 2-fold lower affinity, and malonate and oxalate with a 10- to 30-fold lower affinity.
Figure 7.
Substrate specificity of AgDCAT1 in E. coli. [14C]Malate uptake in CBT315(pAgDCAT1) was measured with 0.5 mm malate (pH 5.9) in the presence of a 3-fold molar excess of non-labeled competing substrates. Mean values of three independent experiments in triplicate are shown. Error bars = se; n = 9.
Malate Transport via AgDCAT1 Is Dependent on Membrane Potential
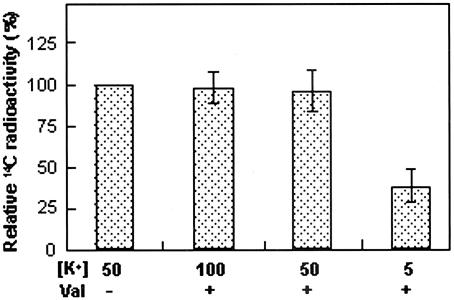
In the case of barley (Hordeum vulgare) mesophyll vacuoles (Martinoia et al., 1991), malate transport depends on the membrane potential. Thus, to determine whether the membrane potential could promote the AgDCAT1-mediated malate flux, CBT315(pAgDCAT1) cells were treated with the K+ ionophore valinomycin in the presence of different concentrations of extracellular K+. This method of altering the membrane potential is well established in E. coli (Ahmed and Booth, 1983; Li and Pajor, 2001). Malate uptake by CBT315(pAgDCAT1) in the presence of valinomycin was significantly lower at 5 mm of external K+, which should hyperpolarize the membrane potential, than at 50 or 100 mm external K+, which should depolarize the membrane potential (Fig. 8). A control experiment in which the osmolarity of the external solutions had been adjusted yielded similar results (data not shown). These data indicate that a depolarized membrane potential promotes dicarboxylate uptake via AgDCAT1.
Figure 8.
Effect of membrane potential on the malate transport of AgDCAT1 in E. coli. CBT315(pAgDCAT1) cells were pretreated for 2 min with 50 μm valinomycin (Val) in the presence of 5, 50, or 100 mm external K+, and then the [14C]malate solution was added to a final malate concentration of 1 mm (pH 5.9). The assays were repeated three times in triplicate, and mean values were compared with the control ([K+] = 50 mm, without valinomycin) are shown. Error bars = se; n = 9.
DISCUSSION
In this paper, we have characterized AgDCAT1, a nodule-specific gene from alder that encodes a member of the PTR family of plasma membrane transporters. Its expression in E. coli showed that AgDCAT1 mediates the transport of dicarboxylates, including malate, succinate, fumarate, and oxaloacetate. Dicarboxylates have been suggested previously to be the carbon sources for Frankia sp. in the infected cells of alder nodules (Huss-Danell, 1997). We found that AgDCAT1 is specifically expressed in the alder nodules and that the protein product of the gene is localized at the symbiotic interface. Thus, AgDCAT1 appears to participate in the supply of carbon sources from alder to its nitrogen-fixing microsymbiont.
AgDCAT1 has similarities and differences to the dicarboxylate transporters found in the PBM of soybean symbiosomes. They both transport many different dicarboxylates and are energized by depolarized membrane potential, which suggests that they transport anionic forms of dicarboxylates. However, they differ somewhat in their substrate preference and in their affinity for various substrates. AgDCAT1 shows a similar affinity for malate, fumarate, and succinate. In contrast, the dicarboxylate transporter of PBM prefers malate and oxaloacetate over succinate and fumarate, and its affinity for the dicarboxylates is much lower than that of AgDCAT1 (Ou Yang et al., 1990). It would be very interesting to compare the structures of the dicarboxylate transporters of these different symbiotic systems when the dicarboxylate transporter of the soybean PBM is identified.
In vivo, the prevailing conditions favor malate efflux from the plant cell because malate concentrations are higher in the cell than in the apoplast, and the membrane potential is negative in the cytoplasmic side. So far, however, we have shown that AgDCAT1 participates in the uptake of malate into cells. To be able to use our findings to understand the physiologically relevant function of AgDCAT1, we need to postulate that AgDCAT1 is capable of the bidirectional transport of malate. Supporting this is that many transporters are not mechanically fixed in the direction of transport; rather, they are capable of bidirectional transport depending on the prevailing conditions (Yu and Choi, 1997; Sensi et al., 1997; Schwartz et al., 2002). To test if AgDCAT1 can export malate, we assayed malate efflux via AgDCAT1 from malate-preloaded E. coli and yeast. However, we could not detect any AgDCAT1-mediated efflux from these cells (data not shown), perhaps because these cells metabolize malate too efficiently. Thus, further experiments are necessary to determine whether AgDCAT1 is capable of exporting dicarboxylates. The heterologous expression of a zinc transporter from soybean nodules has resulted in a similar topological problem. When it was expressed in yeast, it catalyzed the uptake of Zn2+, but when it was in the PBM, it catalyzed the export of Zn from the cytoplasm (Moreau et al., 2002). It was suggested that this Zn transporter is capable of bidirectional transport and that the direction of the transport is determined by the membrane potential and the concentration gradient of the substrate. This may also be true for AgDCAT1. The negative membrane potential of the plasma membrane of the infected cell, which is the interface between the host and the actinorhizal symbiont, should inhibit malate uptake into the infected cell and should instead promote malate efflux from the cell to the microsymbiont. Consistent with this explanation is our experiment using valinomycin in combination with low external K+, which showed that hyperpolarization of the membrane potential decreased malate uptake via AgDCAT1 (Fig. 8). In addition, the concentration gradient of dicarboxylates, which is established by the uptake of malate by the microsymbiont, should promote the malate efflux from the cell.
Because AgDCAT1 is expressed specifically in nodules, the question arises whether it evolved from a gene for a transporter with different substrate specificity or whether it represents a nodule-specific duplication product of a malate transporter gene expressed elsewhere. AgDCAT1 is not homologous to any known dicarboxylate transporter, including a plant mitochondrial di- and tricarboxylate transporter (Grobler et al., 1995; Fiermonte et al., 1999; Palmieri et al., 2000; Janausch et al., 2002; Picault et al., 2002). Because other PTR family dicarboxylate transporters have not been identified to date, we suggest that AgDCAT1 may have evolved from a PTR transporter with another substrate specificity to accommodate the need for a dicarboxylate transporter in nodule symbiosis.
To our knowledge, AgDCAT1, a nodule-specific transporter from alder, is the first plasma membrane dicarboxylate transporter that has been characterized in higher plants. Although the molecular details of the dicarboxylate transport via AgDCAT1 remain to be understood, the present data suggest that AgDCAT1 is the first transporter to be identified that is likely to be responsible for supplying an intracellular nitrogen-fixing microsymbiont with carbon sources. Thus, our study improves the understanding of the nutrient exchange that takes place during root nodule symbioses and sheds new light on the diversity of substrates that are transported by members of the PTR family.
MATERIALS AND METHODS
Plant Material
Alder (Alnus glutinosa) seeds were collected from a local source (Weerribben, The Netherlands). The plants were grown in a greenhouse at 25°C under cycles of 16 h of light and 8 h of dark. Seeds were germinated in trays containing sterile gravel that were wetted with sterile tap water. After 3 weeks, the seedlings were transferred to sterile gravel that had been wetted with one-quarter-strength Hoagland solution (Hoagland and Arnon, 1938), and each plantlet was infected with 1 mL of a 1:5 diluted dispersed culture of Frankia sp. HFPArI3 (Berry and Torrey, 1979) that had been grown in phosphorus medium without nitrogen (Meesters et al., 1985). The nodules were harvested 6 to 8 weeks after infection. For control experiments, roots were harvested from noninoculated seedlings 2 to 3 weeks after germination.
Isolation of Nucleic Acids, Cloning, and Sequencing Procedures
DNA and RNA isolation from alder were performed as described previously (Ribeiro et al., 1995). DNA manipulations were carried out as described by Sambrook et al. (1989). Nucleotide sequences were determined using an automatic sequencer (model 373A, Applied Biosystems, Foster City, CA). Sequence data were analyzed using the programs of the Wisconsin Genetics Computer Group (Devereux et al., 1984). Database searches were performed by using the BLAST algorithm (Altschul et al., 1990) on the nucleotide sequence databases of the National Center of Biotechnology Information, National Library of Medicine, National Institutes of Health (Bethesda, MD).
Nucleic Acid Hybridization Methods
Total RNA was denatured in dimethyl sulfoxide (DMSO)/glyoxal and electrophoresed on 1.2% (w/v) agarose gels (Sambrook et al., 1989). DNA was separated on 0.8% (w/v) agarose gels (Sambrook et al., 1989). Nucleic acids were transferred to GeneScreen (New England Nuclear, Boston) filters (RNA) or to Amersham Hybond N+ (Amersham, Little Chalfont, UK) filters (DNA). RNA-blot hybridizations were performed in buffer containing 50% (w/v) formamide at 42°C (Sambrook et al., 1989). DNA-blot hybridizations were performed according to the protocol provided by the manufacturer for Amersham Hybond N+. Filters were washed at 65°C with decreasing salt concentrations down to 0.5× SSC and 0.1% (w/v) SDS.
Western Blot of AgDCAT1
Two anti-AgDCAT1 antisera were produced by Peptron (Taejun, South Korea) by immunizing rabbits with peptides corresponding to the C terminus of AgDCAT1 (CEDAHQKINGKEEKV, denoted as CED15) or the loop between transmembrane domains 6 and 7 (TGMDRGADGLTIS, denoted as TGM13). Plasma membrane proteins were isolated from 10 g of alder nodules using a two-phase system according to Robinson and Hinz (2001), concentrated using 72% (w/v) tricholoroacetic acid, and separated on SDS-polyacrylamide gels. Protein transfer and immunodetection were performed as described by Sambrook et al. (1989).
Immunolocalization of AgDCAT1 in Alder Nodule
The fixation of nodules was carried out according to Stadler et al. (1995). Parts of nodules were briefly degassed in 3 mL of fixative (3:1 [v/v] ethanol:acetic acid), and the tissues were fixed at room temperature for 1 h. After three 30-min washes in 70% (v/v) ethanol containing 1 mm dithiothreitol (DTT and one overnight washing step, the tissues were dehydrated on ice with 80%, 85%, 90%, and 95% (v/v) ethanol containing 1 mm DTT for 20 min with each solution and finally incubated twice for 20 min in 99.8% (v/v) ethanol containing 10 mm DTT. The tissue was infiltrated with methacrylate in three sequential incubations at 4°C using a methacrylate mix (75% [v/v] butyl methacrylate, 25% [v/v] methyl methacrylate, 0.5% [w/v] benzoine ethyl ether, and 10 mm DTT) at increasing methacrylate mix to ethanol ratios (first incubation, overnight, 1:2 [v/v]; second incubation, 6 h, 1:1 [w/v]; and third incubation, overnight, 2:1 [w/v]). After one additional incubation for 6 h and two final overnight incubations at 4°C, all in 100% (w/v) methacrylate mix, the samples were transferred to ultrathin PCR tubes. The methacrylate was polymerized by incubation at 4°C for 15 h under UV light (310 nm) in 100% (v/v) methacrylate mix. Semithin sections (2 μm) were cut with an ultramicrotome (Ultracut R, Leica, Bensheim, Germany) and placed on poly-l-Lys-coated slides.
To remove the methacrylate from the semithin sections, the coverslips were incubated for 2 min in 100% (v/v) acetone. Rehydration involved incubations with an ethanol series (100%, 70%, and 30% [v/v]) for 30 s each. The coverslips were then washed with Tris-buffered saline buffer (50 mm Tris-HCl [pH 7.5] and 150 mm NaCl) for 30 s and incubated in blocking buffer (1% [w/v] nonfat milk powder in Tris-buffered saline) for 45 min. After an overnight incubation with anti-TGM13 antiserum (diluted 1:100 or 1:500 [v/v] in blocking buffer), the coverslips were washed three times with blocking buffer and incubated for 1 h with the anti-rabbit IgG-fluorescein isothiocyanate isomer 1 conjugate (Sigma, St. Louis; diluted 1:300 [v/v] in blocking buffer). After five final 5-min washes with blocking buffer, the coverslips were rinsed with water and mounted with the 10-μL ProLong-Antifade Kit (Molecular Probes, Leiden, Netherlands). Photographs were taken with a fluorescence microscope (Carl Zeiss, Axioskope 2, Zeiss, Göttingen, Germany) equipped with filter blocks for excitation at 455 to 495 nm and emission at 505 to 555 nm.
Functional Characterization of AgDCAT1 in Xenopus laevis Oocytes
AgDCAT1 was functionally expressed in oocytes and was performed as described by Tsay et al. (1993). Female X. laevis were obtained from Xenopus I (Merkle Road, Dexter, MI). The AgDCAT1 coding region from the original cDNA clone was subcloned in the EcoRI site of the X. laevis oocyte expression vector pGEMHE (Liman et al., 1992). pGEMHE-AgDCAT1 was linearized with NheI, and capped cRNA was transcribed in vitro using the T7 RNA transcription kit (Ambion, Austin, TX). Fifty nanoliters of the cRNA (1 μg μL-1) were injected into collagenase-digested oocytes. Current measurements were performed 2 to 3 d after injection.
The conventional two-electrode voltage clamp technique was performed using a TEV200A amplifier (Dagan, Minneapolis). Oocytes were incubated in a solution containing 230 mm mannitol, 0.3 mm CaCl2, and 10 mm MES/Tris (pH 7.4), then perfused with 10 mm malate or another substrate plus 220 mm mannitol, 0.3 mm CaCl2, and 10 mm MES/Tris (pH 5.5 or 7.4). The oocytes were held at -60 mV, and the currents were recorded and measured using Axotape (Axon Instruments, Inc., Union City, CA).
Expression and [14C]Malate Transport in Yeast
AgDCAT1 was cloned into the NotI site of pFL61 (Minet et al., 1992) to produce pFL61-AgDCAT1, and this was introduced into yeast (Saccharomyces cerevisiae) strain DTY165 using the lithium acetate method (Schiestl and Gietz, 1989). As a negative control, pFL61 vector alone was transformed into the same strain. DTY165 cells expressing AgDCAT1 or vector alone were cultured in SD-ura broth and harvested at mid-log phase. The cells were then washed twice with 100 mm potassium phosphate buffer (pH 5.9), resuspended in the same buffer to a final concentration of 1 mg protein mL-1, and kept on ice until the uptake assays. [14C]Malate transport assays were performed according to Camarasa et al. (2001), with modifications. Thus, the cell suspensions were pre-incubated at 28°C for 2 min; then, uptake was initiated by adding l-[1,4(2,3)-14C] malic acid (Amersham) diluted with 1 mm cold malate to a specific activity of 0.1 μCi nmol-1. After 2 min, the reaction was stopped by adding ice-cold 100 mm LiCl. The samples were immediately filtered through 0.45-μm nitrocellulose membrane filters, washed with 4 mL of ice-cold 100 mm LiCl, and incubated in scintillation cocktail solution (Research Products International Corp., Mount Prospect, IL). The radioactivity was measured by using a liquid scintillation counter (Tri-CARB2100TR, Packard Bioscience Co., Meriden, CT).
Complementation of Escherichia coli
E. coli K-12 (DCT) and its dicarboxylate transport mutant strain CBT315 (CGSC5269) were obtained from the E. coli Genetic Stock Center (Yale University, New Haven, CT). AgDCAT1 cDNA was cloned into the EcoRI site of the pKK223-3 vector, yielding pAgDCAT1, and CBT315 then was transformed with pAgDCAT1. The pKK223-3 vector alone was transformed into CBT315 as a negative control and into K-12 as a positive control. The phenotypes were compared on M9 medium (Sambrook et al., 1989) with malate as the sole carbon source.
[14C]Malate Transport Assays in E. coli
[14C]Malate transport assays were performed as described (Labarre et al., 1996) with modifications. CBT315(pKK223-3) and CBT315(pAgDCAT1) cells were cultured to mid-log phase in Luria-Bertani medium, washed twice with 50 mm potassium phosphate buffer (pH 5.9), and 0.75 mg protein mL-1 was resuspended in the same buffer for the assays. After pre-incubation for 3 min at 30°C, uptake was initiated by adding l-[1,4(2,3)-[14C] malate] diluted with the appropriate concentration of cold malate to a specific activity of 0.05 to 0.1 μCi nmol-1. Further steps were performed as described in the assays in yeast.
The Km and Vmax values for malate were determined according to the Lineweaver-Burk plot based on AgDCAT1-mediated malate uptake at external concentrations of 0.025 to 10 mm. To test the effect of membrane potential on malate uptake via AgDCAT1, membrane potential was clamped at different levels using valinomycin at different concentrations of extracellular K+. After the pre-incubation step, the cells were treated with valinomycin in different concentrations of potassium phosphate buffer (pH 5.9) for 2 min; then, uptake was initiated as described above. Valinomycin was dissolved to a final concentration of 50 μm in 0.1% (v/v) DMSO. This concentration is in the range used for experiments with bacteria (Lee et al., 1999; Fischer et al., 2000). DMSO (0.1% [v/v]) alone did not have any effect on malate uptake. Cell viability was tested by the BacLight bacteria kit (Molecular Probes, Eugene, OR), and it did not differ between control cells and cells treated with valinomycin.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We thank Tony van Kampen (Department of Molecular Biology, Agricultural University, Wageningen, The Netherlands) for DNA sequencing and Gary Stacey and Serry Koh (Department of Microbiology, University of Tennessee, Knoxville) for testing AgDCAT1 for peptide transport activity in yeast. We thank Christian Knop (Göttingen University, Göttingen, Germany) for help with protein isolation and immunodetection. We would also like to thank Arie Moran (Department of Physiology, Ben Gurion University, Beer-Sheva, Israel) for helpful suggestions and comments.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.032102.
This work was supported in part by the National Science Council of Taiwan (grant no. NSC86–2811–B–001–076R to Y.L. when at Academia Sinica), by the National Research Laboratory program of Ministry of Science and Technology of Korea (to Y.L.), and by the Dutch Organization for Scientific Research (to C.G. and K.P. when at Wageningen University).
References
- Ahmed S, Booth IR (1983) The use of valinomycin, nigericin and trichlorocarbanilide in control of the proton motive force in Escherichia coli cells. Biochem J 212: 105-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 304-310 [DOI] [PubMed] [Google Scholar]
- Arima K, Hines ER, Kiela PR, Drees JB, Collins JF, Ghishan FK (2002) Glucocorticoid regulation and glycosylation of mouse intestinal type IIb Na-P-i cotransporter during ontogeny. Am J Physiol 283: G426-G434 [DOI] [PubMed] [Google Scholar]
- Berry AM, Torrey JG (1979) Isolation and characterization in vivo and in vitro of an actinomycetous endophyte from Alnus rubra Bong. In JC Gordon, DT Wheeler, DA Perry, eds, Symbiotic Nitrogen Fixation in the Management of Temperate Forests. Oregon State University, Corvallis, pp 69-84
- Brosius J, Holy A (1984) Regulation of ribosomal RNA promoters with a synthetic lac operator. Proc Natl Acad Sci USA 81: 6929-6933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarasa C, Bidard F, Bony M, Barre P, Dequin S (2001) Characterization of Schizosaccharomyces pombe malate permease by expression in Saccharomyces cerevisiae. Appl Environ Microbiol 67: 4144-4151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean RM, Rivers RL, Zeidel ML, Roberts DM (1999) Purification and functional reconstitution of soybean nodulin 26: an aquaporin with water and glycerol transport properties. Biochemistry 38: 347-353 [DOI] [PubMed] [Google Scholar]
- de Faria SM, McInroy SG, Sprent JI (1987) The occurrence of infected cells, with persistent infection threads in legume root nodules. Can J Bot 65: 553-558 [Google Scholar]
- Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12: 387-395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiermonte G, Dolce V, Arrigoni R, Runswick MJ, Walker JE, Palmieri F (1999) Organization and sequence of the gene for the human mitochondrial dicarboxylate carrier: evolution of the carrier family. Biochem J 344: 953-960 [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Graber P, Turina P (2000) The activity of the ATP synthase from Escherichia coli is regulated by the transmembrane proton motive force. J Biol Chem 275: 30157-30162 [DOI] [PubMed] [Google Scholar]
- Frommer WB, Hummel S, Rentsch D (1994) Cloning of an Arabidopsis histidine transporting protein related to nitrate and peptide transporters. FEBS Lett 347: 185-189 [DOI] [PubMed] [Google Scholar]
- Grobler J, Bauer F, Subden RE, Van Vuuren HJ (1995) The mae1 gene of Schizosaccharomyces pombe encodes a permease for malate and other C4 dicarboxylic acids. Yeast 11: 1485-1491 [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DT (1938) The water-culture method for growing plants without soil. University of California, Berkeley, California Agriculture Experiment Station Circular 347
- Husain J, Lo R, Grbavec D, Stifani S (1996) Affinity for the nuclear compartment and expression during cell differentiation implicate phosphorylated Groucho/TLE1 forms of higher molecular mass in nuclear functions. Biochem J 317: 523-531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss-Danell K (1997) Actinorhizal symbioses and their N2 fixation. New Phytol 136: 375-405 [DOI] [PubMed] [Google Scholar]
- Janausch IG, Zientz E, Tran QH, Kröger A, Unden G (2002) C4-dicarboxylate carriers and sensors in bacteria. Biochim Biophys Acta 1553: 39-56 [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kwak JM, Uozumi N, Schroeder JI (1998) AtKUP1: an Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell 10: 51-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchi H, Hata S (1993) Isolation and characterization of novel nodulin cDNAs representing genes expressed at early stages of soybean nodule development. Mol Gen Genet 238: 106-119 [DOI] [PubMed] [Google Scholar]
- Labarre C, Guzzo J, Cavin J-F, Divies C (1996) Cloning and characterization of the genes encoding the malolactic enzyme and the malate permease of Leuconostoc oenos. Appl Environ Microbiol 62: 1274-1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AI, Delgado A, Gunsalus RP (1999) Signal-dependent phosphorylation of the membrane-bound NarX two-component sensor-transmitter protein of Escherichia coli: nitrate elicits a superior anion ligand response compared to nitrite. J Bacteriol 181: 5309-5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Pajor AM (2001) Functional characterization of CitM, the Mg2+-citrate transporter. J Membr Biol 185: 9-16 [DOI] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P (1992) Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9: 861-871 [DOI] [PubMed] [Google Scholar]
- Lin F, Lester HA, Mager S (1996) Single-channel currents produced by the serotonin transporter and analysis of a mutation affecting ion permeation. Biophys J 71: 3126-3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo TCY, Rayman MK, Sanwal BD (1972) Transport of succinate in Escherichia coli: I. Biochemical and genetic studies of transport in whole cells. J Biol Chem 247: 6323-6331 [PubMed] [Google Scholar]
- Martinoia E, Vogt E, Rentsch D, Amrhein N (1991) Functional reconstitution of the malate carrier of barley mesophyll vacuoles in liposomes. Biochim Biophys Acta 1062: 271-278 [DOI] [PubMed] [Google Scholar]
- Meesters TM, van Genesen ST, Akkermans ADL (1985) Growth, acetylene reduction activity and localization of nitrogenase in relation to vesicle formation in Frankia strains Cc1.17 and Cp1.2. Arch Microbiol 143: 137-794 [Google Scholar]
- Minet M, Dufour ME, Lacroute F (1992) Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsis thaliana cDNAs. Plant J 2: 417-422 [DOI] [PubMed] [Google Scholar]
- Moreau S, Thomson RM, Kaiser BN, Trevaskis B, Guerinot ML, Udvardi MK, Puppo A, Day DA (2002) GmZIP1 encodes a symbiosis-specific zinc transporter in soybean. J Biol Chem 277: 4738-4746 [DOI] [PubMed] [Google Scholar]
- Mylona P, Pawlowski K, Bisseling T (1995) Symbiotic nitrogen fixation. Plant Cell 7: 869-885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas KB, Nicholas HB Jr, Deerfield DW II (1997) GeneDoc: analysis and visualization of genetic variation. EMBnet NEWS 4: 14 [Google Scholar]
- Ou Yang LJ, Udvardi MK, Day DA (1990) Specificity and regulation of the dicarboxylate carrier on the peribacteroid membrane of soybean nodules. Planta 182: 437-444 [DOI] [PubMed] [Google Scholar]
- Ou Yang LJ, Whelan J, Weaver CD, Roberts DM, Day DA (1991) Protein phosphorylation stimulates the rate of malate uptake across the peribacteroid membrane of soybean nodules. FEBS Lett 293: 188-190 [DOI] [PubMed] [Google Scholar]
- Palmieri L, Runswick MJ, Fiermonte G, Walker JE, Palmieri F (2000) Yeast mitochondrial carriers: bacterial expression, biochemical identification and metabolic significance. J Bioenerg Biomembr 32: 67-77 [DOI] [PubMed] [Google Scholar]
- Pawlowski K, Bisseling T (1996) Rhizobial and actinorhizal symbioses: what are the shared features? Plant Cell 8: 1899-1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picault P, Palmieri L, Pisano I, Hodges M, Palmieri F (2002) Identification of a novel transporter for dicarboxylates and tricarboxylates in plant mitochondria. J Biol Chem 277: 24204-24211 [DOI] [PubMed] [Google Scholar]
- Ribeiro A, Akkermans ADL, van Kammen A, Bisseling T, Pawlowski K (1995) A nodule-specific gene encoding a subtilisin-like protease is expressed in early stages of actinorhizal nodule development. Plant Cell 7: 785-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers RL, Dean RM, Chandy G, Hall JE, Roberts DM, Zeidel ML (1997) Functional analysis of nodulin 26, an aquaporin in soybean root nodule symbiosomes. J Biol Chem 272: 16256-16261 [DOI] [PubMed] [Google Scholar]
- Robinson DG, Hinz G (2001) Organelle isolation. In B Satiat-Jeunemaitre, C Hawes, eds, Plant Cell Biology: A Practical Approach, Ed 2. IRL Press, Oxford, pp 295-324
- Sambrook J, Fritsch E, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schiestl RH, Gietz RG (1989) High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet 16: 339-346 [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Tsuruoka S, Vijayakumar S, Petrovic S, Mian A, Al-Awgati Q (2002) Acid incubation reverses the polarity of intercalated cell transporters, an effect mediated by hensin. J Clin Invest 109: 89-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Canzoniero LMT, Yu SP, Ying HS, Koh J-Y, Kerchner GA, Choi DW (1997) Measurements of intracellular free Zn in living cortical neurons: routes of entry. J Neurosci 17: 9554-9564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonders MA, Amara SG (1996) Channels in transporters. Curr Opin Neurobiol 6: 294-302 [DOI] [PubMed] [Google Scholar]
- Sonders MS, Zhu S-J, Zahniser NR, Kavanaugh MP, Amara SG (1997) Multiple ionic conductances of the human dopamine transporter: the action of dopamine and psychostimulants. J Neurosci 17: 960-974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Wolf K, Hilgarth C, Tanner W, Sauer N (1995) Subcellular localization of the inducible Chorella HUP1 monosaccharide-H+ symporter and cloning of a co-induced galactose-H+ symporter. Plant Physiol 107: 33-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner HY, Naider F, Becker JM (1995) The PTR family: a new group of peptide transporters. Mol Microbiol 16: 825-834 [DOI] [PubMed] [Google Scholar]
- Streeter JG (1995) Recent developments in carbon transport and metabolism in symbiotic systems. Symbiosis 19: 175-196 [Google Scholar]
- Takagi T (1991) New aspects of electrophoresis of membrane proteins: electrophoretic light shattering and polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate analogues. In A Chrambach, MJ Dunn, BJ Radola, eds, Advances in Electrophoresis, Vol 4. VCH Publishers, New York, pp 391-406 [Google Scholar]
- Tsay Y-F, Schroeder JI, Feldmann KA, Crawford NM (1993) The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 12: 705-713 [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Whitehead LF, Day DA (1995) A channel-like transporter for NH4+ on the symbiotic interface of N2-fixing plants. Nature 378: 629-632 [Google Scholar]
- Uozumi N (2001) E. coli as an expression system for K+ transport systems from plants. Am J Physiol 281: C733-C739 [DOI] [PubMed] [Google Scholar]
- Verma DPS (1992) Signals in root nodule organogenesis and endocytosis of Rhizobium. Plant Cell 4: 1039-1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L, Miller A (2001) Transporters responsible for the uptake and partitioning of nitrogenous solutes. Annu Rev Plant Physiol Plant Mol Biol 52: 659-688 [DOI] [PubMed] [Google Scholar]
- Yu SP, Choi DW (1997) Na+/Ca2+ exchange currents in cortical neurons: concomitant forward and reverse operation and effect of glutamate. Eur J Neurosci 9: 1273-1281 [DOI] [PubMed] [Google Scholar]