Abstract
Purpose: Extracellular matrix metalloproteinase inducer (EMMPRIN) was reported to involve in the invasion and metastasis of malignancies by regulating the expression of vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in stromal and cancer cells. The study aimed to clarify the role of EMMPRIN expression in tumorigenesis and progression of ovarian epithelial carcinomas.
Methods: EMMPRIN siRNA were transfected into ovarian carcinoma cells with the phenotypes and their related molecules examined. EMMPRIN expression was determined in ovarian normal tissue, benign and borderline tumors, and epithelial carcinomas by real-time PCR, western blot, and immunohistochemisty.
Results: EMMPRIN siRNA treatment resulted in a lower growth, G1 arrest, apoptotic induction, decreased migration, and invasion. The transfectants showed reduced expression of Wnt5a, Akt, p70s6k, Bcl-xL, survivin, VEGF, and MMP-9 than mock and control cells at both mRNA and protein levels. According to real-time PCR and western blot, EMMPRIN mRNA or protein level was higher in ovarian borderline tumor and carcinoma than normal ovary and benign tumors (P < 0.05), and positively correlated with dedifferentiation and FIGO staging (P < 0.05). Immuhistochemically, EMMPRIN expression was positively correlated with FIGO staging, dedifferentiation, Ki-67 expression, the lower cumulative and relapse-free survival rate (P < 0.05).
Conclusions: Upregulated expression of EMMPRIN protein and mRNA might be involved in the pathogenesis, differentiation, and progression of ovarian carcinomas, possibly by modulating cellular events, such as proliferation, cell cycle, apoptosis, migration, and invasion.
Keywords: ovarian cancer, EMMPRIN, cellular phenotypes, carcinogenesis, progression, prognosis
Introduction
Invasion and metastasis are key events in the aggressive biology of carcinomas and major obstacles to the treatment of malignancies. Proteolysis of the extracellular matrix (ECM) by matrix metalloproteinases (MMPs) is one of the most critical steps for growth, invasion, and metastasis of malignancies. The search for MMP-inducing factors in tumor cells led to the identification of EMMPRIN, whose name reflects its extracellular matrix metalloproteinase inducer activity1,2 although MMP-9 was reported to be transcriptionally modulated by miR-211.3
EMMPRIN (also known as CD147, basigin, M6, and tumor cell-derived collagenase stimulatory factor) was purified from the surface of LX-1 lung carcinoma cells. The gene is localized to human chromosome 19p13.3, spans 12 169 bp, and encodes a 1566 bp transcript. The encoded protein is a glycosylated cell surface transmembrane protein that belongs to the immunoglobin superfamily. It is composed of a 185 amino acid (aa) extracellular region, a single 22 aa residue transmembrane domain, and a short 39 aa residue cytoplasmic domain.1,4,5 The extracellular region contains 3 N-linked glycosylation sites, associated with 5–35 kDa glycosylation content, which is responsible for the MMP- stimulating activity. Co-transfection of EMMPRIN-expressing vectors with different tags and cross-linking experiments have suggested that the molecules could associate with each other on the plasma membrane, forming homo-oligomers in a cis-dependent manner via N-terminally located Ig-like domains.6,7
EMMPRIN has a broad tissue distribution, including activated T cells, differentiated macrophages, retinal pigment epithelium, endometrium, and normal human keratinocytes. Its expression is also frequently elevated in breast cancers, hepatocellular carcinomas, esophageal and cervical squamous cell carcinomas, non-small cell lung carcinomas, genitourinary, gastric, colorectal, prostate, and ovarian carcinomas. EMMPRIN is expressed in various forms, including highly (HG, 45–65 kDa) and lowly glycosylated (LG, 32–44 kDa) forms, as well as a core protein (approx 27 kDa). In vivo and in vitro evidences indicated that activated EMMPRIN might increase tumor invasion by inducing MMP synthesis in the surrounding stromal cells, including membrane type 1 and type 2 MMP, MMP-1, MMP-2, and MMP-3, and the endogenous activators of MMP-2.8-22 Recently, EMMPRIN was reported to stimulate tumor angiogenesis by elevating vascular endothelial growth factor (VEGF) and MMP expression in neighboring fibroblasts and epithelial cells via microvesicle shedding.12 It was reported that integrin α3β1 colocalized with EMMPRIN in human hepatoma cells and enhanced the effect of EMMPRIN on adhesion, invasion, and MMPs secretion. EMMPRIN overexpression also increases the expression of molecules downstream of integrins, including focal adhesion kinase (FAK), phospho-FAK, paxillin, and phospho-paxillin.23,24 Upregulated EMMPRIN expression is induced by TGF-β coupled with epithelial–mesenchymal transition of hepatocellular carcinoma cells. The EMMPRIN expression is controlled by the cell survival PI3K/Akt/GSK3β signaling pathway and is directly regulated by the transcription factor Slug.25 EMMPRIN overexpression in lung cancer cells upregulated the β-catenin signaling pathway, and silencing EMMPRIN inhibited β-catenin signaling, cell migration, proliferation, anchorage-independent growth, and tumor growth in a mouse xenograft model. Taken together, it is believed that EMMPRIN has a strong impact on the invasion and metastasis of malignant tumors.26
Ovarian cancer is the second leading cancer in women and the fifth leading cause of cancer-related deaths in women. More than 90% of ovarian cancers are classified as “epithelial” and are believed to arise from the ovarian epithelium, due to such risk factors as family history of ovarian carcinoma, mutation (p53, BRCA1, and BRCA2), epigenetic alteration (RUNX1 hypomethylation), estrogen replacement therapy, infertile women, times of pregnancy, endometriosis, and postmenopausal condition. Although several agents targeting the induction of apoptosis or autophagy, and the inhibition of proliferation are widely applied after surgical treatment, ovarian cancer is disproportionately deadly due to the usually late onset of symptoms.27-32 Here, we investigated the effects and related mechanisms of EMMPRIN knockdown on the phenotypes of ovarian carcinoma cells. Additionally, the protein and mRNA expression of EMMPRIN was examined in ovarian normal tissue, benign and borderline tumors, primary and metastatic epithelial carcinomas. They were compared with the clinicopathological and prognostic parameters of tumors, including expression of Ki-67 protein.
Results
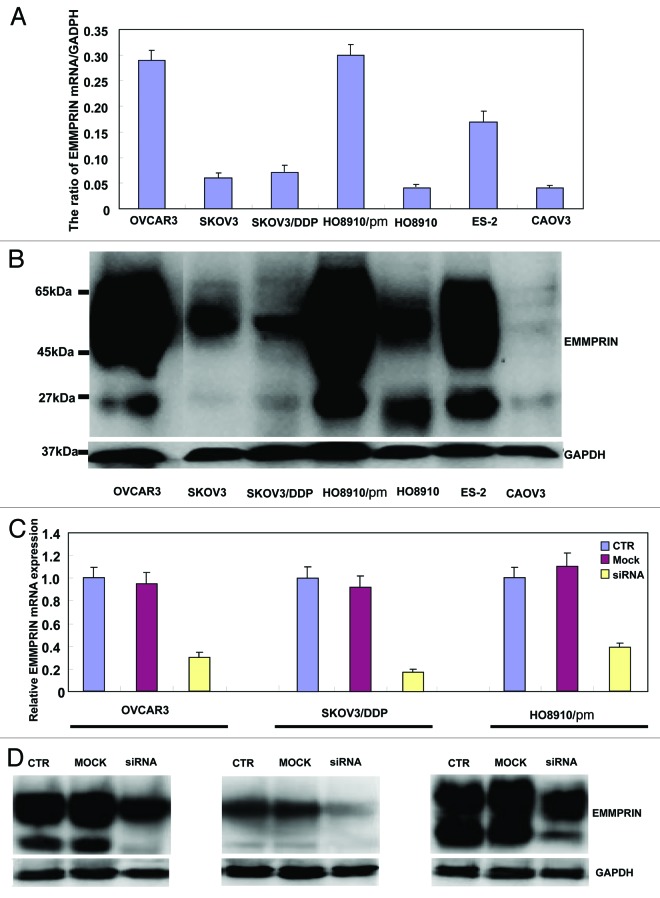
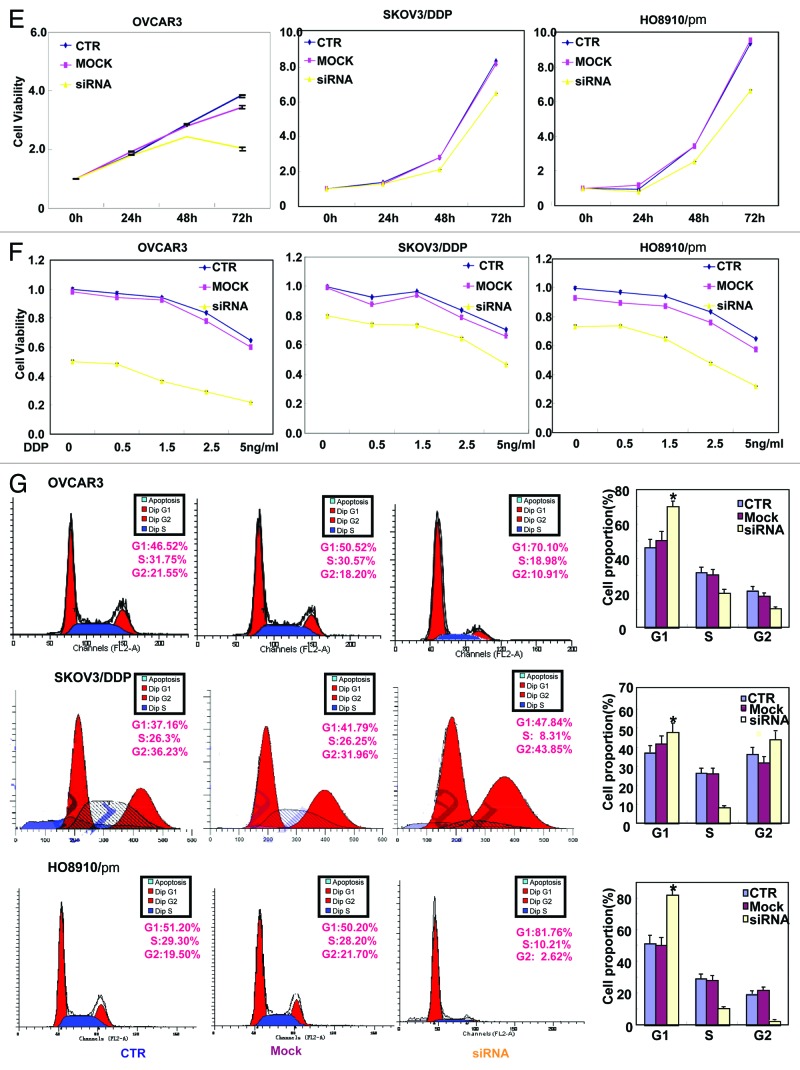
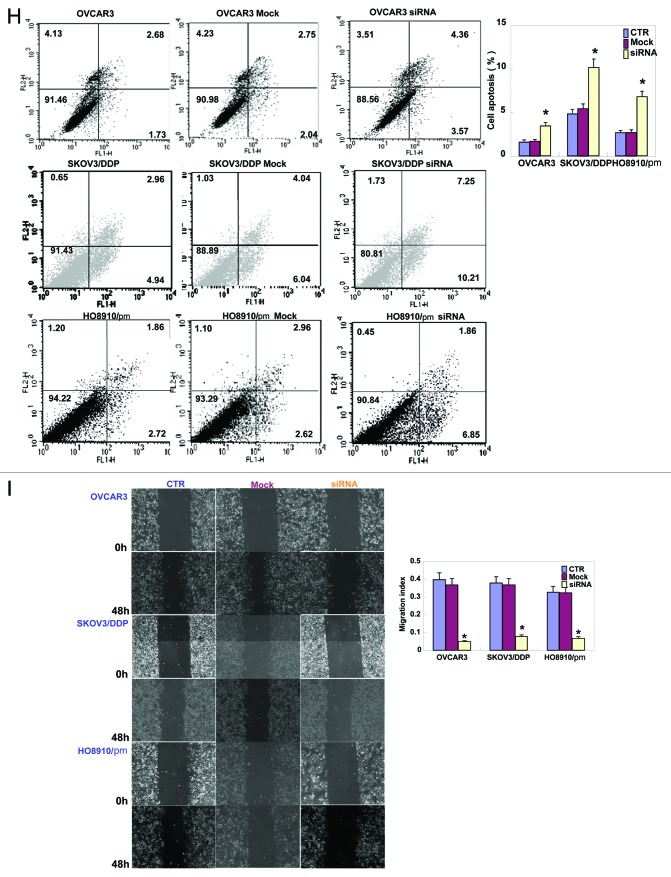
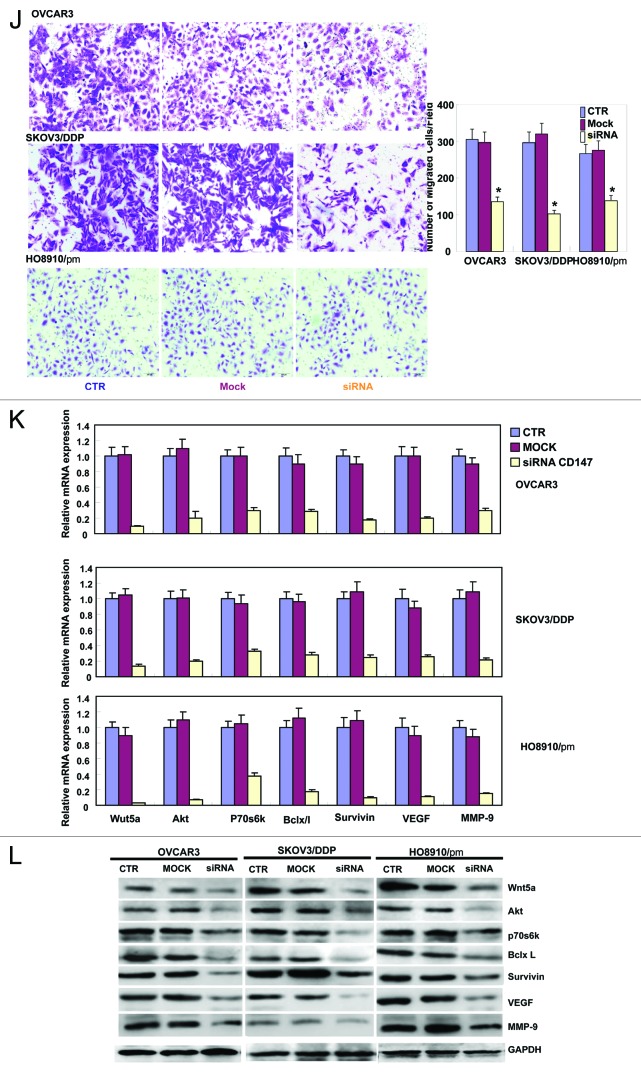
As Figure 1A and B indicates, OVCAR3, HO8910/pm, and ES-2 cells exhibited strong EMMPRIN mRNA expression by real-time PCR and western blot. EMMMPRIN was highly expressed in OVCAR3, HO8910/pm, and ES-2 cells with high glycosylation. However, there was no significance in the expression of EMMPRIN mRNA and protein between SKOV3 and its cisplatin-resistant SKOV3/DDP. To clarify the role of EMMPRIN, its expression was successfully knocked down in OVCAR3, SKOV3/DDP, and HO8910/pm cells by siRNA interference, evidenced by real-time PCR (Fig. 1C), and western blot (Fig. 1D). The transfectants showed a lower growth by CCK-8 (Fig. 1E, P < 0.05), no significantly decreased proliferation with cisplatin treatment (Fig. 1F, P > 0.05), G1 arrest by PI staining (Fig. 1G, P < 0.05) in comparison with the control or mock. There was an apoptosis-induced effect of EMMPRIN knockdown in the 3 transfectants, evidenced by Annexin V-FITC staining (Fig. 1H, P < 0.05). At the same time, we also found the transfectants exhibited lower migration by wound healing (Fig. 1I, P < 0.05) and slower invasion by transwell chamber assay (Fig. 1J, P < 0.05) than the control and mock cells. Additionally, EMMPRIN siRNA transfectants showed reduced expression of Wnt5a, Akt, p70s6k, Bcl-xL, survivin, VEGF, and MMP-9, compared with the control and mock cells by real-time PCR (Fig. 1K) and western blot (Fig. 1L). There was no difference in GST-π and MDR expression between the transfectants, mock, and control cells (data not shown) by real-time PCR.
Figure 1A–D. The effects of EMMPRIN knockdown on the aggressive phenotypes of ovarian carcinoma cells. The mRNA and protein expressions of EMMPRIN were screened in ovarian carcinoma cells (OVCAR3, SKOV3, SKOV3/DDP, HO8910, HO8910/pm, HO8910, ES-2, and CAOV3) by real-time PCR (A) and western blot (B), respectively. EMMPRIN proteins were classified into Core protein (27kDa), less-glycosylated (~32–44 kDa, LG) and highly glycosylated form (~45–65 kDa, HG). After transfection of EMMPRIN siRNA, its expression was reduced in OVCAR3, SKOV3/DDP, and HO8910/pm cells by real-time RT-PCR (C) and western blot (D) compared with control (CTR), and mock cells. The transfectants showed lower growth by CCK-8 (E), no significantly lower proliferation with DDP treatment by CCK-8 (F), G1 arrest by PI staining (G), higher apoptosis by Annexin V staining (H), lower migration by wound healing (I), and weaker invasion by transwell (J) than the control and mock cells. After the treatment of EMMPRIN siRNA, there was reduced expression of Wnt5a, Akt, p70s6k, Bcl-xL, survivin, VEGF, and MMP-9 at both mRNA and protein levels in OVCAR3, SKOV3/DDP, and HO8910/pm cells by real-time PCR (K) and western blot (L). * P < 0.05. Results are representative of 3 different experiments, and data are expressed as mean ± standard deviation with the control as “1”.
Figure 1E–G.
See Figure 1A–D legend.
Figure 1H and I. See Figure 1A–D legend.
Figure 1J–L. See Figure 1A–D legend.
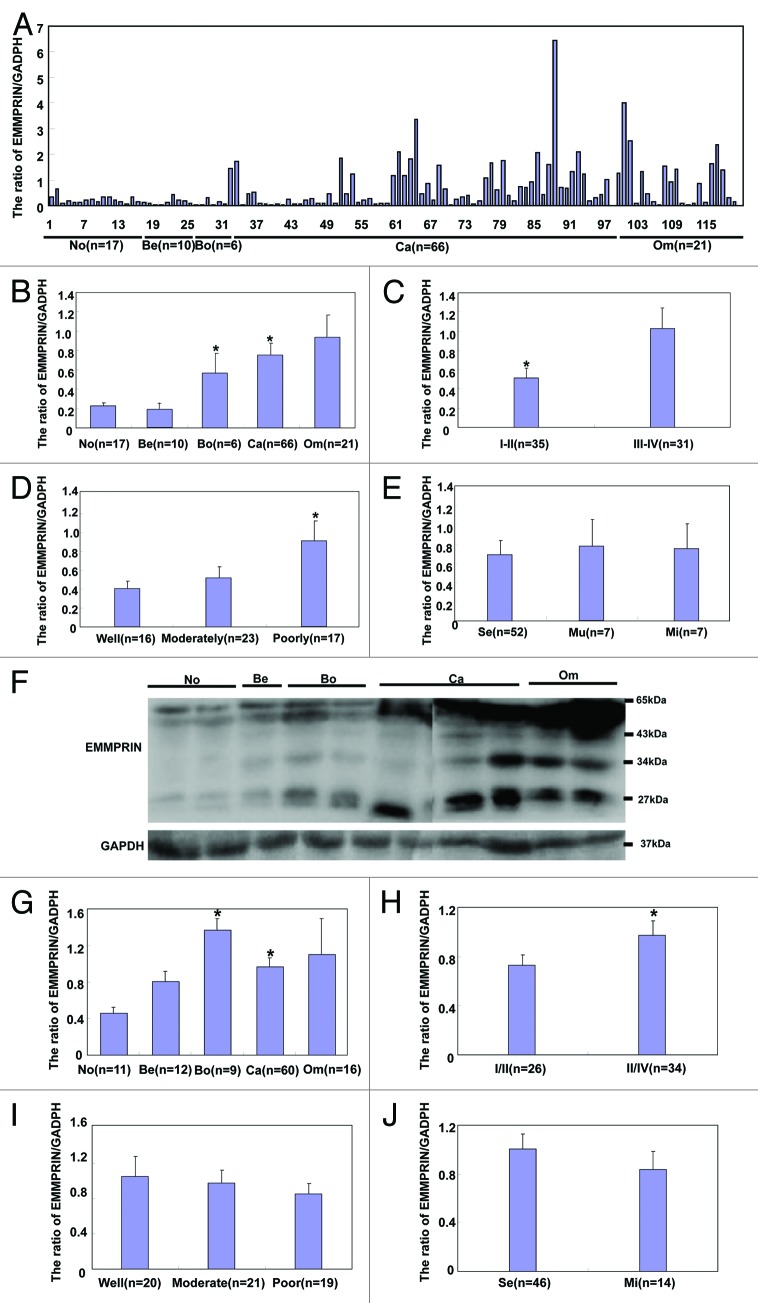
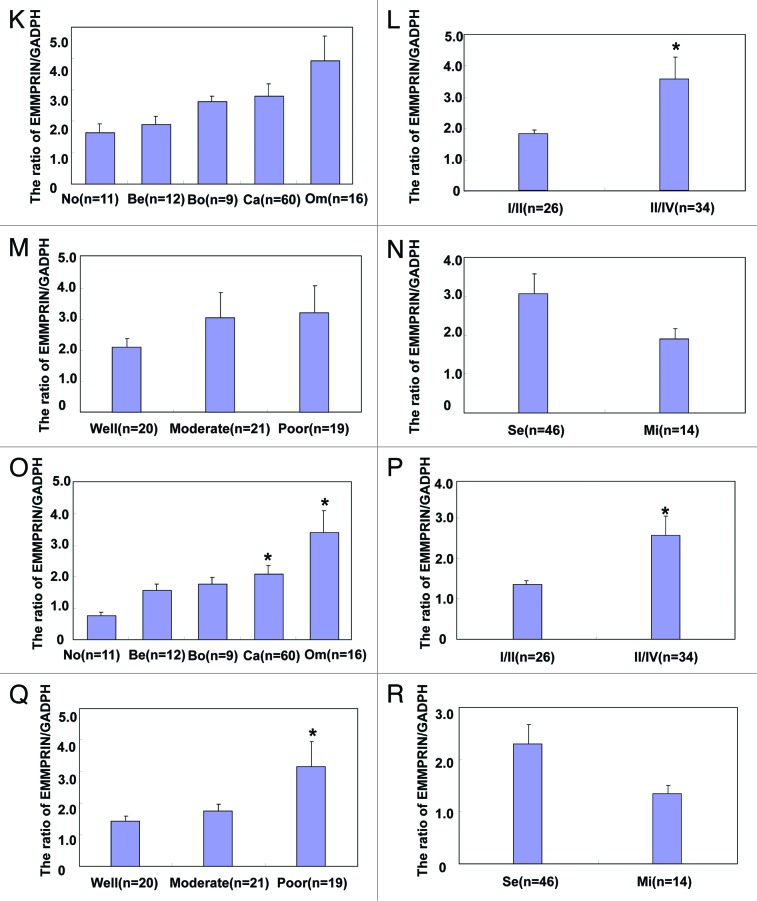
As shown in Figure 2, EMMPRIN mRNA level was higher in ovarian borderline tumor and carcinoma than normal ovary and benign tumors (Fig. 2A and B, P < 0.05) by real-time RT-PCR. High-staging carcinoma showed more EMMPRIN mRNA expression than low-staging one (Fig. 2C, P < 0.05). Decreased expression of EMMPRIN mRNA was observed in well- and moderately-differentiated carcinoma in comparison with poorly-differentiated carcinoma (Fig. 2D, P < 0.05). However, there was no correlation between EMMPRIN mRNA expression and pathological classification (Fig. 2E, P > 0.05). According to western blot (Fig. 2F), core protein expression level was significantly higher in borderline tumor and carcinoma than normal ovary and benign tumors (Fig. 2G, P < 0.05), whereas HG form was more expressed in carcinoma than normal ovary (Fig. 2K, P < 0.05), and metastatic than primary carcinoma (Fig. 2O, P < 0.05). There was greater expression of the 3 EMMPRIN forms in III-IV carcinomas in comparison with I-II ones (P < 0.05, Fig. 2H, L, and P). Additionally, higher HG EMMPRIN expression was observed in poorly-differentiated carcinomas than well- and moderately- differentiated ones (Fig. 2Q, P < 0.05).
Figure 2A–J.EMMPRIN mRNA and protein expression in ovarian carcinogenesis and its correlation with clinicopathological features of ovarian carcinoma. EMMPRIN mRNA was quantified in ovarian normal tissue (No, n = 17), benign (Be, n = 10) and borderline (Bo, n = 6) tumor, primary carcinoma (Ca, n = 66), and metastatic carcinoma in omentum (Om, n = 21) by real-time PCR. EMMPRIN mRNA expression level was significantly higher in ovarian borderline tumors and carcinoma than in ovarian normal tissue and benign tumors (B), positively with FIGO staging (C) inversely linked to differentiation degree of ovarian carcinoma (D), but not to subtyping (E). Tissue lysate of ovarian normal tissue (n = 11), benign (n = 12), and borderline (n = 9) tumor, primary carcinoma (n = 60), and metastatic carcinoma in omentum (n = 16) was loaded and probed with anti-EMMPRIN antibody (27–65 kDa, panel 1) with GAPDH (37 kDa, panel 2) as an internal control (F) by western blot. According to the molecular weight, EMMPRIN proteins were classified and respectively subjected to densitometric analysis in core protein (27 kDa, G–J), less-glycosylated (~32–44 kDa, LG, K–N) and highly glycosylated form (~45–65 kDa, HG, O–R). Normal ovary lowly expressed its core form, in comparison to the borderline tumors and carcinoma (G). Its HG form was less expressed in normal tissue than in primary carcinoma that showed less HG EMMPRIN expression than metastatic carcinoma (O). There appeared higher expression of core (H), LG (L), and HG (P) EMMPRIN proteins in I/II than III/IV ovarian carcinomas respectively. The poorly-differentiated carcinoma showed more HG EMMPRIN expression than the well- or moderately-differentiated carcinoma (Q). *P < 0.05.
Figure 2K–R. See Figure 2A–J legend.
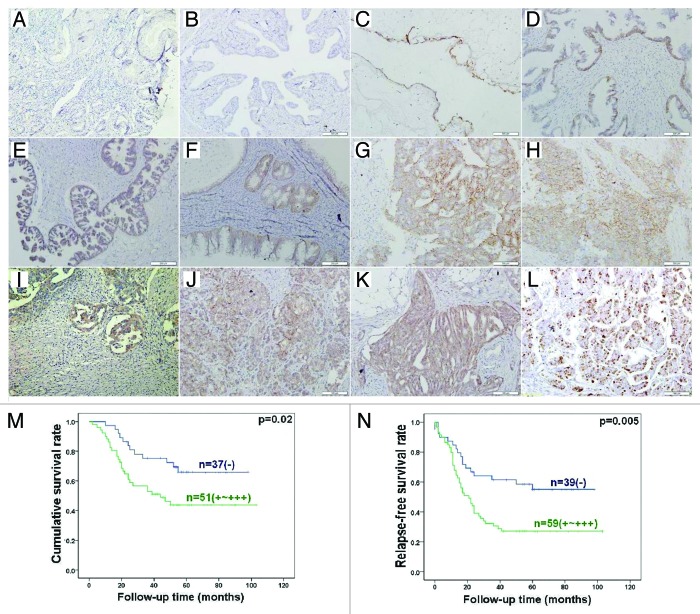
Immunostaining revealed strong EMMPRIN expression in the cytoplasm and membrane of serous adenoma, serous and mucinous borderline tumor, serous, and mucinous adenocarcinoma, endometriod carcinoma, serous adenocarcinoma in omentum, but not or very weak in normal fiber cells and in fallopian tube (Fig. 3A–K). As shown in Table 1, EMMPRIN protein expression was detectable in ovarian normal tissue (8%, 2/25), benign tumor (26.7%, 4/15), borderline tumor (60.0%, 15/25), carcinoma (64.5%, 156/242), and metastatic carcinoma in omentum (76.8%, 43/56), respectively. Statistically, EMMPRIN expression was stronger in borderline tumor and primary carcinoma than that in ovarian normal tissue and benign tumors (P < 0.05), whereas metastatic carcinoma in was stronger omentum than primary carcinoma (P < 0.05). EMMPRIN expression was positively correlated with FIGO staging, dedifferentiation and Ki-67 expression (P < 0.05), but not with age or histological classification (P > 0.05, Table 2).
Figure 3. EMMPRIN expression and its prognostic significance in ovarian samples by immunohistochemistry. Note: EMMPRIN protein is distributed to the membrane and cytoplasm, while Ki-67 protein to the nucleus. EMMPRIN was not expressed in normal fiber cells (A) and in fallopian tube (B), but strongly in serous (C) adenoma, serous (D and E), and mucinous (F) borderline tumor, serous (G and H) and mucinous (I) adenocarcinoma, endometriod carcinoma (J), serous adenocarcinoma in omentum (K). Furthermore, Ki-67 protein showed higher expression in ovarian adenocarcinoma (L). Kaplan–Meier analysis indicated that EMMPRIN expression was positively correlated with lower cumulative (M) and relapse-free (N) survival rate of patients with ovarian carcinomas.
Table 1. EMMPRIN protein expression in ovarian epithelial carcinogenesis.
| Groups | n | EMMPRIN protein expression | ||||
|---|---|---|---|---|---|---|
| − | + | ++ | +++ | PR(%) | ||
| Normal ovary | 25 | 23 | 1 | 1 | 0 | 8.0 |
| Ovarian benign tumors | 15 | 11 | 1 | 3 | 0 | 26.7 |
| Ovarian borderline tumor | 25 | 10 | 4 | 4 | 7 | 60.0* |
| Ovarian carcinoma | 242 | 86 | 46 | 47 | 63 | 64.5* |
| Metastatic carcinoma in omentum | 56 | 13 | 8 | 12 | 23 | 76.8** |
Abbreviation: PR, positive rate. *Compared with normal ovary and ovarian benign tumors, P < 0.01; **Compared with primary carcinoma, P < 0.05.
Table 2. Relationship between EMMPRIN protein expression and clinicopathological features of ovarian carcinoma.
| Clinicopathological features |
n | EMMPRIN protein expression | |||||
|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | PR(%) | P value | ||
| Age(years) | 0.774 | ||||||
| <56 | 120 | 46 | 20 | 21 | 33 | 61.7 | |
| ≥56 | 122 | 40 | 26 | 26 | 30 | 67.2 | |
| Pathological classification | 0.229 | ||||||
| Serous adenocarcinoma | 177 | 63 | 36 | 34 | 44 | 64.4 | |
| Mucinous adenocarcinoma | 27 | 13 | 3 | 5 | 6 | 51.9* | |
| Miscellaneous subtypes | 38 | 10 | 7 | 8 | 13 | 73.7 | |
| FIGO staging | 0.027 | ||||||
| I-II | 94 | 47 | 12 | 17 | 18 | 50.0 | |
| III-IV | 148 | 47 | 34 | 30 | 37 | 68.2 | |
| Differentiation | 0.032 | ||||||
| Well-differentiated | 58 | 27 | 13 | 6 | 12 | 53.4 | |
| Moderately-differentiated | 98 | 36 | 16 | 22 | 24 | 63.3 | |
| Poorly-differentiated | 86 | 23 | 17 | 19 | 27 | 73.3 | |
| Ki-67 expression | 0.040 | ||||||
| − | 31 | 9 | 10 | 9 | 3 | 71.0 | |
| + | 23 | 11 | 3 | 5 | 4 | 52.2 | |
| ++ | 25 | 6 | 6 | 1 | 12 | 76.0 | |
| +++ | 25 | 3 | 8 | 4 | 10 | 88.0 | |
| Serum Ca125 level(U/mL) | 0.303 | ||||||
| <500 | 44 | 20 | 9 | 7 | 8 | 54.5 | |
| ≥500 | 48 | 18 | 7 | 12 | 11 | 62.5 | |
*Compared with other pathological subtypes, P < 0.01.
Follow-up information was available on 88 ovarian carcinoma patients for periods ranging from 1 to 103 months (median = 52 mo, there were, altogether, 98 cases, while 10 of them lost follow-up). Survival curves for ovarian carcinomas were stratified according to EMMPRIN protein expression (Fig. 3M and N). Univariate analysis using Kaplan–Meier method indicated an inverse relationship between EMMPRIN expression and cumulative or relapse-free survival rate of patients with ovarian carcinoma (P < 0.05). Multivariate analysis using Cox proportional hazard model indicated that only FIGO staging was an independent prognostic factor for overall survival of the ovarian carcinoma patients (P < 0.05, Table 3).
Table 3. Multivariate analysis of clinicopathological variables for the overall survival of the patients with ovarian carcinomas.
| Clinicopathological parameters | Overall survival | Relapse-free survival | ||
|---|---|---|---|---|
| Relative risk (95%CI) | P value | Relative risk (95%CI) | P value | |
| Age (≥56 y) | 1.949(0.954–3.982) | 0.067 | 1.716(0.949–3.102) | 0.074 |
| Pathological subtypes | 1.063(0.428–2.641) | 0.895 | 0.882(0.415–1.874) | 0.744 |
| FIGO staging(III-IVI) | 2.671(1.004–7.105) | 0.049 | 2.071(0.958–4.479) | 0.064 |
| Differentiation degree (poorly) | 1.579(0.762–3.269) | 0.219 | 1.082(0.602–1.945) | 0.791 |
| EMMPRIN protein expression (+~+++) | 1.542(0.763–3.232) | 0.251 | 1.625(0.882–2.993) | 0.120 |
| Remanant foci size (≥1 cm) | 1.931(0.862–4.512) | 0.129 | 1.431(0.717–2.857) | 0.309 |
| Serum CA125 level (≥500) | 0.976(0.494–1.928) | 0.944 | 1.147(0.648–2.033) | 0.638 |
Abbreviation: CI, confidence interval.
Discussion
The interactions between tumor cells and microenvironment orchestrate angiogenesis, growth, dissemination, and metastasis of tumors. The cross-talk between tumor cells and adjacent stromal cells via paracrine and autocrine signals participates in tumor immune system avoidance, spreading, and angiogenesis. These biological events are mediated by a number of soluble and membrane molecules, including growth factors, soluble Fas, Fas ligand, soluble MMPs, soluble VEGF, EMMPRIN, and ADAM9.13,21,22,34-37
Numerous studies have indicated that the presence and modulation of EMMPRIN might play some roles in normal physiological processes.4,38 In the present study, EMMPRIN protein expression was seldom or weakly observed in ovarian fiber cells and fallopian tube. Gabison et al.4 reported EMMPRIN to be predominantly expressed in corneal epithelium, but markedly elevated in the anterior stroma of ulcerated corneas. Therefore, we speculate that EMMPRIN might be involved in stromal remodeling and epithelial repair of ovarian epithelial tissue. Ectopic EMMPRIN overexpression in relatively less aggressive carcinoma cell lines results in an acquired ability to form large and malignant tumors with a more invasive phenotype in nude mice.39 In this study, we detected stronger EMMPRIN expression in carcinomas and borderline tissue than benign tumor and normal ovary at both mRNA and protein levels, in line with other malignancies.8-16,18-22 Additionally, there was higher expression of core and HG EMMPRIN in ovarian carcinoma than normal ovary, suggesting their important role in ovarian carcinogenesis. Caveolin-1 partly associates with EMMPRIN and forms caveolin-1-LG-EMMPRIN complex on the cell surface during glycosylation procedure.40 Taken together, it is suggested that the alteration in the expression and the protein glycosylation of EMMPRIN might play an essential role in ovarian carcinogenesis.
Elevated EMMPRIN expression has also been shown to correlate with the progression of various malignancies.4,8-12,21,22 Here, it was found that EMMPRIN mRNA, in situ EMMPRIN expression, the expression level of core, LG, and HG EMMPRIN was positively linked to FIGO staging of ovarian carcinoma. Metastatic carcinoma showed higher EMMPRIN glycosylation level than primary carcinoma. These findings suggested that mRNA, protein expression, and glycosylation level of EMMPRIN might be involved in the progression of ovarian carcinoma and be considered as a potential marker for the aggressiveness of ovarian carcinoma. Additionally, higher mRNA expression and high glycosylation were found in poorly differentiated carcinoma, opposite to the finding of gastric carcinoma,21 indicating that they might have impact on the differentiation of ovarian carcinoma cells. The positive correlation between EMMPRIN and Ki-67 expression was found in ovarian carcinoma, consistent with a previous report.22 Experimentally, blockade of host EMMPRIN expression, but not cancer cell-derived EMMPRIN, suppresses tumor growth in human colon cancer xenografts.39 Here, we also observed that EMMPRIN knockdown could cause the low proliferation, G1 arrest and low migration and invasion of 3 ovarian carcinoma cells, supporting the promoting effects of EMMPRIN expression on proliferation, migration, and invasion of ovarian carcinoma cells.
Yang et al.40 found that EMMPRIN expression rendered breast carcinoma cells resistant to anoikis, a form of apoptosis triggered by a lack of or improper cell–matrix interactions, mediated by downregulation of the pro-apoptotic BH3-only protein, Bim, through a MAP kinase-dependent pathway. Our data showed that EMMPRIN siRNA treatment promoted apoptosis with the deceased expression of anti-apoptotic gene and encoding proteins (Bcl-xL and survivin). Marieb et al.10 documented that upregulated EMMPRIN expression stimulated hyaluronan production by elevating hyaluronan synthases, which had been linked to anchorage-independent growth of cancer cells. Because Wnt5a, VEGF, and MMP-9 are involved in invasion or metastasis of several cancers,41,42 ovarian carcinoma cells with EMMPMRIN knockdown exhibited weak ability to migrate and invade by downregulating these 3 molecules. In agreement with our finding, a large body of evidence indicates that increased soluble EMMPRIN released from cancer cells can stimulate the MMP synthesis and activation of surrounding stromal cells, promoting the progression of tumors.4,8-12 Recently, Tang et al.43 found that the elevation of MMPs mediated by EMMPRIN could result in more proteolytic cleavage of membrane-associated EMMPRIN, forming a positive feedback between tumor–stroma interaction. Furthermore, EMMPRIN transfection of tumor cells, or treating tumor cells with the recombinant protein, increased expression of MMPs.44 These findings support the opinion that EMMPRIN might enhance tumor growth and invasion of ovarian carcinoma by disrupting apoptotic and invasive events.
The subpopulations of tumor cell lines constitutively expressing high levels of cell-surface EMMPRIN exhibit cancer stem-like cell properties, such as much greater invasiveness, anchorage-independent growth, spheroid formation, and drug resistance.45 EMMPRIN induces unfolded protein response to inhibit apoptosis and chemosensitivity by increasing the transcription of Bip in hepatocellular carcinoma.46 There was also a decrease of monocarboxylate transporter 1 expression in the invasion and metastasis potential of pancreatic cancer cells, as well as increased chemosensitivity to gemcitabine in Panc-1 cells.47 Membranous EMMPRIN expression, but not the overall EMMPRIN expression, was associated with poor response to cisplatin-based chemotherapies and a poor prognosis in advanced patients with non-small-cell lung carcinoma.48 Silencing EMMPRIN increased the proliferation-inhibiting effect of cisplatin to lung cancer A549/DDP,42 laryngeal carcinoma Hep2,49 and gastric carcinoma SG790150 cells. However, we found no difference in EMMPRIN expression between ovarian carcinoma SKOV3 and SKOV3/DDP. The EMMPRIN downregulation didn’t cause the higher sensitivity to cisplatin and the alteration in the expression of drug resistance genes (GST-π and MDR) in OVCAR3, SKOV3/DDP, and HO8910/PM cells. These data indicated that EMMPRIN expression might not be linked to the chemosensitivity to cisplatin in ovarian cancer cells.
Although Ishibashi et al.9 reported that EMMPRIN expression was not associated with the recurrence-free survival of esophageal squamous cell carcinoma, we found that EMMPRIN expression might be used as a maker to indicate the worse prognosis of gastric carcinoma, albeit not an independent factor.21 Reportedly, positive EMMPRIN staining was correlated significantly with decreased tumor-specific survival of human seminoma,51 medulloblastoma,52 bladder cancer,53 and differentiated thyroid carcinoma54 as an independent prognosticator. In hepatocellular carcinoma, the patients with EMMPRIN expression had poorer tumor recurrence-free survival than those with negative expression of EMMPRIN.55 In the present study, we analyzed the relation between EMMPRIN expression and survival of 98 patients with ovarian carcinoma. The results revealed a negative link between EMMPRIN expression and relapse-free or cumulative survival rate of ovarian carcinoma patients. The multivariate analysis demonstrated that only FIGO staging was an independent prognostic factor for overall ovarian carcinomas. These findings suggested that EMMPRIN protein expression was closely linked to the adverse prognosis of the patients with ovarian carcinoma, albeit not independent.
In summary, the upregulated expression and protein glycosylation of EMMPRIN might contribute to pathogenesis, differentiation, and progression of ovarian epithelial carcinoma, possibly by modulating cellular events such as proliferation, cell cycle, apoptosis, migration, and invasion. It could thus be considered as a good marker to predict tumorigenesis and aggressiveness of ovarian epithelial carcinoma.
Materials and Methods
Cell culture and transfection
Ovarian carcinoma cell lines, CAOV-3 (serous adenocarcioma), OVCAR3 (serous cystic adenocarcinoma), SKOV-3 (serous papillary cystic adenocarcinoma), cisplatin-resistant SKOV3 (SKOV3 /DDP), HO8910 (serous cystic adenocarcinoma), highly-invasive HO8910 (HO8910/pm), and ES-2 (clear cell carcinoma), were purchased from ATCC. They were maintained in RPMI 1640 (ES-2, H08910, H08910/pm, OVCAR3, and SKOV3/DDP), DMED (CAOV3), and McCoy 5A(SKOV3) medium supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, and 100 μg/mL streptomycin in a humidified atmosphere of 5% CO2 at 37 °C. The cells were transfected with EMMPRIN siRNA at 70% confluence 24 h after seeding on dishes according to the manufacturer’s instructions (QIAGEN). The target EMMPRIN sequences were GGUCCUUGCCCUUUGUGGAdT dT (sense) and UCC ACAAAGGGCAAGGACCdTdT (antisense). The negative siRNA control sequences were UUCUCCG AACGUGUCAC GUTT (sense) and ACGUGACACG UUCGGAGAATT (anti-sense). The cells were harvested by centrifugation, rinsed with phosphate buffered saline (PBS), and subjected to total RNA or protein extraction.
Proliferation assay
Cell counting Kit-8 (CCK-8) was employed to determine the number of viable cells. In brief, 2.5 × 103 cells/well were seeded on 96-well plate and allowed to adhere. At different time points, 10 μL of CCK-8 solution was added into each well of the plate, and the plates were incubated for 3 h in the incubator and measured at 450 nm.
Cell cycle analysis
The cells were trypsinized, collected, washed by PBS twice, and fixed in cold 10 mL ethanol for more than 2 h. Then, the cells were washed by PBS twice and incubated with 1 mL RNase (0.25 mg/mL) at 37 °C for 1 h. The cells were pelleted and resuspended in propidium iodide (PI) at a concentration of 50 µg/mL and incubated at 4 °C in the dark for 30 min. Finally, flow cytometry was employed to examine PI signal.
Apoptosis assay by flow cytometry
Flow cytometry was performed with 7-amino-actinomycin (7-AAD) and FITC-labeled annexin V (BD PharMingen) to detect phosphatidylserine externalization as an endpoint indicator of early apoptosis according to the manufacturer’s instructions.
Wound-healing assay
Cells were seeded at a density of 1.0 × 106 cells/well in 6-well culture plates. After they had grown to confluence, the cell monolayer was scraped with a pipette tip to create a scratch, washed by PBS 3 times, and cultured in the FBS-free medium. Cells were photographed at 24 h, and the scratch area was measured using Image software.
Cell invasion assays
For invasive assay, 2.5 × 105 cells were resuspended in serum-free RPMI 1640, and seeded in the matrigel-coated insert on the top portion of the chamber (BD Bioscience, 354481). The lower compartment of the chamber contained 10% v/v FBS as a chemoattractant. After incubated at 37 °C and 5% CO2 for 24 h, cells on the membrane were scrubbed, washed with PBS, fixed in 100% methanol, and stained with Giemsa dye for the measurement.
Subjects
Between January 2005 and December 2011, ovarian normal tissue, benign and borderline tumors, primary epithelial carcinomas, and their metastatic carcinomas in omentum were collected from surgical resection at Department of Gynecology, The First Hospital Affiliated of China Medical University. The average age at surgery was 51.2 y (range 20–81 y). The parts of ovarian tissues were subjected to the routine preparation of pathological block. Some samples were frozen immediately in liquid nitrogen and stored at −80°C until use. None of the patients underwent chemotherapy, radiotherapy or adjuvant treatment before surgery. We followed up the patients by consulting their case documents and by telephone. Informed consent was obtained from all subjects, and the study protocol was approved by China Medical University Ethics Committee.
Real-time PCR
Total RNA was extracted from ovarian carcinoma cell lines and ovarian tissue using Trizol (Takara). Two micrograms of total RNA was subjected to cDNA synthesis using the AMV reverse transcriptase and random primer (Takara). According to the GenBank, oligonucleotide primers for PCR were shown in Table S1. Real-time PCR amplification of cDNA was performed in 20 µL mixtures according to the protocol of SYBR Premix Ex Taq™ II kit (Takara) with GAPDH as an internal control.
Western blot
Protein was extracted in RIPA lysis buffer and its concentration was determined by protein assay kit (Bio-Rad Laboratories). The denatured protein was separated on a sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred to Hybond membrane, which was then blocked overnight in 5% skim milk in Tris Buffered Saline with Tween 20 (TBST, 10 mM TRIS-HCl, 150 mM NaCl, 0.1% Tween 20). For immunoblotting, the membrane was incubated for 1 h with an antibody against EMMPRIN (Novocastra Laboratories Ltd; 1:100), Wnt5a (Santa Cruz), phosphorylated p70 s6k (T421/s424, Cell Signaling), Akt (Santa Cruz), Bcl-xL (Santa Cruz), survivin (Santa cruz), VEGF (Santa Cruz), or MMP-9 (Santa Cruz) protein. The blot was then was then rinsed with TBST, and incubated with anti-mouse, anti-rabbit, or anti-goat IgG conjugated to horseradish peroxidase (DAKO, 1:1000) for 1 h. Bands were visualized with Fuji 4000 using ECL detection reagents (Santa Cruz). After the detection, membranes were washed with WB Stripping Solution (pH2–3, Nacalai) for 20 min and treated as described above except with mouse GAPDH antibody (Sigma, 1:10 000) as an internal control. Densitometric quantification of EMMPRIN protein in ovarian samples was performed with an internal control of GAPDH using Scion Image software (Scion Corporation).
Pathology, tissue microarray (TMA), and immunohistochemistry (IHC)
All tissues were fixed in 10% neutral formalin, embedded in paraffin and sections cut at 4 μm. These sections were stained by hematoxylin-and-eosin (HE) to confirm their histological diagnosis and other microscopic characteristics. Each ovarian carcinoma was evaluated according to the international Federation of Gynecology and Obstetrics (FIGO) staging system. Histological architecture of ovarian carcinoma was expressed in terms of WHO classification.
Representative areas of solid tumors were identified in HE stained sections of the selected tumor cases, and a 2 mm diameter tissue core was punched out from each donor block and transferred to a recipient block with a maximum of 48 cores using a Tissue Microarrayer (AZUMAYA KIN-1). Four-μm-thick sections were cut from the recipient block and transferred to poly-lysine-coated glass slides. HE staining was performed on TMA for confirmation of tumor tissue.
Consecutive sections were deparaffinized with xylene, rehydrated with alcohol, and subjected to antigen retrieval by irradiation in target retrieval solution (TRS, DAKO) for 15 min with a microwave oven (Oriental Rotor Lmt Co). Five percent bovine serum albumin was then applied for 1 min to prevent non-specific binding. The sections were incubated with primary antibodies for 15 min, then treated with anti-mouse or anti-rabbit Envison-PO (DAKO) antibody for 15 min. All incubations were performed in a microwave oven to allow intermittent irradiation as described previously.33 After each treatment, the slides were washed 3 times with TBST for 1 min. Mouse anti-EMMPRIN (Novocastra; 1:50) or rabbit anti-Ki-67 (DAKO; 1:50) antibodies were employed for the detection of the respective proteins. Binding sites were visualized with 3, 3′-diaminobenzidine and counterstained with Mayer’s hematoxylin. Omission of the primary antibody was used as a negative control.
The immunoreactivity to EMMPRIN was localized in the cytoplasm and membrane, and Ki-67 in the nucleus (Fig. 3). One hundred cells were randomly selected and counted from 5 representative fields of each section blindly by 2 independent observers (Zhao Y and Zheng HC). The inconsistent data were confirmed by both persons until final agreements were reached. The expression positivity was graded and counted as follows: 0 = negative; 1 = 1–50%; 2 = 50–74%; 3 ≥ 75%. The staining intensity score was graded as follows: 1, weak; 2, intermediate; 3, strong. The scores for EMMPRIN or Ki-67 positivity and staining intensity were multiplied to obtain a final score, which determines their expression as (− = 0; + = 1–2; ++ = 3–4; +++ = 6–9).
Measurement of CA125
Serum CA125 was determined by Quantitative Chemiluminescence Immunoassay Kit (Gentaur, France). Briefly, 50 μL of standard (0–1000 U/ml), specimens, and controls was dispensed into appropriate wells. Then, 100 μL of Enzyme Conjugate Reagent was added into each well, gently mixed, and incubated the plate at room temperature for 60 min. The microtiter wells were rinsed and flicked with wash buffer. After that, residual water droplets were removed by striking the well sharply onto absorbent paper. Finally, 100 μL hemiluminescence substrate solution was dispensed into each well, mixed gently, and subjected to absorbance determination.
Statistical analysis
Statistical evaluation was performed using Spearman correlation test to analyze the rank data, and Mann–Whitney U to differentiate the means of different groups. Kaplan–Meier survival plots were generated, and comparisons were made with log-rank statistic. Cox’s proportional hazards model was employed for multivariate analysis. P < 0.05 was considered as statistically significant. SPSS 10.0 software was employed to analyze all data.
Supplementary Material
Acknowledgments
This study was supported by Shenyang Outstanding Talent Foundation of China; Shenyang Science and Technology Grant (F11-264-1-10; F12-277-1-01); A Project Supported by Scientific Research Fund of Liaoning Provincial Education Department (L2010633); Liaoning Science and Technology Grant (2009225008-11); Natural Scientific Foundation of China (No. 81172371; 81202049); Grant-in aid for Scientific Research from the Ministry of Education, Culture, Sports and Technology of Japan (23659958).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/25950
References
- 1.Voigt H, Vetter-Kauczok CS, Schrama D, Hofmann UB, Becker JC, Houben R. CD147 impacts angiogenesis and metastasis formation. Cancer Invest. 2009;27:329–33. doi: 10.1080/07357900802392675. [DOI] [PubMed] [Google Scholar]
- 2.Ueda M, Terai Y, Kanda K, Kanemura M, Takehara M, Futakuchi H, Yamaguchi H, Yasuda M, Nishiyama K, Ueki M. Tumor angiogenesis and molecular target therapy in ovarian carcinomas. Hum Cell. 2005;18:1–16. doi: 10.1111/j.1749-0774.2005.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 3.Asuthkar S, Velpula KK, Chetty C, Gorantla B, Rao JS. Epigenetic regulation of miRNA-211 by MMP-9 governs glioma cell apoptosis, chemosensitivity and radiosensitivity. Oncotarget. 2012;3:1439–54. doi: 10.18632/oncotarget.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabison EE, Mourah S, Steinfels E, Yan L, Hoang-Xuan T, Watsky MA, De Wever B, Calvo F, Mauviel A, Menashi S. Differential expression of extracellular matrix metalloproteinase inducer (CD147) in normal and ulcerated corneas: role in epithelio-stromal interactions and matrix metalloproteinase induction. Am J Pathol. 2005;166:209–19. doi: 10.1016/S0002-9440(10)62245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nabeshima K, Iwasaki H, Koga K, Hojo H, Suzumiya J, Kikuchi M. Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol Int. 2006;56:359–67. doi: 10.1111/j.1440-1827.2006.01972.x. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Hemler ME. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 2001;61:2276–81. [PubMed] [Google Scholar]
- 7.Yoshida S, Shibata M, Yamamoto S, Hagihara M, Asai N, Takahashi M, Mizutani S, Muramatsu T, Kadomatsu K. Homo-oligomer formation by basigin, an immunoglobulin superfamily member, via its N-terminal immunoglobulin domain. Eur J Biochem. 2000;267:4372–80. doi: 10.1046/j.1432-1327.2000.01482.x. [DOI] [PubMed] [Google Scholar]
- 8.Davidson B, Givant-Horwitz V, Lazarovici P, Risberg B, Nesland JM, Trope CG, Schaefer E, Reich R. Matrix metalloproteinases (MMP), EMMPRIN (extracellular matrix metalloproteinase inducer) and mitogen-activated protein kinases (MAPK): co-expression in metastatic serous ovarian carcinoma. Clin Exp Metastasis. 2003;20:621–31. doi: 10.1023/A:1027347932543. [DOI] [PubMed] [Google Scholar]
- 9.Ishibashi Y, Matsumoto T, Niwa M, Suzuki Y, Omura N, Hanyu N, Nakada K, Yanaga K, Yamada K, Ohkawa K, et al. CD147 and matrix metalloproteinase-2 protein expression as significant prognostic factors in esophageal squamous cell carcinoma. Cancer. 2004;101:1994–2000. doi: 10.1002/cncr.20593. [DOI] [PubMed] [Google Scholar]
- 10.Marieb EA, Zoltan-Jones A, Li R, Misra S, Ghatak S, Cao J, Zucker S, Toole BP. Emmprin promotes anchorage-independent growth in human mammary carcinoma cells by stimulating hyaluronan production. Cancer Res. 2004;64:1229–32. doi: 10.1158/0008-5472.CAN-03-2832. [DOI] [PubMed] [Google Scholar]
- 11.Li HG, Xie DR, Shen XM, Li HH, Zeng H, Zeng YJ. Clinicopathological significance of expression of paxillin, syndecan-1 and EMMPRIN in hepatocellular carcinoma. World J Gastroenterol. 2005;11:1445–51. doi: 10.3748/wjg.v11.i10.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sier CF, Zuidwijk K, Zijlmans HJ, Hanemaaijer R, Mulder-Stapel AA, Prins FA, Dreef EJ, Kenter GG, Fleuren GJ, Gorter A. EMMPRIN-induced MMP-2 activation cascade in human cervical squamous cell carcinoma. Int J Cancer. 2006;118:2991–8. doi: 10.1002/ijc.21778. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, Bugelski P, Yan L. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005;65:3193–9. doi: 10.1158/0008-5472.CAN-04-3605. [DOI] [PubMed] [Google Scholar]
- 14.van der Jagt MF, Sweep FC, Waas ET, Hendriks T, Ruers TJ, Merry AH, Wobbes T, Span PN. Correlation of reversion-inducing cysteine-rich protein with kazal motifs (RECK) and extracellular matrix metalloproteinase inducer (EMMPRIN), with MMP-2, MMP-9, and survival in colorectal cancer. Cancer Lett. 2006;237:289–97. doi: 10.1016/j.canlet.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Hakuma N, Betsuyaku T, Kinoshita I, Itoh T, Kaga K, Kondo S, Nishimura M, Dosaka-Akita H. High incidence of extracellular matrix metalloproteinase inducer expression in non-small cell lung cancers. Association with clinicopathological parameters. Oncology. 2007;72:197–204. doi: 10.1159/000112826. [DOI] [PubMed] [Google Scholar]
- 16.Han ZD, Bi XC, Qin WJ, He HC, Dai QS, Zou J, Ye YK, Liang YX, Zeng GH, Chen ZN, et al. CD147 expression indicates unfavourable prognosis in prostate cancer. Pathol Oncol Res. 2009;15:369–74. doi: 10.1007/s12253-008-9131-z. [DOI] [PubMed] [Google Scholar]
- 17.Han ZD, He HC, Bi XC, Qin WJ, Dai QS, Zou J, Ye YK, Liang YX, Zeng GH, Zhu G, et al. Expression and clinical significance of CD147 in genitourinary carcinomas. J Surg Res. 2010;160:260–7. doi: 10.1016/j.jss.2008.11.838. [DOI] [PubMed] [Google Scholar]
- 18.Dang D, Atakilit A, Ramos DM. EMMPRIN modulates migration and deposition of TN-C in oral squamous carcinoma. Anticancer Res. 2008;28(4B):2049–54. [PubMed] [Google Scholar]
- 19.Hanata K, Yamaguchi N, Yoshikawa K, Mezaki Y, Miura M, Suzuki S, Senoo H, Ishikawa K. Soluble EMMPRIN (extra-cellular matrix metalloproteinase inducer) stimulates the migration of HEp-2 human laryngeal carcinoma cells, accompanied by increased MMP-2 production in fibroblasts. Arch Histol Cytol. 2007;70:267–77. doi: 10.1679/aohc.70.267. [DOI] [PubMed] [Google Scholar]
- 20.Riethdorf S, Reimers N, Assmann V, Kornfeld JW, Terracciano L, Sauter G, Pantel K. High incidence of EMMPRIN expression in human tumors. Int J Cancer. 2006;119:1800–10. doi: 10.1002/ijc.22062. [DOI] [PubMed] [Google Scholar]
- 21.Zheng HC, Takahashi H, Murai Y, Cui ZG, Nomoto K, Miwa S, Tsuneyama K, Takano Y. Upregulated EMMPRIN/CD147 might contribute to growth and angiogenesis of gastric carcinoma: a good marker for local invasion and prognosis. Br J Cancer. 2006;95:1371–8. doi: 10.1038/sj.bjc.6603425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng HC, Wang W, Xu XY, Xia P, Yu M, Sugiyama T, Takano Y. Up-regulated EMMPRIN/CD147 protein expression might play a role in colorectal carcinogenesis and its subsequent progression without an alteration of its glycosylation and mRNA level. J Cancer Res Clin Oncol. 2011;137:585–96. doi: 10.1007/s00432-010-0919-3. [DOI] [PubMed] [Google Scholar]
- 23.Tang J, Wu YM, Zhao P, Yang XM, Jiang JL, Chen ZN. Overexpression of HAb18G/CD147 promotes invasion and metastasis via alpha3beta1 integrin mediated FAK-paxillin and FAK-PI3K-Ca2+ pathways. Cell Mol Life Sci. 2008;65:2933–42. doi: 10.1007/s00018-008-8315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu YM, Tang J, Zhao P, Chen ZN, Jiang JL. Enhanced expression of Hab18g/CD147 and activation of integrin pathway in HCC cells under 3-D co-culture conditions. Cell Biol Int. 2009;33:199–206. doi: 10.1016/j.cellbi.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Wu J, Ru NY, Zhang Y, Li Y, Wei D, Ren Z, Huang XF, Chen ZN, Bian H. HAb18G/CD147 promotes epithelial-mesenchymal transition through TGF-β signaling and is transcriptionally regulated by Slug. Oncogene. 2011;30:4410–27. doi: 10.1038/onc.2011.149. [DOI] [PubMed] [Google Scholar]
- 26.Sidhu SS, Nawroth R, Retz M, Lemjabbar-Alaoui H, Dasari V, Basbaum C. EMMPRIN regulates the canonical Wnt/beta-catenin signaling pathway, a potential role in accelerating lung tumorigenesis. Oncogene. 2010;29:4145–56. doi: 10.1038/onc.2010.166. [DOI] [PubMed] [Google Scholar]
- 27.Piek JM, van Diest PJ, Verheijen RH. Ovarian carcinogenesis: an alternative hypothesis. Adv Exp Med Biol. 2008;622:79–87. doi: 10.1007/978-0-387-68969-2_7. [DOI] [PubMed] [Google Scholar]
- 28.Bandera CA. Advances in the understanding of risk factors for ovarian cancer. J Reprod Med. 2005;50:399–406. [PubMed] [Google Scholar]
- 29.Kandala PK, Srivastava SK. Regulation of macroautophagy in ovarian cancer cells in vitro and in vivo by controlling glucose regulatory protein 78 and AMPK. Oncotarget. 2012;3:435–49. doi: 10.18632/oncotarget.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acquati F, Monti L, Lualdi M, Fabbri M, Sacco MG, Gribaldo L, Taramelli R. Molecular signature induced by RNASET2, a tumor antagonizing gene, in ovarian cancer cells. Oncotarget. 2011;2:477–84. doi: 10.18632/oncotarget.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keita M, Bachvarova M, Morin C, Plante M, Gregoire J, Renaud MC, Sebastianelli A, Trinh XB, Bachvarov D. The RUNX1 transcription factor is expressed in serous epithelial ovarian carcinoma and contributes to cell proliferation, migration and invasion. Cell Cycle. 2013;12:972–86. doi: 10.4161/cc.23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang CI, Choi J, Zhou Z, Flesken-Nikitin A, Tarakhovsky A, Nikitin AY. MET-dependent cancer invasion may be preprogrammed by early alterations of p53-regulated feedforward loop and triggered by stromal cell-derived HGF. Cell Cycle. 2011;10:3834–40. doi: 10.4161/cc.10.22.18294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumada T, Tsuneyama K, Hatta H, Ishizawa S, Takano Y. Improved 1-h rapid immunostaining method using intermittent microwave irradiation: practicability based on 5 years application in Toyama Medical and Pharmaceutical University Hospital. Mod Pathol. 2004;17:1141–9. doi: 10.1038/modpathol.3800165. [DOI] [PubMed] [Google Scholar]
- 34.Zheng HC, Sun JM, Wei ZL, Yang XF, Zhang YC, Xin Y. Expression of Fas ligand and caspase-3 contributes to formation of immune escape in gastric cancer. World J Gastroenterol. 2003;9:1415–20. doi: 10.3748/wjg.v9.i7.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabison EE, Hoang-Xuan T, Mauviel A, Menashi S. EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie. 2005;87:361–8. doi: 10.1016/j.biochi.2004.09.023. a. [DOI] [PubMed] [Google Scholar]
- 36.Lorusso G, Rüegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol. 2008;130:1091–103. doi: 10.1007/s00418-008-0530-8. [DOI] [PubMed] [Google Scholar]
- 37.Wong HL, Cao R, Jin G, Chan KM, Cao Y, Zhou Z. When MT1-MMP meets ADAMs. Cell Cycle. 2012;11:2793–8. doi: 10.4161/cc.20949. [DOI] [PubMed] [Google Scholar]
- 38.Toole BP. Emmprin (CD147), a cell surface regulator of matrix metalloproteinase production and function. Curr Top Dev Biol. 2003;54:371–89. doi: 10.1016/S0070-2153(03)54015-7. [DOI] [PubMed] [Google Scholar]
- 39.Abraham D, Zins K, Sioud M, Lucas T, Aharinejad S. Host CD147 blockade by small interfering RNAs suppresses growth of human colon cancer xenografts. Front Biosci. 2008;13:5571–9. doi: 10.2741/3100. [DOI] [PubMed] [Google Scholar]
- 40.Yang JM, O'Neill P, Jin W, Foty R, Medina DJ, Xu Z, et al. Emmprin (CD147) confers resistance of breast cancer cells to anoikis through inhibition of bim. J Biol Chem. 2006;281:97619–27. doi: 10.1074/jbc.M508421200. [DOI] [PubMed] [Google Scholar]
- 41.Nishita M, Enomoto M, Yamagata K, Minami Y. Cell/tissue-tropic functions of Wnt5a signaling in normal and cancer cells. Trends Cell Biol. 2010;20:346–54. doi: 10.1016/j.tcb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, Tsuneyama K, Takano Y. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006;26(5A):3579–83. [PubMed] [Google Scholar]
- 43.Tang W, Chang SB, Hemler ME. Links between CD147 function, glycosylation, and caveolin-1. Mol Biol Cell. 2004;15:4043–50. doi: 10.1091/mbc.E04-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang Y, Kesavan P, Nakada MT, Yan L. Tumor-stroma interaction: positive feedback regulation of extracellular matrix metalloproteinase inducer (EMMPRIN) expression and matrix metalloproteinase-dependent generation of soluble EMMPRIN. Mol Cancer Res. 2004;2:73–80. [PubMed] [Google Scholar]
- 45.Dai L, Guinea MC, Slomiany MG, Bratoeva M, Grass GD, Tolliver LB, Maria BL, Toole BP. CD147-dependent heterogeneity in malignant and chemoresistant properties of cancer cells. Am J Pathol. 2013;182:577–85. doi: 10.1016/j.ajpath.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang J, Guo YS, Zhang Y, Yu XL, Li L, Huang W, Li Y, Chen B, Jiang JL, Chen ZN. CD147 induces UPR to inhibit apoptosis and chemosensitivity by increasing the transcription of Bip in hepatocellular carcinoma. Cell Death Differ. 2012;19:1779–90. doi: 10.1038/cdd.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan Y, He B, Song G, Bao Q, Tang Z, Tian F, Wang S. CD147 silencing via RNA interference reduces tumor cell invasion, metastasis and increases chemosensitivity in pancreatic cancer cells. Oncol Rep. 2012;27:2003–9. doi: 10.3892/or.2012.1729. [DOI] [PubMed] [Google Scholar]
- 48.Zeng HZ, Qu YQ, Liang AB, Deng AM, Zhang WJ, Xiu B, Wang H, Wang H. Expression of CD147 in advanced non-small cell lung cancer correlated with cisplatin-based chemotherapy resistance. Neoplasma. 2011;58:449–54. doi: 10.4149/neo_2011_05_449. [DOI] [PubMed] [Google Scholar]
- 49.Zhu C, Pan Y, He B, Wang B, Xu Y, Qu L, Bao Q, Tian F, Wang S. Inhibition of CD147 gene expression via RNA interference reduces tumor cell invasion, tumorigenicity and increases chemosensitivity to cisplatin in laryngeal carcinoma Hep2 cells. Oncol Rep. 2011;25:425–32. doi: 10.3892/or.2010.1088. [DOI] [PubMed] [Google Scholar]
- 50.Wang B, Xu YF, He BS, Pan YQ, Zhang LR, Zhu C, Qu LL, Wang SK. RNAi-mediated silencing of CD147 inhibits tumor cell proliferation, invasion and increases chemosensitivity to cisplatin in SGC7901 cells in vitro. J Exp Clin Cancer Res. 2010;29:61. doi: 10.1186/1756-9966-29-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bi XC, Liu JM, He HC, Ye YK, Han ZD, Dai QS, Liang YX, Cai C, Chen JH, Chen XB, et al. Extracellular matrix metalloproteinase inducer: a novel poor prognostic marker for human seminomas. Clin Transl Oncol. 2012;14:190–6. doi: 10.1007/s12094-012-0783-5. [DOI] [PubMed] [Google Scholar]
- 52.Chu T, Chen X, Yu J, Xiao J, Fu Z. Extracellular matrix metalloproteinase inducer is a negative prognostic factor of pediatric medulloblastoma. Pathol Oncol Res. 2011;17:705–11. doi: 10.1007/s12253-011-9373-z. [DOI] [PubMed] [Google Scholar]
- 53.Xue YJ, Lu Q, Sun ZX. CD147 overexpression is a prognostic factor and a potential therapeutic target in bladder cancer. Med Oncol. 2011;28:1363–72. doi: 10.1007/s12032-010-9582-4. [DOI] [PubMed] [Google Scholar]
- 54.Tan H, Ye K, Wang Z, Tang H. CD147 expression as a significant prognostic factor in differentiated thyroid carcinoma. Transl Res. 2008;152:143–9. doi: 10.1016/j.trsl.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Q, Zhou J, Ku XM, Chen XG, Zhang L, Xu J, Chen GS, Li Q, Qian F, Tian R, et al. Expression of CD147 as a significantly unfavorable prognostic factor in hepatocellular carcinoma. Eur J Cancer Prev. 2007;16:196–202. doi: 10.1097/01.cej.0000236245.40619.c3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.