Abstract
Conventional asthma and COPD treatments include the use of bronchodilators, mainly β2-adrenergic agonists, muscarinic receptor antagonists and corticosteroids or leukotriene antagonists as anti-inflammatory agents. These active drugs are administered either separately or given as a fixed-dose combination medication into a single inhaler. ASM-024, a homopiperazinium compound, derived from the structural modification of diphenylmethylpiperazinium (DMPP), has been developed to offer an alternative mechanism of action that could provide symptomatic control through combined anti-inflammatory and bronchodilator properties in a single entity. A dose-dependent inhibition of cellular inflammation in bronchoalveolar lavage fluid was observed in ovalbumin-sensitized mice, subsequently treated for 3 days by nose-only exposure with aerosolized ASM-024 at doses up to 3.8 mg/kg (ED50 = 0.03 mg/kg). The methacholine ED250 values indicated that airway hyperresponsivenness (AHR) to methacholine decreased following ASM-024 administration by inhalation at a dose of 1.5 mg/kg, with a value of 0.145±0.032 mg/kg for ASM 024-treated group as compared to 0.088±0.023 mg/kg for untreated mice. In in vitro isometric studies, ASM-024 elicited dose-dependent relaxation of isolated mouse tracheal, human, and dog bronchial preparations contracted with methacholine and guinea pig tracheas contracted with histamine. ASM-024 showed also a dose and time dependant protective effect on methacholine-induced contraction. Overall, with its combined anti-inflammatory, bronchodilating and bronchoprotective properties, ASM-024 may represent a new class of drugs with a novel pharmacological approach that could prove useful for the chronic maintenance treatment of asthma and, possibly, COPD.
Introduction
Asthma is a respiratory disease characterised by airway inflammation and hyperresponsiveness resulting in reversible bronchoconstriction that affects between 8 to 10% of the population in industrialised countries [1]. Current treatment is mostly based on inhaled corticosteroids and β2 receptor agonists used individually or in combination [2]. Despite these effective treatments, half of asthmatics are not adequately controlled [3]. The reduced efficacy associated with the overuse of β2 agonists leading to β2-adrenergic receptor tachyphylaxis may play role in this inadequate control in chronically-treated asthmatics [4], [5]. Moreover, about 5 to 10% of patients with severe asthma fail to respond to inhaled or oral glucocorticosteroids [6]–[8]. Most importantly, high doses and long term use of inhaled corticosteroids have been associated with significant and sometimes serious side effects [2]. Because of these unmet needs, the development of new treatments for asthma is warranted, especially for drugs that have a different mode of action, thus potentially bypassing the limitations of current medication [9].
ASM-024 is an analogue of dimethylphenylpiperazinium (DMPP), a well-described nicotinic receptor agonist. ASM-024 is currently in Phase II trials for the treatment of asthma and COPD. The pharmacological target, scientific rationale and working hypothesis for the development of ASM-024 were based on the key role of the cholinergic system in the respiratory tract, not only for the control of airway smooth muscle tone, but also in the regulation of inflammation [10]. Nicotinic receptors (nAChRs) are expressed on parasympathetic nerves but also on airway structural, inflammatory and smooth muscle cells support their putative regulatory role in lung inflammatory diseases and particularly asthma [11], [12]. Several studies have demonstrated that acetylcholine is also synthesized by nonneuronal cells including inflammatory and epithelial cells and is involved in the modulation of inflammation through interaction with nicotinic [13], [14] and muscarinic receptors [15]. Epidemiological studies showing a lower incidence of several inflammatory diseases in smokers and anecdotal clinical observations of asthma exacerbations or even de novo asthma development after smoking cessation [16], [17] also support this hypothesis. The mechanisms underlying the protective effects of nicotine on the development of these inflammatory diseases may be linked to the well documented nicotine-induced anti-inflammatory cascade [18], [19]. Based on the above, the pharmacological approach targeting nAChRs and subsequent activation of the anti-inflammatory pathway was proposed for the control of inflammation in asthma pathogenesis.
Early investigations demonstrated that DMPP is highly effective in decreasing lung inflammation and lung tissue resistance in a mouse model of allergic airway inflammation and hyperreactivity [20], presents smooth muscle relaxant properties [21] and inhibits eosinophil migration [22]. Following these proofs of concept studies, ASM-024, a small positively charged homopiperazinium compound, was selected for drug development for the treatment of respiratory inflammatory diseases such as asthma. Due to its quaternary ammonium structure, ASM-024 does not penetrate the blood brain barrier thereby limiting any potential for central effects including addiction and/or dependence. In standard radioligand receptor binding competition assays, ASM-024 showed 60% inhibition at 30 µM with an IC50 of 19 µM and a Ki of 13 µM for nonselective nAChR subtypes, and low binding affinity for most of the nAChR subtypes tested, except for the human α3β4 receptor, for which the Ki was 0.88 µM. Recent observations from whole cell voltage clamp experiments have revealed that ASM-024 inhibits acetylcholine-evoked responses on human α3β4 and α7 subtypes expressed in Xenopus oocytes, indicating a potential antagonist effect. However, when co-applied with the type II α7 positive allosteric modulator, PNU-120596, ASM-024 appears to function as an agonist and effectively activates the α7 ion channel [23]. Compounds with similar properties are defined as “silent agonists” and were reported to have anti-inflammatory effects [24]. In addition, although a receptor binding assay indicated low binding affinity for the various muscarinic receptor (mAChRs) subtypes, ASM-024 was shown to decrease muscarinic responses to acetylcholine of M1, M2, and M3 mAChR expressed in Xenopus oocytes. These observations indicate a complex multifunctional mechanism of action which is still being investigated. The rather recent finding of the effect of ASM-024 at the muscarinic level has led to the effort to explore the potential clinical use of the compound in the treatment of COPD. The objective of this paper is to describe the pharmacological properties of this new molecule. For this purpose we investigated the effects of ASM-024 on inflammatory response and airway smooth muscle contractility and relaxation using in vivo and in vitro preclinical models.
Materials and Methods
ASM-024 Pharmacological Activity
To evaluate the pharmacological effects of ASM-024, studies were conducted in vivo with a mouse model of allergic airway inflammation and hyperreactivity and in vitro with tracheal or bronchial preparations obtained from mice, guinea pigs, dogs, and humans. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Canadian Council on Animal Care (CCAC) regarding acclimation, environmental enrichment, and analgesic and anesthesia procedures. The protocols were approved by the Committee on the Ethics of Animal Experiments of Université Laval. Tissues from human donors were obtained after written informed consent from all individuals in accordance with an Internal Review Board-approved protocol at IUCPQ Research Center (Institut universitaire de cardiologie et de pneumologie de Québec) in Québec, QC (Canada).
Mouse Model of allergic airway inflammation and hyperreactivity
Balb/c mice (n = 7–9 for each group) were intraperitoneally sensitized with 100 µg/100 µL ovalbumin (OVA) in 2% (w/v) aluminium hydroxide on days 0 and 7. OVA-sensitized mice were intranasally challenged with the allergen OVA (50 µg/50 µL) on days 20–23. On day 24, mice were sacrificed and bronchoalveolar alveolar lavage (BAL) was performed; the number of total cells recovered in the BAL was used as a measure of the severity of lung inflammation. Cells were stained with Hema 3 Stain Set (Fisher Scientific) and differential cell counts were performed by two observers. Lymphocytes were distinguished from macrophages based on shape, coloration and morphology of the cells and nuclei. Appropriate positive and negative controls were done in parallel with the treated groups. Separate groups of OVA-sensitized mice were used for the measure of hyperreactivity.
Oral Drug Delivery. OVA-sensitized animals were treated with various doses of ASM-024 dissolved in water (0.03 to 30 mg/kg) by oral gavage (p.o.) of 100 µL on days 21–23, 30 minutes before the OVA intranasal challenge. In some experiments aimed at measuring airway responsiveness (AR), the drug was given for 3 or 7 days p.o. and the AR measured. Control mice were treated with water only.
Inhalation Drug Delivery. Mice were sensitized and challenged with OVA as described above. Aerosolized ASM-024 or saline (vehicle control) was administered using the inExpose system, a nose-only aerosol exposure tower from Scientific Respiratory Equipment Inc. (SCIREQ) (Montréal, QC, Canada). Aerosol was delivered with the Aeroneb® Lab Micropump Nebulizer, producing particle size of 1.8 µm MMAD, 2.0 µm GSD. The animals were exposed to different doses of ASM-024 for 25 minutes on days 20–23. OVA challenges were done 30 minutes after the end of exposure of ASM-024 aerosol. Target doses were 0.03, 0.3 and 3 mg/kg. Actual administered doses were calculated according to the following formulas:
Estimation of inhaled volume per mouse 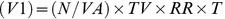
Estimation of ASM-024 administered dose 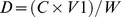
Where N is nebulisation rate, VA is air flow (2 L/min), TV is tidal volume (estimated at 170 µl), RR is respiratory rate (estimated at 150 breath/min), T is the duration of treatment, C is ASM-024 concentration solution and W is mouse weight (18–20 g).
Based on these parameters, the estimated administered doses were, 0.038, 0.38, 3.80 mg/kg.
In Vivo Airway Responsiveness in Allergen-Sensitized Mice. On day 24, OVA sensitized and challenged mice were exposed to a single dose of 1.5 mg/kg aerosolized ASM-024, 10 minutes prior the methacholine challenge. AR was measured using the FlexiVent apparatus (SCIREQ, Montréal, QC, Canada). Mice were anesthetised with ketamine-xylazine, tracheotomised and intubated with an 18 G catheter and paralysed with pancuronium (0.5 mg/kg). Respiratory frequency was set at 160 breaths/min, a tidal volume of 0.2 mL and a positive end-expiratory pressure of 3 mL H2O. Increasing concentrations of methacholine (0–1 mg/kg) were administered intravenously. The Snapshot perturbation maneuver was imposed to measure airway resistance (R). A lower resistance response to methacholine (R) value for OVA-sensitized and ASM-024-treated compared to untreated mice indicates lower airway responsiveness. Results are expressed as percentage of increase resistance. The effective dose of methacholine (mg/kg) required to induce a 250% (ED250) increase of airway resistance was calculated.
In vitro Smooth Muscle Relaxant and Bronchoprotective Effects
The effects of ASM-024 on airway smooth muscle were investigated in isometric studies performed on tracheal and bronchial preparations. Tracheas were isolated from naïve mice and guinea pigs. Human bronchi were isolated from healthy lung tissue obtained from patients undergoing resection for lung cancer. Human tissues were obtained from the IUCPQ site of the Respiratory Health Network Tissue Bank of the FRQS (www.tissuebank.ca), subjects were non-smokers or ex-smokers for more than 6 months with normal respiratory functions. Bronchi were also isolated from lung of euthanized healthy dogs.
Tracheal or bronchial preparations devoid of adherent connective tissue were suspended in 5 mL organ baths containing Krebs solution. Krebs composition in mM: NaCl 118, KCl 4.7, KH2PO4 1.18, MgSO4 1.18, NaHCO3 25, glucose 5.6, CaCl2 2.5. The tissues were maintained at 37°C and continually gassed with 5% CO2 in O2. A passive tension of 0.5 g was applied to mouse and guinea pig tracheas and dog bronchi and 1 g was applied for human bronchial preparations. Tension was maintained for a 30–60 minutes equilibration period until it achieved a steady state. Tissues were then contracted with methacholine (10−5 M), a muscarinic agonist, or histamine (10−5 M) (for guinea pig tracheas), cumulative concentrations of ASM-024 (10−7 to 10−3 M) were added and changes of tension recorded. The results are expressed as a percentage of maximal contraction.
To assess the potential protective effect on smooth muscle contraction, isolated mouse tracheas were first exposed to 10−5 M methacholine and the reference contraction was recorded. Once a plateau was reached, the tracheas were washed and re-equilibrated for 30 minutes and then treated for 10 minutes with ASM-024 (10−6 to 10−3 M) or vehicle, and contractile response to increasing concentrations of methacholine (10−8 to 10−5 M) were recorded; contraction is expressed as percentage of the maximal reference tension induced by 10−5 M methacholine. In another set of experiment, ASM-024 pre-treated tracheas were washed extensively and after a period of 15 or 60 minutes were contracted with increasing concentrations of methacholine.
In some experiments, mouse tracheas were exposed to 10−2 M and 10−1 M hexamethonium, a non-selective nAChR antagonist, for 10 minutes before the addition of increasing concentrations of ASM-024.
Statistical Analysis
Data in the text and figure legends are expressed as mean ± SD. For isometric studies, sample size (n values) equal the number of animals from which tracheas and bronchi were taken. EC50 and ED250 values were estimated by linear regression of individual concentration-responses curves using XLfitTM (IDBS). Statistical significance of differences between means was determined by one-way ANOVA followed by Tukey test. For AR and isometric experiments, data were analyzed using a repeated mixed model. The multivariate normality assumptions were verified with the Shapiro-Wilk test after a Cholesky factorization. The Brown and Forsythe's variation of Levene's test statistic was used to verify the homogeneity of variances (statistical package SAS, version 9.1.3, SAS Institute Inc., Cary, NC, U.S.A.). The results were considered significant with p-values < 0.05.
Results
In Vivo Anti-inflammatory Effects in Allergen-Sensitized Mice
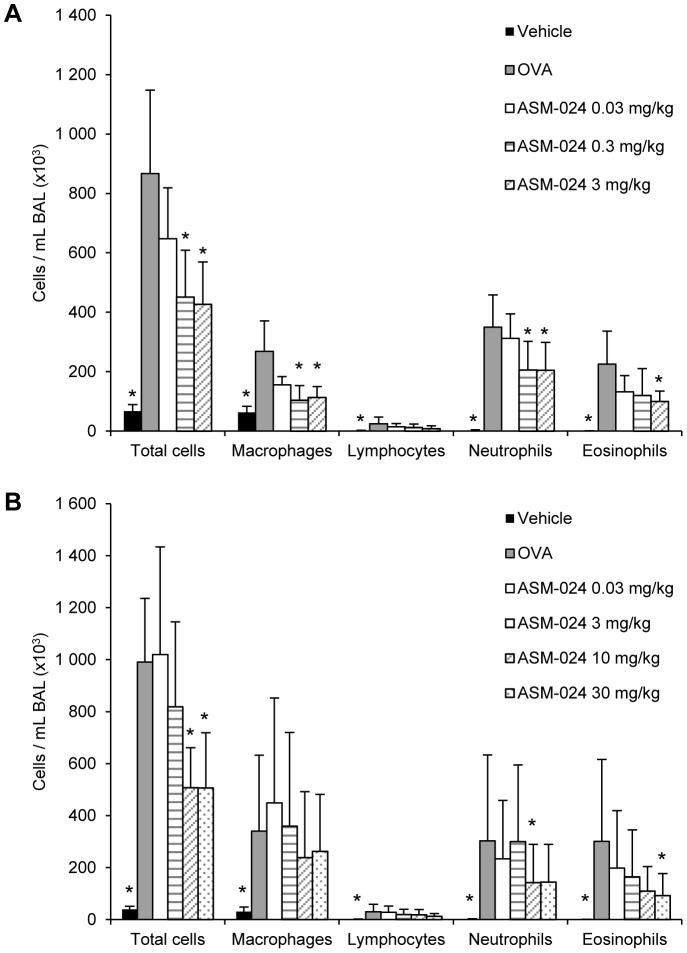
Mice that received ASM-024 at doses up to 30 mg/kg p.o. showed no clinical evidence of side effects. The pharmacological effects of ASM-024 are presented in figures 1a and b. ASM-024, given by oral or nebulisation to OVA-sensitized mice decreased lung inflammation as demonstrated by a dose dependent decrease in the number of inflammatory cells recovered by BAL. Although the number of cells did not decrease to the levels found in control non-sensitized animals, the decrease was dose proportional and highly significant (p < 0.002) when compared to OVA-sensitized mice which did not receive ASM-024. BAL cell differential counts showed that all cell populations, including eosinophils, were decreased and that the relative percentages remained similar.
Figure 1. Effect of ASM-024 on cellular inflammation.
ASM-024 was administered by (A) nebulization of a solution and (B) orally to OVA-sensitized mice. A dose-related inhibition of inflammation is observed, no further inhibitory effect observed at a dose higher than 10 mg/kg p.o.; Values are mean ± SD. * Significantly different from vehicle control groups; p≤0.001; n = 8–9; ED50 p.o. = 3.55 mg/kg; ED50 inhalation = 0.03 mg/kg.
In Vivo Airway Responsiveness in Allergen-Sensitized Mice
The methacholine ED250 values indicated that airway responsiveness (AR) to methacholine was significantly decreased following administration of ASM-024 by inhalation at doses of 1.5 and 3 mg/kg but not at 0.3 mg/kg. A statistically significant attenuation of AR was also observed after intranasal instillation of ASM-024 at a dose of 3 mg/kg (Table 1).
Table 1. Effective dose of methacholine (mg/kg) required to induce a 250% increase of airway resistance.
| Group | Mean ± SD ED250 |
| Control naive mice | 0.234±0.043 * |
| OVA_Vehicle | 0.089±0.031 |
| OVA_ ASM-024 0.3 mg/kga | 0.111±0.027 |
| OVA_ ASM-024 1.5 mg/kga | 0.145±0.032* |
| OVA_ ASM-024 3.0 mg/kga | 0.128±0.037* |
| OVA_ ASM-024 3.0 mg/kgb | 0.159±0.043* |
Inhalation, b Intranasal
p<0.05 compared to vehicle-treated OVA-sensitized mice.
ASM-024 administered by intranasal or inhalation routes immediately prior to the methacholine challenge to OVA-sensitized mice attenuated airway hyper-responsiveness.
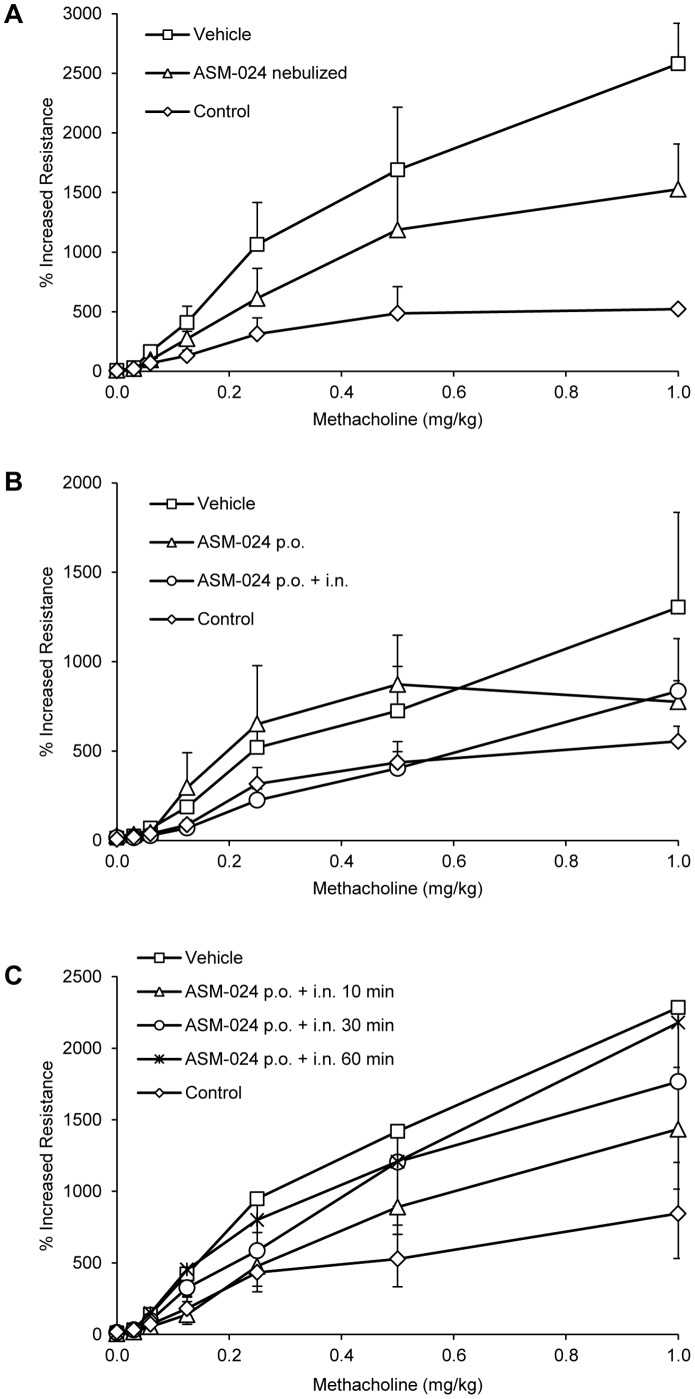
No significant effect on AR was observed following oral administration of 4 mg/kg during 7 days. A previous experiment where a dose of 10 mg/kg was used showed no effect on AR (data not shown). However, a significant reduction was observed when the drug was administered by intranasal instillation at a dose of 4 mg/kg, 15 minutes before the methacholine challenge (Figure 2b), and this effect was time-dependant (Figure 2c).
Figure 2. Effect of ASM-024 on lung tissue resistance in response to methacholine.
ASM-024 was administered by (A) nebulization of a solution and (B and C) orally to OVA-sensitized mice. A single 5 minutes exposure to 1.5 mg/kg nebulized ASM-024 prior to the methacholine challenge significantly decreased airway resistance in OVA-sensitized mice; p<0.05 for 0.06, 0.125, 0.25 and 1 mg/kg methacholine (A). Oral administration of 4 mg/kg ASM-024 during 7 days has no significant effect on AR. An additional intranasal instillation of 4 mg/kg ASM-024, 15 minutes before the methacholine challenge reduced AR; p<0.05 for 0.06 to 0.5 mg/kg methacholine. Values are expressed as a percentage of increased resistance; mean ± SD; n = 3–8 for control, n = 6–8 each for untreated and ASM-024-treated groups (B). The local effect on airway resistance is time-dependant; maximal relaxation is observed at about 10 minutes before the methacholine challenge (C).
In vitro Smooth Muscle Relaxant and Protective Effects
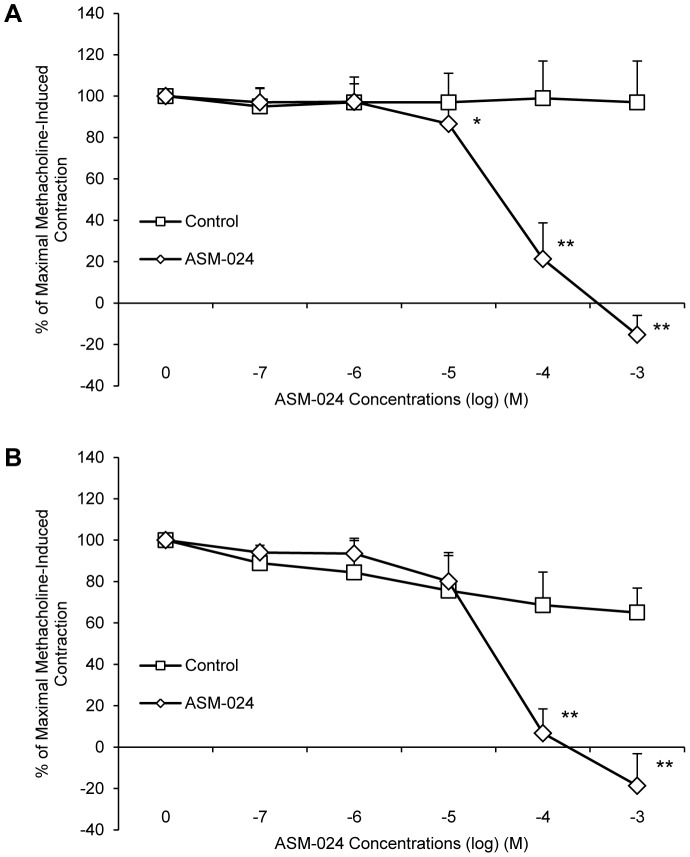
ASM-024 produced a significant dose-related relaxation of methacholine-contracted mouse and guinea-pig tracheas with an overall mean EC50 value of 62.23±17.54 µM for mouse and 43.39±19.19 for guinea pig. Control tracheas maintained their contraction (Figures 3a and 3b).
Figure 3. In vitro ASM-024-induced relaxation of methacholine-contracted (A) mouse and (B) guinea pig tracheas.
Values (mean ± SD) are expressed as% of maximal methacholine-induced contraction; n = 10–18. Mouse tracheas EC50 = 54.15±24.15 µM, guinea pig trachea EC50 = 43.39±19.19 µM. * Significantly different from vehicle control, p<0.05; ** significantly different from vehicle control, p<0.0001.
Similar experiments were performed using dog bronchi and human bronchial preparations obtained from patients undergoing lung resection. A dose-dependent smooth muscle relaxation of dog (Figure 4a) and human (Figure 4b) pre-contracted bronchi was observed.
Figure 4. In vitro ASM-024-induced relaxation of methacholine-contracted (A) dog and (B) human bronchi preparations and representative tension recording depicting relaxation response of dog bronchial rings and partial reversion after washing (C).
Values expressed as% of maximal methacholine-induced contraction are means ± SD; n = 8–18. * Significantly different from vehicle control, p<0.05; ** significantly different from vehicle control, p≤0.0001. Dog bronchi EC50 = 60.51±30.76 µM, human bronchi EC50 = 110.28±36.96 µM. Eq: equilibrium, MCh: methacholine.
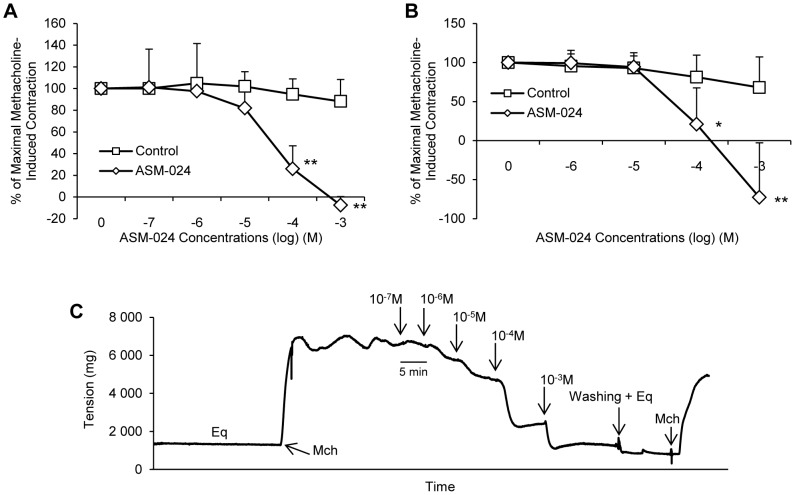
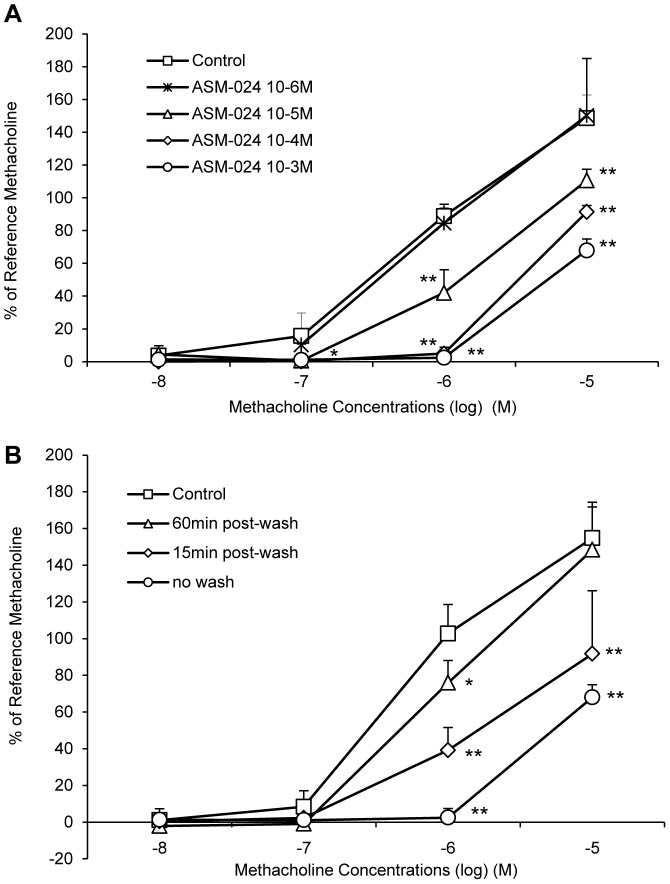
ASM-024-induced relaxation occurs within minutes depending on the concentration. Steady state is obtained within 3 to 5 minutes and was maintained for as long as the compound was in the organ bath. It was however partially reversible as extensive washing of the tracheas followed by the addition of methacholine resulted in the return of the contraction at a magnitude lower than the initial contraction (Figure 4c). Once the relaxation effect of ASM-024 was well demonstrated, we verified if the drug could block the contraction induced by methacholine. The results of this experiment are presented in Figure 5 where it can be seen that ASM-024 at a 10−3 to 10−5 M concentration attenuated the contractile effect of methacholine (Figure 5a). This effect was transient since it had waned after ASM-024 had been removed 15 minutes prior to the addition of methacholine and almost completely disappeared after 60 minutes of the removal of ASM-024 (Figure 5b).
Figure 5. Protective effect of ASM-024 on methacholine-induced contraction of mouse tracheas.
(A) A dose-related inhibition of contraction is observed. (B) A time-dependant decrease in methacholine-induced contraction is observed up to 60 minutes after ASM-024 removal from the milieu. Application of ASM-024 alone did not affect basal tension. Values expressed as% of maximal methacholine-induced contraction (10−5 M) recorded prior to ASM-024 treatments are means ± SD; n = 3–4.
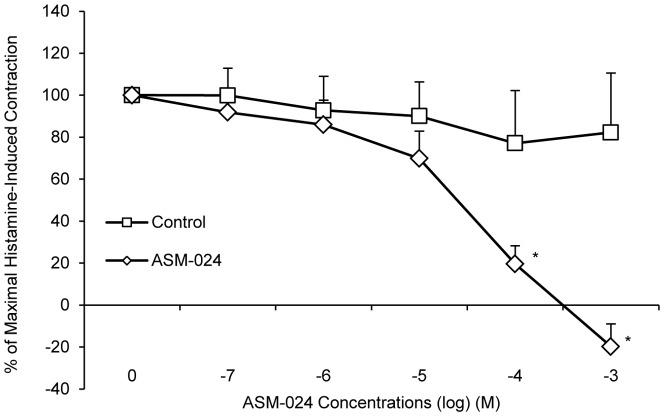
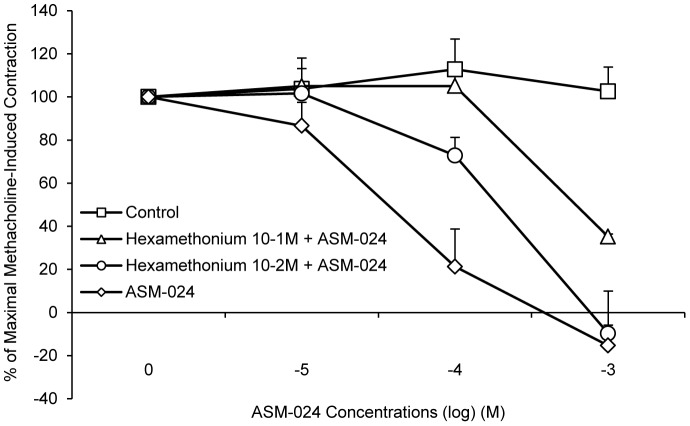
To evaluate whether ASM-024 is able to induce relaxation of a non-muscarinic contractile agent, its effect on histamine-induced contraction was evaluated. The experiments were conducted on guinea pig tracheas which, contrary to mouse tracheas, express histamine receptors (Figure 6). ASM-024 dose dependently reduced the contractile response to histamine (EC50 = 39.06±29.10 µM). The addition of hexamethonium, a subtype non-specific non-competitive nAChR antagonist, resulted in partial reversal of ASM-024-induced myorelaxation in methacholine pre-contracted mouse tracheas. The EC50 values were 239.13±41.37 µM and 540.60±1.00 µM for hexamethonium (10−2 M) + ASM-024, and hexamethonium (10−1 M) + ASM-024, respectively (figure 7).
Figure 6. In vitro ASM-024-induced relaxation of histamine-contracted guinea pig tracheas.
Values expressed as% of maximal histamine-induced contraction are means ± SD; n = 5–9. * Significantly different from vehicle control, p<0.001. EC50 = 39.06±29.10 µM.
Figure 7. Partial reversal of ASM-024-induced relaxation.
A dose-dependent shift to the right in the dose-response curve of ASM-024-induced relaxation is observed upon pre-treatment with hexamethonium, a nAChR antagonist (EC50 = 239.13±41.37 µM and 540.60±1.00 µM vs 62.23±17.54 µM for ASM-024). Values expressed as% of maximal methacholine-induced contraction (10−5 M) after addition of hexamethonium recorded prior to ASM-024 treatments are means ± SD; n = 2–4.
Discussion
Although this study was not designed to specifically address the toxicology profile of ASM-024, the results show that this drug can induce significant pharmarcological activities relevant to asthma at doses that showed no evidence of toxicity either in in vivo or in vitro models. In the course of the clinical development of ASM-024, a comprehensive nonclinical safety program was conducted with ASM-024 in rats and dogs including safety pharmacology, toxicology, and genotoxicity studies. Under single oral administration, ASM-024 was well tolerated at oral doses up to 320 mg/kg in rats and 120 mg/kg in dogs and up to 40 mg/kg in both rats and dogs by nebulization [35]. These doses are well above the pharmacological active doses determined in mouse models of allergic airway inflammation and hyperreactivity. ASM-024 showed no evidence of genotoxicity in the standard battery of genotoxicity studies and no evidence of producing hypersensitisation in an inhalation model in the guinea pig. Results of the studies reported here show that ASM-024 has anti-inflammatory, smooth muscle relaxant and bronchoprotective properties. Airway cellular inflammation was attenuated following ASM-024 administration both via oral and inhalation routes, indicating that the drug displays both local and systemic anti-inflammatory activity, although the doses for which a pharmacological effect is observed were higher by the oral administration. This may be due to poor oral systemic absorption and/or rapid hepatic first pass metabolism. The pharmacological effects observed with ASM-024 were comparable to those observed with DMPP [20] and ASM-002, another ASM-024 analogue (unpublished data). These effects were associated with an in vivo reduction of serum IgE, lung tissue inflammation and in vitro reduction of eosinophil migration and LTC4 release. However these latest experiments were not repeated with ASM-024.
Moreover, direct short term local administration either through intranasal treatment or by nose-only nebulization just before a methacholine challenge led to a significant reduction in airway tissue resistance which was not observed after oral or nebulized administration suggesting that ASM-024 has a direct local effect on airway smooth muscle relaxation. These results indicate that the decrease in inflammatory response is not necessarily associated with a reduction of airway hyperresponsiveness, but indicate a dual mechanism of action on cellular inflammation independent of the direct airway relaxation effect.
In isometric in vitro studies, ASM-024 inhibited the contractile response to methacholine and histamine in a dose-dependent and reversible manner. Of interest is that a similar smooth muscle relaxation was observed in the four species studied, including man. Not only does ASM-024 induced relaxation it also had a protective effect on methacholine-induced constriction.
Most structural and inflammatory cells present in the respiratory tract express nicotinic and/or muscarinic receptors and both types have been involved in the regulation of inflammatory responses [12], [25]. Activation of nicotinic receptors by acetylcholine and nicotinic agonists, particularly the α7 nAChR subtype [13] but also other subtypes, can limit inflammation through signal transduction pathways that do not involve ion channel activation [26]–[28]. Activation of muscarinic receptors by acetylcholine has pro-inflammatory and airway remodelling effects that are associated with COPD and asthma [15], and indeed there is now evidence that muscarinic receptor antagonists are also associated with anti-inflammatory, anti-proliferative, and anti-remodeling effects [25], [29].
The precise pharmacological target and mode of action of ASM-024 on inflammation and smooth muscle relaxation has not been fully elucidated. ASM-024 is a DMPP derivative and although it is a weak agonist, a modulatory role via nicotinic receptors is probable. Recent observations have indicated that ASM-024 may function as a "silent nicotinic receptor agonist" since alone it does not induce activation of the nicotinic ion channel but when co-applied with the positive α7 receptor allosteric modulator PNU-120596, it is able to activate the α7 receptor ion channel currents [23]. Ongoing studies are being conducted to demonstrate that ASM-024 and other similar pharmacophore(s) may act on signaling pathways independently of ion channel activation. Indeed, other signal transduction mechanisms not involving channel opening have been shown to occur via certain subtype-selective nAChRs [30]–[33].
In isometric studies, the effectiveness of ASM-024 to relax both methacholine- and histamine-induced tracheal contraction suggest that a mechanism other than specific antagonism of cholinergic muscarinic receptors is involved in the antispasmodic action. Recent observations have indicated that ASM-024 dose-dependently inhibited intracellular Ca2+ increase on cultured tracheo-bronchial human smooth muscle cells stimulated with histamine (unpublished data). The precise mechanism of ASM-024 effect on intracellular calcium decrease has yet to be defined: Ca2+ influx through other membrane Ca2+ channels, Ca2+ mobilisation, Ca2+ reuptake and/or Ca2+ efflux are other important pathways being investigated. ASM-024 may have a different pharmacological activity than currently-available compounds for the treatment of asthma and COPD. Since the epithelium was not removed, an indirect effect through epithelium derived factors cannot be ruled out. However the rapidity of the relaxation may suggest a direct effect at the airway smooth muscle level. Nicotinic receptors are expressed on airway smooth muscle [21], [30] but their role on these cells is unknown. In a previous study using the nicotinic agonist DMPP [21], the relaxant effect was partially reversed with hexamethonium a non-selective, nAChR antagonist, suggesting a potential role of nicotinic receptors in the relaxation response, similar results were observed with ASM-024. nAChRs are permeable to Na+ and K+ and to a lesser extent to Ca2+ so a direct modulatory role of cation conductance by ASM-024 through these receptors is possible.
Although the concentrations used to achieve pharmacological responses in vitro were relatively high, the in vivo doses were within well tolerated range and suggest that the amount of drug that would be required to achieve clinically relevant pharmacological effects would not preclude its potential use in humans to treat asthma. One explanation for this disconnect between in vitro and in vivo data may be related to the rapid desensitization of the nicotinic receptor upon binding of the ligand, essentially resulting in the need for continuous exposure to higher concentrations in order to achieve a pharmacological effect in vitro whereas in vivo recovery from the desensitized state may occur more rapidly [34].
Further studies are planned to define the precise mechanisms of action for ASM-024. ASM-024 appears to be a modulator of cholinergic receptor function. Its action seems to be multi-mediated as it has demonstrated a novel three-fold mechanism of action of i) non-competitive inhibition nicotinic receptor ion channel function, ii) potential orthosteric modulation of α7-mediated signal transduction via non-conducting states and iii) blockade of muscarinic activity by a mechanism independent of a direct specific antagonism of cholinergic muscarinic receptors.
Overall, this compound displays anti-inflammatory, smooth muscle relaxant and bronchoprotective properties, with a different pharmacological profile than currently available compounds for the treatment of asthma and COPD and may represent an interesting and new approach to treat these pulmonary diseases.
Acknowledgments
The authors thank Dr Papke and his team at the University of Florida for their collaboration to further elucidate the molecular mechanisms of action of ASM024.
The authors thank the staff at the Respiratory Health Network Tissue Bank for their valuable assistance.
Funding Statement
This work was supported by grants from Asmacure Ltée, 2600 boul. Laurier, Suite 880, Québec, QC. G1V 4W2. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization (2008) Asthma fact sheet No. 307. Updated November 2013. Available: http://www.who.int/mediacentre/factsheets/fs307/en/index.html. Accessed 2013 Dec 31.
- 2.Global Initiative for Asthma (GINA) (2011) Global Strategy for Asthma Management and Prevention. Available: http://www.ginasthma.org/. Accessed 2013 Dec31.
- 3. Bousquet J, Bachert C, Canonica GW, Casale TB, Cruz AA, et al. (2009) Extended Global Allergy and Asthma European Network, World Allergy Organization and Allergic Rhinitis and its Impact on Asthma Study Group. Unmet needs in severe chronic upper airway disease (SCUAD). J Allergy Clin. Immunol 124: 428–433. [DOI] [PubMed] [Google Scholar]
- 4. Yates DH, Worsdell M, Barnes PJ (1997) Effect of regular salmeterol treatment on albuterol-induced bronchoprotection in mild asthma. Am J Respir Crit Care Med 156: 988–991. [DOI] [PubMed] [Google Scholar]
- 5. Sears MR (2002) Adverse effects of β-agonists. J Allergy Clin Immunol 110: S322–328. [DOI] [PubMed] [Google Scholar]
- 6. Barnes PJ, Adcock IM (2009) Glucocorticoid resistance in inflammatory diseases. Lancet 373 (9678): 1905–1917. [DOI] [PubMed] [Google Scholar]
- 7. O′Byrne PM, Naji N, Gauvreau GM (2012) Severe asthma: future treatments. Clinical & Experimental Allergy 42 (5): 706–711. [DOI] [PubMed] [Google Scholar]
- 8. Kupczyk M, Wenzel S (2012) U.S. and European severe asthma cohorts: what can they teach us about severe asthma? J Intern Med 272 (2): 121–32. [DOI] [PubMed] [Google Scholar]
- 9.Durham A, Adcock IM, Tliba O (2011) Steroid resistance in severe asthma: current mechanisms and future treatments. Curr Pharm Des 17 ((7) ) 674–684. [DOI] [PubMed] [Google Scholar]
- 10. Pieper MP (2012) The non-neuronal cholinergic system as novel drug target in the airways. Life Sci 91 (21–22) 1113–1118. [DOI] [PubMed] [Google Scholar]
- 11. Pavlov VA (2008) Cholinergic modulation of inflammation. Int J Clin Exp Med 1: 203–212. [PMC free article] [PubMed] [Google Scholar]
- 12. Gwilt CR, Donnelly LE, Rogers DF (2007) The non-neuronal cholinergic system in the airways: an unappreciated regulatory role in pulmonary inflammation? Pharmacol Ther 115: 208–222. [DOI] [PubMed] [Google Scholar]
- 13. Wang H, Yu M, Ochani M (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 42: 384–388. [DOI] [PubMed] [Google Scholar]
- 14. Hurst R, Rollema H, Bertrand D (2013) Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther 137(1): 22–54. [DOI] [PubMed] [Google Scholar]
- 15. Karakiulakis G, Roth M (2012) Muscarinic Receptors and Their Antagonists in COPD: Anti-Inflammatory and Anti-remodeling Effects. Mediators Inflamm 2012: 409580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Troisi RJ, Speizer FE, Rosner B, Trichopoulos D, Willett WC (1995) Cigarette smoking and incidence of chronic bronchitis and asthma in women. Chest 108: 1557–1561. [DOI] [PubMed] [Google Scholar]
- 17. Godtfredsen NS, Lange P, Prescott E, Osler M, Vestbo J (2001) Changes in smoking habits and risk of asthma: a longitudinal population based study. Eur Respir J 18: 549–554. [DOI] [PubMed] [Google Scholar]
- 18. Sopori M (2002) Effects of cigarette smoke on the immune system. Nat Rev Immunol 2(5): 372–377. [DOI] [PubMed] [Google Scholar]
- 19. Floto RA, Smith KG (2003) The vagus nerve, macrophages, and nicotine. Lancet 361 (9363): 1069–1070. [DOI] [PubMed] [Google Scholar]
- 20. Blanchet MR, Israël-Assayag E, Cormier Y (2005) Modulation of airway inflammation and resistance in mice by a nicotinic receptor agonist. Eur Respir J 26: 21–27. [DOI] [PubMed] [Google Scholar]
- 21. Dorion G, Israël-Assayag E, Beaulieu MJ, Cormier Y (2005) Effect of 1,1-dimethylphenyl 1,4-piperazinium on mouse tracheal smooth muscle responsiveness. Am J Physiol Lung Cell Mol Physiol 288(6): L1139–45. [DOI] [PubMed] [Google Scholar]
- 22. Blanchet MR, Langlois A, Israël-Assayag E, Beaulieu MJ, Ferland C, et al. (2007) Modulation of eosinophil activation in vitro by a nicotinic receptor agonist. J Leukoc Biol 81: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 23.Stokes C, Israël-Assayag E, Papke RL (2013) A minimal pharmacophore for a nicotinic alpha7 silent agonist, relevance for the therapeutic development of treatments for asthma. Abstract presented at the Society for Neuroscience, November, San-Diego.
- 24.Chojnacka K, Papke RL, Horenstein NA (2013) Synthesis and evaluation of a conditionally-silent agonist for the α7 nicotinic acetylcholine receptor. Bioorganic and Medicinal Chemistry Letters; 23 4145–4149. [DOI] [PMC free article] [PubMed]
- 25. Kolahian S, Gosens R (2012) Cholinergic regulation of airway inflammation and remodelling. J Allergy (Cairo) 2012: 681258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hosur V, Loring RH (2011) α4β2 nicotinic receptors partially mediate anti-inflammatory effects through Janus kinase 2-signal transducer and activator of transcription 3 but not calcium or cAMP signaling. Mol Pharmacol 79(1): 167–74. [DOI] [PubMed] [Google Scholar]
- 27. Mikulski Z, Hartmann P, Jositsch G, Zasłona Z, et al. (2010) Nicotinic receptors on rat alveolar macrophages dampen ATP-induced increase in cytosolic calcium concentration. Respiratory Research 11: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simard AR, Gan Y, St-Pierre S, Kousari A, Patel V, et al. (2013) Differential modulation of EAE by α9*- and β2*-nicotinic acetylcholine receptors. Immunology and Cell Biology 91(3): 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kistemaker LE, Oenema TA, Meurs H, Gosens R (2012) Regulation of airway inflammation and remodeling by muscarinic receptors: perspectives on anticholinergic therapy in asthma and COPD. Life Sci 91(21–22): 1126–1133. [DOI] [PubMed] [Google Scholar]
- 30.Wessler I, Kirkpatrick CJ (2008) Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. British Journal of Pharmacology 154: , 1558–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suzuki T, Hide I, Matsubara A, Hama C, Harada K, et al. (2006) Microglial alpha7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J Neurosci Res 83(8): 1461–1470. [DOI] [PubMed] [Google Scholar]
- 32. de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, et al. (2005) Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol 6(8): 844–851. [DOI] [PubMed] [Google Scholar]
- 33. Marrero MB, Bencherif M (2009) Convergence of alpha 7 nicotinic acetylcholine receptor-activated pathways for anti-apoptosis and anti-inflammation: central role for JAK2 activation of STAT3 and NF-kappaB. Brain Res 1256: 1–7. [DOI] [PubMed] [Google Scholar]
- 34. Buccafusco JJ, Beach JW, Terry AV Jr (2009) Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exp Ther 328(2): 364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Israël-Assayag E, Vachon L, Aubin S, Cormier Y (2010) Targeting Nicotinic Acetylcholine Receptors To Treat Asthma: Pharmacokinetics And Metabolism Of ASM-024, A New Nicotinic Receptor Agonist AJRCCM conference.181.1 Meeting Abstracts.A5398.