Abstract
Elevated atmospheric CO2 can change foliar tissue chemistry. This alters leaf litter palatability to macroinvertebrate detritivores with consequences for decomposition, nutrient turnover, and food-web structure. Currently there is no consensus on the link between CO2 enrichment, litter chemistry, and macroinvertebrate-mediated leaf decomposition. To identify any unifying mechanisms, we presented eight invertebrate species from aquatic and terrestrial ecosystems with litter from Alnus glutinosa (common alder) or Betula pendula (silver birch) trees propagated under ambient (380 ppm) or elevated (ambient +200 ppm) CO2 concentrations. Alder litter was largely unaffected by CO2 enrichment, but birch litter from leaves grown under elevated CO2 had reduced nitrogen concentrations and greater C/N ratios. Invertebrates were provided individually with either (i) two litter discs, one of each CO2 treatment (‘choice’), or (ii) one litter disc of each CO2 treatment alone (‘no-choice’). Consumption was recorded. Only Odontocerum albicorne showed a feeding preference in the choice test, consuming more ambient- than elevated-CO2 birch litter. Species’ responses to alder were highly idiosyncratic in the no-choice test: Gammarus pulex and O. albicorne consumed more elevated-CO2 than ambient-CO2 litter, indicating compensatory feeding, while Oniscus asellus consumed more of the ambient-CO2 litter. No species responded to CO2 treatment when fed birch litter. Overall, these results show how elevated atmospheric CO2 can alter litter chemistry, affecting invertebrate feeding behaviour in species-specific ways. The data highlight the need for greater species-level information when predicting changes to detrital processing–a key ecosystem function–under atmospheric change.
Introduction
Global concentrations of atmospheric carbon dioxide (CO2) could more than double by 2100 [1]. Typically, CO2 enrichment leads to increased plant photosynthesis, resulting in greater biomass and production [2]. Plant tissue chemistry is typically modified, with decreasing nitrogen concentrations and increasing carbon-nitrogen (C/N) ratios affecting herbivore life-history and feeding responses [3].
Approximately 90% of primary production in forest ecosystems escapes herbivory and forms detritus [4], providing a crucial energy pool that underpins the trophic structure of soils and adjacent freshwaters [5]. The effect of elevated CO2 on the chemical composition of green foliar tissues reduces its palatability to detritivores when it falls as litter [6]. In particular, elevated CO2 can reduce litter resource quality by decreasing litter nitrogen content [7], [8], subsequently increasing C/N ratios [9], [10]. Increases in structural [6], [8], [9] and defensive [10], [11] compounds have also been reported, along with both increases and decreases in phosphorus concentrations [12], [13]. The potential for rising CO2 concentrations to alter litter chemical composition is established, but the consequences for invertebrate-mediated decomposition – an important ecosystem function – remain unclear [14].
Detritivorous macroinvertebrates are functionally important in detritus-based ecosystems, as they are responsible for both comminution and consumption of litter, releasing nutrients for other organisms, such as saprophagous fungi [15], [16]. To maintain optimal body nutrient concentrations, theoretical predictions and empirical evidence suggest that invertebrates can increase feeding rates of reduced-quality material (e.g. [17], [18]), a process known as ‘compensatory feeding’ (as defined by [19]). Despite this, poor quality litter has also been shown to increase handling times [20], while reducing nutrient assimilation, slowing development rates, and increasing mortality [6], [21]. These conflicting responses have resulted from studies focusing on a small number of species (e.g. [13], [18]), which also fail to incorporate aquatic and terrestrial invertebrates, despite differences in detrital accumulation and energy flow between these habitats [22]. A broad-scale study incorporating a range of invertebrate species from different habitats is essential to identify the unifying mechanisms that govern invertebrate feeding responses to elevated-CO2 litter.
We investigated the feeding preferences and consumption rates of eight detritivorous macroinvertebrate species presented with Alnus glutinosa (Linnaeus) Gaertner (common alder) and Betula pendula Roth (silver birch) leaf litter produced under ambient and elevated atmospheric CO2. We tested the hypotheses that: (1) CO2 enrichment will reduce leaf chemical quality and, given nitrogen-fixing ability in alder, responses will differ by tree species; (2) when presented with a choice between ambient and elevated CO2 litter, invertebrates will prefer ambient material due to its higher quality; (3) when given litter of one CO2 treatment only, consumption of elevated-CO2 litter will be greater, to compensate for its reduced quality.
Methods
Leaf Litter Preparation
Alder and birch litters were produced at the BangorFACE facility, Bangor, UK [23] (Fig. 1). Trees were grown in eight identical plots (four ambient-CO2 and four elevated-CO2) to minimise infrastructure-induced artefacts. CO2 enrichment was carried out using high velocity pure CO2 injection, controlled using equipment and software modified from EuroFACE [24]. Elevated CO2 concentrations, measured at 1 min intervals, were within 30% deviation from the pre-set target concentration of 580 ppm CO2 (ambient +200 ppm) for 75–79% of the photosynthetically-active period (daylight hours from budburst until leaf abscission) of 2005–2008. Vertical profiles of CO2 concentration measured at 50 cm intervals through the canopy showed a maximum difference of +7% from reference values obtained at the top of the canopy [23]. From the beginning of leaf senescence, fallen leaf litter was collected weekly until all leaves had abscised (October to December). Litter within each CO2 treatment was homogenised and air-dried.
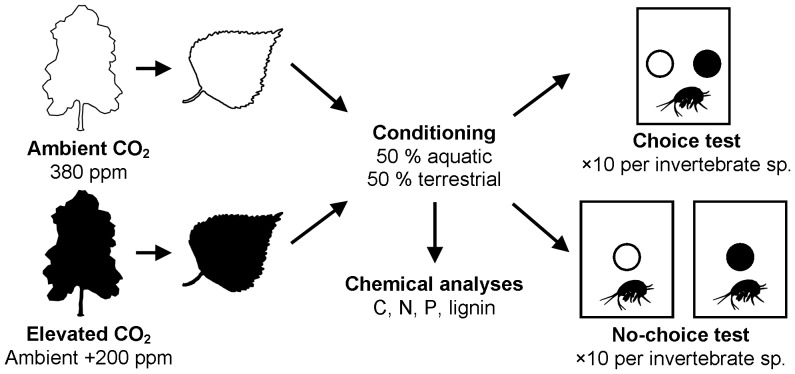
Figure 1. Overview of the experimental approach.
Litter was produced under ambient- and elevated-CO2 atmospheres at BangorFACE, UK. Half of the litter from each CO2 treatment was conditioned aquatically and half terrestrially. Chemical analyses of the conditioned litter were undertaken, and litter discs were presented to aquatic and terrestrial invertebrates in choice and no-choice tests. Only one tree and one invertebrate species have been shown for clarity. Not to scale.
Initial chemical leaching and microbial colonisation of litter (‘conditioning’) are crucial steps in making litter palatable to detritivorous macroinvertebrates [25], [26]. Prior to the start of the experiment, litter was conditioned in fine mesh bags (100 µm to permit microorganisms only) placed in plastic containers (29×29×10 cm; Fig. 1). For each tree species ×CO2 treatment combination, one bag was placed in aerated stream water that was inoculated with stream-collected litter of mixed-species origin (‘aquatic conditioning’); a second bag per tree species ×CO2 treatment combination was inserted between field-collected soil and mixed deciduous leaf litter (‘terrestrial conditioning’). Containers were maintained at 11±1°C with a 12∶12 h light-dark cycle and terrestrial containers were sprayed with deionised water every three days to maintain humidity (∼50%). These conditions were selected to represent natural conditioning processes in aquatic and terrestrial habitats in a controlled manner. After two weeks, leaf discs were cut using a 9 mm diameter cork-borer (avoiding the mid-vein), which were air-dried and weighed (±0.1 mg) prior to experimental use.
Litter samples allocated to chemical analyses (Fig. 1) were stored at –80°C before being oven-dried (50°C for 24 h) and ground into powder (120 s, 50 beats s–1; Pulverisette 23 ball mill, Fritsch GmbH, Idar-Oberstein, Germany). Each sample was composed of litter from three separate leaves. For carbon, nitrogen and phosphorus analyses, five samples were processed per tree × CO2 treatment × conditioning type combination; for lignin analysis, four samples were used. The percentage leaf dry mass (% leaf DM) of carbon and nitrogen, and the carbon-nitrogen (C/N) ratio, were determined by flash combustion and chromatographic separation of ∼1.5 mg leaf powder using an elemental analyser (Elemental Combustion System 4010 CHNS-O Analyzer, Costech Analytical Technologies, Inc., Milan, Italy), calibrated against a standard (C26H26N2O2S). Phosphorus concentrations (% leaf DM) were quantified using X-ray fluorescence (see [27] for detailed methodology). The percentage Acetyl-Bromide-Soluble Lignin (% ABSL) was determined following the acetyl bromide spectrophotometric method [28]. Lignin-nitrogen (lignin/N) ratios were calculated for each tree species × CO2 treatment × conditioning treatment combination.
Invertebrates
Eight macroinvertebrate species were selected for study (Table 1), representing a taxonomic range of litter consumers found in temperate forest habitats [29], [30]. Aquatic species were collected from streams in the Brecon Beacons National Park, South Wales, UK (51°50′53″N, 3°22′16″W and 51°50′55″N, 3°33′43″W) and Roath Park, Cardiff, UK (51°30′00″N, 3°10′10″W); terrestrial species were collected from soil-litter interfaces in Bute Park, Cardiff, UK (51°48′49″N, 3°18′24″W). The National Park Authority granted general permission to access sites on common land in the Brecon Beacons National Park, South Wales, UK. Cardiff Council granted permission for access to sites in Cardiff, UK. No endangered or protected species were involved in collections from the field. All individuals were adults, apart from larval Odontocerum albicorne and Sericostoma personatum caddisflies. Individuals from within each species were selected for size similarity. Prior to experimental use, invertebrates were maintained for at least four weeks in single-species containers (11±1°C, 12∶12 h light-dark cycle) and were fed Fagus sylvatica Linnaeus (common beech) litter conditioned as for experimental litter, preventing habituation to experimental alder and birch litter. Feeding was ceased two days prior to the experiments to allow for gut clearance.
Table 1. Detritivorous macroinvertebrate species used in the study.
| Habitat | Name | Authority | Order: Family |
| Aquatic | Asellus aquaticus | (Linnaeus 1758) | Isopoda: Asellidae |
| Gammarus pulex | (Linnaeus 1758) | Amphipoda: Gammaridae | |
| Odontocerum albicorne | (Scopoli 1763) | Trichoptera: Odontoceridae | |
| Sericostoma personatum | (Kirby & Spence 1826) | Trichoptera: Sericostomatidae | |
| Terrestrial | Blaniulus guttulatus | (Bosc 1792) | Julida: Blaniulidae |
| Oniscus asellus | Linnaeus 1758 | Isopoda: Oniscidae | |
| Porcellio scaber | Latreille 1804 | Isopoda: Porcellionidae | |
| Tachypodoiulus niger | (Leach 1815) | Julida: Julidae |
Experimental Arenas
All experiments were conducted in 11×16.5×3.5 cm lidded plastic arenas (Cater For You Ltd, High Wycombe, UK) lined with compacted sterilised aquarium gravel (Unipac, Northampton, UK) and were maintained at 11±1°C with a 12∶12 h light-dark cycle. Aquatic microcosms were filled with 400 ml of filtered (100 µm mesh) stream water (circumneutral pH; collected from 51°50′53″N, 3°22′16″W) and aerated through a pipette tip (200 µl Greiner Bio-One) attached to an air-line. Terrestrial microcosms were sprayed with deionised water every three days to maintain moisture content and humidity (∼50%). All arenas were uniquely labeled (‘microcosm ID’). These standardised conditions were chosen to mimic natural habitats, while minimising the availability of supplementary organic material that could act as a confounding resource during the feeding trials.
For litter of each tree species, detritivores were presented with: (i) a choice between ambient- and elevated-CO2 material, to provide a direct comparison of detritivore preferences, and (ii) a no-choice situation with each CO2 treatment presented on its own, approximating litter consumption in current (ambient-CO2) and future (elevated-CO2) atmospheric conditions (Fig. 1). In each experiment, ten microcosms were set up for each invertebrate and tree species combination (n = 160). A single invertebrate was added to each arena and was placed in the end opposite the airline in aquatic arenas and equidistant to both discs in the choice test. In the choice test, one disc of each CO2 treatment was pinned to the centre of the arena, 4 cm apart. Discs were replenished when at least 50% of the existing disc had been consumed. In the no-choice test, half of the microcosms contained one ambient-CO2 disc and the other half one elevated-CO2 disc, pinned to the centre of the arena. Both experiments ended after 14 days, or when five (50%) of the individuals of a specific species consumed at least 50% of one disc (choice experiment only). For each invertebrate, the total mass of litter consumed was calculated (±0.1 mg). For choice experiment data, this value was divided by the number of days over which the test had taken place.
Additionally, control microcosms were set up to ensure that differences in mass loss between CO2 treatments were due to invertebrate activity alone. For each experiment, ten microcosms were set up for each habitat type × tree species combination. Controls for the choice test each contained one disc of each CO2 treatment; half of the no-choice control microcosms contained one ambient-CO2 disc and the other half contained one elevated-CO2 disc. Leaf discs were air-dried and weighed (±0.1 mg) after 14 days and their total mass loss calculated.
Data Analysis
Statistical analyses were performed separately for alder and birch litter using R version 3.0.1 [31]. Data available from http://dx.doi.org/10.6084/m9.figshare.791634. were checked for normality and homogeneity of variance following Crawley [32]; response variables were transformed using Box-Cox power transformations when assumptions were not met (car package [33]). Significance was set at α = 0.05 for all analyses.
Two-way analysis of variance (ANOVA) was used to test the main and interactive effects of CO2 treatment and microcosm type on each chemical variable (carbon, nitrogen, phosphorus and lignin concentrations, and C/N ratio). Planned contrasts (lsmeans package [34]) were used to compare the effects of CO2 treatments for each conditioning treatment.
The main and interactive effects of CO2 treatment and microcosm type were tested on the mass loss of control discs. Linear mixed-effects models were used to analyse choice control data (nlme package [35]), where non-independence of discs sharing the same microcosm was accounted for by including microcosm ID as a random term. The same fixed terms were used to analyse control data from the no-choice test using two-way ANOVA.
In the choice test, litter consumption per day was analysed using linear mixed-effects models (nlme package [35]) with the main and interactive effects of CO2 treatment and invertebrate species as fixed effects and microcosm ID as a random effect. Planned contrasts were performed to compare consumption of ambient- and elevated-CO2 discs within (i) each invertebrate species, and (ii) invertebrate species grouped by habitat of origin (contrast package [36]).
In the no-choice test, the main and interactive effects of CO2 treatment and invertebrate species on litter consumption were tested using two-way ANOVA. Planned contrasts were performed to test the effects of CO2 treatment on disc consumption within (i) each invertebrate species (lsmeans package [34]) and (ii) invertebrate species grouped by habitat of origin (gmodels package [37]).
Results
Litter Chemistry
CO2 enrichment altered leaf litter chemistry, but effects differed between tree species. For birch, CO2-enriched litter contained lower nitrogen concentrations, and higher lignin concentrations and C/N ratios than ambient-CO2 litter (Tables 2 and 3). Litter chemistry varied between conditioning types, with higher carbon concentrations in aquatically-conditioned litter and lower nitrogen concentrations in terrestrially-conditioned litter (Table 2). For both conditioning types, elevated-CO2 litter contained lower nitrogen concentrations (aquatic, estimate = 0.76% DM, P<0.001; terrestrial, estimate = 1.17% DM, P<0.001; Table 3) and higher C/N ratios (aquatic, estimate = 8.31, P<0.001; terrestrial, estimate = 10.28, P<0.001; Table 3). For alder litter, the effect of CO2 treatment was less predictable, with differential responses between conditioning types (Table 2). Elevated CO2 increased alder nitrogen concentrations when conditioned terrestrially (estimate = 0.29% DM, P = 0.036; Table 3), although there was no concurrent effect in aquatically-conditioned litter (estimate = 0.1% DM, P = 0.44; Table 3). No treatment or species effects on litter phosphorus concentrations were observed (Tables 2 and 3).
Table 2. ANOVA summary table of main and interactive effects of CO2 treatment (CO2) and conditioning type (CT) on litter chemistry.
| Carbon | Nitrogen | Phosphorus | Lignin | C/N | |||||||
| Tree species | Variables | F 1,16 | P | F 1,16 | P | F 1,16 | P | F 1,12 | P | F 1,16 | P |
| Alder | CO2 | 0.6 | 0.435 | 1.1 | 0.305 | 2.8 | 0.117 | 0.04 | 0.543 | 1.3 | 0.271 |
| CT | 0.3 | 0.577 | 4.1 | 0.059 | 0.2 | 0.684 | 0.2 | 0.673 | 3.8 | 0.071 | |
| CO2 × CT | 1.5 | 0.241 | 4.7 | 0.045 | 0.4 | 0.387 | 3.6 | 0.082 | 4 | 0.064 | |
| Birch | CO2 | 0.1 | 0.712 | 791 | <0.001 | 3.1 | 0.098 | 4.8 | 0.048 | 605.3 | <0.001 |
| CT | 12.1 | 0.003 | 95 | <0.001 | 0.04 | 0.848 | 1 | 0.331 | 62.5 | <0.001 | |
| CO2 × CT | 3.6 | 0.077 | 36.4 | <0.001 | 0.3 | 0.566 | 0.1 | 0.756 | 6.8 | 0.019 | |
P values <0.05 are emboldened.
Table 3. Chemical composition of leaf litter (mean ±1 SEM).
| Chemical composition | Chemical ratios | |||||||
| Tree species | CT | CO2 | Carbon(% DM) | Nitrogen(% DM) | Phosphorus(% DM) | Lignin(% ABSL) | C/N | Lignin/N |
| Alder | Aquatic | Ambient | 48.61±0.37a | 3.73±0.16a | 0.074±0.009a | 22.17±2.64a | 13.11±0.16a | 5.94 |
| Elevated | 48.48±0.25a | 3.63±0.091a | 0.064±0.009a | 19.56±2.74a | 13.37±0.36a | 5.38 | ||
| Terrestrial | Ambient | 48.04±0.22a | 3.35±0.016a | 0.084±0.009a | 19.16±1.01a | 14.33±0.02a | 5.71 | |
| Elevated | 48.68±0.40a | 3.65±0.026b | 0.062±0.01a | 24.34±1.14a | 13.35±0.10a | 6.68 | ||
| Birch | Aquatic | Ambient | 51.22±0.13a | 2.54±0.018a | 0.09±0.008a | 22.10±3.28a | 20.17±0.11a | 8.7 |
| Elevated | 50.84±0.13a | 1.79±0.004b | 0.066±0.01a | 27.76±1.69a | 28.47±0.08b | 15.55 | ||
| Terrestrial | Ambient | 49.86±0.24a | 3.08±0.017a | 0.082±0.01a | 25.09±2.07a | 16.19±0.04a | 8.15 | |
| Elevated | 50.44±0.41a | 1.91±0.063b | 0.07±0.006a | 29.32±1.52a | 26.47±0.74b | 15.33 | ||
Abbreviations: percent dry mass (% DM), percent acetyl-bromide-soluble lignin (% ABSL), conditioning type (CT).
Different lowercase letters indicate significant differences (P<0.05) between CO2 treatments for each tree species × CT combination.
Invertebrate Responses
For both tree species in the choice and no-choice control arenas, disc mass loss in the absence of invertebrates was unaffected by CO2 treatment and conditioning type (P>0.05). Litter mass loss in the presence of invertebrates was therefore assumed to be a result of invertebrate feeding alone.
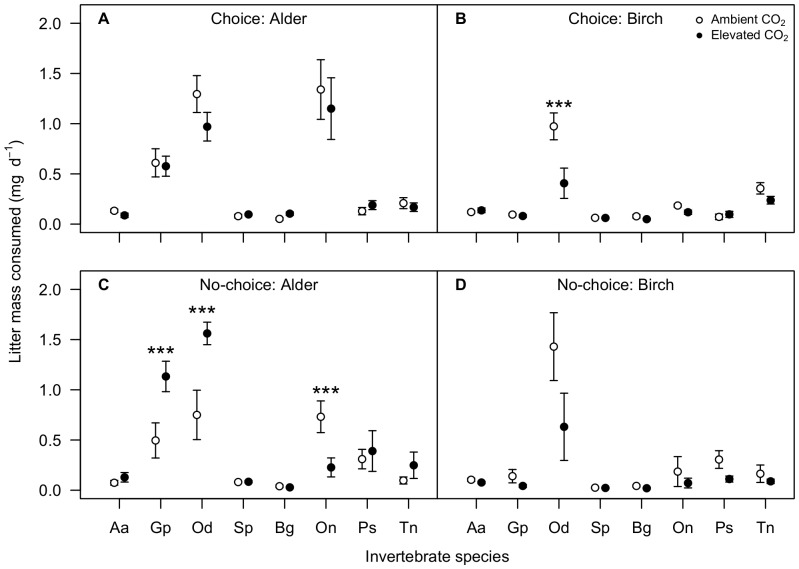
In the choice test, leaf palatability affected invertebrate feeding, but this was dependent on tree species. Birch litter consumption was higher for ambient- than elevated-CO2 discs overall (F 1,72 = 10.48, P = 0.002); there was no effect of CO2 on consumption of alder discs (F 1,72 = 187.21, P = 0.34). Consumption also varied between invertebrate species (alder, F 7,72 = 0.92, P<0.001; birch, F 7,72 = 30.05, P<0.001). The effect of CO2 on birch consumption varied by invertebrate species (F 7,72 = 3.44, P = 0.003), where O. albicorne preferred ambient-CO2 discs (estimate = 1.29 mg d−1, P<0.001; Fig. 2B). The effect of CO2 on litter preference did not vary between invertebrates feeding on alder (F 1,72 = 0.5, P = 0.83; Fig. 2A). When grouped, aquatic species preferred ambient-CO2 birch discs over those grown under elevated CO2 (estimate = 1.09 mg d−1, P = 0.008), but no other preferences were exhibited (aquatic species fed alder, estimate = 0.02 mg d−1, P = 0.585; terrestrial species fed alder, estimate = 0.03 mg d−1, P = 0.496; terrestrial species fed birch, estimate = 0.06 mg d−1, P = 0.061).
Figure 2. Effects of CO2 treatment on feeding responses of each invertebrate species.
The mean litter consumption (±1 SE) of each invertebrate species is shown for (A) alder and (B) birch in the choice test, and (C) alder and (D) birch in the no-choice test. Asterisks indicate significant differences between CO2 treatments within each invertebrate species (***P<0.001). Species are arranged by habitat of origin: aquatic species are Asellus aquaticus (Aa), Gammarus pulex (Gp), Odontocerum albicorne (Oa) and Sericostoma personatum (Sp); terrestrial species are Blaniulus guttulatus (Bg), Oniscus asellus (On), Porcellio scaber (Ps) and Tachypodoiulus niger (Tn).
In the no-choice test, consumption rates were higher when invertebrates fed on ambient- rather than elevated-CO2 birch discs (F 1,64 = 6.39, P = 0.014). The trend was consistent across all invertebrate species, but no individual species showed a significant response (CO2 treatment × invertebrate species: F 7,64 = 0.341, P = 0.932; Fig. 2D). This overall effect of CO2 did not occur in alder leaves (F 1,64 = 3.6, P = 0.062), but the effect of CO2 varied significantly between species (F 7,64 = 4.56, P<0.001); more of the elevated-CO2 discs were consumed by G. pulex (estimate = 2.89 mg, P = 0.002) and O. albicorne (estimate = 3.22 mg, P<0.001), while O. asellus consumed more of the ambient-CO2 discs (estimate = 2.86 mg, P = 0.0022; Fig. 2C). When grouped by habitat, aquatic invertebrates ate more elevated-CO2 than ambient-CO2 alder (estimate = 1.965 mg, P<0.001) but there was no effect on birch (estimate = 0.1 mg, P = 0.073). CO2 treatment had no effect on consumption by terrestrial species fed either alder (estimate = 0.22 mg, P = 0.306) or birch (estimate = 0.1 mg, P = 0.085).
Discussion
Elevated atmospheric CO2 and microbial conditioning type modified leaf litter chemistry, though effects differed between tree species (supporting Hypothesis 1). Individual invertebrate species varied in their responses, suggesting that caution has to be taken when extrapolating general trends from single-species studies.
Elevated atmospheric CO2 reduced birch litter quality: the concentration of nitrogen decreased and the C/N ratio increased, regardless of conditioning type. Most species did not respond to this change; O. albicorne was the only species with behaviour that supported Hypothesis 2, showing a strong preference for ambient-CO2 litter. Prior work supports this response: Ferreira et al. [13] showed that low C/N ratios reduced birch litter consumption by the caddisfly Sericostoma vittatum Rambur, while Cotrufo et al. [17] found that the woodlouse P. scaber preferred high quality (lower C/N ratio and lignin concentration) Fraxinus excelsior Linnaeus litter grown under ambient CO2. Alder litter showed negligible chemical change as a result of elevated CO2, perhaps due to symbiosis with nitrogen-fixing bacteria that help maintain nutrient supplies [38]. Unexpectedly, a slight increase in quality (increased nitrogen concentration) under elevated CO2 occurred when alder litter was conditioned terrestrially, but this did not result in any feeding preferences. Effects of conditioning type on litter chemistry may have occurred due to differences in chemical leaching and microorganism activity between aquatic and terrestrial environments [39]. Our data indicate that CO2 enrichment will affect litter palatability to macroinvertebrate detritivores as a result of chemical change, though these effects will be plant and invertebrate species-specific.
In the no-choice test, invertebrates were expected to compensate for low-quality litter by increasing consumption relative to high-quality litter. In contrast to this expectation, compensatory feeding was not observed in either tree species. There was no clear pattern for alder; invertebrate responses were highly idiosyncratic, with O. asellus being the only species to consume more of the low-quality resource (terrestrially-conditioned alder litter contained lower nitrogen when grown under ambient-CO2). Hättenschwiler et al. [18] detected a similar compensatory response for O. asellus and another woodlouse, P. scaber: higher consumption rates were recorded on low-quality, CO2-enriched F. sylvatica litter (low nitrogen concentration, high C/N ratio). The current study showed that G. pulex and O. albicorne consumed more elevated-CO2 than ambient-CO2 alder, despite no observed chemical differences. It is possible that elevated CO2 reduced litter palatability by altering chemical constituents that were not quantified here, such as secondary metabolites. For example, phenolics and tannins have been shown to be affected by CO2 levels [40]. Birch litter responses appeared less idiosyncratic, with no individual species increasing consumption of elevated-CO2 litter. These results suggest that litter species identity determines the predictability of invertebrate feeding responses, but that compensatory feeding is not a unifying trend amongst detritivorous macroinvertebrates.
Feeding rates may have varied due to increased handling times associated with low quality birch litter (e.g. [20]), or because of differences in species’ body chemistry and their ability to cope with elemental imbalances with CO2-enriched resources [41], [42]. Heterotrophs, such as the detritivores in our study, tend to maintain constant body elemental composition [43] and may alter feeding behaviour to achieve optimum chemical balance. Our results show that individual invertebrate species rarely demonstrated significant responses to CO2 treatments in either test. This suggests that although individual species responses appear idiosyncratic, when considered as a whole, the invertebrate community generally shows consistent and predictable behavioural and functional responses to litter chemical changes induced by elevated CO2.
Altered consumption of litter by macroinvertebrates will affect energy release from detritus, in turn affecting secondary production, and food-web structure and functioning [5]. Specifically, on the basis of invertebrate responses in our study, mineralisation of carbon and nutrients could slow down in forests dominated by birch or other tree species with similar chemistry. This is reinforced by our observations of high lignin/N and C/N ratios of elevated-CO2 birch litter, which are predictors for slow decomposition rates [44]. Conversely, stands containing a lot of alder, or other species with lower C/N ratios, may show little response in terms of detrital processing and nutrient turnover. Differences between tree species make it difficult to predict overall decomposition rates, a task made more difficult by the prevalence of litter mixtures in temperate deciduous forests, which tend to exhibit non-additive decay [45].
Changes to litter quality as a result of elevated CO2 may also affect invertebrate community composition, a potentially important determinant of decomposition rates [19]. This could be caused by changes to food selection [46] and increased patchiness of resource quality in litter mixtures on the forest floor [47]. Differential changes to feeding rates may alter competitive dynamics between invertebrate species, with advantages for species whose dietary breadth extends beyond leaf litter, such as G. pulex and S. personatum [48], [49].
Our study provides, to date, the broadest assessment of detritivorous invertebrate species’ feeding responses to CO2-enriched litter, improving our mechanistic understanding of a key ecosystem process in temperate woodland ecosystems. Future elevations of atmospheric CO2 are predicted to affect the breakdown of detritus indirectly by reducing leaf litter quality for macroinvertebrate detritivores. The study highlights that this process is highly tree species-specific, and there will be strong responses in some forest stands and minimal effects in others. Identifying the mechanisms governing such ecosystem variation in functional responses to climate change is essential if we are to predict the consequences of elevated CO2 for forest carbon dynamics and nutrient cycling at regional and landscape-scales.
Acknowledgments
Stefan Reidinger, Deborah Coldwell and Simon McQueen-Mason at the University of York for assistance with chemical profiling. Adriana De Palma for comments on early versions of the manuscript. Jaimie Crowther for technical assistance.
Funding Statement
Funding by Postgraduate Research Studentships to MWD (Cardiff University President’s Research Scholarship) and ADA (NERC/I527861); a Yale Climate and Energy Institute Fellowship to TWC; and a Knowledge Economy Skills Scholarship (KESS) studentship to SMT. Bangor FACE facility development funded by the Science Research Investment Fund (SRIF), with Forestry Commission Wales and the Centre for Integrated Research in the Rural Environment (CIRRE) supporting running costs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Websites for funding bodies as follows: Cardiff University President’s Scholarship, http://www.cardiff.ac.uk/presidents/; NERC, http://www.nerc.ac.uk. Yale Climate and Energy Institute Fellowship, http://climate.yale.edu/grants-fellowships/postdoctoral-fellowships; KESS, http://www.higherskillswales.co.uk/kess/; SRIF, http://www.delni.gov.uk/index/further-and-higher-education/higher-education/role-structure-he-division/he-research-policy/research-capital-funding-srif.htm; Forestry Commission Wales, http://naturalresourceswales.gov.uk/splash?orig=/; and CIRRE, http://www.cirre.ac.uk/.
References
- 1.IPCC (2007) Climate Change 2007: The physical science basis. In: Solomon SQD, Manning M, Chen Z, Marquis M, Averyt KB, et al.., editors. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Report on Climate Change. Cambridge: Cambridge University Press. 749–845.
- 2. Curtis PS, Wang X (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form and physiology. Oecologia 113: 219–313. [DOI] [PubMed] [Google Scholar]
- 3. Robinson EA, Ryan GD, Newman JA (2012) A meta-analytical review of the effects of elevated CO2 on plant-arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol 194: 321–336. [DOI] [PubMed] [Google Scholar]
- 4. Cebrian J (1999) Patterns in the fate of production in plant communities. Am Nat 154: 449–468. [DOI] [PubMed] [Google Scholar]
- 5. Moore JC, Berlow EL, Coleman DC, de Ruiter PC, Dong Q, et al. (2004) Detritus, trophic dynamics and biodiversity. Ecol Letters 7: 584–600. [Google Scholar]
- 6. Tuchman NC, Wetzel RG, Rier ST, Wahtera KA, Teeri JA (2002) Elevated atmospheric CO2 lowers leaf litter nutritional quality for stream ecosystem food webs. Glob Change Biol 8: 163–170. [Google Scholar]
- 7. Coûteaux M, Kurz C, Bottner P, Raschi A (1999) Influence of increased atmospheric CO2 concentration on quality of plant material and litter decomposition. Tree Physiol 19: 301–311. [DOI] [PubMed] [Google Scholar]
- 8. Norby RJ, Cotrufo MF, Ineson P, O’Neill EG, Canadell JG (2001) Elevated CO2, litter chemistry, and decomposition: A synthesis. Oecologia 127: 153–165. [DOI] [PubMed] [Google Scholar]
- 9. Cotrufo MF, Ineson P, Rowland AP (1994) Decomposition of tree leaf litters grown under elevated CO2: Effect of litter quality. Plant Soil 163: 121–130. [Google Scholar]
- 10. Tuchman NC, Wahtera KA, Wetzel RG, Teeri JA (2003) Elevated atmospheric CO2 alters leaf litter quality for stream ecosystems: An in situ leaf decomposition study. Hydrobiologia 495: 203–211. [Google Scholar]
- 11. Parsons WFJ, Lindroth RL, Bockheim JG (2004) Decomposition of Betula papyrifera leaf litter under the independent and interactive effects of elevated CO2 and O3 . Glob Change Biol 10: 1666–1667. [Google Scholar]
- 12. Liu L, King JS, Giardina P (2007) Effects of elevated atmospheric CO2 and tropospheric O3 on nutrient dynamics: decomposition of leaf litter in trembling aspen and paper birch commuities. Plant Soil 299: 65–82. [DOI] [PubMed] [Google Scholar]
- 13. Ferreira V, Gonçalves AL, Godbold DL, Canhoto C (2010) Effect of increased atmospheric CO2 on the performance of an aquatic detritivore through changes in water temperature and litter quality. Glob Change Biol 16: 3284–3296. [Google Scholar]
- 14. Prather CM, Pelini SL, Laws A, Rivest E, Woltz M, et al. (2012) Invertebrates, ecosystem services and climate change. Biol Rev 88: 327–348. [DOI] [PubMed] [Google Scholar]
- 15. Wallace JB, Webster JR (1996) The role of macroinvertebrates in stream ecosystem function. Annu Rev Entomol 41: 115–139. [DOI] [PubMed] [Google Scholar]
- 16. Lavelle P, Decaëns T, Aubert M, Barot S, Blouin M, et al. (2006) Soil invertebrates and ecosystem services. Eur J Soil Biol 42: S3–S15. [Google Scholar]
- 17. Cotrufo MF, Briones MJI, Ineson P (1998b) Elevated CO2 affects field decomposition rate and palatability of tree leaf litter: Importance of changes in substrate quality. Soil Biol Biochem 30: 1565–1571. [Google Scholar]
- 18. Hättenschwiler S, Bühler S, Körner C (1999) Quality, decomposition and isopod consumption of tree litter produced under elevated CO2 . Oikos 85: 271–281. [Google Scholar]
- 19. Gessner MO, Swan CM, Dang CK, McKie BG, Bardgett RD, et al. (2010) Diversity meets decomposition. Trends Ecol Evol 25: 372–380. [DOI] [PubMed] [Google Scholar]
- 20. Ott D, Rall BC, Brose U (2012) Climate change effects on macrofaunal litter decomposition: the interplay of temperature, body masses and stoichiometry. Philos T Roy Soc B 367: 3025–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frost PC, Tuchman NC (2005) Nutrient release rates and ratios by two stream detritivores fed leaf litter grown under elevated atmospheric CO2 . Archiv Hydrobiol 163: 463–477. [Google Scholar]
- 22. Shurin JB, Gruner DS, Hillebrand H (2006) All wet or dried up? Real differences between aquatic and terrestrial food webs. Proc Roy Soc B 273: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smith AR, Lukac M, Hood R, Healy JR, Miglietta F, et al. (2013) Elevated CO2 enrichment induces a differential biomass response in a mixed species temperate forest plantation. New Phytol 198: 156–168. [DOI] [PubMed] [Google Scholar]
- 24. Miglietta F, Peressotti A, Vaccari FP, Zaldei A, deAngelis P, et al. (2001) Free-air CO2 enrichment (FACE) of a poplar plantation: the POPFACE fumigation system. New Phytol 150: 465–476. [Google Scholar]
- 25. Daniel O, Schonholzer F, Ehlers S, Zeyer J (1997) Microbial conditioning of leaf litter and feeding by the wood-louse Porcellio scaber . Pedobiologia 41: 397–401. [Google Scholar]
- 26. Graça MAS, Cressa C, Gessner MO, Feio MJ, Callies KA, et al. (2001) Food quality, feeding preferences, survival and growth of shredders from temperate and tropical streams. Freshwater Biol 46: 947–957. [Google Scholar]
- 27. Reidinger S, Ramsey M, Hartley SE (2012) Rapid and accurate analyses of silicon and phosphorus in plants using a portable X-ray fluorescence spectrometer. New Phytol 195: 699–706. [DOI] [PubMed] [Google Scholar]
- 28. Foster CE, Martin TM, Pauly M (2010) Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) part I: Lignin. J Vis Exp 37: e1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moog O (2002) Fauna Aquatica Austriaca. Vienna: Federal Ministry of Agriculture, Forestry, Environment and Water Management.
- 30.Wurst S, De Deyn GB, Orwin K (2012) Soil biodiveristy and functions. In: Wall DH, editor. Soil Ecology and Ecosystem Services. Oxford: Oxford University Press. 28–44.
- 31. R Development Core Team (2013) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- 32.Crawley MJ (2007) The R Book. Chichester: John Wiley & Sons, Ltd. 942 p. [Google Scholar]
- 33.Fox J, Weisberg S (2011) car: Companion to applied regression. R package version 2.0–18.
- 34.Lenth RV (2013) lsmeans: Least-squares means. R package version 1.06–5.
- 35.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team (2013) nlme: Linear and nonlinear mixed effects models. R package version 3.1–109.
- 36.Kuhn M, Weston S, Wing J, Forester J (2011) contrast: A collection of contrast methods. R package version 0.1.
- 37.Warnes GR (2012) gmodels: Various R programming tools for model fitting. R package version 2.15.4.
- 38. Temperton VM, Grayston SJ, Jackson G, Barton CVM, Millard P, et al. (2003) Effects of elevated carbon dioxide concentration on growth and nitrogen fixation in Alnus glutinosa in a long-term field experiment. Tree Physiol 23: 1051–1059. [DOI] [PubMed] [Google Scholar]
- 39. Taylor M, Zimmer M (2012) Drowned or dry: A cross-habitat comparison of detrital breakdown processes. Ecosystems 15: 477–491. [Google Scholar]
- 40.Lindroth RL (2012) Atmospheric change, plant secondary metabolites and ecological interactions. In: Iason GR, Dicke M, Hartley SE, editors. The Ecology of Plant Secondary Metabolites: From Genes to Global Processes. Cambridge: Cambridge University Press. 120–153.
- 41. Martinson HM, Schneider K, Gilbert J, Hines JE, Hambäck PA, et al. (2008) Detritivory: stoichiometry of a neglected trophic level. Ecol Res 23: 487–491. [Google Scholar]
- 42. Hladyz S, Gessner MO, Giller PS, Pozo J, Woodward G (2009) Resource quality and stoichiometric constraints on stream ecosystem functioning. Freshwater Biol 54: 957–970. [Google Scholar]
- 43.Sterner RW, Elser JJ (2002) Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton: Princeton University Press. 584 p. [Google Scholar]
- 44. Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63: 621–626. [Google Scholar]
- 45. Gartner TB, Cardon ZG (2004) Decomposition dynamics in mixed-species leaf litter. Oikos 104: 230–246. [Google Scholar]
- 46. Hättenschwhiler S, Bretscher D (2001) Isopod effects on decomposition of litter produced under elevated CO2, N deposition and different soil types. Glob Change Biol 7: 565–579. [Google Scholar]
- 47. Swan CM, Palmer MA (2006) Preferential feeding by an aquatic consumer mediates non-additive decomposition of speciose leaf litter. Oecologia 149: 107–114. [DOI] [PubMed] [Google Scholar]
- 48. MacNeil C, Dick JTA, Elwood RW (1997) The trophic ecology of freshwater Gammarus spp. (Crustacea: Amphipoda): problems and perspectives concerning the functional feeding group concept. Biol Rev 72: 349–364. [Google Scholar]
- 49. Friberg N, Jacobsen DJ (1999) Variation in growth of the detritivore-shredder Sericostoma personatum (Trichoptera). Freshwater Biol 32: 133–142. [Google Scholar]