Abstract
Background
Isoniazid (INH) resistance is now the most common type of tuberculosis (TB) infection resistance worldwide. The aim of this study was to evaluate the clinical characteristics and treatment outcomes of patients with low- and high-concentration INH-monoresistant TB.
Methods
One hundred and thirty-four patients with culture-confirmed INH-monoresistant TB during 2006 January to 2007 December were retrospectively enrolled. INH resistance was classified as either low-concentration or high-concentration resistance according to the critical concentrations of 0.2 µg/mL or 1 µg/mL of INH, respectively. The patients’ clinical outcomes, treatment regimens, and treatment duration were analyzed.
Results
The treatment success rates between low- and high-concentration INH-resistant TB were similar (81.8% vs. 86.7%). The treatment regimens and treatment duration were similar between both groups. Only a minor percentage of the patients in both groups received 6-month treatment regimens (low vs. high concentration resistance, 9.1% vs. 13.3%; respectively, p = 0.447) The most common reason for treatment duration longer than 6 months was pyrazinamide given for less than 6 months, followed by a delay in clinical response to treatment. Multivariable analysis showed that prior tuberculosis treatment (Odds ratio, 2.82, 95% C.I., 1.02–7.77, p = 0.045) was the only independent risk factor for unsuccessful treatment outcome.
Conclusion
Different levels of INH resistance did not affect the treatment outcomes of patients with INH-monoresistant tuberculosis. Prolonged Rifampin-containing regimens may achieve those good outcomes in patients with low- and high-concentration INH-monoresistant TB.
Introduction
Tuberculosis (TB) is the leading infectious cause of death worldwide, with 9 million new cases and nearly 2 million deaths annually [1]. Recent global surveys have reported that drug-resistant TB exists in every location [2]. Compounding the challenges of an already lengthy and complicated treatment course, the World Health Organization reported a trend toward an increasing number of cases of drug-resistant TB [3]. Isoniazid (INH) is an important first-line agent for the treatment of TB because of its potent early bactericidal activity. However, resistance to INH is very common, with a prevalence rate of 28% among previously treated cases and 10% among new cases [4]. Due to the increasing number of INH-resistant tuberculosis cases, the effect of such resistance on treatment outcomes is of particular interest.
Recent large-scale cohort studies have demonstrated that INH monoresistant TB did not decline in recent years despite the downward trends observed in overall TB cases [4], [5]. The treatment successful rates were similar in patients with INH-monoresistant and susceptible TB, however, patients with INH-monoresistant TB required longer treatment periods than those with INH-susceptible TB [4], [5]. Previous studies have reported a low rate of treatment failure (2%) for INH-resistant strains treated with an initial regimen of 4 to 5 drugs containing rifampin for at least 6 months [6]. Therefore, the American Thoracic Society (ATS), Centers for Disease Control and Prevention (CDC), and Infectious Diseases Society of America (IDSA) issued guidelines recommending treatment with a standard 4-drug regimen (INH, rifampin, pyrazinamide, and ethambutol) for 6 months, with discontinuation of INH after the results of drug susceptibility tests are known [7]. A 6-month short-course regimen of first-line drugs including INH, rifampin, pyrazinamide, and ethambutol has been reported to be as effective in drug-susceptible patients as in INH-monoresistant patients. [8] However, emerging evidence suggests that duration of treatment for longer than 6 months is required in more than half of the patients with INH-monoresistant TB [4]. In addition, highly heterogeneous treatment regimens for INH-monoresistant TB have been reported in many reports [4], [9]–[11]. Therefore, further studies are needed to evaluate the efficacy of the current treatment regimens and outcomes for INH-monoresistant TB.
INH resistance is classified as either low- or high-concentration resistance according to the critical concentrations of 0.2 µg/mL or 1 µg/mL of INH, respectively. According to previous studies, different genetic mutations are responsible for low- and high-concentration INH resistance [10], and they can therefore be thought of as two distinct entities. However, comprehensive studies are still lacking to address the differences between patients with low-and high-concentration monoresistant TB in terms of baseline characteristics, treatment regimen, treatment-related adverse effects, and outcomes. In addition, the in vitro sensitivity tests show that INH can inhibit the growth of low-concentration INH monoresistant Mycobacterium tuberculosis at the concentration of 1 µg/mL. INH is usually dosed at 5mg/kg/day, up to 300 mg/day, yielding a peak level in serum of 3–5 µg/ml [12]. Although INH-monoresistant TB can be successfully treated with at least 6-month Rifampin containing regimens, it is not clear whether this outcome is independent of the degree of high or low-concentration INH resistance. Since previous studies did not stratify the results according to low- and high-concentration INH resistance, outcomes for patients with high-concentration INH monoresistant TB may not be as good for patients with low-concentration INH monoresistant TB. The aim of this study was to evaluate the patients’ demographic characteristics, treatment regimens, and treatment outcomes for those with different concentrations of INH-monoresistant TB.
Materials and Methods
Study Population
We retrospectively recruited patients with culture-confirmed INH-monoresistant Mycobacterium tuberculosis during January 2006 to December 2007 in Chang Gung Memorial Hospital, a tertiary hospital in Taiwan. Patients were excluded if INH resistance was acquired during treatment or if resistance to any other first-line anti-TB medication was documented [4]. The Chang Gung Medical Foundation Institutional Review Board approved the study and waived the requirement for informed consent due to the retrospective nature of this study.
Study Design
Each patient’s medical records were reviewed to collect the clinical characteristics and laboratory results. In addition, information including prior tuberculosis treatment, treatment regimens, adverse drug reactions, adherence to therapy, and clinical follow-up for 1 year after completion of treatment was analyzed.
Definitions
Drug susceptibility was confirmed in all cases at the laboratory by the agar-proportion method [13]. INH resistance was classified as either low concentration or high concentration when there was >1% growth of Mycobacterium tuberculosis complex in the presence of 0.2 µg/mL or 1 µg/mL of INH, respectively [4]. A patient was defined as being cured if conversion from positive to negative sputum culture was achieved after the start of treatment and the patient remained culture-negative throughout the period of treatment. Sputum culture conversion was defined as the time in months from the time treatment was started to the time at which the first negative sputum culture was obtained. Treatment completion was defined as the patients who had completed treatment but did not meet the criteria to be classified as instances of cure or failure. In this study, both cure and treatment completion were regarded as treatment successes [9]. In accordance with the ATS/CDC/IDSA guidelines, a patient was considered to have treatment failure if culture results remained positive after 4 months of treatment, and if they had a relapse when a second episode of TB was diagnosed within 1 year after treatment completion [7]. The definition of default was a patient who missed >20% of their total doses or >2 months of consecutive therapy.
An adverse drug reaction was defined as any symptom or laboratory abnormality leading to an interruption of ≥1 dose of antituberculosis medication [4]. Patients were considered to have non-adherence to treatment if any of the following conditions were met: (1) more than 14 consecutive days of treatment were missed; (2) more than 2 consecutive visits to the clinic were missed; or (3) more than 20% of doses were missed in any month by a patient receiving directly observed therapy [4].
Statistical Analysis
Data were expressed as mean ± SEM (standard error of the mean). The Student’s t test was used for comparisons of continuous variables between the two groups, while the Mann-Whitney test was used for non-normal distributions. Categorical variables were compared by x2 or Fisher’s exact tests. Univariable associations were reported and multivariable logistic regression was used to identify independent associations between measured covariates and the probability of unsuccessful treatment. The final multivariable model was constructed first by including all variables considered in the univariable analysis, then by sequentially removing explanatory variables with the greatest P value. If the effect size of the other explanatory variables changed by less than 10%, the variable was dropped from the model, otherwise it was retained. The log-rank test was used for analysis of the proportion of those remaining on therapy. A p value less than 0.05 was considered statistically significant. Analysis was carried out using SPSS (version 13.0; SPSS; Chicago, IL) statistical software.
Results
Demographic and Clinical Characteristics of Patients
A total of 1229 culture-positive tuberculosis patients were identified in our hospital from January 2006 to December 2007, of whom 134 (10.9%) had INH monoresistance and were included in this study. The baseline demographics and clinical characteristics of these patients are listed in Table 1. The mean ages of the patients with low- and high-concentration monoresistant TB were 53.2 and 58.8 years, respectively, with 77.3% and 73.3% males, respectively. Other characteristics including prior tuberculosis treatment, adherence to treatment and treatment duration were similar between the two groups.
Table 1. Demographic and clinical characteristics of the patients.
| Characteristic | INH low concentration resistancen = 44 | INH high concentration resistancen = 90 | Odds ratio(95%CI) | p value |
| Male, n | 34(77.3%) | 66(73.3%) | 1.24(0.53–2.88) | 0.623 |
| Age, years | 53.2±3.7 | 58.8±3.0 | 0.264 | |
| Prior tuberculosis treatment | 12(27.3%) | 29(32.2%) | 0.79(0.36–1.25) | 0.559 |
| Pulmonary tuberculosis | 38(86.4%) | 86(95.6%) | 0.88(0.16–5.04) | 0.889 |
| Positive AFB smear test | 30(68.2%) | 60(66.7%) | 1.50(0.65–3.47) | 0.342 |
| Cavitary chest radiograph | 10(22.7%) | 22(24.4%) | 1.03(0.44–2.44) | 0.946 |
| Received initial isoniazid | 42(95.5%) | 88(97.8%) | 0.48(0.06–3.51) | 0.458 |
| Directly observed therapy | 44(100%) | 85(93.3%) | 5.73(0.31–106) | 0.111 |
| Adherence to treatment | 43(95.5%) | 87(96.7%) | 1.48(0.15–14.69) | 0.735 |
| Adverse reaction | 22(50%) | 40(44.4%) | 1.25(0.61–2.58) | 0.545 |
| Sputum culture conversion at ≤2 months | 14(31.8%) | 18(20%) | 1.87(0.82–4.23) | 0.132 |
| Treatment duration, days | 297.8±19.0 | 289.9±14.6 | 0.750 |
Abbreviations: INH: isoniazid; AFB: acid fast bacilli; CI: confidence interval.
Categorical data are expressed as number (%).
Continuous data are expressed as mean±SEM.
Treatment Regimens for INH-monoresistant TB and the Reasons for Extension of Treatment Beyond 6 Months
The treatment regimens of the patients are shown in Table 2. Highly heterogeneous treatment regimens were used in both groups, and the treatment regimens and duration were similar between both groups. The most common regimen was 2 months of INH, rifampin, ethambutol, and pyrazinamide, followed by 5 to 7 months of rifampin, ethambutol and pyrazinamide treatment in both groups. Only a minor percentage of patients in both groups received 6-month treatment regimens (INH low-concentration resistance vs. high-concentration resistance, 9.1% vs. 13.3%, respectively). Table 3 lists the reasons for extension of treatment beyond 6 months in the patients with INH monoresistant TB. The most common reason for a treatment duration longer than 6 months was pyrazinamide (PZA) given for less than 6 months followed by a delay in clinical response to treatment. The reasons for extending treatment beyond 6 months was not significantly different between the two groups.
Table 2. Treatment regimens for isoniazid-monoresistant tuberculosis.
| Treatment regimens | INH low concentrationresistance,n = 44 | INH high concentrationresistance,n = 90 | Odds ratio(95% CI) | p value |
| 6 Months, n(%) | 4(9.1%) | 12(13.3%) | 0.65(0.2–2.15) | 0.477 |
| HREZ (2), REZ (4) | 4(9.1%) | 10(11.1%) | 0.80(0.24–2.71) | 0.720 |
| Other | 0(0%) | 2(2.2%) | 0.4(0.02–8.47) | 0.993 |
| 7–12 Months | 28(63.6%) | 51(56.7%) | 1.34(0.64–2.81) | 0.441 |
| HREZ (2), REZ (5–7) | 10(22.7%) | 21(23.3%) | 0.97(0.41–2.28) | 0.938 |
| HREZ (9) | 5(11.4%) | 10(11.1%) | 1.03(0.33–3.21) | 0.965 |
| HRE (9–12) | 3(6.8%) | 4(4.4%) | 1.57(0.34–7.36) | 0.562 |
| HREZ (2), RE (7–10) | 4(9.1%) | 2(2.2%) | 2.90(0.62–13.57) | 0.160 |
| HREZ (2), REZ (7–10) | 2(4.5%) | 4(4.4%) | 1.38(0.22–8.58) | 0.728 |
| HREZ (2), HRE (7–10) | 2(4.5%) | 4(4.4%) | 1.02(0.18–5.82) | 0.979 |
| Other | 2(4.5%) | 6(6.7%) | 0.38(0.08–1.82) | 0.221 |
| >12 months | 12(27.3%) | 27(30%) | 0.88(0.39–1.95) | 0.744 |
| HREZ (2), RE (>10) | 5(11.4%) | 13(14.4%) | 0.76(0.25–2.28) | 0.623 |
| HREZ (2), RZ (>10) | 4(9.1%) | 12(13.3%) | 0.65(0.20–2.15) | 0.477 |
| Other | 3(6.8%) | 2(2.2%) | 3.22(0.52–20.02) | 0.187 |
Abbreviations: INH: isoniazid; CI: confidence interval.
E, ethambutol; H, isoniazid; R, rifampin; Z, pyrazinamide.
Table 3. Reasons for extension of treatment beyond 6 months.
| Treatment regimens | INH low concentrationresistance, n = 44 | INH high concentrationresistance, n = 90 | Odds ratio(95% CI) | p value |
| Pyrazinamide given for <6 months, all | 19(47.5%) | 32(41.0%) | 1.30(0.60–2.80) | 0.502 |
| Because of physician preference | 3(7.5%) | 6(7.7%) | 0.97(0.23–4.11) | 0.970 |
| Because of adverse reaction | 16(40.0%) | 26(33.3%) | 1.33(0.61–2.93) | 0.474 |
| Hepatotoxicity | 8(20.0%) | 8(10.3%) | 2.19(0.75–6.35) | 0.143 |
| Hyperuricemia/gout | 8(20.0%) | 16(20.5%) | 0.97(0.37–2.51) | 0.948 |
| Rash | 0(0%) | 2(2.6%) | 0.38(0.02–8.06) | 0.307 |
| Treatment noncompliance | 2(5.0%) | 3(3.8%) | 1.32(0.21–8.22) | 0.768 |
| Extrapulmonary tuberculosis | 5(12.5%) | 4(5.1%) | 2.64(0.67–10.46) | 0.153 |
| Delayed clinical response to treatment | 8(20.0%) | 24(30.8%) | 0.56(0.23–1.40) | 0.213 |
| Delayed culture conversion | 6(15.0%) | 15(19.2%) | 0.74(0.26–2.09) | 0.570 |
Abbreviations: INH: isoniazid; CI: confidence interval.
Clinical Outcomes of Tuberculosis Patients with INH Monoresistant TB
The treatment success rates were high in the patients with INH monoresistant TB (Table 4), and the success rates between the patients with low- and high-concentration INH monoresistant TB were similar (81.8% vs. 86.7%, respectively). For the patients with unsuccessful treatment, death was the most common cause. According to the log-rank test (Figure 1), the proportion of those remaining on therapy was also similar between the two groups (p = 0.761, hazard ratio = 1.06, 95% confidence interval, 0.73–1.55).
Table 4. Clinical outcomes of the tuberculosis patients with INH monoresistance.
| Treatment regimens | INH low concentration resistance, n = 44 | INH high concentration resistance, n = 90 | Odds ratio(95% CI) | p value |
| Successful | 36(81.8%) | 78(86.7%) | ||
| Cure | 35(79.5%) | 75(83.3%) | 0.78(0.31–1.95) | 0.591 |
| Completed | 1(2.3%) | 3(3.3%) | 0.68(0.07–6.68) | 0.735 |
| Unsuccessful | 8(18.2%) | 12(13.3%) | ||
| Default | 1(2.3%) | 2(2.2%) | 2.07(0.13–33.91) | 0.603 |
| Failure | 2(4.5%) | 2(2.2%) | 1.38(0.22–8.58) | 0.728 |
| Death | 4(9.1%) | 7(7.8%) | 1.03(0.29–3.61) | 0.969 |
Abbreviations: INH: isoniazid; CI: confidence interval.
Figure 1. Kaplan-Meier analysis for patients with isoniazid (INH)-resistant tuberculosis (TB) remaining on treatment.
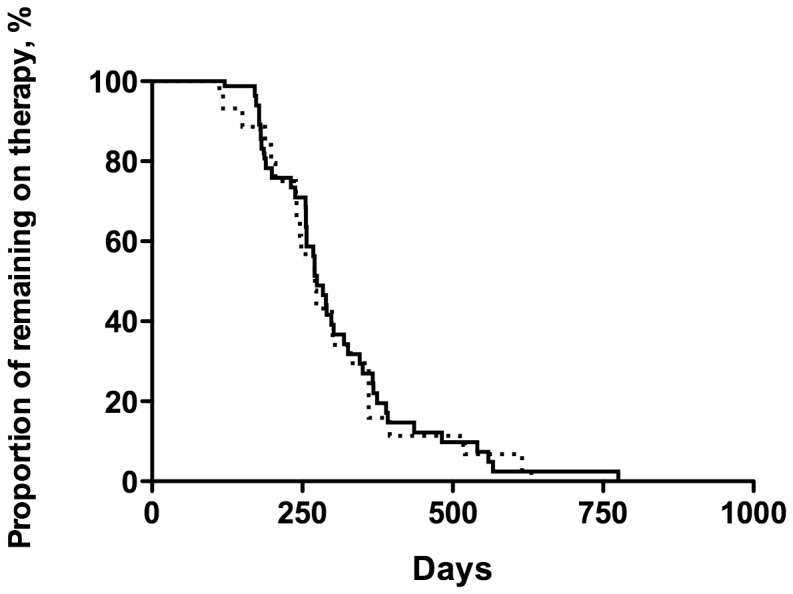
Patients with low-concentration INH-monoresistant TB (dashed line) and high-concentration INH-monoresistant TB (solid line) received a similar duration of anti-TB therapy (p = 0.761 by the log-rank test, hazard ratio = 1.06, 95% confidence interval, 0.73–1.55).
In the study population, 28 patients received INH treatment for longer than 6 months while 106 patients received INH shorter than 6 months. In patients received INH treatment for longer than 6 months, their unsuccessful treatment rates were similar to that in patients received INH treatment shorter than 6 months (5/28, 17.9% vs. 15/106, 14.2%, Odds ratio, 1.32, 95% C.I., 0.43–4.01, p = 0.625). The unsuccessful treatment rates between the patients with low- and high-concentration INH monoresistant TB (2/10, 20% vs. 3/18, 16.7%, Odds ratio, 1.25, 95% C.I., 0.17–9.90, p = 0.825) were similar in those who have received INH treatment longer than 6 months.
There were 41 patients who had received prior anti-TB treatment while 93 patients were treatment naive TB. The unsuccessful treatment rates were higher in patients received prior TB treatment than those without prior TB treatment (10/41, 24.4% vs. 10/93, 10.8%, Odds ratio, 2.68, 95% C.I., 1.02–7.06, p = 0.041). The unsuccessful treatment rates between the patients with low- and high-concentration INH monoresistant TB (4/12, 33.3% vs. 6/29, 20.6%, Odds ratio, 1.92, 95% C.I., 0.43–8.59, p = 0.391) were similar in those who have received prior TB treatment.
Multivariable Analysis
A multivariable analysis was performed to identify risk factors for unsuccessful treatment outcomes among patients with INH-monoresistant TB (table 5). In this multivariable analysis, prior tuberculosis treatment (Odds ratio, 2.82, 95% C.I., 1.02–7.77, p = 0.045) was the only independent risk factor for unsuccessful treatment outcome. INH high-concentration resistance was not an independent risk factor for unsuccessful treatment outcome in the multivariable analysis model.
Table 5. Univariable and multivariable associations with unsuccessful treatment outcome.
| Variables | Univariate analysis | Multivariate analysis | ||
| Odds Ratio (95% C.I.) | p value | Odds Ratio (95% C.I.) | p value | |
| Age>65 year-old | 1.14(0.43–3.02) | 0.788 | 1.24(0.42–3.64) | 0.696 |
| Prior tuberculosis treatment | 2.68(1.02–3.05) | 0.041 | 2.82(1.02–7.77) | 0.045 |
| Positive AFB smear test | 1.17(0.42–3.28) | 0.770 | 0.96(0.32–2.95) | 0.947 |
| Sputum culture conversion at ≤2 months | 1.07(0.36–3.23) | 0.899 | 0.96(0.29–3.18) | 0.945 |
| INH high-concentration resistance | 0.69(0.26–1.84) | 0.459 | 0.62(0.22–1.72) | 0.357 |
Abbreviations: INH: isoniazid; AFB: acid fast bacilli; CI: confidence interval.
Discussion
The findings of the present study demonstrated a high treatment success rate in patients with INH monoresistant TB in Taiwan. The unsuccessful treatment rates were higher in patients received prior TB treatment than those without prior TB treatment. In addition, different levels of INH monoresistance did not affect the treatment course and outcomes of these patients. In spite of highly heterogeneous treatment regimens, most of the patients with INH monoresistant TB received anti-TB therapy for a duration longer than 6 months. The prolonged treatment duration in these patients was mostly caused by PZA given less than 6 months and a delay in clinical response to treatment. Prior tuberculosis treatment was the only independent risk factor for unsuccessful treatment outcome in multivariable analysis.
The patients with low- and high-concentration INH monoresistant TB had similar demographic and clinical characteristics. In addition, the treatment outcomes, treatment regimens, and the reasons for extension of treatment beyond 6 months were not different between the two groups. Although originating from different gene mutations [10], [14], low- and high-concentration INH monoresistant TB had similar responses to therapy and clinical course, and therefore both groups should be treated aggressively. A multi-national study reported a treatment success rate of INH-resistant TB of 82% in new tuberculosis cases, compared to 54% in retreated TB cases [15]. In the present study, around 70% of the recruited patients were new tuberculosis cases. Thus, the treatment success rates were high in both the low- and high-concentration resistance groups.
Compatible with previous studies, the treatment success rate in this study was higher than 80% [10], [15]. One of the possible reasons for this is the high percentage (129/134, 96.3%) of directly observed therapy (DOT). The CDC of Taiwan has enforced the DOT program since 2006, and the implementation rate of DOT for TB case now exceeds 90% [16]. In Taiwan, once a TB case is verified, the patient is invited to participate in the DOT program. Among those who agree, an official DOT observer is assigned to the patient. The observer then monitors the ingestion of anti-TB medications, adverse events, and treatment complications during home visits and provides patients with food coupons as incentives. The DOT program has been documented to be effective in increasing the treatment success rate in TB patients [17]–[19]. The benefits of the DOT strategy are encouraging patients to continue treatment, and identifying those who miss treatment thereby preventing the occurrence of resistant strains. Another possible factor for the high success rate may be attributed to high patient compliance with the anti-TB therapy in the study subjects. In turn, the high treatment compliance rate may be associated with enforcement of the DOT program among these patients.
The Taiwan guidelines recommend a treatment regimen of 6 to 9 months of rifampin, pyrazinamide, and ethambutol, with or without INH [20]. Therefore, the most common treatment regimens in the study population were an initial combination of 4 drugs including INH, rifampin, ethambutol, and pyrazinamide for 2 months, followed by 5 to 7 months of rifampin, ethambutol, and pyrazinamide treatment. Since the bactericidal anti-TB drug, rifampicin, was used in the majority of patients in the study, the critical factor for treatment success in these patients may be the strong sterilizing ability of rifampicin [6]. In this study, adverse effects of the anti-TB drugs frequently developed in patients with INH-monoresistant TB. The high prevalence of drug-induced adverse effects contributed to PZA being used for less than 6 months in these patients. The patients subsequently received an extended period of anti-TB therapy. In addition, a delay in clinical response to treatment and delayed culture conversion also contributed to the prolonged treatment course in the patients with INH-monoresistant TB.
The study has demonstrated good clinical outcomes of patients with INH-monoresistant TB treated with long-course of Rifampin-containing regimens, regardless of the degree of resistance. However, there are two relevant questions unsolved. First, a prolonged course treatment of Rifampin containing regimens is the cornerstone for good outcomes in patients with INH-monoresistant TB and most of the patients in the study were treated accordingly. Therefore, the role of INH therapy in INH monoresistant TB patients received prolonged Rifampin containing treatment remains unresolved. Second, only 13% of patients with high-concentration INH monoresistant TB were successfully treated for 6 months. The 6-month treatment regimen was effective in minor population of those patients and the effects may be contributed from the combination of 4 anti-TB drugs.
The major limitations of the present study are its retrospective nature, which may have led to bias in patient selection. Second, the sample size of the study is small, and therefore the results of the study should be interpreted with caution. A prospective study with larger a sample size is warranted to further confirm the clinical outcomes in patients with different concentrations of INH-monoresistant TB. Third, the DOT program was commonly used in the study subjects. Therefore, the results of the study may only be applied to countries, which enforce a DOT program in the treatment of TB patients. Lastly, genetic analysis on the types of mutation was not performed in the study. The mutations in the katG and inhA gene are associated with high- and low- concentration INH resistance, respectively [10]. In Taiwan, a previous study has showed 48.5% of katG mutation, 30.3% of inhA, and 9.6% of katG and inhA double mutation in INH resistance [21]. The study investigating the mutation analysis and clinical outcome in INH monoresistant TB may provide important information.
The results of this study, if further confirmed by large-scaled studies, suggest that most of patients with INH-monoresistant TB can be successfully treated with prolonged Rifampin containing regimens. In addition, INH-monoresistant TB patients with history of prior tuberculosis treatment may have higher incidence of treatment failure. Those patients should receive aggressive treatment and closely monitoring of their response to anti-TB therapy.
In conclusion, different levels of INH resistance did not affect the treatment outcomes of patients with INH-monoresistant tuberculosis. In spite of relatively complicated treatment courses and heterogeneous treatment regimens, the patients with INH-monoresistant TB had a high treatment success rate. Prolonged Rifampin-containing regimens may achieve those good outcomes in patients with low- and high-concentration INH-monoresistant TB.
Funding Statement
The authors have no support or funding to report.
References
- 1. Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC (1999) Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA: the journal of the American Medical Association 282: 677–686. [DOI] [PubMed] [Google Scholar]
- 2. Pablos-Mendez A, Raviglione MC, Laszlo A, Binkin N, Rieder HL, et al. (1998) Global surveillance for antituberculosis-drug resistance, 1994–1997. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. The New England journal of medicine 338: 1641–1649. [DOI] [PubMed] [Google Scholar]
- 3. Espinal MA, Laszlo A, Simonsen L, Boulahbal F, Kim SJ, et al. (2001) Global trends in resistance to antituberculosis drugs. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. The New England journal of medicine 344: 1294–1303. [DOI] [PubMed] [Google Scholar]
- 4. Cattamanchi A, Dantes RB, Metcalfe JZ, Jarlsberg LG, Grinsdale J, et al. (2009) Clinical characteristics and treatment outcomes of patients with isoniazid-monoresistant tuberculosis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 48: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoopes AJ, Kammerer JS, Harrington TA, Ijaz K, Armstrong LR (2008) Isoniazid-monoresistant tuberculosis in the United States, 1993 to 2003. Arch Intern Med 168: 1984–1992. [DOI] [PubMed] [Google Scholar]
- 6. Mitchison DA, Nunn AJ (1986) Influence of initial drug resistance on the response to short-course chemotherapy of pulmonary tuberculosis. The American review of respiratory disease 133: 423–430. [DOI] [PubMed] [Google Scholar]
- 7. Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, et al. (2003) American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. American journal of respiratory and critical care medicine 167: 603–662. [DOI] [PubMed] [Google Scholar]
- 8. Five-year follow-up of a controlled trial of five 6-month regimens of chemotherapy for pulmonary tuberculosis. Hong Kong Chest Service/British Medical Research Council. The American review of respiratory disease 136: 1339–1342. [DOI] [PubMed] [Google Scholar]
- 9. Kim YH, Suh GY, Chung MP, Kim H, Kwon OJ, et al. (2008) Treatment of isoniazid-resistant pulmonary tuberculosis. BMC infectious diseases 8: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bang D, Andersen PH, Andersen AB, Thomsen VO (2010) Isoniazid-resistant tuberculosis in Denmark: mutations, transmission and treatment outcome. The Journal of infection 60: 452–457. [DOI] [PubMed] [Google Scholar]
- 11. Gegia M, Cohen T, Kalandadze I, Vashakidze L, Furin J (2012) Outcomes among tuberculosis patients with isoniazid resistance in Georgia, 2007–2009. Int J Tuberc Lung Dis 16: 812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weber WW, Hein DW (1979) Clinical pharmacokinetics of isoniazid. Clinical pharmacokinetics 4: 401–422. [DOI] [PubMed] [Google Scholar]
- 13. Canetti G, Froman S, Grosset J, Hauduroy P, Langerova M, et al. (1963) Mycobacteria: Laboratory Methods for Testing Drug Sensitivity and Resistance. Bull World Health Organ 29: 565–578. [PMC free article] [PubMed] [Google Scholar]
- 14. Ando H, Kondo Y, Suetake T, Toyota E, Kato S, et al. (2010) Identification of katG mutations associated with high-level isoniazid resistance in Mycobacterium tuberculosis. Antimicrobial agents and chemotherapy 54: 1793–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Espinal MA, Kim SJ, Suarez PG, Kam KM, Khomenko AG, et al. (2000) Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA: the journal of the American Medical Association 283: 2537–2545. [DOI] [PubMed] [Google Scholar]
- 16. Bloss E, Chan PC, Cheng NW, Wang KF, Yang SL, et al. (2012) Increasing directly observed therapy related to improved tuberculosis treatment outcomes in Taiwan. Int J Tuberc Lung Dis 16: 462–467. [DOI] [PubMed] [Google Scholar]
- 17. Mushtaque A, Chowdhury R (1999) Success with the DOTS strategy. Lancet 353: 1003–1004. [DOI] [PubMed] [Google Scholar]
- 18. Newell JN, Baral SC, Pande SB, Bam DS, Malla P (2006) Family-member DOTS and community DOTS for tuberculosis control in Nepal: cluster-randomised controlled trial. Lancet 367: 903–909. [DOI] [PubMed] [Google Scholar]
- 19. Results of directly observed short-course chemotherapy in 112,842 Chinese patients with smear-positive tuberculosis. China Tuberculosis Control Collaboration. Lancet 347: 358–362. [PubMed] [Google Scholar]
- 20. Guidelines for chemotherapy of tuberculosis in Taiwan. J Microbiol Immunol Infect 37: 382–384. [PubMed] [Google Scholar]
- 21. Huang WL, Chen HY, Kuo YM, Jou R (2009) Performance assessment of the GenoType MTBDRplus test and DNA sequencing in detection of multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol 47: 2520–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]


