Abstract
The photosynthetic cyanobacterium, Synechocystis sp. strain 6803, is a potential platform for the production of various chemicals and biofuels. In this study, direct photosynthetic production of a biopolymer, polyhydroxyalkanoate (PHA), in genetically engineered Synechocystis sp. achieved as high as 14 wt%. This is the highest production reported in Synechocystis sp. under photoautotrophic cultivation conditions without the addition of a carbon source. The addition of acetate increased PHA accumulation to 41 wt%, and this value is comparable to the highest production obtained with cyanobacteria. Transcriptome analysis by RNA-seq coupled with real-time PCR was performed to understand the global changes in transcript levels of cells subjected to conditions suitable for photoautotrophic PHA biosynthesis. There was lower expression of most PHA synthesis-related genes in recombinant Synechocystis sp. with higher PHA accumulation suggesting that the concentration of these enzymes is not the limiting factor to achieving high PHA accumulation. In order to cope with the higher PHA production, cells may utilize enhanced photosynthesis to drive the product formation. Results from this study suggest that the total flux of carbon is the possible driving force for the biosynthesis of PHA and the polymerizing enzyme, PHA synthase, is not the only critical factor affecting PHA-synthesis. Knowledge of the regulation or control points of the biopolymer production pathways will facilitate the further use of cyanobacteria for biotechnological applications.
Introduction
Cyanobacteria are believed to be one of the oldest groups of photosynthetic organisms on Earth and played a significant role in the development of the oxygenic atmosphere we breathe today [1]. In modern day, cyanobacteria continue to play a pivotal role in global carbon recycling, the nitrogen cycle and most importantly, the maintenance of the composition of the atmosphere [2], [3]. Cyanobacteria are considered to be ideal producers of various fine chemicals and biofuels because they fix carbon dioxide into biomass using solar energy. Fluctuations of nutrient concentrations constantly occur in natural environments and microorganisms respond to nutrient starvation by accumulating various carbon and energy storage compounds [4]. The study of these storage polymers, particularly polyhydroxyalkanoate (PHA), has gained considerable interest in recent years in an attempt to address the waste disposal problems caused by petrochemical plastics [5].
At present, the major biological processes utilized for industrial production of PHA are fermentations of heterotrophic bacteria. Nevertheless, the economic viability of PHA as a commodity polymer is limited by high production costs due to costly carbon substrates and requirements during the fermentation processes. Substantial effort has been devoted to investigating PHA production processes that are more cost-effective [6]. An interesting and promising approach is the use of photosynthetic cyanobacteria as the host for PHA production. The cyanobacteria, as ‘microbial factories’, can fix carbon dioxide from the atmosphere into high molecular weight PHA directly via photosynthesis. Besides being photoautotrophic, cyanobacteria require minimal nutrients for growth, eliminating the cost of carbon sources and complex growth media [7]. Thus, the application of cyanobacteria offers the potential of a cost-competitive and sustainable approach for the production of this environmentally friendly polymer.
The presence of PHA in cyanobacteria was first described by Carr whom analyzed PHA in Chloroglea fritschii based on acid hydrolysis of poly(3-hydroxybutyrate), P(3HB), to crotonic acid followed by UV spectroscopic measurement of the hydrolysis product [8]. Since then, much research has demonstrated the presence of PHA in several other cyanobacteria including Aphanothece sp. [9], Oscillatoria limosa [10], some species of the genus Spirulina [11], [12] and the thermophilic strain Synechococcus sp. MA19 [13]. So far, cyanobacteria are characterized by their ability to produce PHA containing only 3-hydroxybutyrate (3HB) and/or 3-hydroxyvalerate (3HV) monomers [9], [10], [14]. Although there are many reports on the occurrence of PHA in cyanobacteria, most of these studies explored the physiology and fermentation aspects of PHA accumulation in cyanobacteria. The biochemical and molecular basis of PHA synthesis in cyanobacteria are not well understood.
The model cyanobacterium Synechocystis sp. strain PCC 6803 is considered as a promising candidate for various biotechnological productions because of the availability of its genome sequence information [15] and the ease of genetic manipulation of this strain due to its naturally transformable feature [16]. In this study, Synechocystis sp. was metabolically engineered by increasing the flux of intermediates to PHA biosynthesis and introducing a PHA synthase with higher activity. RNA-seq analysis was carried out to examine the differential expression involved in the global biological processes and metabolic pathways during the improved photoautotrophic production of PHA. This information will facilitate the potential use of cyanobacteria for the sustainable production of this ‘green’ polymer.
Results
Enhanced PHA Production in Recombinant Cyanobacteria
In the well-studied PHA biosynthetic pathway of Cupriavidus necator, P(3HB) synthesis occurs in a three-step reaction and starts with the condensation of acetyl-CoA to acetoacetyl-CoA by β-ketothiolase [17]. Under photosynthetic conditions, it was hypothesized that the acetyl-CoA pool in cyanobacteria is insufficient to drive the thermodynamically unfavorable condensation reaction forward [18]. Instead of relying solely on the native β-ketothiolase-mediated condensation to form acetoacetyl-CoA, an acetoacetyl-CoA synthase (nphT7S s) from Streptomyces sp. CL190 was incorporated in the P(3HB) pathway design. The nphT7S s gene catalyzes the irreversible condensation of acetyl-CoA and malonyl-CoA to give acetoacetyl-CoA, driving the reaction toward the formation of PHA. The evolution of carbon dioxide from the condensation reaction effectively pushes the reaction toward the formation of acetoacetyl-CoA [19]. A highly active PHA synthase from Chromobacterium sp. USM2 (phaCC s) [20] was co-expressed with nphT7S s to improve the photosynthetic production of P(3HB) in Synechocystis sp. In view of the stimulatory effects of nutrient limitation, carbon supplementation and air-exchange limitation on PHA accumulation [7], [21], biosynthesis experiments were designed under these cultivation conditions: N- or P-deficient (nitrogen- or phosphorus-deficient), air-exchange limitation, and/or in the presence of carbon sources (CO2, acetate and/or fructose).
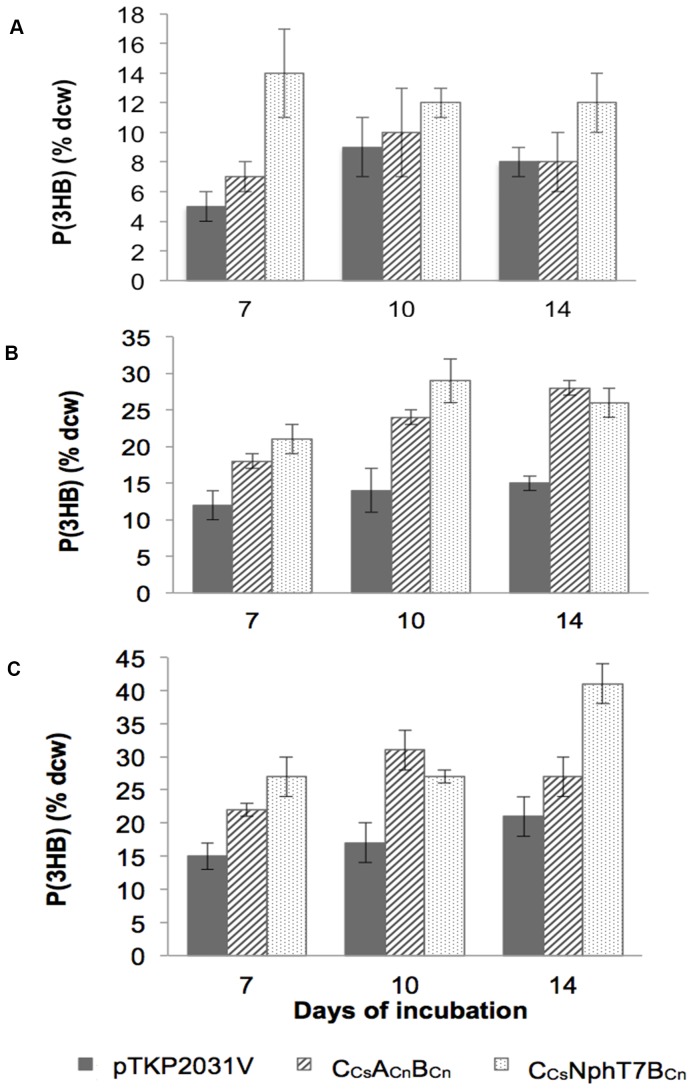
For the design of PHA production pathway in Synechocystis sp., the vector plasmid pTKP2031V was used for the insertion of transgenes into the genome via homologous recombination between sites slr2030 and slr2031 [22]. Synechocystis sp. was transformed with a plasmid harboring phaCC s, nphT7S s and C. necator acetoacetyl-CoA reductase (phaBCn) genes under the control of the light-inducible psbAII promoter. The successful transformant strain CCsNphT7BCn was analyzed for PHA production under a two-stage culture system consisting of sequential cell growth and PHA accumulation phases. The strain CCsNphT7BCn achieved an encouraging direct photosynthetic production of PHA from CO2, with a maximum of 14 wt% P(3HB) content on day 7 of cultivation (Fig. 1A). In comparison, strain CCsACnBCn expressing phaCC s, C. necator β-ketothiolase (phaACn) and phaBCn recorded a reduction in P(3HB) content (7 wt%) under the same cultivation conditions. The strain pTKP2031V, with only a kanamycin resistance cassette integrated into the genome, showed the lowest P(3HB) production potential (5 wt%). Prolonged incubation until day 14, however, did not exert any significant impact on the P(3HB) accumulation potential of the cyanobacteria under photoautotrophic conditions. At higher cell densities, P(3HB) accumulation may be limited by competition for carbon dioxide and light.
Figure 1. Comparison of P(3HB) accumulation in Synechocystis PCC 6803 strains pTKP2031V, CCsACnBCn and CCsNphT7BCn.
Cells were cultivated on modified BG-11 media, (A) bubbled with 2-3% CO2 (B) supplemented with 0.4%(w/v) acetate, incubated with shaking and (C) supplemented with 0.4%(w/v) acetate under air-exchange limiting conditions and incubated with shaking. They were harvested after the specified incubation time (7, 10 or 14 days). Data shown are the means and standard deviation of triplicates.
In order to boost P(3HB) production, an exogenous carbon source [0.4%(w/v) acetate] was provided to the cyanobacterial cultures at the PHA accumulation phase. Strain CCsNphT7BCn recorded the highest PHA content of 29 wt% on day 10 of incubation (Fig. 1B). The increase in the P(3HB) pool resulting from the addition of a carbon source affirms earlier findings on the effect of external carbon source supplementation on PHA production [7], [21]. In the case of air-exchange limitation effect, a significant increase in P(3HB) was observed (up to 41 wt%) for strain CCsNphT7BCn (Fig. 1C). These observations imply that the P(3HB) accumulation potential of Synechocystis sp. is affected by the provision of carbon source and air-exchange. Interestingly, the increase in CO2 supply (5%) to photoautotrophic cultures of Synechocystis sp. was found to increase the PHA content up to 16 wt% in strain CCsNphT7BCn (Table 1). The simultaneous addition of acetate and fructose to N- or P-deficient cultures of strain pTKP2031V showed a reduction in P(3HB) accumulation compared to photoautotrophic conditions (5% CO2). There were no significant changes in the P(3HB) content of strains CCsNphT7BCn and CCsACnBCn under the same cultivation conditions. The order of PHA-producing potential of the recombinant Synechocystis sp. on day 7 of incubation and under the cultivation conditions tested in this study is CCsNphT7BCn> CCsACnBCn>pTKP2031V.
Table 1. P(3HB) accumulation in recombinant Synechocystis sp. PCC 6803 under various treatment conditions.
| Treatment | P(3HB) (%) w/w of dry cells |
| pTKP2031V | |
| N-deficiency, CO2 (5%) | 10±1 |
| P-deficiency, Acetate, Fructose | 3±1 |
| N-deficiency, Acetate, Fructose | 6±1 |
| CCsACnBCn | |
| N-deficiency, CO2 (5%) | 10±2 |
| P-deficiency, Acetate, Fructose | 8±1 |
| N-deficiency, Acetate, Fructose | 12±1 |
| CCsNphT7BCn | |
| N-deficiency, CO2 (5%) | 16±4 |
| P-deficiency, Acetate, Fructose | 18±3 |
| N-deficiency, Acetate, Fructose | 15±2 |
Comparison of P(3HB) accumulation in Synechocystis sp. PCC 6803 strains pTKP2031V, CCsACnBCn and CCsNphT7BCn. Cells cultivated on modified BG-11 media under the indicated cultivation conditions were harvested after 7 days of incubation. Data shown are the means and standard deviation of triplicates.
Expression Levels of PHA Synthesis-related Genes
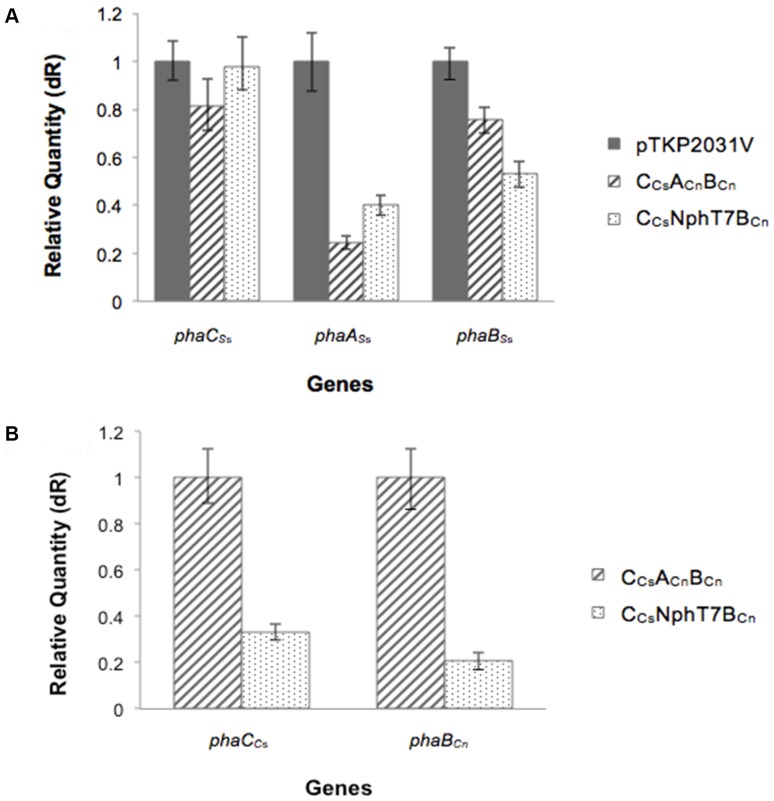
The expression levels of native PHA biosynthetic genes in Synechocystis sp. consisting of phaCSs, phaASs and phaBSs were monitored by real-time PCR analysis (Fig. 2A and 2B). Surprisingly, comparative quantification of phaASs and phaBSs expression levels in Synechocystis sp. strain CCsNphT7BCn relative to pTKP2031V show expression that was approximately 2-fold lower. However, there were no significant differences in the expression level of the native phaCSs gene in the recombinant Synechocystis sp. (pTKP2013V, CCsACnBCn and CCsNphT7BCn) investigated. The expression levels of phaCC s and phaBCn that were introduced into the genome on the same operon showed at least 3-fold lower expression in strain CCsNphT7BCn compared to CCsACnBCn. Despite higher levels of PHA accumulation in CCsNphT7BCn, the expression levels of most PHA synthesis-related genes in this strain were relatively lower compared to CCsACnBCn and pTKP2013V.
Figure 2. Comparative quantification of expression levels of PHA biosynthetic genes in (A) Synechocystis sp. PCC 6803 strains CCsNphT7BCn, CCsACnBCn (target) relative to pTKP2031V (calibrator) and (B) CCsNphT7BCn (target) relative to CCsACnBCn (calibrator).
Cells were cultivated in modified BG-11 media bubbled with 2-3% CO2.
Analysis of Synechocystis sp. Transcriptional Response Under Photoautotrophic PHA Accumulation Conditions
To gain insight into PHA accumulation in cyanobacteria, transcriptomes of recombinant Synechocystis sp. with different PHA-producing potential were analyzed. RNA-seq libraries were prepared from cells cultivated for 7 days in N-deficient BG-11 under photoautotrophic conditions. Sequencing was performed using the Illumina platform yielding a total of 93-million reads for 6 samples, with an average of 15.5-million reads per sample. Scatter plots between the two biological replicates for each recombinant Synechocystis sp. sample show correlation coefficients between 0.96-0.98, indicating the reproducibility of the sequencing data (Fig. S1). The expression levels for each gene were quantified as reads per kilobase of exon model per million mapped reads (RPKM).
The RNA-seq data provide detailed information on the genes that are regulated in response to photoautotrophic PHA accumulation conditions in recombinant Synechocystis sp. strains pTKP2013V, CCsACnBCn and CCsNphT7BCn. In general, the highly expressed genes in Synechocystis sp. were mainly involved in photosynthesis, the electron transport chain, protein metabolic processes and nucleic acid metabolism (Table S1). In particular, the transcript levels of genes involved in photosystem I (psaB, psaA, psaF and psaL) and photosystem II (psbA3, psbA2, psbX, psbY, psbU, psbK and psbD2) activities were among the most abundant. A comparison between gene expression in the recombinant strains that were more efficient in PHA production (CCsACnBCn andCCsNphT7BCn) relative to the reference strain (pTKP2013V) was made (Table 2). The up-regulated genes in strains CCsACnBCn and CCsNphT7BCn significantly enriched photosynthesis, transport and cell communication. In contrast, the down-regulated genes were found to be involved mostly in the metabolism of cofactors and vitamins, protein metabolic processes and DNA-binding. The photosystem I reaction center subunit XII gene (psaM) that is detected only in cyanobacteria was strongly up-regulated in both CCsACnBCn and CCsNphT7BCn. Another photosystem I reaction center subunit gene, psaJ was found to be up-regulated more than 10-fold. Both of these subunits are required to form a functional photosystem I [23]. In addition to genes encoding the photosystem I subunits, photosystem II-associated genes were among the significantly up-regulated genes. PsbX and PsbK, that have been found essential for the stability of photosystem II [24], were induced more than 5-fold in both CCsACnBCn andCCsNphT7BCn. Up-regulation of cytochrome B6-f complex subunits, PetG and PetL that are important for either stability or assembly of the complex, was also observed [25]. Two genes involved in porphyrin and chlorophyll metabolism, magnesium-protoporphyrin IX monomethyl ester cyclase (sll1874) and protoheme IX farnesyltransferase (sll1899) were up-regulated in both strains. Collectively, genes encoding proteins involved in several aspects of photosynthetic activity, e.g. photosystem I and II, cytochrome and chlorophyll metabolism were up-regulated in recombinant Synechocystis sp. that were actively synthesizing PHA.
Table 2. Genes up-regulated in recombinant Synechocystis sp. strains CCsACnBCn and CCsNphT7BCn (compared with pTKP2031V)a.
| Gene ID | Description | Fold change | Expression levelb | Functional category | |||
| (CCsACnBCn vs pTKP2031V) | (CCsNphT7BCn vs pTKP2031V) | pTKP2031V | CCsACnBCn | CCsNphT7BCn | |||
| ssr1169 | salt-stress induced hydrophobic peptide | 31.93 | 29.34 | 22.77 | 726.97 | 668.02 | cation transport |
| slr1064 | mannosyltransferase | 29.17 | 20.04 | 7.85 | 228.9 | 157.24 | polysaccharide metabolic process |
| smr0005 | photosystem I reaction center subunit XII, PsaM | 22.83 | 12.96 | 22.21 | 507.15 | 287.87 | photosynthesis |
| sml0008 | photosystem I reaction center subunit IX, PsaJ | 17.51 | 11.21 | 45.43 | 795.62 | 509.14 | photosynthesis |
| sll1161 | adenylate cyclase | 11.6 | 10.21 | 16.04 | 186.09 | 163.82 | nucleotide metabolic process |
| slr2114 | spore coat polysaccharide biosynthesis protein, SpsC | 10.27 | 9.45 | 7.83 | 80.37 | 73.95 | metabolic process |
| sml0002 | photosystem II protein, PsbX | 10.71 | 7.9 | 186.1 | 1,992.29 | 1,469.60 | photosynthesis |
| sml0005 | photosystem II reaction center protein K, PsbK | 6.98 | 7.61 | 106.49 | 743.15 | 810.34 | photosynthesis |
| smr0010 | cytochrome B6-f complex subunit, PetG | 6.96 | 7.1 | 175.41 | 1,220.93 | 1,245.29 | photosynthesis |
| sll0247 | iron-stress chlorophyll-binding protein | 4.49 | 7 | 61.36 | 275.41 | 429.3 | photosynthesis |
| slr0756 | circadian rhythm protein | 3.21 | 4.93 | 56.8 | 182.13 | 280.03 | circadian rhythm |
| sll0986 | Transposase | 3.57 | 4.68 | 84.36 | 301.33 | 395.05 | DNA-binding |
| slr1318 | iron(III) dicitrate ABC transporter permease | 3.5 | 4.18 | 19.17 | 67.18 | 80.06 | transport |
| sll1270 | glutamine ABC transporter | 2.52 | 4.15 | 91.4 | 230.36 | 379.34 | amino acid transport |
| sll1405 | biopolymer transport protein | 2.25 | 4.02 | 15.07 | 33.95 | 60.53 | protein transport |
| slr1693 | PatA subfamily protein | 3.2 | 4.02 | 55.32 | 177.23 | 222.58 | intracellular signal transduction |
| sll1994a | cytochrome B6f complex subunit, PetL | 3.92 | 3.97 | 17.59 | 68.91 | 69.77 | energy metabolism |
| sll0778 | ABC transporter | 3.39 | 3.8 | 14.72 | 49.95 | 55.89 | lipid transport |
| slr1760 | regulatory components of sensory transduction system | 3.33 | 3.67 | 23.44 | 78.05 | 86.01 | signal transduction |
| sll1874 | magnesium-protoporphyrin IX monomethyl ester cyclase | 3.19 | 3.64 | 21.02 | 67.16 | 76.58 | porphyrin and chlorophyll metabolism |
| slr0312 | NarL subfamily protein | 2.54 | 3.61 | 50.47 | 128.41 | 182.41 | intracellular signal transduction |
| sll0789 | OmpR subfamily protein | 1.84 | 3.58 | 92.86 | 170.51 | 332.85 | intracellular signal transduction |
| slr1755 | NAD(P)H-dependent glycerol-3-phosphate dehydrogenase | 3.02 | 3.32 | 28.65 | 86.61 | 95.08 | glycerophospholipid metabolism |
| sll1821 | 50S ribosomal protein L13 | 2.71 | 3.32 | 86.75 | 235.5 | 288.09 | translation |
| slr0611 | solanesyl diphosphate synthase | 2.52 | 3.21 | 36.57 | 92.07 | 117.33 | metabolic process |
| sll0792 | transcriptional repressor, SmtB | 1.92 | 3.09 | 77.57 | 149.25 | 239.65 | DNA-binding |
| sll0779 | PleD protein | 2.07 | 2.99 | 36.05 | 74.56 | 107.94 | signal transduction |
| sll1740 | 50S ribosomal protein L19 | 1.6 | 2.96 | 356.8 | 572.54 | 1,054.92 | translation |
| slr0984 | CDP-glucose-4,6-dehydratase | 2.7 | 2.88 | 20.43 | 55.12 | 58.88 | amino sugar and nucleotide sugar metabolism |
| sll0790 | sensory transduction histidine kinase | 1.48 | 2.85 | 98.45 | 145.37 | 280.87 | signal transduction |
| slr2079 | glutaminase | 2.31 | 2.79 | 65.16 | 150.48 | 182.1 | cellular amino acid metabolic process |
| slr2123 | D-isomer specific 2-hydroxyacid dehydrogenase | 2.72 | 2.78 | 21.83 | 59.38 | 60.62 | carbohydrate metabolic process |
| sll0643 | urease accessory protein G | 1.57 | 2.73 | 44.07 | 68.98 | 120.44 | GTP catabolic process |
| slr1498 | hydrogenase isoenzyme formation protein, HypD | 2.12 | 2.72 | 33.82 | 71.78 | 91.98 | protein metabolic process |
| sll1041 | ABC transporter | 1.95 | 2.61 | 75.65 | 147.32 | 197.09 | phosphate transport |
| slr1982 | chemotaxis protein, CheY | 1.59 | 2.6 | 298.23 | 474.89 | 774.35 | intracellular signal transduction |
| slr2131 | cation or drug efflux system protein | 2.29 | 2.6 | 26.11 | 59.75 | 67.83 | transport |
| slr1595 | Na/H antiporter | 2.45 | 2.59 | 13.76 | 33.65 | 35.64 | cation transport |
| slr1912 | anti-sigma F factor antagonist | 1.87 | 2.59 | 75.93 | 141.71 | 196.56 | regulation of transcription |
| ssl2296 | pterin-4-alpha-carbinolamine dehydratase | 1.81 | 2.51 | 56.88 | 103.21 | 142.56 | tetrahydrobiopterin biosynthetic process |
| sll1428 | P3 protein | 2.18 | 2.49 | 17.28 | 37.61 | 43.1 | cation transport |
| sll0080 | N-acetyl-gamma-glutamyl-phosphate reductase | 1.6 | 2.48 | 127.18 | 204 | 315.28 | amino acid metabolic process |
| sll1899 | protoheme IX farnesyltransferase | 1.43 | 2.47 | 79.37 | 113.61 | 195.82 | porphyrin and chlorophyll metabolism |
| sll1291 | PatA subfamily protein | 2.03 | 2.45 | 89.47 | 181.58 | 219.06 | signal transduction |
| slr0889 | ABC1-like protein | 1.82 | 2.41 | 33.73 | 61.35 | 81.44 | energy metabolism |
| sll1249 | bifunctional pantoate ligase/cytidylate kinase | 1.5 | 2.39 | 50.72 | 75.95 | 121.45 | pyrimidine base metabolic process |
| slr1909 | NarL subfamily protein | 2.13 | 2.38 | 35.46 | 75.5 | 84.51 | signal transduction |
| slr1805 | sensory transduction histidine kinase | 1.34 | 2.32 | 80.73 | 108.44 | 187.15 | signal transduction |
| sll1229 | hybrid sensory kinase | 1.78 | 2.31 | 49.42 | 88.21 | 113.98 | signal transduction |
a Only the top 50 highest increase in fold-change and genes encoding known proteins are shown.
b The values shown represent the mean of two independent biological replicates.
On the other hand, transcript levels of genes encoding protein metabolism (transcription, translation, amino acid synthesis, etc.) decreased in the recombinant Synechocystis sp. strains CCsACnBCn and CCsNphT7BCn (Table S2). The decrease in transcript levels of genes encoding these proteins [DNA mismatch protein (MutL), methionine sulfoxide reductase B (sll1680), prohibitin (slr1106), exoenzyme S synthesis protein B (ExsB), 3-dehydroquinate dehydratase (AroQ) and hydrogenase (HypA)] may be related to the reduced growth of Synechocystis sp. under N-deficient conditions. Cells response to nutrient-limiting conditions by accumulating PHA and at the same time slowing down metabolic activities. Reductions in expression levels of genes related to the metabolism of cofactors and vitamins [lipopeptide antibiotics iturin a biosynthesis protein (slr0495), cobalamin synthase (CobS), 4-hydroxythreonine-4-phosphate dehydrogenase (PdxA), cobalt-precorrin-6x reductase (CobK), riboflavin biosynthesis protein (RibG), lipolytransferase (LipB) and o-succinylbenzoate synthase (sll0409)] were observed.
A comparison between gene expression in the recombinant Synechocystis sp. strains CCsNphT7BCn and CCsACnBCn was made to gain substantial insights into the global responses of cyanobacteria to accommodate the extensive accumulation of PHA (Table 3). The analysis showed that strain CCsNphT7BCn employed a combination of induced stress response, photosynthesis, energy metabolism and transport during the PHA accumulation phase. Notably, genes encoding proteins involved in several aspects of photosynthetic activity e.g. uroporphyrinogen decarboxylase (HemE), ferredoxin component (slr1205), protochlorophilide reductase subunit (BchB), protohome IX farnesyltransferase (CtaB), photosystem II reaction center protein N (PsbN) and iron-stress chlorophyll-binding protein (IsiA) were up-regulated in strain CCsNphT7BCn compared to CCsACnBCn. The increased photosynthetic activity suggests that the carbon fixing capacity was enhanced to accommodate the increased diversion of carbon to polymer formation. Polyhydroxyalkanoate are bacterial storage compounds synthesized in response to conditions of physiological stress [26]. In the current study, stress-related genes in cyanobacteria include co-chaperonin (groES), Holliday junction resolvase (ruvC), molecular chaperon (groEL), superoxide dismutase (sodB) and heat shock protein 90 (htpG) were modestly up-regulated. As it was proposed that PHA accumulation confers survival and stress tolerance in a changing environment [27], stress conditions may trigger responses that favor PHA production. In addition, the transcript level for the global nitrogen regulator, NtcA was detected at an increased level. NtcA is known to regulate the expression of a large number of genes involved in nitrogen metabolism [28] and induction of the gene encoding this protein can be related to the N-deficient cultivation conditions that were applied to increase PHA biosynthesis in Synechocystis sp. Conversely, down-regulation of the genes involved in DNA-binding, transport, translation and DNA repair were observed in strain CCsNphT7BCn (Table S3).
Table 3. Genes up-regulated in recombinant Synechocystis sp. strain CCsNphT7BCn (compared with CCsACnBCn)a.
| Gene ID | Description | Fold change | Expression levelb | Functional category | |
| (CCsNphT7BCnvsCCsACnBCn) | CCsACnBCn | CCsNphT7BCn | |||
| slr2075 | co-chaperonin, GroES | 3.26 | 328.12 | 1,069.34 | protein folding |
| slr1204 | serine protease, HtrA | 2.73 | 1,046.94 | 2,860.72 | cell communication |
| slr1316 | iron(III) dicitrate ABC transporter permease | 2.37 | 15.33 | 36.3 | iron transport |
| ssr2595 | high light inducible protein | 2.19 | 25.55 | 55.96 | chlorophyll-binding |
| sll0379 | UDP-N-acetylglucosamine acyltransferase | 2.14 | 28.2 | 60.47 | lipopolysaccharide biosynthetic process |
| sll0789 | OmpR subfamily protein | 2.13 | 108.53 | 231.24 | intracellular signal transduction |
| sll0790 | sensory transduction histidine kinase | 2.12 | 90.22 | 191.02 | signal transduction |
| sll0896 | Holliday junction resolvase, RuvC | 2.09 | 17.14 | 35.89 | DNA repair |
| slr2076 | molecular chaperone, GroEL | 2.05 | 733.79 | 1,507.39 | protein folding |
| slr1279 | NADH dehydrogenase subunit A | 2.05 | 25.85 | 52.92 | electron transport chain |
| slr0536 | uroporphyrinogen decarboxylase, HemE | 2.02 | 80.15 | 162.23 | porphyrin and chlorophyll metabolism |
| slr1675 | hydrogenase expression/formation protein, HypA | 2.01 | 111.92 | 225.29 | cellular protein modification process |
| slr1202 | lactose ABC transporter permease | 1.96 | 16.49 | 32.23 | transport |
| sll1740 | 50S ribosomal protein L19 | 1.93 | 427.21 | 825.93 | translation |
| sll1538 | beta-glucosidase | 1.92 | 21.65 | 41.64 | carbohydrate metabolism |
| slr1205 | ferredoxin component | 1.92 | 214.39 | 412.13 | photosynthesis |
| ssr2049 | protochlorophillide reductase 57 kD subunit, BchB | 1.9 | 30.51 | 57.98 | photosynthesis |
| slr1805 | sensory transduction histidine kinase | 1.89 | 63.65 | 120.35 | signal transduction |
| sll1899 | protoheme IX farnesyltransferase, CtaB | 1.88 | 67.28 | 126.68 | porphyrin and chlorophyll metabolism |
| slr1256 | urease subunit gamma | 1.86 | 38.67 | 72.03 | nitrogen metabolism |
| sll0109 | chorismatemutase | 1.84 | 34.12 | 62.78 | amino acid biosynthetic process |
| slr1516 | superoxide dismutase, SodB | 1.83 | 1,356.38 | 2,485.21 | immune system process |
| sll1423 | global nitrogen regulator, NtcA | 1.82 | 376.21 | 682.95 | transcription |
| sll0045 | sucrose phosphate synthase | 1.81 | 22.41 | 40.57 | starch and sucrose metabolism |
| sll0899 | bifunctional N-acetylglucosamine-1-phosphate uridyltransferase/glucosamine-1-phosphate acetyltransferase | 1.8 | 19.64 | 35.42 | carbohydrate metabolism |
| slr1147 | sensory transduction histidine kinase | 1.8 | 34.66 | 62.29 | signal transduction |
| ssl2233 | 30S ribosomal protein S20 | 1.8 | 457.05 | 821.29 | translation |
| smr0009 | photosystem II reaction center protein N, PsbN | 1.79 | 113.3 | 202.33 | photosynthesis |
| slr1728 | potassium-transporting ATPase subunit A | 1.77 | 48.53 | 85.79 | ion transport |
| sll2010 | UDP-N-acetylmuramoyl-L-alanyl-D-glutamate synthetase | 1.77 | 17.31 | 30.59 | peptidoglycan biosynthetic process |
| slr0676 | adenylylsulfate kinase | 1.76 | 18.58 | 32.79 | sulfur metabolism |
| slr1982 | chemotaxis protein, CheY | 1.76 | 344.98 | 605.5 | intracellular signal transduction |
| sll1869 | 3-chlorobenzoate-3,4-dioxygenase | 1.75 | 27.55 | 48.23 | oxidation reduction process |
| sll1085 | glycerol-3-phosphate dehydrogenase | 1.75 | 34.23 | 59.74 | glycerophospholipid metabolism |
| ssl2250 | glycoprotein | 1.74 | 99.29 | 172.65 | drug and analog sensitivity |
| sll1957 | arsenical resistance operon repressor | 1.74 | 19.37 | 33.61 | transcription |
| slr2035 | gamma-glutamyl kinase | 1.73 | 22.05 | 38.08 | amino acid biosynthetic process |
| slr1295 | iron transport protein | 1.72 | 88.89 | 153.22 | iron transport |
| sll0792 | transcriptional repressor, SmtB | 1.71 | 93.19 | 159.74 | transcription regulation |
| sll1405 | biopolymer transport protein | 1.71 | 19.58 | 33.47 | protein transport |
| sll1249 | bifunctionalpantoate ligase/cytidylate kinase | 1.7 | 42.91 | 72.84 | pantothenate biosynthetic process |
| sll1283 | sporulation protein, SpoIID | 1.7 | 47.99 | 81.34 | sporulation |
| ssr1386 | NADH dehydrogenase subunit, NdhL | 1.69 | 51.61 | 87.32 | energy metabolism |
| slr1843 | glucose-6-phosphate 1-dehydrogenase | 1.67 | 64.14 | 107.41 | carbohydrate metabolism |
| sll0247 | iron-stress chlorophyll-binding protein | 1.65 | 186.8 | 308.95 | photosynthesis |
| sll1468 | beta-carotene hydroxylase | 1.65 | 87.04 | 143.75 | carotenoid biosynthetic process |
| slr1476 | aspartate carbamoyltransferase | 1.65 | 57.68 | 95.25 | pyrimidine biosynthetic process |
| sll0648 | lipophilic protein | 1.65 | 26.01 | 42.85 | lipid transport |
| sll0923 | exopolysaccharide export protein | 1.64 | 181.51 | 297.86 | lipopolysaccharide biosynthetic process |
| sll0430 | heat shock protein 90, HtpG | 1.64 | 186.42 | 305.82 | protein folding |
a Only the top 50 highest increase in fold-change and genes encoding known proteins are shown.
b The values shown represent the mean of two independent biological replicates.
Discussion
Current limitation of direct photosynthetic production using cyanobacteria is the relatively low PHA content obtained. In this study, it was encouraging to obtain 14 wt% of P(3HB) from direct photosynthetic fixing of carbon dioxide without the addition of an external carbon source. Although cyanobacteria have simple nutrient requirements, the addition of 0.4%(w/v) acetate was found to increase P(3HB) content up to 41 wt% under air-exchange limiting conditions. Previous studies suggested that enhanced P(3HB) accumulation was the result of direct metabolism of acetate for PHA synthesis by employing an existing pathway operating in cyanobacteria [7], [21]. The provision of exogenous carbon was found to have a positive impact on PHA accumulation albeit at concentrations that were 10- to 20-fold lower than those required by heterotrophic bacteria. Recently, the development of new photobioreactors for mass cultivation of cyanobacteria is in progress and these findings will greatly aid the use of cyanobacteria for potential industrial applications [29], [30].
Early studies indicate that the PHA biosynthetic genes of Synechocystis sp. 6803 do not co-localise together to form an operon [31], [32]. Instead, the PHA synthase of Synechocystis sp. consisting of phaC and phaE subunits are linked in the genome and co-expressed. On the other hand, the β-ketothiolase and acetoacetyl-CoA reductase of Synechocystis sp. do not map close to the PHA synthase locus but are probably clustered together and constitute an operon in a different section of the genome. The expression levels of these two genes were surprisingly lower in the recombinant Synechocystis sp. strains CCsACnBCn and CCsNphT7BCn that had higher PHA production potential compared to strain pTKP2013V that accumulated a lower content of PHA. These results suggest that the endogenous PHA biosynthetic pathway operating in Synechocystis sp. did not have a significant impact on the PHA-synthesizing abilities of strains CCsACnBCn and CCsNphT7BCn.
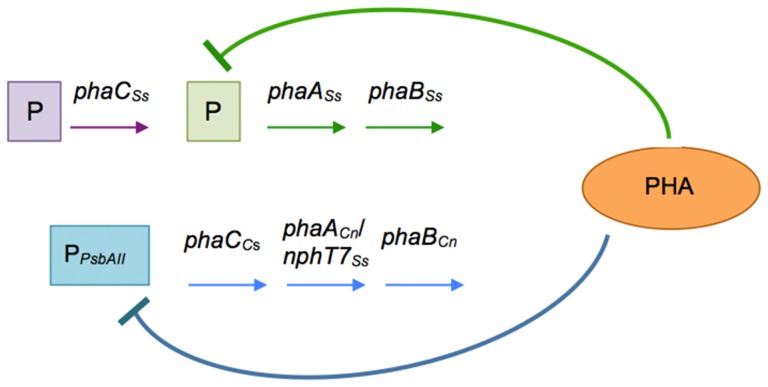
The Chromobacterium sp. PHA synthase and C. necator acetoacetyl-CoA reductase that were introduced into the genome as an operon showed similar lower expression in strain CCsNphT7BCn. The observation that the expression levels of most of the PHA biosynthetic genes were lower in strain CCsNphT7BCn suggests that the concentration of these enzymes is not the limiting factor in achieving higher PHA accumulation. Based on the results presented here, the transcription of genes encoding enzymes involved in PHA biosynthesis is highly regulated and may be affected by the PHA content in the cells (Fig. 3). When the PHA accumulated by the cells has exceeded a certain threshold level, adequate levels of the enzymes may already be present to meet the biosynthetic demand. Thus, the PHA granule itself or some other sensing factors may exert negative feedback on the expression of these enzymes. However, the expression levels of the enzyme catalyzing the last step of PHA biosynthesis, Synechocystis sp. PHA synthase, remained grossly constant in all recombinant Synechocystis sp. because negative feedback regulations are likely exerted in the upper part of the pathway.
Figure 3. The scheme shows the regulation of PHA synthesis-related gene expression in recombinant Synechocystis sp.
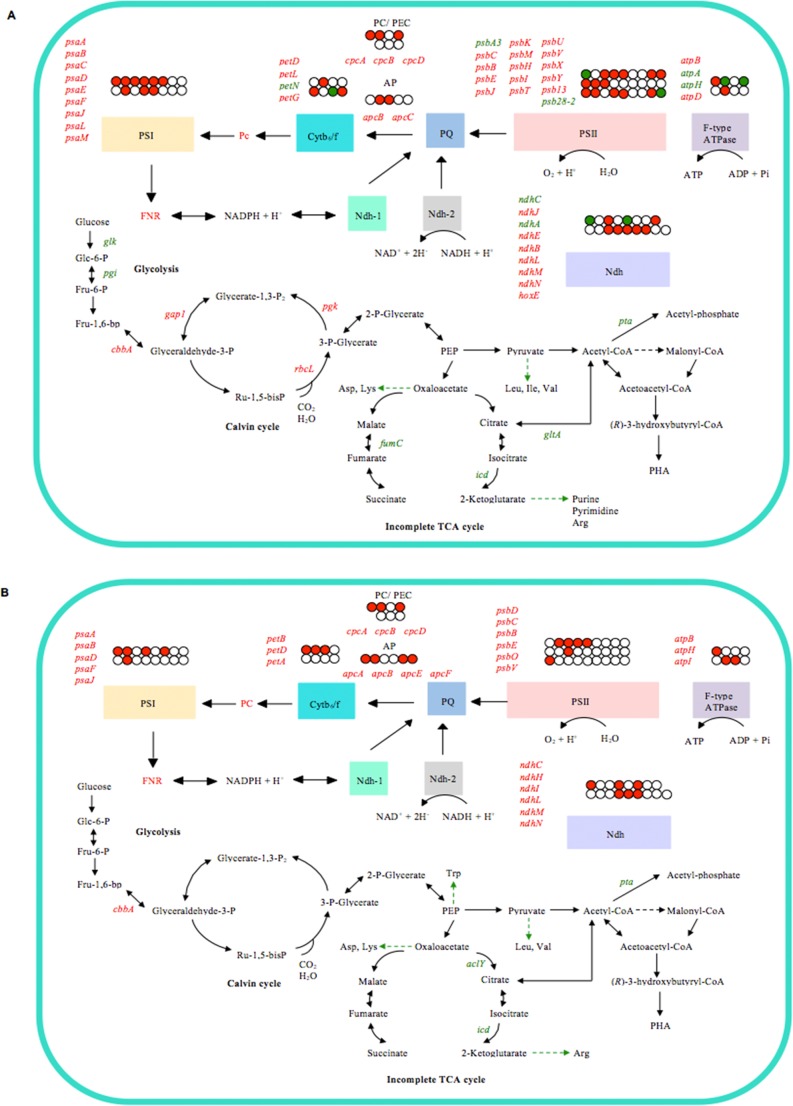
Previous genetic studies have focused on the engineering of various bacteria or plant hosts for PHA production, but less is known about the global transcriptional changes of the recombinant host under a PHA-synthesizing environment. A comprehensive view of the cyanobacterial transcriptome during cultivation under conditions favorable for PHA synthesis was generated using RNA-seq analysis. One particularly interesting observation is the up-regulation of photosynthetic activity in recombinants Synechocystis sp. with higher PHA-synthesizing potential (Fig. 4 A and B). In recent years, there has been tremendous interest in strategies to improve photosynthetic activity in crops [33], [34]. It has been suggested that an increase in photosynthetic activity will improve the yield of crops and provide a potential solution to future food shortages [35]. In this context, the increase of photosynthetic activity in cyanobacteria may explain the higher PHA accumulation observed in recombinant Synechocystis sp. strains CCsNphT7BCn and CCsACnBCn.
Figure 4. The scheme shows the cellular changes in recombinant Synechocystis sp. strains (a) CCsACnBCn and CCsNphT7BCn (compared with pTKP2031V) (b) CCsNphT7BCn (compared with CCsACnBCn) under photoautotrophic PHA biosynthesis conditions.
Only a selection of cellular changes is shown. The genes or pathways that are up-regulated are in red; the downregulated ones are in green. Black dashed lines indicate the engineered route. AP, allophycocyanin; PC/PEC, phycocyanin/phycoerythrocyanin; Cytb6/f, cytochrome b6/f complex; PQ, plastoquinone; FNR, ferredoxin-NADP(+) reductase; Pc, plastocyanin; PSI, photosystem I; PSII, photosystem II; Ndh, NADH dehydrogenase; Glc-6-P, glucose-6-phosphate; Fru-6-P, fructose-6-phosphate; Fru-1,6-bp; fructose-1,6-biphosphate; Glycerate-1,3-P2, 1,3-biphosphoglycerate; 3-P-Glycerate, 3-phosphoglycerate; Ru-1,5-bisP, ribulose-1,5-biphosphate; PEP, phosphoenolpyruvate.
The gene encoding one of the most important enzymes in carbon fixation, the ribulose-1,5 biphosphate carboxylase/oxygenase (RuBisCo) large subunit (rbcL), was up-regulated in both CCsNphT7BCn and CCsACnBCn. RuBisCo is a biologically important enzyme that catalyzes the first step of the reaction that converts atmospheric carbon dioxide into organic carbon [36]. Besides RuBisCo, genes encoding proteins involved in different aspects of photosynthesis and electron transport chain were significantly induced in both CCsNphT7BCn and CCsACnBCn. In particular, the induction of photosynthesis and electron transport chain-related genes was most prominent in strain CCsNphT7BCn with the highest PHA accumulation, suggesting the possible correlation of photosynthetic activity with PHA content. The Synechocystis sp. cells may utilize enhanced photosynthesis, carbon fixation and electron transport chain activities as a means to provide precursors that are necessary to drive the production of PHA. The increased photosynthetic production of PHA reveals that similar metabolic engineering approaches can be applied to the production of biofuels or chemicals using this versatile organism. As cyanobacteria and plants share similar photosynthetic machinery, it is likely that the strategy can be extended in future efforts to improve PHA production in higher plants.
In living cells, catabolic reactions that produce energy and anabolic biosynthetic reactions are regulated to maintain a balance of supply and demand. To cope with the higher PHA production demand, carbon dioxide fixing was enhanced to replenish the pool of carbon that was lost to PHA formation. Concomitant with the increase in photosynthetic activity, the flow of newly fixed carbon dioxide into biosynthetic reactions other than PHA was reduced. Genes encoding metabolism of cofactors and vitamins as well as protein metabolic process were found to be down-regulated in strains CCsNphT7BCn and CCsACnBCn. The reduced growth of recombinant Synechocystis sp. under nutrient-deficient cultivation conditions may account for the depression of these metabolic processes. These cellular anabolic reactions were regulated to maintain the balance of resources in cells. The expression levels of genes involved in the tricarboxylic acid cycle (TCA) were shown to be down-regulated in strains CCsNphT7BCn and CCsACnBCn. These observations agree well with previous finding that reported on the repressed of the TCA cycle genes in C. necator H16 during PHA production [37].
RNA-seq transcriptome analysis reveals that the heterologous expression of PHA synthesis-related genes in Synechocystis sp. affect not only the regulation of PHA biosynthesis but also the preceding pathways that are involved in the provision of precursors for this biosynthesis. The direct photosynthetic production of 14 wt% of P(3HB) from strain CCsNphT7BCn is the highest value achieved for Synechocystis sp. 6803 so far. This work suggests the use of carbon flux as a possible driving force for the biosynthesis of intracellular inclusions e.g. PHA. Future work can be done to confirm this finding by enhancing carbon fixation in cyanobacteria through engineering or overexpressing the enzymes involved in the process.
Materials and Methods
Chemicals and Reagents
All chemicals were purchased from Nacalai Tesque (Tokyo, Japan) or Wako Pure Chemical (Tokyo, Japan) unless otherwise specified. KOD Plus high-fidelity DNA polymerase was purchased from Toyobo (Tokyo, Japan). Restriction enzymes and the DNA ligation kits used were from Takara (Shiga, Japan).
Organism and Culture Conditions
All Synechocystis sp. PCC6803 strains (Table S4) were cultivated at 30°C in BG-11 medium [38] buffered with 20 mM HEPES-KOH, pH 8.0, under continuous illumination of 100 µmol photons m−2 s−1. Liquid cultures were incubated with shaking (100 r.p.m.) or bubbled with air enriched with 2-3% (v/v) CO2. Escherichia coli DH5α used for plasmid cloning was grown with shaking (180 r.p.m) at 37°C in Lysogeny broth. For the selection and maintenance of plasmids, kanamycin (50 µg/mL) or ampicillin (100 µg/mL) were added. To promote PHA biosynthesis in cyanobacteria, a two-stage cultivation was performed. The cultures were first grown in BG-11 medium until the late exponential phase and then harvested, washed and transferred to BG-11 medium devoid of sodium nitrate. P-deficiency was achieved by cultivating cells in BG-11 medium without K2HPO4. Different carbon sources [0.2% (w/v) and 0.4% (w/v) of fructose and/or acetate] were added to study the effects of carbon supplementation on PHA accumulation. Air-exchange limiting conditions on cultures were imposed by sealing the mouth of culture vessels with cotton plugs and covering with aluminium foil [7]. The cyanobacterial cultures were cultivated in the above culture conditions for 7, 10 or 14 days, harvested by centrifugation (8000 g, 10 min) and then lyophilized.
Plasmid Construction and Transformation of Synechocystis sp
The constructs used for transformation of Synechocystis sp. were derived from pTKP2031V (Table S4). pTKP2031V was designed for insertion into the genome via homologous recombination between sites slr2030 and slr2031 together with a kanamycin resistance cassette [22]. The expression of all cyanobacterial constructs was under the psbAII promoter. The gene cluster containing β-ketothiolase (phaACn) and acetoacetyl-CoA reductase (phaBCn) were amplified from chromosomal DNA of C. necator H16 using primers phaABCn (F; NdeI) and phaABCn (R; HpaI) (Table S5). The resulting PCR product was digested with NdeI and HpaI and inserted into NdeI- and HpaI-digested pTKP2031V to obtain pTKP2031V-phaABCn. The PHA synthase (phaCC s) was prepared from chromosomal DNA of Chromobacterium sp. USM2 using primers phaCC s (F; SfuI) and phaCC s (R; AatI). This PCR fragment was digested with SfuI and AatI and subcloned into the appropriate restriction sites of pTKP2031V-phaABCn by ligation to yield pTKP2031V-phaCC s ACnBCn. pTKP2031V-phaCC s nphT7phaBCn was constructed by replacing phaACn in pTKP2031V-phaCC s ACnBCn with nphT7. The nphT7 gene from Streptomyces sp. CL190 was amplified from a previously prepared template, pHis_nphT7 [19] using primers nphT7 (F; SfuI) and nphT7 (R; AatI). Transformation of Synechocystis sp. was performed as described previously [39]. Briefly, 100-200 µL of an exponentially growing culture were mixed with a plasmid solution to a final concentration of 1-2.5 µg/mL. The mixture was then spread onto a nitrocellulose membrane filter placed on a BG-11 plate and incubated overnight (∼12 h) at 30°C under continuous white light (75-100 µmol photons/m2s). The membrane filter was transferred onto a new BG-11 plate containing 50 µg/mL kanamycin. Kanamycin-resistant colonies were isolated and replated three times. The presence and complete segregation of the transgene in the cyanobacterial genome were confirmed by PCR analysis and sequencing.
Quantitative Analysis of PHA
Approximately 25-30 mg of lyophilized cyanobacterial cells were washed with methanol and dried at 65°C overnight. The dry cells were subsequently extracted with chloroform at 65°C for 48 h. The chloroform extract was subjected to methanolysis with a solution consisting of 85%(v/v) methanol and 15%(v/v) concentrated sulphuric acid at 100°C for 140 min [40]. The organic phase comprising the hydroxyacyl methyl esters was analyzed by gas chromatography-mass spectrometry (GC-MS) using the Agilent 7890A GC/5975 MSD system equipped with a HP−5 column (Agilent, USA).
RNA Preparation
Total RNA was extracted from cells using Trizol reagent (Invitrogen, USA) in combination with the PureLink RNA Mini Kit (Invitrogen, USA) according to manufacturer’s protocol. Any traces of DNA remaining in the RNA samples were removed by digestion with DNase I (Takara, Japan). The quality and quantity of the RNA samples were analyzed using a Bioanalyzer 2100 (Agilent, USA).
Real-time PCR Analysis
cDNA synthesis was performed with 250 ng of RNA using the QuantiTect Reverse Transcription Kit (Qiagen, USA). Real-time PCR quantification was performed using Thunderbird SYBR qPCR Mix (Toyobo, Japan) and gene-specific primers with the Mx3000P QPCR system (Agilent, USA). The cycling conditions were as follows: 95°C for 10 min, 40 cycles: 95°C for 15 s and 60°C for 1 min. A melting curve analysis (60°C-95°C) was performed after each amplification to ensure specificity of the reaction. Transcript levels were quantified based on determination of the quantification cycle (Ct). The transcript levels of genes of interest were normalized to the level of the housekeeping gene (16S rRNA) used in this study. Comparative quantification was used to compare the expression levels of genes of interest in Synechocystis sp. PCC 6803 strains CCsNphT7BCn and CCsACnBCn (target) relative to pTKP2031V (calibrator) and CCsNphT7BCn (target) relative to CCsACnBCn (calibrator).
RNA-seq Library Preparation, Illumina Sequencing and Data Analysis
For each sample, 2 µg total RNA was subjected to ribosomal RNA depletion using the Ribo-Zero rRNA removal kit (Epicentre, USA). The cDNA libraries for RNA-seq were constructed from total RNA depleted of rRNA using the Illumina TruSeq Stranded mRNA Sample Preparation Kit (Illumina, USA) according to the manufacturer’s specifications. In brief, preparation of the cDNA libraries included the following steps: RNA fragmentation, cDNA synthesis, 3′ ends adenylation, adapter ligation and cDNA template enrichment. Quantification of the libraries was carried out using a Bioanalyzer 2100 (Agilent, USA) and 4 to 6 pM of the template was used for cluster generation. Libraries were sequenced on a Miseq (Illumina, USA) instrument using the 2×250 paired end protocol. The sequence data has been submitted to the NCBI Gene Expression Omnibus (GEO) under accession number GSE50688. The RNA-seq data analysis was performed using CLC Genomics Workbench 6 software (CLC bio, Denmark). Sequence reads were pre-processed to trim low-quality reads and filter reads shorter than 20 bp. The qualified sequence reads were mapped to the Synechocystis sp. PCC 6803 genome (NC_000911), allowing a maximum of two mismatches. The reference genome sequences and annotations were downloaded from NCBI (downloaded on May 23, 2013). Sequence reads that mapped to non-coding RNA and reads that did not map to unique positions were excluded from further analysis. The transcript levels were expressed as reads per kilobase of exon model per million mapped reads in which the read count for a gene was normalized by the length of the gene and the total number of reads mapped in the sample [41]. Statistical analysis was performed and genes with a False Discovery Rate (FDR) p-value correction <0.05 were determined as differentially regulated genes [42].
Supporting Information
Correlation of RNA-Seq data between biological replicates. Normalized expression values from each sample were used. Correlation coefficients are indicated inside the plots.
(TIF)
Highly expressed genes based on normalized expression level (RPKM values)a.
(DOCX)
Genes down-regulated in recombinant Synechocystis sp. strain CCsACnBCn and CCsNphT7BCn (compared with pTKP2031V)a.
(DOCX)
Genes down-regulated in recombinant Synechocystis sp. strain CCsNphT7BCn (compared with CCsACnBCn)a.
(DOCX)
Strains and plasmids used in this study.
(DOCX)
Primers used in this study.
(DOCX)
Acknowledgments
We are grateful to Takeharu Tsuge of the Tokyo Institute of Technology for assistance with GC-MS analysis, Takashi Osanai for providing the Synechocystis sp. 6803 culture and pTKP2013V plasmid, Tomohisa Kuzuyama for providing the nphT7S s gene and Setsuko Shimada for help with real-time PCR analysis.
Funding Statement
This research is supported by Biomass Engineering Program research by RIKEN and International Program Associate fellowship to N.-S.L. and C.P.F. organized by RIKEN. N.-S.L. is a recipient of the Universiti Sains Malaysia Fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rasmussen B, Fletcher IR, Brocks JJ, Kilburn MR (2008) Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 455: 1101–1104. [DOI] [PubMed] [Google Scholar]
- 2. Tian F, Toon OB, Pavlov AA, De Sterck H (2005) A hydrogen-rich early Earth atmosphere. Science 308: 1014–1017. [DOI] [PubMed] [Google Scholar]
- 3. Raven JA, Falkowski PG (1999) Oceanic sinks for atmospheric CO2 . Plant Cell Environ 22: 741–755. [Google Scholar]
- 4. Madison LL, Huisman GW (1999) Metabolic engineering of poly(3-hydroxyalkanoates): From DNA to plastic. Microbiol Mol Biol R 63: 21–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sudesh K, Iwata T (2008) Sustainability of biobased and biodegradable plastics. CLEAN – Soil, Air, Water 36: 433–442. [Google Scholar]
- 6. Chen G-Q (2009) A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev 38: 2434–2446. [DOI] [PubMed] [Google Scholar]
- 7. Panda B, Mallick N (2007) Enhanced poly-β-hydroxybutyrate accumulation in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. Lett Appl Microbiol 44: 194–198. [DOI] [PubMed] [Google Scholar]
- 8. Carr NG (1966) The occurrence of poly-beta-hydroxybutyrate in the blue-green alga, Chlorogloea fritschii . Biochim Biophys Acta 120: 308–310. [DOI] [PubMed] [Google Scholar]
- 9. Capon RJ, Dunlop RW, Ghisalberti EL, Jefferies PR (1983) Poly-3-hydroxyalkanoates from marine and freshwater cyanobacteria. Phytochemistry 22: 1181–1184. [Google Scholar]
- 10.Stal LJ, Heyer H, Jacobs G (1990) Occurrence and role of poly-hydroxy-alkanoate in the cyanobacterium Oscillatoria limosa. Novel Biodegradable Microbial Polymers: Springer. 435–438.
- 11. Campbell J, Stevens SE Jr, Balkwill DL (1982) Accumulation of poly-beta-hydroxybutyrate in Spirulina platensis . J Bacteriol 149: 361–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vincenzini M, Sili C, de Philippis R, Ena A, Materassi R (1990) Occurrence of poly-beta-hydroxybutyrate in Spirulina species. J Bacteriol 172: 2791–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miyake M, Erata M, Asada Y (1996) A thermophilic cyanobacterium, Synechococcus sp. MA19, capable of accumulating poly-β-hydroxybutyrate. J Ferment Bioeng 82: 512–514. [Google Scholar]
- 14. Stal LJ (1992) Poly(hydroxyalkanoate) in cyanobacteria: an overview. FEMS Microbiol Rev 103: 169–180. [Google Scholar]
- 15. Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, et al. (1996) Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res 3: 109–136. [DOI] [PubMed] [Google Scholar]
- 16. Williams JGK (1988) Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Method Enzymol 167: 766–778. [Google Scholar]
- 17. Tsuge T (2002) Metabolic improvements and use of inexpensive carbon sources in microbial production of polyhydroxyalkanoates. Journal of Bioscience and Bioengineering 94: 579–584. [DOI] [PubMed] [Google Scholar]
- 18. Lan EI, Liao JC (2012) ATP drives direct photosynthetic production of 1-butanol in cyanobacteria. Proc Natl Acad Sci USA 109: 6018–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okamura E, Tomita T, Sawa R, Nishiyama M, Kuzuyama T (2010) Unprecedented acetoacetyl-coenzyme A synthesizing enzyme of the thiolase superfamily involved in the mevalonate pathway. Proc Natl Acad Sci USA 107: 11265–11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhubalan K, Chuah JA, Shozui F, Brigham CJ, Taguchi S, et al. (2011) Characterization of the highly active polyhydroxyalkanoate synthase of Chromobacterium sp. strain USM2. Appl Environ Microbiol 77: 2926–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharma L, Mallick N (2005) Accumulation of poly-β-hydroxybutyrate in Nostoc muscorum: regulation by pH, light-dark cycles, N and P status and carbon sources. Bioresour Technol 96: 1304–1310. [DOI] [PubMed] [Google Scholar]
- 22. Satoh S, Ikeuchi M, Mimuro M, Tanaka A (2001) Chlorophyll b expressed in cyanobacteria functions as a light-harvesting antenna in photosystem I through flexibility of the proteins. J Biol Chem 276: 4293–4297. [DOI] [PubMed] [Google Scholar]
- 23. Xu W, Tang H, Wang Y, Chitnis PR (2001) Proteins of the cyanobacterial photosystem I. Biochim Biophys Acta. 1507: 32–40. [DOI] [PubMed] [Google Scholar]
- 24. Hankamer B, Morris E, Nield J, Carne A, Barber J (2001) Subunit positioning and transmembrane helix organisation in the core dimer of photosystem II. FEBS Lett 504: 142–151. [DOI] [PubMed] [Google Scholar]
- 25. Schwenkert S, Legen J, Takami T, Shikanai T, Herrmann RG, et al. (2007) Role of the low-molecular-weight subunits PetL, PetG, and PetN in assembly, stability, and dimerization of the cytochrome b6f complex in tobacco. Plant Physiol 144: 1924–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54: 450–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pham TH, Webb JS, Rehm BH (2004) The role of polyhydroxyalkanoate biosynthesis by Pseudomonas aeruginosa in rhamnolipid and alginate production as well as stress tolerance and biofilm formation. Microbiology 150: 3405–3413. [DOI] [PubMed] [Google Scholar]
- 28. Bradley RL, Reddy KJ (1997) Cloning, sequencing, and regulation of the global nitrogen regulator gene ntcA in the unicellular diazotrophic cyanobacterium Cyanothece sp. strain BH68K. J Bacteriol 179: 4407–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalontarov M, Doud DFR, Jung EE, Angenent LT, Erickson D (2013) In situ hollow fiber membrane facilitated CO2 delivery to a cyanobacterium for enhanced productivity. RSC Advances 3: 13203–13209. [Google Scholar]
- 30. Kumar K, Dasgupta CN, Nayak B, Lindblad P, Das D (2011) Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria. Bioresour Technol 102: 4945–4953. [DOI] [PubMed] [Google Scholar]
- 31. Hein S, Tran H, Steinbuchel A (1998) Synechocystis sp. PCC6803 possesses a two-component polyhydroxyalkanoic acid synthase similar to that of anoxygenic purple sulfur bacteria. Arch Microbiol 170: 162–170. [DOI] [PubMed] [Google Scholar]
- 32. Taroncher-Oldenburg G, Nishina K, Stephanopoulos G (2000) Identification and analysis of the polyhydroxyalkanoate-specific β-ketothiolase and acetoacetyl coenzyme A reductase genes in the cyanobacterium Synechocystis sp. strain PCC6803. Appl Environ Microbiol 66: 4440–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raines CA (2011) Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: current and future strategies. Plant Physiol 155: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Richards RA (2000) Selectable traits to increase crop photosynthesis and yield of grain crops. J Exp Bot 51: 447–458. [DOI] [PubMed] [Google Scholar]
- 35. Covshoff S, Hibberd JM (2012) Integrating C4 photosynthesis into C3 crops to increase yield potential. Curr Opin Biotechnol 23: 209–214. [DOI] [PubMed] [Google Scholar]
- 36. Parry MAJ, Keys AJ, Madgwick PJ, Carmo-Silva AE, Andralojc PJ (2008) Rubisco regulation: a role for inhibitors. J Exp Bot 59: 1569–1580. [DOI] [PubMed] [Google Scholar]
- 37. Brigham CJ, Speth DR, Rha C, Sinskey AJ (2012) Whole-genome microarray and gene deletion studies reveal regulation of the polyhydroxyalkanoate production cycle by the stringent response in Ralstonia eutropha H16. Appl Environ Microbiol 78: 8033–8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Journal of General Microbiology 111: 1–61. [Google Scholar]
- 39. Osanai T, Oikawa A, Azuma M, Tanaka K, Saito K, et al. (2011) Genetic engineering of group 2 sigma factor SigE widely activates expressions of sugar catabolic genes in Synechocystis species PCC 6803. J Biol Chem 286: 30962–30971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Braunegg G, Sonnleitner B, Lafferty RM (1978) A rapid gas chromatographic method for the determination of poly-β-hydroxybutyric acid in microbial biomass. Eur J Appl Microbiol Biotechnol 6: 29–37. [Google Scholar]
- 41. Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Meth 5: 621–628. [DOI] [PubMed] [Google Scholar]
- 42. Baggerly KA, Deng L, Morris JS, Aldaz CM (2003) Differential expression in SAGE: accounting for normal between-library variation. Bioinformatics 19: 1477–1483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation of RNA-Seq data between biological replicates. Normalized expression values from each sample were used. Correlation coefficients are indicated inside the plots.
(TIF)
Highly expressed genes based on normalized expression level (RPKM values)a.
(DOCX)
Genes down-regulated in recombinant Synechocystis sp. strain CCsACnBCn and CCsNphT7BCn (compared with pTKP2031V)a.
(DOCX)
Genes down-regulated in recombinant Synechocystis sp. strain CCsNphT7BCn (compared with CCsACnBCn)a.
(DOCX)
Strains and plasmids used in this study.
(DOCX)
Primers used in this study.
(DOCX)