Abstract
Plant growth-promoting rhizobacteria, in association with plant roots, can trigger induced systemic resistance (ISR). Considering that low-molecular weight volatile hormone analogues such as methyl jasmonate and methyl salicylate can trigger defense responses in plants, we examined whether volatile organic compounds (VOCs) associated with rhizobacteria can initiate ISR. In Arabidopsis seedlings exposed to bacterial volatile blends from Bacillus subtilis GB03 and Bacillus amyloliquefaciens IN937a, disease severity by the bacterial pathogen Erwinia carotovora subsp. carotovora was significantly reduced compared with seedlings not exposed to bacterial volatiles before pathogen inoculation. Exposure to VOCs from rhizobacteria for as little as 4 d was sufficient to activate ISR in Arabidopsis seedlings. Chemical analysis of the bacterial volatile emissions revealed the release of a series of low-molecular weight hydrocarbons including the growth promoting VOC (2R,3R)-(-)-butanediol. Exogenous application of racemic mixture of (RR) and (SS) isomers of 2,3-butanediol was found to trigger ISR and transgenic lines of B. subtilis that emitted reduced levels of 2,3-butanediol and acetoin conferred reduced Arabidopsis protection to pathogen infection compared with seedlings exposed to VOCs from wild-type bacterial lines. Using transgenic and mutant lines of Arabidopsis, we provide evidence that the signaling pathway activated by volatiles from GB03 is dependent on ethylene, albeit independent of the salicylic acid or jasmonic acid signaling pathways. This study provides new insight into the role of bacteria VOCs as initiators of defense responses in plants.
Plant growth-promoting rhizobacteria (PGPR) are a wide range of root-colonizing bacteria with the capacity to enhance plant growth by increasing seed emergence, plant weight, and crop yields (Kloepper, 1992). Seed treatment with PGPR has been used to enhance growth of several crops (Glick, 1995; Cleyet-Marcel et al., 2001) and to suppress the growth of plant pathogens and deleterious rhizosphere microorganisms.
Application of some PGPR strains to seeds or seedlings has also been found to lead to a state of induced systemic resistance (ISR) in the treated plant (van Loon et al., 1998; Kloepper et al., 1999). ISR occurs when the plant's defense mechanisms are stimulated and primed to resist infection by pathogens (Van Loon, 1997). Such plant microbe interactions lack any visible hypersensitive response with colonization of the roots by PGPR (Wei et al., 1991). This activation of ISR by benign microorganisms in close proximity with roots is distinct from systemic acquired resistance (SAR) in which the response is triggered by pathogenic microorganisms associated with the aerial portions of the plant. For SAR, a hypersensitive response and several pathogenesis-related (PR) genes are triggered that serve as hallmarks of this inducible plant defense response (Ryals et al., 1996). PGPR-elicited ISR was initially observed in carnation (Dianthus caryophyllus) with a reduced susceptibility to Fusarium sp. wilt (van Peer et al., 1991), in common bean (Phaseolus vulgaris) with reduced susceptibility to halo blight (Alström, 1991), and in cucumber (Cucumis sativus) with reduced susceptibility to Colletotrichum orbiculare (Wei et al., 1991). PGPR-mediated ISR has since been reported for several other plant-pathogen systems (Maurhofer et al., 1994; Zhou and Paulitz, 1994; Liu et al., 1995; Leeman et al., 1996; Benhamou et al., 1998). PGPR that colonize root systems with seed applications and protect plants against foliar diseases include Pseudomonas fluorescens, Pseudomonas putida, Bacillus pumilus, and Serratia marcescens (Liu et al., 1995; Raupach et al., 1996; Kloepper et al., 1999; Pieterse et al., 2002).
The phenyl propanoid component, salicylic acid (SA), appears to be a critical plant messenger of pathogen exposure and disease resistance (Durner et al., 1997; Wildermuth et al., 2001), whereas jasmonic acid (JA), a lipoxygenase pathway product, is a potent regulator that mediates plant responses to mechanical damage and pathogenesis (Liechti and Farmer, 2002; Diaz et al., 2003). The airborne natural product derivatives, methyl salicylate (MeSA) and methyl jasmonate (MeJA), and the gaseous hormone ethylene also have been identified as potent activators of defense responses; these volatile components released from leaf tissue can be released at pharmacologically active concentration triggering responses in neighboring plants. MeJA is known to trigger induction of proteinase inhibitors and polyphenol oxidases in tomato (Lycopersicon esculentum; Fidantsef et al., 1999) and induce phytoalexin accumulation in bean and barley (Hordeum vulgare; Weidhase et al., 1987; Croft et al., 1993). Other examples of volatile plant chemicals triggering plant defenses include the expression of defense-related genes in tobacco (Nicotiana tabacum) by MeSA (Shulaev et al., 1997) and control of the amplitude and development of disease symptoms in Arabidopsis and soybean (Glycine max) by ethylene (Bent et al., 1992; Hoffman et al., 1999). C6 plant volatiles also have been identified as signal molecules triggering defense responses in tomato (Farag and Paré, 2002).
Little has been reported as to the role of microbial volatile organic compounds (VOCs) in regulating plant growth and development, although it has been observed recently that bacterial volatile components can serve as agents for triggering growth promotion in Arabidopsis (Ryu et al., 2003). Here, we report that a blend of air-borne chemicals released from specific bacterial strains of PGPR triggers ISR in Arabidopsis seedlings. Several genera of PGPR strains were assessed for eliciting ISR by volatiles under in vitro conditions. The PGPR strains were shown previously to elicit ISR on several crops against fungal, bacterial, and viral pathogens under greenhouse and field conditions (Raupach and Kloepper, 1998, 2000; Murphy et al., 2000; Zehnder et al., 2000). We evaluated the effects of volatiles produced by PGPR strains for eliciting ISR at different exposure times and the response of the volatiles to different mutant lines of Arabidopsis. The volatiles produced by selected PGPR strains Bacillus subtilis GB03 and Bacillus amyloliquefaciens IN937a were characterized. To our knowledge, this is the first report of ISR elicited by volatile chemicals released from PGPR and ascribes a new role for bacterial VOCs in triggering plant defense responses.
RESULTS
ISR by Air-Borne Bacterial Signal(s)
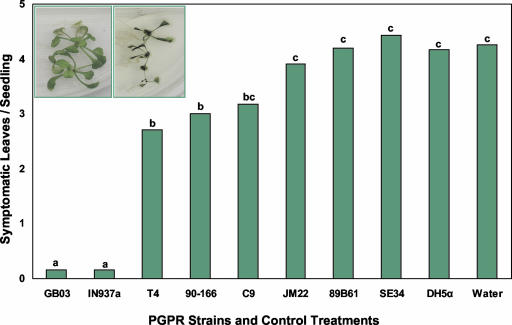
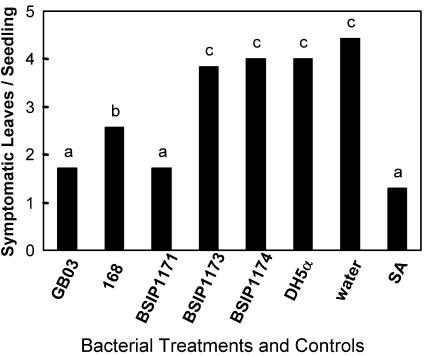
ISR activated in Arabidopsis by PGPR VOCs was assayed in the laboratory by physically separating seedlings from PGPR on divided petri dishes (referred to as I plates) so as to allow only airborne signals to be transmitted between bacterial cultures and the plant seedlings. Arabidopsis Columbia (Col)-0 seedlings exposed to certain PGPR strains for 10 d developed significantly less symptomatic leaves 24 h after inoculation with the soft rot-causing pathogen Erwinia carotovora subsp. carotovora. Rhizobacterial strains that triggered ISR by VOC signaling included B. subtilis GB03, B. amyloliquefaciens IN937a, S. marcescens 90-166, or B. pumilus T4 as compared with the water-treated controls (Fig. 1). The maximum level of protection resulted from treatment with strains GB03 and IN937a, whereas four other PGPR strains that cause ISR when inoculated onto crop seeds in soil failed to induce resistance in the I-plate test (Bacillus pasteurii C9, Enterobacter cloacae JM22, P. fluorescens 89B61, and B. pumilus SE34). The E. coli strain DH5α that has not been observed to activate ISR was used as a negative control. The selective ISR triggered by particular PGPR strains indicated that the release of bacterial VOCs is not the common mechanism for activating defenses for all rhizobacteria, although VOC-mediated ISR was observed for three Gram-positive Bacillus spp. (GB03, IN937a, and T4) and a Gram-negative S. marcescens strain (90-166). Treatment with strains GB03 and IN937a, in which seedlings exhibited the lowest number of symptomatic leaves with pathogen inoculation, were selected for further study (Fig. 1). To confirm that the number of symptomatic leaves in Arabidopsis seedlings is a representative indicator of overall plant infection, cell counts of E. carotovora subsp. carotovora present in 24-h infected leaf tissue was taken from leaf aliquots 10 d subsequent to different VOC incubation treatments as indicated: GB03 (1.1 ± 0.2 × 108 colony-forming units [CFU] g tissue fresh weight-1), IN937a (2.6 ± 0.5 × 108 CFU g tissue fresh weight-1), DH5α (9.5 ± 0.5 × 108 g tissue fresh weight-1), and water control (1.03 ± 0.05 × 109 g tissue fresh weight-1). These numbers reflect the cell count of E. carotovora subsp. carotovora for each treatment 24 h subsequent to infection. There was a significant difference in cell numbers for the GB03 and IN937a compared with both the water and DH5α control treatments.
Figure 1.
PGPR VOCs can modulate infection severity of Arabidopsis seedlings by E. carotovora subsp. carotovora strain SCC1. Plants exposed to airborne chemicals released from eight ISR-inducing PGPR strains compared with a non-ISR Escherichia coli strain DH5α and water treatment; different letters indicate significant differences between treatments according to lsd at P = 0.05. Inset, Representative seedlings 24 h after E. carotovora subsp. carotovora infection and exposure to VOCs from GB03 (left) or DH5α (right).
Effect of Exposure Time of Bacterial VOCs on ISR
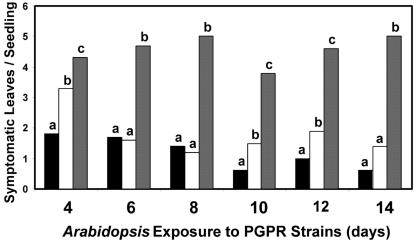
To determine how activation of ISR in plants might be dependent on VOC exposure time, the period in which Arabidopsis seedlings were exposed to bacterial VOCs was varied from 4 to 14 d. Strains GB03 and IN937a resulted in significant reductions in disease severity in seedlings compared with those in the water control for all exposure times tested (Fig. 2). However, the magnitude of disease protection with IN937a was significantly less than the protection of GB03 at an exposure time of 4 d.
Figure 2.
Induced disease resistance in Arabidopsis seedlings within 4 d of PGPR VOC exposure: PGPR strains GB03 (black boxes), IN937a (white boxes), or water treatment (gray boxes). Differences in letters indicates a significant difference between treated and control samples based on Fisher's lsd test at P = 0.05.
Bacterial VOCs Mimic ISR Triggered by PGPR
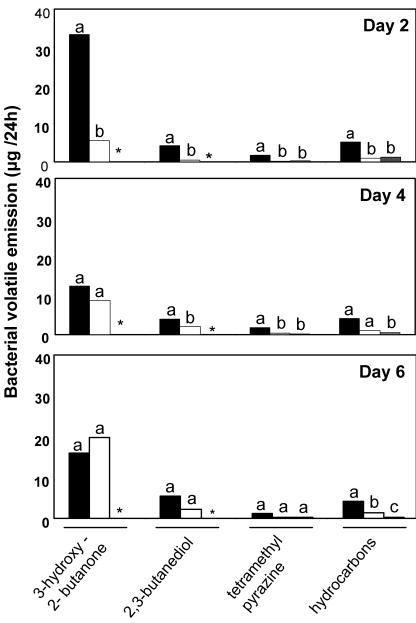
Gas chromatographic analysis of volatiles collected for 24-h intervals revealed consistent differences in the composition of volatile blends released by the ISR-activating bacterial strains GB03 and IN937a compared with the non-ISR-activating bacterial strain DH5α (Fig. 3). Compounds 2,3-butanediol and 3-hydroxy-2-butanone (also referred to as acetoin) were released consistently from the GB03 and IN937a strains, whereas these metabolites were not released from the DH5α and 89B61 (data not shown) or water control. Dodecane, 2-undecanone, 2-tridecanone, and 2-tridecan-1-ol were produced only from the GB03 strain, whereas tetramethyl pyrazine was detected at significantly higher levels from GB03 than these released from IN937a and DH5α. Decane and undecane were released at low levels from all bacterial strains, whereas decanal was detected in the medium even without bacterial exposure. For the strains that trigger ISR by VOC emissions, IN937a and GB03 DH5α, during a 24-h collection on d 4, 3-hydroxy-2-butanone and 2,3-butanediol were the two most abundant VOCs detected at 12 ± 5 and 3.9 ± 0.7 μg, respectively, for GB03 and 8.8 ± 2.2 and 1.9 ± 0.5 μg per 24-h period, respectively, for IN937a. These volatile alcohols are products of an alternative reductive pathway originating from pyruvate that provides an alternative source of NAD+ under anaerobic conditions. With 3-hydroxy-2-butanone and 2,3-butanediol, the qualitative and quantitative composition of volatile blends emitted by the ISR-activating strains differed significantly from non-ISR-activating bacteria DH5α.
Figure 3.
VOC profile of ISR-inducing strains GB03 (black boxes) and IN937a (white boxes) compared with the noninducing strain DH5α (gray boxes). Compounds positively identified include 3-hydroxy-2-butanone, 2,3-butanediol, tetramethyl pyrazine, and the hydrocarbons decane, undecane, decanal, dodecane, 2-undecanone, 2-tridecanone, and 2-tridecanol. Different letters indicate significant differences between treatments according to lsd at P = 0.05. An asterisk indicates that emissions were below detection limits.
The Role of 2,3-Butanediol in ISR
Because of an insertional knockout of the acetolactate synthase operon that controls the penultimate step in acetoin formation (pyruvate to acetolactate conversion) and acetolactate dehydrogenase, the enzymatic step that converts acetoin to 2,3-butanediol, mutant B. subtilis lines have reduced levels of 2,3-butanediol and acetoin (Ramos et al., 2000). In the case of the mutant lines BSIP1173 and BSIP1174, significantly lower levels of acetoin (0.7 ± 0.2 and 0.8 ± 0.3 μg) were emitted than the parental strain 168 (17 ± 5 μg) or the overexpresser line BSIP1171 (11 ± 3 μg; Fig. 4). An attenuation in VOC emissions was also observed for 2,3-butanediol with emissions levels of 13 ± 3 ng for BSIP1173 and 35 ± 18 ng for BSIP1174 compared with 575 ± 80 and 400 ± 90 ng for wild-type 168 and overexpressing BSIP1171 lines respectively. These mutant lines with reduced levels of acetoin and butanediol VOC emissions were tested directly against the wild-type strain 168 that is fully functional in acetoin and 2,3-butanediol synthesis in providing Arabidopsis seedlings protection against soft rot infection. With comparable growth for all strains on one-half-strength Murashige and Skoog media with 1.5% (w/v) Suc and 0.4% (w/v) tryptic soy agar (TSA) data not shown, volatiles of strain GB03 from wild-type (2,3-butanediol-producing) strain 168 and from strain BSIP1171 (overproducing 2,3-butanediol) exhibited significant reductions in disease severity (Fig. 5), whereas disease protection was not observed with two mutant lines emitting much lower levels of 2,3-butanediol and acetoin (BSIP1173 and BSIP1174) or with E. coli DH5α. Significant reduction in disease severity resulted from treatment with the chemical control SA, a signaling molecule known to activate disease resistance in Arabidopsis (Delaney et al., 1995).
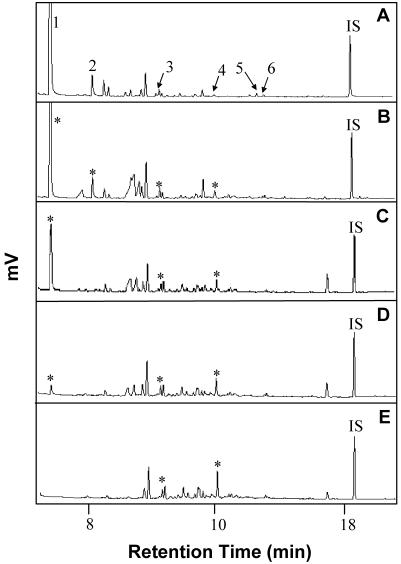
Figure 4.
Chromatographic profiles of volatiles released on d 2 from B. subtilis parental strain 168 (A), an overexpresser line BSIP1171 (B), mutant lines BSIP1173 (C) and BSIP1174 (D), and uninoculated media (E). Compounds positively identified include 3-hydroxy-2-butanone (1), 2,3-butanediol (2), decanal (3), decane (4), tetramethyl pyrazine (5), and undecane (6); nonyl acetate was added as an internal standard (IS). Asterisks in the lower chromatograms designate compounds that align with numbered peaks above.
Figure 5.
Infection of Arabidopsis by E. carotovora subsp. carotovora strain SCC1 after exposure to wild (GB03 and 168) overproducing 2,3-butanediol (BSIP1171) and mutants with attenuated 2,3-butanediol synthesis (BSIP1173 and BSIP1174). E. coli strain DH5α and water treatment were used as negative controls. Different letters indicate significant differences between treatments according to lsd at P = 0.05.
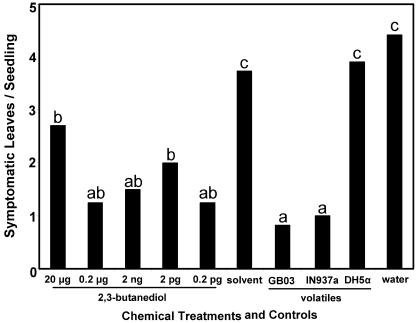
A dose response curve using a racemic mixture of 2,3-butanediol at a range from 20 μg to 0.2 pg in increments of 100-fold dilutions were tested in triggering ISR in Arabidopsis. Seedlings were exposed to the synthetic material for 10 d and then inoculated with the pathogen E. carotovora subsp. carotovora. By measuring the number of symptomatic leaves 24 h after inoculation, the pharmacologically effective airborne doses for triggering ISR were determined. When seedlings had been pre-exposed to 0.2 μg or 0.2 pg of 2,3-butanediol, the greatest resistance to pathogen infection was observed as measured by symptomatic leaf counts (Fig. 6). To minimize the initial burst of 2,3-butanediol released into the petri dish headspace and to extend the plant exposure to the test compound, a lanolin/2,3-butanediol mixture was administered (Kessler and Baldwin, 2001). The fact that differences in the number of symptomatic leaves were not statistically significant suggested that either the emission of 2,3-butanediol alone does not contain the full compliment of ISR-inducing factors to trigger a dose-dependent response or that the precision of the bioassay was not adequate to detect subtle differences in ISR.
Figure 6.
A dose response curve of 2,3-butanediol on the susceptibility of Arabidopsis to infection by E. carotovora subsp. carotovora strain SCC1. Volatile headspace collections from GB03 and IN977a were provided as positive controls, whereas solvent (CH2Cl2) provided a negative control. Different letters indicate significant differences between treatments according to lsd at P = 0.05.
Volatile extracts collected from strains GB03 and IN937a were tested for biological activity and were found to significantly reduce disease severity compared with the dichloromethane (solvent) control (Fig. 6). Arabidopsis exposure to volatile extracts collected from DH5α had no effect on ameliorating disease severity, which was comparable with the solvent control.
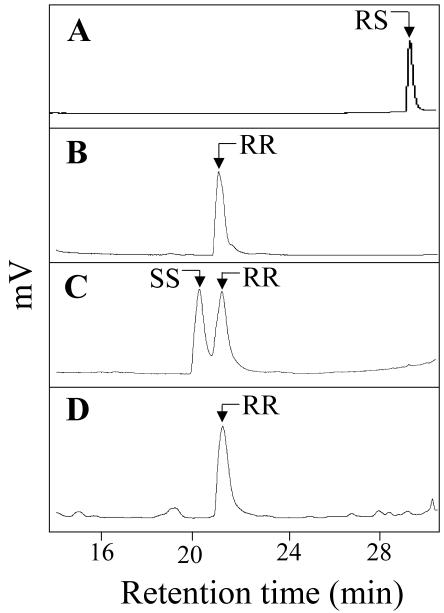
The stereochemistry of GB03 synthesized (2R,3R)-(-)-2,3-butanediol was determined by retention time (rt) comparisons with authentic standards (2S,3S)-(+)-2,3-butanediol, (2R,3R)-(-)-2,3-butanediol, and the meso (RS)-2,3-butanediol on a chiral gas chromatography (GC) column (Fig. 7). The S,S and R,R isomers were separated by approximately 1 min, whereas the meso form came over 7 min later. Acetoin, the oxidized precursor of (2R,3R)-(-)-2,3-butanediol, is enzymatically converted in Bacillus sp. by an NADH-mediated acetoin reductase reaction (Ramos et al., 2000). Assuming an absence of a racemase enzyme, the acetoin intermediate also would be present in the R conformation as (3R)-hydroxy-2-butanone.
Figure 7.
GB03 produced (2R,3R)-(-)-2,3-butanediol identification based on rt matches of authentic standards. GC-flame ionization detector (FID) trace of synthetic (RS)-2,3-butanediol (rt 28.6 min; A), synthetic (2R,3R)-(-)-2,3-butanediol (rt 21.1 min; B), a mixture of (2S,3S)-(+)-2,3-butanediol (rt 20.3 min) and (2R,3R)-(-)-2,3-butanediol (C), and GB03 volatile profile containing (2R,3R)-(-)-2,3-butanediol (rt 21.03 min; D).
Screening Signaling Pathway Mutants and Transgenic Plants for Regulatory Control of ISR
To begin to elucidate the signaling pathway(s) that relates to ISR, a series of mutant and transgenic plant lines were exposed to PGPR VOCs that we found to trigger ISR. Disease severity was reduced by exposure to VOCs from both strains GB03 and IN937a for mutant lines including a coronatine-/JA-insensitive line Coi1, an SA-degrading line NahG, a constitutively producing PR line Crp1, and a line that is SA insensitive or nonexpresser of PR genes Npr1. Of the mutants tested, only in the ethylene-insensitive line ein2 when exposed to VOCs from strain GB03 was the severity of disease symptoms not ameliorated (Table I).
Table I.
Disease severity for different Arabidopsis lines with pre-exposure to bacterial VOCs (lower nos. indicate fewer symptomatic leaves) Arabidopsis lines include: Col-0 (wild type), Ein2 (ethylene insensitive), Coi1 (jasmonic acid insensitive), NahG (degrades SA), Cpr1 (constitutively produces salicylic acid), and Npr1 (SA insensitive or nonexpresser of PR genes). Induced pathogen resistance with GB03 VOC exposure is lost only in the Ein2 line. Arabidopsis seedlings were infected with E. carotovora subsp. carotovora strain SCC1. Different letters indicate significant differences between treatments for a given plant line according to lsd at P = 0.05.
| Treatments
|
Disease Severity Measured by Symptomatic Leaves per Seedling
|
|||||
|---|---|---|---|---|---|---|
| Col-0 | Ein2 | Coi1 | NahG | Cpr1 | Npr1 | |
| GB03 | 2.3b | 4.5b | 3.2b | 0.9a | 1.5a | 0.3a |
| IN937a | 1.3a | 2.4a | 1.8a | 2.7a | 1.2b | 2.2b |
| DH5α | 3.3c | 4.4b | 4.3c | 4.4b | 4.4c | 4.6c |
| Water control | 4.2c | 5.0b | 4.8c | 4.7b | 3.5c | 4.4c |
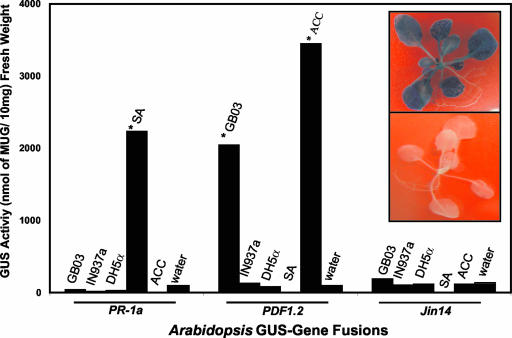
To further probe whether bacterial VOCs elicit known signaling pathways in Arabidopsis, transgenic plant lines with β-glucuronidase (GUS) fusions to Pr-1a, a gene activated by SA, Pdf1.2, a gene activated by JA and ethylene, and Jin14 a gene activated by JA, were exposed to VOCs released from several bacterial strains. The positive chemical controls of SA and the ethylene precursor 1-aminocyclopropane-1-carboxylic acid triggered significant increases in GUS activity for the SA-activated gene (Pr-1a) and the ethylene-/JA-activated gene (Pdf1.2), respectively (Fig. 8). With bacterial VOC exposure, the SA-activated gene (Pr-1a) did not show any increased transcriptional activity as measured by 4-methylumbelliferyl-β-d-glucuronide (MUG) fluorescence, although elevated GUS activity was detected for the ethylene/JA-activated gene (Pdf1.2) with exposure to GB03 VOCs. Because the JA-activated gene (Jin14) was unaffected by GB03 VOCs, ethylene signaling may prove to be essential in the activation of ISR in Arabidopsis.
Figure 8.
Activation of an ethylene/JA::GUS transgenic line of Arabidopsis (Pdf1.2) by GB03 VOCs, whereas other reporter transgenic lines (Pr-1a activated by SA and Jin14 activated by JA) were not activated by bacterial VOCs; the chemical elicitors SA and 1-aminocyclopropane-1-carboxylic acid (activate the Pr-1a gene and the Pdf1.2 gene respectively) were employed as positive controls, and DH5α VOCs and water treatment served as negative controls. An asterisk indicates a significant difference between treated and control samples according to lsd at P = 0.05. Inset, Transgenic seedlings Pdf 1.2 exposed to GB03 volatiles (top) and water control (bottom).
DISCUSSION
Plants have evolved the capacity to release and detect VOCs in their environment. The emission of plant odors has been shown to signal other organisms and members of its own species. Volatile odors released from flowers are well-known attractants of insect pollinators and leaf volatiles induced with tissue damage are potent semiochemicals for aphids and other herbivorous insects (Birkett et al., 2000). Plant VOCs can serve as signals between neighbors, whereby defense mechanisms are induced in undamaged plants in response to volatiles produced by nearby infested plants. Specific plant volatiles, MeSA, MeJA, and cis-jasmone, have been implicated in those mechanisms (Shulaev et al., 1997; Weber, 2002). Plant components containing six carbon atoms, e.g. (E)-2-hexenal, which are rapidly emitted from damaged plant tissue, also have been shown to induce the expression of defense-related genes in intact plants (Arimura et al., 2001; Gatehouse, 2002). In addition, a defensive role for terpenes as volatile elicitors has been proposed in excised lima bean (Phaseolus lunatus) plants (Arimura et al., 2000). However, given the interaction between excision and elicitation, increasing chemical evidence warns against extrapolation of data derived from excised tissue to intact plant models (Schmelz et al., 2001).
Although there are numerous examples of microorganisms interacting to trigger salubrious plant responses (e.g. nitrogen fixation, SAR, and growth promotion) or a deleterious outcome (e.g. soft rot and chlorosis), few reports have probed the role microbial VOCs may play in triggering biochemical changes of either primary or secondary plant metabolism.
Results presented here suggest that selected Bacillus PGPR strains emit VOCs that can elicit plant defenses. These released VOCs and the subset of collected VOCs that are re-applied as airborne chemicals to Arabidopsis seedlings contain sufficient chemical information to trigger ISR as measured by the seedlings ability to resist infection.
Quantitative measurement for VOCs released from agricultural plant species under laboratory conditions are published (Paré and Tumlinson, 1999) and in vitro PGPR emissions are now becoming available (Ryu et al., 2003); however, reasonable comparisons between trophic levels will require further data. Biotic and/or abiotic elicitors are known to induce elevated VOC emissions in many plant species. Can bacteria be induced to trigger elevated levels of VOCs? Does the medium that is used optimize for VOC emissions from the bacteria strains that were tested? We are interested in examining how changes in bacterial growth conditions influence emission profiles. The medium conditions that we have tested thus far optimize for low-medium VOC emissions. This medium is abundant in sugar, and O2 is initially not limiting. Because the petri dishes were covered and sealed with parafilm, O2 could have become limiting with microbial growth. The environment in which these bacteria reside in the soil may also be low in O2, thereby redirecting pyruvate metabolism into the production of 2,3-butanediol and acetoin (Ramos et al., 2000).
The major components detected in headspace collections from GB03 and IN937a were 3-hydroxy-2-butanone (acetoin) and (2R,3R)-(-)-2,3-butanediol, components that are generated from an alternative pathway for pyruvate catabolism that is favored under low pH or oxygen-limiting conditions (Ramos et al., 2000). In this metabolic sequence, acetolactate synthase catalyzes the condensation of two pyruvate molecules into acetolactate, which is decarboxylated to acetoin. Reduction of acetoin to 2,3-butanediol regenerates NAD+ in its oxidized form. It is interesting to note that the enzymes for generating 2,3-butanediol and acetoin have been identified in tobacco, corn (Zea mays), carrot (Daucus carota), and rice (Oryza sativa) cultures, although neither their function nor conditions of regulation have been identified (Forlani et al., 1999).
A model for signal transduction in PGPR-mediated ISR has been proposed by Pieterse et al. (1996, 2002) using mutant lines of Arabidopsis and P. fluorescens strain WCS417r. In their proposed signal transduction pathway, ISR triggered by PGPR is dependent on JA, ethylene, and Npr1, a regulatory gene that encodes salicylate dehydrogenase, whereas ISR activation is independent of SA. Our strategy was to test for possible links between mutant lines of Arabidopsis and ISR triggered by airborne PGPR signals based on the current signal transduction pathway for PGPR activation of ISR in which the bacteria make physical contact with the Arabidopsis roots. Results from our experiments with well-characterized Arabidopsis mutants differed from this model in that ISR resulting from exposure to VOCs of strains GB03 and IN937a involved signal transduction pathways that were independent of SA, JA, and NPR1. In addition, it appears that VOCs from strain IN937a triggered ISR through an ethylene-independent signaling pathway, whereas VOCs from strain GB03 appear to operate through an ethylene-dependent pathway in triggering ISR. Redundancy between SA, JA, and ethylene signaling pathways in ISR may come to explain some of these findings.
Our results with GUS fusions confirm this pattern of ethylene-dependent and -independent regulation in ISR mediated by GB03 VOC emissions. Characterization by Ton el al. (2001) of an ISR1 locus involved in ISR signaling in Arabidopsis by genetic and inhibitor studies found that rhizobacteria-mediated ISR does not require JA signaling, although ISR1 does encode a component of the ethylene response that is required for the expression of rhizobacteria-activated ISR. We are interested particularly in whether ISR activation by VOC emissions is mediated by the same signaling sequence that is triggered when rhizobacteria make physical contact of with Arabidopsis roots (such as in the model system P. fluorescens WCS417r), or if ISR activation by rhizobacteria VOCs is mediated by a novel signaling pathway.
MATERIALS AND METHODS
Chemicals
3-hydroxy-2-butanone, (RS)-2,3-butanediol, (2R,3R)-(-)-2,3-butanediol, (2S,3S)-(+)-2,3-butanediol, and racemic mixture of 2,3-butanediol were obtained from Sigma-Aldrich (Milwaukee, WI) and were of ≥99% purity as determined by capillary GC-FID analysis. Solvents used were of GC grade purity.
Plant Material
All mutant and transgenic lines were derived from parental Arabidopsis ecotype Col-0 that was obtained from the Ohio State University Stock Center (Columbus) except Pr-1a::GUS, which was in the Nossen background (Shah et al., 1997). Mutant lines included Npr1 (SA insensitive or nonexpresser of PR genes; Cao et al., 1994), cpr1 (constitutively expresses PR genes; Bowling et al., 1994; provided by Xinnian Dong, Duke University, Durham, NC), Ein2 (ethylene-insensitive; Alonso et al., 1999; provided by Joseph R. Ecker, University of Pennsylvania, Philadelphia), and Coi1 (insensitive to coronatine and JA; Xie et al., 1998; provided by John G. Turner, University of East Anglia, Norwick, UK). NahG transgenic plants encode salicylate dehydrogenase and degrade SA (Delaney et al., 1995). Arabidopsis promoter fusion GUS transgenic lines included SA-activated Pr-1a::GUS (Shah et al., 1997; provided by Daniel F. Klessig, Rutgers State University of New Jersey, Piscataway), JA-activated Jin14::GUS (provided by Norbert Nass, Institute of Plant Biochemistry, Department of Stress and Developmental Biol., Halle/Saale, Germany), and JA-/ethylene-activated Pdf1.2::GUS (provided by Willem F. Broekaert, Katholieke Universiteit Leuven, Heverlee-Leuven, Belgium).
Arabidopsis seeds were surface sterilized (2-min 70% [v/v] ethanol soaking followed by a 20-min 1% [v/v] sodium hypochlorite soaking), rinsed (4×) in sterile distilled water (SDW) and placed on petri dishes containing one-half-strength Murashige and Skoog medium (Murashige and Skoog salt, Gibco-BRL, Gaithersburg, MD), which consisted of 0.8% (w/v) agar and 1.5% (w/v) Suc, adjusted to pH 5.7. Seeds were then vernalized for 2 d at 4°C in the absence of light followed by placement in growth cabinets (Sanyo Scientific, Itasca, IL) set to a 12-h-light/12-h-dark cycle under 40-W fluorescent lights. The growth cabinet temperature was maintained at 22°C ± 1°C with a relative humidity of 50% to 60%. Two days after vernalization, seedlings were transferred to specialized plastic petri dishes (100 × 15 mm) that contained a center partition (I plates, Fisher Scientific, Pittsburgh); both sides contained one-half-strength Murashige and Skoog solid media with 1.5% (w/v) Suc, and the seedlings were placed on one side of the plate.
Bacterial Cultures
Seven strains of PGPR (Auburn University, AL) that lead to significant reduction in foliar diseases in Arabidopsis were tested for their capacity to elicit ISR. The strains were Pseudomonas fluorescens 89B61, Bacillus pumilus T4, Bacillus pasteurii C-9, Bacillus subtilis GB03, Bacillus amyloliquefaciens IN937a, Serratia marcescens 90-166, Enterobacter cloacae JM22, and B. pumilus SE34. Escherichia coli DH5α (QIAGEN Inc., Valencia, CA), which does not trigger ISR in Arabidopsis, was used as a control. Other bacteria used included B. subtilis 168 (2,3-butanediol producing; Bacillus Genetic Stock Center, Ohio State University) and B. subtilis strains BSIP1171 (2,3-butanediol overproducing), BSIP1173 (2,3-butanediol nonproducing), and BSIP1174 (2,3-butanediol nonproducing) provided by D. Jahn (Braunschweig University, Germany). The Arabidopsis pathogen Erwinia carotovora subsp. carotovora SCC1 was obtained from Dr. E. Tapio Palva (University of Helsinki). For experimental use, all bacteria were streaked onto TSA plates (Difco Laboratories, Detroit) and incubated at 28°C in the absence of light for 24 h. PGPR cells were harvested from TSA plates in SDW to yield 109 CFU mL-1 as determined by optical density and serial dilutions with plate counts. The concentrations of E. carotovora strain SCC1 were adjusted to 108 CFU mL-1 for inoculum preparations. For long-term storage, bacterial cultures were maintained at -80°C in TSA that contained 20% (v/v) glycerol. ISR was chemically initiated in plants by applying 20 μL of SA (20 nmol in water) to the crown of each seedling.
Plant VOC Exposure
One day before plant experiments, the bacterial strains were cultured on TSA plates as described above and scraped into SDW. I plates prepared with one-half-strength Murashige and Skoog solid media containing 1.5% (w/v) Suc, and 2-d-old emerging Arabidopsis seedlings (five–six seedlings/plate) were inoculated with 20 μL (109 CFU mL-1) of a given rhizobacterial strain or SDW applied drop wise to the non-plant side of the petri dish. For chemical treatments, the volatile bacterial extract, diluted 2,3-butanediol as a racemic mixture of RR and SS isomers (99+% purity; Aldrich, Milwaukee, WI), or solvent alone (CH2Cl2) was mixed with lanolin (Sigma, St. Louis) in a ratio of 0.08 g lanolin mL-1 test solution, and 20 μL of the resulting suspension was applied to a sterile paper disc (d = 1 cm; Whatman, Clifton, NJ) on the opposite side of the I plate from the plant seedlings. Plates were covered and sealed with parafilm to minimize air and VOC exchange and arranged in a randomized design within the growth cabinets. Treated I plates were incubated at 22°C with a 12-light/12-h-dark photoperiod.
Pathogen Inoculation and Leaf Soft Rot Determinations
Fourteen days after initial media inoculation, 5-μl suspensions of E. carotovora subsp. carotovora (108 CFU mL-1) were drop inoculated onto five leaves per Arabidopsis seedling. Leaves exhibiting soft rot symptoms were determined by visual inspections 24 h after inoculation. Numbers of symptomatic leaves per seedling were counted as a measure of disease severity. To measure population densities of E. carotovora subsp. carotovora at 24 h after inoculation, plant leaf tissue was weighed, macerated in SDW (1 mL), and plated on TSA plates containing 20 μg mL-1 kanamycin as a selection marker. The strain of E. carotovora used is kanamycin resistant, which reduces the risk of contamination by other bacterial species.
Volatile Collection and Analysis
Volatiles were collected from B. subtilis strains 168 (2,3-butanediol producing), BSIP1171 (2,3-butanediol overproducing), BSIP1173 (2,3-butanediol nonproducing), and BSIP1174, and strains GB03, IN937a, E. coli DH5α, and uninoculated media. The strains were grown on Murashige and Skoog medium containing 1.5% (w/v) agar, 1.5% (w/v) Suc, and 0.4% (w/v) TSA for 48 h at 28°C before collection of volatiles. Individual plates were placed on a sliding glass plate inside a closed Teflon-framed chamber (18 × 18 × 3 cm). Charcoal-purified air was humidified by bubbling through a super-saturated NaCl solution and passed over the bacterial culture at a rate of 1 L min-1. A sterile cotton plug was placed at the inlet of each chamber. Bacterial volatiles were collected by pulling 0.5 L min-1 by vacuum through Super-Q adsorbent traps (ARS, Gainesville, FL) located at the other end of the chamber. Collection chambers were placed under metal halide and sodium lamps for a 16-h-light/8-h-dark photoperiod with a total intensity of 700 μmol m-2 s-1 and kept at 28°C. Volatiles were collected at intervals of 24 h over a period of 6 d. Compounds were extracted from filters with 100 μL of dichloromethane. Nonyl acetate (400 ng) was added as an internal standard. Extracts used for biological testing were pooled without the addition of the internal standard.
Extracts were analyzed by capillary GC on a 30-m- × 0.25-mm-i.d. fused silica column with a 0.25-μm-thick bonded (5% [w/v] phenyl) methylpolysiloxane (J&W, New Castle, DE). Injections were made in the splitless mode for 30 s. The gas chromatograph was operated under the following conditions: injector, 200°C; detector, 210°C; and column oven, 28°C for 3 min, then programmed at a rate of 10°C min-1 to 180°C and finally ramped at a rate of 40°C min-1 to 220°C for 4 min. The velocity of the carrier gas linear flow was 50 cm s-1. Quantification was based on comparison of area under the GC-FID peak with the internal standard. For comparisons of the same compound under different treatments, response factors for individual compounds were assumed to be equal. Selected samples were also analyzed by GC-MS on a (ion trap) mass spectrometer (GCQ plus, Thermoquest, Austin, TX) interfaced to a gas chromatograph (Trace GC2000) and operated in the electron impact mode. Injections were made in the splitless mode for 30 s, and samples were analyzed on a 30-m- × 0.25-mm-i.d. DB5 column (J&W Scientific, Folsom, CA) under the same conditions previously described in GC/FID analysis. The transfer line and ion source temperature were adjusted to 220°C and 180°C, respectively. The components of the bacterial volatile emission were identified by comparison of GC retention times with those of authentic standards and by comparison of mass spectra with spectra of an Environmental Protection Agency/National Institutes of Health database.
The stereochemical configuration of GB03-synthesized 2,3-butanediol was determined by rt comparisons of authentic standards on an Alpha Dex 120 fused silica capillary chiral GC column with 30-m- × 0.25-mm-i.d. × 0.25-μm film thickness (Supelco, Bellefonte, PA). Injections were made in the splitless mode for 30 s. The gas chromatograph was operated under the following conditions: injector, 220°C; detector, 250°C; and column oven, 73°C for 24 min, then programmed at a rate of 10°C min-1 to 120°C and finally ramped at a rate of 60°C min-1 to 220°C. The velocity of the carrier gas linear flow was 20 cm s-1.
Arabidopsis GUS Fusion Assays
GUS activity was measured in seedlings 10 d after exposure to PGPR VOCs or water treatment by using a fluorometric assay. For this assay, approximately 20 mg of plant tissue from each treatment was macerated in an Eppendorf tube (Eppendorf Scientific, Westbury, NY) with 300 μL of GUS extraction buffer. The extracted samples were centrifuged twice at 8,000g for 10 min at 5°C, and 20 μL of the separated supernatant was incubated with 200 μL of 2 mm MUG at 37°C for 2 h; the reaction was stopped by adding 10 μL of 0.2 m sodium carbonate solution. GUS activity was measured with a TKO 100 fluorimeter (Hoefer Scientific Instruments, San Francisco) at an excitation wavelength of 365 nm and an emission wavelength of 455 nm. GUS activity is expressed in nanomoles of MUG per 10 ng-1 fresh weight.
Statistical Analysis
Analysis of variance for experimental data sets was performed using JMP software version 4.0 (SAS Institute Inc., Cary, NC). Significant treatment effects were determined by the magnitude of the F value (P = 0.05). When a significant F test was obtained for treatments, separation of means was accomplished by Fisher's protected lsd at P = 0.05. Bioassays were conducted three times with 12 replications per treatments and one seedling per replication; for VOC analyses, four replicate measurements of each bacterial culture were made.
Acknowledgments
We acknowledge Xinnian Dong, Joseph R. Ecker, John G. Turner, Daniel F. Klessig, Norbert Nass, Willem F. Broekaert, and the Ohio State University Stock Center (Columbus) for providing Arabidopsis seeds; David Jahn and the Bacillus Genetic Stock Center (Ohio State University) for kindly providing Bacillus spp.; and E. Tapio Palva for the E. carotovora subsp. carotovora SCC1. We also thank Nabil Saheb for assistance in VOC collections.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.026583.
This work was supported by the U.S. Department of Agriculture (grant no. 35320–9378), by the Herman Frasch Foundation for Chemical Research, and by the Robert A. Welch Foundation (grant no. D–478).
References
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) Ein2 a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148-2152 [DOI] [PubMed] [Google Scholar]
- Alström S (1991) Induction of disease resistance in common bean susceptible to halo blight bacterial pathogen after seed bacterization with rhizosphere pseudomonads. J Gen Appl Microbiol 37: 495-501 [Google Scholar]
- Arimura G, Ozawa R, Horiuchi JI, Nishioka T, Takabayashi J (2001) Plant-plant interactions mediated by volatiles emitted from plants infested by spider mites. Biochem System Ecol 29: 1049-1061 [Google Scholar]
- Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J (2000) Herbivory-induced volatiles elicit defense genes in lima bean leaves. Nature 406: 512-515 [DOI] [PubMed] [Google Scholar]
- Benhamou N, Kloepper JW, Tuzun S (1998) Induction of resistance against Fusarium wilt of tomato by combination of chitosan with an endophytic bacterial strain: ultrastructure and cytochemistry of the host response. Planta 204: 153-168 [Google Scholar]
- Bent AF, Innes RW, Ecker JR, Staskawicz BJ (1992) Disease development in ethylene-insensitive Arabidopsis thaliana infected with virulent and avirulent Pseudomonas and Xanthomonas pathogens. Mol Plant-Microbe Interact 5: 372-378 [DOI] [PubMed] [Google Scholar]
- Birkett MA, Campbell CAM, Chamberlain K, Guerrieri E, Hick AJ, Martin JL, Matthes M, Napier JA, Pettersson J, Pickett JA et al. (2000) New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc Natl Acad Sci USA 97: 9329-9334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, Dong X (1994) A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell 6: 1845-1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Bowling SA, Gordon SA, Dong X (1994) Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6: 1583-1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleyet-Marcel JC, Larcher M, Bertrand H, Rapior S, Pinochet X (2001) Plant growth enhancement by rhizobacteria. In J-F Morot-Gaudry, ed, Nitrogen Assimilation by Plants, Physiological, Biochemical and Molecular Aspects. Science Publishers, Inc., Enfeld, NH, pp 185-197
- Croft K, Juttner F, Slusarenko AJ (1993) Volatile Products of the lipoxygenase pathway evolved from Phaseolus vulgaris (L.) leaves inoculated with Pseudomonas syringae pv phaseolicola. Plant Physiol 101: 13-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney TP, Friedrich L, Ryals JA (1995) Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc Natl Acad Sci USA 92: 6602-6606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M, Achkor H, Titarenko E, Martinez MC (2003) The gene encoding glutathione-dependent formaldehyde dehydrogenase/GSNO reductase is responsive to wounding, jasmonic acid and salicylic acid. FEBS Lett 543: 136-139 [DOI] [PubMed] [Google Scholar]
- Durner J, Shah J, Klessig DF (1997) Salicylic acid and disease resistance in plants. Trends Plant Sci 2: 266-274 [Google Scholar]
- Farag MA, Paré PW (2002) C-6 Green leaf volatiles trigger local and systemic VOC emissions in tomato. Phytochemistry 61: 545-554 [DOI] [PubMed] [Google Scholar]
- Fidantsef AL, Stout MJ, Thaler JS, Duffey SS, Bostock RM (1999) Signal interactions in pathogen and insect attack: expression of lipoxygenase, proteinase inhibitor II, and pathogenesis-related protein P4 in the tomato Lycopersicon esculentum. Physiol Mol Plant Pathol 54: 97-114 [Google Scholar]
- Forlani G, Mantelli M, Nielsen E (1999) Biochemical evidence for multiple acetoin-forming enzymes in cultured plant cells. Phytochemistry 50: 255-262 [Google Scholar]
- Gatehouse JA (2002) Plant resistance towards insect herbivores: a dynamic interaction. New Phytol 156: 145-169 [DOI] [PubMed] [Google Scholar]
- Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41: 109-117 [Google Scholar]
- Hoffman T, Schmidt J, Zheng X, Bent A (1999) Isolation of ethylene insensitive soybean mutants that are altered in pathogen susceptibility and gene-for-gene resistance. Plant Physiol 119: 935-949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141-2144 [DOI] [PubMed] [Google Scholar]
- Kloepper JW (1992) Plant growth-promoting rhizobacteria as biological control agents. In FB Metting Jr, ed, Soil Microbial Ecology: Applications in Agricultural and Environmental Management. Marcel Dekker Inc., New York, pp 255-274
- Kloepper JW, Rodriguez-Kabana R, Zehnder GW, Murphy J, Sikora E, Fernandez C (1999) Plant root-bacterial interactions in biological control of soilborne diseases and potential extension to systemic and foliar diseases. Aust J Plant Pathol 28: 27-33 [Google Scholar]
- Leeman M, den Ouden FM, van Pelt JA, Dirkx FPM, Steijl H, Bakker PAHM, Schippers B (1996) Iron availability affects induction of systemic resistance to fusarium wilt of radish by Pseudomonas fluorescens. Phytopathology 86: 149-155 [Google Scholar]
- Liechti R, Farmer EE (2002) The jasmonate pathway. Science 296: 1649-1650 [DOI] [PubMed] [Google Scholar]
- Liu L, Kloepper JW, Tuzun S (1995) Induction of systemic resistance in cucumber against bacterial angular leaf spot by plant growth-promoting rhizobacteria. Phytopathology 85: 843-847 [Google Scholar]
- Maurhofer M, Hase C, Meuwly P, Metraux JP, Defago G (1994) Induction of systemic resistance of tobacco necrosis virus by the root-colonizing Pseudomonas fluorescens CHA0: influence of the gacA gene of pyoverdine production. Phytopathology 84: 139-146 [Google Scholar]
- Murphy JF, Zehnder GW, Schuster DJ, Sikora EJ, Polstan JE, Kloepper JW (2000) Plant growth-promoting rhizobacteria mediated protection in tomato against tomato mottle virus. Plant Dis 84: 779-784 [DOI] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121: 325-332 [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Van Wees SCM, Hoffland E, Van Pelt JA, Van Loon LC (1996) Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell 8: 1225-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Van Wees SCM, Ton J, Van Pelt JA, Van Loon LC (2002) Signaling in rhizobacteria-induced systemic resistance in Arabidopsis thaliana. Plant Biol 4: 535-544 [Google Scholar]
- Ramos HC, Hoffmann T, Marino M, Nedjari H, Presecan-Siedel E, Dreesen O, Glaser P, Jahn D (2000) Fermentative metabolism of Bacillus subtilis: physiology and regulation of gene expression. J Bacteriol 182: 3072-3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raupach GS, Kloepper JW (1998) Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology 88: 1158-1164 [DOI] [PubMed] [Google Scholar]
- Raupach GS, Kloepper JW (2000) Biocontrol of cucumber diseases in the field by plant growth-promoting rhizobacteria with and without methyl bromide fumigation. Plant Dis 84: 1073-1075 [DOI] [PubMed] [Google Scholar]
- Raupach GS, Liu L, Murphy JF, Tuzun S, Kloepper JW (1996) Induced systemic resistance in cucumber and tomato against cucumber mosaic virus using plant growth-promoting rhizobacteria. Plant Dis 80: 891-894 [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8: 1809-1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu C-M, Farag MA, Hu C-H, Reddy MS, Wei HX, Paré PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA 100: 4927-4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Alborn HT, Tumlinson JH (2001) The influence of intact plant and excised leaf bioassay designs on volicitin and jasmonic acid induced sesquiterpene volatile release in Zea mays. Planta 214: 171-179 [DOI] [PubMed] [Google Scholar]
- Shah J, Tsui F, Klessig DF (1997) Characterization of a salicylic acid-insensitive mutant (sai1) of Arabidopsis thaliana, identified in a selective screen utilizing the SA-inducible expression of the tms2 gene. Mol Plant-Microbe Interact 10: 69-78 [DOI] [PubMed] [Google Scholar]
- Shulaev V, Silverman P, Raskin I (1997) Airborne signaling by methyl salicylate in plant pathogen resistance. Nature 387: 718-721 [Google Scholar]
- Ton J, Davison S, Van Wees SCM, Van Loon LC, Pieterse CMJ (2001) The Arabidopsis ISR1 locus controlling rhizobacteria-mediated induced systemic resistance is involved in ethylene signaling. Plant Physiol 125: 652-661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loon LC (1997) Induced resistance in plants and the role of pathogenesis-related proteins. Eur J Plant Pathol 103: 753-765 [Google Scholar]
- van Loon LC, Bakker PAHM, Pierterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36: 453-483 [DOI] [PubMed] [Google Scholar]
- van Peer R, Niemann GJ, Schippers B (1991) Induced resistance and phytoalexin accumulation in biological control of fusarium wilt of carnation by Pseudomonas sp. strain WCS417r. Phytopathology 81: 728-734 [Google Scholar]
- Weber H (2002) Fatty acid-derived signals in plants. Trends Plant Sci 7: 217-224 [DOI] [PubMed] [Google Scholar]
- Wei G, Kloepper JW, Tuzun S (1991) Induction of systemic resistance of cucumber to Colletotrichum orbiculare by select strains of plant growth-promoting rhizobacteria. Phytopathology 81: 1508-1512 [Google Scholar]
- Weidhase RA, Kramell HM, Lehmann J, Liebisch HW, Lerbs W, Parthier B (1987) Methyl jasmonate-induced changes in the polypeptide pattern of senescing barley leaf segments. Plant Sci 51: 177-186 [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defense. Nature 414: 562-565 [DOI] [PubMed] [Google Scholar]
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091-1094 [DOI] [PubMed] [Google Scholar]
- Zehnder GW, Yao C, Murphy JF, Sikora EJ, Kloepper JW (2000) Induction of resistance in tomato against cucumber mosaic virus by plant growth-promoting rhizobacteria. Biol Control 45: 127-137 [Google Scholar]
- Zhou T, Pauliz TC (1994) Induced resistance in the biocontrol of Pythium aphanidermatum by Pseudomonas spp. on cucumber. J Phytopathol 142: 51-63 [Google Scholar]