Abstract
We have previously shown that cationic-β-CD:R-poly(vinyl alcohol)-poly(ethylene glycol) pendant polymer host:guest complexes are safe and efficient vehicles for nucleic acid delivery, where R = benzylidene-linked adamantyl or cholesteryl esters. Herein, we report the synthesis and biological performance of a family of PVA-PEG pendant polymers whose pendant groups have a wide range of different affinities for the β-CD cavity. Cytotoxicity studies revealed that all of the cationic-β-CD:pendant polymer host:guest complexes have 100 – 1000-fold lower toxicity than bPEI, with pDNA transfection efficiencies that are comparable to branched polyethylenimine (bPEI) and Lipofectamine 2000 (L2K). Complexes formed with pDNA at N/P ratios greater than 5 produced particles with diameters in the 100 – 170 nm range and ζ-potentials of 15 – 35 mV. Gel shift and heparin challenge experiments showed that the complexes are most stable at N/P ≥ 10, with adamantyl- and noradamantyl-modified complexes displaying the best resistance toward heparin-induced decomplexation. Disassembly rates of fluoresceinated-pDNA:CD+:R-PVA-PEG-rhodamine complexes within HeLa cells showed a modest dependence on host:guest binding constant, with adamantyl-, noradamantyl-, and dodecyl-based complexes showing the highest FRET efficiency 9 h after cellular exposure. These findings suggest that the host:guest binding constant has a significant impact on the colloidal stability in the presence of serum, cellular uptake efficiency, endosomal disassembly, and transfection performance of cationic-β-CD:R-poly(vinyl alcohol)-poly(ethylene glycol) pendant polymer complexes.
Keywords: Gene delivery, Cyclodextrins, Pendant Polymers, Transfection, pDNA
1. Introduction
The ability to specifically deliver nucleic acids to target cells has enormous therapeutic potential.1 Despite extensive research in the area of gene therapy and vector development, very little clinical success has been achieved to date.2 Slow progress in this field can be attributed, in part, to the absence of robust, effective, and scalable vehicles that can safely and efficiently deliver nucleic acids to the target cells. Non-viral vectors are attracting considerable attention due to their modest host immunogenicity and manufacturability.3 A variety of cationic polymers have been investigated as non-viral vectors such as polyethylenimines (PEI),4 poly-(L-lysine),5 PAMAM dendrimers,6,7 poly(lactic-co-glycolic acid) (PLGA),8,9 chitosan,10,11 cyclodextrin (CD) oligomers12–17 and CD polyrotaxanes.18–21 Of these materials, the CD-oligomers developed by Davis et al., have been the most successful with encouraging results in a clinical trial for melanoma therapy in humans.17 Recently, our group has reported a delivery vector based on the self-assembly of cationic β-CD (CD+) with pendant polymers (R-PVA-PEG).22,23 This family of materials was shown to have toxicity profiles 100 – 1000-fold lower than commercial transfection standards such as branched PEI and transfection efficiencies that are similar or superior to branched polyethyleimine bPEI and Lipofectamine2000 (L2k). The pendant polymer class of was shown to have efficacy for the delivery of both pDNA22 and siRNA23. It was observed that nucleic acid compaction could be achieved via complexation with the self-assembled β-CD+:R-PVA-PEG host:guest pendant polymer complexes via multivalent electrostatic interactions.22,23
This strategy enabled the compaction of nucleic acid cargo into stable nanometer-size particles that could then be internalized by target cells and routed to acidic endosomes. It was also observed that the PEG molecular weight and grafting density on the pendant polymer backbone was responsible for lowering the surface charge and improving the colloidal stability of the complexes.22,23 Our studies further revealed the importance of formulation method for improved nanoparticle stability. We found that higher PEG loadings and the use of PEG2000 instead of PEG750 produced complexes with lower surface charge, smaller nanoparticle diameters, improved serum stability, and greater in vitro efficacy. It was also observed that pre-complexation of pendant polymers and CD+, followed by complexation with pDNA, resulted in nanoparticles that had smaller sizes and superior efficacy than those that were formulated by pre-complexation of the CD+ and pDNA.22
pDNA release, arising from pDNA:CD+:pendant polymer decomplexation, could be due to a combination of factors, including degradation of the acetal linkages to the PVA main chain polymer, ester cleavage of the adamantyl ester linkages within the pendant group, and/or exchange of the CD+’s from the adamantyl pendant groups. To investigate the effects of pendant group binding affinity on the physical and biological properties of the complexes, a family of five R-PVA-PEG pendant polymers was synthesized. β-CD-PEI2.5k was used as the cationic β- CD+ species for all the experiments reported herein due to its superior performance, relatively low toxicity compared to high MW bPEI, and capacity to solubilize highly hydrophobic pendant groups. The pendant groups were chosen such that their putative binding affinities for the CD cavity varied by roughly two orders of magnitude.24
The pendant polymers were studied for their relative complexation ability, colloidal stability, cell viability, transfection efficiency and intracellular trafficking. Due to the difference in binding affinities of the various pendant groups towards β-CD, it was anticipated that all the polymers would have different intracellular disassembly rates. These were investigated using a flow cytometry-based FRET assay wherein, the polymer was labeled with a FRET acceptor (tetramethylrhodamine - Rh) and pDNA was labeled with a FRET donor (fluorescein - Fl) (Figure 1).
Figure 1.
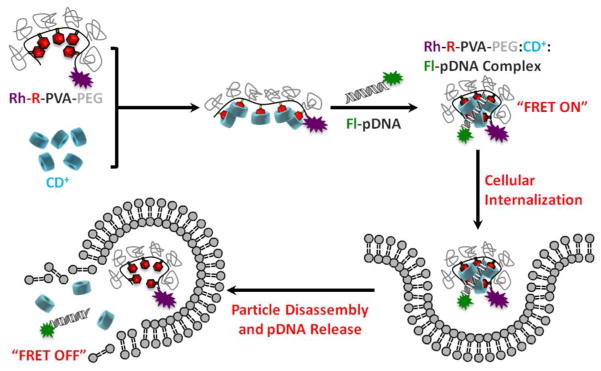
Conceptual diagram of assembly, cellular uptake and disassembly of fluorescein-pDNA (Fl-pDNA):CD+:rhodamine-R-PVA-PEG-rhodamine (Rh-R-PVA-PEG) complexes within the endosomes of target cells.
2. Experimental
Materials
All solvents were of reagent grade, purchased from commercial sources, and used without further purification, except DMF and toluene, which were dried with CaH2 under N2, filtered and distilled under reduced pressure. β-CD, NaOH, I2, Ph3P 1-adamantanecarbonyl chloride, 4-hydroxybenzaldehyde, Na2CO3, 1,1-carbonyldiimidazole (CDI), β-CD, and p-toluenesulfonyl acid monohydrate (TSA) and p-toluenesulfonyl chloride were obtained from Sigma-Aldrich, Inc. 1H NMR spectra were recorded on a 300 MHz Varian INOVA 300 NMR spectrometer at 30 °C. Chemical shifts were referenced to the residual protonated solvent peak. Tetramethylrhodamine-5-carbonyl azide, Alexa Fluor® 680 WGA and LysoTracker® Red DND-99 were purchased from Life Technologies. The fluorescein plasmid labeling kit was purchased from Mirus Biosciences. mhGFP plasmid vector was purchased from Promega. Plasmid DNA was amplified in E. coli and purified according to the supplier’s protocol (Qiagen, Hilden, Germany). The purity and concentration of the purified plasmid DNA was determined by absorption at 260 and 280 nm and by agarose gel electrophoresis. The purified plasmid DNA was resuspended in TE buffer (10 mM Tris-Cl, pH 7.5, 1 mM EDTA) and kept frozen in aliquots at a concentration of 0.5 mg/mL. The synthesis details of the polymers and cationic cyclodextrin can be found in the supporting information for this manuscript.
Particle Size and Zeta Potential Measurements
The diameters, size distributions and zeta potentials of the materials were evaluated by dynamic light scattering using a particle size analyzer (Zetasizer Nano S, Malvern Instruments Ltd.) at 20 °C with a scattering angle of 90°. All the samples were diluted up to 1mL in sterile filtered NanoPure water before analysis.
Atomic Force Microscopy Analysis
AFM imaging of the nanoparticles was conducted in tapping mode (MultiMode, Veeco, USA) using dry samples on mica. The AFM tips (PPP-NCH, Nanoscience Instruments, Inc., USA) had a typical radius of 7 nm or less and a force constant of 46 N/m. The images were recorded at a scan rate of 0.5 or 1 Hz. Samples were prepared by transferring 2 LL of sample solution on a mica surface followed by overnight drying at 20 °C.
Gel Retardation Assay
The pDNA complexation ability of CD+:R-PVA-PEG pendant polymers were determined by 1% agarose (low melting point) gel electrophoresis. The agarose gels were precast in TBE buffer with GelRed dye at 1:10000 dilution. Bulk mixed complexes containing 0.2 μg of pDNA in 20 LL water at different N/P ratios were loaded onto the gel. A 1:5 dilution of loading dye was added to each well and electrophoresis was carried out at a constant voltage of 55 V for 1 h in TBE buffer. The pDNA bands were then visualized under a UV transilluminator at a wavelength of 365 nm.
PicoGreen Competitive Binding Assay
The polyplex stability was studied by PicoGreen competition assay. When bound to dsDNA, fluorescence enhancement of PicoGreen is exceptionally high; little background occurs since the unbound dye has virtually no fluorescence. Increasing amounts of heparin were added to the different polyplexes to study their disassembly in presence of a negatively charged polymer. Upon heparin challenge, PicoGreen binds to dsDNA that has been released from the pDNA:CD+:R-PVA-PEG complex. Consequently, the increase of fluorescence serves an indication of the level of decomplexation between DNA and polymers. Increasing amounts of heparin were added to the complexes and allowed to incubate for 30 min, followed by addition of the Quant-iT PicoGreen reagent (Invitrogen, Carlsbad, CA) and further incubation for 15 min. The fluorescence was measured in a 96-well plate using a plate reader with an excitation maximum of 480 nm and an emission maximum at 520 nm. The fluorescence intensity was then corrected for background fluorescence. Results from three independent triplicate experiments were analyzed and reported along with the standard error of the measurements.
MTS Cell Viability Assay
The cytotoxicity of the CD+:R-PVA-PEG pendant polymer complexes was evaluated using the MTS assay in HeLa cells using bPEI (25 kDa) as a benchmark. The relative cell viabilities were measured as a function of amine densities of the CD+ and bPEI species. HeLa cells were cultured in complete DMEM medium supplemented with 10% FBS at 37 °C, 5% CO2, and 95% relative humidity. The cells were seeded in a 96-well microtiter plates (Nunc, Wiesbaden, Germany) at densities of 7,500 cells/well. After 24 h, the culture media was replaced with serum-free culture media containing increasing amine concentrations of bPEI complexes or CD+:R-PVA-PEG complexes and the cells were incubated for 24 h. After 24 h, 15LL of MTS reagent was added to each well and incubated for 2 h. Following the incubation period, the absorbance was measured using a microplate reader (Spectra Plus, TECAN) at a wavelength of 492 nm. The cell viability (%) relative to control cells cultured in media without polymers was calculated with [A]test/[A]control × 100%, where [A]test is the absorbance of the wells with polymers and [A]control is the absorbance of the control wells. All experiments were conducted for three samples and averaged. In this study, LD50 was the concentration of the carrier at which the relative cell viability decreased to 50%.
LDH Release Assay
LDH is a cytosolic enzyme that is secreted upon damage to cell membranes and thus serves as a cytotoxicity indicator. HeLa cells were seeded in 96-well microtiter plates (Nunc, Wiesbaden, Germany) at densities of 7,500 cells/well and cultured at 37 °C (5% CO2 and 95% relative humidity) in complete DMEM medium supplemented with 10% FBS. After 24 h, the culture media was replaced with serum-free culture media containing increasing amine densities of CD+:R-PVA-PEG complexes and the cells were incubated for 24 h. CytoTox 96® Non-Radioactive Cytotoxicity Assay from Promega was used for the analysis. After incubation, 50 μL of the solution was transferred to a new 96-well plate and allowed to equilibrate to 20 °C. To this, 50 μL of substrate mix was added and incubated for 30 min at 20 °C with protection from light. Stop mix (50 μL) was then added and the absorbance measured at 492 nm. The maximum LDH release was determined by addition of lysis buffer, provided by the manufacturer, to untreated cells. Cytotoxicity was determined as a percentage of the maximum LDH release caused by the lysis buffer.
Cellular Uptake Studies
HeLa cells were used to study the uptake of the complexes by plating 60,000 cells per well in 24-well plates and incubating for 24 h before the experiment. Complexes of fluorescein-labeled pDNA (1 μg per well) with the CD+:R-PVA-PEG complexes at different N/P ratios were incubated with cells for 4 h at 37 °C in 10% serum-supplemented media. After 4 h, the spent media was removed and the cells were washed with PBS and trypsinized. These cells were then collected and analyzed by flow cytometry using the FL1 channel.
In Vitro Transfection/Cell Viability Experiment
HeLa cells were cultured in complete DMEM medium supplemented with 10% FBS at 37 °C, 5% CO2, and 95% relative humidity respectively at a cell density of 60,000 cells/well in 24-well plates. After 24 h, the culture media was replaced with serum-supplemented media containing the CD+:R-PVA-PEG complexes containing 1μg AcGFP1 pDNA at N/P ration of 10, 20 and 30. The cells were incubated with the complexes for 4 h, after which the spent media was aspirated and fresh serum-supplemented media was added. After a further 36 h incubation, the media was aspirated and the cells were washed with PBS, trypsinized and added to FACS analysis tubes. SYTOX 7AAD dead cell stain (1 μL) was added to each sample and incubated on ice for 15 min before analysis by FACS. FL1 channel was used for the GFP fluorescence and FL4 for the 7AAD fluorescence. %GFP mean fluorescence intensity was calculated relative to L2k, which was considered as 100%. The experiment was performed in conditions that were optimized for L2k use.
Intracellular Trafficking Studies by Confocal Microscopy
Confocal microscopy was used to study the intracellular trafficking of the pDNA:CD+:R-PVA-PEG complexes. Alexa Fluor® 680 WGA was used for staining the plasma membrane, and LysoTracker® Red DND-99 was used for staining the lysosomes. HeLa cells were cultured in complete DMEM medium supplemented with 10% FBS at 37 °C, 5% CO2, and 95% relative humidity at a cell density of 30,000 cells/well in chambered coverglass slides. After 24 h, the culture media was replaced with serum-supplemented media containing the pDNA:CD+:R-PVA-PEG complexes labeled with 0.5 μg Fl-pDNA at an N/P ratio of 20 The cells were incubated with the complexes for 2, 4, and 9 h, after which they were stained with the dyes and live imaging was performed using a Nikon A1R MP Multiphoton microscope. Images were recorded at 60x magnification.
FRET-Based Disassembly Studies by Flow Cytometry
HeLa cells were cultured in complete DMEM medium supplemented with 10% FBS at 37 °C, 5% CO2, and 95% relative humidity at a cell density of 60,000 cells/well in 24-well plates. After 24 h, the culture media was replaced with serum-supplemented media containing the pDNA:CD+:R-PVA-PEG-Rh complexes labeled with 1μg Fl-pDNA at an N/P ratio of 20. The complexes were washed free from the cells with buffer at time points of 2, 4, 9, 18, and 24 h. The samples were trypsinized, washed with buffer and fixed with 0.5% paraformaldehyde. The cells were then analyzed by a Beckmann-Coulter FC500 flow cytometer. For FRET analysis, the samples were excited with FL1 and their emission was observed by FL1 and FL2. Each channel was compensated using single fluorophore controls.
3. Results and Discussion
3.1. Synthesis of R-PVA-PEG and CD+ Host:Guest Polymer Components
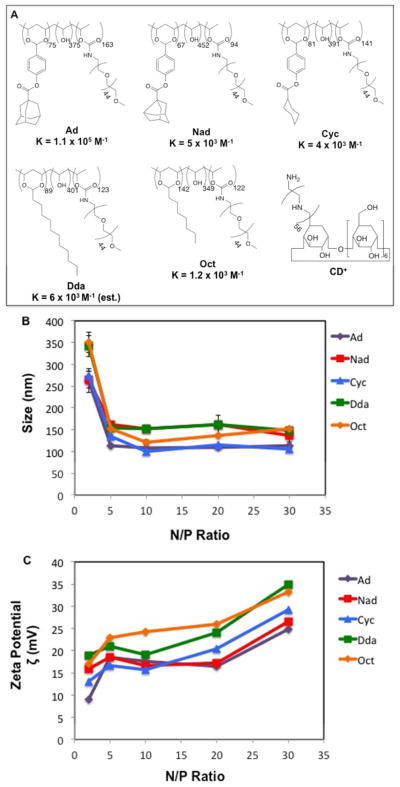
Based on the design principle shown in Figure 1, we synthesized a family of five statistical mPEG-grafted copolymers with pendant groups of adamantyl (R = Ad), noradamantyl (R = Nad), cyclohexyl (R = Cyc), dodecyl (R = Dda), and octyl (R = Oct) (Figure 2A). The Ad, Nad, and Cyc derivatives were synthesized using a method described previously22,23 by first converting the commercially available acid chlorides into the corresponding 4-benzaldehyde alkyl esters. These intermediates were then condensed with PVA (27k) in presence of acid catalyst to give the acetal-linked R-PVA polymer. These precursors were then PEGylated with mPEG2000-NH2 by CDI-activated coupling to give the final R-PVA-PEG pendant polymers shown in Figure 2A.
Figure 2.
(A) Structures of R-PVA-PEG and CD+ with their reported monomeric host:guest binding affinities for the methyl ester form of the guest ligand [24]. The value for Dda is estimated based on the reported binding constant between β-CD and decanoic acid. (B) DLS measurements; and (C) ζ potentials of pDNA:CD+:R-PVA-PEG complexes formulated at various N/P ratios.
We sought to prepare a family of R-PVA-PEG pendant polymers with pendant group modifications of ~12 mol % and mPEG2000 modifications of ~20 mol %. The Ad-PVA-PEG polymer had 12 mol % Ad and 26 mol % PEG modifications (MW ~ 373 kDa), while the Nad-PVA-PEG derivative had 11 mol % Nad and 15 mol % PEG modifications (MW ~ 232 kDa), and the Cyc-PVA-PEG derivative had 13 mol % Cyc and 23 mol % PEG modifications (MW ~ 326 kDa).
In the case of the Dda- and Oct-modified PVA-PEG pendant polymers, dodecanal and octanal were directly attached to the 27 kDa PVA main chain polymer by acetal formation in the presence of acid before subsequent PEGylation in a manner similar to that described above. The Dda-PVA-PEG material had 14 mol % Dda and 20 mol % PEG modifications (MW ~ 288 kDa), while the Oct-PVA-PEG derivative had 27 mol % Oct and 20 mol % PEG modifications (MW ~ 288 kDa). Our results show that the hydrophobic pendant group loadings were more easily targeted than the mPEG modifications (Figure 2A), possibly due to different degrees of water association with the isolated R-PVA intermediate that could interfere with the CDI/mPEG2000- NH2 coupling and/or self-association of the R-PVA intermediate during the PEGylation reaction.
β-CD was modified with linear PEI 2.5k to give the CD+ species shown in Figure 2A. This was done by first converting the β-CD to its monotosylated form as previously reported.25 Briefly, β-CD was treated with toluenesulfonyl imidazole to yield a mixture of unreacted, mono-, and di-6-tosyl-β-CD derivatives. This mixture was then subjected to HP20 C18 column chromatography to isolate the pure 6-monotosylated β-CD. This precursor was then treated with an excess of linear PEI2.5k to give the CD+ after purification by dialysis. This resulted in the cationic cyclodextrin CD+, which was modified at one hydroxyl group of one of the pyranose rings with the PEI 2.5k.
3.2. Physical Characterization of pDNA:CD+:R-PVA-PEG Complexes
The relative abilities of the family of pendant polymers to form host:guest complexes with CD+ and condense pDNA was evaluated by analyzing their surface charge and particle diameter. Pre-assembly of the CD+:R-PVA-PEG host:guest pendant polymer complexes before pDNA condensation was used for the preparation of all transfection complexes described below, since previous work showed that this was superior to the approach where the plasmid was first condensed with CD+ before adding the R-PVA-PEG component.22 The complexes were formulated at a ratio of one equivalent of CD+ for every pendant group on the polymer.
Dynamic light scattering (DLS) was used to determine the number-averaged transfection complex diameters produced using the different pendant polymers. Our data shows that all of the polymers could form complexes between 100 – 170 nm. At low N/P ratios (e.g., 2) (N/P ratio is the ratio of positive charges on the polymer to negative charges on the nucleic acid in the complex), complexes in the 250 – 300 nm size regime were produced; however, the observed diameters dropped below 170 nm when N/P ≥ 5, showing no appreciable change in diameter as the N/P ratio increased (Figure 2B).
Interestingly, while the diameters of the transfection complexes formed by the different pendant polymers showed little variation, the observed PDIs (Polydispersity Index) were quite different, with Ad-, Nad-, and Cyc-based complexes all displaying PDIs less than 0.2, whereas those formed by Dda- and Oct-PVA-PEG had PDIs as high as 0.35 in some cases. This can be attributed to the inefficient binding of the weaker inclusion ligand: β-CD interactions, leading to a more heterogeneous population of transfection complexes than the Ad-, Nad-, and Cyc-PVA-PEG derivatives.
Zeta potentials (ζ) were also measured to determine the extent of charge development on the pDNA:CD+:R-PVA-PEG transfection complexes (Figure 2C). We observed that all the complexes followed a trend, wherein increasing N/P ratios produced an increase in the observed ζ-potential. It is worth noting that the transfection complexes displayed ζ-potentials that were within 10 mV of each other, with Ad-based particles among the lowest and Oct-based particles among the highest ζ-potential complexes. The sharp increase in ζ-potential observed when increasing the N/P from 20 to 30 for all the polymers may suggest the onset of uncomplexed CD+:R-PVA-PEG as is known to occur for bPEI at high charge ratios.26
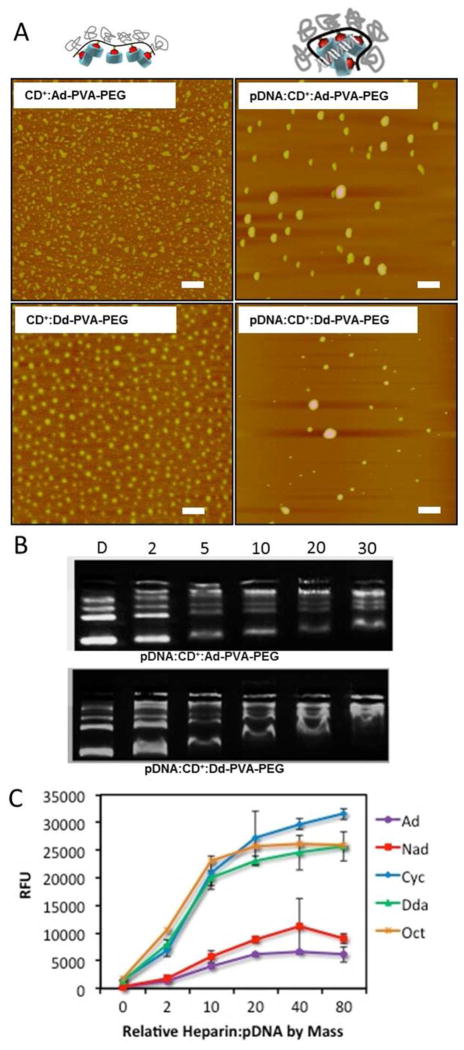
AFM images of the complexes formed by all the CD+:R-PVA-PEG and pDNA:CD+:R-PVA-PEG pendant polymers revealed similar trends as those observed by DLS, indicating the presence of particles in the 50 – 200 nm size regime (Figure 3A and SI Figure S3 – S5). A narrower size distribution was observed for CD+:R-PVA-PEG complexes, with diameters ranging between 50 – 100 nm. We also found that the pDNA:CD+:R-PVA-PEG complexes were approximately spherical, with sizes that decreased when the N/P ratio was increased from 5 to 20. As observed from the DLS studies, Ad-, Nad-, and Cyc-modified polymers formed complexes that were smaller in size and more homogenous in nature than those formed by Dda and Oct polymers. We infer from these results that pendant groups with higher binding affinities such as Ad and Nad contribute to the formation of smaller, more homogeneous particles with lower surface charges. Presumably, the higher binding affinity of the host:guest CD:pendant polymer complex is able to better shield the positive charges emanating from the CD+ due to tighter binding of the PEGylated polymer, leading to lower apparent surface charges.
Figure 3.
(A) AFM images of CD+:R-PVA-PEG and pDNA:CD+:R-PVA-PEG complexes at N/P = 20, scale bar = 500 nm; (B) Gel Shift Assay showing complexation ability of Ad and Dda; and (C) PicoGreen competitive binding assay showing colloidal stability of Ad, Nad, Cyc, Dda, and Oct complexes at N/P = 10.
3.3. Complexation Properties and Colloidal Stability of pDNA:CD+:R-PVA-PEG Complexes
The complexation properties and colloidal stabilities of the pendant polymer complexes were determined by gel retardation assays and PicoGreen competitive binding assays, respectively.
Gel retardation assay results showed that the pDNA complexation ability improved with increasing N/P ratio for all the pendant polymers (Figure 3B, SI Figure S2). For N/P = 2, there is very little condensation observed; it increases only marginally at N/P = 5. N/P ratios of 10, 20, and 30 display much better condensation ability for all the pendant polymer assemblies. Interestingly, no significant differences were observed in the complexation ability of the different polymers, with all essentially being very similar with regard to their gel shift behavior (SI, Figure S2).
The PicoGreen competitive binding assay was performed to study the colloidal stability of complexes in presence of a negatively-charged polymer that can promote decomplexation (Figure 3C and SI Figure S6). PicoGreen selectively binds to free dsDNA and displays exceptionally high fluorescence enhancement. In this experiment, increasing amounts of heparin were added at weight ratios of 0, 2, 10, 20, 40, and 80 relative to the pDNA content in the complexes.
It was anticipated that the fluorescence would increase with increasing heparin concentration due to enhanced decomplexation and release of free dsDNA from the pDNA:CD+:R-PVA-PEG complexes. It was observed that Ad- and Nad-modified R-PVA-PEG complexes had the highest colloidal stability across N/P ratios of 10, 20 and 30, with negligible disassembly observed even at very high heparin:pDNA ratios of 80:1 (Figure 3C). We attribute this observation to the much stronger binding of the Ad- and Nad- pendant groups of the R-PVA-PEG polymer to the β-CD cavity than the Cyc-, Dda-, and Oct-PVA-PEG derivatives.
It was also observed that higher N/P ratios for all the pDNA:CD+:R-PVA-PEG complexes disassembled more easily than the same host:guest pendant polymer complexes at lower N/P ratios. We infer from these findings that the higher surface charge of the N/P = 30 complexes bind heparin more avidly than the corresponding N/P = 10 and 20 complexes that display lower surface charges, thus accelerating the disassembly of the more highly charged N/P = 30 complexes.
These results indicate that the condensation efficiency of all the complexes is similar; however, their colloidal stabilities in the presence of negatively charged polymers differ substantially. Based on our findings, we propose that electrostatic interactions with the CD+ units drives pDNA condensation, whereas host:guest complexation promotes the formation of discrete particles with the colloidal stability to resist complex disassembly as a function of the magnitude of the host:guest binding constant and the density of the PEG corona, respectively, of the R-PVA-PEG pendant polymer. These results also indicate that CD+:Ad-PVA-PEG complexes at N/P ≤ 20 are the most stable in the presence of heparin, suggesting that they should have the highest serum stability compared to the other members of the pendant polymer library.
3.4. Biological Performance of CD+:R-PVA-PEG and pDNA:CD+:R-PVA-PEG Complexes
The in vitro cytotoxicities of CD+:R-PVA-PEG complexes were assessed as a function of the amine density within the complexes using bPEI as a benchmark. This enabled the direct comparison of the amine content in the materials so that compounds with different molecular weights could be readily compared.
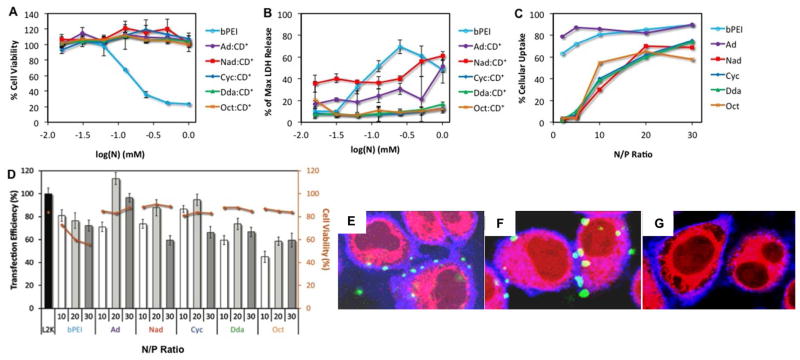
The relative cell viability profiles of the CD+:R-PVA-PEG complexes and bPEI were determined by MTS assay in HeLa cells (Figure 4A). Figure 4A shows that all pendant polymer complexes have a negligible effect on cell viability, even after incubation with cells for 24 h. Given that bPEI has an LD50 of 0.15mM under similar conditions, these data indicate that each of the pendant polymer complexes is nearly 100 – 1000-fold less toxic than bPEI.
Figure 4.
Biological performance of pDNA:CD+:R-PVA-PEG complexes. (A) MTS viability assay as a function of CD+:R-PVA-PEG and bPEI concentration after 24 h exposure to cationic polymer; (B) LDH cytotoxicity analysis of CD+:R-PVA-PEG and bPEI as a function of CD+:R-PVA-PEG and bPEI concentration after 24 h exposure to cationic polymer; (C) cellular uptake levels of CD+:R-PVA-PEG and bPEI after incubation for 4 h as a percentage of all cells exposed to transfection complexes; at N/P ratio of 10, 20 and 30 (D) two-color flow cytometry in vitro transfection efficiency and 7AAD cytotoxicity analysis of R-PVA-PEG:CD+:pDNA complexes at N/P ratio of 10, 20 and 30 with bPEI and L2k as controls (4 h incubation followed by readout after 24 h with 1 μg AcGFP1 pDNA); confocal microscopy z-stack images of Fl-pDNA:CD+:Ad-PVA-PEG complexes at N/P = 20 incubated at (E) 2 h, (F) 4 h and (G) 9 h. Blue – Alexa 689 WGA-labeled plasma membrane; Red – Lysotracker DND-99-labeled lysosomes, Green – transfection complexes. All experiments were performed using HeLa cells in 10% serum-supplemented media.
The in vitro cytotoxicities of the pendant polymer complexes were also assessed by lactate dehydrogenase (LDH) assay (Figure 4B). LDH, a cytosolic enzyme that is secreted when the cell membrane is damaged, is commonly used as an indicator of cytotoxicity. We used the LDH assay to determine the relative cytotoxicities of the pendant polymer complexes and bPEI. LDH assay results confirmed the MTS assay findings, indicating that the pendant polymer complexes were less toxic than bPEI. Treatment with bPEI resulted in 50% LDH release at ~ 0.1mM, in close agreement with the LD50 obtained from the MTS assay. CD+:Ad-PVA-PEG and CD+:Nad-PVA-PEG complexes resulted in 50% LDH release at a concentration of ~1 mM, while CD+:Cyc-PVA-PEG, CD+:Dda-PVA-PEG, and CD+:Oct-PVA-PEG did not induce more than 20% LDH release, even at concentrations as high as 1mM. Since the MTS and LDH assays measure cell viability using very different mechanisms, it was expected that the LD50 values obtained from both experiments would be slightly different, nonetheless, both assays confirm that the CD+:R-PVA-PEG complexes are substantially less toxic than bPEI.
The in vitro cellular uptake of transfection complexes generated by complexation of fluoresceinated-pDNA (Fl-pDNA) with CD+:R-PVA-PEG were assessed in HeLa cells at different N/P ratios in 10% serum-supplemented media (Figure 4C). The complexes were incubated for 4h and then analyzed by flow cytometry. L2k complexes demonstrated an uptake efficiency of almost 90%, which is in agreement with literature reports.27 We found that Ad-modified complexes had the highest cellular uptake amongst all pendant polymers and was very similar to bPEI at higher N/P ratios. Nad-, Cyc-, Dda-, and Oct-modified complexes all had cellular uptakes that were very similar, but almost 20 – 30% lower than bPEI and Ad. Interestingly, while bPEI and Ad-PVA-PEG showed greater than 50% uptake even at N/P ratios as low as 2, all the other polymers had negligible uptake (< 10%) at N/P < 10. Additionally, for Nad-, Cyc-, Dda-, and Oct-modified complexes, a sharp increase in uptake was observed at N/P > 10. The cellular uptake for all the complexes, including bPEI, seemed to saturate at N/P > 20.
The significant difference between the cellular uptake efficiencies of Ad-PVA-PEG and all the other pendant polymers can be attributed to the colloidal stability of the complexes in the presence of serum. As observed in the PicoGreen assay, CD+:Ad-PVA-PEG materials form much more stable complexes than Nad-, Cyc-, Dda-, and Oct-modified complexes, primarily due to the higher binding affinity of the β-CD:Ad host:guest interaction. Thus, the other pendant polymer complexes may be disrupted in the presence of serum at lower N/P ratios, resulting in the low cellular uptake. At higher N/P ratios, Nad-, Cyc-, Dda-, and Oct-modified complexes are able to form more stable complexes due to greater overall pendant group occupancy, thereby resulting in lower disassembly in presence of serum, which in turn leads to improved cellular uptake.
The transfection efficiency of the complexes also was assessed in HeLa cells in presence of 10% serum-supplemented medium (Figure 4D). The transfection experiment was run in tandem with a cell viability stain to enable the measurement of both the transfection efficiency and the cell viability of the complexes in the same experiment (Fig. S7 – S12). The experiment was performed with L2k and bPEI as positive controls and L2k transfection efficiency was considered as 100% at conditions that were optimized for L2k based transfection of pDNA.
The experiments reveal that all the pDNA:CD+:R-PVA-PEG complexes had transfection efficiencies in the range of 50 – 120% of L2k, regardless of the N/P ratio of the formulation. We observed that CD+:Ad-PVA-PEG complexes had the best performance of all compositions tested – superior to both L2k and bPEI by almost 15% and 35%, respectively. CD+:Nad-PVA-PEG and CD+:Cyc-PVA-PEG complexes had performance profiles inferior to CD+:Ad-PVA-PEG, but comparable to L2k and superior to bPEI. CD+:Dda-PVA-PEG and CD+:Oct-PVA-PEG complexes had performances that were far lower than L2k and CD+:Ad-PVA-PEG, and marginally lower than bPEI. These results are in agreement with the results obtained from the PicoGreen, DLS, and cellular uptake studies. The CD+:Ad-PVA-PEG complexes have the best colloidal and serum stability, leading to higher cellular uptake. Additionally, the small sizes and the slight positive charges of these complexes also result in more efficient endocytosis. The combination of all these attributes makes the CD+:Ad-PVA-PEG complexes the best performing material in this compound library. The CD+:Nad-PVA-PEG and CD+:Cyc-PVA-PEG complexes had similar attributes as the Ad materials, but slightly lower serum stability, leading to lower transfection efficiency. Furthermore, the CD+:Dda-PVA-PEG and CD+:Oct-PVA-PEG materials were almost 30 – 50 nm larger in size than the other complexes and had the poorest colloidal stability. We attribute the poor performance of these materials to their poor cellular uptake because of their comparatively large size and colloidal instability.
Interestingly, it was observed that the transfection efficiencies of all the complexes improved when the N/P ratio increased from 10 to 20, but then dropped upon further increase to 30. This could be attributed to an increase in cytotoxicity at higher N/P. In addition, the higher charge may cause the complexes to disassemble more slowly within the cell, leading to lower transgene expression at the 36 h readout time.
Additional studies were performed using a 2-color flow cytometry experiment wherein the cells were labeled with the SYTOX 7AAD viability stain to evaluate both the viability of transfected cells and the transfection performance of the complexes by GFP expression level analysis. Cells treated with each of the R-PVA-PEG complexes displayed viability in the 80 – 90% range, which was comparable to the viability of cells treated with L2k complexes (~85%). bPEI treated cells showed significantly higher toxicity, with the cell viabilities ranging between 50 – 70%. These results are in agreement with the results obtained from the MTS and LDH cytotoxicity assays, which indicated that the CD+:R-PVA-PEG complexes have significantly lower cytotoxicity than bPEI.
3.5. Intracellular Trafficking of pDNA:CD+:R-PVA-PEG Complexes
Binding and intracellular trafficking of the complexes was studied as a function of time by confocal microscopy (Figure 4E-F and SI Figure S21 - 24). For all the complexes, it was observed that a very small number of complexes co-localized with the plasma membrane at 2 h. At the 4 h time-point, it was observed that a much larger population of complexes localized with the plasma membrane, with a small number of complexes that were internalized and co-localized with the lysosomes. As noted in the cellular uptake studies (Figure 4C), CD+:Ad-PVA-PEG complexes produced the largest co-localization with the plasma membrane at the 4 h time-point, followed by CD+:Nad-PVA-PEG and CD+:Cyc-PVA-PEG complexes; the lowest co-localization was observed with CD+:Dda-PVA-PEG and CD+:Oct-PVA-PEG. We observed that most of the complexes were internalized at the 9 h time-point, with only a very small amount of co-localization observed with the plasma membrane at that time. Interestingly, the confocal images suggested that disassembly of the Ad-modified complexes was the slowest, followed by Nad- and Cyc-modified complexes. The Dda- and Oct-modified complexes had disassembled almost completely by the 9 h time-point. The images showed a much larger number of internalized complexes that were intact in the case of Ad-modified complexes than for the other pendant group complexes. In the case of Dda- and Oct- modified complexes, almost no intact assemblies were observed at the 9 h time-point, indicating that most of them had disassembled.
3.6. FRET-Based Complex Disassembly Studies by Flow Cytometry
To more closely monitor the rate of intracellular disassembly of the pDNA:CD+:R-PVA-PEG complexes, a time-dependent FRET experiment was performed (Figure 5, S17 – S25). The FRET pair of fluorescein (Fl) and tetramethylrhodamine (Rh) was used to study the disassembly of the complexes. Commercially available tetramethylrhodamine carbonyl azide was used for labeling the pendant polymers and fluorescein was used to label the plasmid. It was anticipated that when Fl-pDNA was condensed with a CD+:R-PVA-PEG-Rh complex, a FRET signal would be observed and the signal would decay over time as a function of the disassembly rate of the complexes within the cells.
Figure 5.
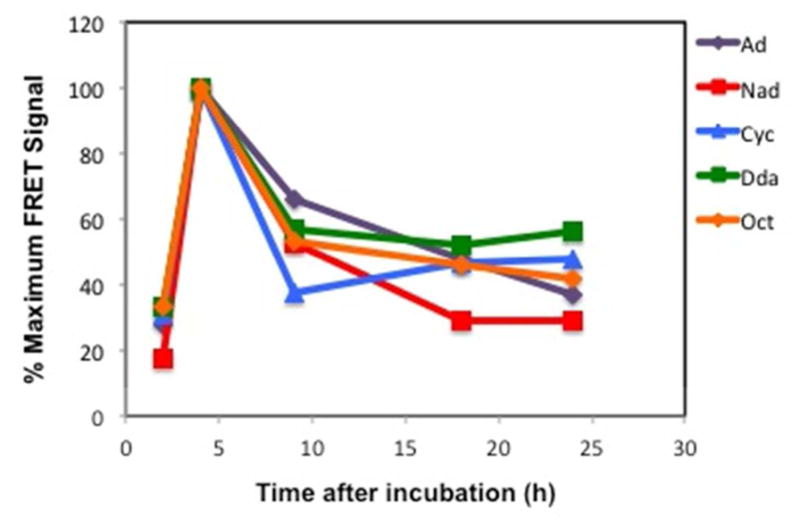
FRET disassembly studies of Fl-pDNA:CD+:R-PVA-PEG-Rh complexes at N/P = 20, where R = Ad, Nad, Cyc, Dda, and Oct. The experiments were performed in HeLa cells in 10% serum-supplemented media and analyzed by flow cytometry.
We observed that the cell-associated FRET signal increased until the 4 h time-point, when cellular uptake of the complexes is maximized, before undergoing a steady decay in the FRET signal within 24 h. While the FRET signal gradually decayed for all the polymers between 4 h and 24 h, it was observed that the decay slope was the sharpest at 4 – 9 h, indicating that majority of the complexes disassembled within this timeframe.
As expected, a trend was observed in the decay of the FRET signal as a function of the pendant group. CD+:Ad-PVA-PEG complexes had a much more gradual decay in the FRET signal than the other polymers, resulting in a lower initial slope for FRET decay. This could be attributed to the high Ad ↔β -CD binding affinity, thus resulting in a slower exchange of the β-CD+ units and slower complex disassembly. Surprisingly, while all the other pendant polymers had expectedly faster disassembly rates than the Ad derivative, the difference in their disassembly rates was less marked, except for the fastest decaying species, CD+:Cyc-PVA-PEG. These observations are in general agreement with the PicoGreen assay findings.
Additionally, it was also observed that the maximum FRET signal of the Fl-pDNA:CD+:Ad-PVA-PEG-Rh complexes, observed at the 4h timepoint for all complexes, was much higher than that of the other polymers (Figure S25). This could be attributed to a combination of factors. The higher FRET signal could be an indication of higher cellular uptake of the Ad-based due to greater colloidal stability of these complexes in serum than the complexes formed from the Nad-, Cyc-, Dda-, and Oct-modified pendant polymers. Another possible reason for the higher FRET signal could be an improved FRET efficiency in the Fl-pDNA:CD+:Ad-PVA-PEG-Rh complexes due to tighter binding (i.e., improved condensation, leading to closer approach of the FRET probes). Since the Nad-, Cyc-, Dda-, and Oct-modified pendant polymers have a lower binding affinity for the CD cavity, the FRET efficiency arising from these complexes may be lower due to the formation more weakly bound complexes that engage in greater dynamic exchange of host:guest partners, thereby leading to an effective increase in the distances between the Fl and Rh probes in these weaker binding complexes (i.e., poor condensation leads to greater probe separation, that is accompanied by lower FRET efficiency).
4. Conclusions
In summary, we observe that the R-PVA-PEG pendant group has significant effects on the physical and biological properties of the pDNA:CD+:R-PVA-PEG transfection complexes. We found that a higher binding affinity of the pendant group to the β-CD+ cavity produced complexes with lower polydispersities, smaller diameters, higher colloidal stabilities, higher cellular uptake, and higher transfection efficiencies. Additionally, the intracellular trafficking experiments and FRET studies indicated that higher affinity of the pendant group resulted in a slower rate of disassembly, such that Ad-modified complexes displayed the slowest rate of disassembly, followed by Nad-, Cyc-, Dda- and Oct-modified complexes. Taken together, our results suggest that pDNA:CD+:R-PVA-PEG complex disassembly rates show a modest dependence on the magnitude of the host:guest binding constant; however, this parameter has a significant impact on the stability of the transfection complexes in serum, leading to greater pDNA uptake and transgene expression. In addition, these CD+:R-PVA-PEG complexes are almost 100 – 1000-fold less toxic than the commercial standard bPEI, with a transfection performance that is superior to bPEI and L2k. Since the pDNA:CD+:Ad-PVA-PEG complexes are stable toward heparin exposure, they may be more stable toward disassembly in the glomerular basement membrane of the kidney where there is a high concentration of heparin sulfate.28 This physical attribute is very attractive since it could potentially translate into a longer circulation time in vivo.
Supplementary Material
Acknowledgments
Funding Sources
Any funds used to support the research of the manuscript should be placed here (per journal style).
The authors would like to acknowledge the support of this work by NIH GM087016, with summer salary support of KD by the Indiana Elks Foundation. NMR and MS data were acquired in the Purdue University Center for Cancer Research Interdepartmental NMR Facility and Campus-Wide Mass Spectrometry Center (supported by NCI CCSG CA23168 to the Purdue Center for Cancer Research). The assistance of Aaron Taylor with the confocal microscopy experiments and of Justin L. Meyers with the FRET flow cytometry experiments is also gratefully acknowledged.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
ASSOCIATED CONTENT
Supporting Information. DLS size distributions, Gel shift assay, AFM images, AFM statistics, Picogreen data, FACS raw data, additional confocal microscopy images. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ryther RCC, Flynt AS, Phillips JA, III, Patton JG. Gene Ther. 2005;12:5. doi: 10.1038/sj.gt.3302356. [DOI] [PubMed] [Google Scholar]
- 2.Whitehead KA, Langer R, Anderson DG. Nat Rev Drug Discovery. 2009;8:129. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eltoukhy AA, Chen D, Alabi CA, Langer R, Anderson DG. Adv Mater (Weinheim, Ger) 2013;25:1487. doi: 10.1002/adma.201204346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boussif O, Lezoualch F, Zanta MA, Mergny DM, Scherman D, Demeneix B, Behr JP. Proc Natl Acad Sci U S A. 1995;92:7297. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner E, Ogris M, Zauner W. Adv Drug Delivery Rev. 1998;30:97. doi: 10.1016/s0169-409x(97)00110-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Wu J, Hafdi N, Behr JP, Erbacher P, Peng L. Chem Commun (Cambridge, U K) 2006;22:2362. doi: 10.1039/b601381c. [DOI] [PubMed] [Google Scholar]
- 7.Liu XX, Rocchi P, Qu FQ, Zheng SQ, Liang ZC, Gleave M, Iovanna J, Peng L. ChemMedChem. 2009;4:1302. doi: 10.1002/cmdc.200900076. [DOI] [PubMed] [Google Scholar]
- 8.Patil Y, Panyam Y. Int J Pharm (Amsterdam, Neth) 2009;367:195. doi: 10.1016/j.ijpharm.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Nat Mater. 2009;8:526. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katas H, Alpar HO. J Control Rel. 2006;115:216. doi: 10.1016/j.jconrel.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Romøren K, Pedersen S, Smistad G, Evensen Ø, Thu BJ. Int J Pharm (Amsterdam, Neth) 2003;261:115. doi: 10.1016/s0378-5173(03)00301-6. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez H, Hwang SJ, Davis ME. Bioconjugate Chem. 1999;10:1068. doi: 10.1021/bc990072j. [DOI] [PubMed] [Google Scholar]
- 13.Reineke TM, Davis ME. Bioconjugate Chem. 2003;14:247. doi: 10.1021/bc025592k. [DOI] [PubMed] [Google Scholar]
- 14.Reineke TM, Davis ME. Bioconjugate Chem. 2003;14:255. doi: 10.1021/bc025593c. [DOI] [PubMed] [Google Scholar]
- 15.Popielarski SR, Mishra S, Davis ME. Bioconjugate Chem. 2003;14:672. doi: 10.1021/bc034010b. [DOI] [PubMed] [Google Scholar]
- 16.Davis ME. Mol Pharmaceutics. 2009;6:659. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 17.Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Nature (London, U K) 2010;464:1067. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ooya T, Choi HS, Yamashita A, Yui N, Sugaya Y, Kano A, Maruyama A, Akita H, Ito R, Kogure K, Harashima H. J Am Chem Soc. 2006;128:3852. doi: 10.1021/ja055868+. [DOI] [PubMed] [Google Scholar]
- 19.Tamura A, Yui N. Biomaterials. 2013;34:2480. doi: 10.1016/j.biomaterials.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni A, DeFrees K, Schuldt RA, Vlahu A, VerHeul R, Hyun S-H, Deng W, Thompson DH. Integr Biol (Camb) 2013;5:115. doi: 10.1039/c2ib20107k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulkarni A, DeFrees K, Schuldt RA, Hyun SH, Wright KJ, Yerneni CK, VerHeul R, Thompson DH. Mol Pharmaceutics. 2013;10:1299. doi: 10.1021/mp300449t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni A, Deng W, Hyun S-H, Thompson DH. Bioconjugate Chem. 2012;23:933. doi: 10.1021/bc2005158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulkarni A, DeFrees K, Hyun SH, Thompson DH. J Am Chem Soc. 2012;134:7596. doi: 10.1021/ja300690j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rekharsky MV, Inoue Y. Chem Rev (Washington, DC, U S) 1998;98:1875. doi: 10.1021/cr970015o. [DOI] [PubMed] [Google Scholar]
- 25.Tang W, Ng SC. Nat Protoc. 2008;3:691. doi: 10.1038/nprot.2008.37. [DOI] [PubMed] [Google Scholar]
- 26.Godbey WT, Wu KK, Hirasaki GJ, Mikos AG. Gene Ther. 1999;6:1380. doi: 10.1038/sj.gt.3300976. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed M, Deng Z, Liu S, Lafrenie R, Kumar A, Narain R. Bioconjugate Chem. 2009;20:2169. doi: 10.1021/bc900350c. [DOI] [PubMed] [Google Scholar]
- 28.Zuckerman JE, Choi CHJ, Davis ME. Proc Natl Acad Sci U S A. 2012;109:3137. doi: 10.1073/pnas.1200718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.