Abstract
Objective
Although depression is a risk and prognostic factor for cardiovascular disease (CVD), depression trials involving cardiac patients have not observed the anticipated cardiovascular benefits. To test our hypothesis that depression treatment delivered before clinical CVD onset reduces risk of CVD events, we conducted an 8-year follow-up study of the Indiana sites of the Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) randomized controlled trial.
Methods
Participants were 235 primary care patients aged ≥60 years with major depression or dysthymia who were randomized to a 12-month collaborative care program involving antidepressants and psychotherapy (85 without and 35 with baseline CVD) or usual care (83 without and 32 with baseline CVD). Hard CVD events (fatal/nonfatal) were identified using electronic medical record and Medicare/Medicaid data.
Results
119 patients (51%) had a hard CVD event. As hypothesized, the Treatment x Baseline CVD interaction was significant (p = .021). IMPACT patients without baseline CVD had a 48% lower risk of an event than Usual Care patients (28% vs. 47%, HR = 0.52, 95% CI: 0.31–0.86). The number needed to treat to prevent one event over five years was 6.1. The likelihood of an event did not differ between IMPACT and Usual Care patients with baseline CVD (86% vs. 81%, HR = 1.19, 95% CI: 0.70–2.03).
Conclusions
Collaborative depression care delivered before CVD onset halved the excess risk of hard CVD events among older, depressed patients. Our findings raise the possibility that the IMPACT intervention could be used as a CVD primary prevention strategy.
Trial Registration
clinicaltrials.gov Identifier: NCT01561105 (http://clinicaltrials.gov/ct2/show/NCT01561105)
Keywords: depression, coronary disease, cerebrovascular disorders, follow-up studies, prevention
Introduction
Thirty years of epidemiologic evidence indicates that depression is an independent risk and prognostic factor for cardiovascular disease (CVD), including coronary artery disease (CAD) and cerebrovascular disease (CBV) (1, 2). Despite this evidence, few clinical trials have evaluated whether pharmacological or psychological depression treatments reduce the likelihood of CVD events (3–7). In general, these trials have not observed the anticipated cardiovascular benefits. Although other potential reasons for these null results have been offered (8), a novel and unexplored explanation is that the late timing of depression treatment in the natural history of CVD may have also played a role. Critically, all of the past trials involved patients with preexisting CVD. We previously hypothesized that treating depression before, versus after, the onset of clinical CVD could reduce the risk of CVD events (9) because: (a) evidence suggests that earlier treatment of another CVD risk factor, hypercholesterolemia, yields more pronounced benefits (10–13), (b) depression begins to exert a deleterious influence early in the pathogenesis of CVD (14–17), (c) the prevalence of vascular depression (18), which tends to respond poorly to treatment (19, 20), is likely to be lower in depressed patients free of CVD, and (d) conventional prognostic factors may override the effect of depression during the later stages of CVD (21, 22).
To test this hypothesis, we conducted an 8-year follow-up study of patients from the Indiana sites of the Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) randomized controlled trial (23, 24). In that multisite trial, 1,801 depressed primary care patients aged ≥60 years were randomly assigned to either a 12-month collaborative stepped care program for late-life depression involving antidepressants and brief psychotherapy or usual care. Because the IMPACT trial was positive for the depression outcomes (23), it provides a good opportunity to evaluate the long-term health effects of successful depression treatment. For the 235 patients from the Indiana sites, we leveraged a unique set of resources – i.e., local electronic medical record data (including death certificate data) linked with Medicare and Medicaid claims – to identify hard CVD events. This set of resources is not currently available at the other IMPACT sites.
Method
Participants
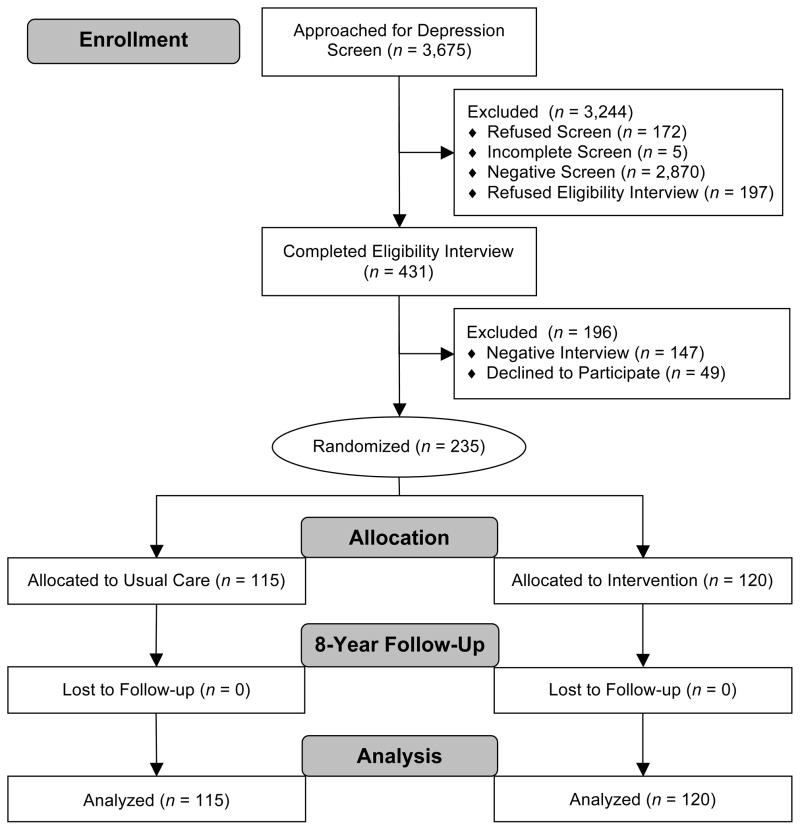
Participants were 235 depressed patients recruited between July 1999-August 2001 from two primary care clinics in an academic group practice in Indianapolis, IN. Recruitment details are available elsewhere (23, 24). Potential participants underwent a depression screen (25), followed by an eligibility interview (26) (Figure 1). Inclusion criteria were age ≥60 years and a current major depressive disorder or dysthymia diagnosis. Patients were excluded if they had a drinking problem (27), had bipolar disorder/psychosis, were in psychiatric treatment, had severe cognitive impairment (28), or were at acute risk of suicide. Our follow-up study was approved by the IUPUI Institutional Review Board and the Centers for Medicare and Medicaid Services Privacy Board. Participants provided written informed consent to the IMPACT procedures, and a waiver of consent was obtained to link electronic medical record and Medicare/Medicaid data.
Figure 1.
Flowchart of participants from the Indiana sites of the Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) Randomized Controlled Trial.
To identify participants with baseline CVD, we merged data from Regenstrief Medical Record System (29), one of largest and longest operating electronic medical records (earliest data from 1978), with data from Medicare and Medicaid claims (earliest data from 1999). Baseline CVD was defined as the occurrence of any event listed in categories b, c, or e (see Outcome Measures) or any of the following procedures before the IMPACT enrollment date: percutaneous coronary intervention (ICD-9 codes 00.66, 36.03, 36.06, 36.07, 36.09; CPT codes 92980-92984, 92995, 92996), coronary artery bypass graft (ICD-9 codes 36.10-36.19; CPT codes 33510-33536), or thrombolytic therapy (CPT code 37195). Of note, we employed this definition of baseline CVD – i.e., a history of myocardial infarction (MI), stroke, revascularization, or thrombolytic therapy – so that our participants with baseline CVD resembled those of past trials examining the effect of depression treatment on CVD events (e.g., post-MI infarction patients) (3, 7). In a sensitivity analysis, we utilized a broader definition of baseline CVD, which likely had higher sensitivity but lower specificity for clinical CVD. Among patients with baseline CVD, the median time from initial CVD event to IMPACT enrollment was 2.6 years (IQR: 1.5–7.5 years). Patients with baseline CVD were almost equally distributed across the IMPACT and Usual Care groups, and no significant differences in the baseline characteristics were detected between the treatment groups (Table 1).
Table 1.
Participant Characteristics by Treatment Group and Baseline Cardiovascular (CVD) Status (N = 235)
| Characteristic | No Baseline CVD | Baseline CVD | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| IMPACT (n = 85) | Usual Care (n = 83) | p value | IMPACT (n = 35) | Usual Care (n = 32) | p value | |
| Demographic Factors | ||||||
| Age (years), mean (SD) | 66.8 (6.3) | 67.8 (6.6) | .33 | 67.9 (6.9) | 67.8 (7.8) | .97 |
| Male, % | 18.8 | 22.9 | .52 | 31.4 | 31.2 | .99 |
| African-American†, % | 42.4 | 53.0 | .17 | 45.7 | 46.9 | .92 |
| Baseline Cardiovascular Risk Factors | ||||||
| Hypertension, % | 68.2 | 75.9 | .27 | 91.4 | 78.1 | .13 |
| Diabetes, % | 34.1 | 34.9 | .91 | 40.0 | 43.8 | .76 |
| Smoker, % | 29.4 | 41.0 | .12 | 28.6 | 31.2 | .81 |
| Body-Mass Index (kg/m2), mean (SD) | 33.2 (10.0) | 30.5 (9.5) | .066 | 31.2 (9.1) | 30.3 (8.2) | .67 |
| Baseline Depression Variables | ||||||
| MDD only, % | 12.9 | 10.8 | .67 | 17.1 | 6.2 | .17 |
| Dysthymia only, % | 36.5 | 38.6 | .78 | 37.1 | 37.5 | .98 |
| MDD and Dysthymia, % | 50.6 | 50.6 | .99 | 45.7 | 56.3 | .39 |
| SCL-20 score, mean (SD) (range: 0–4) | 1.4 (0.5) | 1.5 (0.5) | .17 | 1.5 (0.5) | 1.8 (0.5) | .070 |
| Antidepressant Use in Past 3 Months, % | 51.8 | 48.2 | .64 | 48.6 | 59.4 | .38 |
| Depression Outcomes and Care | ||||||
| SCL-20 Change, mean (SD) | −0.4 (0.7) | 0.1 (0.6) | <.001 | −0.2 (0.6) | −0.1 (0.8) | .83 |
| Antidepressants during the trial, % | 75.3 | 60.2 | .037 | 79.4 | 83.9 | .64 |
| Psychotherapy during the trial, % | 62.4 | 14.5 | <.001 | 58.8 | 25.8 | .007 |
Note. Independent samples t tests were used to compare groups on age, baseline SCL-20, and SCL-20 change (12-month score –baseline score). All other group comparisons were made using chi-square tests. IMPACT = Improving Mood-Promoting Access to Collaborative Treatment. MDD = major depressive disorder. SCL-20 = Symptom Checklist-20.
Because only 6 (3%) patients and 4 (2%) patients fell into the Hispanic and Other categories, respectively, we created a dichotomous race variable (0 = White, Hispanic, and Other; 1 = African American).
Treatment Groups
Patients were randomized to treatment groups (stratified by clinic) using computer-generated random number sequences (23, 24). This information was then enclosed in a set of numbered, sealed envelopes for each clinic that were opened sequentially when a new patient was enrolled. Personnel who conducted the assessment interviews and the data manager who computed the CVD outcomes were blind to treatment assignment.
IMPACT Intervention
This intervention has been described elsewhere (23, 24, 30). Collaborating with the patients and their primary care providers, the depression clinical specialists (DCSs) developed a treatment plan following the IMPACT algorithm (30), which was based on guidelines that were current when the trial was designed (31, 32). This algorithm recommends a Step 1 treatment of 8–12 weeks of an antidepressant (usually a selective serotonin reuptake inhibitor; SSRI) or Problem-Solving Treatment in Primary Care (a brief cognitive-behavioral therapy) (33) depending on the patient’s preference. In addition to providing psychotherapy, DCSs encouraged patients to adhere to antidepressant medication regimens and referred patients to other health/social services as indicated. Patients were followed for up to 12 months, while treatment response was monitored (34). For patients who achieved remission, the DCS developed a relapse prevention plan and followed up on a monthly basis. Step 2 treatment –which involved augmenting Step 1 treatment with a second antidepressant or psychotherapy or switching to another antidepressant or psychotherapy – was delivered to patients who did not achieve remission. A psychiatric consultation was initiated for patients with persistent depression. If remission was not achieved in 6–10 additional weeks, Step 3 treatment was initiated, which consisted of additional medications and psychotherapy, hospitalization, or other mental health services. DCSs discussed new cases and treatment plan changes during supervision with a psychiatrist and a geriatrician.
Usual Care
Patients were informed of their diagnosis, were encouraged to follow-up with their provider, and were followed for 12 months while they received services that were part of usual care. Providers received a letter indicating that their patient has a depressive disorder and was randomized to usual care.
Outcome Measures
A hard CVD event, the primary outcome, was defined as the occurrence of any of the following events in the medical record or Medicare/Medicaid data between IMPACT enrollment date and December 31, 2008: (a) fatal MI (ICD-10 codes I21-I22 the first-listed cause of death), (b) laboratory evidence of acute MI (creatine kinase-myocardial band isoenzyme value >3.0 ng/ml or troponin value >0.3 μg/L), (c) acute MI diagnosis (ICD-9 code 410), (d) fatal stroke (ICD-10 codes I60-I64 the first-listed cause of death), or (e) hemorrhagic (ICD-9 codes 430-432) or nonhemorrhagic (ICD-9 codes 433.01, 433.11, 433.21, 433.31, 433.91, 434.01, 434.11, and 434.91) stroke diagnosis. Secondary outcomes were fatal/nonfatal MI (categories a–c), fatal/nonfatal MI – cardiac enzyme confirmed (categories a and b), fatal/nonfatal stroke (categories d and e), and all-cause mortality. Death dates were extracted from the Medicare data, and causes of death were obtained from death certificates provided by the Indiana State Department of Health. Because the 2007–2008 death certificate data have not been released, cause of death is available only through 2006, which includes 59 of the 91 deaths (65%) in our cohort. Patients who died but did not fall into categories a–e were coded as deaths not due to an MI or stroke, including those with missing cause of death. Patients were followed for a maximum of 7.5–9.5 years (median = 8.1); however, for cause of death (categories a and d), patients were followed for a maximum of 5.5–7.5 years (median = 6.2).
Other Variables
Baseline characteristics (except baseline CVD, smoking, and body mass index) were assessed during the eligibility interview (24) (Table 1). Patients diagnosed with or treated for hypertension or diabetes in the past 3 years were coded as having these conditions. Patients completed the 20 depression items of the Symptom Checklist-90 (SCL-20) (35) to measure symptom severity and reported their use of antidepressants during the preceding 3 months. The SCL-20 is a reliable and valid measure of depressive symptom severity that has been used as outcome measure in several primary care trials (36–40). In our sample, the SCL-20 exhibited good internal consistency at baseline and 12 months (Cronbach’s α = 0.81 and 0.91), which is consistent with previous findings (Cronbach’s α = 0.84–0.86) (41, 42). Regarding validity, the SCL-20 has been found to be moderately correlated (r = 0.54) with another established depression scale, the Patient Health Questionnaire-9 (41). In addition, O’Conner et al. (43) observed that a 50% reduction in SCL-20 score accurately identified 79% of patients who no longer met criteria for MDD after 12 weeks of collaborative care, concluding that this cut point is a conservative measure of change in depression status. Medical record data were used to compute baseline smoking and body mass index. At 3, 6, and 12 months, interviewers readministered the SCL-20 and inquired about antidepressant and psychotherapy use (24).
Data Analysis
We constructed Kaplan-Meier survival curves to illustrate the time from enrollment to first CVD event in the Treatment (IMPACT, Usual Care) x Baseline CVD (yes, no) groups. Patients were censored at date of death or December 31, 2008. We ran Cox proportional hazards models for the full sample to test the Treatment main effect (no covariates) and the Treatment x Baseline CVD interaction (Treatment and Baseline CVD main effects were covariates). We then ran Cox models testing the Treatment main effect separately among patients with and without baseline CVD. We tested the proportional hazards assumption by adding a Time x Randomization status interaction term to the models. For all outcomes, the proportional hazards assumption was not rejected. To illustrate clinical significance, we performed a number needed to treat (NNT) analysis, which determined the number of depressed patients that would need to be treated to prevent one hard CVD event over a 5-year period. This analysis was based on the hazard ratio for the Treatment main effect and the event rates after 5 years among patients without baseline CVD. We also conducted four sets of subgroup/sensitivity analyses: for men and women, for the secondary outcomes, adjusted for baseline characteristics, and using a broader definition of baseline CVD. Finally, we performed exploratory analyses in which we coded the 8 cases who had missing cause of death data and were also negative for all other CVD event markers as MI/stroke deaths. Reversing the coding for these cases did not alter the results (see Table 2).
Table 2.
Cox Proportional Hazards Models Predicting Hard Cardiovascular Disease (CVD) Events, Fatal or Nonfatal Myocardial Infarction (MI), Fatal or Nonfatal Stroke, and All-Cause Mortality
| Outcome | Events (%) | Treatment | Treatment × Baseline CVD | Treatment: No Baseline CVD (n = 168) | Treatment: Baseline CVD (n = 67) | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HR | 95% CI | p value | HR | 95% CI | HR | 95% CI | ||
| Hard CVD Events | 119 (51%) | 0.73 | 0.51–1.05 | .021 | 0.52* | 0.31–0.86 | 1.19 | 0.70–2.03 |
| Men Only | 35 (63%) | 0.61 | 0.31–1.21 | .062 | 0.30* | 0.10–0.93 | 1.12 | 0.43–2.92 |
| Women Only | 84 (47%) | 0.81 | 0.53–1.25 | .10 | 0.63 | 0.35–1.12 | 1.23 | 0.64–2.35 |
| Adjusted† | 119 (51%) | 0.86 | 0.59–1.25 | .033 | 0.58* | 0.35–0.99 | 1.24 | 0.68–2.25 |
| Broad Baseline CVD‡ | 119 (51%) | 0.73 | 0.51–1.05 | .20 | 0.60 | 0.30–1.21 | 1.01 | 0.66–1.55 |
| Missing Cause of Death§ | 127 (54%) | 0.71 | 0.50–1.01 | .031 | 0.53* | 0.32–0.86 | 1.12 | 0.67–1.86 |
| Fatal or Nonfatal MI | 103 (44%) | 0.82 | 0.56–1.20 | .083 | 0.61 | 0.35–1.06 | 1.26 | 0.73–2.18 |
| Fatal or Nonfatal MI – Enzyme Confirmed | 73 (31%) | 0.79 | 0.50–1.25 | .022 | 0.47* | 0.24–0.93 | 1.49 | 0.76–2.90 |
| Fatal or Nonfatal Stroke | 37 (16%) | 0.56 | 0.29–1.08 | .038 | 0.25** | 0.08–0.75 | 1.10 | 0.45–2.71 |
| All-Cause Mortality | 91 (39%) | 0.95 | 0.63–1.44 | .14 | 0.76 | 0.45–1.29 | 1.44 | 0.74–2.80 |
Note. N = 235 (179 women, 56 men). HR = hazard ratio. CI = confidence interval.
Adjusted for age, sex, race, hypertension, diabetes, smoking status, body mass index, baseline Symptom Checklist-20 (SCL-20), and baseline antidepressant use. Sex (HR = 1.81, 95% CI: 1.21–2.72, p = .004), diabetes (HR = 1.49, 95% CI: 1.02–2.18, p = .04), and baseline SCL-20 (HR = 1.64, 95% CI: 1.14–2.35, p = .01) were independent predictors of hard CVD events in the expected directions.
Baseline CVD (no: n = 112, yes: n = 123) defined as the occurrence of any of the following before IMPACT enrollment: (a) ischemic heart disease diagnosis (ICD-9 codes 410–414, 429.2), (b) laboratory evidence of an acute MI (creatine kinase-myocardial band isoenzyme value >3.0 ng/ml or troponin value >0.3 μg/L), (c) percutaneous coronary intervention (ICD-9 codes 00.66, 36.03, 36.06, 36.07, 36.09; CPT codes 92980-92984, 92995, 92996), (d) coronary artery bypass graft (ICD-9 codes 36.10-36.19; CPT codes 33510-33536), (e) cerebrovascular disease diagnosis (ICD-9 codes 430-434, 436-438), or (f) thrombolytic therapy (CPT code 37195).
Exploratory analyses in which the 8 cases who had missing cause of death data and were negative for all other CVD event markers were coded as MI/stroke deaths.
p < .05.
p < .01.
To evaluate whether treatment effects were mediated by depression outcome or care variables, SCL-20 change, trial antidepressant use (yes, no), and trial psychotherapy (yes, no) were added to Cox models that included the Treatment main effect. Change in the treatment effect after adding each variable was computed as (BT+M – BT) / BT × 100, where BT+M is the unstandardized coefficient for the Treatment main effect in the presence of the potential mediator, and BT is the unstandardized coefficient for the same variable alone. Sobel tests were performed to determine whether the potential mediators statistically mediated treatment effects. Analyses were performed using SAS statistical software, version 9.3 (SAS Institute, Cary, NC).
Results
Effect of the IMPACT Intervention on Depression Outcomes and Care
Among patients without baseline CVD, the IMPACT group exhibited a greater reduction in SCL-20 score than the Usual Care group (p < .001, d = .69; Table 1), and 35% of the IMPACT patients, versus 10% of the Usual Care patients, achieved a 50% reduction in SCL-20 score (p < .001). In contrast, among patients with baseline CVD, there was no group difference in SCL-20 change (p = .83, d = .06; Table 1) or the percentage achieving a 50% reduction in SCL-20 score (IMPACT: 16%, Usual Care: 17%, p = .91). Similarly, IMPACT patients without, but not with, baseline CVD were more likely to have taken antidepressants during the trial than Usual Care patients. Among those with and without baseline CVD, IMPACT patients were more likely to have received psychotherapy. These results are comparable to those of the entire IMPACT trial (23).
Effect of the IMPACT Intervention on Hard Cardiovascular Disease Events
A total of 119 patients experienced a hard CVD event during the 8-year follow-up period (first event: 93 nonfatal MIs, 25 nonfatal strokes [6 hemorrhagic, 18 nonhemorrhagic, 1 both], and 1 fatal MI). CVD event rates were high over the follow-up period (Table 2), likely due to the use of a combined CAD and CBV outcome, the high prevalence of major CVD risk factors at baseline, and the elevated severity of depression at baseline (>50% had both major depressive disorder and dysthymia; see Table 1 for participant characteristics). Not surprisingly, events were concentrated in patients with preexisting CVD; the mean annual event rate was 22.8% and 6.2% for those with and without baseline CVD, respectively. These event rates are consistent with those from past studies of elderly depressed patients with (44) and without (9) baseline CVD.
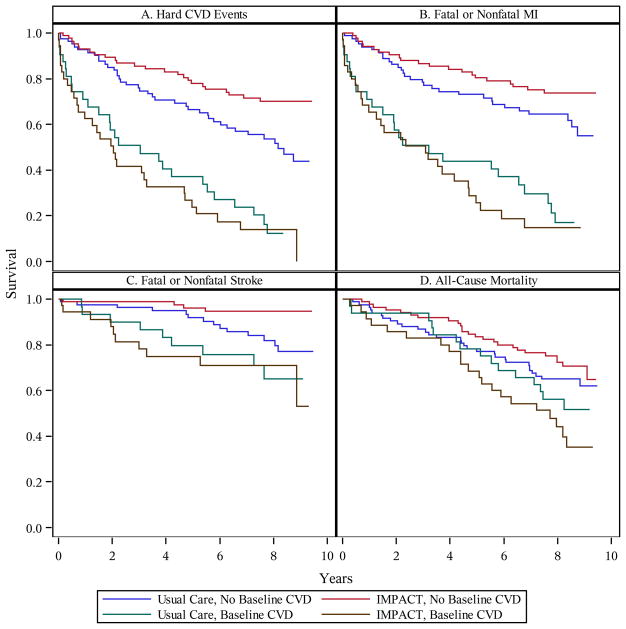
Survival curves indicated that the time to CVD event varied across the Treatment x Baseline CVD groups (Figure 2, Panel A). Among patients without baseline CVD, the CVD event rate was 28% (24/85) for the IMPACT group versus 47% (39/83) for the Usual Care group (log-rank χ2 = 6.71, p = .010), yielding an absolute risk reduction at the end of the follow-up period of 19%. In contrast, among patients with baseline CVD, the CVD event rate was 86% (30/35) for IMPACT versus 81% (26/32) for Usual Care (log-rank χ2 = 0.41, p = .52).
Figure 2.
Kaplan-Meier survival curves for time to (A) hard CVD events, (B) fatal or nonfatal MI, (C) fatal or nonfatal stroke, and (D) all-cause mortality. CVD = cardiovascular disease. MI = myocardial infarction. IMPACT = Improving Mood-Promoting Access to Collaborative Treatment.
Cox models involving the full sample revealed that the Treatment main effect fell short of significance for hard CVD events (p = .092); IMPACT patients had a 27% lower risk of a CVD event than Usual Care patients (Table 2). As was hypothesized, there was evidence of moderation by baseline CVD, as the Treatment x Baseline CVD interaction was significant (p = .021). Separate Cox models for patients with and without baseline CVD indicated that Treatment main effect was driven by the patients without baseline CVD (Table 2). IMPACT patients without baseline CVD had a 48% lower risk of a CVD event (p = .011), whereas the likelihood of a CVD event did not differ between IMPACT and Usual Care patients with baseline CVD (p = .52). An NNT analysis indicated that 6.1 depressed patients need to be treated to prevent one hard CVD event over a 5-year period.
As is shown in Table 2, similar results were obtained in the subgroup/sensitivity analyses, although some effects did not achieve significance in part due to reduced statistical power caused by a drop in CVD events. First, the IMPACT intervention was associated with a significant CVD risk reduction for men without baseline CVD (70%, p = .037); however, this risk reduction for women (37%, p = .12) fell short of significance. Second, IMPACT patients without baseline CVD had a significantly reduced risk of fatal/nonfatal MI – cardiac enzyme confirmed (53%, p = .030) and fatal/nonfatal stroke (75%, p = .014), although the reduced risk of fatal/nonfatal MI fell short of significance (39%, p = .081; Figure 2, Panels B and C). The Treatment main effect was not significant for all-cause mortality among patients without baseline CVD (p = .30; Figure 2, Panel D) but IMPACT patients did have a numerically lower risk. Third, adjusting for baseline characteristics did not alter the results; IMPACT patients without baseline CVD had a 42% lower risk of a CVD event (p = .044). Fourth, we observed a similar pattern of results when we utilized a broader definition of baseline CVD (no: n = 112, yes: n = 123); however, the lower risk of CVD events among patients without baseline CVD fell short of significance (40%, p = .16). When the broad definition was used, only 32 of the 119 events occurred in the cohort without baseline CVD. Across the subgroup/sensitivity analyses, the Treatment main effect was not significant among patients with baseline CVD (all ps > .26).
Mediation Analyses
As is shown in Table 1, we observed a treatment effect on the three candidate mediators among patients without baseline CVD. IMPACT patients exhibited greater SCL-20 reductions and were more likely to have taken antidepressants and received psychotherapy. Adding SCL-20 change to the models predicting hard CVD events decreased the treatment effect by only 8% (HR = 0.55, 95% CI: 0.32–0.94, p = .028), and SCL-20 change did not predict CVD events (HR = 1.12, 95% CI: 0.75–1.66, p = .58). Similarly, adding trial antidepressants decreased the treatment effect by only 2% (HR = 0.52, 95% CI: 0.31–0.88, p = .014), and trial antidepressants was not a predictor of CVD events (HR = 0.90, 95% CI: 0.54–1.53, p = .71). In contrast, adding trial psychotherapy increased the treatment effect by 17% (HR = 0.46, 95% CI: 0.26.-0.82, p = .009), but trial psychotherapy also did not predict CVD events (HR = 1.27, 95% CI: 0.71–2.27, p = .42). Sobel tests revealed that there was no evidence of statistical mediation by any of the candidate mediators (all ps > .42).
Discussion
The present findings (a) support our novel hypothesis that depression treatment delivered before the onset of clinical CVD reduces the risk of CVD events and (b) suggest an alternative or complement to the current paradigm of initiating depression treatment after clinical CVD onset to improve cardiovascular prognosis. In this long-term follow-up study of the IMPACT trial, depressed patients without baseline CVD who received collaborative care for depression had a 48% lower risk of a hard CVD event than patients who received usual care (19% absolute risk reduction). This degree of risk reduction is comparable to that of major CVD prevention approaches (45, 46). Further highlighting the clinical significance of our findings, we determined that approximately six depressed patients aged ≥60 years need to be treated with the IMPACT intervention to prevent one fatal/nonfatal MI or stroke over five years. In contrast, among depressed patients with baseline CVD, the risk of a hard CVD event was comparable in both treatment groups. Our findings are robust; a similar pattern of results was found for men and women, for fatal/nonfatal MI and stroke, after adjustment for potential confounders, and when a broader definition of baseline CVD was employed. We did, however, observe evidence that the cardioprotective effect of the IMPACT intervention may be greater for men and for fatal/nonfatal stroke. Although our study is by no means definitive due to its post hoc nature, we do report unique results that strengthen the case that depression is a risk factor for CVD. Moreover, our findings suggest that evidence-based depression treatment can bring about a clinically meaningful reduction in incident CVD events.
Previous trials, in particular the adequately powered ENRICHD trial (3) and Myocardial Infarction and Depression-Intervention Trial (7), have not detected a cardioprotective effect of depression treatment. The Coronary Psychosocial Evaluation Studies trial (4) is an intriguing exception; however, the number of cardiovascular events was low. Of relevance, all of these trials involved patients with preexisting CVD. Here, we propose and report evidence suggesting that the late timing of depression treatment in the natural history of CVD may have contributed to the null results of past trials. In other words, the cardiovascular benefits of depression treatment may be larger in magnitude earlier in the natural history of CVD. Gallo and colleagues (47) found that collaborative care for depression reduced all-cause mortality, but not CVD deaths, among older, depressed patients. While their findings may seem at odds with ours, nearly half of their patients had CVD at baseline, and analyses stratified by baseline CVD were not reported. Consequently, it is unknown whether collaborative depression care exerted a cardioprotective effect among patients without baseline CVD in that trial.
There are several reasons why depression treatment may exert a cardioprotective effect before, versus after, the onset of clinically manifest CVD. First, emerging evidence suggests that earlier versus later treatment of another CVD risk factor, hypercholesterolemia, yields more pronounced benefits (10–12), possibly by slowing atherosclerotic progression (13). Combining data from prospective studies, Law and colleagues (11) estimated that the risk reduction in CAD events of a 1.8 mmol/l decrease in LDL cholesterol was greater for patients in their 50s (77%) than those in their 60s (61%) and 70s (49%). Other evidence suggests that earlier treatment is associated with slower atherosclerotic progression, as younger age at initiation of statin treatment predicted smaller increases in carotid intima-media thickness over 4.5 years among children with familial hypercholesterolemia (13). A recent meta-analysis of genetic studies also bolsters the earlier treatment rationale; it was found that lower LDL cholesterol beginning early in life (due to a mutation) confers a three times greater decrease in CAD risk than the same cholesterol reduction later in life (due to statin treatment) (10). Collectively, these findings raise the possibility that earlier treatment of other modifiable CVD risk factors, including depression, may also retard atherosclerotic progression to a greater extent, which should translate into a lower rate of CVD events.
Second, depression treatment may have cardioprotective effect prior to clinical CVD onset because depression begins to exert a deleterious influence early in the pathogenesis of CVD. Depression has been associated with endothelial dysfunction (14) and more rapid atherosclerotic progression (15, 16) in humans free of CVD and predicts early atherogenesis in primates (17). Therefore, intervening on depression earlier would minimize the duration of exposure to this risk factor. Third, vascular depression (18), which has been associated with poor treatment response (19, 20), is likely to be less prevalent among depressed patients free of clinical CVD. Consistent with this notion, the antidepressive efficacy of the IMPACT intervention in our study was greater among patients without versus with baseline CVD (d = .69 versus .06). Similar to our study, most trials of depressed patients with CVD have observed relatively small treatment effect sizes for depression outcomes (d = 0.20–0.38) (48), which highlights the need for more effective interventions for these patients. It is worth noting that two recent trials of stepped depression care have reported more promising (moderate) effect sizes in patients with acute coronary syndrome (4, 49). Fourth, conventional prognostic factors, such as disease severity or medical treatment, may override the effect of depression during the later stages (21, 22). Due to their speculative nature, future studies are needed to evaluate these four potential reasons for why depression treatment may exert a cardioprotective effect before clinical CVD onset.
A key next step is elucidating the mechanisms underlying the cardioprotective effect of collaborative care for depression among patients without baseline CVD. We found that symptomatic improvement during the 12-month treatment phase explained only 8% of the cardioprotective effect of the IMPACT intervention, and this variable was not a significant mediator. However, because depression data during the follow-up period are not available, it remains an open question as to whether the cardioprotective effect is depression dependent. We also found that antidepressant and psychotherapy use during the trial did not appreciably reduce the treatment effect and were not significant mediators. Unfortunately, we do not have long-term data for depression care, and the yes-no questions assessing treatment received may have failed to capture important aspects, such as dose and duration of antidepressant treatment and number of psychotherapy sessions. Because depression has been linked with atherogenic physiologic (e.g., autonomic dysfunction and systemic inflammation) and behavioral (e.g., smoking, physical inactivity, and poor medication adherence) factors (50), successful treatment of depression could lead to improvements in these factors, thereby reducing CVD risk. Of relevance here, receiving problem-solving therapy, a cognitive-behavioral therapy, may have decreased the likelihood of depression relapse during the follow-up period (51). The direct inhibitory influence of SSRIs on platelet reactivity (52) could also be responsible for the cardioprotective effect of the IMPACT intervention. Moreover, among patients with baseline CVD, the lack of a treatment group difference in antidepressant use during the trial, possibly due to the high rate of treatment, may have contributed to the absence of a cardioprotective effect of the intervention. Indeed, a secondary analysis of the ENRICHD trial revealed that SSRI use was associated with a lower likelihood of CVD events among post-MI patients (53). Finally, given that IMPACT patients were referred for other health/social services when indicated, these services may have resulted in improvements in CVD risk profiles. Furthermore, the effect of these additional services on incident CVD events may have been greater among patients without baseline CVD, as it is likely that these patients were not being monitored as closely and receiving as much medical care at the start of the trial as patients with baseline CVD. To determine the relative contribution of depression-dependent and depression-independent pathways and to identify the underlying mechanisms, future trials should obtain measures of depression outcomes and care and of other services throughout the follow-up period, as well as repeatedly assess the atherogenic physiological and behavioral factors.
Our follow-up study has limitations. First, the IMPACT trial was not designed to test our hypothesis, and our analyses are post hoc in nature and should be interpreted with caution. Consequently, randomization was not stratified by CVD status, and hard CVD events was not a pre-specified endpoint of the trial. Even so, patients with baseline CVD were almost equally distributed across the treatment groups, no group differences in potential confounders were detected, and analyses adjusted for potential confounders yielded similar results. Second, the cohort of patients with baseline CVD was small, and the dividing line between groups with and without baseline CVD was not sharp. Thus, our finding that the IMPACT intervention did not reduce the likelihood of CVD events among patients with preexisting CVD should be interpreted cautiously, especially considering the almost nil effect of the intervention on depressive symptoms. Of relevance, a sensitivity analysis using a broader definition of baseline CVD, which increased the size of the baseline CVD cohort to 123, yielded similar findings. In addition, the absence of a depression intervention effect on cardiovascular outcomes among CVD patients is consistent with results of much larger trials (3, 7). Third, we were able to identify incident CVD events only in a subgroup of patients from the multisite IMPACT trial. We were restricted to patients from the Indiana sites because we took advantage of existing data sources – namely, a local electronic medical record system and Medicare/Medicaid claims data obtained for a large group of older adults in Indianapolis area (including IMPACT patients) as part of another project. Scientifically, our approach is reasonable, given that the methodological features of a randomized controlled trial were in place at each site (23, 24). However, replicating our project at other IMPACT sites where similar data sources are available or could be obtained is an important next step that would increase statistical power, as well as the external validity of our findings. Fourth, we did not have cause of death data for the 32 deaths that occurred in 2007–2008, which may have led us to misclassify some deaths as not due to MI or stroke. However, it is unlikely that this affected our results, given that 24 of these patients (75%) were positive for a nonfatal event marker, thereby correctly establishing their time to first CVD event. In addition, coding the 8 cases who had missing cause of death data and were negative for all nonfatal event markers as MI/stroke deaths did not alter the results. Fifth, while we were adequately powered to detect a Treatment x Baseline CVD interaction and an intervention effect for our primary outcome, clinically meaningful effects in the subgroup/sensitivity analyses (e.g., in women and when we used the broader baseline CVD definition) fell short of significance, indicating that statistical power was likely low or inadequate in these analyses. Future trials could utilize our effect size estimates to ensure that they are adequately powered for the subgroups or outcomes of interest. Sixth, the use of the SCL-20 to assess depressive symptom severity is a potential limitation, given that its specificity may not be as high as other depression scales (41) and its utility in predicting CVD outcomes has not been demonstrated (1).
In summary, we found that collaborative care for depression delivered before the onset of CVD halved the excess risk of hard CVD events among depressed primary care patients aged ≥60 years. Although our results raise the possibility that the IMPACT intervention could be employed as a primary prevention strategy for CAD and CBV, there is now a need to conduct a well-powered randomized controlled trial designed to definitively test our hypothesis that treating depression earlier in the natural history of CVD reduces the risk of CVD events. If this trial confirms our hypothesis, it would support providers using the IMPACT intervention as both a depression intervention and cardiovascular risk reduction strategy among older, depressed patients without clinically manifest CVD.
Acknowledgments
Funding Sources
This research was supported by the National Institute on Aging grants AG031222 and AG024078 awarded to Dr. Callahan.
Some data management tasks were performed by Joseph G. Kesterson, MA, Regenstrief Institute, Inc., Indianapolis, IN.
Acronyms
- CVD
cardiovascular disease
- CAD
coronary artery disease
- CBV
cerebrovascular disease
- IMPACT
Improving Mood-Promoting Access to Collaborative Treatment
- ICD-9
International Classification of Diseases, 9th Revision
- CPT
current procedural terminology
- MI
myocardial infarction
- DCS
depression clinical specialist
- SSRI
selective serotonin reuptake inhibitor
- ICD-10
International Classification of Diseases, 9th Revision
- SCL-20
Symptom Checklist-20
- NNT
number needed to treat
Footnotes
Potential Conflicts of Interest
None.
References
- 1.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J of Geriatr Psychiatry. 2007;22:613–26. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 2.Meijer A, Conradi HJ, Bos EH, Thombs BD, van Melle JP, de Jonge P. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33:203–16. doi: 10.1016/j.genhosppsych.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289:3106–16. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 4.Davidson KW, Rieckmann N, Clemow L, Schwartz JE, Shimbo D, Medina V, Albanese G, Kronish I, Hegel M, Burg MM. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Arch of Intern Med. 2010;170:600–8. doi: 10.1001/archinternmed.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, Jr, Krishnan KR, van Zyl LT, Swenson JR, Finkel MS, Landau C, Shapiro PA, Pepine CJ, Mardekian J, Harrison WM, Barton D, McLvor M Sertraline Antidepressant Heart Attack Randomized Trial Group. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–9. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 6.Rollman BL, Belnap BH, LeMenager MS, Mazumdar S, Houck PR, Counihan PJ, Kapoor WN, Schulberg HC, Reynolds CF. Telephone-delivered collaborative care for treating post-CABG depression: a randomized controlled trial. JAMA. 2009;302:2095–103. doi: 10.1001/jama.2009.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Melle JP, de Jonge P, Honig A, Schene AH, Kuyper AM, Crijns HJ, Schins A, Tulner D, van den Berg MP, Ormel J investigators M-I. Effects of antidepressant treatment following myocardial infarction. B J Psychiatry. 2007;190:460–6. doi: 10.1192/bjp.bp.106.028647. [DOI] [PubMed] [Google Scholar]
- 8.Goldston K, Baillie AJ. Depression and coronary heart disease: a review of epidemiological evidence, explanatory mechanisms, and management approaches. Clin Psychol Rev. 2008;28:288–306. doi: 10.1016/j.cpr.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Brown JM, Stewart JC, Stump TE, Callahan CM. Risk of coronary heart disease events over 15 years among older adults with depressive symptoms. Am J Geriatr Psychiatry. 2011;19:721–9. doi: 10.1097/JGP.0b013e3181faee19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ference BA, Yoo W, Alesh I, Mahajan N, Mirowska KK, Mewada A, Kahn J, Afonso L, Williams KA, Sr, Flack JM. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol. 2012;60:2631–9. doi: 10.1016/j.jacc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994;308:367–72. doi: 10.1136/bmj.308.6925.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodenburg J, Vissers MN, Wiegman A, van Trotsenburg AS, van der Graaf A, de Groot E, Wijburg FA, Kastelein JJ, Hutten BA. Statin treatment in children with familial hypercholesterolemia: the younger, the better. Circulation. 2007;116:664–8. doi: 10.1161/CIRCULATIONAHA.106.671016. [DOI] [PubMed] [Google Scholar]
- 14.Cooper DC, Tomfohr LM, Milic MS, Natarajan L, Bardwell WA, Ziegler MG, Dimsdale JE. Depressed mood and flow-mediated dilation: a systematic review and meta-analysis. Psychosom Med. 2011;73:360–9. doi: 10.1097/PSY.0b013e31821db79a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssen I, Powell LH, Matthews KA, Cursio JF, Hollenberg SM, Sutton-Tyrell K, Bromberger JT, Everson-Rose SA. Depressive symptoms are related to progression of coronary calcium in midlife women. Am Heart J. 2011;161:1186–91. doi: 10.1016/j.ahj.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart JC, Janicki DL, Muldoon MF, Sutton-Tyrrell K, Kamarck TW. Negative emotions and 3-year progression of subclinical atherosclerosis. Arch Gen Psychiatry. 2007;64:225–33. doi: 10.1001/archpsyc.64.2.225. [DOI] [PubMed] [Google Scholar]
- 17.Shively CA, Register TC, Adams MR, Golden DL, Willard SL, Clarkson TB. Depressive behavior and coronary artery atherogenesis in adult female cynomolgus monkeys. Psychosom Med. 2008;70:637–45. doi: 10.1097/PSY.0b013e31817eaf0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. The “vascular depression” hypothesis. Arch Gen Psychiatry. 1997;54:915–22. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien J, Ames D, Chiu E, Schweitzer I, Desmond P, Tress B. Severe deep white matter lesions and outcome in elderly patients with major depressive disorder: follow up study. BMJ. 1998;317:982–4. doi: 10.1136/bmj.317.7164.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson S, Baldwin RC, Jackson A, Burns AS. Is subcortical disease associated with a poor response to antidepressants? Neurological, neuropsychological and neuroradiological findings in late-life depression. Psychol Med. 1998;28:1015–26. doi: 10.1017/s003329179800693x. [DOI] [PubMed] [Google Scholar]
- 21.Scheier MF, Bridges MW. Person variables and health: personality predispositions and acute psychological states as shared determinants for disease. Psychosom Med. 1995;57:255–68. doi: 10.1097/00006842-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 23.Unutzer J, Katon W, Callahan CM, Williams JW, Jr, Hunkeler E, Harpole L, Hoffing M, Della Penna RD, Noel PH, Lin EH, Arean PA, Hegel MT, Tang L, Belin TR, Oishi S, Langston C IMPACT Investigators. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–45. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 24.Unutzer J, Katon W, Williams JW, Jr, Callahan CM, Harpole L, Hunkeler EM, Hoffing M, Arean P, Hegel MT, Schoenbaum M, Oishi SM, Langston CA. Improving primary care for depression in late life: the design of a multicenter randomized trial. Med Care. 2001;39:785–99. doi: 10.1097/00005650-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, 3rd, Hahn SR, Brody D, Johnson JG. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749–56. [PubMed] [Google Scholar]
- 26.First MB, Spitzer A, Gibbon M, Williams JB. Structured clinical interview for DSM-IV axis I disorders (SCID) Washington, DC: American Psychiatric Press, Inc; 1996. [Google Scholar]
- 27.Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry. 1974;131:1121–3. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- 28.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–81. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 29.McDonald CJ, Tierney WM, Overhage JM, Martin DK, Wilson GA. The Regenstrief Medical Record System: 20 years of experience in hospitals, clinics, and neighborhood health centers. MD Comput. 1992;9:206–17. [PubMed] [Google Scholar]
- 30.Unutzer J IMPACT Investigators. IMPACT Intervention Manual. Los Angeles, CA: Center for Health Services Research, UCLA Neuropsychiatric Institute; 1999. [Google Scholar]
- 31.Agency for Health Care Policy and Research Depression Guideline Panel. Clinical Practice Guideline No. 5. AHCPR Publication Nos. 93-0550 & 93-0551. Rockville, MD: U.S. Dept of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; 1993. Depression in Primary Care. Volume 1. Detection and Diagnosis; Volume 2. Treatment of Major Depression. [Google Scholar]
- 32.Lebowitz BD, Pearson JL, Schneider LS, Reynolds CF, 3rd, Alexopoulos GS, Bruce ML, Conwell Y, Katz IR, Meyers BS, Morrison MF, Mossey J, Niederehe G, Parmelee P. Diagnosis and treatment of depression in late life. Consensus statement update. JAMA. 1997;278:1186–90. [PubMed] [Google Scholar]
- 33.Hegel MT, Barrett JE, Oxman TE, Mynors-Wallis LM, Gath D. Problem-Solving Treatment for Primary Care (PST-PC): A Treatment Manual for Depression. Hanover, NH: Dartmouth University; 1999. [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale-- preliminary report. Psychopharmacol Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- 36.Katon W, Robinson P, Von Korff M, Lin E, Bush T, Ludman E, Simon G, Walker E. A multifaceted intervention to improve treatment of depression in primary care. Arch Gen Psychiatry. 1996;53:924–32. doi: 10.1001/archpsyc.1996.01830100072009. [DOI] [PubMed] [Google Scholar]
- 37.Katon W, Von Korff M, Lin E, Simon G, Walker E, Unutzer J, Bush T, Russo J, Ludman E. Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Arch Gen Psychiatry. 1999;56:1109–15. doi: 10.1001/archpsyc.56.12.1109. [DOI] [PubMed] [Google Scholar]
- 38.Katon W, Von Korff M, Lin E, Walker E, Simon GE, Bush T, Robinson P, Russo J. Collaborative management to achieve treatment guidelines. Impact on depression in primary care. JAMA. 1995;273:1026–31. [PubMed] [Google Scholar]
- 39.Kroenke K, West SL, Swindle R, Gilsenan A, Eckert GJ, Dolor R, Stang P, Zhou XH, Hays R, Weinberger M. Similar effectiveness of paroxetine, fluoxetine, and sertraline in primary care: a randomized trial. JAMA. 2001;286:2947–55. doi: 10.1001/jama.286.23.2947. [DOI] [PubMed] [Google Scholar]
- 40.Williams JW, Jr, Barrett J, Oxman T, Frank E, Katon W, Sullivan M, Cornell J, Sengupta A. Treatment of dysthymia and minor depression in primary care: A randomized controlled trial in older adults. JAMA. 2000;284:1519–26. doi: 10.1001/jama.284.12.1519. [DOI] [PubMed] [Google Scholar]
- 41.Lee PW, Schulberg HC, Raue PJ, Kroenke K. Concordance between the PHQ-9 and the HSCL-20 in depressed primary care patients. J Affect Disord. 2007;99:139–45. doi: 10.1016/j.jad.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Williams JW, Jr, Stellato CP, Cornell J, Barrett JE. The 13- and 20-item Hopkins Symptom Checklist Depression Scale: psychometric properties in primary care patients with minor depression or dysthymia. International Journal of Psychiatry in Medicine. 2004;34:37–50. doi: 10.2190/U1B0-NKWC-568V-4MAK. [DOI] [PubMed] [Google Scholar]
- 43.O’Connor M, Butcher I, Hansen CH, Kleiboer A, Murray G, Sharma N, Thekkumpurath P, Walker J, Sharpe M. Measuring improvement in depression in cancer patients: a 50% drop on the self-rated SCL-20 compared with a diagnostic interview. Gen Hosp Psychiatry. 2010;32:334–6. doi: 10.1016/j.genhosppsych.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Shiotani I, Sato H, Kinjo K, Nakatani D, Mizuno H, Ohnishi Y, Hishida E, Kijima Y, Hori M Osaka Acute Coronary Insufficiency Study G. Depressive symptoms predict 12-month prognosis in elderly patients with acute myocardial infarction. J Cardiovasc Risk. 2002;9:153–60. doi: 10.1177/174182670200900304. [DOI] [PubMed] [Google Scholar]
- 45.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, de Craen AJ, Knopp RH, Nakamura H, Ridker P, van Domburg R, Deckers JW. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376. doi: 10.1136/bmj.b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallo JJ, Bogner HR, Morales KH, Post EP, Lin JY, Bruce ML. The effect of a primary care practice-based depression intervention on mortality in older adults: a randomized trial. Ann Intern Med. 2007;146:689–98. doi: 10.7326/0003-4819-146-10-200705150-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thombs BD, de Jonge P, Coyne JC, Whooley MA, Frasure-Smith N, Mitchell AJ, Zuidersma M, Eze-Nliam C, Lima BB, Smith CG, Soderlund K, Ziegelstein RC. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA. 2008;300:2161–71. doi: 10.1001/jama.2008.667. [DOI] [PubMed] [Google Scholar]
- 49.Davidson KW, Bigger JT, Burg MM, Carney RM, Chaplin WF, Czajkowski S, Dornelas E, Duer-Hefele J, Frasure-Smith N, Freedland KE, Haas DC, Jaffe AS, Ladapo JA, Lesperance F, Medina V, Newman JD, Osorio GA, Parsons F, Schwartz JE, Shaffer JA, Shapiro PA, Sheps DS, Vaccarino V, Whang W, Ye S. Centralized, stepped, patient preference-based treatment for patients with post-acute coronary syndrome depression: CODIACS Vanguard randomized controlled trial. JAMA Intern Med. 2013:1–8. doi: 10.1001/jamainternmed.2013.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joynt KE, Whellan DJ, O’Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry. 2003;54:248–61. doi: 10.1016/s0006-3223(03)00568-7. [DOI] [PubMed] [Google Scholar]
- 51.Gloaguen V, Cottraux J, Cucherat M, Blackburn IM. A meta-analysis of the effects of cognitive therapy in depressed patients. J Affect Disord. 1998;49:59–72. doi: 10.1016/s0165-0327(97)00199-7. [DOI] [PubMed] [Google Scholar]
- 52.Shimbo D, Davidson KW, Haas DC, Fuster V, Badimon JJ. Negative impact of depression on outcomes in patients with coronary artery disease: mechanisms, treatment considerations, and future directions. J Thromb Haemost. 2005;3:897–908. doi: 10.1111/j.1538-7836.2004.01084.x. [DOI] [PubMed] [Google Scholar]
- 53.Taylor CB, Youngblood ME, Catellier D, Veith RC, Carney RM, Burg MM, Kaufmann PG, Shuster J, Mellman T, Blumenthal JA, Krishnan R, Jaffe AS ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62:792–8. doi: 10.1001/archpsyc.62.7.792. [DOI] [PubMed] [Google Scholar]