Abstract
Background
The influence of different hospital and surgeon volumes on short-term survival after hepatic resection is not clearly clarified. By taking the known prognostic factors into account, the purpose of this study is to assess the combined effects of hospital and surgeon volume on short-term survival after hepatic resection.
Methods
13,159 patients who underwent hepatic resection between 2002 and 2006 were identified in the Taiwan National Health Insurance Research Database. Data were extracted from it and short-term survivals were confirmed through 2006. The Cox proportional hazards model was used to assess the relationship between survival and different hospital, surgeon volume and caseload combinations.
Results
High-volume surgeons in high-volume hospitals had the highest short-term survivals, following by high-volume surgeons in low-volume hospitals, low-volume surgeons in high-volume hospitals and low-volume surgeons in low-volume hospitals. Based on Cox proportional hazard models, although high-volume hospitals and surgeons both showed significant lower risks of short-term mortality at hospital and surgeon level analysis, after combining hospital and surgeon volume into account, high-volume surgeons in high-volume hospitals had significantly better outcomes; the hazard ratio of other three caseload combinations ranging from 1.66 to 2.08 (p<0.001) in 3-month mortality, and 1.28 to 1.58 (p<0.01) in 1-year mortality.
Conclusions
The combined effects of hospital and surgeon volume influenced the short-term survival after hepatic resection largely. After adjusting for the prognostic factors in the case mix, high-volume surgeons in high-volume hospitals had better short-term survivals. Centralization of hepatic resection to few surgeons and hospitals might improve patients’ prognosis.
Introduction
The discussion about the association between volume and mortality after the high-risk surgical procedures is ongoing. High-volume hospitals have better long-term survival rates for some operations [1], [2]. Numerous studies have explored the association between surgeon volume and mortality for certain procedures [3],[4],[5],[6], but few addressed on the relationship between surgeon volume for hepatic resection and short-term mortality [7],[8],[9]. Besides, most volume-outcome relationship studies explored the association between the two at hospital or surgeon level.
Hepatic surgery is not an unusual operation and is now performed at many hospitals worldwide [10]. In Asia, hepatocellular carcinoma (HCC) has a high prevalence [11]. Due to donor shortage remains a main problem in Asia, hepatic resection being considered the first line curative methods for some patients with HCC [12],[13]. In addition, intra-hepatic cholangiocarcinoma, metastatic malignancies, and benign diseases, such as, trauma, intra-hepatic bile duct stone and benign tumors, also require hepatic resection [10].
However, a general limitation of most of the available studies, and those addressing survival after hepatic resection, is the lack of important prognostic factors, such as, indication for surgery, comorbidities, hepatitis/cirrhosis and cirrhosis-related complications and the combined effect of hospital and surgeon volume, which makes it difficult to take into account the relative effects of these correlated factors [7],[8],[9].
Therefore, by taking the known prognostic factors into consideration, the purpose of this study was to explore the combined effects of hospital and surgeon volume and the association between different surgeon volumes in high- and in low-volume hospitals in relation to short-term survival after hepatic resection by using a population-based national database of Taiwan patients between 2002 and 2006.
Materials and Methods
Ethics Statements
This study was initiated after being approved by the Institutional Review Board of the Buddhist Dalin Tzu Chi General Hospital, Taiwan. Because the identification numbers and personal information of the individuals included in the study were not included in the secondary files, the review board stated that written consent from patients was not required.
Patients and Study Design
We used data between 2002 and 2006 from the National Health Insurance (NHI) Research Database, which covered medical benefit claims for over 23 million people in Taiwan (approximately 99 percent of Taiwan’s population) [14]. Taiwan’s NHI has the characteristics of universal insurance coverage, providing comprehensive services, and a single-payer system with the government as sole insurer. The database was monitored for completeness and accuracy by Taiwan’s Department of Health. Patients who underwent hepatic resection for cancer disease (HCC, cholangiocarcinoma, metastatic malignancy), and benign disease (eg, trauma, intra-hepatic bile duct stone, benign tumors) between 2002 and 2006 were included. A total of 13,159 patients were identified. The mortality was identified from the National Register of Deaths Database. Hospitals and surgeons were sorted using similar methods as previous studies [15],[16]. The method for defining high and low hospital and surgeon volume of hepatic resection was: (1) Hospitals were categorized by their total patient volume by using unique hospital identifiers in this database. The 13,159 patients were sorted into two approximately equal groups based on the cumulative hospital volume of hepatic resections performed [7],[8]. The cumulative hospital volume of 245 and more cases was defined as high-volume hospital. By this definition, there were 13 high-volume centers for hepatic resection. (Table S1 in Appendix S1). (2) Surgeons were categorized by their total patient volume by using unique identifiers in this database. We initially divided patients into two approximately equal groups by cumulative surgeon volume, but many surgeons in Taiwan performed few hepatic resections annually, the volume cutoff was low. Surgeon volume cutoff was therefore chosen as roughly one-third of all patients undergoing hepatic resection by high-volume surgeons [9]. The cumulative surgeon volume of 25 and more cases was defined as high-volume surgeon. By this definition, there were 59 high-volume surgeons. (Table S2 in Appendix S1).
Measurements
The key dependent variable of interest was 30-day, 3-month and 1-year survival of these patients. Mortality was the outcome measure. Overall mortality included all causes of death occurring after the surgery. The key independent variables were the hospital volume, surgeon volume and the combination of surgeon and hospital volume, which were sorted into four groups based on volume (high-volume surgeons in high-volume hospitals, low-volume surgeons in high-volume hospitals, high-volume surgeons in low-volume hospitals, and low-volume surgeons in low-volume hospitals). Patient demographics included age, gender, indication for surgery, comorbidity, surgical procedure (major: lobectomy or more; minor:<lobectomy), and individual socioeconomic status (SES), geographic region, and urbanization level of residence. The comorbidities included hypertension, ischemic heart disease, arrhythmia, cerebrovascular disease, chronic obstructive pulmonary disease and associated conditions including chronic bronchitis and asthma, renal disease, and diabetes. Patients’ severity of underlying liver disease was evaluated by presence or absence of hepatitis/cirrhosis and cirrhosis-related complications. The cirrhosis-related complications included portal hypertension, esophageal/gastric varices bleeding, ascites, pleural effusion, encephalopathy and hepatorenal syndrome. We identified the presence or absence of these comorbidities and cirrhosis-related complications for each patient by querying the Taiwan NHI database using the International Classification of Diseases 9 codes (ICD-9). Any cirrhosis-related complication existed before the admission for surgery was defined as pre-operative cirrhosis-related complication. It was identified from the ICD-9 codes for inpatients within 6 months before surgery. Post-operative cirrhosis-related complication was identified from the ICD-9 codes for each discharge from surgery in the cohort.
This study used income-related insurance payment amount as a proxy measure of individual SES, which is an important prognostic factor for survival [17],[18]. The individuals were classified into three groups: (1) low SES, lower than US$528 per month (New Taiwan Dollars (NT) 0, $1 to $15,840), (2) moderate SES, between US$528 to $833 per month (NT $15,841 to $25,000), and (3) high SES, US$833 per month (NT $25,001) or more [19]. We selected NT$15,840 as the low income level cutoff point because this was the government-stipulated minimum wage for full-time employees in Taiwan from 2002 to 2006.
Statistical Analysis
The SAS statistical package (version 9.2; SAS Institute, Inc., Cary, N.C.) and SPSS (version 15, SPSS Inc., Chicago, IL, USA) were used to analyze the data. A p-value of P<0.05 was used to determine statistical significance. The cumulative 30-day, 3-month, and 1-year survival rates and the survival curves were constructed and compared using a log-rank test. Survival was measured from the time after hepatic resection by using overall death as censoring variables. The Cox proportional hazard regression model was used to assess the hospital and the surgeon volume and the combined effects of surgeon and hospital volume on short-term survival after adjusting for patient demographic variables.
Results
Of all hepatic resections between 2002 and 2006 in Taiwan, 61.2% was for HCC, 25.2% for benign disease, 8.8% for metastatic malignancy and 4.8% for cholangiocarcinoma. Patients’ characteristics are summarized in Table 1. Among these patients, 29.1% of them were operated by high-volume surgeons in high-volume hospitals, 23.1% by low-volume surgeons in high-volume hospitals, 7.7% by high-volume surgeons in low-volume hospitals and 40.1% by low-volume surgeons in low-volume hospitals.
Table 1. Baseline characteristics according to hospital volume and surgeon volume (n = 13159).
| Variable | High-volume hospital | Low-volume hospital | P value | ||||||
| High-volumesurgeon (n = 3830) | Low-volume surgeon (n = 3035) | High-volume surgeon (n = 1010) | Low-volume surgeon (n = 5284) | ||||||
| n (%) | n (%) | n (%) | n (%) | ||||||
| Age, years (mean ±SD) | 55.06±13.95 | 56.56±15.08 | 57.17±12.96 | 58.00±14.50 | <0.001 | ||||
| Gender | 0.002 | ||||||||
| Male | 2561 | (66.9) | 1926 | (63.5) | 637 | (63.1) | 3347 | (63.3) | |
| Female | 1269 | (33.1) | 1109 | (36.5) | 373 | (36.9) | 1937 | (36.7) | |
| Indication for surgery | <0.001 | ||||||||
| Hepatocellular carcinoma | 2667 | (69.6) | 1718 | (56.6) | 621 | (61.5) | 3046 | (57.6) | |
| Cholangiocarcinoma | 166 | (4.3) | 143 | (4.7) | 51 | (5.0) | 276 | (5.2) | |
| Metastatic malignancy | 159 | (4.2) | 441 | (14.5) | 74 | (7.3) | 486 | (9.2) | |
| Benign disease | 838 | (21.9) | 733 | (24.2) | 264 | (26.1) | 1476 | (27.9) | |
| Surgical procedure | 0.007 | ||||||||
| Lobectomy or more | 983 | (25.7) | 732 | (24.1) | 232 | (23.0) | 1423 | (26.9) | |
| < Lobectomy | 2847 | (74.3) | 2303 | (75.9) | 778 | (77.0) | 3861 | (73.1) | |
| With hepatitis/cirrhosis | 1629 | (42.5) | 1113 | (36.7) | 423 | (41.9) | 1992 | (37.7) | <0.001 |
| Pre-operative cirrhosis-related complication | 352 | (9.2) | 281 | (9.3) | 90 | (8.9) | 679 | (12.9) | <0.001 |
| Post-operative cirrhosis-related complication | 166 | (4.3) | 140 | (4.6) | 47 | (4.7) | 331 | (6.3) | <0.001 |
| Comorbidity | |||||||||
| Hypertension | 440 | (11.5) | 433 | (14.3) | 135 | (13.4) | 725 | (13.7) | 0.003 |
| Ischemic heart disease | 38 | (1.0) | 52 | (1.7) | 12 | (1.2) | 95 | (1.8) | 0.009 |
| Arrhythmia | 33 | (0.9) | 34 | (1.1) | 5 | (0.5) | 68 | (1.3) | 0.066 |
| Heart failure | 5 | (0.1) | 18 | (0.6) | 3 | (0.3) | 24 | (0.5) | 0.012 |
| Cerebrovascular disease | 14 | (0.4) | 23 | (0.8) | 6 | (0.6) | 58 | (1.1) | 0.001 |
| COPD and associated condition | 30 | (0.8) | 31 | (1.0) | 8 | (0.8) | 69 | (1.3) | 0.085 |
| Renal disease | 43 | (1.1) | 83 | (2.7) | 18 | (1.8) | 147 | (2.8) | <0.001 |
| Diabetes | 364 | (9.5) | 384 | (12.7) | 125 | (12.4) | 733 | (13.9) | <0.001 |
| Socioeconomic status | <0.001 | ||||||||
| High | 1051 | (27.4) | 651 | (21.4) | 220 | (21.8) | 911 | (17.2) | |
| Moderate | 1439 | (37.6) | 1163 | (38.3) | 433 | (42.9) | 2308 | (43.7) | |
| Low | 1340 | (35.0) | 1221 | (40.2) | 357 | (35.3) | 2065 | (39.1) | |
| Geographic region | <0.001 | ||||||||
| Northern | 1872 | (48.9) | 1393 | (45.9) | 413 | (40.9) | 2311 | (43.7) | |
| Central | 730 | (19.1) | 591 | (19.7) | 185 | (18.3) | 922 | (17.4) | |
| Southern/Eastern | 1228 | (32.1) | 1045 | (34.4) | 412 | (40.8) | 2051 | (38.8) | |
| Urbanization level of residence | <0.001 | ||||||||
| Urban | 1212 | (31.6) | 1013 | (33.4) | 298 | (29.5) | 1188 | (22.5) | |
| Suburban | 1586 | (41.4) | 1253 | (41.3) | 421 | (41.7) | 2254 | (42.7) | |
| Rural | 1032 | (26.9) | 769 | (25.3) | 291 | (28.8) | 1842 | (34.9) | |
Abbreviation: SD, standard deviation; COPD, chronic obstructive pulmonary disease.
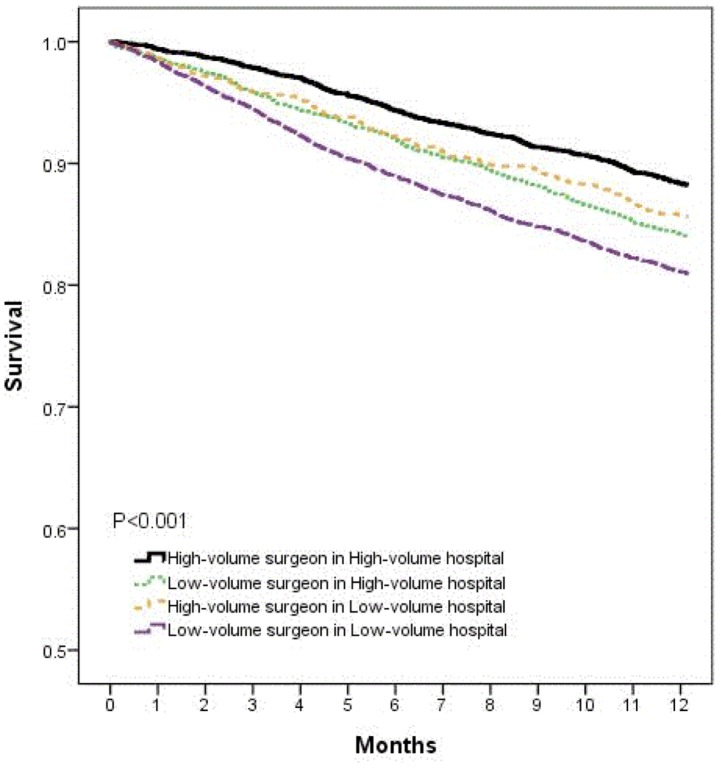
Figure 1 shows the Kaplan-Meier survival probabilities after hepatic resection in each volume group. The high-volume surgeon in high-volume hospital had the highest survival and the low-volume surgeon in low-volume hospital had the lowest.
Figure 1. Short-term survival after hepatic resection by combined effect of surgeon and hospital volume.
Table 2 shows the adjusted hazard ratios based on the Cox proportional hazards regression models after adjusting for patients’ age, gender, indication for surgery, surgical procedure, comorbidity, hepatitis/cirrhosis status, presence of pre-operative and post-operative cirrhosis-related complication, socioeconomic status, geographic region, and urbanization level of residence. In model 1, examining the relationships between hospital volume and short-term survival; after adjusting for other factors, low-volume hospitals had the hazard ratio of 1.50 (95% CI, 1.09–2.07) in 30-day mortality, 1.56 (95% CI, 1.30–1.86) in 3-month mortality and 1.33 (95% CI, 1.21–1.46) in 1-year mortality compared to high-volume hospitals. In model 2, after adjusting for other factors, the relationships between surgeon volume and short-term survival showed that low-volume surgeons had the hazard ratio of 1.64 (95% CI, 1.12–2.41) in 30-day mortality, 1.62 (95% CI, 1.31–2.00) in 3-month mortality and 1.41 (95% CI, 1.27–1.56) in 1-year mortality compared to high volume surgeons. In model 3, examining the combined effects of hospital and surgeon volume, after adjusting for other factors, low-volume surgeons in high- and in low-volume hospitals were associated with higher 30-day mortality; the hazard ratios were 1.81 (95% CI, 1.06–3.08) and 2.15 (95% CI, 1.33–3.46) respectively compared to high-volume surgeons in high-volume hospitals. In 3-month mortality, patients not operated by high-volume surgeons in high-volume hospitals had the hazard ratios ranging from 1.66 to 2.08 (p<0.001). In 1-year mortality, patients not operated by high-volume surgeons in high-volume hospitals had the hazard ratios ranging from 1.28 to 1.58 (p<0.01).
Table 2. The adjusted hazard ratios of provider categories in different regression models.
| Variable | 30-day mortality risk | 3- month mortality risk | 1-year mortality risk | ||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Model 1 | |||||||||
| High-volume hospital | 1 | 1 | 1 | ||||||
| Low-volume hospital | 1.50 | 1.09–2.07 | 0.013 | 1.56 | 1.30–1.86 | <0.001 | 1.33 | 1.21–1.46 | <0.001 |
| Model 2 | |||||||||
| High-volume surgeon | 1 | 1 | 1 | ||||||
| Low-volume surgeon | 1.64 | 1.12–2.41 | 0.010 | 1.62 | 1.31–2.00 | <0.001 | 1.41 | 1.27–1.56 | <0.001 |
| Model 3 | |||||||||
| High-volume surgeon inHigh-volume hospital | 1 | 1 | 1 | ||||||
| Low-volume surgeon inHigh-volume hospital | 1.81 | 1.06–3.08 | 0.028 | 1.66 | 1.24–2.22 | 0.001 | 1.33 | 1.16–1.53 | <0.001 |
| High-volume surgeon inLow-volume hospital | 1.98 | 0.99–3.96 | 0.051 | 1.90 | 1.30–2.79 | 0.001 | 1.28 | 1.05–1.56 | 0.013 |
| Low-volume surgeon inLow-volume hospital | 2.15 | 1.33–3.46 | 0.002 | 2.08 | 1.61–2.69 | <0.001 | 1.58 | 1.40–1.78 | <0.001 |
Abbreviation: HR, hazard ratio; 95% CI, 95% confidence interval.
Adjusted for patients’ age, gender, indication for surgery, surgical procedure, comorbidity, hepatitis/cirrhosis, pre-and post-operative cirrhosis-related complication, socioeconomic status, geographic region and urbanization level of residence.
Table 3 demonstrates other factors associated with short-term survival at three different time points after hepatic resection. Those had a negative influence on survival included increased age, undergoing major surgical procedure (lobectomy or more), presence of cirrhosis-related complication, ischemic heart disease, renal disease and with a low SES. Cholangiocarcinoma and metastatic malignancy had a poor outcome than HCC.
Table 3. The adjusted hazard ratios of patient demographic variables.
| Variable | 30-day mortality risk | 3- month mortality risk | 1-year mortality risk | ||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Age, year | 1.00 | 0.99–1.01 | 0.541 | 1.01 | 1.00–1.02 | 0.001 | 1.008 | 1.004–1.01 | <0.001 |
| Gender | |||||||||
| Male | 1 | 1 | 1 | ||||||
| Female | 0.94 | 0.67–1.32 | 0.728 | 1.04 | 0.86–1.26 | 0.640 | 1.05 | 0.95–1.16 | 0.306 |
| Indication for surgery | |||||||||
| Hepatocellular carcinoma | 1 | 1 | 1 | ||||||
| Cholangiocarcinoma | 0.89 | 0.45–1.76 | 0.743 | 1.52 | 1.13–2.06 | 0.005 | 2.06 | 1.78–2.40 | <0.001 |
| Metastatic malignancy | 1.20 | 0.65–2.19 | 0.556 | 1.34 | 0.98–1.82 | 0.060 | 1.34 | 1.14–1.56 | <0.001 |
| Benign disease | 0.88 | 0.56–1.38 | 0.599 | 0.49 | 0.36–0.66 | <0.001 | 0.27 | 0.22–0.32 | <0.001 |
| Surgical treatment | |||||||||
| Lobectomy or more | 1 | 1 | 1 | ||||||
| < Lobectomy | 0.66 | 0.47–0.93 | 0.019 | 0.71 | 0.59–0.86 | 0.001 | 0.64 | 0.58–0.71 | <0.001 |
| With hepatitis/cirrhosis | 1.29 | 0.90–1.84 | 0.160 | 1.01 | 0.83–1.23 | 0.876 | 0.92 | 0.83–1.02 | 0.156 |
| Pre-operative cirrhosis-related complication | 0.44 | 0.16–1.20 | 0.110 | 3.11 | 2.43–3.98 | <0.001 | 5.93 | 5.29–6.64 | <0.001 |
| Post-operative cirrhosis-related complication | 7.22 | 2.53–20.61 | <0.001 | 1.32 | 0.98–1.79 | 0.065 | 0.44 | 0.37–5.33 | <0.001 |
| Comorbidity | |||||||||
| Hypertension | 0.85 | 0.51–1.41 | 0.536 | 0.63 | 0.47–0.85 | 0.003 | 0.71 | 0.61–0.83 | <0.001 |
| Ischemic heart disease | 3.87 | 1.99–7.52 | <0.001 | 2.73 | 1.76–4.22 | <0.001 | 1.67 | 1.24–2.26 | 0.001 |
| Arrhythmia | 1.87 | 0.68–5.16 | 0.224 | 0.92 | 0.43–1.96 | 0.842 | 0.76 | 0.49–1.18 | 0.226 |
| Heart failure | 1.59 | 0.48–5.21 | 0.440 | 1.95 | 0.98–3.88 | 0.055 | 1.83 | 1.05–3.19 | 0.031 |
| Cerebrovascular disease | 1.57 | 0.38–6.41 | 0.527 | 2.77 | 1.51–5.07 | 0.001 | 1.43 | 0.91–2.27 | 0.118 |
| COPD and associated condition | 2.67 | 0.97–7.32 | 0.056 | 1.02 | 0.48–2.16 | 0.956 | 1.24 | 0.85–1.81 | 0.258 |
| Renal disease | 8.96 | 5.95–13.47 | <0.001 | 5.11 | 3.89–6.70 | <0.001 | 2.62 | 2.14–3.21 | <0.001 |
| Diabetes | 0.91 | 0.56–1.45 | 0.694 | 1.05 | 0.82–1.35 | 0.684 | 0.92 | 0.80–1.06 | 0.306 |
| Socioeconomic status | |||||||||
| Low | 1 | 1 | 1 | ||||||
| Moderate | 0.78 | 0.55–1.11 | 0.183 | 1.05 | 0.87–1.29 | 0.569 | 1.14 | 1.02–1.27 | 0.014 |
| High | 0.40 | 0.22–0.73 | 0.003 | 0.42 | 0.30–0.59 | <0.001 | 0.77 | 0.67–0.89 | 0.001 |
| Geographic region | |||||||||
| Northern | 1 | 1 | 1 | ||||||
| Central | 1.65 | 1.08–2.51 | 0.019 | 1.18 | 0.92–1.50 | 0.174 | 1.05 | 0.93–1.20 | 0.389 |
| Southern/Eastern | 0.91 | 0.61–1.35 | 0.647 | 0.85 | 0.68–1.05 | 0.137 | 0.89 | 0.80–1.01 | 0.060 |
| Urbanization level of residence | |||||||||
| Urban | 1 | 1 | 1 | ||||||
| Suburban | 1.11 | 0.72–1.71 | 0.633 | 0.89 | 0.71–1.12 | 0.337 | 0.96 | 0.85–1.09 | 0.586 |
| Rural | 1.49 | 0.93–2.37 | 0.091 | 0.99 | 0.77–1.28 | 0.987 | 1.04 | 0.91–1.19 | 0.500 |
Abbreviation: HR, hazard ratio; 95% CI, 95% confidence interval; COPD, chronic obstructive pulmonary disease.
Discussion
The current study from a national database identifying 13,159 hepatic resections between 2002 and 2006 in Taiwan revealed that short-term survival after hepatic resection was largely influenced by the combined effects of hospital and surgeon volume. Surgeon volume had a significant impact on 30-day mortality, high-volume surgeons in high- and in low-volume hospitals demonstrated better outcomes than low-volume surgeons. When looking at 3-month and 1-year mortality, high-volume surgeons in high-volume hospitals demonstrated a significantly better outcome than other three.
The strength of this study are based on the fact that it was a nationwide population-based cross-sectional study, include almost all patients undergoing hepatic resection in Taiwan. At the end of 2006, the NHI covered 99.0% of Taiwan’s population [14], with nearly complete follow-up information of mortality among the whole study population, as well as the fact that the dataset was routinely monitored for diagnostic accuracy by the National Health Insurance Bureau of Taiwan. The current study not only took most of the known prognostic factors into account but also analyzed short-term survival at three time points after hepatic resection. Furthermore, hepatic resection had not been centralized in Taiwan to a significant degree during the study period, which allowed for investigation of the effect of volume.
Patient-related difference is important in the volume-outcome relationship study. Some studies revealed the minority, older, and low SES patients are more likely to be treated at low-volume hospitals [20],[21]. And there is a negative association between SES and cancer survival rate [22],[23],[24]. A study from the United States also revealed that patients who underwent cancer operations for lung, esophagus, and pancreas tumors with more comorbidities were more likely to receive their cancer surgery at low-volume hospitals [25]. Although these trends were also seen in this current study, several of these variables were associated with increased short-term mortality and were entered into the multivariate analysis. After adjusting for case-mix in this fashion, there were no changes in the trends for mortality that low-volume providers had inferior outcomes.
A great body of literature has addressed the relation between hospital volume for hepatic resection and mortality. Most previous studies reported a substantial inverse relation between hospital volume and mortality, but the roll of the surgeon volume was not fully established yet. A study from the United States reported that the surgeon volume for hepatic resection was not associated with in-hospital mortality [9], but the present study found contradictory results. In that study, it did not adjust for the known prognostic factors, eg, indication for surgery, hepatitis/cirrhosis, cirrhosis-related complications, socioeconomic status, and lacked an analysis for the combined effect of hospital and surgeon volume.
In agree with previous studies of volume-outcome relationship in some other procedures, surgeon volume was inversely associated with short-term mortality [3],[4],[6]. However, this association did not remain in 3-month and 1-year mortality after hepatic resection for high-volume surgeons in low-volume hospitals after taking hospital volume into account. This finding indicates that hospital-volume factors, eg, more specialties for perioperative care of liver diseases, skilled nursing staff and developed intensive care units, account for the effect of surgeon volume on short-term mortality, might influence the risk of mortality after hepatic resection.
In line with previous studies, high-volume hospital or high-volume surgeon was a predictor of survival [7],[8],[9]. In the present study, it provides an additional insight on the volume-outcome relationship based on the combination of surgeon and hospital volume that high-volume surgeons in high-volume hospitals was a predictor of short-term survival after hepatic resection; high-volume surgeons in low-volume hospitals as well as low-volume surgeons in high-volume hospitals were not. Nevertheless, the positive association remained in high-volume surgeons when comparing with low-volume surgeons. These results indicated the effect of surgeon volume on patients’ short-term survival after hepatic resection. Surgeons should maintain a higher volume of hepatic resections and increase experience for a better outcome.
It also has been suggested that the referral of patients to high-volume hospitals will provide a better application of recommended processes of care [2],[26]. In the present study, patients who underwent hepatic resection by low-volume surgeons in high-volume hospitals had an increased risk of mortality compared to those who underwent hepatic resection by high-volume surgeons in high-volume hospitals. Our finding suggests that in addition to referring patients to a high-volume hospital, referring patients to a high-volume surgeon in high-volume hospital may be another important consideration.
There are some limitations to this study. First, diagnosis and any comorbidity were completely dependent on ICD codes, any coding errors in patients’ underlying diseases could lead to disparities in comorbidity and cirrhosis-related complication. Nonetheless, the National Health Insurance Bureau of Taiwan randomly reviews the charts and interviews patients in order to verify diagnostic accuracy [27]. Second, instead of surgical mortality, the all-cause mortality was used. But the short-term surgical and all-cause mortality differ only to a small degree and it is unlikely that the differences would be systemically different for high- and low-volume providers. Third, the cutoff used for assessing surgeon volume can be considered a little low; however, they are comparable to those of previous large studies that addressed hospital and surgeon volume from the United States [7],[8],[28].
In summary, our findings provide supports for the combined effects of hospital and surgeon volume with regard to short-term survival after hepatic resection. This population-based study revealed that short-term survival after hepatic resection was largely influenced by the combined effects of hospital and surgeon volume. There was a clear association between high-volume surgeons in high-volume hospitals and better short-term survival. Centralization of hepatic resection to few surgeons and hospitals might improve patients’ prognosis.
Supporting Information
The process of defining the hospital and the surgeon volume. Table S1, defining the category of hospital volume. Table S2, defining the category of surgeon volume.
(DOC)
Acknowledgments
This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by the National Health Research Institutes (Registered number 101115). The interpretation and conclusions contained herein do not represent the opinions of the Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
Funding Statement
The authors have no support or funding to report.
References
- 1. Birkmeyer JD, Sun Y, Wong SL, Stukel TA (2007) Hospital volume and late survival after cancer surgery. Ann Surg 245: 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fong Y, Gonen M, Rubin D, Radzyner M, Brennan MF (2005) Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg 242: 540–544 discussion 544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hannan EL, Radzyner M, Rubin D, Dougherty J, Brennan MF (2002) The influence of hospital and surgeon volume on in-hospital mortality for colectomy, gastrectomy, and lung lobectomy in patients with cancer. Surgery 131: 6–15. [DOI] [PubMed] [Google Scholar]
- 4. Harmon JW, Tang DG, Gordon TA, Bowman HM, Choti MA, et al. (1999) Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg 230: 404–411 discussion 411–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hannan EL, Siu AL, Kumar D, Kilburn H Jr, Chassin MR (1995) The decline in coronary artery bypass graft surgery mortality in New York State. The role of surgeon volume. JAMA 273: 209–213. [PubMed] [Google Scholar]
- 6. Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, et al. (2003) Surgeon volume and operative mortality in the United States. N Engl J Med 349: 2117–2127. [DOI] [PubMed] [Google Scholar]
- 7. Glasgow RE, Showstack JA, Katz PP, Corvera CU, Warren RS, et al. (1999) The relationship between hospital volume and outcomes of hepatic resection for hepatocellular carcinoma. Arch Surg 134: 30–35. [DOI] [PubMed] [Google Scholar]
- 8. Dimick JB, Cowan JA Jr, Knol JA, Upchurch GR Jr (2003) Hepatic resection in the United States: indications, outcomes, and hospital procedural volumes from a nationally representative database. Arch Surg 138: 185–191. [DOI] [PubMed] [Google Scholar]
- 9. Nathan H, Cameron JL, Choti MA, Schulick RD, Pawlik TM (2009) The Volume-Outcomes Effect in Hepato-Pancreato-Biliary Surgery: Hospital Versus Surgeon Contributions and Specificity of the Relationship. J Am Coll Surg 208: 528–538. [DOI] [PubMed] [Google Scholar]
- 10. Dimick JB, Wainess RM, Cowan JA, Upchurch Jr GR, Knol JA, et al. (2004) National Trends in the Use and Outcomes of Hepatic Resection. J Am Coll Surg 199: 31–38. [DOI] [PubMed] [Google Scholar]
- 11. Tanaka M, Katayama F, Kato H, Tanaka H, Wang J, et al. (2011) Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures. J Epidemiol 21: 401–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, et al. (1996) Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334: 693–699. [DOI] [PubMed] [Google Scholar]
- 13. Bigourdan JM, Jaeck D, Meyer N, Meyer C, Oussoultzoglou E, et al. (2003) Small hepatocellular carcinoma in Child A cirrhotic patients: hepatic resection versus transplantation. Liver Transpl 9: 513–520. [DOI] [PubMed] [Google Scholar]
- 14.National Health Insurance Administration. Minister of Health and Welfare website. Available: http://www.nhi.gov.tw/webdata/webdata.aspx?menu=17&menu_id=1023&WD_ID=1043&webdata_id=1359. Accessed 2013 Nov 18.
- 15. Lin CC, Lin HC (2008) Effects of surgeon and hospital volume on 5-year survival rates following oral cancer resections: the experience of an Asian country. Surgery 143: 343–351. [DOI] [PubMed] [Google Scholar]
- 16. Chang CM, Huang KY, Hsu TW, Su YC, Yang WZ, et al. (2012) Multivariate analyses to assess the effects of surgeon and hospital volume on cancer survival rates: a nationwide population-based study in taiwan. PLoS One 7: e40590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Braaten T, Weiderpass E, Lund E (2009) Socioeconomic differences in cancer survival: the Norwegian Women and Cancer Study. BMC Public Health 9: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kwok J, Langevin SM, Argiris A, Grandis JR, Gooding WE, et al. (2010) The impact of health insurance status on the survival of patients with head and neck cancer. Cancer 116: 476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin HC, Chao PZ, Lee HC (2008) Sudden sensorineural hearing loss increases the risk of stroke: a 5-year follow-up study. Stroke 39: 2744–2748. [DOI] [PubMed] [Google Scholar]
- 20. Liu JH, Zingmond DS, McGory ML, SooHoo NF, Ettner SL, et al. (2006) Disparities in the utilization of high-volume hospitals for complex surgery. JAMA 296: 1973–1980. [DOI] [PubMed] [Google Scholar]
- 21. Epstein AJ, Gray BH, Schlesinger M (2011) Racial and ethnic differences in the use of high-volume hospitals and surgeons. Arch Surg 145: 179–186. [DOI] [PubMed] [Google Scholar]
- 22. Boyd C, Zhang-Salomons JY, Groome PA, Mackillop WJ (1999) Associations between community income and cancer survival in Ontario, Canada, and the United States. J Clin Oncol 17: 2244–2255. [DOI] [PubMed] [Google Scholar]
- 23. Mackillop WJ, Zhang-Salomons J, Groome PA, Paszat L, Holowaty E (1997) Socioeconomic status and cancer survival in Ontario. J Clin Oncol 15: 1680–1689. [DOI] [PubMed] [Google Scholar]
- 24. Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, et al. (2006) Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol 24: 1342–1349. [DOI] [PubMed] [Google Scholar]
- 25. Al-Refaie WB, Muluneh B, Zhong W, Parsons HM, Tuttle TM, et al. (2011) Who receives their complex cancer surgery at low-volume hospitals? J Am Coll Surg 214: 81–87. [DOI] [PubMed] [Google Scholar]
- 26. Gruen RL, Pitt V, Green S, Parkhill A, Campbell D, et al. (2009) The effect of provider case volume on cancer mortality: systematic review and meta-analysis. CA Cancer J Clin 59: 192–211. [DOI] [PubMed] [Google Scholar]
- 27.National Health Insurance Administration. Minister of Health and Welfare website. Available: http://www.nhi.gov.tw/information/bulletin_file/421_0890036465-19.doc. Accessed 2006 May 2.
- 28. Scarborough JE, Pietrobon R, Clary BM, Marroquin CE, Bennett KM, et al. (2008) Regionalization of Hepatic Resections Is Associated with Increasing Disparities among Some Patient Populations in Use of High-Volume Providers. J Am Coll Surg 207: 831–838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The process of defining the hospital and the surgeon volume. Table S1, defining the category of hospital volume. Table S2, defining the category of surgeon volume.
(DOC)