Abstract
White-nose Syndrome (WNS) is an emerging infectious mycosis that has impacted multiple species of North American bats since its initial discovery in 2006, yet the physiology of the causal agent, the psychrophilic fungus Pseudogymnoascus destructans ( = Geomyces destructans), is not well understood. We investigated the ability of P. destructans to secrete enzymes that could permit environmental growth or affect pathogenesis and compared enzyme activity across several Pseudogymnoascus species isolated from both hibernating bats and cave sediments. We found that P. destructans produced enzymes that could be beneficial in either a pathogenic or saprotrophic context, such as lipases, hemolysins, and urease, as well as chitinase and cellulases, which could aid in saprotrophic growth. The WNS pathogen showed significantly lower activity for urease and endoglucanase compared to con-generic species (Pseudogymnoascus), which may indicate a shift in selective pressure to the detriment of P. destructans’ saprotrophic ability. Based on the positive function of multiple saprotrophic enzymes, the causal agent of White-nose Syndrome shows potential for environmental growth on a variety of substrates found in caves, albeit at a reduced level compared to environmental strains. Our data suggest that if P. destructans emerged as an opportunistic infection from an environmental source, co-evolution with its host may have led to a reduced capacity for saprotrophic growth.
Introduction
White-nose Syndrome is a fungal disease that has killed millions of hibernating bats in North America since its discovery in 2006 [1], [2]. The causal agent of WNS was identified as a novel fungal pathogen, Geomyces destructans [1], [3], [4], which was recently reclassified into the genus Pseudogymnoascus (Pseudeurotiaceae, incertae sedis, Leotiomycetes) [5]. While important details of physiology in this organism have been established such as growth temperature [6] and its ability to invade wing tissue [7], [8], many important aspects of its disease ecology remain unknown.
Invasive fungal pathogens are devastating plant and animal populations across the globe, and understanding their ecology is crucial in predicting disease outcomes [9]. The ability to carry out saprotrophic growth outside a host has important implications for disease management in emergent mycoses; models of host-pathogen dynamics indicate that high levels of saprotrophic growth can lead to host extinction [9]. For example, the emergent amphibian mycosis Batrachochytryium dendrobatidis (Bd) may be capable of growth outside its host, as it can be cultured on multiple carbon and nitrogen sources [10], including feathers [11]. Although definitive evidence of Bd environmental growth has not been obtained, models of the Bd infection cycle indicate that in addition to spore persistence, environmental growth could increase the risk of local host extinction [12]. The saprotrophic growth of P. destructans could permit year-round growth in caves, increasing both the chance of infection of naïve bats from an environmental reservoir, for which anecdotal evidence already exists (A. Hicks, pers. comm. 2011), and the risk of spread of this pathogen to new caves through contaminated sediment. Lorch et al. 2013 [13] found Pd DNA and viable Pd spores in cave sediments after the departure of the WNS-infected bat hosts; however, it is unknown whether the presence of Pd in these sediments is due to environmental propagation or to the persistence of spores shed from infected hosts. If the latter were the case, an obligate bat pathogen would be limited to growth in the hibernation season when bat body temperatures during torpor permit fungal growth, reducing the likelihood of environmental spread.
Current evidence suggests that P. destructans may be a facultative pathogen; it can grow on a variety of laboratory media, while obligate pathogens often cannot be cultured outside of their hosts on such media [4], [14]. While the current evidence is consistent with an introduction from Europe, the caves in which North American bats hibernate contain diverse, presumably native Pseudogymnoascus and Geomyces spp. [5], [16]–[19]. However, their role in the cave environment remains unknown [17], [19]. The partially annotated P. destructans genome indicates the presence of numerous genes coding for saprotrophic enzymes, including cellulases and chitinases [15]. If these enzymes confer a functional phenotype, they could allow P. destructans to grow saprotrophically in cave microhabitats and provide an environmental reservoir for infection of susceptible hosts. Questions regarding saprotrophic Pseudogymnoascus enzyme activity and how it compares to the activity of P. destructans may not only indicate the potential of an environmental reservoir for White-nose Syndrome, but may help us understand the natural history of this pathogen.
In this research, we examined enzymes that could play a role in fungal growth on various cave microhabitats, some of which could also be exploited in pathogenesis. We compared enzyme activity of P. destructans with that of a suite of Pseudogymnoascus spp. isolated from cave environments and the close relative P. pannorum var. pannorum. As additional controls, we included the established saprotroph Penicillium pinophilum (Trichocomaceae, Eurotiales, Eurotiomycetes), and Oidiodendron maius (Myxotrichaceae, incertae sedis, Leotiomycetes), which can form mutualistic associations with ericoid plants and has also been found as a saprobe in multiple locales [20], including cave sediments [16]. Our results indicate that P. destructans produces several enzymes that could permit environmental growth and support pathogenesis, but that P. destructans shows lower saprotrophic potential than closely related environmental isolates.
Materials and Methods
Cultures and Growth Conditions
Unless otherwise noted, all reagents were purchased from Sigma Aldrich (St. Louis, MO). Pseudogymnoascus species were isolated from Cudjo’s Cave, Cumberland Gap National Historic Park (NPS collection permit # CUGA-2009-SCI-0007) by swabbing hibernating little brown bats (Myotis lucifugus) and cave surfaces with pre-moistened (dH2O) sterile cotton swabs. This was followed by swabbing on Emmon’s modification of Sabouraud dextrose agar (SDA) augmented with cycloheximide (100 mg/L) [21]. Cultures were incubated at 5°C to select for psycrophilic and psychrotolerant Pseudogymnoascus species, and a library of 668 isolates was generated. Preliminary identification was performed using colony morphology and microscopy, and PCR amplification and sequencing of the internal transcribed sequence (ITS), which is considered the most robust genetic sequence for phylogenetic inference at the species level in the fungi [22], was used to confirm the identity of putative Pseudogymnoascus spp. and to assess the phylogenetic diversity of strains used in physiological assays. The ZR Soil Microbe DNA Miniprep Kit (Zymo Research, Irvine, CA) was used to extract DNA from cultures. Samples were prepared for PCR using 20–50 ng of template genomic DNA, 2X Taq Master Mix (New England Biolabs, Ipswich, MA), and 500 nM each of the primers ITS5 and ITS4 in a total volume of 20 µL [23]. PCR conditions were: initial denaturation at 94°C (2 min), 36 cycles of denaturation at 94°C (45 s), annealing at 52°C (45 s), elongation at 72°C (1 min 30 s), and a final extension at 72°C (10 min). To assess the diversity of strains used in enzyme analysis, two protein-coding genes were amplified: the transcription elongation factor EF1-α (TEF1) using the primers 983F/2218R [24] and DNA replication licensing factor MCM7 (MCM7) using the primers Mcm7-709for/Mcm7-1348 [25]. PCR conditions were the same as for the ITS, with 200 nM of each primer and the Taq MasterMix was used at 1.2X concentration. Collection information and Genbank accession numbers for the strains used in physiological tests are provided in Table 1, and full primer sequences are available in Table S2.
Table 1. Origin of isolates used in this study*.
| Isolate | Sp. | Collection | Origin | Location | Test | ITS | MCM7 | TEF1 |
| BL308 | 1 | Barton | Mlf | Tennessee, USA | 10x | KF686750 | KF686779 | KF686764 |
| BL549 | 1 | Barton | Mlf | Tennessee, USA | 10x | KF686751 | KF686771 | KF686767 |
| BL578 | 1 | Barton | Mlf | Tennessee, USA | 10x | KF686752 | KF686772 | NA |
| BL606 | 1 | Barton | Mlf | Tennessee, USA | 10x | KF686753 | KF686780 | NA |
| MYA 4855 | 3 | ATCC | Mlf | New York, USA | 10x | KF686759 | KF686773 | KF686768 |
| 16222 | 2 | ATCC | wfs | Germany | 10x | NA | KF686777 | KF686766 |
| MYA 4765 | 4 | ATCC | Vmr | Poland | 10x | NA | NA | NA |
| 9644 | 5 | ATCC | radio | Papua New Guinea | 10x | NA | NA | NA |
| FI204 | 1 | Barton | cs | Tennessee, USA | 3x | KF686754 | NA | KF686763 |
| FI590 | 1 | Barton | cs | Tennessee, USA | 3x | KF686755 | NA | NA |
| FI606 | 1 | Barton | cs | Tennessee, USA | 3x | KF686756 | NA | NA |
| FI609 | 1 | Barton | cs | Tennessee, USA | 3x | NA | KF686778 | KF686761 |
| FI687 | 1 | Barton | cs | Tennessee, USA | 3x | KF686757 | KF686775 | KF686769 |
| FI698 | 1 | Barton | cs | Tennessee, USA | 3x | KF686758 | KF686776 | KF686765 |
| 13PA1 | 1 | FCMR | cs | Pennsylvania, USA | 3x | NA | KF686774 | KF686770 |
| 4NY16 | 1 | FCMR | cs | New York, USA | 3x | JX270377 | KF017653 | KF017762 |
| 4NY17A | 1 | FCMR | cs | New York, USA | 3x | JX270378 | KF017654 | KF017763 KF017763 |
| 5NY8 | 1 | FCMR | cs | New York, USA | 3x | JX270387 | KF017656 | KF017765 KF017765 |
Species codes: 1 Pseudogymnoascus sp.; 2 Pseudogymnoascus pannorum,; 3 Pseudogymnoascus destructans; 4 Oidiodendron maius; 5 Penicillium pinophilum. Origin codes: cs, cave sediment; Mlf Myotis lucifugus fur; rs radio set; wfs wheat field soil; Vmr Vaccinium myrtilis roots.
Lab strains used in analysis were compared to species isolated from bat hibernacula from Lorch et al [13] and sequenced in Minnis et al [5] (Table S1). Sequences were visually inspected and assembled in Geneious version 6.0 [26] and aligned using MUSCLE version 3.7 [27] on the CIPRES server [28]. The alignment was visually examined in Mesquite version 2.75 [29] to exclude ambiguously aligned regions. Trees were generated for individual genes and a concatenated dataset, which included all taxa, even those with missing data. Evolution models were selected using the AIC criterion in jModelTest version 2.1.4 [30], [31], and were determined to be GTR+I+gamma for each of the three genes. Maximum likelihood trees were generated using RaxML [32] using the default settings plus GTRGAMMA+I for RAxML-HPC BlackBox version 7.27 on the CIPRES server; the best ML tree and 500 bootstrap replicates were produced in a single run.
For long-term storage at −80°C, spores were collected by washing sporulating cultures with 5 mL of 0.1% deoctylsulfosuccinate (DSS) in Sabouraud Dextrose Broth (containing 25% glycerol v/v) to suspend the hydrophobic spores. Five genetically distinct [5], unnamed Pseudogymnoascus species originally isolated by Lorch et al. [13] were obtained from the Center for Forest Mycology Research (CFMR; Madison, WI). Cultures of P. destructans ATCC MYA-4855 and P. pannorum var. pannorum ATCC 16222 were obtained from the American Type Culture Collection (ATCC, Manassas, VA), where they are stored under the prior name Geomyces. Control species, also obtained from the ATCC, were the ascomycetes Penicillium pinophilum ATCC MYA-9644 and Oidiodendron maius ATCC MYA-4765, an ericoid mycorrhizoid fungus in the Myxotrichaceae [33]. Pseudogymnoascus, though long thought to be a member of the Myxotrichaceae with Oidiodendron [34]–[36], has recently shown to be in a different Leotiomycete family [37]–[39], while Penicillium is a distantly related filamentous ascomycete in the class Eurotiomycetes [40]. Fungi were cultured at room temperature (20°C), except for P. destructans, which was cultured at 10°C on potato dextrose agar (PDA; 20 g/L dextrose, 4 g/L potato extract, 15 g/L agar, pH 6.8) from frozen spore suspensions prior to use in enzyme assays. For plate-based assays, point inoculation was used to transfer fungal spores from sporulating cultures to the center of prepared media. To inoculate filter paper cultures, spores suspensions (diluted to 107 spores/mL) were prepared by washing cultures on PDA with 5 mL of DSS, which has previously been shown to be non-toxic to both Geomyces and Pseudogymnoascus species [41].
Plate-based Assays
Enzyme tests in solid media were performed at pH 6.8 in agar-based media (15 g/L). To assess the temperature optima for Pseudogymnoascus growth, 10 replicates of each species were cultivated on PDA at 5, 10, 15, 20, 25, and 30°C, with the diameter of each colony checked weekly for six weeks. The weekly growth rate for each single replicate was calculated in using the lmList function in R version 2.15.1 [42], and the mean and standard deviation for each species at a given temperature was calculated. To assess enzyme activity, both the colony and clearance zone diameters were used to calculate the Relative Enzyme Activity (REA), which is the ratio of the clearance zone to colony size. To determine the best medium for hemolysis analysis, a pilot study was conducted on four media, each with 5% sheep’s blood: brain heart infusion agar and tryptic soy agar (TSA) from Hardy Diagnostics (Santa Maria, CA), and lab-prepared TSA and PDA. The PDA was prepared using 10 mL of PDA and 5 mL of a PDA/blood overlay. Based on the results of this pilot study, hemolysis was assessed at 10°C for the six Pseudogymnoascus species using TSA amended with 5% defibrillated sheep’s blood (Cleveland Scientific, Bath, OH). Lipase assays were performed on Spirit Blue Agar (BD Difco, Franklin Lakes, NJ); the agar mix was sterilized by autoclaving 20 min and cooled to 55°C before adding 30 mL of sterilized lipase reagent (100∶1 olive oil:Tween 80 in 400 mL deionized water). Colloidal chitin agar was prepared from shrimp shell chitin following the protocol in Hsu and Lockwood [43], with the following changes: the chitin was dissolved overnight rather than for 30 minutes in concentrated HCl, and was used immediately in agar preparation as a wet cake in sufficient quantity to yield 4 g/L dry weight. The production of urease was tested using Christensen’s urea agar [44]. To test cellulose degradation, media containing one of three different types of cellulose – carboxymethyl-cellulose (CMC), crystalline cellulose (Avicel), and D-cellobiose – were prepared following Yoon et al 2007 [45] with the following alterations: yeast extract was used rather than yeast nitrogen base, and dyes were used as post-stains based on Saczi et al [46] rather than being included in the media. Cellulase tests were repeated for Pseudogymnoascus spp. using yeast nitrogen base rather than yeast extract, with the cellulose as the sole carbon source. While the chitin, Avicel, Spirit Blue, and blood plates show distinct clearance zones, the CMC-agar and D-cellobiose agar require further processing. Based on Saczi et al [46], the CMC-agar was post-stained with Congo red for 1 hour and destained with 0.7 M NaCl for 15 minutes. Bromcresol purple (pH 6.8) was selected as a post-stain for D-cellobiose agar due to its ability to detect pH changes under mildly acidic to near-neutral conditions.
Humic and fulvic acids were extracted from potting soil as described in Bhullar et al [47] and used to prepare agar media with ∼20 mg/L of either humic or fulvic acid as the sole source of nutrients. Fungal cultures were inoculated using point inoculation and kept for one week at either 20°C or 10°C, with 10 replicates for each temperature/medium combination. Growth was recorded as the increase in colony diameter over time.
Liquid Assays
Further tests of cellulase ability were performed in 50 mL liquid media with 9 cm Whatman #4 filter paper cut into 1 cm2 pieces. An inoculating dose of 106 spores was added to this liquid assay, which also contained 0.1 M NaNO3, 11.48 µM K2HPO4, 7.35 µM KH2PO4, 6.10 µM MgSO4, 30 µM FeCl3, 1.24 µM ZnSO4, and 1.22 µM MnSO4. Two flasks were inoculated with P. destructans while one was used for each of the BL308, BL549, BL578, BL606, P. pannorum and Penicillium pinophilum cultures. After one month of shaking incubation (120 rpm) at 10°C, the cultures were centrifuged at 6000×g. The supernatant was filtered to remove cellular material and stored at 4°C prior to use in liquid cellulase assays. The presence of reducing and non-reducing sugars was assessed with a modification of the dinitrosalicylic acid (DNSA) reagent method from Miller [48]. Briefly, 2 mL of cellulose solution (1% CMC or Avicel in 0.05 M sodium citrate) and 2 mL of culture filtrate were incubated in a 50°C water bath for one hour. Samples were then subdivided; the first half of the sample was used directly to determine the presence of reducing sugars by adding 1 mL DNSA reagent, incubating at 95°C for 15 min, and adding of 1 mL of 40% potassium sodium tartrate. The other half of the sample was used to assess the presence of non-reducing sugars: before proceeding with the DNSA reagent, the sample was first hydrolyzed at 95°C for 5 minutes using HCl (final concentration: 0.24 M) and neutralized with KOH (final concentration: 0.25 M). Absorbance at 575 nm was measured using a DR2800 Spectrophotometer (Hach Co., Loveland, CO). Triplicate tests were performed for each culture filtrate and for control tests using either the enzyme solution or the cellulose solution in isolation, with standardized curves generated for glucose and sucrose using 0, 2.5, 5, 7, 9, and 10 mM solutions. Linear regression equations were used to calculate the mM of reducing and non-reducing sugars produced from each cellulase/enzyme assay.
The activity of β-glucosidase was assessed with the standard p-nitrophenyl-β-glucopyranoside (pNPβG) method. Briefly, 0.1 mL cellulose filtrate was added to 0.9 mL of a 0.02% solution of pNPβG in 0.1 M sodium acetate buffer (pH 4.8) and incubated at 50°C for 30 min before adding 2 mL of Clark and Lubs buffer (pH 9.8) [49] and measuring absorbance at 430 nm. A standard curve was prepared using 10−1, 10−2, 10−3, 10−4, 10−5, and 10−6 µM p-nitrophenol solutions, and the exponential regression equation was used to calculate the amount of p-nitrophenol released from pNPβG, an indication of β-glucosidase activity.
Statistical Analysis
All statistical tests of enzyme activitiy were performed in R [42] using an ANOVA to test temperature and species effects and the Tukey’s Highly Signfiicant Differences (Tukey’s HSD) test to assess significant differences between each REA.
Results
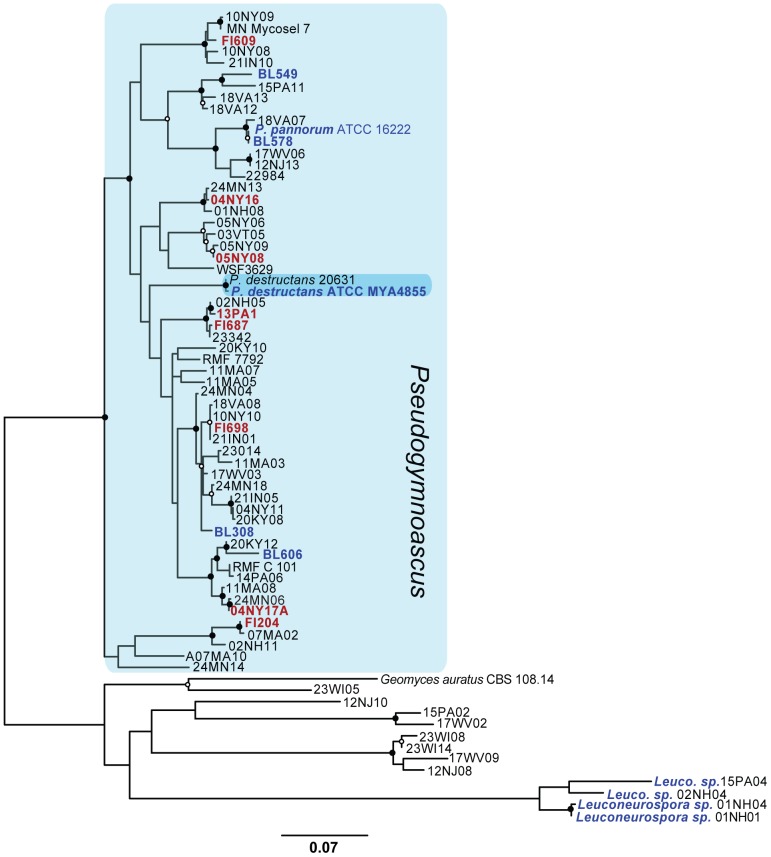
Before beginning our comparative enzymatic analyses, it was important to determine the relatedness of the bat and environmental Pseudogymnoascus isolates to each other and P. destructans. In order to do this, we used RAxML [32] to generate a phylogenetic tree of these isolates, including P. destructans, using a concatenated sequence of three genetic loci (ITS region: 876, MCM7: 486, TEF1: 806). This tree included 72 taxa (Tables 1 and S1), which allowed us to reliably place our identified isolates in each of the Pseudogymonascus clades [5]. The phylogeny (Figure 1) confirms the results of other investigators, in that P. destructans remains in a distinct clade from other North American Pseudogymnoascus isolates [4], [5]. The results also suggest that our environmental isolate BL578 is closely related to P. pannorum ATCC 16222, possibly as a subtype or strain.
Figure 1. Best maximum-likelihood tree for the Pseudogymnoascus spp. used in this study (lnL = −10622.006982).
A concatenated alignment of the TEF-1, MCM7, and partial ITS genes was used to generate a Maximum-likelihood tree in RAxML using strains identified in Minnis and Lindner [5]. Values show bootstrap support from 500 bootstrap replicates; closed circles indicate ≥95% support at each branch point, while open circles indicate >70% support. The taxa shown in red were used in 10X tests, while the taxa in blue were used in triplicate tests. Geomyces auratus and members of the Leuconeurospora were used as the outgroups. Accession numbers for each sequence can be found in Tables 1 and S1.
Based on the phylogeny for our Pseudogymnoascus strains, two genetically diverse subsets of Pseudogymnoascus spp were selected for physiological tests: (1) a set of six Pseudogymnoascus spp. representing five clades, including four of our isolates (BL308, BL549, BL578 and BL606), P. destructans, the control species P. pannorum, Penicillium pinophilum, and O. maius (used in 10x replicate tests); and (2) a set of eight isolates from bat fur and cave sediments representing eight additional Pseudogymnoascus lineages (13PA1 to FI698) used in triplicate tests. The 10x set was used to permit statistical analysis comparing REA among species, while the 3x set was used to gain a broader assessment of the range of REA in Pseudogymnoascus.
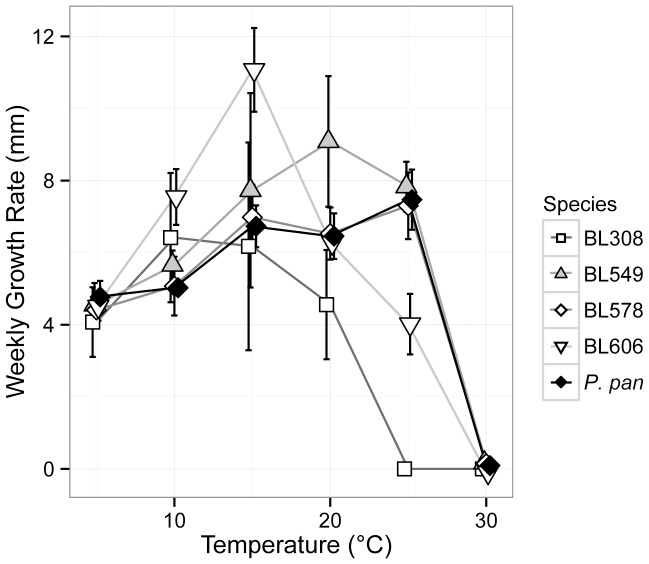
The optimal temperature of P. destructans growth ranges from 12.5–15.8°C, where it grows both radially and produces asexual spores, although growth on Myotis lucifugus bats in the environmental has been observed as low as 2°C [6]. To examine the role temperature might play in enzyme activity, we examined the growth of our strains at various temperatures and calculated the linear growth rates based on the weekly growth (Figure 2). All of the species examined grew between 5 and 25°C, with optimal growth varying between 10–25°C. Bat isolate BL308 was more restricted in its growth, showing an optimum growth of 10°C with no growth at 25°C, similar to P. destructans. Strains that were closely related to each other, such as BL578 and P. pannorum ATCC 16222, showed similar responses to temperature, but there was otherwise no immediately evident correlation between physiology and strain relationships. The bat-isolated strains in our study thus demonstrated both psychrophilic and psychrotolerant growth, in line with other members of Pseudogymnoascus and Geomyces. Based on these growth temperatures and our understanding of the temperature range of hibernacula (<12°C), we examined enzyme activity for the saprotrophic Pseudogymnoascus spp. at two temperatures: 10°C, which is close to the average temperature of bat hibernacula and the optimal growth temperature for P. destructans and strain BL308; and 20°C, the optimal growth temperature of some of our bat-isolated Pseudogymnoascus species. Using these temperatures allowed us to examine whether fungi show a higher REA at their optimal growth temperature or the temperature of the environment from which they were isolated.
Figure 2. Temperature-dependent growth of environmental isolates of Pseudogymnoascus.
The plot shows the average growth rate of each taxon over 6 weeks. Error bars indicate the significant difference of each assay (n = 10). P. pan = P. pannorum.
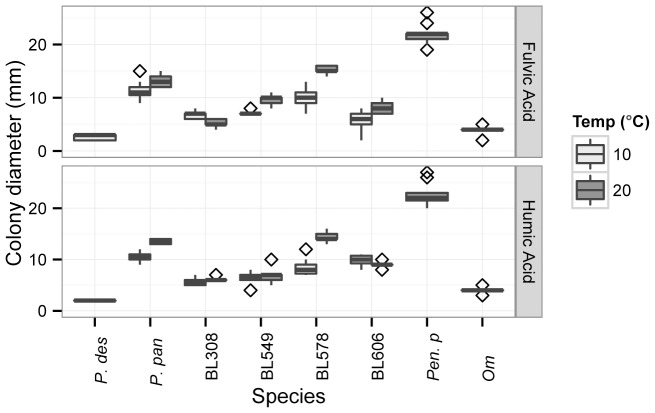
Fulvic and humic acids are complex aromatic and polyaromatic organic molecules rich in carboxyl and phenolate groups produced by cellulose decomposition in the environment [50]. We tested the ability of Pseudogymnoascus spp. to grow on these acids which, as the dominant form of dissolved organic carbon (DOC) in both soils and caves [51], [52], may support fungal growth in hibernacula. All fungi tested showed growth over one week on both fulvic and humic acid media; P. destructans and O. maius had the slowest growth rates, while the saprotroph Penicillium pinophilum had the highest growth rate (Figure 3). Both the temperature and species tested significantly affected growth rate on both fulvic and humic acids (ANOVA results in Table S3). A Tukey’s HSD test of the temperature and species effects found that P. destructans had significantly lower growth than most other Pseudogymnoascus species, but was not significantly different from environmental isolates BL308 (10°C and 20°C), BL549 (10°C), or from O. maius (10°C) (significance values are shown in Table S4). A comparison of psychrotolerant Pseudogymnoascus species at ambient (20°C) and cool temperatures (10°C) indicated that for most species, temperature did not affect growth rate on this medium, while strain BL578 and BL549 grew significantly better at 20°C than at 10°C on fulvic acid, and BL578 and P. pannorum grew significantly better at 20°C than at 10°C on humic acid (significance values are shown in Table S6).
Figure 3. Growth of Pseudogymnoascus spp. on fulvic and humic acid extracts.
Boxplot boundaries show the first and third quartiles, with the mean as the centerline and whiskers as 1.5 times the inter-quartile distance. Outliers are plotted as points. P. des = P. destructans; P. pan = P. pannorum; Pen. p = Penicillium pinophilum; and Om = Oidiodendron maius.
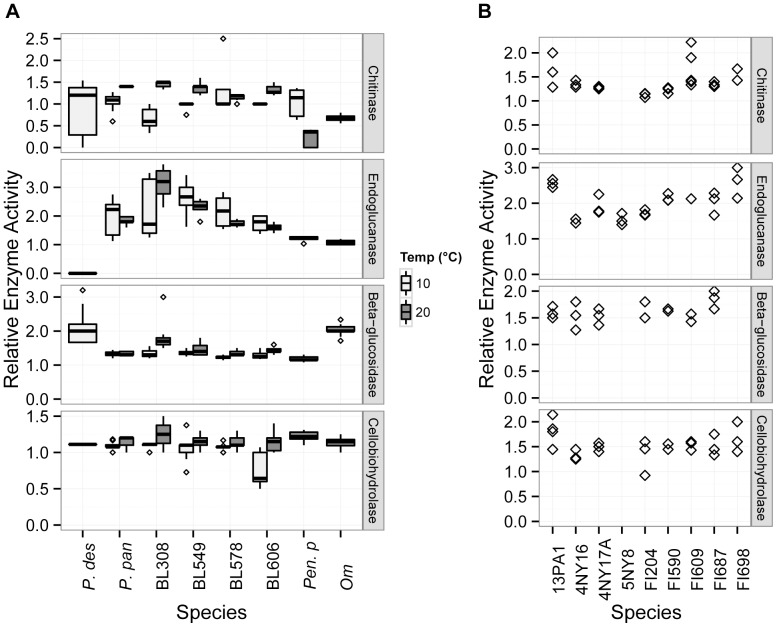
The presence of cellulose and chitin in caves may be dependent on environmental effects that vary among sites, such as flooding or the presence of bat and/or insect populations [53], [54]; caves that experience periodical flooding can accumulate large quantities of plant debris [55] and the guano of insectivorous bats contains a high amount of chitinous insect cuticle [56]. We therefore investigated the ability of P. destructans to produce cellulases and chitinases (Figure 4), which could provide an environmental carbon and energy source for the growth of P. destructans. While most of the species examined could be assessed for chitinase at one week, the REA of O. maius and P. destructans were assessed at two and four weeks, respectively. All examined species produced chitinases, with P. destructans showing similar activity to a suite of related fungal species. Four of the five environmental Pseudogymnoascus isolates showed lower chitinase REA at 10°C than at 20°C, but this difference was significant only for BL308 (Tukey’s adjusted p-value <0.001; see Table S6 for other values). The lowest chitinase activity observed was for the Penicillium pinophilum at 20°C. At 10°C, P. destructans chitinase activity did not differ significantly from that of the other species (Table S5), but chitinase activity for P. destructans at 10°C was significantly lower than that of BL308 and BL549 and higher than that of P. pinophilum at 20°C. While P. destructans and P. pinophilum showed similar REA to the other fungi, the clearance zone for these two species remained slightly cloudy, suggesting an incomplete hydrolysis. Growth remained scant for these species, with little aerial mycelia.
Figure 4. Relative enzyme activity for saprotrophic enzymes in P. destructans and other fungi.
Boxplot boundaries show the first and third quartiles, with the mean as the centerline and whiskers as 1.5 times the inter-quartile distance. Outliers are plotted as points. P. des = P. destructans; P. pan = P. pannorum; Pen. p = Penicillium pinophilum; and Om = Oidiodendron maius.
Due to the potential role of plant detritus in promoting fungal growth in caves, we assessed the activity of three different functional categories of cellulases: endoglucanases, which cleave internal cellulose bonds, exoglucanases, which cleave the non-reducing end of the cellulose polymer, and β-glucosidases, which break down the disaccharide cellulose by-product. Due to varying growth rates, P. destructans was assessed for endoglucanase at three weeks, β-glucosidase at two weeks, and cellobiohydrolase at four weeks. The other Pseudogymnoascus spp. were assessed at one week for endoglucanase at both temperatures, while the REA of β-glucosidase and cellobiohydrolase was measured at one week for the 20°C assay and two weeks for the 10°C assay. P. pinophilum and O. maius were assessed at one week and two weeks, respectively, for all cellulase categories. The endoglucanase assay (using CMC) was negative for P. destructans, but positive for all other species examined (Figure 4). This assay did not yield the classic red-to-black color change that indicates medium acidification, but showed a pale red zone indicating where cellulose had been degraded surrounding colonies. When the tests were repeated using CMC as the sole carbon source, the REA increased for each species, but remained negative for P. destructans. Despite its apparent lack of endoglucanase activity, P. destructans was able to grow and sporulate on CMC media. The β-glucosidase assay indicated that P. destructans had significantly higher REA than all other fungi examined except for BL308 and the plant mutualist O. maius, which were not significantly different (Table S5). Cellobiohydrolase REA was positive for each fungus, and, though much slower to develop in P. destructans, not significantly different from the other fungi (Table S5), except BL606, which had significantly lower REA at 10°C (Tukey’s adjusted p-value = 0.001). Temperature was a significant factor overall for β-glucosidase (ANOVA F-value = 16.005, p-value <0.001) and cellobiohydrolase activity (ANOVA F-value = 35.871, p<0.001), but not for endoglucanase (ANOVA F-value = 0.004, p = 0.948). Nevertheless, BL308 showed significantly higher endoglucanase activity at 20°C than at 10°C (Tukey’s adjusted p-value = 0.001), as it did for β-glucosidase activity (Tukey’s adjusted p-value <0.001). BL606 was the only strain showing significant differences for cellobiohydrolase activity at these two temperatures, with higher activity at 20°C (Tukey’s adjusted p-value = 0.004) (Table S6).
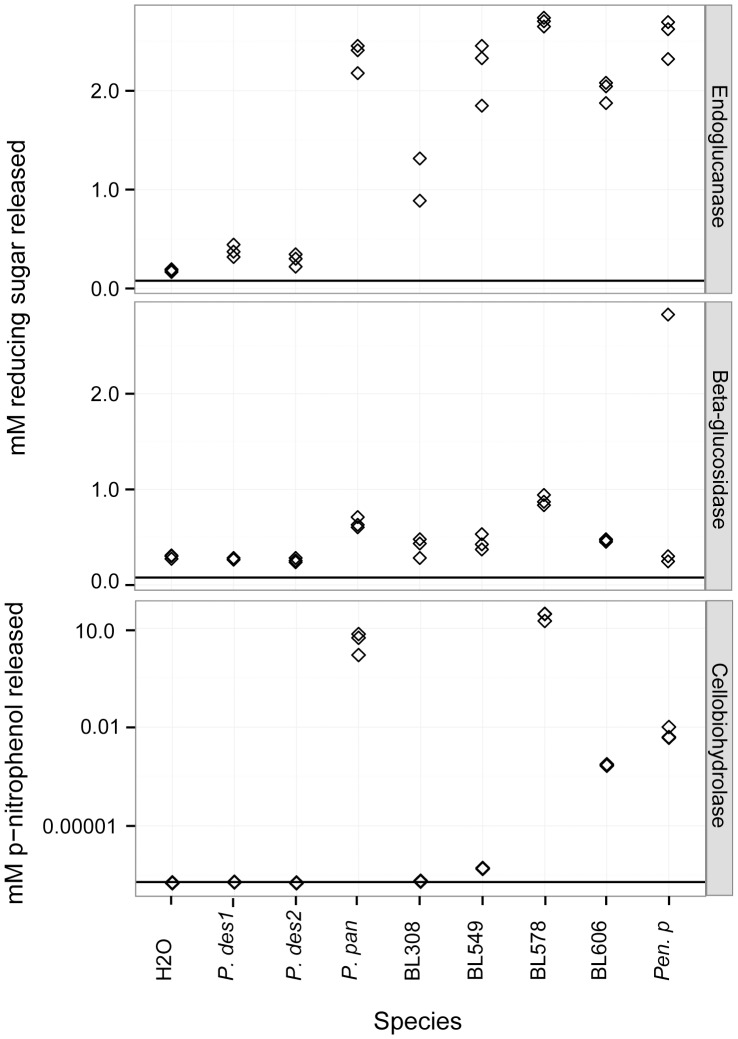
To determine which cleavage products were released from cellulose for potential fungal growth, we examined the production of reducing and non-reducing sugars in liquid assays. Using CMC, these assays were negative for the production of non-reducing sugars by all the fungi tested (data not shown), while reducing sugars were produced (Figure 5). The P. destructans culture filtrate released a small amount of reducing sugars from CMC (mean ± SD = 0.333±0.008 µM), which was double that of the negative control (0.182±0.014 µM). The other fungi assayed released much higher levels of reducing sugars than P. destructans, with BL308 as the least active (mean = 1.03±0.247 µM) and BL578 as the most active (mean = 2.70±0.045 µM). A subsequent assay using the insoluble cellulose Avicel tested negative for reducing sugars using P. destructans filtrate (0.265±0.017 µM) compared to the negative control (0.295±0.019 µM). The other Pseudogymnoascus spp. tested positive for reducing sugars on Avicel, ranging from 0.398±0.103 µM (BL308) to 0.882±0.054 µM (BL578), while Penicillium pinophilum tested negative (0.274±0.038 µM). The cellobiohydrolase assay using pNPβG was negative for P. destructans and BL308, but positive for the other Pseudogymnoascus spp. and Penicillium pinophilum (Figure 5).
Figure 5. Liquid assays of endoglucanase, β-glucosidase, and cellobiohydrolase.
Reducing sugars released from CMC (endoglucanase activity) and Avicel (β-glucosidase activity) when treated with fungal filter paper culture supernatant and p-nitrophenol release from pNPβG (cellobiohydrolase activity). Tests were performed in triplicate, and the results were plotted individually. The horizontal line indicates results for negative controls using culture filtrates and H2O.
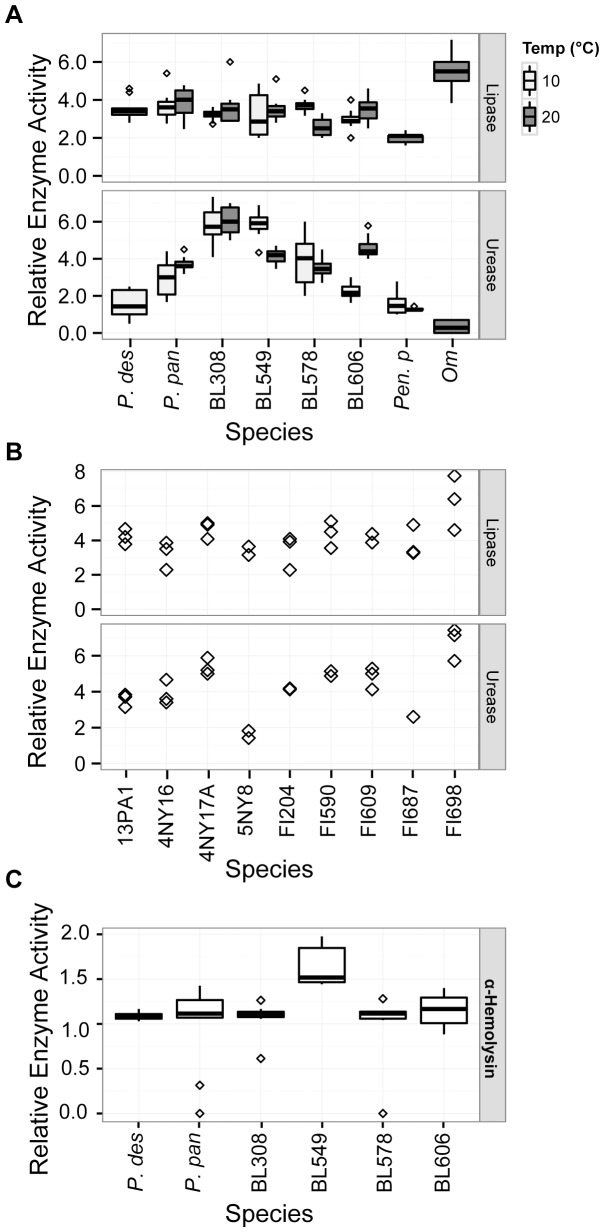
In addition to examining potential saptrotrophic enzyme activity in our Pseudogymnoascus isolates, we compared them for enzymatic activity that could aid in pathogenesis. To determine whether P. destructans produces enzymes that could play a role in breaking down host tissues, we tested whether members of Pseudogymnoascus could produce lipases (Figure 6A & B) and hemolases (Figure 6C). We initially tested hemolysis on four complex media containing 0.5% sheep’s blood, each of which contain different levels of nutrients. Hemolysis was noted only in TSA/blood plates, and not in the BHI/blood or PDA/blood preparations (data not shown). Lipase REA was not significantly affected by temperature (ANOVA F-value = 0.002, p = 0.961), although REA for BL578 was significantly higher at 10°C than at 20°C (Tukey’s adjusted p-value = 0.025). While lipase activity was rapid (visible in one week for all species save P. destructans and O. maius, which were assessed at two weeks), hemolysis was slow. Alpha-hemolysis, which indicates the partial lysis of red blood cells, was observed in our fungal cultures only after several weeks: 11 weeks for P. destructans and 8 weeks for the other Pseudogymnoascus spp., which grew more rapidly. The REA of P. destructans α-hemolysis was not significantly different from that of the other Pseudogymnoascus spp. (Table S4). The complete lysis of red blood cells and breakdown of hemoglobin (β-hemolysis) was not observed for any of the tested fungi.
Figure 6. Relative enzyme activity of enzymes that may have pathogenic and saprotrophic function in environmental Pseudogymnoascus spp. and P. destructans.
A) 10x replicates testing lipase and urease activity at hibernaculum and ambient temperature. B) 3x replicates testing lipase and urease activity at ambient temperature. C) 10x replicates testing α-hemolysis activity on on tryptic soy agar/5% sheep’s blood after 8 weeks (environmental isolates) and 11 weeks (P. destructans). Boxplot boundaries show the first and third quartiles, with the mean as the centerline and whiskers as 1.5 times the inter-quartile distance. Outliers are plotted as points.
If degraded by urease, the urea in bat urine and guano could serve as a source of nitrogen for fungal growth. Urease could also play an important role in pathogenesis, as the enzyme has been implicated in virulence pathways in other pathogenic microorganisms [57]. We tested urease activity on Christensen’s urea agar, and found that all the fungi tested except O. maius rapidly produced the enzyme (Figure 6A & B). The 20°C assays were assessed at one week, but the 10°C assays for the Pseudogymnoascus spp. and P. destructans were measured at two weeks, save for BL606, which was assessed at one week. The plant mutualist O. maius developed weakly positive REA for urease only after seven weeks. The Pseudogymnoascus spp. showed wide variation in their urease ability, ranging from average REAs of 1.54 for P. destructans to 6.03 for BL308 (20°C). The Pseudogymnoascus spp. tended to show high urease activity, with some species producing urease zones over six-fold greater than the colony diameter. While P. destructans showed significantly lower urease activity than did all other examined Pseudogymnoascus spp., it produced urease with an activity zone double its colony size, similar to BL606 at 10°C and P. pannorum at 10°C and 20°C. Temperature was not a significant factor in urease REA overall (ANOVA F-value = 2.839, p = 0.0947), but BL549 produced significantly higher urease REA at 10°C than at 20°C (Tukey’s adjusted p-value <0.001), while BL606 produced significantly higher urease REA at 20°C (Tukey’s adjusted p-value <0.001).
Discussion
As top predators in their ecosystems, bats play a critical role in controlling insect populations. Thus, the high mortality in multiple bat species from White-nose Syndrome renders P. destructans a major threat not only to these wildlife populations, but also to agriculture and public health [1], [58], [59]. The recent findings of the persistence of P. destructans in cave sediments suggest that the fungus can survive in bat hibernacula even in the absence of its host, which has a profound impact on both disease management and the epidemiology of the disease [13]. We therefore compared the function of several enzymes that could serve in a saprotrophic niche for the pathogenic P. destructans as well as resident Pseudogymnoascus species. The enzymes selected for this study were not intended to be an exhaustive survey of Pseudogymnoascus metabolism; rather, they were chosen to represent enzymes that might be required for growth on major components of the cave ecosystem. We also examined a subset of enzymes that could have dual function for these fungi, supporting saprotrophic growth and potentially contributing to a pathogenic lifestyle.
Cave environments are generally carbon and energy-limited due to geologic isolation and the lack of light driven photosynthesis [60]. As a result, the substrates available to fungi in caves are primarily in the form of allochthonous input, such as soil-derived dissolved organic carbon (DOC) or detritus, including leaf litter from entering surface streams. Another source of energy is the organic waste of trogloxenes, such as crickets and other arthropods and bats and their chitin- and urea-rich bat guano [53]–[54]. We therefore assessed the activity of chitinase and cellulase activity in Pseudogymnoascus, as well as the ability to grow on DOC in the form of fulvic and humic acids. Our results suggest that while P. destructans demonstrates enzymatic activity in each of these groups, testing positive for growth on fulvic and humic acids (Figure 3) and for chitinase and cellulase activity (Figure 4), it shows slower growth and lower cellulase activity relative to other Pseudogymnoascus spp. The chitinase relative enzyme activity in P. destructans was similar to other tested Pseudogymnoasci, while actual growth of the pathogen on chitin was limited. The chitinase production by other Pseudogymnoasci indicates that these species can use chitin as a nutrition source, which may allow growth on insects and/or the insect remnants found in bat guano, although such growth by P. destructans would likely be comparatively slow.
Assays of cellulases that would allow growth on plant debris were contradictory. In plate assays, P. destructans showed the highest β-glucosidase REA of all examined species, but was slow to develop cellobiohydrolase and was negative for endoglucanase (Figure 4). Liquid assays found that P. destructans using filter paper as a source of carbon produced endoglucanases, but not β-glucosidases or cellobiohydrolases (Figure 5). The negative result on solid media for endoglucanase may have been due to the assay’s reliance on visible color differences in the surrounding media, which may thus have a higher minimum detection limit than the liquid assay. Strain BL308 and Penicillium pinophilum, like P. destructans, tested negative in liquid tests for Avicel, a synthetic microcrystalline cellulose, but positive for growth on the substrate on solid media. These results suggest that P. destructans does produce each class of cellulase, but only to a limited degree and under restricted conditions. Compared to the bat-isolated and environmental Pseudogymnoascus spp., P. destructans appears capable of degrading cellulose, albeit at a reduced capacity.
Although occasional, superficial mycoses in humans and animals by members of Pseudogymnoascus have been observed, members of this genus are considered to be predominately saprotrophic [61], [62]. To assess the expression of enzymes that might function in either a saprotrophic or pathogenic context, we examined lipase, hemolase and urease activity. Our data demonstrated that P. destructans was positive for all three enzymes, with lipase activity similar to the other members of the genus (Figure 6). Lipolytic fungi are known to play an important role in the beginning stages of leaf litter decomposition [63], and lipases can also aid infectious mycoses in host tissue adhesion and invasion [64]. All Pseudogymnoascus spp. examined were positive for α-hemolysis after several weeks of incubation, although P. destructans hemolysis occurred more slowly. Compared to the rapid hemolysis seen in true pathogens, such as the bacterial pathogens Staphylococcus aureus and Streptococcus pyogenes, which generate visible zones of hemolysis on blood agar within hours [65], [66], the hemolytic activity in P. destructans appears to begin when nutrients become limiting. Thus, hemolytic activity by P. destructans may not be optimized for systemic growth within its host, but rather may aid in survival on the nutrient-limited landscape of a bat wing membrane. This high lipase activity but low hemolysin activity corresponds with the histological evidence of WNS infection, where the fungus degrades the wing epithelium and basal membrane to invade connective tissue [8], [67], but does so without directly invading the blood vessels [67]. Thus, hemolysis may not play an important role in disease pathology, but may be necessary to obtain the iron needed to support fungal growth.
All of the fungi examined in this study save O. maius showed high urease activity. Urease would be advantageous for growth in bat guano, but as this enzyme is broadly distributed among soil microbes [68], [69], urease proficiency is probably not a specific adaption to cave life. Nonetheless, urease has been implicated in tissue invasion and virulence signaling pathways [57], [70], [71], including in Coccidioides posadasii, where ammonia produced by urease damages host tissues [72], [73] and Cryptococcus neoformans, where urease plays an important role in crossing the blood-brain barrier [74]. Urease may thus play two roles in WNS: permitting propagation of P. destructans on guano and potentially aiding in pathology. Further research is needed to understand the possible involvement of these enzymes in WNS disease ecology, as well as other saprotrophic pathways that may have been coopted for pathogenesis.
We had examined the effect of temperature on enzyme activity to test whether species would show higher REA at their optimal growth temperature or at the temperature of the environment from which they had been isolated. In fact, we found that temperature rarely caused a significant difference in relative enzyme activity, and in the majority of cases that did show a significant difference, the REA was higher at 20°C, regardless of the temperature preferences of the organism or site of origin. For instance, BL308, which has an optimal growth at 10°C and was isolated from a cold cave, showed significantly higher chitinase, endoglucanase, and β-glucosidase activity at 20°C. The only two instances of significantly higher REA at 10°C were for BL549 urease and BL578 lipase; these two species have optimal growth at 20°C and 25°C respectively. Thus, while some of the cave-isolated fungi grow more quickly at the cool temperatures found in northern bat hibernacula, their enzyme function indicates that they are not likely restricted to these environments.
The results of this study indicate that the Pseudogymnoascus spp. found in caves can degrade a variety of organic molecules for growth in multiple microhabitats, including insect detritus, decaying plant material, and bat guano. Such results suggest that rather than having a specific niche, members of the genus Pseudogymnoascus can function as generalist decomposers, a finding supported by the presence of Pseudogymnoascus and related Geomyces species worldwide [75]–[82], including Antarctica and the Arctic. Our finding that P. destructans shows similar saprotrophic potential to other members of this genus may indicate a potential environmental origin for this pathogen; however, the reduced growth rate and saprotrophic enzyme activity suggest that P. destructans may have gained functionality in pathogenesis at the expense of an environmental lifestyle. Such a diminished saprotrophic capacity could be explained if P. destructans had entered into an evolutionary host-pathogen relationship at sometime in the past; a significant length of coevolution between host and pathogen could have resulted in a decrease in the selective pressure on P. destructans to maintain enzymes needed for decomposition. To determine if this is indeed the case, a phylogenetic comparison of the genes involved in both saprotrophic and pathogenic pathways may be able to detect changes in either expression pathways and/or enzymatic activity and could potentially provide a molecular clock for the timing of the movement of P. destructans out of the soil/cave sediment environment and into its host.
Supporting Information
Collection information and Genbank accession numbers for strains used in assessing phylogenetic diversity.
(XLSX)
Primers used for sequencing Pseudogymnoascus isolates.
(XLSX)
Results of two-way ANOVA assessing temperature and species effects in relative enzyme activity.
(DOCX)
Tukey’s Highly Significant Differences Test for growth on organic acids comparing P. destructans to other species.
(DOCX)
Tukey’s Highly Significant Differences Test for relative enzyme activity comparing multiple fungal species to P. destructans.
(DOCX)
Tukeys’ Highly Significant Differences Test comparing intraspecific growth or relative enzyme activity at 20°C and 10°C.
(DOCX)
Acknowledgments
We thank the US Fish and Wildlife Service for funding this study and the Cumberland Gap National Historic Park for providing collection permits. We would also like to thank Daniel Lindner and Andrew Minnis at the Center for Forest Mycology Research (CFMR) for providing cultures used in this study, Tonisha Henry and Josephine Landenberger for assistance on temperature-dependent growth and lipase studies, and Matìas Cafaro for advice regarding cellulase assays.
Funding Statement
US Fish and Wildlife Services (www.fws.gov). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Blehert DS, Hicks AC, Behr M, Meteyer CU, Berlowski-Zier BM, et al. (2009) Bat white-nose syndrome: an emerging fungal pathogen? Science 323: 227. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds HT, Barton HA (2013) White-nose Syndrome: Human activity in the emergence of an extirpating mycosis. Microbiology Spectrum in press. [DOI] [PubMed]
- 3. Lorch JM, Meteyer CU, Behr MJ, Boyles JG, Cryan PM, et al. (2011) Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature 480: 376–378. [DOI] [PubMed] [Google Scholar]
- 4. Gargas A, Trest MT, Christensen M, Volk TJ, Blehert DS (2009) Geomyces destructans sp. nov. associated with bat white-nose syndrome. Mycotaxon 108: 147–154. [Google Scholar]
- 5. Minnis AM, Lindner DL (2013) Phylogenetic evaluation of Geomyces and allies reveals no close relatives of Pseudogymnoascus destructans, comb. nov., in bat hibernacula of eastern North America. Mycological Research 117: 638–649. [DOI] [PubMed] [Google Scholar]
- 6. Verant ML, Boyles JG, Waldrep W Jr, Wibbelt G, Blehert DS (2012) Temperature-dependent growth of Geomyces destructans, the fungus that causes bat White-nose Syndrome. PLoS One 7: e46280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pikula J, Bandouchova H, Novotny L, Meteyer CU, Zukal J, et al. (2012) Histopathology confirms white-nose syndrome in bats in Europe. Journal of Wildlife Diseases 48: 207–211. [DOI] [PubMed] [Google Scholar]
- 8. Meteyer CU, Buckles EL, Blehert DS, Hicks AC, Green DE, et al. (2009) Histopathologic criteria to confirm white-nose syndrome in bats. Journal of Veterinary Diagnostic Investigation 21: 411–414. [DOI] [PubMed] [Google Scholar]
- 9. Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, et al. (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484: 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piotrowski JS, Annis SL, Longcore JE (2004) Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96: 9–15. [PubMed] [Google Scholar]
- 11. Johnson ML, Speare R (2005) Possible modes of dissemination of the amphibian chytrid Batrachochytrium dendrobatidis in the environment. Disease of Aquatic Organisms 65: 181–186. [DOI] [PubMed] [Google Scholar]
- 12. Mitchell KM, Churcher TS, Garner TWJ, Fisher MC (2008) Persistence of the emerging pathogen Batrachochytrium dendrobatidis outside the amphibian host greatly increases the probability of host extinction. Proceedings of the Royal Society B 275: 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lorch JM, Muller LK, Russell RE, O’Connor M, Lindner DL, et al. (2013) Distribution and environmental persistence of the causative agent of White-nose Syndrome, Geomyces destructans, in bat hibernacula of the eastern United States. Applied and Environmental Microbiology 79: 1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaturvedi V, Springer D, Behr M, Ramani R, Li X, et al. (2010) Morphological and molecular characterizations of psychrophilic fungus Geomyces destructans from New York bats with white-nose syndrome (WNS). PLoS One 5: e10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geomyces destructrans Sequencing Project. http://www.broadinstitute.org: Broad Institute of Harvard and MIT.
- 16.Lorch JM, Lindner DL, Gargas A, Muller LK, Minnis AM, et al. (2013) A culture-based survey of fungi in soil from bat hibernacula in the eastern United States and its implications for detection of Geomyces destructans, the causal agent of bat white-nose syndrome. Mycologia. [DOI] [PubMed]
- 17.Johnson LJ, Miller AN, McCleery RA, McClanahan R, Kath JA, et al. (2013) Psychrophilic and psychrotolerant fungi on bats: Geomyces a common fungus on bat wings prior to the arrival of White Nose Syndrome. Applied and Environmental Microbiology in press. [DOI] [PMC free article] [PubMed]
- 18. Warnecke L, Turner JM, Bollinger TK, Lorch JM, Misra V, et al. (2012) Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proc Natl Acad Sci U S A 109: 6999–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vanderwolf KJ, McAlpine DF, Malloch D, Forbes GJ (2013) Ectomycota associated with hibernating bats in eastern Canadian caves prior to the emergence of White-nose Syndrome. Northeastern Naturalist 20: 115–130. [Google Scholar]
- 20.Rice AV, Currah RS (2006) Oidiodendron maius: saprobe in sphagnum peat, mutualist in ericaceous roots? In: Schulz B, Sieber TN, editors. Microbial Root Endophytes. Berlin Heidelberg: Springer-Verlag. 227–246. [Google Scholar]
- 21.Emmons CW, Binford CH, Utz JP, Kwon-Chung KJ (1977) Medical mycology. Philadelphia, Pennsylvania, USA: Lea & Febiger. [Google Scholar]
- 22. Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, et al. (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi . Proceedings of the National Academy of Sciences 109: 6241–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White TJ, Bruns T, Lee S, Taylor JW, editors (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. New York: Academic Press, Inc.
- 24. Rehner SA, Buckley E (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. [DOI] [PubMed] [Google Scholar]
- 25. Schmitt I, Crespo A, Divakar PK, Fankhauser JD, Herman-Sackett E, et al. (2009) New primers for promising single-copy genes in fungal phylogenetics and systematics. Persoonia 23: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geneious version 6.0 created by Biomatters. Available from http://www.geneious.com.
- 27. Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller MA, Holder MT, Vos R, Midford PE, Liebowitz T, et al. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE). New Orleans, LA. 1–8.
- 29.Maddison WP, Maddison DR (2011) Mesquite: a modular system for evolutionary analysis. Version 2.75. http://mesquiteproject.org.
- 30. Darrida D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guindon S, Gascuel O (2003) A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Systematic Biology 52: 696–704. [DOI] [PubMed] [Google Scholar]
- 32. Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- 33. Perotto S, Girlanda M, Martino E (2002) Ericoid mycorrhizal fungi: some new perspectives on old acquaintances. Plant and Soil 244: 41–45. [Google Scholar]
- 34. Currah RS (1985) Taxonomy of the Onygenales: Arthrodermataceae, Gymnoascaceae, Myxotrichaceae, and Onygenaceae. Mycotaxon 24: 1–216. [Google Scholar]
- 35. Udagawa S, Uchiyama S, Kamiya S (1993) Gymnostellatospora, a new genus of the Myxotrichaceae. Mycotaxon 48: 157–164. [Google Scholar]
- 36. Rice AV, Currah RS (2006) Two new species of Pseudogymnoascus with Geomyces anamorphs and their phylogenetic relationship with Gymnostellatospora. . Mycologia 98: 307–318. [DOI] [PubMed] [Google Scholar]
- 37. Sogonov MV, Schroers H-J, Gams W, Dijksterhuis J, Summerbell RC (2005) The hyphomycete Teberdinia hygrophila gen. nov., sp. nov. and related anamorphs of Pseudeurotium species. Mycologia 97: 695–709. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Binder M, Schoch CL, Johnston PR, Spatafora JW, et al. (2006) Evolution of helotialean fungi (Leotiomycetes, Pezizomycotina): A nuclear rDNA phylogeny. Molecular Phylogenetics and Evolution 41. [DOI] [PubMed]
- 39.Wang Z, Johnston PR, Takamatsu S, Spatafora JW, Hibbett DS (2006) Toward a phylogenetic classification of the Leotiomycetes based on rDNA data. Mycologia 98. [DOI] [PubMed]
- 40. Geiser DM, Gueidan C, Miadlikowska J, Lutzoni F, Kauff F, et al. (2006) Eurotiomycetes: Eurotiomycetidae and Chaetothyriomycetidae. Mycologia 98: 1053–1064. [DOI] [PubMed] [Google Scholar]
- 41. Shelley V, Kaiser S, Williams T, Kramer M, Haman K, et al. (2012) Evaluation of strategies for the decontamination of equipment for Geomyces destructans, the causative agent of White-nose Syndrome (WNS). Journal of Cave and Karst Studies 75: 1–10. [Google Scholar]
- 42.Team RC (2012) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- 43. Hsu SC, Lockwood JL (1975) Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Applied Microbiology 29: 422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Christensen WB (1946) Urea decomposition as a means of differentiating Proteus and paracolon cultures from each other and from Salmonella and Shigella types. Journal of Bacteriology 52: 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yoon JH, Park JE, Suh DY, Hong SB, Ko SJ, et al. (2007) Comparison of dyes for easy detection of extracellular cellulases in fungi. Mycobiology 35: 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saczi A, Radford A, Erenler K (1986) Detection of cellulolytic fungi by using Congo red as an indicator: a comparative study with the dinitrosalycilic acid reagent method. Journal of Applied Bacteriology 61.
- 47. Bhullar K, Waglechner N, Pawlowski A, Koteva K, Banks ED, et al. (2012) Antibiotic resistance is prevalent in an isolated cave microbiome. PLoS One 7: e34953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31: 426–428. [Google Scholar]
- 49. Bower VE, Bates RG (1955) pH values of the Clark and Lubs buffer solutions at 25°C. Journal of Research at the National Bureau of Standards 55: 197–200. [Google Scholar]
- 50. Clapp CE (2001) Humic substances: considerations of compositions, aspects of structure, and environmental influences. Soil Science 166: 723–737. [Google Scholar]
- 51.Saiz-Jiminez C, Hermosin B (1999) Thermally assisted hydrolysis and methylation of dissolved organic matter in dripping waters from the Altamira Cave. Journal of Analytical and Applied Pyrolysis 49.
- 52. Mudarra M, Andreo B, Baker A (2011) Characterisation of dissolved organic matter in karst spring waters using intrinsic fluorescence: Relationship with infiltration processes. Science of the Total Environment 409: 3448–3462. [DOI] [PubMed] [Google Scholar]
- 53. Weckerly FW (2012) Cave cricket exit counts: environmental influences and duration of surveys. Journal of Cave and Karst Studies 74: 1–6. [Google Scholar]
- 54. Polseela R, Vitta A, Nateeworanant S, Apiwathnasorn C (2011) Distribution of cave-dwelling phlebotomine sand flies and their nocturnal and diurnal activity in Phitsanulok Province, Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 42: 1395–1404. [PubMed] [Google Scholar]
- 55. Kelley RH, Jack JD (2002) Leaf litter decomposition in an ephemeral karst lake (Chaney Lake, Kentucky, U.S.A.). Hydrobiologia 482: 41–47. [Google Scholar]
- 56. Chroňáková A, Horák A, Elhottová D, Krištůfek V (2009) Diverse Archaeal community of a bat guano pile in Domica Cave (Slovak Karst, Slovakia). Folia Microbiologica 54: 436–446. [DOI] [PubMed] [Google Scholar]
- 58. Frick WF, Pollock JF, Hicks AC, Langwig KE, Reynolds DS, et al. (2010) An emerging disease causes regional population collapse of a common North American bat species. Science 329: 679–682. [DOI] [PubMed] [Google Scholar]
- 59. Dzal Y, McGuire LP, Veselka N, Fenton MB (2011) Going, going, gone: the impact of white-nose syndrome on the summer activity of the little brown bat (Myotis lucifugus). Biology Letters 7: 392–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Simon KS, Pipan T, Culver DC (2007) A conceptual model of the flow and distribution of organic carbon in caves. Journal of Cave and Karst Studies 69: 279–284. [Google Scholar]
- 61. Christen-Zaech S, Patel S, Mancini AJ (2008) Recurrent cutaneous Geomyces pannorum infection in three brothers with ichthyosis. J Am Acad Dermatol 58: S112–113. [DOI] [PubMed] [Google Scholar]
- 62. Gianni C, Caretta G, Romano C (2003) Skin infection due to Geomyces pannorum var. pannorum . Mycoses 46: 430–432. [DOI] [PubMed] [Google Scholar]
- 63. Dilly O, Bartsch S, Rosenbrock P, Buscot F, Munch JC (2001) Shifts in physiological capabilities of the microbiota during the decomposition of leaf litter in a black alder (Alnus glutinosa (Gaertn.) L.) forest. Soil Biology and Biochemistry 333: 921–930. [Google Scholar]
- 64. Stehr F, Felk A, Gácser A, Kretschmar M, Mahnβ B, et al. (2004) Expression analysis of the Candida albicans lipase gene family during experimental infections and in patient samples. FEMS Yeast Research 4: 401–408. [DOI] [PubMed] [Google Scholar]
- 65. Bhakdi S, Tranum-Jensen J (1991) Alpha-toxin of Staphylococcus aureus. . Microbiology and Molecular Biology Reviews 55: 733–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nizet V (2002) Streptococcal beta-hemolysins: genetics and role in disease pathogenesis. Trends in Microbiology 10: 575–580. [DOI] [PubMed] [Google Scholar]
- 67.Cryan PM, Meteyer CU, Boyles JG, Blehert DS (2010) Wing pathology of white-nose syndrome in bats suggests life-threatening disruption of physiology. BMC Biology 8: doi:10.1186/1741-7007-1188-1135. [DOI] [PMC free article] [PubMed]
- 68. Mobley HL, Hausinger RP (1989) Microbial ureases: significance, regulation, and molecular characterization. Microbiology and Molecular Biology Reviews 53: 85–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Taylor JP, Wilson B, Mills MS, Burns RG (2002) Comparison of microbial numbers and enzymatic activities in surface soils and subsoils using various techniques. Soil Biology and Biochemistry 34: 387–401. [Google Scholar]
- 70. Ghosh S, Navarathna DHMLP, Roberts DD, Cooper JT, Atkin AL, et al. (2009) Arginine-induced germ tube formation in Candida albicans is essential for escape from murine macrophage line RAW 264.7. Infection and Immunity 77: 1596–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lee IR, Morrow CA, Fraser JA (2013) Nitrogen regulation of virulence in clinically prevalent fungal pathogens. FEMS Microbiology Letters 345: 77–84. [DOI] [PubMed] [Google Scholar]
- 72. Mirbod-Donovan F, Schaller R, Hung CY, Xue J, Reichard U, et al. (2006) Urease produced by Coccidioides posadii contributes to the virulence of this respiratory pathogen. Infection and Immunity 74: 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cole GT (1997) Ammonia production by Coccidioides immitis and its possible significance to the host-fungus interplay. In: Bossche H, Stevens DA, Odds FC, editors. Host-Fungus Interplay. New York, NY: Plenum Press. 247–263.
- 74. Olszewski MA, Noverr MC, Chen GH, Toews GB, Cox GM, et al. (2004) Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. American Journal of Pathology 164: 1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Anbu P, Hilda A, Gopinath SC (2004) Keratinophilic fungi of poultry farm and feather dumping soil in Tamil Nadu, India. Mycopathologia 158: 303–309. [DOI] [PubMed] [Google Scholar]
- 76. Kochkina GA, Ivanushkina NE, Akimov VN, Gilichinskii DA, Ozerskaya SM (2007) Halo- and psychrotolerant Geomyces fungi from arctic cryopegs and marine deposits. Microbiology 76: 39–47. [PubMed] [Google Scholar]
- 77. Marshall WA (1998) Aerial transport of keratinaceous substrate and distribution of the fungus Geomyces pannorum in Antarctic soils. Microbial Ecology 36: 212–219. [DOI] [PubMed] [Google Scholar]
- 78. Rice AV, Currah RS (2006) Two new species of Pseudogymnoascus with Geomyces anamorphs and their phylogenetic relationship with Gymnostellatospora. . Mycologia 98: 307–318. [DOI] [PubMed] [Google Scholar]
- 79. Fenice M, Selbmann L, Zucconi L, Onofri S (1997) Production of extracellular enzymes by Antarctic fungal strains. Polar Biology 17: 275–280. [Google Scholar]
- 80.Duncan SM, Minasaki R, Farrell R, Thwaites JM, Held BW, et al. (2008) Screening fungi isolated from historic Discovery Hut on Ross Island, Antarctica for cellulose degradation. Antarctic Science 20 5.
- 81. Rosa LH, L AVMd, Santiago IF, Rosa CA (2010) Endophytic fungi community associated with the dicotyledonous plant Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) in Antarctica. FEMS Microbiology Ecology 73: 178–189. [DOI] [PubMed] [Google Scholar]
- 82. Vohník M, Fendrych M, Albrechtová J, Vosátka M (2007) Intracellular colonization of Rhododendron and Vaccinium roots by Cenococcum geophilum, Geomyces pannorum and Meliniomyces variabilis. . Folia Microbiologica 52: 407–414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Collection information and Genbank accession numbers for strains used in assessing phylogenetic diversity.
(XLSX)
Primers used for sequencing Pseudogymnoascus isolates.
(XLSX)
Results of two-way ANOVA assessing temperature and species effects in relative enzyme activity.
(DOCX)
Tukey’s Highly Significant Differences Test for growth on organic acids comparing P. destructans to other species.
(DOCX)
Tukey’s Highly Significant Differences Test for relative enzyme activity comparing multiple fungal species to P. destructans.
(DOCX)
Tukeys’ Highly Significant Differences Test comparing intraspecific growth or relative enzyme activity at 20°C and 10°C.
(DOCX)