Abstract
Background
About 60% of Pheochromocytoma (PCC) and Paraganglioma (PGL) patients have either germline or somatic mutations in one of the 12 proposed disease causing genes; SDHA, SDHB, SDHC, SDHD, SDHAF2, VHL, EPAS1, RET, NF1, TMEM127, MAX and H-RAS. Selective screening for germline mutations is routinely performed in clinical management of these diseases. Testing for somatic alterations is not performed on a regular basis because of limitations in interpreting the results.
Aim
The purpose of the study was to investigate genetic events and phenotype correlations in a large cohort of PCC and PGL tumours.
Methods
A total of 101 tumours from 89 patients with PCC and PGL were re-sequenced for a panel of 10 disease causing genes using automated Sanger sequencing. Selected samples were analysed with Multiplex Ligation-dependent Probe Amplification and/or SNParray.
Results
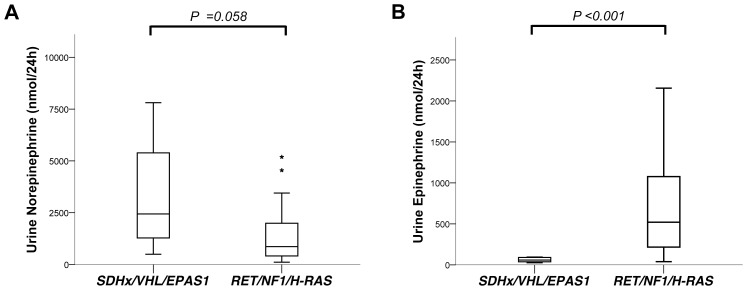
Pathogenic genetic variants were found in tumours from 33 individual patients (37%), 14 (16%) were discovered in constitutional DNA and 16 (18%) were confirmed as somatic. Loss of heterozygosity (LOH) was observed in 1/1 SDHB, 11/11 VHL and 3/3 NF1-associated tumours. In patients with somatic mutations there were no recurrences in contrast to carriers of germline mutations (P = 0.022). SDHx/VHL/EPAS1 associated cases had higher norepinephrine output (P = 0.03) and lower epinephrine output (P<0.001) compared to RET/NF1/H-RAS cases.
Conclusion
Somatic mutations are frequent events in PCC and PGL tumours. Tumour genotype may be further investigated as prognostic factors in these diseases. Growing evidence suggest that analysis of tumour DNA could have an impact on the management of these patients.
Introduction
Pheochromocytoma (PCC) and paraganglioma (PGL) are rare neural crest-derived tumours arising in the adrenal medulla (PCC) or autonomic ganglia (PGL). A majority of patients present with a focal tumour lesion and may be cured with R0 resection [1]–[3]. However, even following an apparently successful surgical resection, there may be a risk of local or metastatic recurrence that motivates a long follow up period [2], [4], [5]. Age, familial disease and tumour size correlate to increased risk of malignancy and recurrence [2] and histologic criteria may also aid in predicting risk for malignant disease [6], [7]. Translational studies show that approximately 60% of PCC and PGL cases have either germline or somatic mutations in one of 13 suggested disease causing loci; SDH subunits A, B, C and D, SDHAF2, VHL, EPAS1, RET, NF1, TMEM127, MAX and H-RAS [8]–[20]. In the clinical setting, genetic screening of these genes by fragment prioritization of germline DNA is regarded as golden standard of care, and may have a substantial impact on patient management [21], [22]. Depending on the affected gene, the risk of local recurrence and/or metastatic disease can be estimated and guide in the selection of appropriate preventive measures [22]. Screening for tumour specific genetic events is not recommended in clinical practice due to a lack of genotype-phenotype correlations that could justify the necessary resource allocation [8], [23]. However, as most genetic screening studies of PCC and PGL tumours have been performed on small cohorts, we hypothesized that a comprehensive genetic screening in a large clinically annotated cohort could be informative. The aim of this study was, to describe the genetic landscape of PCC and PGL tumours and to correlate tumour genotype with patient characteristics and disease outcome.
Patients and Methods
Patients
This is a single centre, retrospective study of 101 tumour samples from 89 patients with PCC and PGL treated at the Department of Surgery, Uppsala university hospital, Sweden. Selected patients were previously screened for mutations in H-RAS describing somatic genetic variants (n = 4) and MAX describing no pathogenic genetic variant [20], [24]. Fourteen patients were clinically diagnosed with hereditary syndromes; familial paraganglioma type 4 (PGL4; n = 2), Von Hippel Lindau syndrome (VHL; n = 4) and Multiple Endocrine Neoplasia type 2 (MEN2; n = 8) by certified genetic testing laboratories. Four additional patients had been diagnosed with Neurofibromatosis type 1 (NF1) by presence of clinical criteria. DNA samples from 195 healthy and unrelated individuals were utilized as a control to determine frequency of germline variants with unknown significance (VUS) in Swedish population.
Ethical Statement
Ethical approval was obtained from the regional ethics committee in Uppsala as well as written informed consent from the individual patients. All patients were above 18 years of age at the time of inclusion.
Clinical Data
Age at diagnosis was set at the time for radiological diagnosis. Tumour size was calculated as the mean of two diameters. Cases were classified as metastatic by the presence of invasion into nonchromaffin organs determined by histological examination or radiological/molecular imaging. Preoperative urinary catecholamines measurements analysed in a clinical setting were included for evaluation. Norepinephrine assays had been performed with two different reference intervals, <350 nmol/24 h and <400 nmol/24 h depending on utilized assays. Reference intervals for urinary epinephrine was <90 nmol/24 h, for plasma normetanephrine <0,6 nmol/L and for plasma metanephrine <0,3 nmol/L.
DNA Extraction & Sequencing
DNA was extracted from cryosections of tumour samples, peripheral blood and/or normal tissue, using DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) as previously described [25]. Cryosections from included tumours were analysed for tissue morphology and selected samples were macrodissected in order to reduce contamination of normal cells. Using a phenotype guided fragment prioritization approach [1], [26], exons and intron-exon boundaries of SDHB (NM_003000.2), SDHC (NM_003001.3), SDHD (NM_003002.2), SDHAF2 (NM_017841.2), VHL (NM_000551.3), EPAS1 (exons 9 and 12, NM_001430.4), RET (exons 10–11 and 13–16, NM_020975.4), TMEM127 (NM_017849.3), MAX (NM_002382.3) and H-RAS (exons 2 and 3, NM_176795.3) were amplified by PCR and sequenced using automated Sanger sequencing (Beckman Coulter Genomics, Takeley, UK). In patients with pathogenic germline variants, all exons and intron-exon boundaries of the affected gene were sequenced in order to investigate a potentially inactivating variant on the 2nd allele. Ultimately, patients without detected pathogenic mutations in this study had been screened for all ten above mentioned genes. Primer sequences can be obtained by request.
Mutational Analysis
Chromatograms generated by Sanger Sequencing were reviewed using CLC genomics workbench 5.5 (CLC bio, Aarhus, Denmark). Genetic variants were annotated for overlapping information available in public databases; Catalogue of Somatic Mutations in Cancer (COSMIC) [27], the Single Nucleotide Polymorphism database (dbSNP), Human Genome Mutation Database (HGMD public) [28] and Leiden Open source Variation Database (LOVD). In silico analysis was performed using Sorting Intolerant From Tolerant (SIFT) [29] and Polymorphism Phenotyping v2 (Polyphen-2) [30].
Multiplex Ligation-dependent Probe Amplification
Inclusion criteria for analysis were absence of a pathogenic germline variant. Only DNA extracted from blood/normal tissue was selected for Multiplex ligation-dependent probe amplification (MLPA). Included samples were analysed with SALSA MLPA P226 SDH and P016-B2 VHL probe mixes (MRC-Holland, Amsterdam, Netherlands) that have coverage of the SDHA, SDHB, SDHC, SDHD, SDHAF2 and VHL loci. Reactions were carried out as previously described [31] and an ABI3130xl Genetic Analyzer (Life Technologies, Carlsbad, CA) was used for fragment separation. The MLPA data were analyzed using GeneMapper 4.0 genotyping software (Applied Biosystem) and SeqPilot version 3.3.2 (JSI medical systems GmBH, Kippenheim, Germany). The experiments were carried out at a laboratory certified for clinical use and analysed by an experienced clinical investigator (MN).
Single Nucleotide Polymorphism Array
Inclusion criteria were presence of pathogenic or unknown variants in SDHB, SDHC, VHL or clinical criteria of NF1. Tumour DNA from the selected samples were subjected to SNParray analysis; using Illumina Omni1-Quad or Omni2,5-Quad chips (Illumina Inc, CA, USA), containing 1,140,419 and 2,379,855 probes respectively. Hybridization and sequencing was performed by university core facilities, SNP&SEQ Technology Platform in Uppsala, Sweden (http://molmed.medsci.uu.se/SNPSEQTechnologyPlatform/). Generated data was imported into the Illumina BeadStudio (Illumina, CA, USA) software and analysed using Nexus Copy Number Variation 7.0 Build 7887 (Biodiscovery Inc, CA, USA) to detect copy number variation and allelic imbalance using default thresholds The fraction of DNA derived from tumour cells were estimated by analysing the B-allele frequency in regions showing copy number alterations [32]. Samples with tumour cell purity <70% were analysed with adjusted settings.
Statistics
SPSS 19 (IBM, Armonk NY, US) was used for statistical calculations. Chi2 test was performed for analysis of nominal variables. As age at diagnosis, tumour size and catecholamine output were not normally distributed, hence a non-parametric test (Mann-Whitney U test) was selected for analysis of these scaled values. Patients with variants of unknown significance (VUS) were classified as wild type. Multiple regression test of phenotype correlation to carrier status and genotypes were not possible to perform due to the low number of observations. P-values <0.05 were considered as significant and <0.1 as borderline significant.
Results
Patient characteristics are described in table 1. The median age at diagnosis was 49 years (range 15–85) and there were 35 males and 53 females. Eighty patients had adrenal PCC (31 left adrenal, 37 right adrenal, 10 bilateral), eight had thoracoabdominal PGLs and there were one head and neck PGL. The median tumour size was 55 mm (range 8–170). There were wide discrepancies in catecholamine output; urinary norepinephrine range 112–19130 nmol/24 h (ref <400/<350 nmol/24 h), urinary epinephrine range 19–33322 nmol/24 h (ref <90 nmol/24 h), plasma normetanephrine range 1–37 nmol/L (ref <0,6 nmol/L), and plasma metanephrine range 0–140 nmol/L (ref <0,3 nmol/L). There were 13 patients with recurrent disease, eight of whom recurred with distant metastases and five with local recurrences. One additional patient had distant metastases at the time of diagnosis. The median follow up time was 106 months (range 0–714 months).
Table 1. Clinical characteristics and carrier status.
| Cohort (n = 89) | No discovered mutations (n = 52)* | Germline variants (n = 18)** | Somatic variants (n = 16) | Germline and no mutation | Somatic and no mutation | Germline and Somatic mutation | ||||||
| Range | Range | Range | Range | P | ||||||||
| Median age, years | 49,5 | 15–85 | 53 | 22–85 | 29,5 | 15–66 | 48 | 25–81 | 0.002§ | 0.622§ | 0.025§ | |
| Gender, Male/Female | 35/53 | 19/32 | 7/11 | 8/8 | 0.902§§ | 0.365§§ | 0.515§§ | |||||
| Tumour specifications | ||||||||||||
| Median size, mm | 55 | 8–170 | 60 | 20–140 | 51 (n = 14) | 20–170 | 45 | 8–100 | 0.166§ | 0.305§ | 0.755§ | |
| Localization, left adrenal/right adrenal | 31/37 | 22/21 | 3/5 | 5/9 | 0.478§§ | 0.315§§ | 0.933§§ | |||||
| Adrenal/extra adrenal tumour | 80/9 | 47/5 | 16/2 | 14/2 | 0.855§§ | 0.740§§ | 0.900§§ | |||||
| Multifocal tumours | 10 | 1 | 9 | 0 | <0.001§§ | 0.561§§ | <0.001§§ | |||||
| Biochemistry | ||||||||||||
| Median Urine Norepinephrine, nmol/24 h | 1687 | 112–19130 | 2181,5 | 230–19130 | 836 | 112–4545 | 2439 | 225–14244 | 0.033§ | 0.962§ | 0.049§ | |
| Median Urine Epinephrine, nmol/24 h | 189 | 19–33322 | 247 | 19–33322 | 195 | 27–641 | 96 | 25–11664 | 0.2§ | 0.541§ | 0.622§ | |
| Median Plasma Normetanephrine, nmol/L | 6,4 | 1–37 | 7,1 | 4–16 | 1,4 | 1–2 | 11,6 | 1–37 | 0.004§ | 0.640§ | 0.136§ | |
| Median Plasma Metaepinephrine, nmol/L | 0,5 | 0–140 | 1,65 | 0–34 | 0,45 | 0–1 | 1,05 | 0–140 | 0.579§ | 0.961§ | 0.391§ | |
| Recurrent disease | 13 | 8 | 5 | 0 | 0.376§§ | 0.070§§ | 0.022§§ | |||||
| Metastatic disease*** | 9 | 7 | 2 | 0 | 0.797§§ | 0.121§§ | 0.169§§ | |||||
Statistical correlation of clinical variables and carriers status. Multifocal tumours was defined as bilateral pheochromocytoma or multiple paraganglioma tumours.
Patients with no available germline DNA were excluded.
Included 14 germline variants and 4 patients with clinical criteria of Neurofibromatosis type 1.
Nine patients had metastatic disease, one of these had metastatic disease at the time of diagnosis and the remaining had metastatic recurrences later on in the disease course. §Mann-Whitney U Test and §§Chi Square test.
Genetic Screening
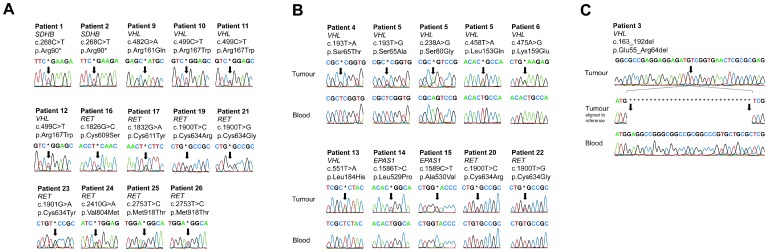
A total of 33 patients (37%) had a pathogenic genetic variant in included disease causing loci. We could confirm 14 of these mutations in constitutional DNA (Figure 1a) and 16 were confirmed as somatic by absence in constitutional DNA (Figures 1b and c). There was no constitutional DNA available for three of the patients with pathogenic mutations. All genetic variants previously clinically diagnosed by the Department of Clinical Genetics (n = 14) were verified in the present study. There were four additional patients with clinical criteria of Neurofibromatosis type 1 but with no genetic testing. Cases with discovered mutations and corresponding clinical characteristics are presented in Table 2.
Figure 1. Chromatograms exported from CLC Genomics Workbench 5.5 displaying (A) Pathogenic genetic variants available in constitutional DNA, (B) confirmed somatic variants and (C) 30 base pair somatic deletion in VHL.
Table 2. Pathogenic and unknown genetic varaints and their corresponding patient characteristics.
| Patient no. | Diagnosis | Gender | Age at diagnosis | Hereditary | Syndrome criteria | Size (mm) | Unilateral/multiple | Recurrent | Metastatic | Gene | Exon | Somatic/Germline | cDNA | Amino acid substitution | Concluded Pathogenicity |
| 1 | TA PGL | M | 15 | + | PGL4 | - | Multiple | + | − | SDHB | 3 | Germline | c.268C>T | p.Arg90* | Pathogenic |
| 2 | TA PGL | F | 26 | + | PGL4 | - | Multiple | + | − | SDHB | 3 | Germline | c.268C>T | p.Arg90* | Pathogenic |
| 3 | PCC | M | 76 | − | − | 70 | Uni | − | − | VHL | 1 | Somatic | c.163_192del | p.Glu55_Arg64del | Pathogenic |
| 4 | PCC | M | 58 | − | − | 90 | Uni | − | − | VHL | 1 | Somatic | c.193T>A | p.Ser65Thr | Pathogenic |
| 5 | PCC | F | 49 | − | − | 25 | Uni | − | − | VHL | 1 | Somatic | c.193T>G | p.Ser65Ala | Pathogenic |
| 6 | PCC | F | 25 | − | − | 25 | Uni | − | − | VHL | 1 | Somatic | c.238A>G | p.Ser80Gly | Pathogenic |
| 7 | PCC | F | 47 | − | − | 8 | Uni | − | − | VHL | 2 | Somatic | c.458T>A | p.Leu153Gln | Pathogenic |
| 8 | PCC | M | 47 | − | − | 100 | Uni | − | − | VHL | 3 | Somatic | c.475A>G | p.Lys159Glu | Pathogenic |
| 9 | PCC | F | 66 | NA | VHL | 65/60 | Multiple | − | − | VHL | 3 | Germline | c.482G>A | p.Arg161Gln | Pathogenic |
| 10 | PCC | F | 25 | + | VHL | 60/40 | Multiple | − | − | VHL | 3 | Germline | c.499C>T | p.Arg167Trp | Pathogenic |
| 11 | PCC | M | 21 | + | VHL | 40/30 | Multiple | − | − | VHL | 3 | Germline | c.499C>T | p.Arg167Trp | Pathogenic |
| 12 | PCC | F | 25 | + | VHL | 30 | Uni | − | − | VHL | 3 | Germline | c.499C>T | p.Arg167Trp | Pathogenic |
| 13 | PCC | F | 31 | − | − | 40 | Uni | − | − | VHL | 3 | Somatic | c.551T>A | p.Leu184His | Pathogenic |
| 14 | TA PGL | F | 64 | − | − | 20 | Uni | − | − | EPAS1 | 12 | Somatic | c.1586T>C | p.Leu529Pro | Pathogenic |
| 15 | PCC | F | 81 | − | − | 45 | Uni | − | − | EPAS1 | 12 | Somatic | c.1589C>T | p.Ala530Val | Pathogenic |
| 16 | PCC | F | 27 | + | MEN2A | 100 | Uni | − | − | RET | 10 | Germline | c.1826G>C | p.Cys609Ser | Pathogenic |
| 17 | PCC | F | 57 | + | MEN2A | 23 | Multiple | + | − | RET | 10 | Germline | c.1832G>A | p.Cys611Tyr | Pathogenic |
| 18 | PCC | F | 61 | − | − | 15 | Uni | − | − | RET | 11 | NA | c.1891G>T | p.Asp631Tyr | Pathogenic |
| 19 | PCC | F | 29 | + | MEN2A | 30/47 | Multiple | − | − | RET | 11 | Germline | c.1900T>C | p.Cys634Arg | Pathogenic |
| 20 | PCC | F | 47 | − | − | 80 | Uni | − | − | RET | 11 | Somatic | c.1900T>C | p.Cys634Arg | Pathogenic |
| 21 | PCC | M | 29 | + | MEN2A | 20/NA | Multiple | + | − | RET | 11 | Germline | c.1900T>G | p.Cys634Gly | Pathogenic |
| 22 | PCC | F | 57 | − | − | 60 | Uni | − | − | RET | 11 | Somatic | c.1900T>G | p.Cys634Gly | Pathogenic |
| 23 | PCC | F | 30 | + | MEN2A | 20/NA | Multiple | − | − | RET | 11 | Germline | c.1901G>A | p. Cys634Tyr | Pathogenic |
| 24 | PCC | M | 65 | - | MEN2A | 95 | Uni | + | + | RET | 14 | Germline | c.2410G>A | p.Val804Met | Pathogenic |
| 25 | PCC | F | 18 | − | MEN2B | 25 | Uni | + | − | RET | 16 | Germline | c.2753T>C | p.Met918Thr | Pathogenic |
| 26 | PCC | M | 34 | − | MEN2B | 60/60 | Multiple | − | − | RET | 16 | Germline | c.2753T>C | p.Met918Thr | Pathogenic |
| 27 | PCC | M | 45 | − | − | 30 | Uni | NA | NA | RET | 16 | NA | c.2753T>C | p.Met918Thr | Pathogenic |
| 28 | PCC | F | 31 | − | − | 55 | Uni | − | − | RET | 16 | NA | c.2753T>C | p.Met918Thr | Pathogenic |
| 29 | PCC | M | 54 | − | − | 45 | Uni | − | − | H-RAS | 2 | Somatic | c.37G>C | p.Gly13Arg | Pathogenic |
| 30 | PCC | M | 76 | − | − | 76 | Uni | − | − | H-RAS | 3 | Somatic | c.181C>A | p.Gln61Lys | Pathogenic |
| 31 | PCC | M | 36 | − | − | 30 | Uni | − | − | H-RAS | 3 | Somatic | c.181C>A | p.Gln61Lys | Pathogenic |
| 32 | TA PGL | M | 31 | − | − | 100 | Uni | − | − | H-RAS | 3 | Somatic | c.182A>G | p.Gln61Arg | Pathogenic |
| 33 | PCC | M | 45 | − | − | 100 | Uni | − | − | H-RAS | 3 | Somatic | c.182A>G | p.Gln61Arg | Pathogenic |
| 34 | PCC | F | 61 | − | − | 25 | Uni | − | − | SDHC | 5 | Germline | c.328C>T | p.Pro110Ser | Unknown |
| 35 | PCC | M | 55 | − | − | 65 | Uni | − | − | SDHC | 6 | Germline | c.490A>T | p.Met164Val | Unknown |
| 36 | TA PGL | M | 28 | − | − | 20 | Uni | − | − | VHL | 3 | Germline | c.548C>T | p.Ser183Leu | Unknown |
| 37 | PCC | F | 27 | − | − | 50 | Uni | − | − | RET | 13 | Germline | c.2372A>T | p.Tyr791Phe | Unknown |
PCC; Pheochromocytoma, TA PGL; Thoracoabdominal Paraganglioma, NA; Not Available, F; Female, M; Male, MEN2; Multiple Endocrine Neoplasia type 2, PGL4; Familial Paraganglioma type 4, VHL; Von Hippel Lindau, Uni; Unilateral or focal tumour lesion.
Two related patients (mother and son) presented with multiple abdominal PGL. Both had several local recurrences that were not classified as metastatic lesions. Re-sequencing revealed a pathogenic nonsense mutation in SDHB; c.268C>T, p.Arg90* [13] that was present in DNA from blood.
There were 12 cases with pathogenic mutations in the VHL gene. Patient number 9 with bilateral PCC (index case) had a pathogenic germline missense mutation in VHL; c.482G>A p.Arg161Gln. A family comprising of three siblings with bilateral or unilateral PCC had pathogenic germline missense mutation in VHL; c.499C>T, p.Arg167Trp [33]. Both p.Arg161Gln and p.Arg167Trp had previously been reported as pathogenic [33]. Seven patients had somatic mutations in VHL. There were six unique SNVs; c.193T>G, p.Ser65Ala; c.193T>A, p.Ser65Thr; c.238A>G, p.Ser80Gly; c.458T>A, p.Leu153Gln; c.475A>G, p.Lys159Glu and c.551T>A, p.Leu184His in one patient each. Patient number 4 had a 30 base pair deletion c.163_192del, p.Glu55_Arg64del that was absent in DNA from peripheral blood. All carriers of somatic VHL mutations had unilateral PCC, sporadic disease presentation and there were no apparent signs or symptoms of VHL syndrome.
Two patients had mutations in EPAS1 which were absent in DNA from their blood; one c.1586T>C, p.Leu529Pro and one c.1589C>T, p.Ala530Val. These mutations are previously described as pathogenic [16], [34]. Both patients had sporadic disease presentation. Patient 16 had borderline polycytemia with a haemoglobin level of 150 g/L (reference interval 120–150 g/L).
There were 13 patients with pathogenic mutations in RET. Eight had germline pathogenic mutations and clinical characteristics of MEN2 syndrome; c.1826G>C, p.Cys609Ser; c.1832G>A, p.Cys611Tyr; c.1900T>C, p.Cys634Arg; c.1900T>G, p.Cys634Gly; c.1901G>A, p. Cys634Tyr; c.2410G>A, p.Val804Met in one patient each and c.2753T>C, p.Met918Thr in tow different patients. Two patients with unilateral PCC and sporadic disease presentation had somatic mutation in RET; c.1900T>G, p.Cys634Gly and c.1900T>C, p.Cys634Arg. For three of the patients with SNVs in RET there were no constitutional DNA available; c.1891G>T, p.Asp631Tyr in one patient and c.2753T>C, p.Met918Thr in two patients. All three cases had sporadic disease presentation and there were no signs or symptoms suggesting MEN2 syndrome. All these RET mutations are described as pathogenic in the literature [8], [33].
Four patients had previously been described with somatic H-RAS mutations [20]. One additional somatic mutation in H-RAS; c.181C>A, p.Gln61Lys; was detected in a male patient that had sporadic disease presentation.
Variants of Unknown Significance
Four patients had germline variants of unknown significance (VUS). There were two VUS in SDHC; c.328C>T, Pro110Ser [35] and c.490A>T, Met164Leu in two different patients with unilateral PCC and sporadic disease presentation. Both mutations were available in constitutional DNA. Succinate dehydrogenase subunit C Met164Leu has been reported to have impact in functional models but was classified as benign in vivo [36]. The pathogenicity of Pro110Ser has not been investigated in detail [35]. Succinate dehydrogenase subunit C Codon 110 is conserved among vertebrate orthologs whereas codon 164 is not a conserved residue. In silico analysis determined the variants as benign, SIFT (0,93 and 0,96) and Polyphen2 (0,231 and 0,0).
Patient 36, a 28 year old male, was referred to the local hospital due to hypertension, headache and multiple episodes of syncope. Urinary norepinephrine was elevated, 1752 nmol/24 h (<350), but epinephrine was within reference margins 37 nmol/24 h (<90). A computed tomography revealed an abdominal mass located in close proximity to the left renal artery and vein. The lesion was surgically resected and the pathology report showed a PGL, 20 mm in size with a Ki67 index of <1%. Genetic screening of RET as well as Succinate Dehydrogenase subunits B and D was normal. The proband remains free of recurrence 21 months following the initial diagnosis. There were no apparent signs or symptoms of von Hippel Lindau syndrome. Sanger sequencing revealed a single nucleotide polymorphism in VHL; c.548C>T, p.Ser183Leu. Multiplex ligation-dependent probe amplification of constitutional DNA did not show any pathological imbalances. In tumour tissue, loss of heterozogosity and copy number loss was observed on the whole arm of chromosome 3p by Omni-1-quad SNP array (Illumina, CA, USA).VHL; c.548C>T, p.Ser183Leu has been classified as pathogenic in a functional model but the individual contribution of the allele to patient phenotype is not fully described [37]. Screening of 190 healthy individuals revealed homozygous C allele in all cases. Codon 183 is conserved among mammalian orthologs and in silico analysis determined the variant as probably pathogenic: SIFT (0,18) and Polyphen2 (1,0).
Patient 37 was diagnosed with a unilateral PCC at 27 years of age having apparently sporadic presentation. Re-sequencing revealed a RET mutation c.2372A>T, p.Tyr791Phe that was found in constitutional DNA. The pathogenicity of RET p.Tyr791Phe is disputed [38], [39].
There were no pathogenic variants discovered in SDHAF2, TMEM127 and MAX.
Structural Variations
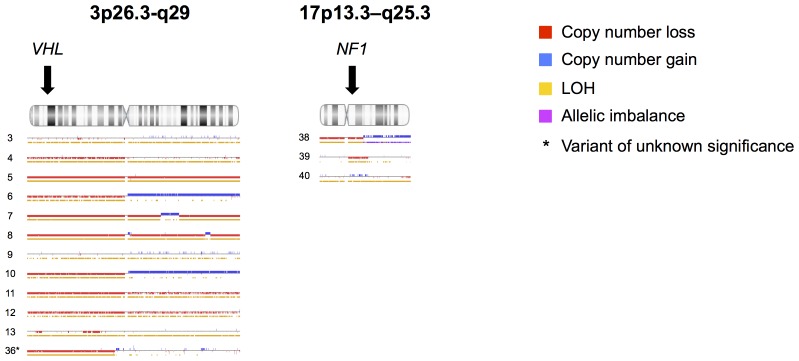
Multiplex ligation-dependent probe amplification analysis did not reveal any copy number gains or losses. Analysis of SNP array data showed loss of heterozygosity (LOH) located to the genomic coordinates of the respective gene in 1/1 SDHB, 11/11 VHL and 3/3 NF1-related tumours (table 3, figure 2). There were no LOH at coordinates corresponding to SDHC loci in tumours from patients with germline SDHC Pro110Ser and Met164Leu variants. Patient 36 with a germline VHL p.Ser183Leu had LOH at the VHL loci. Analysis failed due to corrupted data in two cases, one SDHB and one NF1-related tumour.
Table 3. Copy number variation and loss of heterozygosity by SNP array.
| Copy number variation | Loss of heterozogozity | |||||||
| Patient no. | Gene | Amino acid substitution | Chromosome | Start position | End position | Start position | End position | Nexus 7.0 Quality score |
| 3 | VHL | p.Glu55_Arg64del | 3 | Not detected | 1 | 194000000 | 0,018 | |
| 4 | VHL | p.Ser65Thr | 3 | 1 | 85000000 | 1 | 194000000 | 0,046 |
| 5 | VHL | p.Ser65Ala | 3 | 1 | 194000000 | 1 | 194000000 | 0,016 |
| 6 | VHL | p.Ser80Gly | 3 | 1 | 85000000 | 1 | 85000000 | 0,017 |
| 7 | VHL | p.Leu153Gln | 3 | 1 | 120000000 | 1 | 120000000 | 0,074 |
| 8 | VHL | p.Lys159Glu | 3 | 1 | 194000000 | 1 | 194000000 | 0,028 |
| 9 | VHL | p.Arg161Gln | 3 | Not detected | 1 | 194000000 | 0,024 | |
| 10 | VHL | p.Arg167Trp | 3 | 1 | 87000000 | 1 | 87000000 | 0,022 |
| 11 | VHL | p.Arg167Trp | 3 | 1 | 194000000 | 1 | 194000000 | 0,025 |
| 12 | VHL | p.Arg167Trp | 3 | 1 | 194000000 | 1 | 194000000 | 0,033 |
| 13 | VHL | p.Leu184His | 3 | 9000000 | 13000000 | 1 | 194000000 | 0,022 |
| 36 | VHL | p.Ser183Leu | 3 | 1 | 81000000 | 1 | 81000000 | 0,027 |
| 38 | NF1 | NA | 17 | 1 | 39000000 | 1 | 39000000 | 0,026 |
| 39 | NF1 | NA | 17 | 25000000 | 43000000 | 25000000 | 43000000 | 0,03 |
| 40 | NF1 | NA | 17 | Not detected | 1 | 81000000 | 0,046 | |
Tumours with loss of heterozygosity at loci of mutated tumour suppressor. Patients with diagnostic criteria of NF1 were considered as potential carriers of a pathogenic variant in the NF1 gene. NA, Not Available.
Figure 2. Detected copy number events from SNP array as displayed by Nexus copy number 7.0.
Results are separated by chromosome and presented for each individual tumour with patient id at the left margin. Presented data constitute cases harbouring pathogenic genetic variants in VHL (n = 11) as well as patients with clinical criteria of NF1 (n = 3). Data is also presented for patient 36 that harboured a VHL mutation of unknown significance. Colour annotation indicates copy number loss (red), copy number gain (blue), loss of heterozygosity (yellow) and allelic imbalance (magenta). Arrows indicates VHL (chromosome 3) and NF1 (chromosome 17) loci. Loss of heterozygosity at VHL locus was detected in 11/11 tumours with pathogenic VHL mutations and in 3/3 tumours from patients with clinical criteria of NF1.
Statistical Correlation
Statistical analyses of carrier status and genotype correlations to phenotype are presented in Tables 1 and 4. Stratified into groups accordingly to mutation status, germline carriers had a age at diagnosis that were significantly lower (median 29,5 years) compared to those with somatic aberrations (median 48 years, P = 0.025) as well as patients without known mutations (median 53 years, P = 0.002). The frequency of mutifocal tumours were also different in germline carriers (53%) compared to patients with somatic carrier status (0%, P<0.001) as well as those without known mutations (2%, P<0.001).
Table 4. Clinical characteristics and genotype.
| No discovered mutations (n = 52) | Cluster 1 SDHx/VHL/EPAS1 (n = 15) | Cluster 2 RET/NF1/H-RAS (n = 22) | Cluster 1 and no mutation | Cluster 2 and no mutation | Cluster 1 and Cluster 2 | ||||
| Range | Range | P | |||||||
| Median age, years | 53 | 22–85 | 47 | 15–81 | 45 | 18–76 | 0.089§ | 0.036§ | 0.699§ |
| Gender, Male/Female | 19/32 | 5/10 | 11/11 | 0.781§§ | 0.310§§ | 0.315§§ | |||
| Tumour specifications | |||||||||
| Median size, mm | 60 | 20–140 | 40 | 8–100 | 51 | 15–170 | 0.086§ | 0.213§ | 0.658§ |
| Localization, left adrenal/right adrenal | 22/21 | 1/8 | 8/8 | 0.028§§ | 0.937§§ | 0.052§§ | |||
| Adrenal/extra adrenal tumour | 47/5 | 12/3 | 21/1 | 0.275§§ | 0.465§§ | 0.137§§ | |||
| Multifocal tumours | 1 | 4 | 5 | <0.001§§ | 0.004§§ | 0.693§§ | |||
| Biochemistry | |||||||||
| Median Urine Norepinephrine, nmol/24 h | 2181,5 | 230–19130 | 2439 | 495–14244 | 862 | 112–5189 | 0.960§ | 0.018§ | 0.03§ |
| Median Urine Epinephrine, nmol/24 h | 247 | 19–33322 | 58 | 25–96 | 520 | 39–11664 | 0.002§ | 0.232§ | <0.001§ |
| Median Plasma Normetanephrine, nmol/L | 7,1 | 4–16 | 19,3 | 2–37 | 1,5 | 1–34 | 1.0§ | 0.076§ | 0.327§ |
| Median Plasma Metaepinephrine, nmol/L | 1,65 | 0–34 | 0,25 | 0,25 | 0,7 | 0–140 | 0.304§ | 0.914§ | 0.073§ |
| Recurrent disease | 8 | 2 | 3 | 0.689§§ | 0.723§§ | 0.925§§ | |||
| Metastatic disease | 7 | 0 | 2 | 0.133§§ | 0.599§§ | 0.230§§ | |||
Statistical analysis of correlation between clinical characteristics and genotype. § Mann-Whitney U Test, §§ Chi-square test, * Patients with clinical criteria of NF1 defined as having germline carrier status.
No cases of recurrent disease were observed in somatic carriers (0%), in contrast to germline carriers (28%, P = 0.022) and borderline significant compared to patients without discovered mutations (15%, P = 0.07). Preoperative levels of urine norepinephrine were lower in patients with germline carrier status compared to somatic carriers and those without mutation (P = 0.049 and P = 0.033 respectively). Gender, tumour size, tumour localization, metastatic disease as well as urine and plasma epinephrine output were not different among the three carrier status groups.
Stratification according to genotype into cluster 1; SDHx/VHL/EPAS1 mutants and cluster 2; RET/NF1/H-RAS mutants, resulted in a difference in age at diagnosis between cluster 2 carriers (median 45) and patients without mutations (median 53, P = 0.036). A borderline significance difference was also noted for PCC localization, there were one left adrenal and eight right adrenals affected in cluster 1 compared to eight left and eight right-sided tumours in cluster 2 (P = 0.052). A difference in PCC lateralization was also noted between cluster 1 patients and those without discovered mutations (P = 0.028). In patients without mutation there were one case with multiple PGL and none bilateral PCC, different than in cluster 1 (4 cases total, P<0.001) and cluster 2 (5 cases total, P = 0.004). Urine norepinephrine levels (Figures 3a and b) were higher in cluster 1 patients (median 2439/24 h, P = 0.03) and those without mutation (median 2181,5, P = 0.018) compared to cluster 2 (median 862). Reversely Epinephrine levels were lower in cluster 1 (median 58/24 h, P<0.001) and in those without mutation (median 247 nmol/24 h, P = 0.002) compared to cluster 2 patients (median 520 nmol/24 h). Age at diagnosis, gender, plasma cathecolamines as well as recurrent and metastatic disease were not different among the three groups.
Figure 3. Box plots illustrating preoperative levels of urinary (A) norepinephrine and (B) epinephrine stratified accordingly to genotype clusters.
Discussion
Genetics
We have analysed 101 PCC and PGL tumours for SNVs in nine different genes, complemented by selective MLPA and SNP array analysis. A total of 33 patients (37%) had pathogenic variants in SDHB, VHL, EPAS1, RET and H-RAS. Including patients with clinical criteria of Neurofibromatosis type 1 and loss of heterozygosity at the NF1 locus, 41% of the cohort could be associated with genetic aberrations in known genes. We did not find any pathogenic germline mutations that had not been previously discovered by clinical screening, this low frequency of germline mutations in apparently sporadic patients have previously been described in Swedish patients [40]. Considering the characteristics of the cohort with a predominance of benign and unilateral PCC having sporadic presentation, the frequencies of pathogenic genetic variants and patient carrier status were similar to those previously reported [8]. Loss of heterozygosity could be detected in tumour DNA from 11/12 patients having somatic or germline mutations in the SDHB or VHL genes. SNParray analysis of tumour DNA from patient 10 (VHL p.Arg161Gln) did not show LOH at any locus. This tumour sample will have to be carefully reviewed for contaminating wild type cells and re-analysed. Patients with clinical criteria of NF1 syndrome did not have a clinical diagnostic genetic test performed, probably due to the size of the NF1 gene and the number of possible loci. Three of these patients tumours were analysed with SNParray that revealed loss of heterozygosity at the NF1 loci in tumour DNA in all investigated cases. This strongly suggest that these patients do have a germline mutation in NF1 [15], [18]. The interpretation of clinical correlations in sporadic patients presented by this study is limited by the absence of analysis of the NF1 gene that was recently found to be commonly affected in patients with sporadic PCC and PGL [15], [18]. To analyse this extensive loci, further studies may utilize Next Generation Sequencing or SNP arrays [35], [41].
Multiplex ligation-dependent probe amplification analysis of constitutional DNA showed no copy number gains or losses in any of the investigated cases. Both the MLPA laboratory workflow and result analysis were performed using robust workflows by experienced clinical investigators, ensuring high reliability of these results.
Variants of Unknown Significance
Multiple factors contribute to determine the disease causing impact of a specific genetic variant; variant deleteriousness, probands phenotype, family history, molecular and in silico characterization as wells as the allele frequency in a population without disease [42]. For SDHC Pro110Ser and Met164Leu, clinical presentation and family history did not indicate familial paraganglioma type 3. Analysis of the biochemical phenotype revealed a high norepinephrine to epinephrine ratio, pointing to a disease causing mutation in the SDHx or VHL loci [43]. Available literature did not support neither classification of pathogenic nor benign status of SDHC Pro110Ser and Met164Leu. In silico analysis determined these variants to have a benign effect on protein structure. But as in silico methods have a high rate of false negative predictions [44] and the fact that the alleles are not reported in any normal population we classified the variants as VUSs.
In patient 37 with the germline VHL p.Ser183Leu variant there was no family history suggesting VHL syndrome. The patient phenotype with a focal PGL did not strongly indicate a germline mutation even though up to 10% of patients may present with PGL [26]. The ratio of norepinephrine to epinephrine was high indicating a mutation in SDHx or VHL genes [43]. Screening of 190 healthy subjects did not find this variant nor was it found by a database search, supporting that the variant is not a common polymorphism. In silico calculation determined the variant as probable pathogenic. This variant’s contribution to patient disease was classified as unknown. However, several indicators point out this variant as potentially pathogenic and as most variants in the VHL gene are pathogenic, this variant should influence the clinical management of the proband.
Genotype Clustering
The molecular phenotype of EPAS1 mutated tumours has been disputed [16], [17]. However, clinical correlations and experimental investigations have indicated similarities of EPAS1 lesions to SDHx and VHL mutated tumours. Catecholamine production detected in EPAS1 carriers in this study suggested cluster 1 differentiation. The available literature strongly suggests a common pathway for H-RAS with cluster 2 genes RET and NF1 [20], [43]. Additionally, the catecholamine output observed in H-RAS mutated tumours resemble that of RET and NF1 [43]. We included EPAS1 in cluster 1 and H-RAS in cluster 2 as well as patients with clinical criteria of NF1.
Genotype Phenotype Correlations
As previously described, germline carriers were significantly younger at time of diagnosis, and had a higher frequency of multifocal disease [26]. Among carriers with somatic mutations there were no recurrences, nor were there any cases of metastatic disease. Stratified into cluster 1 and 2 genotypes, differences in biochemistry output was observed, in line with previous studies investigating germline carriers [43]. A difference in the location of adrenal tumours was noted with cluster 1 patients having the right adrenal affected in all but one case. No obvious explanation to this lateralisation was found in the literature and studies of larger cohorts is needed to confirm this observation.
Five of 13 patients with recurrent disease were carriers of germline mutations in SDHB or RET, the remaining eight patients had sporadic disease presentation. The frequency of discovered variants in included loci were different in metastatic (22%) compared to non-metastatic cases (40%). Further genetic investigations of sporadic patients with recurrent and/or metastatic tumours could help to identify novel disease causing genes associated with a high risk of recurrence. Genetic prognostic markers could potentially be of value in a clinical context in order to personalize the extent of follow up [5], [45]. Planned studies aiming at identifying factors that might predict recurrent disease would favourably include tumour genotype characterization.
Tumour Genotype in Clinical Management
There is a growing rationale for analysing somatic events in PCC and PGL tumours as a diagnostic test: (1) EPAS1 mutations may occur early in embryogenesis and these mosaic carriers are not found by analysis of DNA in peripheral blood [16], [46]; (2) Translational studies have suggested using genotype as predictive markers for sensitivity to targeted therapy; SDHx/VHL mutations might benefit from antiangiogenic treatment whereas RET/NF1/TMEM127/MAX driven tumours could benefit from inhibitions of kinase pathways [47], [48]. The present study further suggests that tumour genotype might have additional prognostic implications. Even though methods for using formalin fixed archived tissue are evolving rapidly, analysis of tumour DNA is favourably performed using high quality fresh frozen genetic material thus discussions regarding the current routines, archiving only formalin fixed tissue, are warranted.
Conclusion
Somatic mutations are frequent events in PCC and PGL tumours. In patients with somatic carrier status there were no cases of recurrent nor metastatic disease. These findings suggest that analysis of tumour DNA could have an impact on the management of PCC and PGL patients and should be further investigated as prognostic factors in these diseases.
Acknowledgments
We thank Prof. Gunnar Westin for generously sharing research facilities and express our gratitude to Mrs. Birgitta Bondesson and Mr. Johan Tegerup for their excellent technical assistance. Bodil Svennblad and Johan Lindbäck at Uppsala Clinical Research Center (http://www.ucr.uu.se/) contributed excellent statistical support.
Funding Statement
The study was supported by grants from Swedish Cancer Society (PB), Selander Foundation (PB, PH, PS), Lions Cancer Foundation, Uppsala (JC, PH) and the Swedish state under the LUA/ALF agreement concerning research and education of doctors, (MN, id no. 76310). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mannelli M, Castellano M, Schiavi F, Filetti S, Giacche M, et al. (2009) Clinically guided genetic screening in a large cohort of italian patients with pheochromocytomas and/or functional or nonfunctional paragangliomas. J Clin Endocrinol Metab 94: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 2.Amar L, Servais A, Gimenez-Roqueplo AP, Zinzindohoue F, Chatellier G, et al.. (2005) Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab 90: 2110–2116. Epub 2005 Jan 2111. [DOI] [PubMed]
- 3. Darr R, Lenders JW, Hofbauer LC, Naumann B, Bornstein SR, et al. (2012) Pheochromocytoma - update on disease management. Ther Adv Endocrinol Metab 3: 11–26 doi: 10.1177/2042018812437356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scott HW Jr, Halter SA (1984) Oncologic aspects of pheochromocytoma: the importance of follow-up. Surgery 96: 1061–1066. [PubMed] [Google Scholar]
- 5. Van Slycke S, Caiazzo R, Pigny P, Cardot-Bauters C, Arnalsteen L, et al. (2009) Local-regional recurrence of sporadic or syndromic abdominal extra-adrenal paraganglioma: incidence, characteristics, and outcome. Surgery 146: 986–992 doi: 910.1016/j.surg.2009.1010.1055 [DOI] [PubMed] [Google Scholar]
- 6. Agarwal A, Mehrotra PK, Jain M, Gupta SK, Mishra A, et al. (2010) Size of the tumor and pheochromocytoma of the adrenal gland scaled score (PASS): can they predict malignancy? World J Surg 34: 3022–3028 doi: 3010.1007/s00268-00010-00744-00265 [DOI] [PubMed] [Google Scholar]
- 7. Thompson LD (2002) Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol 26: 551–566. [DOI] [PubMed] [Google Scholar]
- 8. Burnichon N, Vescovo L, Amar L, Libe R, de Reynies A, et al. (2011) Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum Mol Genet 20: 3974–3985. [DOI] [PubMed] [Google Scholar]
- 9. Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, et al. (2000) Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287: 848–851. [DOI] [PubMed] [Google Scholar]
- 10.Hao HX, Khalimonchuk O, Schraders M, Dephoure N, Bayley JP, et al.. (2009) SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science 325: 1139–1142. Epub 2009 Jul 1123. [DOI] [PMC free article] [PubMed]
- 11.Qin Y, Yao L, King EE, Buddavarapu K, Lenci RE, et al.. (2010) Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet 42: 229–233. Epub 2010 Feb 2014. [DOI] [PMC free article] [PubMed]
- 12. Comino-Mendez I, Gracia-Aznarez FJ, Schiavi F, Landa I, Leandro-Garcia LJ, et al. (2011) Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet 43: 663–667. [DOI] [PubMed] [Google Scholar]
- 13.Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, et al.. (2001) Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet 69: 49–54. Epub 2001 Jun 2012. [DOI] [PMC free article] [PubMed]
- 14. Niemann S, Muller U (2000) Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet 26: 268–270. [DOI] [PubMed] [Google Scholar]
- 15. Welander J, Larsson C, Backdahl M, Hareni N, Sivler T, et al. (2012) Integrative genomics reveals frequent somatic NF1 mutations in sporadic pheochromocytomas. Hum Mol Genet 24: 24. [DOI] [PubMed] [Google Scholar]
- 16. Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, et al. (2012) Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med 367: 922–930 doi: 910.1056/NEJMoa1205119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favier J, Buffet A, Gimenez-Roqueplo AP (2012) HIF2A mutations in paraganglioma with polycythemia. N Engl J Med 367: 2161; author reply 2161–2162. doi: 2110.1056/NEJMc1211953#SA1211951. [DOI] [PubMed]
- 18. Burnichon N, Buffet A, Parfait B, Letouze E, Laurendeau I, et al. (2012) Somatic NF1 Inactivation is a Frequent Event in Sporadic Pheochromocytoma. Hum Mol Genet 6: 6. [DOI] [PubMed] [Google Scholar]
- 19. Ladroue C, Carcenac R, Leporrier M, Gad S, Le Hello C, et al. (2008) PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med 359: 2685–2692. [DOI] [PubMed] [Google Scholar]
- 20.Crona J, Delgado Verdugo A, Maharjan R, Stålberg P, Granberg D, et al.. (2013) Somatic Mutations in H-RAS in Sporadic Pheochromocytoma and Paraganglioma Identified by Exome Sequencing. Journal of Clinical Endocrinology & Metabolism. [DOI] [PubMed]
- 21.Amar L, Baudin E, Burnichon N, Peyrard S, Silvera S, et al.. (2007) Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab 92: 3822–3828. Epub 2007 Jul 3824. [DOI] [PubMed]
- 22. Buffet A, Venisse A, Nau V, Roncellin I, Boccio V, et al. (2012) A Decade (2001–2010) of Genetic Testing for Pheochromocytoma and Paraganglioma. Horm Metab Res 19: 19. [DOI] [PubMed] [Google Scholar]
- 23.Weber A, Hoffmann MM, Neumann HP, Erlic Z (2012) Somatic Mutation Analysis of the SDHB, SDHC, SDHD, and RET Genes in the Clinical Assessment of Sporadic and Hereditary Pheochromocytoma. Horm Cancer 3: 187–192. Epub 2012 May 2010. [DOI] [PMC free article] [PubMed]
- 24. Crona J, Maharjan R, Delgado Verdugo A, Stalberg P, Granberg D, et al. (2013) MAX mutations status in Swedish patients with pheochromocytoma and paraganglioma tumours. Fam Cancer 7: 7. [DOI] [PubMed] [Google Scholar]
- 25.Akerstrom T, Crona J, Delgado Verdugo A, Starker LF, Cupisti K, et al.. (2012) Comprehensive re-sequencing of adrenal aldosterone producing lesions reveal three somatic mutations near the KCNJ5 potassium channel selectivity filter. PLoS One 7: e41926. Epub 42012 Jul 41927. [DOI] [PMC free article] [PubMed]
- 26. Welander J, Soderkvist P, Gimm O (2011) Genetics and clinical characteristics of hereditary pheochromocytomas and paragangliomas. Endocr Relat Cancer 18: R253–276. [DOI] [PubMed] [Google Scholar]
- 27. Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, et al. (2011) COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res 39: D945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, et al. (2003) Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat 21: 577–581. [DOI] [PubMed] [Google Scholar]
- 29.Kumar P, Henikoff S, Ng PC (2009) Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4: 1073–1081. Epub 2009 Jun 1025. [DOI] [PubMed]
- 30. Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, et al. (2010) A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arkblad EL, Darin N, Berg K, Kimber E, Brandberg G, et al.. (2006) Multiplex ligation-dependent probe amplification improves diagnostics in spinal muscular atrophy. Neuromuscul Disord 16: 830–838. Epub 2006 Oct 2017. [DOI] [PubMed]
- 32.Banck MS, Kanwar R, Kulkarni AA, Boora GK, Metge F, et al.. (2013) The genomic landscape of small intestine neuroendocrine tumors. J Clin Invest 15. [DOI] [PMC free article] [PubMed]
- 33. Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, et al. (2002) Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med 346: 1459–1466. [DOI] [PubMed] [Google Scholar]
- 34.Yang C, Sun MG, Matro J, Huynh TT, Rahimpour S, et al.. (2013) Novel HIF2A mutations disrupt oxygen sensing leading to polycythemia, paragangliomas and somatostatinomas. Blood. [DOI] [PMC free article] [PubMed]
- 35.Crona J, Delgado Verdugo A, Granberg D, Welin S, Stalberg P, et al.. (2013) Next generation sequencing in genetic screening of pheochromocytoma and paraganglioma. Endocrine Connections. [DOI] [PMC free article] [PubMed]
- 36. Panizza E, Ercolino T, Mori L, Rapizzi E, Castellano M, et al. (2013) Yeast model for evaluating the pathogenic significance of SDHB, SDHC and SDHD mutations in PHEO-PGL syndrome. Hum Mol Genet 22: 804–815 doi: 810.1093/hmg/dds1487. Epub 2012 Nov 1021 [DOI] [PubMed] [Google Scholar]
- 37. Bond J, Gale DP, Connor T, Adams S, de Boer J, et al. (2011) Dysregulation of the HIF pathway due to VHL mutation causing severe erythrocytosis and pulmonary arterial hypertension. Blood 117: 3699–3701 doi: 3610.1182/blood-2010-3612-327569 [DOI] [PubMed] [Google Scholar]
- 38.Vaclavikova E, Dvorakova S, Sykorova V, Bilek R, Dvorakova K, et al.. (2009) RET mutation Tyr791Phe: the genetic cause of different diseases derived from neural crest. Endocrine 36: 419–424. Epub 2009 Oct 2014. [DOI] [PubMed]
- 39.Erlic Z, Hoffmann MM, Sullivan M, Franke G, Peczkowska M, et al.. (2010) Pathogenicity of DNA variants and double mutations in multiple endocrine neoplasia type 2 and von Hippel-Lindau syndrome. J Clin Endocrinol Metab 95: 308–313. Epub 2009 Nov 2011. [DOI] [PMC free article] [PubMed]
- 40. Muth A, Abel F, Jansson S, Nilsson O, Ahlman H, et al. (2012) Prevalence of germline mutations in patients with pheochromocytoma or abdominal paraganglioma and sporadic presentation: a population-based study in Western sweden. World J Surg 36: 1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Comino-Mendez I, de Cubas AA, Bernal C, Alvarez-Escola C, Sanchez-Malo C, et al. (2013) Tumoral EPAS1 (HIF2A) mutations explain sporadic pheochromocytoma and paraganglioma in the absence of erythrocytosis. Hum Mol Genet 26: 26. [DOI] [PubMed] [Google Scholar]
- 42.Easton DF, Deffenbaugh AM, Pruss D, Frye C, Wenstrup RJ, et al.. (2007) A systematic genetic assessment of 1,433 sequence variants of unknown clinical significance in the BRCA1 and BRCA2 breast cancer-predisposition genes. Am J Hum Genet 81: 873–883. Epub 2007 Sep 2006. [DOI] [PMC free article] [PubMed]
- 43. Eisenhofer G, Walther MM, Huynh TT, Li ST, Bornstein SR, et al. (2001) Pheochromocytomas in von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2 display distinct biochemical and clinical phenotypes. J Clin Endocrinol Metab 86: 1999–2008. [DOI] [PubMed] [Google Scholar]
- 44. Leslie EJ, Standley J, Compton J, Bale S, Schutte BC, et al. (2012) Comparative analysis of IRF6 variants in families with Van der Woude syndrome and popliteal pterygium syndrome using public whole-exome databases. Genet Med 15: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Heerden JA, Roland CF, Carney JA, Sheps SG, Grant CS (1990) Long-term evaluation following resection of apparently benign pheochromocytoma(s)/paraganglioma(s). World J Surg 14: 325–329. [DOI] [PubMed] [Google Scholar]
- 46. Pacak K, Jochmanova I, Prodanov T, Yang C, Merino MJ, et al. (2013) New Syndrome of Paraganglioma and Somatostatinoma Associated With Polycythemia. J Clin Oncol 18: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wells SA, Jr., Robinson BG, Gagel RF, Dralle H, Fagin JA, et al.. (2012) Vandetanib in Patients With Locally Advanced or Metastatic Medullary Thyroid Cancer: A Randomized, Double-Blind Phase III Trial. J Clin Oncol 30: 134–141. Epub 2011 Oct 2024. [DOI] [PMC free article] [PubMed]
- 48. Favier J, Igaz P, Burnichon N, Amar L, Libe R, et al. (2012) Rationale for Anti-angiogenic Therapy in Pheochromocytoma and Paraganglioma. Endocr Pathol 23: 34–42. [DOI] [PubMed] [Google Scholar]