Abstract
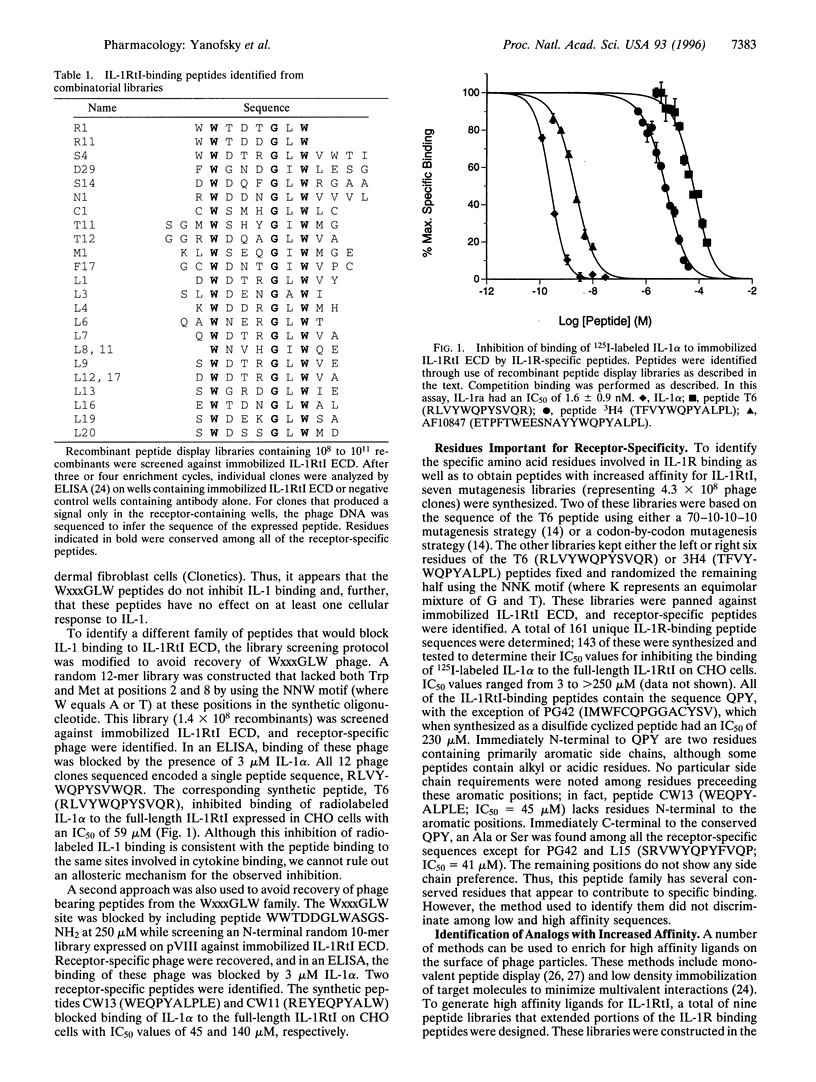
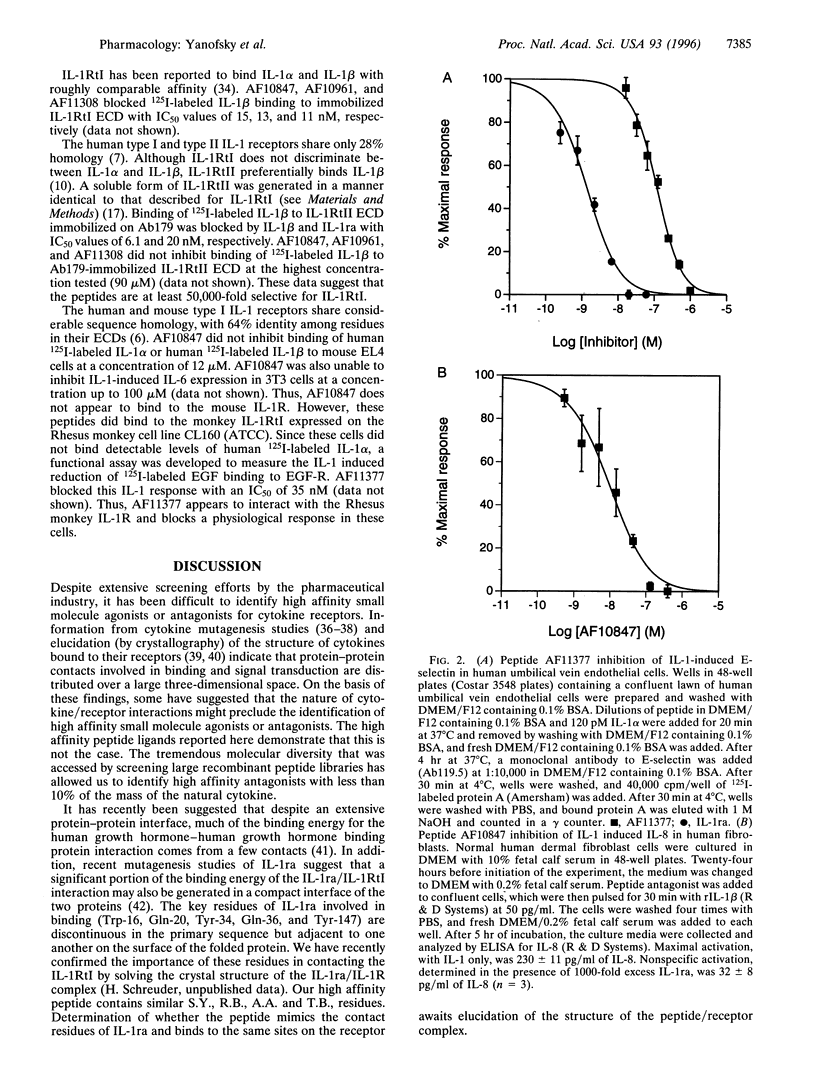
Two families of peptides that specifically bind the extracellular domain of the human type I interleukin I (IL-1) receptor were identified from recombinant peptide display libraries. Peptides from one of these families blocked binding of IL-lalpha to the type I IL-1 receptor with IC50 values of 45-140 microM. Affinity-selective screening of variants of these peptides produced ligands of much higher affinity (IC50 approximately 2 nM). These peptides block IL-1-driven responses in human and monkey cells; they do not bind the human type II IL-1 receptor or the murine type I IL-1 receptor. This is the first example (that we know of) of a high affinity peptide that binds to a cytokine receptor and acts as a cytokine antagonist.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett R. W., Cwirla S. E., Ackerman M. S., Olson A. M., Peters E. A., Dower W. J. Selective enrichment and characterization of high affinity ligands from collections of random peptides on filamentous phage. Anal Biochem. 1992 Aug 1;204(2):357–364. doi: 10.1016/0003-2697(92)90252-3. [DOI] [PubMed] [Google Scholar]
- Bass S., Greene R., Wells J. A. Hormone phage: an enrichment method for variant proteins with altered binding properties. Proteins. 1990;8(4):309–314. doi: 10.1002/prot.340080405. [DOI] [PubMed] [Google Scholar]
- Bird T. A., Saklatvala J. IL-1 and TNF transmodulate epidermal growth factor receptors by a protein kinase C-independent mechanism. J Immunol. 1989 Jan 1;142(1):126–133. [PubMed] [Google Scholar]
- Chua A. O., Gubler U. Sequence of the cDNA for the human fibroblast type interleukin-1 receptor. Nucleic Acids Res. 1989 Dec 11;17(23):10114–10114. doi: 10.1093/nar/17.23.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clackson T., Wells J. A. A hot spot of binding energy in a hormone-receptor interface. Science. 1995 Jan 20;267(5196):383–386. doi: 10.1126/science.7529940. [DOI] [PubMed] [Google Scholar]
- Colotta F., Re F., Muzio M., Bertini R., Polentarutti N., Sironi M., Giri J. G., Dower S. K., Sims J. E., Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993 Jul 23;261(5120):472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Wells J. A. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989 Jun 2;244(4908):1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- Cwirla S. E., Peters E. A., Barrett R. W., Dower W. J. Peptides on phage: a vast library of peptides for identifying ligands. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6378–6382. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Dinarello C. A. Modalities for reducing interleukin 1 activity in disease. Immunol Today. 1993 Jun;14(6):260–264. doi: 10.1016/0167-5699(93)90042-J. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. The role of interleukin-1 in host responses to infectious diseases. Infect Agents Dis. 1992 Oct;1(5):227–236. [PubMed] [Google Scholar]
- Dower S. K., Fanslow W., Jacobs C., Waugh S., Sims J. E., Widmer M. B. Interleukin-I antagonists. Ther Immunol. 1994 Apr;1(2):113–122. [PubMed] [Google Scholar]
- Dower S. K., Kronheim S. R., Hopp T. P., Cantrell M., Deeley M., Gillis S., Henney C. S., Urdal D. L. The cell surface receptors for interleukin-1 alpha and interleukin-1 beta are identical. Nature. 1986 Nov 20;324(6094):266–268. doi: 10.1038/324266a0. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. P., Evans R. J., Arend W. P., Verderber E., Brewer M. T., Hannum C. H., Thompson R. C. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990 Jan 25;343(6256):341–346. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- Ellison J. W., Berson B. J., Hood L. E. The nucleotide sequence of a human immunoglobulin C gamma1 gene. Nucleic Acids Res. 1982 Jul 10;10(13):4071–4079. doi: 10.1093/nar/10.13.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. J., Bray J., Childs J. D., Vigers G. P., Brandhuber B. J., Skalicky J. J., Thompson R. C., Eisenberg S. P. Mapping receptor binding sites in interleukin (IL)-1 receptor antagonist and IL-1 beta by site-directed mutagenesis. Identification of a single site in IL-1ra and two sites in IL-1 beta. J Biol Chem. 1995 May 12;270(19):11477–11483. doi: 10.1074/jbc.270.19.11477. [DOI] [PubMed] [Google Scholar]
- Gallop M. A., Barrett R. W., Dower W. J., Fodor S. P., Gordon E. M. Applications of combinatorial technologies to drug discovery. 1. Background and peptide combinatorial libraries. J Med Chem. 1994 Apr 29;37(9):1233–1251. doi: 10.1021/jm00035a001. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Goodnow C. C., Dudzik K. I., Oi V. T., Davis M. M. Secretion of a chimeric T-cell receptor-immunoglobulin protein. Proc Natl Acad Sci U S A. 1987 May;84(9):2936–2940. doi: 10.1073/pnas.84.9.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum C. H., Wilcox C. J., Arend W. P., Joslin F. G., Dripps D. J., Heimdal P. L., Armes L. G., Sommer A., Eisenberg S. P., Thompson R. C. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990 Jan 25;343(6256):336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- Hawkins R. E., Russell S. J., Winter G. Selection of phage antibodies by binding affinity. Mimicking affinity maturation. J Mol Biol. 1992 Aug 5;226(3):889–896. doi: 10.1016/0022-2836(92)90639-2. [DOI] [PubMed] [Google Scholar]
- Horuk R., McCubrey J. A. The interleukin-1 receptor in Raji human B-lymphoma cells. Molecular characterization and evidence for receptor-mediated activation of gene expression. Biochem J. 1989 Jun 15;260(3):657–663. doi: 10.1042/bj2600657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introna M., Breviario F., d'Aniello E. M., Golay J., Dejana E., Mantovani A. IL-1 inducible genes in human umbilical vein endothelial cells. Eur Heart J. 1993 Dec;14 (Suppl K):78–81. [PubMed] [Google Scholar]
- Isshiki H., Akira S., Tanabe O., Nakajima T., Shimamoto T., Hirano T., Kishimoto T. Constitutive and interleukin-1 (IL-1)-inducible factors interact with the IL-1-responsive element in the IL-6 gene. Mol Cell Biol. 1990 Jun;10(6):2757–2764. doi: 10.1128/mcb.10.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labriola-Tompkins E., Chandran C., Kaffka K. L., Biondi D., Graves B. J., Hatada M., Madison V. S., Karas J., Kilian P. L., Ju G. Identification of the discontinuous binding site in human interleukin 1 beta for the type I interleukin 1 receptor. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11182–11186. doi: 10.1073/pnas.88.24.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowman H. B., Bass S. H., Simpson N., Wells J. A. Selecting high-affinity binding proteins by monovalent phage display. Biochemistry. 1991 Nov 12;30(45):10832–10838. doi: 10.1021/bi00109a004. [DOI] [PubMed] [Google Scholar]
- Maizel A. L., Mehta S. R., Ford R. J., Lachman L. B. Effect of interleukin 1 on human thymocytes and purified human T cells. J Exp Med. 1981 Feb 1;153(2):470–475. doi: 10.1084/jem.153.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Martens C. L., Cwirla S. E., Lee R. Y., Whitehorn E., Chen E. Y., Bakker A., Martin E. L., Wagstrom C., Gopalan P., Smith C. W. Peptides which bind to E-selectin and block neutrophil adhesion. J Biol Chem. 1995 Sep 8;270(36):21129–21136. doi: 10.1074/jbc.270.36.21129. [DOI] [PubMed] [Google Scholar]
- McMahan C. J., Slack J. L., Mosley B., Cosman D., Lupton S. D., Brunton L. L., Grubin C. E., Wignall J. M., Jenkins N. A., Brannan C. I. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO J. 1991 Oct;10(10):2821–2832. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp E. A., Cameron P. M., Ranawat C. S., Schmidt J. A., Bayne E. K. Specific bioactivities of monocyte-derived interleukin 1 alpha and interleukin 1 beta are similar to each other on cultured murine thymocytes and on cultured human connective tissue cells. J Clin Invest. 1986 Sep;78(3):836–839. doi: 10.1172/JCI112649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J. E., Acres R. B., Grubin C. E., McMahan C. J., Wignall J. M., March C. J., Dower S. K. Cloning the interleukin 1 receptor from human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8946–8950. doi: 10.1073/pnas.86.22.8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J. E., Gayle M. A., Slack J. L., Alderson M. R., Bird T. A., Giri J. G., Colotta F., Re F., Mantovani A., Shanebeck K. Interleukin 1 signaling occurs exclusively via the type I receptor. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6155–6159. doi: 10.1073/pnas.90.13.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. P. Filamentous phage assembly: morphogenetically defective mutants that do not kill the host. Virology. 1988 Nov;167(1):156–165. doi: 10.1016/0042-6822(88)90065-7. [DOI] [PubMed] [Google Scholar]
- Symons J. A., Duff G. W. A soluble form of the interleukin-1 receptor produced by a human B cell line. FEBS Lett. 1990 Oct 15;272(1-2):133–136. doi: 10.1016/0014-5793(90)80466-v. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawara T., Shingu M., Nobunaga M., Naono T. Effects of recombinant human IL-1 beta on production of prostaglandin E2, leukotriene B4, NAG, and superoxide by human synovial cells and chondrocytes. Inflammation. 1991 Apr;15(2):145–157. doi: 10.1007/BF00917509. [DOI] [PubMed] [Google Scholar]
- Walter M. R., Windsor W. T., Nagabhushan T. L., Lundell D. J., Lunn C. A., Zauodny P. J., Narula S. K. Crystal structure of a complex between interferon-gamma and its soluble high-affinity receptor. Nature. 1995 Jul 20;376(6537):230–235. doi: 10.1038/376230a0. [DOI] [PubMed] [Google Scholar]
- Whitehorn E. A., Tate E., Yanofsky S. D., Kochersperger L., Davis A., Mortensen R. B., Yonkovich S., Bell K., Dower W. J., Barrett R. W. A generic method for expression and use of "tagged" soluble versions of cell surface receptors. Biotechnology (N Y) 1995 Nov;13(11):1215–1219. doi: 10.1038/nbt1195-1215. [DOI] [PubMed] [Google Scholar]
- Zurawski S. M., Pope K., Cherwinski H., Zurawski G. Expression in Escherichia coli of synthetic human interleukin-1 alpha genes encoding the processed active protein, mutant proteins, and beta-galactosidase fusion proteins. Gene. 1986;49(1):61–68. doi: 10.1016/0378-1119(86)90385-9. [DOI] [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M., Kossiakoff A. A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992 Jan 17;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]


