Abstract
To date, several classes of hormones have been described that influence plant development, including auxins, cytokinins, ethylene, and, more recently, brassinosteroids. However, it is known that many fungal and bacterial species produce substances that alter plant growth that, if naturally present in plants, might represent novel classes of plant growth regulators. Alkamides are metabolites widely distributed in plants with a broad range of biological activities. In this work, we investigated the effects of affinin, an alkamide naturally occurring in plants, and its derivates, N-isobutyl-2E-decenamide and N-isobutyl-decanamide, on plant growth and early root development in Arabidopsis. We found that treatments with affinin in the range of 10-6 to 10-4 m alter shoot and root biomass production. This effect correlated with alteration on primary root growth, lateral root formation, and root hair elongation. Low concentrations of affinin (7 × 10-6–2.8 × 10-5 m) enhanced primary root growth and root hair elongation, whereas higher concentrations inhibited primary root growth that related with a reduction in cell proliferating activity and cell elongation. N-isobutyl-2E-decenamide and N-isobutyl-decanamide were found to stimulate root hair elongation at concentrations between 10-8 to 10-7 m. Although the effects of alkamides were similar to those produced by auxins on root growth and cell parameters, the ability of the root system to respond to affinin was found to be independent of auxin signaling. Our results suggest that alkamides may represent a new group of plant growth promoting substances with significant impact on root development and opens the possibility of using these compounds for improved plant production.
Alkamides are secondary metabolites comprising over 200 related compounds widely distributed in plants. These amides have been found in as many as 10 plant families: Aristolochiaceae, Asteraceae, Brassicaceae, Convolvulaceae, Euphorbiaceae, Menispermaceae, Piperaceae, Poaceae, Rutaceae, and Solanaceae. Several species containing high levels of alkamides are found in the Asteraceae, Piperaceae, and Rutaceae (Christensen and Lam, 1991; Kashiwada et al., 1997; Parmar et al., 1997). The general structure of alkamides originates from the condensation of an unsaturated fatty acid and an amine (Hofer et al., 1986). Although different chain length alkamides have been found in plants, most of them contain a 2E double bond conjugated to the amide group substituted with a N-isobutyl group (Rios-Chavez et al., 2003).
Increasing interest has being paid to alkamides because of their wide range of biological activities (Jacobson, 1971). Many of the plant species containing these compounds have been used in traditional medicine of different civilizations (Ximenez, 1615). They are recognized for their pungent taste and for causing numbing and salivation. Insecticidal properties have also been attributed to alkamides such as affinin (Jacobson, 1971). However, it is completely unknown whether alkamides could have a role in plant growth and differentiation.
Amidenin, a nonsubstituted alkamide isolated from the actinomycete fungus Amycolatopsis sp. was reported to stimulate the growth of rice (Oryza sativa) seedlings (Kanbe et al., 1993), although little information is available about the mode of action of amidenin and no information is available about any potential growth regulating activity of alkamides produced by plants.
In the present work, the effects of three plant alkamides (N-isobutyl-2E,6Z,8E-decatrienamide, N-isobutyl-2E-decenamide, and N-isobutyl-decanamide) on the growth and development of Arabidopsis seedlings are presented. We provide evidence that alkamides can act as plant growth-promoting substances. Developmental alterations induced by alkamides included greater formation and emergence of lateral roots and increased root hair elongation. The involvement of auxin in mediating the observed activity of alkamides was tested using the auxin responsive marker genes DR5:uidA and BA3:uidA and auxin-signaling Arabidopsis mutants.
RESULTS
Isolation and Purification of Alkamides
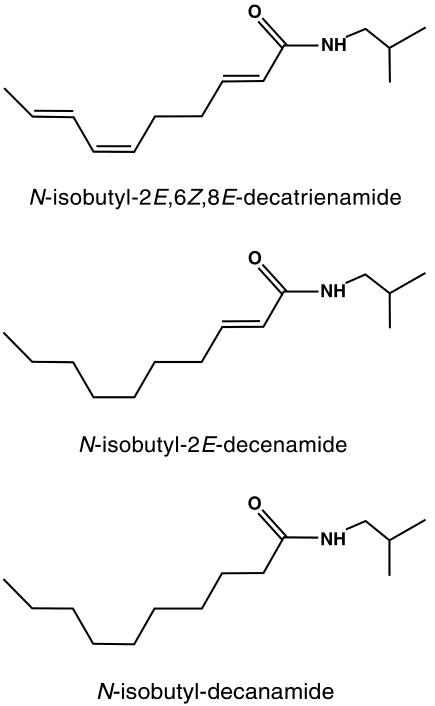
Affinin (N-isobutyl-2E,6Z,8E-decatrienamide), a member of the alkamides, is present in Heliopsis longipes roots in concentrations as high as 1% (w/w) on a fresh weight basis (Molina-Torres et al., 1996), opening the possibility of using this compound to investigate further the effects of alkamides on plant growth and the physiological mechanisms by which they act. The alkamides N-isobutyl-2E-decenamide and N-isobutyl-decanamide were obtained by catalytic reduction of affinin (See “Materials and Methods”). Figure 1 shows the structural features of the molecules whose effects on plant growth and development were analyzed in this work.
Figure 1.
Affinin and major catalytic reduction alkamide structures. A, N-isobutyl-2E,6Z,8E-decatrienamide; B, N-isobutyl-2E-decenamide, major intermediate at 3 min reduction time; and C, N-isobutyl-decanamide, final reduction product.
Effect of Affinin on Growth of Arabidopsis Seedlings
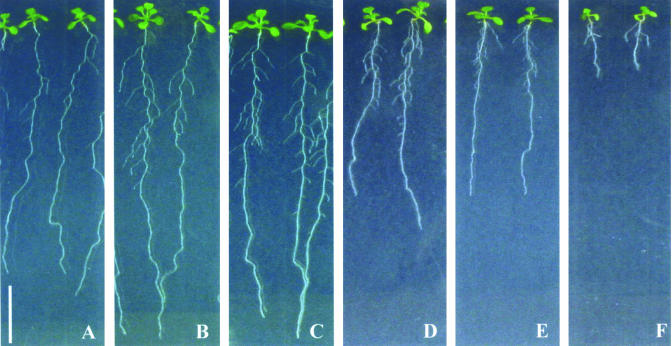
We tested the effect of affinin on the growth of Arabidopsis (Colombia 0 [Col-0] ecotype), by growing plants on 0.1× Murashige and Skoog solid medium containing different concentrations of this compound. After 12 d of growth, treatments of 7 × 10-6 and 1.4 × 10-5 m affinin stimulated shoot and root biomass production (Fig. 2, A–C). Treatments of 2.8 × 10-5, 5.6 × 10-5, and 1.2 × 10-4 m affinin had an inhibitory effect on Arabidopsis growth (Fig. 2, D–F). In particular, affinin treatments had a dramatic effect on the Arabidopsis root system architecture by altering primary root growth and lateral root formation (Fig. 2, A–F).
Figure 2.
Effects of affinin on Arabidopsis growth. Wild-type Col-0 seedlings were grown for 10 d under increasing affinin concentrations on vertically oriented agar plates. Control (A), 7 × 10-6 (B), 1.4 × 10-5 (C), 2.8 × 10-5 (D), 5.6 × 10-5 (E), and 1.2 × 10-4 m (F) affinin. All photographs are at the same magnification. Scale bar = 1 cm.
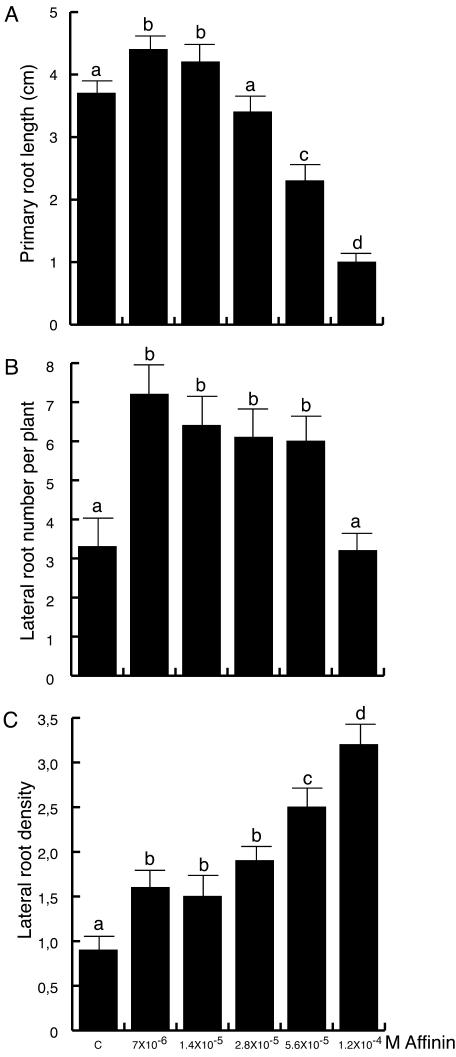
To more closely analyze the effects of affinin on plant development, primary root length, number of emerged lateral roots, and lateral root density were determined in Arabidopsis seedlings grown in 0.1× Murashige and Skoog media supplemented with various concentrations of affinin. After 8 d of growth, primary root growth promotion was observed at concentrations of 7 × 10-6 and 1.4 × 10-5 m affinin, whereas primary root inhibition could be detected at concentration of 5.6 × 10-5 m. At this affinin concentration, the primary root length was 43% lower than that registered for the controls (Fig. 3A). Affinin concentrations in the range of 7 × 10-6 to 5.6 × 10-5 m increased by 2-fold the number of lateral roots (Fig. 3B). The density of lateral roots was also calculated by dividing the number of lateral roots by the length of the primary root to normalize for the effects of affinin on primary root length. Lateral root density dramatically increased with increased affinin concentration in the media, being up to 3.5-fold higher in the 1.2 × 10-4 m affinin treatment when compared with the untreated controls (Fig. 3C).
Figure 3.
Effects of affinin on Arabidopsis root architecture. Wild-type Col-0 seedlings were grown for 10 d under increasing affinin concentrations on vertically oriented agar plates. Data are given for the length of the primary root (A), lateral root number (B), and lateral root density (C). Values shown represent the mean of 20 seedlings ± sd. Different letters represent means statistically different at the 0.05 level.
Effect of Affinin on Lateral Root Primordia Initiation
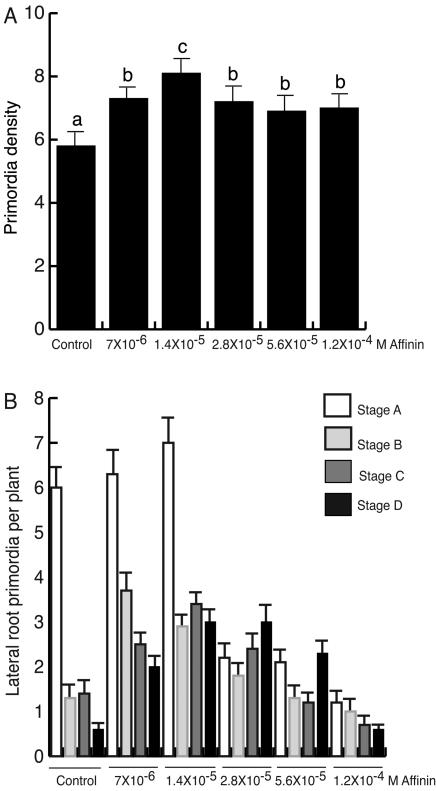
The effect of affinin concentrations increasing the number of lateral roots could be due to a stimulation of the emergence of pre-existing lateral root primordia or to the de novo formation of additional root primordia. To establish the developmental basis for the effect of affinin on lateral root development, lateral root primordia (LRP) originating in the Arabidopsis primary root were quantified at d 6 after germination. Seedling roots were first cleared to enable LRP at early stages of development to be visualized and counted. Each LRP was classified according to its stage of development as reported by Zhang et al. (1999), who consider the following stages: stage A, up to three cell layers; stage B, unemerged, of more than three cell layers; stage C, LR emerged of less than 0.5 mm in length; stage D, lateral root larger than 0.5 mm.
The lateral root density analysis revealed that plants treated with 7 × 10-6 and 1.4 × 10-5 m affinin, respectively, formed 55% and 71% more LRP than the control (Fig. 4A). The stage distribution of primordia was also affected by affinin. Treatments of 7 × 10-6 and 1.4 × 10-5 m affinin increased the number of LRP in stages B through D compared with the control (Fig. 4B). An even greater interval of affinin treatments (7 × 10-6–5.6 × 10-5 m) increased the number of LRP that had emerged from the primary root (stage D; Fig. 4B). The observations that affinin treatments increased primordia density and the transition of LRP from stage A to further stages of development suggest that affinin can promote root branching by inducing lateral root primordia initiation and promoting the emergence of lateral roots.
Figure 4.
Effects of affinin on Arabidopsis lateral root development. A, Density of lateral roots; B, lateral root stage distribution. Six-day-old primary roots were cleared and the number and stage of LRP were recorded. The results show the number of primordia at each developmental stage according to Zhang et al. (1999), for 10 individual roots. This analysis was repeated twice with similar results.
Effect of Alkamides on Root Hair Development
Root hairs are root epidermal cells that increase the total absorptive surface of the root system and participate in nutrient and water uptake. To analyze if alkamides could alter root hair development, we performed experiments in which Arabidopsis plants were grown on the surface of agar plates with 0.1× Murashige and Skoog medium and different concentrations of alkamides.
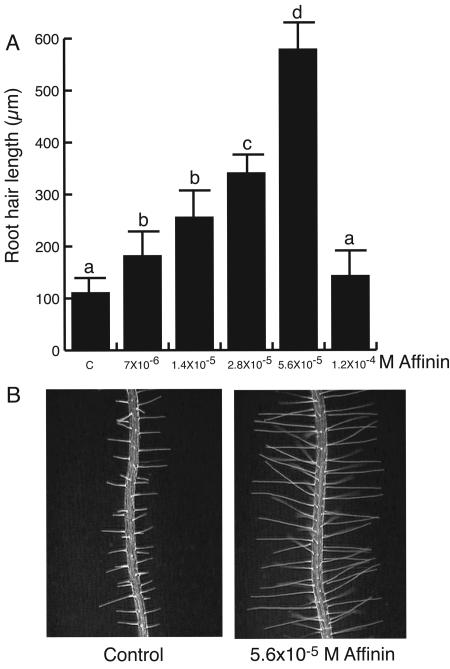
Root hair parameters were analyzed at d 6 after germination on primary roots of control and alkamide-treated plants. Affinin treatments substantially increased root hair length (Fig. 5, A and B). A significant difference in root hair length could be observed at treatment of 7 × 10-6 m affinin. However, the most important effect was observed in roots grown under 5.6 × 10-5 m affinin. Under our experimental conditions, root hairs of control plants were approximately 0.1 mm long, whereas those of plants treated with 5.6 × 10-5 m affinin were approximately 0.5 mm in length, about five times the length of control root hairs.
Figure 5.
Effect of affinin on root hair formation. A, Average root hair length of fully expanded root hairs. B, Light microscope images of control and affinin induced-root hairs (magnification = 60×).
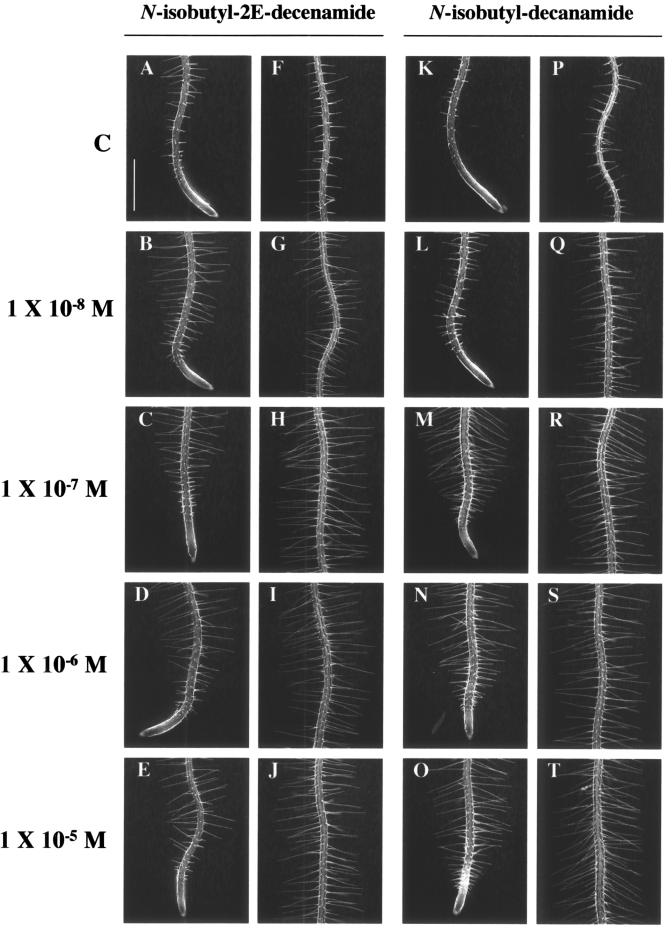
The effect of N-isobutyl-2E-decenamide and N-isobutyl-decanamide on root hair growth was also tested. Both molecules, obtained by chemical reduction of affinin, stimulated root hair elongation at concentrations between 10-8 to 10-5 m (Fig. 6, A–T). Concentrations higher than 10-8 m N-isobutyl-2E-decenamide stimulated root hair elongation close to the root tip (Fig. 6, A–E). The stimulatory effect of this compound on root hair growth was most clearly observed when mature regions of the primary root were compared (Fig. 6, F–J). Similar effects were observed with treatments of N-isobutyl-decanamide (Fig. 6, K–T). Treatments of 1 × 10-7 m N-isobutyl-2E-decenamide and N-isobutyl-decanamide had a 4- to 5-fold increase in hair length when compared with the control (Fig. 6, H and R). These results indicate that several alkamides can regulate root hair growth, some of which have greater activity than affinin on root hair elongation.
Figure 6.
Effects of N-isobutyl-2E-decenamide and N-isobutyl-decanamide on epidermal cell differentiation. The root hairs forming at the primary root tip region (A–E and K–O) and in mature region (F–J and P–T) of the root of 6-d-old Arabidopsis seedlings were photographed. Photographs are representative individuals of at least 40 plants analyzed. Scale bar = 500 μm.
Effects of Affinin on Cell Division and Elongation
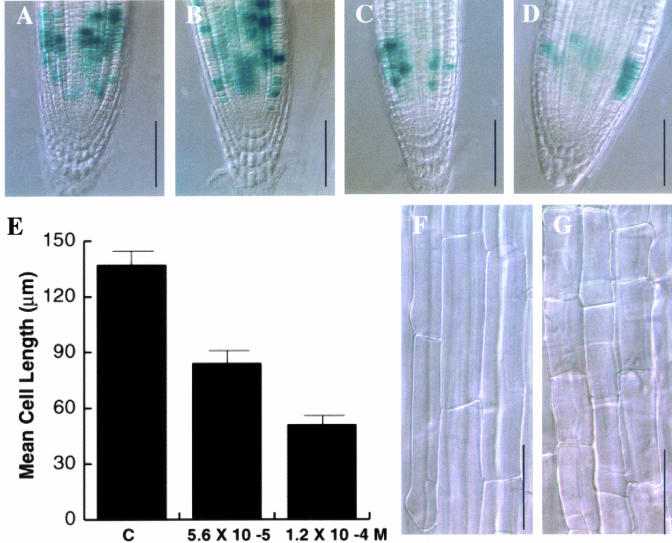
To test whether Alkamide treatments could alter root development by altering cell division and/or elongation, we analyzed the expression of the cell cycle marker CycB1:uidA (Colón-Carmona et al., 1999) in transgenic plants growing under different affinin treatments. The location and intensity of CycB1:uidA expression in the 7 × 10-6 M affinin treatment was similar to that observed in untreated seedlings, showing typical patchy expression in the meristem (Fig. 7, A and B). Interestingly, in the treatments of 5.6 × 10-5 and 1.2 × 10-4 M affinin the number of cells showing CycB1:uidA expression was clearly reduced (Fig. 7, C and D). We also analyzed whether cell elongation was altered by alkamides by measuring the epidermal cell size in the differentiation and maturation regions of primary roots in WT (Col-0) plants. These measurements showed that epidermal cells in plants treated with 5.6 × 10-5 and 1.2 × 10-4 M affinin are in average 49% and 63% shorter than those of wild type (Fig. 7, E–G). Therefore, the observed inhibitory effect of affinine on primary root elongation is caused both by a reduction in the cell proliferating activity in the meristem and by an inhibition of cell elongation.
Figure 7.
Effects of affinin on cell proliferating activity and cell elongation. Wild-type Col-0 and CycB1:uidA seedlings were grown for 7 d under varied affinin concentrations on vertically oriented agar plates, were stained for GUS activity, and were cleared to measure cell size. A, CycB1:uidA primary root meristem of a plant grown in medium without affinin; B, 7 × 10-6 m affinin; C, 5.6 × 10-5 m affinin; D, 1.2 × 10-4 m affinin. E, Mean trichoblast cell length in Col-0 plants treated with or without 5.6 × 10-5 and 1.2 × 10-4 m affinin. F, Light microscope images of control and 1.2 × 10-4 m affinin treated-epidermal cells. CycB1:uidA photographs are representative individuals of at least 20 plants stained. Scale bar in A through D = 100 μm and in F and G = 50 μm.
Effect of Affinin on Auxin-Inducible Gene Expression
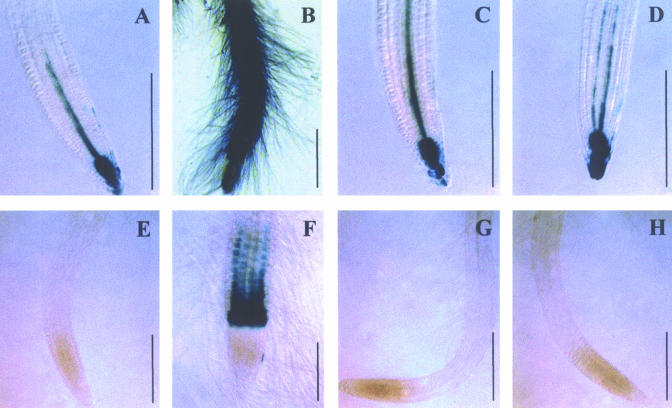
The observed effect of affinin on several aspects of root development is similar to that described for auxin in most plant species, including Arabidopsis. Auxins such as indole acetic acid enhance primary root growth at low concentrations, and at high concentrations, promote root hair and lateral root formation and elongation but inhibit primary root growth (Blakely et al., 1982; Laskowski et al., 1995). To test whether affinin could alter auxin-regulated gene expression in roots, and in this way affect the root system architecture, we conducted analyses of the expression of the β-glucuronidase (GUS) reporter gene in Arabidopsis lines harboring the DR5:uidA and BA3:uidA gene constructs. Both reporter lines have proved to be useful in studying auxin-regulated gene expression in Arabidopsis (Ulmasov et al., 1997; Oono et al., 1998). Figure 8 shows histochemical staining for transgenic DR5:uidA and BA3:uidA plants that were grown 7 d under auxin (2,4-dichlorophenoxyacetic acid [2,4-d]) or affinin treatments. As previously reported (Ulmasov et al., 1997), in untreated control plants, DR5:uidA is expressed primarily in the columella and quiescent center (Fig. 8A). DR5:uidA plants grown under a concentration of 10-8 m 2,4-D showed GUS activity throughout the primary root (Fig. 8B). A similar pattern of expression has been previously reported (Ulmasov et al., 1997; Sabatini et al., 1999). In contrast, the pattern of GUS expression in DR5:uidA seedlings treated with up to 1.2 × 10-4 m affinin remained similar to that observed in untreated controls (Fig. 8, C and D).
Figure 8.
Effect of affinin on auxin-regulated gene expression. A, Twelve hours of GUS staining of DR5:uidA primary roots grown for 7 d in medium without auxin; B, under 10-8 m 2,4-D; C, treatments of 2.8 × 10-5 m affinin; C, 1.2 × 10-4 m affinin. E, Twelve hours of GUS staining of BA3:uidA primary roots grown for 7 d in medium without auxin, under 10-8 m 2,4-D (F), and treatments of 2.8 × 10-5 m affinin (G) and 1.2 × 10-4 m affinin (H). Photographs are representative individuals of at least 20 plants stained. Scale bar = 200 μm.
Untreated BA3:uidA plants did not show detectable levels of GUS activity (Fig. 8E), whereas when treated with 10-8 m 2,4-D, they showed GUS expression mainly in the root elongation zone (Fig. 8F), similarly to that reported by Oono et al., (1998). In contrast, BA3:uidA seedlings failed to show GUS staining in the root elongation zone even at the highest level of affinin tested (10-4 m; Fig. 8, G and H). These results show that affinin treatments can induce root growth alterations without altering the expression of auxin-inducible genes.
Alkamide Response in Auxin-Signaling Mutants
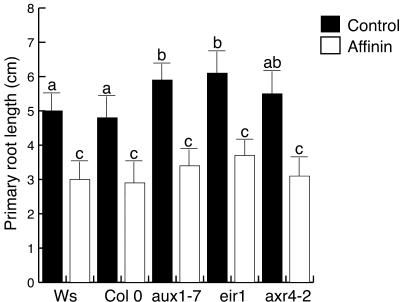
The results from DR5:uidA and BA3:uidA marker expression analyses indicated that affinin effects could not be related to auxin-altered responsive gene expression. To date, different auxin-resistant mutants have been isolated, which represent a valuable tool in investigating at the genetic level any potential role of the auxin-signaling pathway in mediating the root developmental changes induced by alkamides. With this in mind, the effect of 5.6 × 10-5 m affinin on primary root growth of the aux1-7, eir1-1, and axr4-2 auxin mutants was tested. As shown in Figure 9, treatment with 5.6 × 10-5 m affinin caused 40% inhibition in primary root growth in WT plants of the Col-0 and Wassilewskija ecotypes compared with untreated control plants. Under our experimental conditions, in media lacking affinin, the three auxin mutants showed longer primary roots compared with WT plants. Despite this difference in primary root growth, when aux1-7, eir1-1, and axr4-2 plants were grown in the presence of 5.6 × 10-5 m affinin, an inhibition in root elongation was observed (Fig. 9). Because root elongation in auxin-resistant mutants was inhibited to a similar extent than the WT, we conclude that these mutants are equally sensitive to high affinin concentrations than WT plants.
Figure 9.
Primary root growth response of wild-type (Wassilewskija and Col-0) and auxin-resistant mutants to affinin. Seedlings were grown for 12 d on nutrient media with or without 5.6 × 105 m affinin on vertically oriented agar plates. Values shown represent the mean primary root length of 20 seedlings ± sd. Different letters are used to indicate means that differ significantly at the 0.05 level. The experiment was repeated twice with similar results.
DISCUSSION
Effects of Alkamides on Plant Growth and Development
Results from recent research suggest the existence of a wide range of molecules of diverse origin that could alter plant development, and some of these include compounds with fatty acid moieties such as sphingolipids and alkamides (Worral et al., 2003). Kanbe et al. (1993) showed that amidenin, a nonsubstituted alkamide isolated from an actinomycete fungus, could stimulate growth of rice seedlings at concentrations from 0.6 × 10-5 to 1.8 × 10-5 m. However, no information is available about the specific effects of amidenin, and, to the best of our knowledge, no alkamide of plant origin has been tested for plant growth regulating activity. Here, we report that affinin, a plant alkamide alters the growth and development of Arabidopsis seedlings. Affinin treatments in the 7 × 10-6 to 2.8 × 10-5 m range, enhanced early growth of Arabidopsis, stimulated lateral root formation, and promoted root hair elongation. Analyses of LRP in cleared primary roots showed that affinin treatments can induce the formation of lateral roots by inducing perycicle cells to divide and form new LRP, and by stimulating the emergence of existing LRP. The effects of affinin in stimulating biomass production and lateral root development have also been observed in tobacco (Nicotiana tabacum) and rice plants (data not shown), suggesting that some alkamides could have general plant growth regulating activity.
Treatments with 5.6 × 10-5 to 1.2 × 10-4 m affinin inhibited primary root growth and 1.2 × 10-4 m affinin also inhibited root hair elongation. These treatments were found to alter cell division and elongation in Arabidopsis primary roots (Fig. 7, A–G). Because the most important stimulating effect of affinin on root hair elongation occurred at a concentration that inhibits primary root growth (5.6 × 10-5 m), the possibility is raised that the observed enhanced root hair elongation might be due to the formation of shorter epidermal cells. However, our data shows that root hair development is significantly stimulated at low affinin concentrations (7 × 10-6 m) in which primary root growth is stimulated. In addition, we observed that treatment with 1.2 × 10-4 m affinin that showed the greatest inhibitory effect on epidermal cell elongation also inhibited root hair growth when compared with lower affinin treatments (Figs. 5B and 7E). Therefore, the effects of affinin on root hair growth cannot be just explained as an epidermal cell growth compensation effect. Therefore, we propose that the effect of affinin on root hair elongation is, at least in part, mediated by a specific signaling mechanism yet to be identified.
The alkamides N-isobutyl-2E-decenamide and N-isobutyl-decanamide are naturally present in Acmella radicans and Cissampelos glaberrima (Laurerio-Rosario et al., 1996; Rios-Chavez et al., 2003). However, to have enough material for our assays, these amides were obtained by chemical reduction of affinin. The most important difference in the structure of these molecules, compared with affinin, is the absence of the 6, 8-conjugated double bonds in both, and the absence of a 2E-conjugated double bond in the latter (Fig. 1). These compounds were found to stimulate root hair growth at a concentration of 10-8 m, a concentration 100 to 1,000 times lower than that required of affinin to stimulate root hair growth (Fig. 6).
Alkamides Alter Root Development by an Auxin-Independent Mechanism
The phytohormone auxin is a key regulator of lateral root development and root hair formation (Blakely et al., 1982; Laskowski et al., 1995). Auxin is synthesized in the shoot system and is transported to the root through the action of specific polar auxin transport systems. Recently, it has been demonstrated that basipetal and acropetal auxin transport are required during the initiation and emergence phases of lateral root development (Casimiro et al., 2001; Bhalerao et al., 2002). Auxins are active over a very wide range of concentrations: low auxin concentrations (10-10–10-9 m) stimulate primary root growth, whereas higher concentrations (10-8–10-6 m) inhibit primary root growth and stimulate lateral root and root hair formation. An important difference in the mode of action of alkamides compared with auxins is that whereas auxins induce lateral root formation at concentrations that have an inhibitory effect on primary root growth (10-8–10-7 m range), alkamides can induce lateral root formation at a concentration that enhances primary root growth (10-6–10-5 m). These observations suggest that alkamides could alter root growth by a different mechanism to that of auxins.
Further support of the hypothesis that alkamides act by an auxin-independent signaling pathway came from two different lines of evidence: expression studies of the auxin-inducible markers DR5:uidA and BA3:uidA, and the primary root growth inhibition of the auxin mutants aux1-7, eir1-1, and axr4-2 when grown under a high affinin concentration. In our experiments, treatment with 10-8 m 2,4-D induced DR5:uidA and BA3:uidA expression, whereas concentrations of up to 10-4 m affinin failed to induce these auxin-inducible gene markers (Fig. 8). N-isobutyl-2E-decenamide and N-isobutyl-decanamide also failed to induce auxin-regulated expression of DR5:uidA and BA3:uidA (data not shown). These results, together with the finding that the primary root growth of the auxin-resistant mutants aux1-7, eir1-1, and axr4-2 is equally sensitive to affinin than the WT (Fig. 9), indicate that growth effects and developmental changes induced by alkamides are not mediated by the known auxin-signaling pathways.
Alkamides: New Players in Plant Signaling?
Although no data is available about the site of biosynthesis or transport mechanisms of alkamides in plants, taking our data together with those from the literature, it is tempting to speculate that, like endogenous auxins (Abel et al., 1994), alkamides may affect particular signal transduction cascades that modify root growth and differentiation. Because the effect of affinin in stimulating root hair elongation is similar to that reported for ethylene (Tanimoto et al., 1995), one possibility is that alkamides could alter ethylene biosynthesis/perception in some way. However, in contrast to ethylene that induces the ectopic formation of root hairs (Tanimoto et al., 1995; Cao et al., 1999), alkamides apparently only induce root hair elongation in trichoblast cells. Further experimentation to determine whether alkamides influence ethylene production or alter its signaling pathway is required.
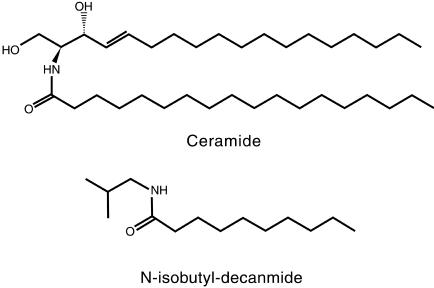
Very recently, the sphingolipids, a group of molecules that include ceramide (Fig. 10) and sphingosine-1-phosphate with well-known regulatory functions in animal and fungal species, have been found to play an important role in plant development (Worral et al., 2003). For example, it has been found that drought and abscisic acid can affect the aperture of the stomatal pore by the action of sphingosine-1-phosphate (Ng and Hetherington, 2001). Based on their chemical structure, the alkamides studied in this work are somewhat structurally related to the sphingolipids ceramide and glucosylceramide (Figs. 1 and 10). In affinin, the decamide resembles the acidic moiety of ceramide, albeit having a shorter C10 instead of the C18 chain in ceramide (Fig. 10). Whether alkamides and sphingolipids have similar biological activities or act through common signaling pathways remains to be determined. The possibility that a breakdown product of ceramide or other sphingolipids may result in metabolites with the observed function of alkamides cannot be excluded.
Figure 10.
Structures of ceramide and N-isobutyl-decanamide.
The data presented in this work and other recent evidence from the literature suggest the existence of new plant growth-regulating substances yet to be characterized that may include alkamides and sphingolipids. To our knowledge, this is the first report of plant-produced alkamides as regulators of root development. Whether these compounds can alter other aspects of plant development would be an interesting field for future research.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Heliopsis longipes (Gray) Blake (Asteraceae) plants were collected at an altitude of 2,500 m above sea level in the municipality of Xichú, located in the Sierra Gorda of Guanajuato State, central México. Voucher specimens (H. longipes Jorge Molina Torres, Instituto de Ecología del Bajío) were deposited at the Instituto de Ecología (Pátzcuaro, México).
Arabidopsis ecotype Col-0 and the Arabidopsis transgenic lines DR5: uidA (Ulmasov et al., 1997), BA3:uidA (Oono et al., 1998), and CycB1:uidA (Colón-Carmona et al., 1999) were used for all experiments unless indicated otherwise. Arabidopsis mutant eir1 (Roman et al., 1995) was kindly provided by Dr. Plinio Guzmán (Departamento de Ingeniería Genética, Centro de Investigación y Estudios Avanzados del Instituto Politécnico Nacional, Irapuato, Gto. México). aux1-7 (Pickett et al., 1990) and axr4-2 (Hobbie and Estelle, 1995) were kindly provided by Dr. Claire Grierson (School of Biological Sciences, University of Bristol, Bristol, UK). Seeds were surface sterilized with 95% (v/v) ethanol for 5 min and with 20% (v/v) bleach for 7 min. After five washes in distilled water, seeds were germinated and grown on agar plates containing 0.1× Murashige and Skoog medium, pH 5.7, 0.5% (w/v) Suc, and 1% (w/v) agar (López-Bucio et al., 2002). The basic medium contained 2.0 mm NH4NO3, 1.9 mm KNO3, 0.3 mm CaCl2.2H2O, 0.15 mm MgSO4.7H2O, 5 μm KI, 25 μm H3BO3, 0.1 mm MnSO4.H2O, 0.3 mm ZnSO4.7H2O, 1 μm Na2Mo04.2H2O, 0.1 μm CuSO4.5H2O, 0.1 μm CoCl2.6H2O, 0.1 mm FeSO4.7H2O, 0.1 mm Na2EDTA.2H2O, inositol (10 mg L-1), and Gly (0.2 mg L-1).
Plates were placed vertically at an angle of 65° to allow root growth along the agar surface and to allow unimpeded growth of the hypocotyl into the air. For plant growth, we used a plant growth cabinet (Percival Scientific, Perry, IA) with a photoperiod of 16 h of light, 8 h of darkness, a light intensity of 300 μmol m-2 s-1, and temperature of 22°C to 24°C.
Isolation of Affinin
H. longipes roots (1 kg) were ground and extracted in 10 L of ethanol at room temperature for 1 week. The alcoholic extract was evaporated to dryness, dissolved in hexane, and fractionated in a 50-cm open glass column packed with silica gel mesh 200 (J.T. Baker, Paris, KY). Different fractions were eluted with 100 mL of hexane and increasing polarity mixtures of hexane:ethyl acetate: 200 mL (90:10, v/v), 200 mL (80:20), 100 mL (70:30), 100 mL (60:40), and 100 mL (50:50), and 100 mL of ethyl acetate. Fifty-milliliter fractions were collected, evaporated, and dissolved in 1 mL of ethanol and were analyzed by capillary gas chromatography (GC) coupled to a mass selective (MS) detector. Fractions eluted with hexane:ethyl acetate (70:30) contained affinin that was further quantified as reported previously (Molina-Torres et al., 1996). Affinin was purified by column chromatography and three times by thin-layer chromatography (TLC) to obtain only one main peak on GC/MS (data not shown). At this stage of purification, affinin comprised 99.6% (w/v) of the total extract, including two affinin stereoisomers (produced by trans-isomerization of one or the two double bonds present in affinin), and methyl-affinin (N-2 methylbutyl 2E,6Z,8E decatrienamide) comprising the remaining 0.4% (w/v) of the extract. No other compounds could be detected in the affinin extract by GC-MS analysis. For the reduced amides, N-isobutyl-2E-decenamide and N-isobutyl-decanamide, purity was 100% because affinin and its stereoisomers were reduced to the same component and were totally separated from methyl-affinin by argented TLC.
GC/MS Analysis
Samples were analyzed in a gas chromatograph (model 5890; Hewlett-Packard, Palo Alto, CA) equipped with a capillary column HP-1MS (30- × 0.25-mm i.d; 0.25-μm film thickness) coupled to a MS detector (model 5972; Hewlett-Packard). Operating conditions were an injector temperature of 200°C, and the oven temperature was programmed with an initial temperature of 150°C for 3 min, increasing at the rate of 4°C min-1 to a final temperature of 300°C, which was maintained for 20 min. Helium was used as carrier gas with a constant flow of 1 mL min-1. One microliter of the sample was injected with a split ratio of 50:1.
Production of N-Isobutyl-2E-Decenamide and N-Isobutyl-Decanamide
To obtain the N-isobutyl-2E-decenamide and N-isobutyl-decanamide, a catalytic reduction of affinin was performed. Ten milligrams of platinum oxide were added to 10 mg of affinin resuspended in 2 mL of ethanol. A hydrogen gas stream was bubbled through the mixture maintained at 80°C. After 3 min of reduction, the 2E-decanamide was collected as major component and was purified by analytical argented TLC 20- × 20-cm glass plates as reported elsewhere (Rios-Chavez et al., 2003). Plates were developed using a hexane:ethyl acetate (2:1, v/v) as a solvent system. Developed plates were solvent freed at room temperature and were then sprayed with a 0.02% (w/v) ethanolic fluorescein solution. The N-isobutyl-2E-decenamide was detected as a fluorescent band on a dark background with a relative retention index of 0.7. This band was collected from the plate and was eluted with ethyl acetate that was later evaporated under a stream of nitrogen. The TLC purification was repeated three times and the fraction containing the intermediary was submitted to GC/MS detection, giving only one peak. The N-isobutyl-decanamide was obtained after a 7-min reduction as the only product. The platinum oxide was centrifuged, and the supernatant was decanted, evaporated to dryness, dissolved in hexane, and submitted to GC/MS detection, giving only one peak. Compounds were initially identified as N-isobutyl-2E-decenamide and N-isobutyl-decanamide by their mass spectra. 1H NMR and 13C NMR analysis on a model Gemini 200 (Varian, Palo Alto, CA) further confirmed the reported structures.
Histological Analysis
For histochemical analysis of GUS activity, Arabidopsis seedlings were incubated overnight at 37°C in a GUS reaction buffer (0.5 mg mL-1 5-bromo-4-chloro-3-indolyl-B-d-glucuronide in 100 mm sodium phosphate, pH 7). The stained seedlings were cleared by the method of Malamy and Benfey (1997). For each marker line and for each treatment, at least 10 transgenic plants were analyzed. A representative plant was chosen for each affinin or auxin treatment and was photographed using the Nomarski optics on a microscope (DMR; Leica, Deerfield, IL).
Data Analysis
Arabidopsis root systems were viewed with a stereomicroscope (AFX-II-A; Nikon, Tokyo). All lateral roots emerged from the primary one, were observed at the 3× objective, and were taken into account for lateral root number data. Primary root length was determined for each root using a plastic ruler. The stages of lateral root primordia were analyzed on cleared roots using a microscope (UW; Nikon) in the 40× objective. For trichoblast measurements, cleared root images from the mature zone were recorded with a digital camera connected with a microscope. From the same zone, the lengths of 10 mature epidermal cells per root were photographed and the cell length was then measured with Image-Pro Plus version 4.0 for Windows (Image-Pro Plus; Media Cybernetics, Silver Spring, MD). The measurements were repeated in at least eight plants from two independent experiments.
For all experiments, the overall data was statistically analyzed with the SPSS 10 program (SPSS Inc., Chicago). Univariate and multivariate analyses with a Tukey's post hoc test were used for testing differences in primary root length, lateral root number, and lateral root density under affinin treatments in WT and mutant plants. Different letters are used to indicate means that differ significantly (P < 0.05).
Acknowledgments
We thank Cristina Reynaga-Peña for her support in microscopy. We also thank Claire Grierson, Athanasios Teologis, Tom Guilfoyle, Peter Doerner, and Plinio Guzmán for kindly providing us with seeds of transgenic and mutant lines.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.034553.
This work was supported in part by the Howard Hughes Medical Institute (grant no. Nbr55003677) and by the European Commission (grant no. ICA–4–CT2000–30017).
References
- Abel S, Oeller PW, Theologis A (1994) Early auxin-induced genes encode short-lived nuclear proteins. Proc Natl Acad Sci USA 91: 326-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalerao RP, Ekloff J, Ljung K, Marchant A, Bennet M, Sandberg G (2002) Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29: 325-332 [DOI] [PubMed] [Google Scholar]
- Blakely LM, Durham M, Evans TA, Blakely RM (1982) Experimental studies on lateral root formation in radish seedling roots: general methods, developmental stages, and spontaneous formation of lateral roots. Bot Gaz 14: 341-352 [Google Scholar]
- Cao XF, Lindstead P, Berger F, Kieber J, Dolan L (1999) Differential ethylene sensitivity of epidermal cells is involved in the establishment of cell pattern in the Arabidopsis root. Physiol Plant 106: 311-317 [DOI] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beekman T, Dhooge S, Swarup R, Graham N, Inze D, Sandberg G, Casero PJ et al. (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843-852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen L, Lam J (1991) Acetylenes and related compounds in Heliantheae. Phytochemistry 30: 11-49 [Google Scholar]
- Colón-Carmona A, You R, Haimovitch-Gal T, Doerner P (1999) Spatio temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J 20: 503-508 [DOI] [PubMed] [Google Scholar]
- Hobbie L, Estelle M (1995) The axr4 auxin resistant mutant of Arabidopsis thaliana defines a gene important for root gravitropism and lateral root initiation. Plant J 7: 211-220 [DOI] [PubMed] [Google Scholar]
- Hofer O, Greger H, Robien W, Werner A (1986) 13C NMR and 1H lanthanide induced shifts of naturally occurring alkamides with cyclic amide moieties: amides from Achillea falcata. Tetrahedron Lett 42: 2707-2716 [Google Scholar]
- Jacobson M (1971) The unsaturated isobutilamides. In M Jacobson, D Crosby, eds, Naturally Occurring Insecticides. Marcel Dekker, New York, pp 137-176
- Kanbe K, Naganawa H, Okamura M, Sasaki T, Hamada M, Okami Y, Takeuchi T (1993) Amidenin, a new plant growth-regulating substance isolated from Amycolatopsis sp. Biosci Biotechnol Biochem 57: 1261-1263 [Google Scholar]
- Kashiwada Y, Ito C, Katagiri H, Mase I, Komatzu K, Namba T, Ikeshiro Y (1997) Amides of the fruit of Zanthoxylum spp. Phytochemistry 44: 1125-1127 [Google Scholar]
- Laskowski MJ, Williams ME, Nusbaum HC, Sussex IM (1995) Formation of lateral root meristems is a 2-stage process. Development 121: 3303-3310 [DOI] [PubMed] [Google Scholar]
- Laurerio-Rosario S, Silva A, Parente J (1996) Alkamides from Cissampelos glaberrima. Plant Med 62: 376-377 [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderon L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L (2002) Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol 129: 244-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33-44 [DOI] [PubMed] [Google Scholar]
- Molina-Torres J, Salgado-Garciglia R, Ramirez-Chavez E, del Rio R (1996) Purely oleofinic alkamides in Heliopsis longipes and Acmella (Spilanthes) oppositifolia. Biochem Syst Ecol 24: 43-47 [Google Scholar]
- Ng CKY, Hetherington AM (2001) Drought-induced guard cell signal transduction involves sphingosine-1-phosphate. Nature 410: 596-599 [DOI] [PubMed] [Google Scholar]
- Oono Y, Chen QG, Overvoorde PJ, Kohler C, Theologis A (1998) age mutants of Arabidopsis exhibit altered auxin-regulated gene expression. Plant Cell 10: 1649-1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar V, Jain S, Bisht K, Jain R, Taneja P, Jha A, Tyagi O, Prasad A, Wengel J, Olsen C, Bool P (1997) Phytochemistry of the genus Piper. Phytochemistry 46: 597-673 [Google Scholar]
- Pickett FB, Wilson AK, Estelle M (1990) The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol 94: 1462-1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Chavez P, Ramirez-Chavez E, Armenta-Salinas C, Molina-Torres J (2003) Acmella radicans var. radicans: in vitro culture establishment and alkamide content. In Vitro Cell Dev Biol Plant 39: 37-41 [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg, Ecker JR (1995) Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics 139: 1393-1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser 0, Bechtold N, Weisbeek P, Scheres B (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463-472 [DOI] [PubMed] [Google Scholar]
- Tanimoto M, Roberts K, Dolan L (1995) Ethylene is a positive regulator of root hair development in Arabidopsis thaliana. Plant J 8: 943-948 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle T (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963-1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worral D, Ng CKY, Hetherington AM (2003) Sphingolipids, new players in plant signaling. Trends Plant Sci 8: 317-320 [DOI] [PubMed] [Google Scholar]
- Ximenez F (1615) Quatro Libros de la Naturaleza, y Animales que Aftan Recevidos en el Vfo de Medicina de la Nueva España. Viuda de Diego Lopez Davalas, Mexico City, Mexico
- Zhang H, Jennings A, Barlow PW, Forde BG (1999) Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA 96: 6529-6534 [DOI] [PMC free article] [PubMed] [Google Scholar]