Abstract
The maize (Zea mays) late pollen gene ZmMADS2 belongs to the MIKC type of MADS box transcription factor genes. Here, we report that ZmMADS2, which forms a homodimer in yeast (Saccharomyces cerevisiae), is required for anther dehiscence and pollen maturation. Development of anthers and pollen was arrested at 1 d before dehiscence in transgenic plants expressing the ZmMADS2-cDNA in antisense orientation. Temporal and spatial expression analyses showed high amounts of ZmMADS2 transcripts in endothecium and connective tissues of the anther at 1 d before dehiscence and in mature pollen after dehiscence. Transient transformation of maize and tobacco (Nicotiana tabacum) pollen with the luciferase reporter gene under the control of different ZmMADS2 promoter deletion constructs demonstrated the functionality and tissue specificity of the promoter. Transgenic maize plants expressing a ZmMADS2-green fluorescent protein fusion protein under control of the ZmMADS2 promoter were used to monitor protein localization during anther maturation and pollen tube growth. High amounts of the fusion protein accumulate in degenerating nuclei of endothecial and connective cells of the anther. A possible function of ZmMADS2 during anther dehiscence and pollen maturation and during pollen tube growth is discussed.
In higher plants, development of the haploid male gametophyte (pollen) is closely related to maturation of the surrounding sporophytic tissues of the anther. In angiosperms, anther tissues arise from three “germ” layers, designated L1 to L3 (Satina and Blakeslee, 1941). In maize (Zea mays), tapetal initials and pollen mother cells are generated by the division of a diploid sporophytic cell. The tapetal initial cells give rise to the outer, middle, and inner (tapetal) layers of the sporangium wall (Kiesselbach, 1999). The sporogenous cells (pollen mother cells) undergo meiosis, giving rise to a tetrad of haploid cells, which are released as free microspores (McCormick, 1993). During microgametogenesis, microspores develop into mature pollen by two mitotic divisions. The first mitotic division results in a vegetative and a generative cell. The latter divides either in the pollen grain or the pollen tube to generate the two haploid sperm cells. In maize, the generative cell within the young pollen divides before maturation of the anther is completed to generate a tricellular mature pollen grain (Bedinger, 1992).
During gametogenesis, the innermost cell layer of the anther, the tapetum, plays a crucial role for the release and nutrition of the microspores. Microspores are supplied with nutrients from the tapetum; therefore, mutations affecting tapetal development lead to abortion of microgametogenesis and male sterility (Cheng et al., 1979; Chaudhury, 1993; Okada et al., 1999; Wilson et al., 2001; Kapoor et al., 2002). Tapetal cells secrete callase to release the meiotic tetrad from an enclosing callose wall. The exact timing and proper function of callase has been shown to be essential for pollen development. Precursors for the biosynthesis of the outer pollen wall (exine) are also supplied by tapetal cells, and even their remnants serve the pollen grain as tryphine or pollenkitt after degeneration (Bedinger, 1992). The structure of maize anthers differs significantly from the body of dicot anthers. For example, in tomato (Lycopersicon esculentum; Bonner and Dickinson, 1989) or tobacco (Nicotiana tabacum; Koltunow et al., 1990), the endothecium develops only in the region adjacent to the stomium in the distal one-third of the anther. In contrast, anther locules of maize are completely surrounded by a single layer of endothecial cells directly attached to the central connective (Cheng et al., 1979). During the final stages of pollen maturation, the tapetal cell layers degenerate completely, with endothecium, epidermis, and connective remaining functional, whereas anther dehiscence proceeds. The dehiscence program is temporally coordinated with the pollen differentiation process and triggers an ordered series of events within the anther culminating with anther burst and release of mature pollen (Goldberg et al., 1993).
Several mutants have been described affecting both anther and pollen maturation in corn and, thus, result in male sterility (e.g. Beadle, 1932; Cheng et al., 1979; Albertsen and Phillips, 1981; Neuffer et al., 1997). For example, Cheng et al. (1979) demonstrated that development of tapetal cells is affected at the young microspore stage in ms10, whereas microspores show cytoplasmic degeneration and failure of pollen wall synthesis in the intermediate microspore stage. The molecular nature of the corresponding genes is mostly unknown. In contrast to maize, several delayed dehiscence mutants have been described in Arabidopsis (for review, see Patterson, 2001). Anthers in the DEFECTIVE IN ANTHER DEHISCENCE mutant, for example, do not dehisce, and pollen grains do not germinate either on medium or on fresh stigmata (Ishiguro et al., 2001). Another mutation, ms35, generates lack of secondary wall thickening in the endothecium; thus, anthers do not open, although pollen are fully fertile (Dawson et al., 1999).
The temporally coordinated degeneration of anther tissues seems to be regulated by a sequential gene expression cascade. Several transcription factor genes haves been reported to be expressed in anthers, including those encoding MYB-related proteins, zinc finger transcription factors, and MADS box transcription factors, namely DEFH125, AGL15, AGL18, ZmMADS1, and ZmMADS2 (Zachgo et al., 1997; Kobayashi et al., 1998; Alvarez-Buylla et al., 2000; Fernandez et al., 2000; Heuer et al., 2000, 2001; Robson et al., 2001; Yang et al., 2001). Kobayashi et al. (1998), for example, reported seven zinc finger transcription factor genes sequentially expressed during anther development in petunia (Petunia hybrida) and suggested that they act in a regulatory cascade. The maize MADS box genes ZmMADS1 and ZmMADS2 may be candidates of a similar system regulating anther and pollen development. ZmMADS1 expression peaks in young microspores and decreases during microgametogenesis (pollen development), whereas ZmMADS2 transcripts accumulate in mature pollen and pollen tubes, when ZmMADS1 expression is almost completely switched off (Heuer et al., 2000). Here, we report the functional analysis of the ZmMADS2 gene of maize, which is required for regulating anther dehiscence and pollen maturation and discuss its role in nuclear degradation during anther dehiscence.
RESULTS
ZmMADS2 Antisense Plants Exhibit Anther Dehiscence and Pollen Maturation Defects
To study the function of the late pollen gene ZmMADS2, we have used an antisense approach to generate loss-of-function mutants. The constitutive maize ubiquitin promoter was chosen to drive full-length ZmMADS2-cDNA transgene expression. This promoter was previously been shown to be expressed at much higher levels in pollen and other tissues compared with the ZmMADS2 promoter (Schreiber and Dresselhaus, 2003).
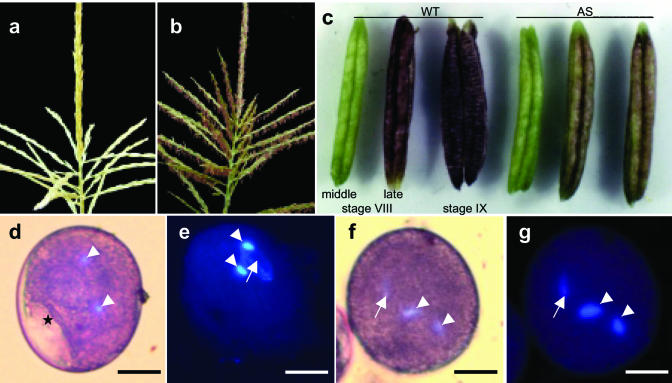
Eighteen BASTA resistant transgenic maize lines were generated of a total of 1,783 bombarded immature maize embryos (transformation efficiency of 1%). Genomic Southern blots showed integrations of the ZmMADS2-cDNA antisense construct pDNS-4 in nine transgenic plants (cotransformation efficiency of 50%). Four plants (all independent lines) showed a full-length integration of the pUbi::ZmMADS2-AS construct, whereas the other five plants, representing two independent transgenic lines, showed a partial integration. All transgenic plants containing full-length integrations showed strong transgene expression in leaves, whereas the other plants showed a lower expression because of an incomplete integration of the ubiquitin promoter (data not shown). Two of the four full-length integration plants and their T1 and T2 progenies showed a wild-type (WT) phenotype, whereas the other seven T0 plants were male sterile. As shown in Figure 1a, all seven sterile plants (representing four independent lines) showed the same phenotype: fully developed tassels but no opening of male florets. Tassels of transgenic plants remained green for several weeks without occurrence of anthesis (Fig. 1a), whereas tassels of WT plants of the same age reached maturity (Fig. 1b). Development of anthers was arrested shortly before opening of the anterior pore without elongation of the filament. Figure 1c shows the development of anthers from T0 plants compared with WT plants from 3 d before until anthesis. Anthers of transgenic plants were arrested at stage VIII of development, whereas anthers of WT plants dehisced and released mature pollen. Anthocyanin levels indicate an arrest between middle and late stage VIII of anther development. Maturation of transgenic pollen corresponded to an arrest of development at 1 d before anthesis. Starch granules were visible inside the still partly vacuolated pollen grain, and nuclei of sperm cells appeared round instead of the sickle-shaped form of mature WT pollen (Fig. 1, d and e). A few hundred pollen of different anthers of both transgenic and WT lines were analyzed. Some 2% pollen of transgenic and WT lines were arrested at the microspore stage, whereas about 98% of WT pollen reached maturity. In contrast, more than 90% pollen of anthers from independent male sterile lines showed the arrested phenotype (see description above), and few pollen reached maturity. In contrast to WT pollen, arrested and fully developed pollen of male sterile plants neither germinated in vitro nor led to progeny kernels after selfing or outcrossing to A188 WT plants. Expression of ZmMADS2 in pollen of male sterile plants could not be detected. Later pollination of cobs from male sterile plants with pollen of WT plants also did not result in progeny kernels. This female “sterility” effect of male sterile plants was probably caused by the late pollination as silks became dry.
Figure 1.
Phenotypes of transgenic maize plants expressing ZmMADS2 cDNA in antisense orientation under control of the constitutive ubiquitin promoter of maize. Development of tassels, anthers, and pollen is arrested at 1 d before anthesis. a, Tassel of a transgenic plant at the latest stage of development. b, Tassel of a WT plant at anthesis. c, Anthers of a WT plant (left) and that of a transgenic antisense (AS) plant (right) at 2 d before anthesis (middle stage VIII), 1 d before anthesis (late stage VIII), and at anthesis (stage IX). Light microscopic (d) and UV microscopic (e) images of pollen of transgenic plants at the latest stage of development. Pollen were stained with 4′,6-diamino-2-phenylindole dihydrochloride (DAPI) and acetic orcein. Note that a vacuole is still visible (asterisk), and sperm cells (arrowheads) are round instead of sickle shaped. Light microscopic (f) and UV microscopic (g) image of a mature WT pollen stained with DAPI and acetic orcein. Vacuoles are no longer visible, and sperm cells (arrowheads) are sickle shaped. Arrows point toward the vegetative nucleus. Bars = 20 μm.
Genomic Structure of the ZmMADS2 Gene
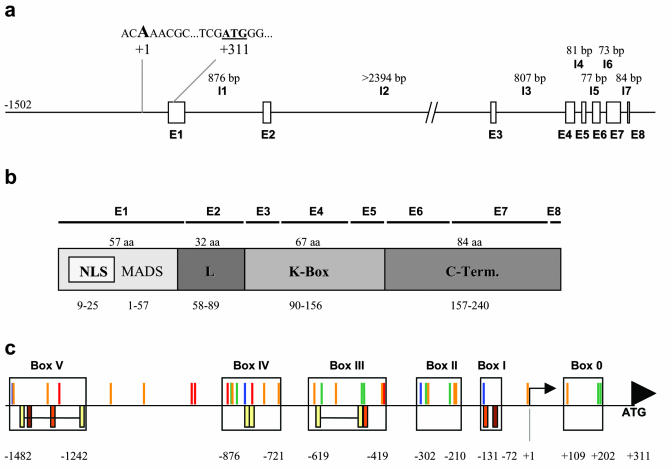
ZmMADS2 is a member of the MIKC type (without N-terminal extension) of MADS box transcription factor genes. As shown in Figure 2a, the ZmMADS2 gene (GenBank accession numbers AY227363 and AY264885) contains eight exons and seven introns, with exon as well as intron positions and sizes comparable with other typical MADS box genes (Riechmann and Meyerowitz, 1997). The second intron (I2 in Fig. 2a), which was cloned incompletely, is an exception, because with more than 2.3 kb, it is unusually long. The MADS box is located within exon 1 (E1 in Fig. 2b) and contains a bipartite nuclear localization signal. Exon 2 contains the linker or intervening sequence (E2 in Fig. 2b). Functional domains are separated by relatively large introns, whereas shorter introns are inserted inside both the K box and C terminus. The context sequence of the translational initiation codon is typical for monocots (Joshi et al., 1997). The transcription start point was defined by primer extension analysis (data not shown) and is located 311 bp upstream of the ATG START codon. The ZmMADS2 promoter was isolated as a 1,502-bp fragment upstream of the transcription start point. A TATA box some 20 to 30 bp upstream of the transcription start point could not be detected. Sequence analyses using the PLACE database (Higo et al., 1999) and the MatInspector software (Genomatrix Software, München, Germany; Quandt et al., 1995) revealed numerous putative cis-acting elements (Fig. 2c) within the promoter sequence, including late pollen-specific elements of tomato and tobacco (Bate and Twell, 1998; Rogers et al., 2001), which are almost equally distributed within the promoter sequence. Root-specific elements (Elmayan and Tepfer, 1995) occur from position -1,301 to -419, sugar starvation-induced elements (Grierson et al., 1994; Hwang et al., 1998; Toyofuku et al., 1998) within boxes 0 and II to IV, and stress/abscisic acid-induced elements (Lopez-Molina et al., 2002) in boxes I, II, IV, and V (Fig. 2c). Sequence analysis using the Gene Quest Software (LASERGENE software, DNASTAR, Inc., Madison, WI) showed multiple repetitive elements within the promoter sequence. Interestingly, the only box lacking repetitive elements, box II, has been shown in transient expression studies (Fig. 3b) to contain the most important cis element(s) for pollen-specific marker gene expression in maize and tobacco.
Figure 2.
Genomic structure of the ZmMADS2 gene. a, The ZmMADS2 gene consists of eight exons (E1–E8) and seven introns (I1–I7) with an unusually large second intron (I2). The transcription start point was determined using a primer extension assay. The ATG START codon is located 312 bp downstream of the transcription start point. The context sequence around the translational initiation codon ATG is typical for monocots. Intron sizes are given in base pairs above the sequence. b, ZmMADS2 belongs to the MIKC type of MADS box proteins. ZmMADS2 contains of a highly conserved MADS box at the N terminus, a linker sequence, a keratine box, and a highly variable C terminus. A putative bipartite nuclear localization signal is found inside the MADS box. Distribution of exons is shown above protein domains. c, Clustering of putative cis-acting elements and repetitive sequences upstream of the ATG START codon. Putative cis elements responsible for sugar starvation induction are displayed in green (ACGTA or AATAGAAAA), those responding to water stress and abscisic acid are displayed in blue (TAACTG and ACACNNG, respectively), putative root-specific elements are shown in red (ATATT), and sequences homologous to motifs responsible for late pollen-specific expression are given in orange (AGAAA or GTGA). Sequence repeats are shown below the horizontal bar. Yellow, Direct repeats (GTATGTAACATA... –... GTATGTAACATA in box V, AGTTATATATTTAT-AGTTATATATTTAT in box IV, and CTCAAATACGTAA... –... CTCAAATACGTAA in box III), brown, inverted repeats (TAATAATTATTA); dark orange, dyad repeats (ATTGAAAAGTTA). Arrow, Transcription start site; arrowhead, position of the start codon.
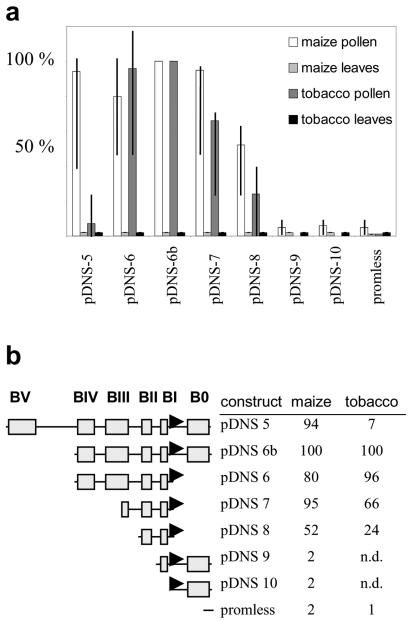
Figure 3.
ZmMADS2 promoter deletion analyses with transiently transformed pollen and leaves of maize and tobacco. a, Relative expression levels of luciferase directed by ZmMADS2 promoter deletion constructs in pollen and leaves of maize and tobacco, respectively. Ten experiments were conducted with pDNS-5, -6, -6b, -7, and -8, whereas three experiments were conducted with pDNS-9 and -10. The promoterless control was used in more than 30 experiments. Vertical lines within bars indicate relative minimum and maximum rates, and bars indicate average expression levels. For a better comparison, expression levels of pDNS-6b were set to 100% for each individual experiment. b, Deletion constructs consist of boxes and putative cis elements as shown in Figure 2c. Relative luciferase expression in pollen of maize and tobacco as an average of 10 experiments is shown. n.d., Not determined.
Transient Expression of ZmMADS2 Promoter Deletion Constructs
Transient transformation of mature maize and tobacco pollen and young leaves was performed with seven ZmMADS2 promoter deletion constructs to elucidate pollen-specific promoter elements of monocot genes. A promoterless luciferase construct was used as a negative control (de Wet et al., 1987). As shown in Figure 3a, pDNS 6b (approximately 1 kb upstream of transcription start point plus 5′-untranslated region [UTR]) showed highest expression in both maize and tobacco pollen. In maize, expression levels of pDNS-5, -6, and -7 were only slightly lower, ranging on average between 80% to 95% compared with the relative expression level of pDNS-6b. Relative expression levels of pDNS-8 ranged from 35% to 70% compared with pDNS-6b (Fig. 3, a and b). In tobacco, pDNS-6 showed an average expression level of 96%. Expression levels of pDNS-7 ranged between 30% and 70% with an average of 66%, and those of pDNS-8 were between 10% and 50% with an average of 24% (Fig. 3b). In contrast to maize, expression levels of pDNS-5, the longest promoter fragment, were very low in tobacco, ranging from 3% to 25% (average of 7%). This may be because of a repression by cis-acting elements located in Box V functioning in tobacco but not in maize (Fig. 2c). Expression levels of pDNS-9 and -10 were only slightly above background in maize compared with the promoterless LUC construct and were not determined in tobacco. Luciferase expression was not measurable above background levels in young leaves of both plants, indicating that the ZmMADS2 promoter is pollen specific. The most important functional cis-acting elements are located in box II between positions -327 and -210 relative to the transcription start point. This region could now be used to identify pollen-specific cis elements functioning in both monocots (maize) and dicots (tobacco).
ZmMADS2 Is Transcribed in Anthers at 1 d before Dehiscence and during Pollen Tube Growth
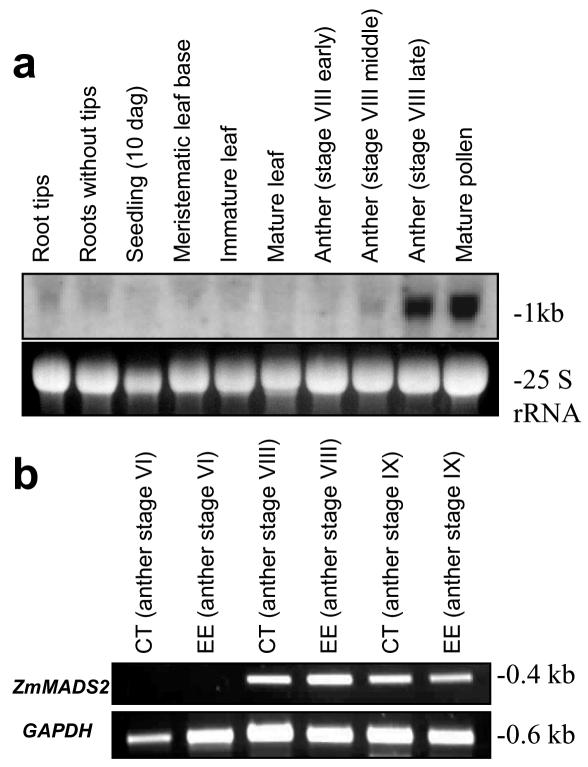
Northern-blot analyses and reverse transcription (RT)-PCR was performed to study the expression of ZmMADS2 during anther maturation and pollen development. As shown in Figure 4a, transcripts were first detected during anther development, in the middle of stage VIII, when anthers reach their final size but are still green. Transcripts are most abundant in anthers at late stage VIII (shortly before opening of the anterior pore, see also Fig. 1c) and in mature pollen. Very weak signals were observed in roots with and without tips but not in other vegetative tissues. To investigate whether the signals obtained from anthers were derived from the maternal tissues of the anther or maturating pollen, we have microdissected anther tissues at different developmental stages and removed microspores/pollen. Figure 4b shows expression of ZmMADS2 starting at stage VIII of anther development in both connective tissue and endothecium, which also contained epidermal cells. Interestingly, relative strong transcript amounts were still detectable in degenerating endothecium and connective tissues after anthesis (stage IX).
Figure 4.
ZmMADS2 is expressed in anther tissues shortly before anther dehiscence and in mature pollen. a, Northern blot showing expression of ZmMADS2 in vegetative tissues and during anther development. Transcripts are first detected in the middle of stage VIII, when anthers reach their final size but are still green (see also Fig. 1c). Highest transcript amounts are visible in anthers at late stage VIII (red color; shortly before opening of the anterior pore) and in mature pollen. Very weak signals were observed in roots with and without tips. An expression in other vegetative tissues could not be detected. b, RT-PCR analysis of microdissected anther tissues. Connective tissue (CT) and endothecium with epidermis (EE) were isolated from anther stages indicated. Forty PCR cycles were conducted to amplify ZmMADS2 transcripts and 35 PCR cycles for GAPDH as a control. Expression of ZmMADS2 is visible in maternal anther tissues at the last stages of anther development (stages VIII and IX).
ZmMADS2 Forms Homodimers and Localizes to Nuclear Fragments in Endothecial and Connective Cells
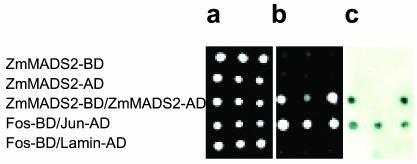
MADS box proteins are known to form dimers in the cytoplasm, which then enter the nucleus. To investigate whether ZmMADS2 forms homodimers in vivo, a prerequisite to enter the nucleus without an additional binding partner, we have used the yeast (Saccharomyces cerevisiae) two-hybrid system with ZmMADS2 both as bait and prey. Of 60 independent yeast clones growing on selective media, all carrying both bait and prey, 44 clones showed a blue staining using the β-galactosidase assay. Figure 5 shows that auto-activation of ZmMADS2 fused to the LexA-binding or B42 activation domain was not detectable. The selection (Fig. 5b) and the β-galactosidase assay (Fig. 5c) demonstrate that ZmMADS2 proteins interact and form homodimers in vivo.
Figure 5.
ZmMADS2 forms homodimers in the yeast two-hybrid system. Three independent clones were each pipetted on agar plates containing full medium without selection (a); selection medium without His, Trp, Lys, and Ura with 300 μg mL-1 Zeocin (b), and the β-galactosidase assay (c). Top row, ZmMADS2 fused to the LexA BD (DNA-binding domain), bait. Second row, ZmMADS2 fused to the B42 activation domain (AD), prey. Third row, ZmMADS2 fused to LexA BD (bait) and ZmMADS2 fused to B42 AD (prey). Fourth row, Fos fused to LexA BD (bait) and Jun fused to B42 AD (prey) as positive control. Fifth row, Fos fused to LexA BD (prey) and Lamin fused to B42 AD (prey) as a negative control.
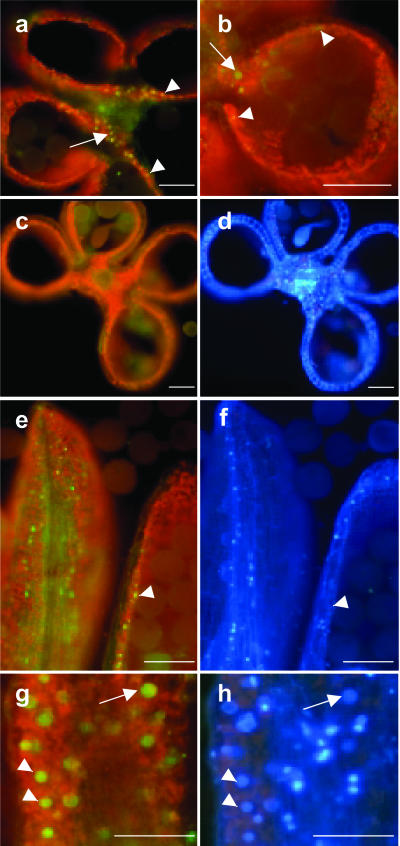
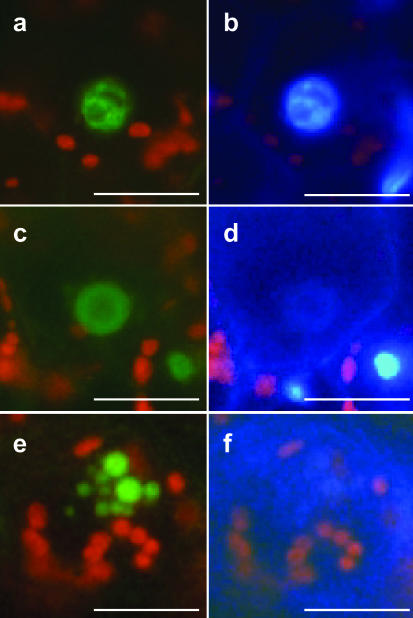
To investigate the subcellular localization of ZmMADS2 and its tissue-specific localization within the anther, transgenic maize plants expressing a ZmMADS2-green fluorescent protein (GFP) fusion protein under control of the ZmMADS2 promoter were generated. Immature embryos (728) were bombarded, and 12 BASTA plants were regenerated (transformation efficiency of 1.6%). Two independent lines containing a full-length integration of the pZmMADS2::ZmMADS2-GFP construct were used for further studies. Both lines showed a strong expression of the chimeric gene (data not shown) and the pattern of GFP fluorescence as identical. Strong GFP fluorescence was detectable exclusively in nuclei of cells of the connective tissue and the endothecial layer of anthers at 1 d before anthesis (Fig. 6, a, b, e, and g). Restricted localization of the fusion protein to the nucleus indicates that ZmMADS2 acts as a DNA-binding protein. At the stage of maximum GFP fluorescence, the tapetum was completely degenerated, whereas inter-microsporangial stripes at the site of separation were still attached to the connective tissue (Fig. 6a). GFP fluorescence was never observed at other developmental stages of the anther nor in WT anthers at comparable stages of development (Fig. 6c). Longitudinal sections showed that the ZmMADS2 fusion protein is expressed in endothecium and connective tissue and that localization of the protein is not restricted to the unopened anterior pore (Fig. 6e). Additional DAPI staining of cross or longitudinal sectioned anthers revealed that nuclei showing brightest GFP fluorescence showed faint DAPI signals, whereas nuclei showing strong DAPI staining gave weaker or no GFP fluorescence. This result indicates that ZmMADS2 protein accumulates during the final stages of anther maturation, when stabilizing anther cells degenerate before merging and dehiscence of the anther locules. Figure 7 illustrates the nuclear degradation process during the final stage of anther maturation within representative endothecial cells and cells of the connective tissue. As cell death in these cells is proceeding, the compartmented structure of the nuclei (Fig. 7, a and b) disintegrates (Fig. 7, c and d). Apoptotic bodies, containing almost completely degraded DNA, appear at the final stage of degeneration (Fig. 7, e and f).
Figure 6.
ZmMADS2-GFP expression in cross and longitudinal sections of anthers from transgenic maize plants at 2 d before anthesis. a, Cross section of a late stage VIII anther of a transgenic maize line expressing the ZmMADS2-GFP-fusion protein under control of the ZmMADS2 promoter (Fig. 3) in connective tissue (arrow) and endothecial cells (arrowheads). b, Close-up of an anther locule; arrow marks nucleus in connective tissue and arrowheads in endothecial cells. c, WT anther of the same stage lacking green fluorescence. d, DAPI staining of c. e and g, Longitudinal section to show expression of the ZmMADS2-GFP-fusion protein in nuclei of endothecial cells. e, Expression of the fusion protein is not restricted to the region of unopened anterior pore. Note that nuclei showing bright GFP-fluorescence display only faint DAPI signals. g, Close-up of a longitudinal section showing expression of the ZmMADS2-GFP fusion protein in connective tissue. f and h, DAPI staining of images shown in e and g. Note that the stronger the GFP fluorescence, the weaker the DAPI signal. Arrowheads point to strong GFP fluorescence and weak DAPI staining; arrow points toward stronger GFP fluorescence and weaker GFP signals. Bars = 100 μm.
Figure 7.
ZmMADS2 marks degrading nuclei of endothecial cells during anther maturation. a, ZmMADS2-GFP fluorescence of an intact nucleus within an endothecial cell. b, DAPI staining of the image shown in a. Note that the nucleus shows compartmentation. c, The ZmMADS2-GFP-fusion protein is visible, whereas most DNA is degraded. The structure of the nucleus is lost. d, DAPI staining of c. Few intact DNA are left. e, ZmMADS2-GFP is detectable in apoptotic bodies of an endothecial nucleus. f, DAPI staining of e. DNA is almost completely degraded. Bars = 10 μm.
DISCUSSION
The majority of plant MADS box genes have been shown to be involved in flowering and flower organ development, although some are also expressed during vegetative development. We have reported here about the functional analysis of the MADS box gene ZmMADS2 from maize, which belongs to the AGL17 subfamily of MADS box transcription factors (Heuer et al., 2000). ZmMADS2 shares highest amino acid sequence identity with DEFH125 (Zachgo et al., 1997). Both genes are predominantly expressed in anthers and pollen, but most other members of this monophyletic clade, such as AGL2, AGL17, and ANR1, are exclusively expressed in roots (Zhang and Forde, 1998; Burgeff et al., 2002). ANR1 is the only member of this clade whose function is known, because it is a regulator of a signal transduction pathway linking the external NO3- concentration in the soil with an increase in the rate of lateral root elongation (Zhang and Forde, 1998).
Many late pollen genes such as ZmMADS2 have been reported to be expressed both in sporophytic anther tissues and in the male gametophyte (Xu et al., 1993). The LAT52 gene of tomato, for example, shows an expression pattern similar to ZmMADS2. This gene is expressed in sporophytic tissues of the anther, mature pollen, and root caps, but also in the endosperm (Twell et al., 1991) and was shown recently to be a ligand of the pollen receptor kinase LePRK2 (Tang et al., 2002). Translational enhancement of the late pollen genes LAT52 and NTP303 has been reported to be mediated by their 5′-UTRs (Bate et al., 1996; Hulzink et al., 2002). Transient transformation assays with ZmMADS2 promoter deletion constructs revealed only slight differences in expression levels of the luciferase reporter gene either with or without 5′-UTR, indicating that the functional role of the 5′-UTR of ZmMADS2 is different.
Distribution of functional domains and localization of intron-exon boundaries within the ZmMADS2 gene resemble those of a typical MADS box gene, except for the large second intron (>2.3 kb). An unusual large second intron (2,985 bp) is also present in the AG (AGAMOUS) gene of Arabidopsis, which is required for tissue-specific expression (Sieburth and Meyerowitz, 1997). This also seems to be different for the ZmMADS2 gene, where pollen-specific elements are present some 330 to 210 bp upstream of the transcription start point.
Bioinformatical analysis of the ZmMADS2 promoter resulted in the identification of numerous putative regulatory elements. Already known cis elements required for pollen- and root-specific expression are distributed equally within the ZmMADS2 promoter sequence, indicating that these known motifs might not be significant for regulation of pollen- and root-specific expression in maize. Regulatory elements identified in the promoter of the pollen-specific maize gene ZM13 (Hamilton et al., 1998), a maize homolog of the previously mentioned LAT52 gene of tomato (Bate et al., 1996), could not be identified in the ZmMADS2 promoter region. Transient transformation studies with the luciferase reporter gene under control of seven ZmMADS2 promoter deletion constructs revealed that cis-acting elements required for pollen-specific expression are located in boxes II and III. Putative cis-acting elements inside this fragment include two G box-like sequences mediating sugar repression in box III (ACGTA), an additional one in box II (AATAGAAAA), and a coupling element of G boxes is located in box II (CGACG; Hwang et al., 1998; Toyofuku et al., 1998). In addition, a Suc-responsive element, an auxin-responsive element, and elements responding to water stress and abscisic acid were identified in this region of the promoter, suggesting a role of ZmMADS2 e.g. in water export of the stabilizing anther tissues (endothecium and connective), which causes the opening of anther locules. Dehydration of the anther is an active process with water being exported along an osmotic gradient generated by starch/sugar conversion (Bonner and Dickinson, 1989). Jasmonic acid was described recently as a factor that might regulate synchronous regulation of pollen maturation, anther dehiscence, and flower opening in Arabidopsis because of active water transportation (Ishiguro et al., 2001), because e.g. water uptake of the filaments from the anther wall (endothecium and epidermis) results in filament elongation and anther dehiscence. Similar to ZmMADS2 antisense plants, the DEFECTIVE IN ANTHER DEHISCENCE jasmonic acid-insensitive mutant of Arabidopsis shows a block in anther dehiscence leading to male sterility (Ishiguro et al., 2001). DELAYED DEHISCENCE encodes another enzyme of the jasmonic acid pathway necessary for timely release of pollen in Arabidopsis (Sanders et al., 2000). Whether ZmMADS2 is a jasmonic acid-responsive gene has to be shown in further experiments.
Increasing amounts of the ZmMADS2 protein were observed in nuclei of endothecial and connective cells during degeneration culminating with highest amounts in apoptotic bodies containing little amounts of DNA. Programmed cell death (PCD) is a very active process occurring during anther maturation (Ku et al., 2003) and requires tight genetic regulation. ZmMADS2 might represent an example of a regulator protein associated with PCD. Interestingly, AGL17, a member of the same monophyletic clade, has been shown to be localized in sloughing cells of the cap of primary and lateral root tips (Burgeff et al., 2002). These cells undergo a process partly similar to PCD.
The finding that the ZmMADS2-GFP fusion protein could not be observed in maize pollen tubes, although transcript amounts have been shown previously to be present (Heuer et al., 2000), indicates that either the transcript is not translated, or GFP signals have not been strong enough for measuring significant fluorescence levels. Maize pollen grows very fast, and cytoplasm is not only found in the tip of the tube, which is the case e.g. for tobacco pollen tubes, but instead along the whole tube. ZmMADS2-GFP fusion protein might be diluted in the large cytoplasm amounts of the tube resulting in insufficient GFP fluorescence. A ZmMADS2-specific antibody is now required to study the presence and localization of the protein in mature pollen and during pollen tube growth.
The role of ZmMADS2 during pollen tube growth is still unclear. Zachgo et al. (1997) suggested that the Antirrhinum majus homolog of ZmMADS2, DEFH125, is secreted from pollen tubes and transported into nuclei of the transmitting tissue. When a pollen tube has passed, cells of the transmitting tract are no longer needed and usually die by desiccation. It remains to be shown in further experiments if Zm-MADS2 is secreted from pollen tubes in vivo to move toward cells of the transmitting tissue or other target cells like the degenerating synergid, which is another example of a plant cell undergoing PCD. The identification of ZmMADS2 target genes and possible interacting partners will now be the next step to elucidate the functional network of this transcriptional regulator during reproduction in both male sporophytic and male gametophytic tissue.
MATERIALS AND METHODS
Plant Material and Stable Transformation of Maize (Zea mays)
Maize plants of the inbred line A188 and tobacco (Nicotiana tabacum; line SR1) were grown in the greenhouse under standard greenhouse conditions at 26°C with 16 h of light and relative humidity of 60%. Anthers of WT and transgenic plants were collected at 1, 2, and 3 d before anthesis and at anthesis. Before DAPI staining, anthers were cut into slices with a razor blade. Isolation and transformation of immature maize embryos of the inbred line A188 was carried out as described by Brettschneider et al. (1997).
Genomic Sequence Analyses, Primer Extension, and DNA Sequencing
The promoter sequence of the ZmMADS2 gene was amplified by genome walking using the GenomeWalker Kit (CLONTECH Laboratories, Palo Alto, CA). Gene-specific primers Tnorf1 (5′-CCTATA GCTAGCTCTCTTCTTGACCCT-3′) and Tnorf2 (5′-TAAGGAGCGAGAGGTTGTGGTTG TGG-3′) were used with a DraI and a PvuII genomic library of maize. DNA fragments were fully sequenced and aligned. The first intron and part of the second intron (see I1 and I2 in Fig. 2a) were amplified by genome walking from a HindII library of maize. Introns 3 to 7 were amplified from genomic DNA of maize inbred line A 188. All fragments were cloned into pCR-Blunt II TOPO (Invitrogen) and sequenced using the ABI PRISM 377 Sequencer (PE-Applied Biosystems, Foster City, CA). Analysis of putative cis-acting elements in the ZmMADS2 promoter was carried out by using the PLACE database (plant cis elements; http://www.dna.affrc.go.jp/htdocs/PLACE/). Repetitive sequences were determined using the DNAStar program (LASERGENE). The ZmMADS2 transcription start point was determined using the Primer Extension System (Promega, Madison, WI). According to the manufacturer's protocol, a (6-fluorescein-6-carboxamido)hexanoate-marked primer (5′-Fam-CAAAGAAGGTAAGGAGGAGGAGAT-3′) was annealed to 1 μg of RNA extracted from mature pollen and the size of the extension product compared with standard markers using an ABI PRISM 377 Sequencer.
The nucleotide sequence data reported are available in the EMBL, GenBank, and DDBJ Nucleotide Sequence Databases under the accession numbers AY227363 and AY264885.
Genomic Southern Blots and Expression Analyses
Extraction of genomic DNA from maize was performed according to Dellaporta et al. (1983). Ten micrograms of genomic DNA was digested with the restriction enzymes indicated and resolved on 0.8% (w/v) agarose gels. DNA was transferred to Hybond N+ membranes (Amersham Biosciences Europe, Freiburg, Germany) with 0.4 m NaOH. Blots were hybridized overnight with radioactive probes prepared using the Prime-It Random Primer Labeling Kit (Stratagene, La Jolla, CA) in QuickHyb (Stratagene) or Church buffer (7% [w/v] SDS, 0.5 m NaH2PO4 [pH 7.2], and 1 mm EDTA) containing 100 μg mL-1 salmon sperm DNA. Filters were washed with decreasing concentrations of SSC with a final wash at 65°C in 0.2× SSC/0.1% (w/v) SDS. Filters were exposed at -70°C to Kodak X-Omat AR (Eastman-Kodak, Rochester, NY) or Hyperfilm MP (Amersham-Pharmacia Biotech) films using intensifier screens. Plant material for northern-blot analyses was collected in the greenhouse from different tissues and organs of the maize inbred line A188. RNA was extracted from all samples with TRIzol (Invitrogen) according to the manufacturer's specification. RNA was separated in 1.5% (w/v) denaturing agarose gels and transferred with 10× SSC onto Amersham Hybond N+ membranes. RNA was cross linked to membranes with 300 mJ of radiation in a UV Stratalinker 1800 (Stratagene). Hybridization, washing, and exposure were performed according to the procedure described for DNA gel blots. For RT-PCR analysis, RNA was prepared using the mRNA DIRECT Micro-Kit (Dynal Biotech, Hamburg, Germany). Anthers stages were freshly collected from the greenhouse, prepared in a petri dish containing Tris-EDTA buffer using fine forceps and a binocular, and immediately frozen in liquid nitrogen. Tissues were collected in 1.5-mL Eppendorf tubes (Eppendorf AG, Hamburg, Germany) and grinded with plastic mortars adding some autoclaved sea sand in the same tubes. RNA preparation was performed following the Dynal protocol. RT was performed using Moloney murine leukemia virus RT (MBI, Fermentas, St. Leon-Rot, Germany) for 1 h at 42°C followed by 10 min at 70°C. Two to 4 μL of the RT reaction was used for a standard PCR with 1 unit of TaqDNA Polymerase (MBI) per reaction.
Constructs for Transformation
ZmMADS2 cDNA was amplified with primers M2Bcl (5′-TGATCATGGGGAGGGGAAA GATC-3′) and M2Xho (5′-CTCGAGTGGAATTAATTGCAATCCTAGC-3′) and integrated in antisense orientation using the BamHI and SalI restriction sites in pUbi-Cass (Christensen and Quial, 1996), resulting in the construct pDNS-4. The ZmMADS2::gfp fusion construct was prepared by amplification of the gfp coding sequence including the ST-LS1 intron and the NOS terminator with primers GfpNco (5′-CCATGGGCAAGGGCGAG-3′) and NoEco (5′-GAATTCCCGATCTA GTAAC-3′) using pMon30049 (Pang et al., 1996) as template. The fragment was integrated in pLitmus 29 (NEB) using NcoI and EcoRI restriction sites. The ZmMADS2 promoter (1.8 kb) including the 5′-UTR was amplified from a genomic library using the primers M2Pf (5′-TCTAGAGAAGCTGTCGTGTTC-3′) and M2Pr1 (5′-GCTAAGGAGCGAGAGGTT-3′). The open reading frame of ZmMADS2 was amplified from pTL1–22 (Heuer et al., 2000) using the primers M2fPE (5′-TCTCGGCTAGCTTCCTC-3′) and M2rBspH (5′-TCATGAGTGGAATTAATTGCAATCCTAGC-3′) and digested with XbaI and BspHI. Promoter and cDNA were cloned into XbaI and NcoI restriction sites of the GFP vector. The resulting plasmid was called pDNS-1. pDNS-4 and pDNS-1 were used for stable transformation of immature maize embryos. ZmMADS2 promoter deletion constructs were generated by inserting fragments of the ZmMADS2 promoter in a slightly modified promoterless luciferase construct (de Wet et al., 1987). An NheI adapter was integrated in the promoterless construct (R. Brettschneider, unpublished data), and the PvuII genome walker fragment (see above) was inserted using the HindIII and NheI restriction sites, respectively. The resulting construct, pDNS-5, was used as a template for amplification of shorter fragments for generation of constructs pDNS-6, -7, and -8. Depending on the template sequence at bp -1,038, -497, and -327, respectively, primers Prom1 (5′-AAGCTTGTAAAGA CCTCGACCGGA A-3′; pDNS-6), Prom2 (5′-AAGCTTAGCTCAAATACGTAACAAAG-3′; pDNS-7), and Prom2′ (5′-AGAAGCTACTACGCCCTGCAG-3′; pDNS-8) were used at the 5′ end to introduce either a PstI or HindIII restriction site. The counterpart primer LUC2 (5′-GCCTTATGCAGTTGCTCTCC-3′) is located in the luciferase coding sequence behind an XbaI site. PCR products were restricted with XbaI and either HindIII or PstI and ligated into the corresponding linearized vector. The ZmMADS2 5′-UTR (255 bp) was isolated with NheI from a cDNA clone of a maize mature pollen library (Heuer et al., 2000) and inserted in correct orientation in pDNS-6. The resulting plasmid, pDNS-6b, was used as a template for amplification of the ZmMADS2 promoter deletion constructs pDNS-9 and -10, respectively. pDNS-9 and pDNS-10 were generated analogously to pDNS-6 to -8 using primers Prom4 (5′-TAGGGGTAATGAGTCTAGACGGC-3′) and Prom5 (5′-GGGGAAAGTCTAGACTCGCA AG-3′), introducing an XbaI site at -210 and -67, respectively. The ZmMADS2-gfp fusion construct used for transient transformation of tobacco pollen was generated by amplification of a ZmMADS2 promoter fragment (corresponding to pDNS-8), the 5′-UTR, the ZmMADS2-cDNA, gfp, and terminator sequence with Prom2′ and a vector primer using pDNS-1 as a template. The resulting product was integrated in pCR-Blunt II TOPO (Invitrogen) as described above. All constructs were fully sequenced before usage. Only constructs without errors were used for transformation experiments.
Transient Transformation of Maize and Tobacco Pollen
Pollen of maize and tobacco were collected from nonstressed greenhouse plants and transformed as described by Schreiber and Dresselhaus (2003). A relatively even spreading of maize pollen was achieved by placing petri dishes containing solid pollen germination medium (PGM) side by side on a tablet about 30 cm under tassels, which were shaken. Best results were obtained when dry pollen was collected in the afternoon from plants that have not been in contact with any pest control after anthesis. Tobacco pollen was collected directly from mature flowers into microfuge tubes and mixed with 2 mL of liquid PGM. We pipetted 200 μL on PGM plates and left them at room temperature for 45 to 60 min. until germination started. PGM was prepared as two times concentrated solution containing 20% (w/v) Suc (Roth), 0.001% (w/v) H3BO3 (Sigma-Aldrich, St. Louis), 20 mm CaCl2 (Sigma-Aldrich), 0.1 mm KH2PO4 (Merck KGaA, Darmstadt, Germany), and 12% (w/v) polyethylene glycol 4000 (Merck KGaA, Darmstadt, Germany).
PGM containing all components was heated to 70°C on a stirring heater, and temperature was held for another 10 min until there was a complete solution of polyethylene glycol. After sterile filtration of 2× PGM, an equal volume of autoclaved 0.6% (w/v) noble agar (Agar Molecular Biology Grade, AppliChem, Darmstadt, Germany) to a final concentration of 0.3% (w/v) was added and poured into petri dishes 3 cm in diameter. Plates were left in a sterile bench with opened lids until PGM was solid and no remnants of liquid remained on the surface of PGM. It was important to stir 2× PGM for at least 10 min at 70°C and to use noble agar instead of other gelling agents like agarose or phytagel. Biolistic transformation and marker gene studies were carried out as described by Schreiber and Dresselhaus (2003).
Analysis of Protein-Protein Interaction
Detection of homodimerization of ZmMADS was carried out using the yeast (Saccharomyces cerevisiae) Hybrid Hunter Two Hybrid System, Version A (Invitrogen) according to the manufacturer's specifications. Yeast transformation was carried out as described by Agatep et al. (1998).
Acknowledgments
We like to thank Reinhold Brettschneider for providing the promoterless luciferase construct and for fruitful discussions. We acknowledge Sigrid Heuer for critical reading of the manuscript, Patricia Lauert for helping with the primer extension analysis, Dr. Rebecca Favaro for helping with the yeasttwo-hybrid experiments, and Dr. Hermann Schmidt (DNA Cloning Service, Hamburg, Germany) for preparing the pDNS-1 and pDNS-4 constructs.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.030577.
This work was supported by the Südwestdeutsche Saatzucht (Rastatt; to D.N.S.) and by the Deutsche Forschungsgemeinschaft (grant DFG Dr 334/2–3 to J.B.).
References
- Agatep R, Kirkpatrick RD, Parchaliuk DL, Woods RA, Gietz RD (1998) Transformation of Saccharomyces cerevisiae by the lithium acetate/single-stranded carrier DNA/polyethylene glycol (LiAc/ss-DNA/PEG) protocol. Technical Tips Online. http://tto.trends.com
- Albertsen MC, Phillips RL (1981) Developmental cytology of 13 genetic male sterile loci in maize. Can J Genet Cytol 23: 195-208 [Google Scholar]
- Alvarez-Buylla ER, Liljegren SJ, Pelaz S, Gold SE, Burgeff C, Ditta GS, Vergara-Silva F, Yanofsky MF (2000) MADS-box gene evolution beyond flowers: expression in pollen, endosperm, guard cells, roots and trichomes. Plant J 24: 457-466 [DOI] [PubMed] [Google Scholar]
- Bate N, Spurr C, Foster GD, Twell D (1996) Maturation-specific translational enhancement mediated by the 5′-UTR of a late pollen transcript. Plant J 10: 613-623 [DOI] [PubMed] [Google Scholar]
- Bate N, Twell D (1998) Functional architecture of a late pollen promoter: pollen-specific transcription is developmentally regulated by multiple stage-specific and co-dependent activator elements. Plant Mol Biol 37: 859-869 [DOI] [PubMed] [Google Scholar]
- Beadle GW (1932) Genes in maize for pollen sterility. Genetics 17: 413-431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedinger P (1992) The remarkable biology of pollen. Plant Cell 4: 879-887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner LJ, Dickinson HG (1989) Anther dehiscence in Lycopersicon esculentum Mill.: I. Structural aspects. New Phytol 113: 97-115 [DOI] [PubMed] [Google Scholar]
- Brettschneider R, Becker D, Lörz H (1997) Efficient transformation of scutellar tissue of immature maize embryos. Theor Appl Genet 94: 737-748 [Google Scholar]
- Burgeff C, Liljegren SJ, Tapia-Lopez R, Yanofsky MF, Alvarez-Buylla ER (2002) MADS-box gene expression in lateral primordia, meristems and differentiated tissues of Arabidopsis thaliana roots. Planta 214: 365-372 [DOI] [PubMed] [Google Scholar]
- Chaudhury AM (1993) Nuclear genes controlling male fertility. Plant Cell 5: 1277-1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PC, Greyson RI, Walden DB (1979) Comparison of anther development in genic male-sterile (ms10) and in male-fertile corn (Zea mays) from light microscopy and scanning electron microscopy. Can J Bot 57: 578-596 [Google Scholar]
- Christensen AH, Quial PH (1996) Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res 5: 213-218 [DOI] [PubMed] [Google Scholar]
- Dawson J, Sozen E, Vizir I, Van Waeyenberge S, Wilson ZA, Mulligan BJ (1999) Characterization and genetic mapping of a mutation (ms35) which prevents another dehiscence in Arabidopsis thaliana by affecting secondary wall thickening in the endothecium. New Phytol 144: 213-222 [Google Scholar]
- Dellaporta SL, Wood VP, Hicks JB (1983) A plant DNA mini-preparation: version II. Plant Mol Biol Rep 1: 19-21 [Google Scholar]
- de Wet JR, Wood KV, DeLuca M, Helinski DR, Subramani S (1987) Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol 7: 725-737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmayan T, Tepfer M (1995) Evaluation in tobacco of the organ specificity and strength of the rol D promoter, domain A of the 35S promoter and the 35S 2 promoter. Transgenic Res 4: 388-396 [DOI] [PubMed] [Google Scholar]
- Fernandez DE, Heck GR, Perry SE, Bleecker AB, Fang S-C (2000) The embryo MADS domain factor AGL15 acts post-embryonically: inhibition of perianth senescence and abscission via constitutive expression. Plant Cell 12: 183-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RB, Beals TP, Sanders PM (1993) Anther development: basic principles and practical applications. Plant Cell 5: 1217-1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson C, Du J-S, de Torres Zabala M, Beggs K, Smith C, Holdsworth M, Bevan M (1994) Separate cis sequences and trans factors direct metabolic and developmental regulation of a potato tuber storage protein gene. Plant J 5: 815-826 [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Schwarz YH, Mascarenhas JP (1998) A monocot pollen-specific promoter contains separable pollen-specific and quantitative elements. Plant Mol Biol 38: 663-669 [DOI] [PubMed] [Google Scholar]
- Heuer S, Lörz H, Dresselhaus T (2000) The MADS box gene ZmMADS2 is specifically expressed in maize pollen and during maize pollen tube growth. Sex Plant Reprod 13: 21-27 [Google Scholar]
- Heuer S, Hansen S, Bantin J, Brettschneider R, Kranz E, Lörz H, Dresselhaus T (2001) The maize MADS box gene ZmMADS3 affects node number and spikelet development and is co-expressed with ZmMADS1 during flower development, in egg cells, and early embryogenesis. Plant Physiol 127: 33-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res 27: 297-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulzink RJM, de Groot PFM, Croes AF, Quaedvlieg W, Twell D, Wullems GJ, van Herpen MMA (2002) The 5′-untranslated region of the ntp303 gene strongly enhances translation during pollen tube growth, but not during pollen maturation. Plant Physiol 129: 342-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y-S, Karrer EE, Thomas BR, Chen L, Rodriguez RL (1998) Three cis-elements required for rice α-amylase Amy3D expression during sugar starvation. Plant Mol Biol 36: 331-341 [DOI] [PubMed] [Google Scholar]
- Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K (2001) The DEFEC- TIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence and flower opening in Arabidopsis. Plant Cell 13: 2191-2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi CP, Zhou H, Huang X, Chiang VL (1997) Context sequences of translation initiation codon in plants. Plant Mol Biol 35: 993-1001 [DOI] [PubMed] [Google Scholar]
- Kapoor S, Kobayashi A, Takatsuji H (2002) Silencing of the tapetum-specific zinc finger gene TAZ1 causes premature degeneration of tapetum and pollen abortion in petunia. Plant Cell 14: 2353-2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesselbach TA (1999) The Structure and Reproduction of Corn: 50th Anniversary Edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 38-48
- Kobayashi A, Sakamoto A, Kubo K, Rybka Z, Kanno Y, Takatsuji H (1998) Seven zinc-finger transcription factors are expressed sequentially during the development of anthers in petunia. Plant J 13: 571-576 [DOI] [PubMed] [Google Scholar]
- Koltunow AM, Truettner J, Cox KH, Wallroth M, Goldberg RB (1990) Different temporal and spatial gene expression patterns occur during anther development. Plant Cell 2: 1201-1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku S, Yoon H, Suh HS, Chung YY (2003) Male-sterility of thermosensitive genic male-sterile rice is associated with premature programmed cell death of the tapetum. Planta 217: 559-565 [DOI] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, McLachlin DT, Chait BT, Chua N-H (2002) ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J 32: 317-328 [DOI] [PubMed] [Google Scholar]
- McCormick S (1993) Male gametophyte development. Plant Cell 5: 1265-1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuffer MG, Coe EH, Wessler SR (1997) Mutants of Maize. Cold Spring Harbor, NY, Cold Spring Harbor Laboratory Press.
- Okada T, Zhang Z, Russel SD, Toriyama K (1999) Localization of the Ca2+-binding protein, Bra r 1, in anthers and pollen tubes. Plant Cell Physiol 40: 1243-1252 [DOI] [PubMed] [Google Scholar]
- Pang S-Z, DeBoer DL, Wan Y, Ye G, Layton JG, Neher K, Armstrong CL, Fry JE, Hinchee MAW, Fromm ME (1996) An improved green fluorescent protein gene as a vital marker in plants. Plant Physiol 112: 893-900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SE (2001) Cutting loose: abscission and dehiscence in Arabidopsis. Plant Physiol 126: 494-500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt K, Frech K, Karas H, Wingender E, Werner T (1995) MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res 23: 4878-4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann JL, Meyerowitz EM (1997) MADS domain proteins in plant development. Biol Chem 378: 1079-1101 [PubMed] [Google Scholar]
- Robson F, Costa MM, Hepworth SR, Vizir I, Pineiro M, Reeves PH, Putterill J, Coupland G (2001) Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J 28: 619-631 [DOI] [PubMed] [Google Scholar]
- Rogers HJ, Bate N, Combe J, Sullivan J, Sweetman J, Swan C, Lonsdale DM, Twell D (2001) Functional analysis of cis-regulatory elements within the promoter of the tobacco late pollen gene g10. Plant Mol Biol 45: 577-585 [DOI] [PubMed] [Google Scholar]
- Sanders PM, Lee PY, Biesgen C, Boone JD, Beals TP, Weiler EW, Goldberg RB (2000) The Arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell 12: 1041-1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satina S, Blakeslee AF (1941) Periclinal chimeras in Datura stramonium in relation to development of leaf and flower. Am J Bot 28: 862-871 [Google Scholar]
- Schreiber DNS, Dresselhaus T (2003) Optimization of in vitro pollen germination and transient transformation of Zea mays and other plant species. Plant Mol Biol Rep 21: 31-41 [Google Scholar]
- Sieburth LE, Meyerowitz EM (1997) Molecular dissection of the AGAMOUS control region shows that cis elements for spatial regulation are located intragenically. Plant Cell 9: 355-365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Ezcurra I, Muschietti J, McCormick S (2002) A cysteine-rich extracellular protein, LAT52, interacts with the extracellular domain of the pollen receptor kinase LePRK2. Plant Cell 14: 2277-2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku K, Umemura T, Yamaguchi J (1998) Promoter elements required for sugar-repression of the RAmy3D gene for alpha-amylase in rice. FEBS Lett 428: 275-280 [DOI] [PubMed] [Google Scholar]
- Twell D, Yamaguchi J, Wing RA, Ushiba J, McCormick S (1991) Promoter analysis of genes that are coordinately expressed during pollen development reveals pollen-specific enhancer sequences and shared regulatory elements. Gen Dev 5: 496-507 [DOI] [PubMed] [Google Scholar]
- Wilson ZA, Morroll SM, Dawson J, Swarup R, Tighe PJ (2001) The Arabidopsis MALE STERILITY1 (MS1) gene is a transcriptional regulator of male gametogenesis, with homology to the PHD-finger family of transcription factors. Plant J 28: 27-39 [DOI] [PubMed] [Google Scholar]
- Xu H, Davies SP, Kwan BYH, O'Brien AP, Singh M, Knox RB (1993) Haploid and diploid expression of a Brassica campestris anther-specific gene promoter in Arabidopsis and tobacco. Mol Gen Genet 239: 58-65 [DOI] [PubMed] [Google Scholar]
- Yang S, Sweetman JP, Amirsadeghi S, Barghchi M, Huttly AK, Chung WI, Twell D (2001) Novel anther-specific myb genes from tobacco as putative regulators of phenylalanine ammonia-lyase expression. Plant Physiol 126: 1738-1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachgo S, Saedler H, Schwarz-Sommer Z (1997) Pollen-specific expression of DEFH125, a MADS-box transcription factor in Antirrhinum with unusual features. Plant J 11: 1043-1050 [DOI] [PubMed] [Google Scholar]
- Zhang H, Forde BG (1998) An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279: 407-409 [DOI] [PubMed] [Google Scholar]